Abstract
Understanding the neurobiological substrates that encode learning about food‐associated cues and how those signals are modulated is of great clinical importance especially in light of the worldwide obesity problem. Inappropriate or maladaptive responses to food‐associated cues can promote over‐consumption, leading to excessive energy intake and weight gain. Chronic exposure to foods rich in fat and sugar alters the reinforcing value of foods and weakens inhibitory neural control, triggering learned, but maladaptive, associations between environmental cues and food rewards. Thus, responses to food‐associated cues can promote cravings and food‐seeking by activating mesocorticolimbic dopamine neurocircuitry, and exert physiological effects including salivation. These responses may be analogous to the cravings experienced by abstaining drug addicts that can trigger relapse into drug self‐administration. Preventing cue‐triggered eating may therefore reduce the over‐consumption seen in obesity and binge‐eating disorder. In this review we discuss recent research examining how cues associated with palatable foods can promote reward‐based feeding behaviours and the potential involvement of appetite‐regulating peptides including leptin, ghrelin, orexin and melanin concentrating hormone. These peptide signals interface with mesolimbic dopaminergic regions including the ventral tegmental area to modulate reactivity to cues associated with palatable foods. Thus, a novel target for anti‐obesity therapeutics is to reduce non‐homeostatic, reward driven eating behaviour, which can be triggered by environmental cues associated with highly palatable, fat and sugar rich foods.
Abbreviations
- BMI
body mass index
- BOLD
blood oxygenation level dependent signal
- CS
conditioned stimulus
- fMRI
functional magnetic resonance imagine
- GHSR
growth hormone secretagogue receptors
- LH
lateral hypothalamus
- MCH
melanin concentrating hormone
- NAc
nucleus accumbens
- NPY
neuropeptide Y
- OFC
orbitofrontal cortex
- OXR
orexin receptor
- PVT
paraventricular nucleus of the thalamus
- US
unconditioned stimulus
- VTA
ventral tegmental area
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Consumption beyond basic homeostatic needs, purely based on the rewarding properties of palatable foods, is proposed to be a central contributor to the current worldwide obesity epidemic (Berthoud, 2012). Until recently, only three anti‐obesity medications were approved by the Food and Drug Administration (USA) for the long‐term clinical management of obesity and related metabolic syndrome. These medications are orlistat (Xenical) which inhibits intestinal lipases to prevent fat absorption, the selective 5‐HT2C receptor agonist lorcaserin (Belviq), which acts by potentiating monoamine signalling in the CNS and the combination of the stimulant phentermine, which suppresses appetite and increases metabolic rate, and extended‐release topiramate (Qsymia). In 2014, the combination of the μ‐opioid antagonist naltrexone plus the noradrenaline and dopamine re‐uptake inhibitor bupropion (both controlled release; Contrave) became the fourth medication approved for long‐term weight management in patients with obesity, and in December 2014, liraglutide (Saxenda), a glucagon‐like peptide 1 receptor agonist, was also approved (Yanovski and Yanovski, 2015).
With increased knowledge of the pathogenesis of obesity, several other potential anti‐obesity agents are being investigated. In humans, cues associated with food powerfully enhance both ratings of appetite and food consumption (Ferriday and Brunstrom, 2008). In particular, improved understanding of the neurocircuitry of appetite and metabolism regulation has provided many potential targets for therapeutic anti‐obesity agents that are currently under development. Thus, a novel target is to reduce non‐homeostatic, reward driven eating behaviour, which can be triggered by environmental cues associated with highly palatable, fat and sugar rich foods.
Eating is essential for survival and is underpinned by the fundamental physiological need to consume energy. However, people often consume in excess of the basic nutrient and energy requirement needed to maintain physiological homeostasis, particularly when there is an abundance of readily available food and drink. Indeed, eating is more often than not controlled by external cues and stimuli, the time of the day and social factors, rather than time since the last meal and the need to replenish energy stores. The modern environment is replete with food‐associated cues that encourage eating in the absence of metabolic demands. In this review, 'cues’ refer to external, environmental cues, such as the sight and smell of particular foods, or locations where certain foods are procured. These cues are capable of generating ‘cue‐reactivity’ – the ability of cues to signal that a reward (or punishment) will be imminently received. Through a process of Pavlovian conditioning, cues that typically predict food intake can trigger cue‐reactivity, a motivational state that may evoke expectations of food or be experienced as an urge to eat (Jansen, 1998), considered separate from the interoceptive cues generated by internal states such as hunger or satiety (Schachter, 1968). Cue‐specific reactions are proposed to evoke cravings for particular foods (Meule et al., 2012; Jastreboff et al., 2013; Meule et al., 2014) in a manner that can be deemed analogous to cue‐evoked cravings in drug addicts (see Perry et al. 2014). People who overeat and have become overweight or obese are thought to develop increased cue‐reactivity through disordered learning histories related to food intake. Thus, obesity is proposed to be associated with abnormal reward processing of palatable foods and cues associated with these foods (Rogers and Hill, 1989; Davis and Fox, 2008; Stice et al.,2008; Stoeckel et al., 2008; Boggiano et al., 2009; Stice et al., 2011b; Stice et al., 2011a; Demos et al., 2012; Murdaugh et al., 2012; Yokum et al., 2014; Jensen and Kirwan, 2015). Furthermore, some of these abnormalities in reward processing may predate obesity while others may be a consequence of overeating (Bello et al., 2002; Yokum et al., 2011; Murdaugh et al., 2012; Burger et al., 2013; Burger et al., 2014).
At a neural level, hypothalamic appetite regulating circuits project to the reward related midbrain dopamine neurocircuitry (DiLeone et al., 2003; Lutter and Nestler, 2009; Yeo and Heisler, 2012) providing an anatomical basis by which the regulation of such reward related feeding might occur. Gut and adipose tissue‐derived signals including peptides such as neuropeptide Y (NPY), cholecystokinin, leptin, insulin and ghrelin, as well as other physical and hormonal signals conveyed by the vagal nerves to the brainstem, are critical determinants of both the metabolic responses to food (post‐ingestive responses such as satiation) and in the initiation of food intake (Havel, 2001). Thus, neural cross talk between homeostatic and reward‐related neurocircuitry permits functional interactions to influence the initiation of ingestive behaviours (Shizgal et al., 2001). This review discusses the behavioural and hormonal correlates of food‐associated cue‐reactivity that may lead to the over‐consumption of foods, and the underlying homeostatic and reward neurocircuitry that interacts to control such reward‐driven feeding behaviours. We discuss modulation of appetite regulatory peptides [ghrelin, orexin, leptin and melanin concentrating hormone (MCH)] as potential therapeutic targets for maladaptive eating behaviours and food cravings that may contribute to obesity.
Food is a natural reinforcer
Reward or positive reinforcement describes the positive value allocated to an object, behavioural act or an internal physical state that induces pleasurable effects. Food and sex are considered primary reinforcers, capable of directing behaviours leading to their procurement, and our desire for them is hardwired due to their intrinsic requirement for survival on a species‐wide basis. Such reinforcers exert many of their effects on behaviour via activation of mesocorticolimbic neurocircuitry, involving projections from the ventral tegmental area (VTA) to areas of the brain such as the nucleus accumbens (NAc), hypothalamus, hippocampus, frontal cortex and central/basomedial amygdala (Fibiger and Phillips, 1988; Kelley and Berridge, 2002; Rada et al., 2005), resulting in the release of neurotransmitters including dopamine, GABA and opioid peptides. Drugs of abuse such as cocaine, alcohol and opioids also activate this reward neurocircuitry, leading to release of dopamine in the mesolimbic pathway (Berridge and Robinson, 1998). Through appetitive (reward‐driven) learning, motivational significance or incentive salience is assigned to cues and responses predictive of rewarding outcomes (Robinson and Berridge, 1993). As such, both food‐ and drug‐associated cues have been shown to activate similar brain regions involved in learning, memory and motivation, including the prefrontal cortex, nucleus accumbens, amygdala, orbitofrontal cortex (OFC) and the striatum (Kalivas, 2000; Vanderschuren and Kalivas, 2000; Hotsenpiller et al., 2001; Kalivas et al., 2005; Tang et al., 2012).
In the addiction literature, a distinction between ‘liking’ and ‘wanting’ rewards has been drawn (Robinson and Berridge, 2003). ‘Wanting’ is the desire to acquire a reward, as opposed to ‘liking’, which is the hedonic (pleasurable) impact of rewards. These constructs are thought to be grounded in distinct neural substrates (Berridge and Robinson, 1998). The neural substrates underlying liking involve the NAc, ventral pallidum and OFC, and primarily GABAergic, opioid and endocannabinoid neurotransmission (Berridge, 2009). ‘Wanting’ is related to motivational influences on behaviour and has been associated with dopamine signalling in the mesolimbic region, as well as its connections with the prefrontal cortex and amygdala (Barbano and Cador, 2007; Salamone and Correa, 2009; Mahler and Berridge, 2012a). In rats, elevating NAc dopamine had no effect on hedonic responding for sucrose, but increased lever pressing for sucrose, indicative of enhanced wanting (Wyvell and Berridge, 2000), supporting the proposal that dopamine can mediate reward‐related cues to trigger ‘wanting’, but not the hedonic appraisal of palatable foods. In contrast, opioid agonists were shown to influence the hedonic appraisal of palatable rewards, but did not alter the motivation to lever press for food rewards (Pecina and Berridge, 2005).
Cues become associated with palatable foods to control behaviour
In Pavlovian conditioning, associations are formed between an initially neutral environmental cue, [the conditioned stimulus (CS)], with a natural reinforcer, such as food, [the unconditioned stimulus (US)]. These associations enable the cue (CS) to promote consumption, even in sated animals, potentially influencing homeostatic signals by increasing feelings of hunger (Weingarten, 1983; Cornell et al., 1989; Petrovich, 2011). Alternatively, cues may override satiety signals (Schachter, 1968; Petrovich et al., 2002), as the food‐associated cues have been imbued with incentive salience – the ‘wanting’ attributed to reward‐predicting stimuli (Robinson and Berridge, 1993; Berridge, 2007), which can then direct behaviour to procure food. Dopamine neurons in the VTA show phasic activations to food delivery and food‐predicting cues (Schultz, 1998a; Schultz, 1998b). Thus, cues associated with foods are assigned incentive value, and motivating events are signalled through phasic dopamine release (Schultz, 1998a; Schultz, 1998b; Brown et al., 2011; Flagel et al., 2011b; McCutcheon et al., 2012).
Through predictive associations with rewarding foods, cues are able to evoke food‐seeking behaviours, including the performance of a specific action, such as pressing a lever to get food. Animals will learn that a certain cue signals that a food reward is available in a food hopper, and will direct behaviour to the hopper when this cue is presented (known as goal‐tracking). Alternatively, animals may direct their behaviour to the cue that predicts food delivery (sign‐tracking). These behaviours typically depend on the relative ‘value’ of the food reward, which determines how much effort will be expended to obtain the outcome. Thus, performance of associated responses is dependent on the internal motivational state to obtain a particular reward. The motivational properties of a food can be reduced, or devalued, by its consumption (sensory‐specific satiety) or by its pairing with toxin‐induced sickness (Dickinson and Balleine, 1990; Balleine and Dickinson, 1998). Following the devaluation procedure, animals typically show reduced levels of food‐seeking behaviours as the food outcome is no longer valued. This food‐value mediated behavioural control enables animals to adapt to changing environmental conditions. The brain regions involved in the attribution of incentive value to cues predictive of food rewards include the basolateral amygdala, OFC and NAc. Excitotoxic lesions to these regions in rats that had learnt to associate particular cues with foods resulted in an inability to control food‐seeking responses according to the value of a food following its devaluation (Pickens et al., 2003, 2005; Ostlund and Balleine, 2007a, 2007b; Johnson et al., 2009). Our recent research has demonstrated that rats exposed to palatable high fat/high sugar foods were impaired at controlling food‐seeking responses to palatable food‐associated cues following devaluation by sensory‐specific satiety (Reichelt et al., 2014). This indicates that excessive consumption of obesogenic diets can impair responding to cues associated with palatable foods based on their current value, suggesting that these diets cause changes to reward systems in the brain.
Neural systems involved in cue‐triggered food‐seeking behaviour
Dopamine signalling is crucial in the formation of associations between cues and food rewards (Bassareo and Di Chiara, 1999a,1999b). In Pavlovian conditioning, delivery of food is not contingent on behaviour; nevertheless, animals, such as rats, typically approach and contact a cue that is paired with the palatable food (Davey and Cleland, 1982). These cue‐directed behaviours (also known as sign‐tracking; Flagel et al., 2011a) are thought to reflect the cue acquiring incentive salience (motivational value) as a result of its pairing with the food (Berridge, 2004). During Pavlovian conditioning, cue‐directed sign‐tracking behaviours and food directed goal‐tracking behaviours (e.g. approaches to the food hopper location) can be expressed differentially within a group of animals, some animals showing more sign‐tracking and others more goal‐tracking behaviours. Pronounced sign‐tracking behaviour is assumed to index a greater incentive value to the outcome predictive cue. Substance abuse and addiction are associated with increased sensitivity to drug‐related cues, perhaps resulting from sensitization of midbrain dopamine neurons in response to drugs that imbues the drug‐related stimuli with excessive incentive salience (Robinson and Berridge, 1993, 2001). Recently, a study demonstrated that rats that showed greatest sensitivity to food‐related cues in the form of sign‐tracking and goal‐tracking responses to a sucrose‐paired cue gained more weight when exposed to a palatable junk food diet (Robinson et al., 2015). The greater levels of cue‐triggered attraction to sucrose‐associated stimuli during Pavlovian conditioning indicated enhanced incentive motivation in rats that became obese, as opposed to rats that were obesity resistant. Thus, those rats that attributed greater incentive value to the sucrose predicting cue were more likely to become obese (Robinson et al., 2015).
Sign‐tracking has been shown to be mediated by the NAc and its dopaminergic inputs from the VTA (Flagel et al., 2011a; Saunders and Robinson, 2012; Lopez et al., 2015). Injection of the dopamine receptor antagonist flupenthixol into the NAc core markedly impaired the expression of a sign‐tracking conditioned response, but not a goal‐tracking conditioned response (Saunders and Robinson, 2012). Thus, dopamine signalling in the NAc core appears particularly important for attributing incentive salience to reward‐predicting cues. Lesions of the NAc in rats, via 6‐OHDA induced dopamine depletion, impaired both acquisition and expression of a cue‐food association, as demonstrated by cue elicited approach behaviour to a food hopper in the form of goal‐tracking (Cardinal et al., 2002). Furthermore, a recent study by Darvas et al. (2014) showed that Slc6a3Cre/+:Th knock‐out mice with severe depletion of striatal dopamine (only ∼5% of wild type levels, but sufficient dopamine for normal feeding behaviour and locomotion), were impaired in the acquisition of a goal‐tracking conditioned food‐seeking response to the food hopper when a food cue was presented. Slc6a3Cre/+:Th knock‐out mice with 30% of wild‐type dopamine levels were able to perform the task comparably to wild type controls (Darvas et al., 2014). This implicates dopamine in the acquisition of a conditioned food‐seeking response; however these observations indicate that dopamine in the accumbens and striatum may play different roles in the mechanisms of acquisition versus expression of food‐seeking responses.
Cue‐potentiated feeding
A range of animal studies has demonstrated that food‐associated cues are capable of promoting eating in the absence of metabolic requirements (Weingarten, 1983; Petrovich, 2005, 2007; Reppucci and Petrovich, 2012). Hungry rats (or mice) are first trained to associate a cue such as a sound or a distinct context with deliveries of a palatable food. The rats are then permitted to freely feed on chow in their home cages until their body weights have returned to normal. To test for cue‐potentiated feeding, rats are returned to the training chambers and undergo explicit satiation on the trained food outcome (Petrovich et al. 2005, 2007), and then tested for cue‐induced feeding by being exposed to the discrete cue in the presence of the palatable food, or they are tested with that food in its associated context. Such rats eat more of the palatable food when the cue is presented compared to no cue, or in a neutral context (Petrovich et al., 2005, 2007; Reppucci and Petrovich, 2012). The cue does not potentiate feeding by promoting conditioned approach behaviours to the food hopper where the food was delivered during training; rats were shown to also overeat when the food was available in a different location in the test chamber (Holland and Gallagher, 2003).
Food‐associated cues can promote eating behaviours in humans
Cues predictive of food availability are powerful modulators of appetite. Food‐related cues generate expectancy of reward delivery through phasic activation of mesocorticolimbic dopaminergic signalling (Schultz, 1998a; Schultz, 1998b), leading to cravings for foods even when sated (Blum et al., 2011). In humans, normal weight participants ate pizza or ice cream, despite reporting satiety after previously consuming these foods to satiety (Cornell et al., 1989). The mere presence of these palatable foods enhanced the reported desire for them and the amount consumed correlated with the reported desire for the food (Cornell et al., 1989) indicating that the wide availability of palatable foods is likely to promote over‐consumption. In pre‐school children, discrete audio‐visual cues (a light and a song) presented immediately prior to the delivery of snack foods in a particular classroom evoked greater consumption than unpaired cues in a different classroom (Birch et al., 1989). More recently, Grenard et al. (2013) reported that food cues increased snacking in adolescents, as did social cues such as being with friends. Moreover, the presence of palatable foods attracted attention and increased craving in adult women with no eating behaviour issues. However, the size of these effects was greatly increased in adults with a history of sub‐clinical binge‐eating behaviours (Sobik et al., 2005), and greater responsiveness to food cues in adult restrained eaters correlated with ratings of cravings and increased consumption of desired foods (Fedoroff et al., 1997; van Koningsbruggen et al., 2012).
Neuroimaging studies indicate differential responsiveness to food‐associated cues in obese and obesity‐prone individuals
Hyper‐responsiveness to food cues may be underpinned by the assignment of an increased incentive value to highly palatable, energy dense foods, driving over‐consumption through augmented reward neurocircuitry (Dagher, 2009; Volkow et al., 2012; Stice et al., 2013). Studies in humans utilizing functional magnetic resonance imaging (fMRI) have enabled monitoring of effects of food‐associated cues on a correlate of neuronal activity (BOLD response) in reward‐related brain regions, allowing investigations into whether obesity modulates measures of brain activation. Increased BOLD responses on presentation of food cues have been observed in obese individuals in reward processing brain regions and in brain regions involved in control over motivated behaviours, such as the insula and ventrolateral prefrontal cortex, indicating hyper‐responsiveness (Rothemund et al., 2007; Stice et al., 2008; Davids et al., 2010; Martin et al., 2010; Gearhardt et al., 2011). Weaker striatal activation was observed during food intake, suggesting that activity in brain regions capable of overriding cue‐induced cravings is also decreased by obesity, potentially permitting overeating (Stice et al., 2008). Obese individuals exhibited increased corticolimbic‐striatal activation in response to food cues that correlated with reports of increased cravings (Jastreboff et al., 2013) and mesolimbic activity following the presentation of food cues positively correlated with BMI (Loeber et al., 2012). Presentation of high‐calorie food cues increased the mesolimbic BOLD response in obese women in comparison to normal weight controls (Rothemund et al., 2007), suggesting obesity changes food cue‐reactivity. However, recent research has indicated that the propensity for learning associations between cues paired with food rewards was predictive of future weight gain (Burger and Stice, 2014). This research thus provides evidence that individuals who show increased neural responsiveness to food cues are more vulnerable to excess weight gain. Similarly, rats who showed greater sign‐tracking responses to sucrose‐paired cues were most likely to gain excessive weight when fed an energy rich junk food diet (Robinson et al., 2015).
Summary: Reward systems in cue‐triggered food‐seeking behaviour
Consumption in response to food‐associated cues in the absence of metabolic need can be viewed in terms of the distinction between ‘wanting’ and ‘liking’ (Robinson and Berridge, 2000), and some comparison may be drawn to the concept of incentive salience in drug addiction. Incentive salience theory suggests that repeated exposure to drugs of abuse alters the neural mechanisms of reward signalling and that natural reinforcers may have similar effects. It has, however, not yet been demonstrated whether abnormal responding to food cues is due to the repeated exposure prior to the onset of obesity or as a result of obesity. Studies suggest that there are differences in food reward processing between overweight and lean individuals and the way they respond to food‐associated cues (Volkow et al., 2008, 2012). Thus maladaptive responses to cues present in the environment may promote consumption and increase food‐cue‐reactivity, driving over‐consumption (Davidson and Swithers, 2004). Importantly cues can become associated with the sensory as well as the affective/motivational properties of the food (Parkinson et al., 2001) and so can direct behaviour in a specific manner. As shown in Figure 1, over‐consumption may be due to a shift to increased wanting following repeated exposure to palatable foods and cues predictive of delivery of food reward. This is perhaps analogous to the cue‐reactivity experienced by abstaining drug addicts when presented with drug associated cues (Tiffany, 1990), and in dietary‐restraining individuals (Green et al., 2000).
Figure 1.
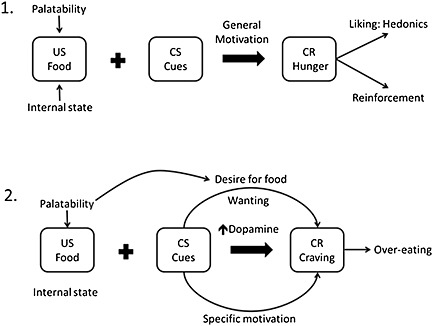
Development of cue‐induced control of non‐homeostatic feeding behaviour that may underpin over‐eating based on incentive salience. 1. Food acts as an US due to its naturally reinforcing capacity. The internal rewarding properties of the food‐reward are influenced by the palatability (hedonics) of the food and also by internal homeostatic state, which is modulated by levels of hunger and motivation to evoke a general motivated state. Pairings of food (US) with a conditioned stimulus (CS = external cue) leads to the stimulation of a conditioned response (CR), in this case hunger, which generates hedonic appraisal of the event and reinforcement. 2. Following repeated pairings with highly palatable and intrinsically rewarding foods, presentation of cues induce phasic dopamine release (Schultz, 1998a) that is capable of generating wanting for the food in the absence of internal homeostatic demands. Cues are therefore able to generate a specific motivational drive in the absence of homeostatic or metabolic demands. This motivational state induces cravings, which can promote food‐seeking behaviours and over‐eating that can lead to obesity.
Role of appetite regulating hormones in the control of reward‐driven feeding
Hunger enhances attention to food‐associated stimuli (Stockburger et al., 2009). Exposure to palatable foods stimulates consummatory reflexes such as salivation, enhances reported hunger sensations and the desire for food in people (Hill et al., 1984; Hardman et al., 2014) and increases the release of dopamine in the dorsal striatum (Volkow et al., 2002). Thus, reward‐driven feeding behaviours and regulation of energy homeostasis are intrinsically linked. In the following sections the roles of peripheral (ghrelin, leptin) and central (orexin, MCH) signalling with respect to cue‐induced stimulation of feeding behaviours will be discussed.
Both human and animal studies demonstrate that environments containing food‐associated cues can generate eating in the absence of metabolic requirements. Homeostatic control of eating is co‐ordinated by a network of appetite‐controlling peptides that link peripheral (gastrointestinal) and central (neural) components of behaviour. The appetite‐controlling system (Schwartz et al., 2000) includes peripherally secreted hormones that stimulate feeding such as ghrelin, and the ‘adipostat’ hormones insulin and leptin, as well as centrally released orexigenic mediators NPY, orexins (hypocretins) and MCH. In the brain, the hypothalamus is thought to function as an energy sensing system interfacing with the reward‐related mesocorticolimbic dopamine system via secretion of hypothalamic neuropeptides including orexins and NPY (Cone et al., 2014). However, despite NPY's potent stimulatory effect on feeding (Hansen and Morris, 2002; Israel et al., 2005; Kohno and Yada, 2012), NPY has no observed excitatory effect on midbrain dopamine neurons (Quarta and Smolders, 2014), and there is no evidence currently that food‐associated cues modulate NPY release.
The interplay with mesolimbic reward‐associated pathways and lateral hypothalamic (LH) regulation of energy homeostasis by endocrine feedback signals has not yet been widely investigated. However, cue‐stimulated peptide release may have modulatory effects on the hedonic system. In particular, the neuropeptides MCH and orexin interact with mesolimbic dopamine transmission to promote phasic dopamine release during food consumption, and ghrelin‐evoked stimulation of the VTA increases the motivation to procure food rewards (Patyal et al., 2012; Cone et al., 2014). Furthermore, leptin has been shown to interact with reward systems and influence drug seeking behaviour including craving induced through alcohol withdrawal (Kiefer et al., 2001). This cue‐stimulated peptide regulation of the activity of the mesolimbic dopamine pathway may represent a potential mechanism for regulating food craving and non‐homeostatic feeding.
Leptin signalling controls satiety and reward‐driven feeding
Leptin, a hormone secreted by fat cells, suppresses food intake and promotes weight loss. Leptin is generally thought to control appetite by activation of receptors in the arcuate nucleus that inhibit release of orexigens such as NPY and agouti releasing peptide, while stimulating the release of anorexigens such as pro‐opiomelanocortin. As obese individuals commonly exhibit increased serum levels of leptin, hyperphagia in obesity is attributed to insensitivity to circulating leptin and disruption of leptin signalling in the hypothalamus (El‐Haschimi et al., 2000; Munzberg et al., 2004; Enriori et al., 2007). Leptin also binds to specific leptin receptors on dopaminergic neurons in the VTA, inhibiting dopamine signalling in the NAc (Figlewicz et al., 2003; Hommel et al., 2006; Trinko et al., 2011) and the central nucleus of the amygdala (Leshan et al., 2010), providing a regulatory link between energy homeostasis and reward‐related behaviour (Fulton et al., 2006).
Although leptin acts as a satiety signal (Trinko et al., 2011; Kanoski et al., 2012), it has also been shown that intraventricular leptin reduces the self‐administration of sucrose (Figlewicz et al., 2003; Hommel et al., 2006) and decreased the rewarding effects of drugs of abuse such as opioids (Lim et al., 2014). Furthermore, intracerebral infusion of leptin has been found to attenuate the reward value of intracranial self‐stimulation in rats (Fulton et al., 2000). However, leptin is not released in response to discrete cues associated with palatable foods, but may modulate cue‐reactivity, providing a potential pharmacological mechanism to reduce cue‐evoked food‐seeking. Plasma leptin concentrations have been shown to positively correlate with reports of withdrawal‐induced craving for alcohol in alcoholic subjects (Kiefer et al., 2001) and nicotine in withdrawn tobacco smokers (von der Goltz et al., 2010), strengthening the hypothesis that leptin interacts with mesolimbic reward pathways. Furthermore, leptin resistance in the VTA might be an important factor in the development of dietary induced obesity; high fat diet fed rats that became obese were less sensitive to the physiological effects of leptin in the VTA than high fat diet fed rats that were resistant to obesity (Bruijnzeel et al., 2013). Thus, leptin resistance in the VTA could be a risk factor for uncontrolled eating, bingeing and food cravings that potentiate the development of obesity (Bruijnzeel et al., 2013). A recent neuroimaging study demonstrated a significant positive correlation between plasma leptin concentration, BMI and the ventral striatal fMRI BOLD response during visual food cue presentations (Loeber et al., 2012). However, this provides only correlational evidence to suggest food cue‐reactivity may be associated with leptin, and increased leptin would typically promote satiety under physiological conditions.
Ghrelin signalling acts as an interface between physiological and reward signalling
Ghrelin is associated with the regulation of appetite and feeding behaviour; in particular, it acts as a hunger signal. Ghrelin is synthesized primarily in the gastrointestinal system and binds to growth hormone secretagogue receptors (GHSRs) that are expressed throughout the brain and also in adipose tissue (Zigman et al., 2006). Systemic administration of ghrelin increases food intake and shifts preference toward diets rich in fat (Egecioglu et al., 2010; Perello et al., 2010). Recent research has demonstrated that ghrelin acts as a key interface between VTA dopamine signalling and physiological (hunger) signals emanating from the LH, provoking food‐seeking behaviour (Cone et al., 2014). Ghrelin controls motivation to eat via its interaction with the mesolimbic dopamine circuitry (Abizaid et al., 2006; Overduin et al., 2012), while leaving the hedonic value of the food eaten unaffected, as measured by analysis of licking microstructure (initial lick rates or lick‐cluster size) (Overduin et al., 2012). GHSRs are expressed throughout the midbrain dopamine system (Guan et al., 1997; Zigman et al., 2006). This suggests that ghrelin may have a role in reward regulation, perhaps via activation of a projection from the laterodorsal tegmental area onto the dopaminergic VTA neurons that project to the NAc (Naleid et al., 2005), thereby affecting the reinforcing properties of both natural rewards and addictive drugs (Engel and Jerlhag, 2014).
Ghrelin mediates the ability of food‐associated cues to evoke feeding behaviours, as blockade of ghrelin signalling through GHSRs by antagonist administration in mice disrupted cue‐potentiated feeding behaviour (Walker et al., 2012). Intriguingly, GHSR knock out mice exhibited cue‐potentiated feeding, indicating that compensatory mechanisms may control motivated food‐cue associations in the absence of ghrelin signalling (Walker et al., 2012). Furthermore, both food‐associated cues and the systemic administration of ghrelin activated dorso‐ and ventromedial hypothalamic nuclei in rats, evoking stereotypical locomotor hyperactivity defined as ‘food anticipatory activity’ (van der Plasse et al., 2013), indicating recruitment of the same medial hypothalamic neuronal circuitry. Recently, it was demonstrated that following acquisition of a Pavlovian discrimination in which one cue predicted the delivery of rewarding food (CS+) and a second cue predicted nothing (CS−), infusion of ghrelin into the lateral ventricle augmented both phasic dopamine measured by voltammetry and phasic increases in the activity of NAc neurons measured by electrophysiology evoked by the presentation of the CS+, but not the CS− (Cone et al., 2015). This indicates that centrally administered ghrelin is able to increase mesolimbic signalling evoked by motivationally significant stimuli (Cone et al., 2014).
Orexinergic signalling modulates cue‐driven food‐seeking responses
Orexins are neuropeptides that stimulate feeding behaviours (Clegg et al., 2002; Barson et al., 2013) and underpin the performance of instrumental behaviours aimed at procurement of both food and drugs (Lawrence et al., 2006; Harris et al., 2007; Choi et al., 2010; Mahler et al., 2014). The lateral hypothalamic orexin/hypocretin system is therefore another candidate in the modulation of reward‐based feeding behaviour (Kay et al., 2014; Williams, 2014). There are two known forms of orexin – orexin A and orexin B, and two known receptors, OX1 and OX2. The OX1 receptor is predominantly involved in reward and feeding behaviours and orexin A has a high affinity for this receptor subtype.
Cue triggered reinstatement of reward seeking following extinction of responding involves activity of VTA dopamine neurons (Mahler and Aston‐Jones, 2012b). This dopaminergic activity is modulated by simultaneous orexin and glutamate transmission in VTA to promote cue‐triggered reinstatement of drug seeking (Mahler et al., 2013). Orexin antagonists reduce cue‐induced reinstatement of nicotine (Plaza‐Zabala et al., 2013), cocaine (Mahler et al., 2013) and heroin seeking behaviours (Smith et al., 2012) but not of ethanol seeking (Brown et al., 2013). Effects of orexin antagonists on sucrose‐seeking have been less clear; systemic OX1 receptor antagonists prevent cue‐induced sucrose seeking when rats are food deprived and therefore are highly motivated to procure food rewards, but had little effect on rats fed ad libitum (Cason et al., 2013). Orexin A promotes motivation for salient reinforcers as antagonism of OX1 receptors reduced responding when high levels of effort were required for seeking cocaine or high fat pellets in self‐administration protocols as opposed to during a less effortful schedule (Borgland et al., 2009).
Orexinergic activity in the LH is induced by a discrete cue associated with food delivery in the absence of food or physiological hunger (Reppucci et al., 2012). This indicates that orexinergic neurons are activated by both hunger and cues that induce feeding in the absence of hunger. Orexin‐secreting neurons in the LH project to the paraventricular nucleus of the thalamus (PVT). The PVT acts as a relay between LH controlled feeding behaviour and reward‐related feeding controlled by the mesolimbic system (Kirouac et al., 2005; Parsons et al., 2007) and the striatum (Martin‐Fardon and Boutrel, 2012). Cues associated with palatable food activate LH orexin neurons that project to the PVT inducing striatal NAc dopamine release and food‐seeking behaviour (Choi et al., 2010). The orexin system appears to drive compulsive consumption of a rewarding stimulus, and binge‐like consumption (Alcaraz‐Iborra and Cubero, 2015). Thus, taken together, the capacity of orexin to regulate the drive to procure food rewards suggests that the orexin system may be a novel target for suppression of feeding behaviour driven by reward‐related cues.
Melanocortin hormone signalling and the control of cue‐evoked food‐seeking behaviour
Subsets of orexin neurons in the LH also secrete the neuropeptide MCH, which, like orexins, innervates a range of structures involved in feeding (Broberger et al., 1998; Hanada et al., 2000). Orexin and MCH both promote palatable food intake and their release is stimulated by food ingestion. However, the ingestive action of orexins and MCH occurs through different, albeit complementary effects on behaviour (Barson et al., 2013; Nishimura et al., 2014), with orexins promoting food‐seeking and motivation to procure palatable foods, indicated by orexin neuron activity in anticipation of feeding (Mizushige et al., 2006), and MCH release occurring once food intake has occurred, so promoting ongoing food intake (Petrovich et al., 2012).
MCH is also capable of promoting food‐seeking behaviours and appears to have a role in the control of reward‐driven feeding. MCH secreting neurons located in the LH have input to reward areas, including the NAc (Georgescu et al., 2005; Sears et al., 2010), where MCH is thought to modulate the rewarding aspects of feeding. By enhancing activity in the NAc, MCH neurons may play a unique role in enhancing the hedonic value of food, and by receiving inputs from the NAc, MCH neurons may participate in a self‐reinforcing circuit that can support feeding (Saper et al., 2002). MCH receptors are important in the motivation to perform food reinforced instrumental responses. However, the role of MCH receptors in cue‐induced reinstatement of food‐seeking following extinction is unclear (Nair et al., 2009). MCH1 receptor knock‐out mice are able to acquire an appetitive Pavlovian discrimination, but showed no preference for responding on a novel nosepoke response for presentations of the cue paired with food rewards (Sherwood et al., 2012). This impairment in conditioned reinforcement was also observed in mice treated with MCH1 receptor antagonists before the same conditioned reinforcement test procedure (Sherwood et al., 2012). These results suggest an important role for MCH in controlling behaviour based on the conditioned reinforcing value of a cue associated with a food reward. However, in a cue‐potentiated feeding setting, very few MCH neurones in the LH showed cFos co‐activation in comparison to orexin neurons, indicating that MCH signalling in the LH may not be activated by food‐associated cues (Reppucci et al., 2012).
Summary – Food cues can influence aspects of appetite‐regulating peptide signalling
Food‐associated cues exert powerful influences on ingestive behaviours; they elicit the desire to procure hedonically attractive foods, overpower homeostatic signals of satiety and even generate hunger, leading to eating outside of basic physiological requirements and promoting over‐consumption. Moreover, evidence indicates that eating in response to such cues leaves intact the eating that occurs in the animal's home cage (Petrovich et al., 2007). However, it is not yet known whether acute responding to food cues can cause long‐term overeating and obesity. As shown in Figure 2, food‐associated cues are capable of influencing dopaminergic reward neurocircuitry, and the hypothalamus acts as a critical relay centre to provide an interface between homeostatic signals and mesolimbic dopamine activity.
Figure 2.
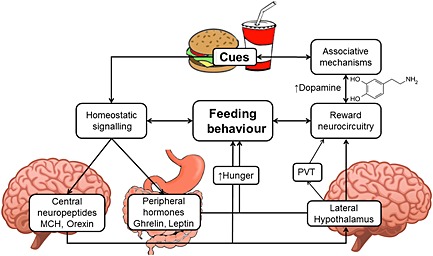
Food‐associated cues evoke feeding behaviours via effects on both homeostatic appetite regulating signals and reward neurocircuitry. Palatable food‐associated cues are assigned incentive salience and are capable of activating reward neurocircuitry (in particular, the mesolimbic dopamine system) leading to cravings and promotion of feeding behaviour in absence of metabolic demands. Appetite‐regulating peptides including ghrelin, orexin and MCH are capable of promoting hunger and evoke feeding behaviour; leptin and insulin signal satiety, and these signals interface with reward neurocircuitry via the lateral hypothalamus and through specific receptors located in the VTA. Satiety promoting hormones leptin and insulin may promote abnormal responsiveness to food cues by enhancing learning about sources of palatable food following ingestion, via specific hippocampal receptors and these are also influenced by orexigenic peptides. However, obesity induces insensitivity to insulin and leptin and little evidence indicates their cue‐conditioned anticipatory release. Food‐associated cues therefore may be capable of influencing appetite promoting peptide signals that are able to interface with reward neurocircuitry.
Appetite‐regulating hormones as potential therapeutic strategies to reduce cue‐related feeding
Environments containing food‐associated cues can generate eating in the absence of metabolic requirements. Reducing this non‐homeostatic, reward driven consumption may provide a novel therapeutic strategy for obesity. Neuroendocrine signals such as leptin, insulin, MCH, orexins and ghrelin are involved in hunger and satiety signalling as well as reward‐related neurocircuitry including mesolimbic dopamine transmission. Neuroimaging studies have shown alterations in neuronal activation when food‐associated cues are presented; these changes in neuronal activity correlate with observed levels of leptin and insulin due to increased energy intake, adipose deposits and obesity. Furthermore, Kroemer et al. (2013) reported that fasting ghrelin levels correlated positively with BOLD responses to food cues in the hypothalamus and midbrain reward associated areas. Ghrelin also correlated with reports of subjective appetite during presentation of food cues, suggesting that it may enhance subjective craving elicited by such cues (Kroemer et al., 2013). The ensuing physiological and neural changes in obesity may therefore lead to a vicious cycle of high energy food consumption, augmenting weight gain and adiposity. The synergistic relationship between homeostatic hormonal signalling may further promote dysregulation of dopamine, increasing cue‐reactivity. Furthermore, hypoactivity in inhibitory brain regions influencing top‐down behavioural control (Stice et al., 2008) may further promote craving via exposure to food‐associated cues leading to a vicious cycle of unrestrained eating.
Studies that have investigated reductions in cue‐evoked feeding using peptide and non‐peptide antagonists have reported conflicting results, and differences in stability and tissue penetration may account for this discrepancy. Blockade of peripheral GHSRs is currently a pharmacological target for treatment of excessive adiposity in obesity, based on their involvement in regulating fat metabolism (Patterson et al., 2011). Blockade of central GHSRs with non‐peptide antagonists may also be useful in reducing reward‐driven consumption by decreasing subjective craving when confronted with palatable food‐associated cues. Preclinical trials have provided evidence that antagonism of GHSRs with orally administered Compound 26 (also known as LXG‐9342) blocked cue potentiated feeding in sated mice (Walker et al., 2012), suggesting that GHSR antagonists may reduce this aspect of cue‐evoked eating behaviours.
Antagonism of orexin signalling is currently being investigated as a potential therapeutic target for drug addiction. Orexin antagonists prevent cue‐induced reinstatement of heroin seeking (Smith and Aston‐Jones, 2012), and there is some evidence that the OX1 receptor antagonist SB334867 attenuates cue‐induced reinstatement of sucrose seeking in male rats (Cason and Aston‐Jones, 2013). However, SB334867 failed to reduce cue‐induced reinstatement of sucrose seeking in female rats, suggesting that food‐seeking induced by cues engages the orexin system differentially in males and females, and sex‐hormone signalling may further mediate cue‐evoked food‐seeking (Cason and Aston‐Jones, 2014).
The peptide MCH1 receptor antagonist PMC‐3881‐PI decreases conditioned responding to food‐associated cues (Sherwood et al., 2012), and systemic injections of the non‐peptide MCH1 receptor antagonist, GW803430, decreased cue‐induced reinstatement of sucrose seeking in rats provided with free access to chow (Karlsson et al., 2012). However, systemic injections of the non‐peptide MCH1 receptor antagonist SNAP 94847 reduced the motivation to lever presses for food pellets, but had no effect on cue induced reinstatement of high fat food‐seeking following extinction of operant responding (Nair et al., 2009). Thus, further studies should investigate the efficacy of different peptide and non‐peptide antagonists as potential therapeutic strategies.
Conclusions
The manner by which food‐associated cues stimulate seeking and eating food involves both reward driven and neuroendocrine mechanisms. From this standpoint, the interaction of homeostatic and reward‐related pathways in the regulation of feeding behaviours should be considered an integrated functional system, and therefore regulation of appetite mediating peptides may provide an alternative approach to the treatment of obesity. However, whether there are alterations in sensitivity between homeostatic mechanisms for sensing energy deficiencies in those who overeat and have become obese compared to those who eat to maintain energy homeostasis presents a significant obstacle to the treatment of obesity through pharmacological approaches. This question poses an additional level of complexity in the modulation of cue‐induced eating behaviour as both underlying reward processing and homeostatic systems are altered in obesity. The satiety promoting peptide leptin also has important roles in feeding behaviour and reward driven behaviours. However, leptin resistance develops in obesity due to receptor insensitivity or down regulation. Thus treatment strategies focusing on leptin as a target have been less successful. From the research presented in this review, modulation of ghrelin, MCH and orexin signalling appears to be critically linked with cue‐driven responsiveness of reward neurocircuitry and thus may provide a novel way of suppressing reward‐driven feeding behaviours in responses to environmental cues. Interventions that target reward systems and appetite‐regulating systems in combination may therefore prove more effective in the treatment of obesity and disordered eating by diminishing the impact of food‐associated cues that can promote overconsumption.
Conflict of interest
Authors declare that they have not any conflict of interest.
Acknowledgements
ACR is the recipient of an Australian Research Council Discovery Early Career Research Award (project number DE140101071).
Reichelt, A. C. , Westbrook, R. F. , and Morris, M. J. (2015) Integration of reward signalling and appetite regulating peptide systems in the control of food‐cue responses. British Journal of Pharmacology, 172: 5225–5238. doi: 10.1111/bph.13321.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD et al. (2006). Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz‐Iborra M, Cubero I (2015). Do Orexins contribute to impulsivity‐driven binge consumption of rewarding stimulus and transition to drug/food dependence? Pharmacol Biochem Behav 134: 31–34. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013). The Concise Guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A (1998). Goal‐directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37: 407–419. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M (2007). Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 191: 497–506. [DOI] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF (2013). Complementary roles of orexin and melanin‐concentrating hormone in feeding behavior. Int J Endocrinol 2013: 983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G (1999a). Differential responsiveness of dopamine transmission to food‐stimuli in nucleus accumbens shell/core compartments. Neuroscience 89: 637–641. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G (1999b). Modulation of feeding‐induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11: 4389–4397. [DOI] [PubMed] [Google Scholar]
- Bello NT, Lucas LR, Hajnal A (2002). Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport 13: 1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2004). Motivation concepts in behavioral neuroscience. Physiol Behav 81: 179–209. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2009). ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 97: 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research . Brain Res Rev 28: 309–369. [DOI] [PubMed] [Google Scholar]
- Berthoud HR (2012). The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 71: 478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, Johnson S (1989). Conditioned meal initiation in young children. Appetite 13: 105–113. [DOI] [PubMed] [Google Scholar]
- Blum K, Liu Y, Shriner R, Gold MS (2011). Reward circuitry dopaminergic activation regulates food and drug craving behavior. Curr Pharm Des 17: 1158–1167. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas JM, Murdaugh DL (2009). The Pavlovian power of palatable food: lessons for weight‐loss adherence from a new rodent model of cue‐induced overeating. Int J Obes (Lond) 33: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB et al. (2009). Orexin A/hypocretin‐1 selectively promotes motivation for positive reinforcers. J Neuroscience :Off J Soc Neuroscience 29: 11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T (1998). Hypocretin/orexin‐ and melanin‐concentrating hormone‐expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene‐related protein systems. J Comp Neurol 402: 460–474. [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF (2011). Primary food reward and reward‐predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 34: 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ (2013). Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self‐administration, but not cue‐conditioned ethanol‐seeking, in ethanol‐preferring rats. Int J Neuropsychopharmacolog Off Sci J Collegium Int Neuropsychopharmacolog 16: 2067–2079. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Qi X, Corrie LW (2013). Anorexic effects of intra‐VTA leptin are similar in low‐fat and high‐fat‐fed rats but attenuated in a subgroup of high‐fat‐fed obese rats. Pharmacol Biochem Behav 103: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E (2013). Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr 97: 1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E (2014). Greater striatopallidal adaptive coding during cue‐reward learning and food reward habituation predict future weight gain. Neuroimage 99: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J et al. (2002). Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci 116: 553–567. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston‐Jones G (2013). Role of orexin/hypocretin in conditioned sucrose‐seeking in rats. Psychopharmacology (Berl) 226: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston‐Jones G (2014). Role of orexin/hypocretin in conditioned sucrose‐seeking in female rats. Neuropharmacology 86: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC (2010). The role of orexin‐A in food motivation, reward‐based feeding behavior and food‐induced neuronal activation in rats. Neuroscience 167: 11–20. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ (2002). Eating elicited by orexin‐a, but not melanin‐concentrating hormone, is opioid mediated. Endocrinology 143: 2995–3000. [DOI] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF (2014). Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neuroscience :Off J Soc Neurosc 34: 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CE, Rodin J, Weingarten H (1989). Stimulus‐induced eating when satiated. Physiol Behav 45: 695–704. [DOI] [PubMed] [Google Scholar]
- Dagher A (2009). The neurobiology of appetite: hunger as addiction. Int J Obes (Lond) 33 (Suppl 2): S30–33. [DOI] [PubMed] [Google Scholar]
- Darvas M, Wunsch AM, Gibbs JT, Palmiter RD (2014). Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc Natl Acad Sci U S A 111: 2764–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GC, Cleland GG (1982). Topography of signal‐centered behavior in the rat: effects of deprivation state and reinforcer type. J Exp Anal Behav 38: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M et al. (2010). Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes (Lond) 34: 94–104. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE (2004). A Pavlovian approach to the problem of obesity. Int J obesity related Metab disorders : J Int Assoc Stud Obesity 28: 933–935. [DOI] [PubMed] [Google Scholar]
- Davis C, Fox J (2008). Sensitivity to reward and body mass index (BMI): evidence for a non‐linear relationship. Appetite 50: 43–49. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM (2012). Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J neurosc Off J Soc Neurosc 32: 5549–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A, Balleine B (1990). Motivational control of instrumental performance following a shift from thirst to hunger. Q J Exp psychologyB, Comp Physiol Psychology 42: 413–431. [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ (2003). Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73: 759–768. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D, Bohlooly YM et al. (2010). Ghrelin increases intake of rewarding food in rodents. Addict Biol 15: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS (2000). Two defects contribute to hypothalamic leptin resistance in mice with diet‐induced obesity. J Clin Invest 105: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014). Role of appetite‐regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs 28: 875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli‐Lemos L, Billes SK et al. (2007). Diet‐induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5: 181–194. [DOI] [PubMed] [Google Scholar]
- Fedoroff IC, Polivy J, Herman CP (1997). The effect of pre‐exposure to food cues on the eating behavior of restrained and unrestrained eaters. Appetite 28: 33–47. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM (2008). How does food‐cue exposure lead to larger meal sizes? Br J Nutr 100: 1325–1332. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG (1988). Mesocorticolimbic dopamine systems and reward. Ann N Y Acad Sci 537: 206–215. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003). Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964: 107–115. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE (2011a). A food predictive cue must be attributed with incentive salience for It to induce c‐Fos mRna expression in cortico‐striatal‐thalamic brain regions. Neuroscience 196: 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I et al. (2011b). A selective role for dopamine in stimulus‐reward learning. Nature 469: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P (2000). Modulation of brain reward circuitry by leptin. Science 287: 125–128. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN et al. (2006). Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD (2011). Neural correlates of food addiction. Arch Gen Psychiatry 68: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ et al. (2005). The hypothalamic neuropeptide melanin‐concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced‐swim performance. J Neurosci Off J Soc Neurosci 25: 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MW, Rogers PJ, Elliman NA (2000). Dietary restraint and addictive behaviors: the generalizability of Tiffany's cue reactivity model. Int J Eat Disord 27: 419–427. [DOI] [PubMed] [Google Scholar]
- Grenard JL, Stacy AW, Shiffman S, Baraldi AN, MacKinnon DP, Lockhart G et al. (2013). Sweetened drink and snacking cues in adolescents: a study using ecological momentary assessment. Appetite 67: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ et al. (1997). Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48: 23–29. [DOI] [PubMed] [Google Scholar]
- Hanada R, Nakazato M, Matsukura S, Murakami N, Yoshimatsu H, Sakata T (2000). Differential regulation of melanin‐concentrating hormone and orexin genes in the agouti‐related protein/melanocortin‐4 receptor system. Biochem Biophys Res Commun 268: 88–91. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Morris MJ (2002). Evidence for an interaction between neuropeptide Y and the melanocortin‐4 receptor on feeding in the rat. Neuropharmacology 42: 792–797. [DOI] [PubMed] [Google Scholar]
- Hardman CA, Scott J, Field M, Jones A (2014). To eat or not to eat. Effects Expectancy react food cues Appetite 76: 153–160. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall‐Thompson JF, Aston‐Jones G (2007). Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel PJ (2001). Peripheral signals conveying metabolic information to the brain: short‐term and long‐term regulation of food intake and energy homeostasis. Exp Biol Med 226: 963–977. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Magson LD, Blundell JE (1984). Hunger and palatability: tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite 5: 361–371. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M (2003). Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus‐potentiated feeding and Pavlovian‐instrumental transfer. Eur J Neurosci 17: 1680–1694. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB et al. (2006). Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME (2001). Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci 14: 1843–1855. [DOI] [PubMed] [Google Scholar]
- Israel Y, Kandov Y, Khaimova E, Kest A, Lewis SR, Pasternak GW et al. (2005). NPY‐induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. Peptides 26: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Jansen A (1998). A learning model of binge eating: cue reactivity and cue exposure. Behav Res Ther 36: 257–272. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN (2013). Neural correlates of stress‐ and food cue‐induced food craving in obesity: association with insulin levels. Diabetes Care 36: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CD, Kirwan CB (2015). Functional brain response to food images in successful adolescent weight losers compared with normal‐weight and overweight controls. Obesity 23: 630–636. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC (2009). The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome‐specific reinforcer devaluation effects. J Neurosci Off J Soc Neurosci 29: 696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2000). A role for glutamate transmission in addiction to psychostimulants. Addict Biol 5: 325–329. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J (2005). Unmanageable motivation in addiction: a pathology in prefrontal‐accumbens glutamate transmission. Neuron 45: 647–650. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC et al. (2012). Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab 303: E496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Zook M, Ciccocioppo R, Gehlert DR, Thorsell A, Heilig M et al. (2012). Melanin‐concentrating hormone receptor 1 (MCH1‐R) antagonism: reduced appetite for calories and suppression of addictive‐like behaviors. Pharmacol Biochem Behav 102: 400–406. [DOI] [PubMed] [Google Scholar]
- Kay K, Parise EM, Lilly N, Williams DL (2014). Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food‐conditioned place preference in rats. Psychopharmacology (Berl) 231: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002). The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci Off J Soc Neurosci 22: 3306–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D et al. (2001). Leptin: a modulator of alcohol craving? Biol Psychiatry 49: 782–787. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S (2005). Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059: 179–188. [DOI] [PubMed] [Google Scholar]
- Kohno D, Yada T (2012). Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides 46: 315–319. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Pilhatsch M, Bidlingmaier M et al. (2013). Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol 18: 855–862. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006). The orexin system regulates alcohol‐seeking in rats. Br J Pharmacol 148: 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ et al. (2010). Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine‐ and amphetamine‐regulated transcript neurons of the extended central amygdala. J Neurosci Off J Soc Neurosci 30: 5713–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Kim H, McCabe MF, Chou CW, Wang SX, Chen LL et al. (2014). A leptin‐mediated central mechanism in analgesia‐enhanced opioid reward in rats. J Neurosci 34: 9779–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstadt‐Klein S, Schneider S et al. (2012). Impairment of inhibitory control in response to food‐associated cues and attentional bias of obese participants and normal‐weight controls. Int J Obes (Lond) 36: 1334–1339. [DOI] [PubMed] [Google Scholar]
- Lopez JC, Karlsson RM, O'Donnell P (2015). Dopamine D2 modulation of sign and goal tracking in rats. Neuropsychopharmacolog Off Publ Am College Neuropsychopharmacolog [DOI] [PMC free article] [PubMed]
- Lutter M, Nestler EJ (2009). Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139: 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC (2012a). What and when to “want”? Amygdala‐based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 221: 407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston‐Jones GS (2012b). Fos activation of selective afferents to ventral tegmental area during cue‐induced reinstatement of cocaine seeking in rats. J Neurosci Off J Soc Neurosci 32: 13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston‐Jones G (2013). Interactions between VTA orexin and glutamate in cue‐induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 226: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston‐Jones G (2014). Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17: 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Fardon R, Boutrel B (2012). Orexin/hypocretin (Orx/Hcrt) transmission and drug‐seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci 6: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR et al. (2010). Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity 18: 254–260. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF (2012). Sucrose‐predictive cues evoke greater phasic dopamine release than saccharin‐predictive cues. Synapse 66: 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Lutz A, Vogele C, Kubler A (2012). Women with elevated food addiction symptoms show accelerated reactions, but no impaired inhibitory control, in response to pictures of high‐calorie food‐cues. Eat Behav 13: 423–428. [DOI] [PubMed] [Google Scholar]
- Meule A, Lutz AP, Vogele C, Kubler A (2014). Impulsive reactions to food‐cues predict subsequent food craving. Eat Behav 15: 99–105. [DOI] [PubMed] [Google Scholar]
- Mizushige T, Kawai T, Matsumura S, Yoneda T, Kawada T, Tsuzuki S et al. (2006). POMC and orexin mRNA expressions induced by anticipation of a corn‐oil emulsion feeding are maintained at the high levels until oil ingestion. Biomed Res 27: 227–232. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Flier JS, Bjorbaek C (2004). Region‐specific leptin resistance within the hypothalamus of diet‐induced obese mice. Endocrinology 145: 4880–4889. [DOI] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE (2012). fMRI reactivity to high‐calorie food pictures predicts short‐ and long‐term outcome in a weight‐loss program. Neuroimage 59: 2709–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams‐Deutsch T, Pickens CL, Smith DG, Shaham Y (2009). Effects of the MCH1 receptor antagonist SNAP 94847 on high‐fat food‐reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology (Berl) 205: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Cummings DE, Levine AS (2005). Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26: 2274–2279. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Mabuchi K, Taguchi S, Ikeda S, Aida E, Negishi H et al. (2014). Involvement of orexin‐A neurons but not melanin‐concentrating hormone neurons in the short‐term regulation of food intake in rats. J physiol sci JPS; 64: 203– 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW (2007a). The contribution of orbitofrontal cortex to action selection. Ann N Y Acad Sci 1121: 174–192. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW (2007b). Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci Off J Soc Neurosci 27: 4819–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin J, Figlewicz DP, Bennett‐Jay J, Kittleson S, Cummings DE (2012). Ghrelin increases the motivation to eat, but does not alter food palatability. American journal of physiology. Regul Integr Comp physiol 303: R259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Crofts HS, McGuigan M, Tomic DL, Everitt BJ, Roberts AC (2001). The role of the primate amygdala in conditioned reinforcement. J Neurosci Off J Soc Neurosci 21: 7770–7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ (2007). Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol 500: 1050–1063. [DOI] [PubMed] [Google Scholar]
- Patterson M, Bloom SR, Gardiner JV (2011). Ghrelin and appetite control in humans—potential application in the treatment of obesity. Peptides 32: 2290–2294. [DOI] [PubMed] [Google Scholar]
- Patyal R, Woo EY, Borgland SL (2012). Local hypocretin‐1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front Behav Neurosci 6: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC (2005). Hedonic hot spot in nucleus accumbens shell: where do mu‐opioids cause increased hedonic impact of sweetness? J Neurosci Off J Soc Neuroscience 25: 11777–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne‐Lawrence S, Rovinsky SA et al. (2010). Ghrelin increases the rewarding value of high‐fat diet in an orexin‐dependent manner. Biol Psychiatry 67: 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ (2014). Role of cues and contexts on drug‐seeking behaviour. Br J Pharmacol 171: 4636–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD (2011). Forebrain circuits and control of feeding by learned cues. Neurobiol Learn Mem 95: 152–158. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M (2005). Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci Off J Soc Neurosci 25: 8295–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ (2012). Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin‐concentrating hormone neurons, by a learned food‐cue that stimulates feeding in sated rats. Neuroscience 224: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M (2002). Amygdalo‐hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci Off J Soc Neurosci 22: 8748–8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC (2007). Learned contextual cue potentiates eating in rats. Physiol Behav 90: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC (2005). Orbitofrontal lesions impair use of cue‐outcome associations in a devaluation task. Behav Neurosci 119: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G (2003). Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci Off J Soc Neurosci 23: 11078–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza‐Zabala A, Flores A, Martin‐Garcia E, Saravia R, Maldonado R, Berrendero F (2013). A role for hypocretin/orexin receptor‐1 in cue‐induced reinstatement of nicotine‐seeking behavior. Neuropsychopharmacolog Off Publ Am College Neuropsychopharmacolog 38: 1724–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Smolders I (2014). Rewarding, reinforcing and incentive salient events involve orexigenic hypothalamic neuropeptides regulating mesolimbic dopaminergic neurotransmission. Eur J Pharm Sci Off J Eur Fed Pharm Sci 57: 2–10. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG (2005). Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 134: 737–744. [DOI] [PubMed] [Google Scholar]
- Reichelt AC, Morris MJ, Westbrook RF (2014). Cafeteria diet impairs expression of sensory‐specific satiety and stimulus‐outcome learning. Front Psychology 5: 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD (2012). Learned food‐cue stimulates persistent feeding in sated rats. Appetite 59: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, et al (2015). Individual differences in cue‐induced motivation and striatal systems in rats susceptible to diet‐induced obesity. Neuropsychopharmacolog Off Publ Am College Neuropsychopharmacolog [DOI] [PMC free article] [PubMed]
- Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive‐sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2000). The psychology and neurobiology of addiction: an incentive‐sensitization view. Addiction 95 (Suppl 2): S91–117. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2001). Incentive‐sensitization and addiction. Addiction 96: 103–114. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54: 25–53. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hill AJ (1989). Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav 14: 387–397. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H et al. (2007). Differential activation of the dorsal striatum by high‐calorie visual food stimuli in obese individuals. Neuroimage 37: 410–421. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2009). Dopamine/adenosine interactions involved in effort‐related aspects of food motivation. Appetite 53: 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK (2002). The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199–211. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE (2012). The role of dopamine in the accumbens core in the expression of Pavlovian‐conditioned responses. Eur J Neurosci 36: 2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S (1968). Obesity and eating, internal and external cues differentially affect the eating behavior of obese and normal subjects. Sci 161: 751–756. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998a). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998b). The phasic reward signal of primate dopamine neurons. Adv Pharmacol 42: 686–690. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000). Central nervous system control of food intake. Nature 404: 661–671. [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M et al. (2010). Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin‐concentrating hormone. J Neurosci Off J Soc Neurosci 30: 8263–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Wosiski‐Kuhn M, Nguyen T, Holland PC, Lakaye B, Adamantidis A et al. (2012). The role of melanin‐concentrating hormone in conditioned reward learning. Eur J Neurosci 36: 3126–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizgal P, Fulton S, Woodside B (2001). Brain reward circuitry and the regulation of energy balance. Int J Obesity relat Metab Disorders J Int Assoc Stud Obesity 25: S17–21. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston‐Jones G (2012). Orexin/hypocretin 1 receptor antagonist reduces heroin self‐administration and cue‐induced heroin seeking. Eur J Neurosci 35: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L (2005). Cue‐elicited craving for food: a fresh approach to the study of binge eating. Appetite 44: 253–261. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Zald D, Dagher A (2011a). Dopamine‐based reward circuitry responsivity, genetics, and overeating. Curr Top Behav Neurosci 6: 81–93. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008). Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117: 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS, Epstein LH, Small DM (2011b). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci Off J Soc Neurosci 31: 4360–4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE (2013). The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev 37: 2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger J, Schmalzle R, Flaisch T, Bublatzky F, Schupp HT (2009). The impact of hunger on food cue processing: an event‐related brain potential study. Neuroimage 47: 1819–1829. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE (2008). Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. Neuroimage 41: 636–647. [DOI] [PubMed] [Google Scholar]
- Tang DW, Fellows LK, Small DM, Dagher A (2012). Food and drug cues activate similar brain regions: a meta‐analysis of functional MRI studies. Physiol Behav 106: 317–324. [DOI] [PubMed] [Google Scholar]
- Tiffany ST (1990). A cognitive model of drug urges and drug‐use behavior: role of automatic and nonautomatic processes. Psychol Rev 97: 147–168. [DOI] [PubMed] [Google Scholar]
- Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, DiLeone RJ (2011). Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS One 6: e27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plasse G, Merkestein M, Luijendijk MC, van der Roest M, Westenberg HG, Mulder AB et al. (2013). Food cues and ghrelin recruit the same neuronal circuitry. Int J Obes (Lond) 37: 1012–1019. [DOI] [PubMed] [Google Scholar]
- van Koningsbruggen GM, Stroebe W, Aarts H (2012). Mere exposure to palatable food cues reduces restrained eaters' physical effort to obtain healthy food. Appetite 58: 593–596. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000). Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151: 99–120. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F (2008). Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc London Ser B, Biological sci 363: 3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R (2012). Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci 11: 1–24. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D et al. (2002). “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44: 175–180. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Koopmann A, Dinter C, Richter A, Rockenbach C, Grosshans M et al. (2010). Orexin and leptin are associated with nicotine craving: a link between smoking, appetite and reward. Psychoneuroendocrinology 35: 570–577. [DOI] [PubMed] [Google Scholar]
- Walker AK, Ibia IE, Zigman JM (2012). Disruption of cue‐potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav 108: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP (1983). Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220: 431–433. [DOI] [PubMed] [Google Scholar]
- Williams DL (2014). Neural integration of satiation and food reward: role of GLP‐1 and orexin pathways. Physiology & behavior. [DOI] [PMC free article] [PubMed]
- Wyvell CL, Berridge KC (2000). Intra‐accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci Off J Soc Neurosci 20: 8122–8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA (2015). Naltrexone extended‐release plus bupropion extended‐release for treatment of obesity. Jama 313: 1213–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, Heisler LK (2012). Unraveling the brain regulation of appetite: lessons from genetics. Nat Neurosci 15: 1343–1349. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E (2011). Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity 19: 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E (2014). Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity 22: 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006). Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]


