Abstract
Background and Purpose
The rise in intracellular Ca2+ stimulates the expression of the transcription factor c‐Fos. Depending on the mode of entry of Ca2+ into the cytosol, distinct signal transducers and transcription factors are required. Here, we have analysed the signalling pathway connecting a Ca2+ influx via activation of transient receptor potential melastatin‐3 (TRPM3) channels with enhanced c‐Fos expression.
Experimental Approach
Transcription of c‐Fos promoter/reporter genes that were integrated into the chromatin via lentiviral gene transfer was analysed in HEK293 cells overexpressing TRPM3. The transcriptional activation potential of c‐Fos was measured using a GAL4‐c‐Fos fusion protein.
Key Results
The signalling pathway connecting TRPM3 stimulation with enhanced c‐Fos expression requires the activation of MAP kinases. On the transcriptional level, three Ca2+‐responsive elements, the cAMP‐response element and the binding sites for the serum response factor (SRF) and AP‐1, are essential for the TRPM3‐mediated stimulation of the c‐Fos promoter. Ternary complex factors are additionally involved in connecting TRPM3 stimulation with the up‐regulation of c‐Fos expression. Stimulation of TRPM3 channels also increases the transcriptional activation potential of c‐Fos.
Conclusions and Implications
Signalling molecules involved in connecting TRPM3 with the c‐Fos gene are MAP kinases and the transcription factors CREB, SRF, AP‐1 and ternary complex factors. As c‐Fos constitutes, together with other basic region leucine zipper transcription factors, the AP‐1 transcription factor complex, the results of this study explain TRPM3‐induced activation of AP‐1 and connects TRPM3 with the biological functions regulated by AP‐1. © 2015 The British Pharmacological Society
Abbreviations
- AP‐1
activator protein‐1
- bZIP
basic region leucine zipper
- CREB
cAMP‐response element‐binding protein
- SRE
serum response element
- SRF
serum response factor
- TCF
ternary complex factor
- TPA
phorbol 12‐myristate 13‐acetate
- TRP
transient receptor potential
Tables of Links
| LIGANDS |
|---|
| Forskolin |
| Pregnenolone sulphate |
| TPA |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 1999) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a, 2013b).
Introduction
Transient receptor potential (TRP) proteins are cation channels that contain six transmembrane domains and a pore domain between the fifth and sixth transmembrane domains. The TRP melastatin‐3 (TRPM3) protein is expressed in various tissues and functions as a Ca2+‐permeable non‐selective ion channel (Grimm et al., 2003; Lee et al., 2003; Oberwinkler et al., 2005; Wagner et al., 2008; Naylor et al., 2010; Wagner et al., 2010; Vriens et al., 2014). Many biological functions have been attributed to TRPM3 activation, including vascular smooth muscle cell contraction (Naylor et al., 2010), glutamatergic transmission of cerebellar Purkinje cells (Zamudio‐Bulcock et al., 2011) and insulin secretion (Wagner et al., 2008). However, TRPM3‐deficient mice did not show alterations in resting blood glucose levels (Vriens et al., 2011), suggesting that TRPM3 may play no or only a marginal role in controlling insulin secretion as previously suggested (Colsoul et al., 2011; Thiel et al., 2013). Instead, the analysis of TRPM3‐deficient mice revealed that TRPM3 is a noxious heat sensor in somatosensory neurons (Vriens et al., 2011). Recently, it has been shown that TRPM3 mutation underlies inherited cataracts and glaucoma (Bennett et al., 2014), indicating that TRPM3 is associated with inherited ocular diseases in humans. The analysis of TRPM3‐deficient mice argues against a major role for TRPM3 in visual processing in the outer retina (Brown et al., 2015). Moreover, TRPM3 channel activation promotes growth of clear cell renal carcinoma (Hall et al., 2014).
In our laboratory, we discovered that stimulation of endogenous TRPM3 channels with pregnenolone sulfate activates a signal cascade in insulinoma cells that leads to an up‐regulation of gene transcription (Mayer et al., 2011; Müller et al., 2011). This observation was confirmed in HEK293 cells expressing a tetracycline‐inducible TRPM3 transcription unit (Lesch et al., 2014). Several transcription factors have been identified as responsive to TRPM3‐initiated signalling, including activator protein 1 (AP‐1), cAMP‐response element‐binding protein (CREB), Egr‐1 and ternary complex factors (TCFs) (Mayer et al., 2011; Müller et al., 2011; Lesch et al., 2014, 2015).
Here, we have analysed the signalling pathway leading to increased transcription of the c‐Fos gene following stimulation of TRPM3 channels. The c‐Fos gene encodes a basic region leucine zipper (bZIP) transcription factor that constitutes, together with proteins of the c‐Jun and ATF families of transcription factors, the AP‐1 transcription factor complex. The c‐Fos gene is activated by a variety of extracellular signalling molecules, including Gαs‐ and Gαq‐coupled receptor ligands, receptor tyrosine kinase ligands, phorbol ester, cytokines, calcium ionophores and others. The c‐Fos gene thus functions as a point of convergence for many intracellular signalling cascades involving the transcription factors CREB, STAT, AP‐1, serum response factor (SRF), and TCFs. In fact, many of these transcription factors have been characterized by analysing the regulation of c‐Fos gene transcription. c‐Fos expression is highly responsive to an increase in free cytoplasmic Ca2+. The nature of the Ca2+ source determines which genetic elements, transcription factors and protein kinases are required to activate c‐Fos expression (Gallin and Greenberg, 1995). In previous studies, Ca2+ influx was induced either by activating voltage‐gated Ca2+ channels, ionotropic NMDA receptors and Gαq‐coupled receptors or by adding Ca2+ ionophores to the cells (Sheng et al., 1990; Lee and Gilman, 1994; Misra et al., 1994; Thompson et al., 1995; Xia et al., 1996; Johnson et al., 1997; Ely et al., 2011; Glidewell‐Kenney et al., 2014). Here, we have identified the signalling molecules that connect TRPM3 Ca2+ channel stimulation with enhanced c‐Fos expression. Previous studies from our laboratory showed that stimulation of endogenous TRPM3 channels in insulinoma cells or TRPM3‐expressing HEK293 cells increases c‐Fos promoter activity and expression (Müller et al., 2011; Lesch et al., 2014). The results of this study show that TRPM3‐mediated up‐regulation of c‐Fos expression and activity requires the activation of MAPKs. At the transcriptional level, the cAMP‐response element (CRE), the AP‐1 and the SRF binding sites are essential genetic elements that connect TRPM3 stimulation with enhanced c‐Fos expression.
Methods
Cell culture
HEK293 cells containing the human TRPM3 coding region under the control of a tetracycline‐regulated promoter were kindly provided by David Beech and Yasser Majeed, University of Leeds, UK, and cultured as described previously (Naylor et al., 2008). TRPM3 expression was induced by adding tetracycline (1 μg·mL−1, Sigma‐Aldrich, Steinheim, Germany, # T7680, dissolved in water) to the culture medium containing 0.05% FBS for 24 h before the stimulation with putative TRPM3 activators. As a control, cells that had not received tetracycline were analysed. Stimulation with pregnenolone sulfate (20 μM, Sigma‐Aldrich # P162, dissolved in DMSO) was performed for 24 h. Stimulation with phorbol 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA, 10 ng·mL−1, Calbiochem # 524400‐1, dissolved in DMSO) or forskolin (Calbiochem, Cat # 344270, dissolved in DMSO) was performed in a medium containing 0.05% FBS, which lacked tetracycline.
Lentiviral gene transfer
The lentiviral transfer vectors pFUW‐REST/Elk‐1∆C, pFUW‐REST/CREB, pFUW‐c‐Jun∆N, pFUW‐MKP‐1, pFUW‐MKP‐5 and pFUW‐GAL4‐c‐Fos have been described previously (Mayer et al., 2008; Mayer and Thiel, 2009; Mayer et al., 2009; Rössler and Thiel, 2009; Müller et al., 2010; Spohn et al., 2010; Thiel and Rössler, 2011, 2014; Thiel et al., 2012). The viral particles were produced as previously described (Keim et al., 2012).
Reporter assays
The lentiviral transfer vectors pFW‐hc‐Fos.luc, pFWc‐Fos(CRE)4luc and pFW‐UAS5Sp12luc have been described elsewhere (Rössler et al., 2008; Thiel and Rössler, 2011; Ekici et al., 2012; Thiel et al., 2012; Kaufmann et al., 2013). The lentiviral transfer plasmid pFWc‐Fos∆TKluc, which encodes the luciferase reporter gene under the control of a minimal thymidine kinase (TK) promoter and a truncated human c‐Fos promoter, was generated by subcloning a PacI/Eco47III fragment of the human c‐Fos promoter, derived from plasmid pFW‐hc‐Fos.luc, in a lentiviral transfer vector upstream of the minimal TK promoter and the luciferase open reading frame. The mouse promoter/luciferase plasmids were kind gifts of Djurdjica Coss, UC San Diego (Ely et al., 2011). Lentiviral transfer vectors were generated by cloning of HindIII/BglII fragments of these plasmids upstream of the luciferase reporter gene in a lentiviral transfer vector. Cell extracts were prepared using reporter lysis buffer (Promega, Mannheim, Germany) and analysed for luciferase activities as described previously (Thiel et al., 2000). Luciferase activity was normalized to the protein concentration. Each experiment was performed at least three times in quadruplicate giving consistent results.
Western blots
Whole cell extracts and nuclear extracts were prepared as described previously (Kaufmann and Thiel, 2002). Proteins were separated by SDS‐PAGE, blotted and incubated with antibodies directed against c‐Fos (Santa Cruz, Heidelberg, Germany, # sc‐52) or histone deacetylase‐1 (loading control). The M2 monoclonal antibody (Sigma‐Aldrich # F3165) was used to detect FLAG‐tagged proteins. Immunoreactive bands were detected via enhanced chemiluminescence as described previously (Spohn et al., 2010; Mayer et al., 2011). Quantification of Western blot data was performed with the ChemiDoc XRS+ imaging system from Bio‐Rad (Bio‐Rad Laboratories GmbH, München, Germany), using the Quantity One 1‐D analysis software.
Statistics
Statistical analysis was carried out by using Student's two‐tailed t‐test. Data shown are mean ± SD from three to four independent experiments performed in quadruplicate. Statistical probability is expressed as *P < 0.05, **P < 0.01 and ***P < 0.001. Values were considered significant when P < 0.05.
Results
The steroid pregnenolone sulfate activates endogenous TRPM3 channels and L‐type voltage‐gated Ca2+ channels in insulinoma cells leading to an up‐regulation of c‐Fos promoter activity (Müller et al., 2011). The stimulation of the c‐Fos promoter was blocked in cells pretreated with the L‐type Ca2+ channel blocker verapamil. To elucidate the signalling pathway connecting TRPM3 stimulation with enhanced c‐Fos gene transcription, we used an engineered HEK293 cell line, in which TRPM3 expression is induced by adding tetracycline to the culture medium. As HEK293 cells do not express L‐type voltage‐gated Ca2+ channels (Wagner et al., 2008; Majeed et al., 2010), interference between TRPM3 and L‐type voltage‐gated Ca2+ channel signalling was avoided. HEK293 cells expressing TRPM3 are frequently used as a cellular model system to analyse TRPM3 stimulation and signalling (Grimm et al., 2003; Lee et al., 2003; Wagner et al., 2008; Majeed et al., 2010; Vriens et al., 2014).
Pregnenolone sulfate triggers an up‐regulation of c‐Fos promoter/luciferase reporter gene transcription in HEK293 cells expressing TRPM3
We used lentiviral gene transfer to integrate either a human c‐Fos promoter/luciferase reporter gene (hc‐Fos.luc) or a murine c‐Fos promoter/luciferase reporter gene (mc‐Fos.luc) into the genome of the cells. The eukaryotic chromatin structure is repressive to transcription that requires accessibility to DNA. The implantation of the reporter genes into the chromatin ensured that these genes were packed into an ordered nucleosomal structure. In contrast, plasmids containing reporter genes that are introduced into the cells via transient transfection may be incompletely organized in comparison with cellular chromatin and thus resemble a prokaryotic gene organisation with a nonrestrictive transcriptional ground state. A schematic depiction of the integrated proviruses encoding the luciferase reporter gene under the control of the human or murine c‐Fos regulatory regions is shown in Figure 1A. The landmark genetic elements within the c‐Fos regulatory regions are indicated. HEK293 cells containing a tetracycline‐responsive TRPM3 expression cassette were infected with lentiviruses encoding one of the c‐Fos promoter/luciferase reporter genes. Cells were treated with tetracycline to induce TRPM3 expression, serum starved for 24 h and stimulated with pregnenolone sulfate for 24 h. Figure 1B shows that pregnenolone sulfate stimulation of HEK293 cells expressing TRPM3 induced an up‐regulation of c‐Fos promoter‐regulated reporter gene transcription.
Figure 1.
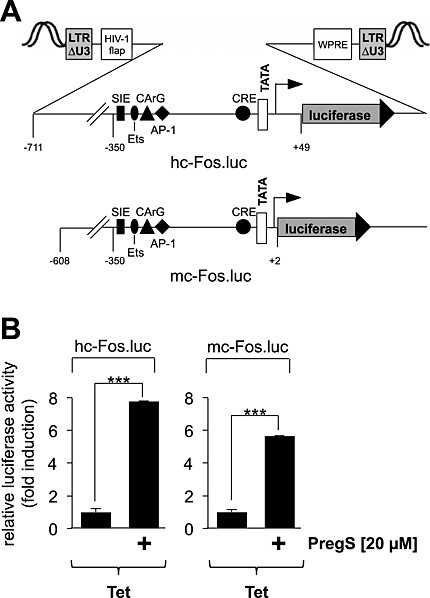
Stimulation of TRPM3 activates transcription of c‐Fos promoter/luciferase reporter genes. (A) Schematic representation of integrated proviruses encoding a human c‐Fos promoter/luciferase reporter gene (hc‐Fos.luc) or a murine c‐Fos promoter/luciferase reporter gene (mc‐Fos.luc). The landmark transcription factor binding sites are depicted, including the SIS‐inducible element (SIE), Ets, the CArG box, the AP‐1 binding site, and the CRE. (B) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with recombinant lentiviruses encoding either a human c‐Fos promoter/luciferase reporter gene (hc‐Fos.luc) or a murine c‐Fos promoter/luciferase reporter gene (mc‐Fos.luc) respectively. The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001).
Protein phosphatases MKP‐1 and MKP‐5 attenuate pregnenolone sulfate‐induced activation of the c‐Fos promoter and c‐Fos expression in HEK293 cells expressing TRPM3
Several studies addressed the role of MAPKs in connecting a rise in the intracellular free Ca2+ concentration with enhanced c‐Fos expression. The activation of ERK was important for neurons to induce gene transcription following activation of L‐type Ca2+ channels (Dolmetsch et al., 2001). Likewise, stimulation of c‐Fos expression via activation of nerve growth factor (NGF) or EGF receptors requires ERK (Johnson et al., 1997). In contrast, ERK‐independent stimulation of c‐Fos gene transcription was observed in ionomycin‐treated PC12 cells and in KCl/FPL64176‐treated AtT20 cells (Johnson et al., 1997). To assess the impact of ERK and other MAPKs as signal transducers for TRPM3, we overexpressed MAPK phosphatases (MKPs) in the cells. MKP‐1, the enzyme that dephosphorylates and inactivates the MAPK ERK, p38 and JNK in the nucleus (Shapiro and Ahn, 1998; Slack et al., 2001), reduced pregnenolone sulfate‐induced up‐regulation of Egr‐1 expression in insulinoma cells (Mayer et al., 2011) and the up‐regulation of AP‐1 activity in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3 (Lesch et al., 2015). Therefore, we asked whether overexpression of MKP‐1 attenuates the pregnenolone sulfate‐induced transcription of c‐Fos promoter/reporter genes as well. Figure 2A and B shows that reporter gene transcription was significantly reduced in pregnenolone sulfate‐stimulated TRPM3‐expressing HEK293 cells that had been infected with an MKP‐1 encoding lentivirus. Moreover, reporter gene transcription was also reduced in cells expressing MKP‐5, the enzyme that dephosphorylates and inactivates the MAPKs, p38 and JNK. Expression of either MKP‐1 or MKP‐5 significantly reduced the up‐regulation of c‐Fos expression following stimulation of TRPM3 channels with pregnenolone sulfate (Figure 2C and D). Probably because of phosphorylation, c‐Fos is visualized as a doublet band on western blots, as reported earlier (Monje et al., 2003; Koga et al., 2009; Kaufmann et al., 2013). These data indicate that activation of MAPKs is essential to connect TRPM3 stimulation with enhanced c‐Fos gene transcription and c‐Fos expression.
Figure 2.
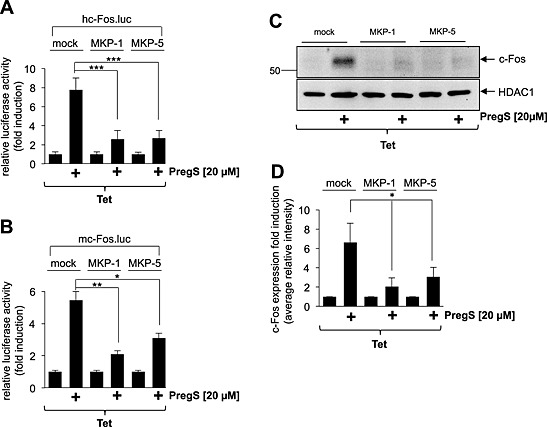
MAPKs connect TRPM3 activation with stimulation of c‐Fos promoter activity and c‐Fos expression. (A, B) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus containing the luciferase reporter gene under the control of either the human (A) or murine (B) c‐Fos promoter. Cells were infected with a lentivirus encoding either MKP‐1 or MKP‐5. The transgenes were expressed under the control of the human ubiquitin‐C promoter. As a control, cells were infected with a lentivirus generated with the lentiviral transfer plasmid pFUW (mock). The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD of three experiments performed in quadruplicate (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Expression of either MKP‐1 or MKP‐5 attenuates c‐Fos expression in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3. HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus encoding either MKP‐1 or MKP‐5. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). Cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM). Nuclear extracts were prepared and subjected to western blot analysis using an antibody directed against c‐Fos. The antibody directed against HDAC1 was used as a loading control. (D) Quantification of c‐Fos expression. The intensity of the c‐Fos signal was normalized to the intensity of the HDAC1 control. Data shown are mean ± SD of three independent experiments (*P < 0.05).
The TRPM3‐induced signalling cascade targets the CRE within the c‐Fos promoter
The CRE within the c‐Fos gene was the first identified genetic element that connects elevated intracellular Ca2+ levels with increased c‐Fos transcription (Sheng et al., 1990). This element was therefore termed ‘calcium‐response element’ (CaRE). However, stimulation of c‐Fos expression in T‐cells does not require the CRE/CaRE (Lee and Gilman, 1994). Recently, we showed that treatment of insulinoma cells with pregnenolone sulfate leads to a significant up‐regulation of CREB‐mediated gene transcription (Müller et al., 2011). The involvement of TRPM3 was verified in these experiments via expression of a TRPM3‐specific shRNA that significantly down‐regulated TRPM3 expression. However, the pregnenolone sulfate‐induced up‐regulation of a CRE‐controlled reporter gene was also impaired by pre‐incubation of the cells with the L‐type voltage‐gated Ca2+ channel inhibitor verapamil (Müller et al., 2011). Thus, both TRPM3 and voltage‐gated Ca2+ channels are involved in regulating pregnenolone sulfate‐induced CRE‐mediated gene transcription in insulinoma cells. The previous experiments have shown that TRPM3 stimulation enhanced c‐Fos gene transcription in HEK293 cells in the absence of L‐type voltage‐gated Ca2+ channels. Now, we asked the question whether the CRE within the c‐Fos promoter functions as a pregnenolone sulfate‐responsive element not only in insulinoma cells but also in HEK293 cells that lack L‐type voltage‐gated Ca2+ channels. First, we confirmed that a rise in the intracellular cAMP concentration stimulated transcription of c‐Fos promoter/luciferase reporter genes (Figure 3A). We used the c‐FosCRE4.luc reporter gene to measure CRE‐regulated gene transcription, because transcription of this gene is only regulated by CREs (Figure 3B). Figure 3C shows that transcription of this CRE‐controlled reporter gene was significantly stimulated in pregnenolone sulfate‐treated HEK293 cells expressing TRPM3. As a control, we showed that this transcription unit is highly responsive to elevated cAMP levels in the cells (Figure 3D). Thus, the CRE functions as CaRE within the c‐Fos gene, when the intracellular Ca2+ concentration is elevated as a result of TRPM3 stimulation.
Figure 3.
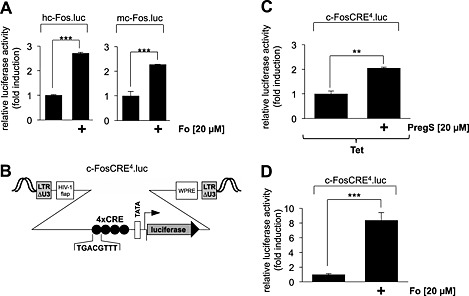
Stimulation of HEK293 cells expressing TRPM3 with pregnenolone sulfate induces transcription mediated by the CRE derived from the human c‐Fos gene. (A) Up‐regulation of c‐Fos promoter activity by elevated intracellular cAMP concentrations. HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with recombinant lentiviruses encoding a luciferase reporter gene under the control of the human (left panel) or murine (right panel) c‐Fos promoter respectively. The cells were serum starved for 24 h and then stimulated with forskolin (Fo, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001). (B) Schematic representation of the integrated provirus encoding a c‐Fos CRE/luciferase reporter gene. The regulatory region of the reporter gene contains a minimal promoter consisting of the human immunodeficiency virus TATA box, the adenovirus major late promoter initiator element and four copies of CRE derived from the human c‐Fos gene. (C) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with recombinant lentiviruses containing the CRE/luciferase reporter gene c‐FosCRE4.luc. The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (**P < 0.01). (D) Cells were infected with recombinant lentiviruses encoding the reporter gene c‐FosCRE4.luc, serum starved for 24 h and stimulated with Fo (20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001).
To corroborate this finding, we expressed a dominant‐negative mutant of CREB, REST/CREB, in HEK293 cells containing a tetracycline‐responsive TRPM3 expression unit. This mutant retains the bZIP domain of CREB but lacks the activation domains (Figure 4A). Nuclear proteins of mock‐infected HEK293 cells or HEK293 infected with a REST/CREB encoding lentivirus were fractionated by SDS‐PAGE, and the fusion protein was identified by Western blot analysis using antibodies targeting the FLAG epitope (Figure 4B). Next, we assessed the functional effects of REST/CREB expression on the pregnenolone sulfate‐induced activation of the c‐Fos promoter. Figure 4C shows that expression of REST/CREB significantly attenuated the up‐regulation of c‐Fos promoter/reporter gene transcription in pregnenolone sulfate‐stimulated HEK293 cells that expressed TRPM3. In addition, expression of REST/CREB attenuated the up‐regulation of c‐Fos expression in HEK293 cells following activation of TRPM3 (Figure 4D and E). These data indicate that CREB is part of the signalling cascade that connects a rise in cytoplasmic Ca2+ via TRPM3 channel stimulation with enhanced c‐Fos promoter activity and c‐Fos expression.
Figure 4.
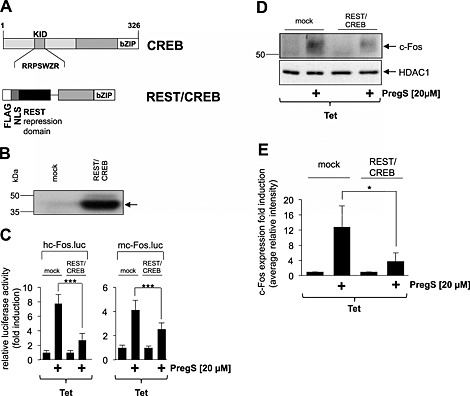
A dominant‐negative CREB mutant attenuates the up‐regulation of c‐Fos promoter activity and c‐Fos expression in HEK293 cells expressing activated TRPM3 channels. (A) Schematic representation of CREB and the dominant‐negative mutant REST/CREB. The phosphorylation‐dependent transcriptional activation domain of CREB [kinase‐inducible domain (KID)] is depicted. The bZIP domain is located on the C‐terminus. The mutant lacks the KID domain but retains the DNA‐binding and dimerization domains. The mutant is expressed as a fusion protein together with a transcriptional repression domain derived from the transcriptional repressor REST. (B) Western blot analysis of HEK293 cells either mock infected or infected with recombinant lentiviruses encoding REST/CREB. Western blots were probed with an antibody against the FLAG tag. Molecular mass markers in kDa are shown on the left. (C) HEK293 cells containing a tetracycline‐inducible transcription unit were double infected with a lentivirus encoding either human c‐Fos promoter‐controlled (left panel) or murine c‐Fos promoter‐controlled luciferase reporter gene (right panel) and with a lentivirus encoding REST/CREB. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001). (D) Expression of REST/CREB attenuates c‐Fos expression in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3. HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus encoding REST/CREB. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). Cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM). Nuclear extracts were prepared and subjected to western blot analysis using an antibody directed against the c‐Fos protein. The antibody directed against HDAC1 was used as a loading control. (E) Quantification of c‐Fos expression. The intensity of the c‐Fos signal was normalized to the intensity of the HDAC1 control. Data shown are mean ± SD from three independent experiments (*P < 0.05).
Mutational analysis of the murine c‐Fos promoter identified the AP‐1 site and the CArG box as pregnenolone sulfate‐responsive elements
To test whether the CRE within the c‐Fos promoter is the only pregnenolone sulfate‐responsive genetic element, we deleted this sequence within the human c‐Fos promoter. A schematic depiction of the integrated proviruses encoding the luciferase reporter gene under the control of the truncated human c‐Fos regulatory region is shown in Figure 5A. Stimulation of HEK293 cells with forskolin revealed that transcription of a reporter gene under the control of the truncated c‐Fos promoter is no longer responsive to elevated cAMP levels in the cells (Figure 5B). However, transcription of the truncated c‐Fos promoter/luciferase reporter gene was still responsive to pregnenolone sulfate stimulation, as long as TRPM3 was expressed (Figure 5C). These data suggest that – in addition to the CRE – other Ca2+‐responsive genetic elements participate in the up‐regulation of c‐Fos expression following stimulation of TRPM3 with pregnenolone sulfate.
Figure 5.
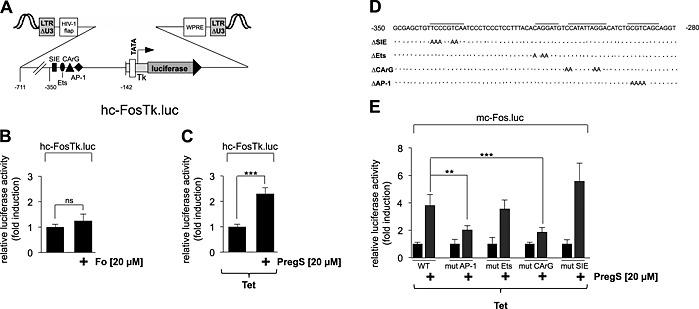
Mutational analysis identifies the AP‐1 binding site and the CArG box as pregnenolone sulfate‐responsive elements within the c‐Fos promoter. (A) Schematic representation of the integrated provirus encoding a truncated c‐Fos promoter/luciferase reporter gene lacking the CRE. (B) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with recombinant lentiviruses containing a luciferase reporter gene under the control of the truncated human c‐Fos promoter. The cells were serum starved for 24 h and then stimulated with forskolin (Fo, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (n.s., not significant). (C) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus containing the truncated human c‐Fos promoter/luciferase reporter gene hc‐FosTK.luc. The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001). (D) Sequence of the murine c‐Fos promoter from −350 to −280, depicting mutated base pairs of the SIS‐inducible element (SIE), Ets and SRF and AP‐1 binding sites respectively. (E) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with recombinant lentiviruses encoding either the wild‐type murine c‐Fos promoter/luciferase reporter gene or one of the mutated murine c‐Fos promoter/luciferase reporter genes. The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P 3< 0.001).
To identify other TRPM3‐responsive elements, we analysed mutants of the murine c‐Fos promoter, which contained base pair mutations of the SIS‐inducible element, and the binding sites for TCFs (Ets), SRF (CArG box) and AP‐1, respectively (Figure 5D). The results show that mutation of the binding sites for AP‐1 and SRF significantly impaired pregnenolone sulfate‐induced up‐regulation of c‐Fos promoter/luciferase gene transcription (Figure 5E). In contrast, mutation of the Ets site and the SIS‐inducible element, which bind TCFs or STAT transcription factors, respectively, had no effect on the TRPM3‐induced up‐regulation of reporter gene transcription.
Expression of a dominant‐negative mutant of the transcription factor Elk‐1 reduces c‐Fos promoter‐regulated reporter gene transcription and c‐Fos expression in HEK293 cells expressing activated TRPM3 channels
Previous experiments performed with insulinoma cells revealed that expression of a dominant‐negative mutant of the TCF Elk‐1, termed REST/Elk‐1∆C, reduced c‐Fos promoter activity following stimulation of endogenous TRPM3 channels with pregnenolone sulfate (Müller et al., 2011). This mutant retains the DNA‐binding and SRF interaction domains but lacks the C‐terminal activation domain of Elk‐1. REST/Elk‐1∆C additionally contains the N‐terminal repression domain of the transcriptional repressor REST (Figure 6A). We expressed the mutant in HEK293 cells containing a tetracycline‐responsive TRPM3 expression unit (Figure 6B) and assessed the impact of this mutant on the regulation of c‐Fos promoter activity. The results show that expression of the mutant reduced the up‐regulation of reporter gene transcription in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3 (Figure 6C). Thus, although the Ets binding site is not required for the up‐regulation of c‐Fos expression by pregnenolone sulfate/TRPM3 (Figure 5E), expression of the dominant‐negative Elk‐1 mutant, which binds to the Ets site of the c‐Fos promoter and also retains the SRF binding domain, attenuates the pregnenolone sulfate‐induced activation of the c‐Fos promoter. In addition, expression of the Elk‐1 mutant attenuated the up‐regulation of c‐Fos expression in HEK293 cells expressing activated TRPM3 channels (Figure 6D and E). These data indicate that Elk‐1 is an important signalling molecule that connects TRPM3 stimulation with enhanced c‐Fos promoter activity and c‐Fos expression.
Figure 6.
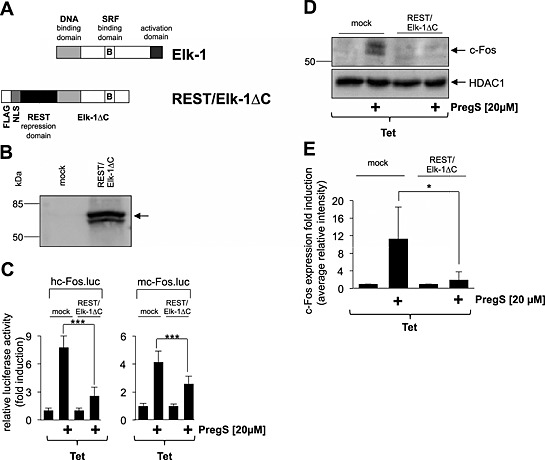
Expression of a dominant‐negative mutant of Elk‐1 attenuates the up‐regulation of c‐Fos promoter activity and c‐Fos expression in HEK293 cells expressing activated TRPM3 channels. (A) Schematic representation of Elk‐1 and the dominant‐negative mutant REST/Elk‐1ΔC. The DNA‐binding domain of Elk‐1 is located on the N‐terminus. The transcriptional activation domain is located on the C‐terminus. A regulatory domain lies within this transcriptional activation domain encompassing the key phosphoacceptor serine residues S383 and S389. Elk‐1 binds with its B‐domain to SRF, leading to the formation of the ternary Elk‐1–SRF complex. The B‐domain also couples the C‐terminal phosphorylation of Elk‐1 with enhanced DNA binding via the Ets domain. The dominant‐negative mutant REST/Elk‐1∆C lacks the phosphorylation‐regulated activation domain but retains the DNA‐binding and SRF‐binding domains. The mutant is expressed as a fusion protein together with a transcriptional repression domain of REST. (B) Western blot analysis of HEK293 cells that were either mock infected or infected with a recombinant lentivirus encoding REST/Elk‐1ΔC. The Western blot was probed with an antibody against the FLAG tag. Molecular mass markers in kDa are shown on the left. (C) HEK293 cells were double infected with a lentivirus containing the luciferase reporter gene under the control of either the human (left panel) or murine (right panel) c‐Fos promoter. In addition, cells were infected with a lentivirus encoding REST/Elk‐1ΔC. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (***P < 0.001). (D) Expression of REST/Elk‐1ΔC attenuates c‐Fos expression in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3. HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus encoding REST/Elk‐1ΔC. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). Cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM). Nuclear extracts were prepared and subjected to Western blot analysis using an antibody directed against the c‐Fos protein. The antibody directed against HDAC1 was used as a loading control. (E) Quantification of c‐Fos expression. The intensity of the c‐Fos signal was normalized to the intensity of the HDAC1 control. Data shown are mean ± SD of three experiments (*P < 0.05).
Expression of a dominant‐negative mutant of the transcription factor c‐Jun reduces c‐Fos promoter‐regulated reporter gene transcription and attenuates c‐Fos expression in HEK293 cells expressing activated TRPM3 channels
The mutational analysis of the murine c‐Fos promoter revealed that the AP‐1 site within the promoter is involved in connecting TRPM3 stimulation with an increase in c‐Fos promoter activity. We also recently showed that stimulation of TRPM3 channels with pregnenolone sulfate induces the biosynthesis of c‐Jun in HEK293 cells expressing TRPM3 (Lesch et al., 2014). To corroborate the role of c‐Jun in the regulation of c‐Fos promoter activity, we expressed the dominant‐negative mutant c‐Jun∆N in HEK293 cells containing a tetracycline‐inducible TRPM3 expression unit. The modular structure of c‐Jun and the dominant‐negative mutant of c‐Jun, c‐Jun∆N, are depicted in Figure 7A. Figure 7B shows that expression of c‐Jun∆N significantly reduced c‐Fos promoter‐regulated reporter gene transcription in pregnenolone sulfate‐stimulated HEK293 cells that expressed TRPM3 channels. Expression of c‐Jun∆N also attenuated the up‐regulation of c‐Fos expression in HEK293 cells expressing activated TRPM3 channels (Figure 7C and D). Thus, c‐Jun is required to connect TRPM3 stimulation with enhanced c‐Fos promoter activity and c‐Fos expression.
Figure 7.
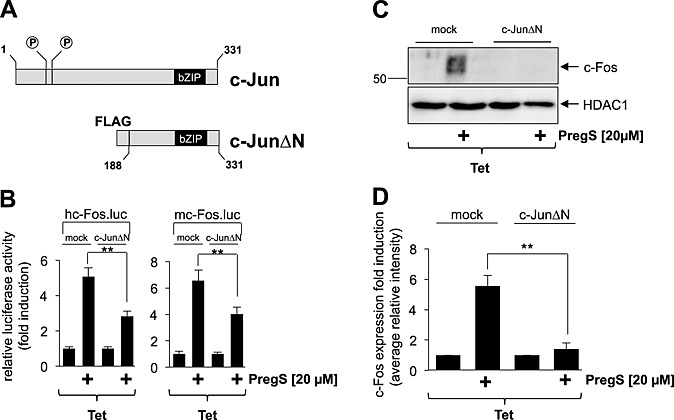
The transcription factor c‐Jun connects TRPM3 activation with increased c‐Fos promoter activity and c‐Fos expression. (A) Modular structure of c‐Jun and the dominant‐negative form c‐JunΔN. The mutant encompasses amino acid residues 188 to 331 of c‐Jun, retaining the bZIP domain responsible for DNA binding and dimerization, but lacking the NH2‐terminal transcriptional activation domain. (B) HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a lentivirus encoding the luciferase reporter gene under the control of the human (left panel) or murine (right panel) c‐Fos promoter. In addition, cells were infected with a lentivirus encoding the c‐Jun mutant c‐JunΔN. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD of three experiments performed in quadruplicate (**P < 0.01). (C) Expression of c‐JunΔN attenuates c‐Fos expression in pregnenolone sulfate‐stimulated HEK293 cells expressing TRPM3. HEK293 cells containing a tetracycline‐inducible TRPM3 transcription unit were infected with a recombinant lentivirus encoding c‐JunΔN. As a control, cells were infected with lentiviral stocks prepared with the lentiviral transfer vector pFUW (mock). Cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM). Nuclear extracts were prepared and subjected to Western blot analysis using an antibody directed against the c‐Fos protein. The antibody directed against HDAC1 was used as a loading control. (D) Quantification of c‐Fos expression. The intensity of the c‐Fos signal was normalized to the intensity of the HDAC1 control. Data shown are mean ± SD of three independent experiments (**P < 0.01).
Stimulation of TRPM3 channels with pregnenolone sulfate increases the transcriptional activation potential of c‐Fos
The previous experiments analysed the intracellular signalling molecules responsible for the up‐regulation of c‐Fos gene transcription following stimulation of TRPM3 channels. Now, we asked whether the transcriptional activation potential of c‐Fos is increased under these conditions as well. We expressed a GAL4‐c‐Fos fusion protein in HEK293 cells containing a tetracycline‐regulated TRPM3 transcription unit as a tool to examine the transcriptional activation potential of c‐Fos (Figure 8A). In addition, we incorporated a GAL4‐responsive reporter gene into the chromatin of the cells to measure the biological activity of the GAL4‐c‐Fos fusion protein. The transcriptional activation potential of c‐Fos was increased in pregnenolone sulfate‐stimulated HEK293 cells that expressed TRPM3, the GAL4‐c‐Fos fusion protein and the GAL4‐responsive reporter gene (Figure 8C). As a control, we stimulated the cells with either forskolin or TPA. In these experiments, cells were not treated with tetracycline, so that TRPM3 was not expressed. Figure 8D and E shows that while the phorbol ester TPA enhanced the transcriptional activation potential of c‐Fos, forskolin treatment did not increase c‐Fos activity.
Figure 8.
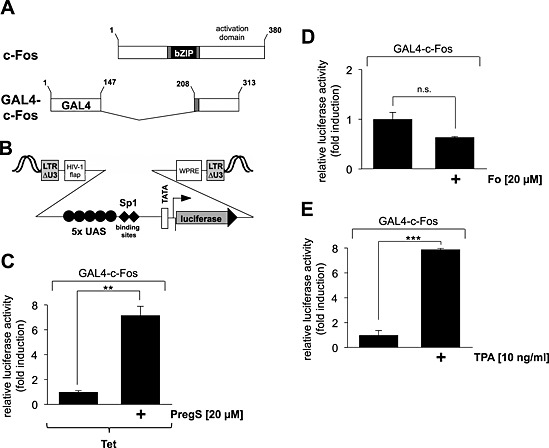
Stimulation of TRPM3 channels up‐regulates the transcriptional activation potential of c‐Fos. (A) Schematic representation of the modular structure of c‐Fos and GAL4‐c‐Fos. The bZIP DNA‐binding and dimerization domains are depicted. The transcriptional activation domain is localized on the C‐terminus. The GAL4‐c‐Fos fusion protein lacks the bZIP domain but retains the C‐terminal activation domain of c‐Fos. The truncated c‐Fos protein is expressed as a fusion protein together with the N‐terminal DNA‐binding domain of GAL4. (B) Schematic representation of the integrated provirus encoding a luciferase reporter gene under the control of the minimal promoter, consisting of five GAL4 binding sites [upstream activating sequence (UAS)], two Sp1 binding sites, a TATA box and an initiator element. (C) HEK293 cells containing a tetracycline‐inducible TRPM3 expression unit were double infected with a lentivirus containing the GAL4‐responsive luciferase reporter gene and a lentivirus encoding GAL4‐c‐Fos. The cells were serum starved for 24 h in the presence of tetracycline (1 μg·mL−1) and then stimulated with pregnenolone sulfate (PregS, 20 μM) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD from three independent experiments performed in quadruplicate (**P < 0.01). (D, E) HEK293 cells containing a tetracycline‐inducible TRPM3 expression unit were double infected with a lentivirus containing the GAL4‐responsive luciferase reporter gene and a lentivirus encoding GAL4‐c‐Fos. The cells were serum starved for 24 h and then stimulated with either forskolin (Fo, 20 μM) (D) or TPA (10 ng·mL−1) (E) for 24 h. Cell extracts were prepared and analysed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean ± SD of three experiments performed in quadruplicate (n.s., not significant; **P < 0.01).
Discussion
The c‐Fos protein is a transcription factor that contains a central ‘core’, a basic region leucine zipper (bZIP) domain, for DNA binding and dimerization. The transactivation domain is located at the C‐terminus. c‐Fos dimerizes with bZIP proteins from the Jun family of transcription factors to generate the AP‐1 transcription factor complex. Targeted inactivation of the c‐Fos gene in transgenic mice resulted in viable mice that were growth retarded and lacked osteoclasts (Johnson et al., 1992). In the nervous system, c‐Fos expression is induced by neuronal activity. Accordingly, an up‐regulation of c‐Fos immunoreactivity is frequently used as an indirect marker to identify activated neurons. Elimination of c‐Fos expression in the hippocampus revealed that c‐Fos is essential for neuronal excitability and survival. The mutant mice exhibited more severe kainic acid‐induced seizures, increased neuronal excitability and neuronal cell death in comparison with wild‐type mice (Zhang et al., 2002).
Expression of the c‐Fos gene is induced by various means, leading to an increase in the intracellular Ca2+ concentration. The route of Ca2+ entry plays a key role in selecting which signal transducers and transcription factor complexes are activated to trigger an up‐regulation of c‐Fos expression. The objective of this study was to investigate the intracellular signalling cascade connecting Ca2+ influx via TRPM3 channels with an up‐regulation of c‐Fos expression and activity. In various cell types, it has been shown that stimulation of L‐type voltage‐gated Ca2+ channels results in an up‐regulation of c‐Fos expression (Thompson et al., 1995; Rajadhyaksha et al., 1999; Jinnah et al., 2003; Zhao et al., 2007). Thus, the use of insulinoma cells as cellular model system, as previously used in our laboratory, would make it difficult to investigate the role of Ca2+ influx via TRPM3 channels on c‐Fos expression, as the effects of pregnenolone sulfate on TRPM3 and L‐type voltage‐gated Ca2+ channels could not be distinguished. We therefore switched to an engineered HEK293 cell line that expresses TRPM3 but not L‐type voltage‐gated Ca2+ channels.
Recent experiments performed with insulinoma cells showed that both TRPM3 channels and L‐type voltage‐dependent Ca2+ channels are required to induce gene transcription following application of pregnenolone sulfate to the cells (Mayer et al., 2011; Müller et al., 2011). We therefore hypothesized that stimulation of TRPM3 with pregnenolone sulfate triggers the depolarization of the plasma membrane of insulinoma cells that, in turn, activates L‐type voltage‐gated Ca2+ channels. As a result, the influx of Ca2+ into the cells increases, which initiates an intracellular signalling cascade, leading to changes in the gene expression pattern of the cells.
The connection between a rise in cytoplasmic Ca2+ and an up‐regulation of c‐Fos gene transcription is accomplished by protein kinases, and MAPKs, PKC and Ca2+/calmodulin‐dependent protein kinases have been proposed to function as signal transducers (Johnson et al., 1997; Dolmetsch et al., 2001; Ely et al., 2011; Glidewell‐Kenney et al., 2014). The experiments described in this study shed light on the importance of MAPKs in the regulation of c‐Fos expression by a TRPM3‐induced signalling cascade. We showed that overexpression of either MKP‐1 or MKP‐5, enzymes that dephosphorylate and inactivate MAPKs in the nucleus, attenuated pregnenolone sulfate‐induced transcription of a c‐Fos promoter‐controlled reporter gene. Thus, the nuclear phosphatases MKP‐1 and MKP‐5 function as nuclear shut‐off devices, as shown for other signalling pathways (Rössler et al., 2008; Rössler and Thiel, 2009; Mayer et al., 2011; Thiel and Rössler, 2011, 2014; Thiel et al., 2012; Kaufmann et al., 2013), which interrupt the signalling cascades induced by pregnenolone sulfate stimulation of TRPM3 channels. Future work will elucidate which of the MAPKs are in fact required to stimulate c‐Fos promoter activity following the influx of Ca2+ ions via activated TRPM3 channels.
Recently, we showed that transcription of a CRE‐controlled reporter gene is up‐regulated in insulinoma cells following pregnenolone sulfate treatment (Müller et al., 2011). We concluded that the CRE functions as a pregnenolone sulfate‐response element. As phosphorylation of CREB and stimulation of CRE‐mediated gene transcription are also induced in various cell types following opening of L‐type Ca2+ channels (Thompson et al., 1995; Rajadhyaksha et al., 1999), the results of the insulinoma study did not unequivocally prove that TRPM3 stimulation is responsible for the activation of CRE‐containing genes in pregnenolone sulfate‐stimulated cells. Using HEK293 cells as a cellular model system that are devoid of L‐type voltage‐gated Ca2+ channels, we showed in this study that stimulation of the cells with pregnenolone sulfate up‐regulated the transcription of a reporter gene that was controlled solely by the CRE derived from the human c‐Fos gene. Thus, elevation of the intracellular Ca2+ concentration, via stimulation of either TRPM3 or L‐type voltage‐gated Ca2+ channels, is sufficient to activate transcription of CRE‐containing genes. Hence, the CRE does not only connect elevated cAMP concentrations with enhanced c‐Fos promoter activity but also mediates the effect of elevated Ca2+ levels upon transcription. The CRE within the c‐Fos promoter has therefore been termed Ca2+ response element (Sheng et al., 1990). Deletion of this motif within the c‐Fos promoter completely abolished the responsiveness to elevated cAMP levels within the cells, but the ability to respond to pregnenolone sulfate stimulation in TRPM3 expressing HEK293 cells was retained. Thus, in addition to the CRE, other Ca2+‐responsive genetic elements function as response elements for the signalling pathway induced by activation of TRPM3.
In addition to the CRE, the SRE of the c‐Fos gene has been identified as a second integrator of Ca2+ signalling pathways within the cells (Lee and Gilman, 1994; Misra et al., 1994; Xia et al., 1996; Johnson et al., 1997). In fact, the molecular biology of SRE‐mediated gene transcription was elucidated in the analysis of the c‐Fos gene (Cahill et al., 1995; Treisman, 1995). In the nervous system, a rise of intracellular Ca2+, either via activating neurotransmitter receptors (i.e. NMDA receptors) or by stimulating L‐type voltage‐gated Ca2+ channels, induces transcription of the c‐Fos gene mediated by the SRE (Misra et al., 1994; Xia et al., 1996). The SRE is composed of the SRF binding site encompassing the consensus sequence CC[A/T]6GG (the CArG box) and TCF binding sites adjacent to the CArG box with the Ets consensus core sequence GGAA/T. Mutational analysis of the c‐Fos promoter revealed that the SRF site is necessary for connecting TRPM3 signalling with enhanced c‐Fos promoter activity. The adjacent TCF binding site could be mutated without having any negative effect upon stimulus‐transcription coupling. Similarly, activation of the c‐Fos promoter by stimulating G protein‐coupled receptors requires SRF, but not the binding of TCFs (Hill and Treisman, 1995; Ely et al., 2011), indicating that Ca2+ influx into the cytosol either via activation of voltage‐gated or ionotropic Ca2+ channels or via the IP3/IP3 receptor pathway activates distinct signalling molecules to up‐regulate c‐Fos expression. Recently, we showed that TRPM3 activation up‐regulates the transcriptional activation potential of the TCF Elk‐1 (Lesch et al., 2014), one of the major MAPK substrates, that connects intracellular signalling cascades with SRE‐mediated transcription. Moreover, we showed that a dominant‐negative mutant of Elk‐1 attenuated TRPM3‐induced transcription of reporter genes under the control of either the Egr‐1, c‐Fos or the collagenase promoter (Mayer et al., 2011; Lesch et al., 2015; this study). Thus, Elk‐1 is a target for the pregnenolone sulfate‐induced signalling cascade via activation of TRPM3. As TCF activation is required, together with an SRF dimer, to activate transcription via the SRE, other cryptic TCF binding sites within the c‐Fos promoter may compensate for the mutation of the Ets site 5´ of the CArG box. Alternatively, Elk‐1 may be recruited to the c‐Fos promoter via protein–protein interaction with SRF, as described recently for the GnRH‐induced activation of c‐Fos expression in gonadotropes (Ely et al., 2011) or the neurokinin B‐stimulated c‐Fos transcription in immortalized GnRH neurons (Glidewell‐Kenney et al., 2014). This scenario would explain the biological effects of the Elk‐1 mutant REST/Elk‐1∆C reported in this study, which retains the B‐domain of Elk‐1 and therefore interfered with the Elk‐1–SRF interaction. The regulation of c‐Fos gene transcription by TCF also explains previously published data showing that TCF activation is essential to connect TRPM3 stimulation with enhanced AP‐1‐mediated gene transcription (Lesch et al., 2015).
Mutational analysis of the c‐Fos promoter revealed that the AP‐1 binding site, in addition to the CRE and the CArG box, confers responsiveness to an increase in cytoplasmic Ca2+ as a result of TRPM3 activation. This site has been shown to mediate c‐Fos gene transcription following stimulation with EGF or phorbol ester (Fisch et al., 1989). In addition, in T‐cells, the AP‐1 site controls c‐Fos mRNA induction by intracellular Ca2+ (Lee and Gilman, 1994). Using a dominant‐negative approach, we have proved that this site contributes to the up‐regulation of reporter gene transcription controlled by the c‐Fos promoter in HEK293 cells expressing activated TRPM3 channels. These experiments shed light on the importance of the bZIP protein c‐Jun within the TRPM3‐induced signalling cascade. Originally, AP‐1 was described as a heterodimer of c‐Jun and c‐Fos. c‐Jun is involved in regulating c‐Fos expression and also regulates its own expression via AP‐1 binding sites within the c‐Jun gene regulatory region (Thiel and ; this study). In addition, our experiments support previous observations that c‐Jun functions as a Ca2+‐responsive transcriptional activator (Cruzalegui et al., 1999; Müller et al., 2011).
In summary, we present here the first study characterizing the signalling molecules required to up‐regulate c‐Fos expression as a result of Ca2+ entry in the cells via activated TRPM3 channels. This study shows that the activation of MAPKs is essential for connecting a rise in cytoplasmic Ca2+ via TRPM3 channels with enhanced c‐Fos promoter activity. In the nucleus, c‐Fos expression requires three Ca2+‐responsive elements, the CRE, the CArG box and the AP‐1 binding site, involving the transcription factors CREB, c‐Jun and SRF following stimulation of TRPM3 expressing cells with pregnenolone sulfate. Experiments employing a dominant‐negative mutant of Elk‐1 additionally suggest an important role for TCFs in the regulation of c‐Fos gene transcription in cells expressing activated TRMP3 channels. The stimulation of c‐Fos expression and activity via pregnenolone sulfate‐induced TRPM3 activation connects TRPM3 with the biological functions of the AP‐1 transcription factor that is involved in the regulation of proliferation, transformation, differentiation and programmed cell death (Shaulian and Karin, 2002).
Author contributions
G. T. designed the research study, contributed essential reagents and wrote the manuscript; S. R. and O. G. R. did the experiments and G. T., O. G. R. and S. R. analysed the data.
Conflict of interest
None declared.
Acknowledgements
We thank David Beech and Yasser Majeed for their generous gift of HEK293 cells containing a tetracycline‐regulated TRPM3 expression unit and Djurdjica Coss for the murine c‐Fos promoter constructs. We thank Libby Guethlein for critical reading of the manuscript. This work was supported by Saarland University.
Rubil, S. , Rössler, O. G. , and Thiel, G. (2016) CREB, AP‐1, ternary complex factors and MAP kinases connect transient receptor potential melastatin‐3 (TRPM3) channel stimulation with increased c‐Fos expression. British Journal of Pharmacology, 173: 305–318. doi: 10.1111/bph.13372.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al (2013a). The Concise Guide to PHARMACOLOGY 2013/14:ligand‐gated ion channels. Br J Pharmacol 170: 1582–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14:enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TM, Mackay DS, Siegfried CJ, Shiels A (2014). Mutation of the melastatin‐related cation channel, TRPM3, underlies inherited cataract and glaucoma. PLoS One 9: e104000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Xiong W‐H, Peters JH, Tekmen‐Clark M, Strycharska I, Reed BT, et al. (2015). TRPM3 expression in mouse retina. PLoS One. 10: e0117615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill MA, Janknecht R, Nordheim A (1995). Signal uptake by the c‐fos serum response element In: Baeuerle PA. Inducible Gene Expression, Vol. 2 Birkhäuser: Boston/Basel/Berlin, pp. 39–72. [Google Scholar]
- Colsoul B, Vennekens R, Nilius B (2011). Transient receptor potential cation channels in pancreatic beta cells. Rev Physiol Biochem Pharmacol 161: 87–110. [DOI] [PubMed] [Google Scholar]
- Cruzalegui FH, Hardingham GE, Bading H (1999). c‐Jun functions as a calcium‐regulated transcriptional activator in the absence of JNK/SAPK1 activation. EMBO J 18: 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME (2001). Signaling to the nucleus by an L‐type calcium channel‐calmodulin complex through the MAP kinase pathway. Science 294: 333–339. [DOI] [PubMed] [Google Scholar]
- Ekici M, Keim A, Rössler OG, Hohl M, Thiel G (2012). Chromatin structure and expression of the AMPA receptor subunit GluR2 in human glioma cells: major role of REST and Sp1. J Cell Biochem 113: 528–543. [DOI] [PubMed] [Google Scholar]
- Ely HA, Mellon PL, Coss D (2011). GnRH induces the c‐Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol 25: 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch TM, Prywes R, Roeder RG (1989). An AP‐1 binding site in the c‐fos gene can mediate induction by epidermal growth factor and 12‐O‐tetradecanoyl phorbol‐13‐acetate. Mol Cell Biol 9: 1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin WJ, Greenberg ME (1995). Calcium regulation of gene expression in neurons: the mode of entry matters. Curr Opin Neurobiol 5: 367–374. [DOI] [PubMed] [Google Scholar]
- Glidewell‐Kenney CA, Trang C, Shao PP, Gutierrez‐Reed N, Uzo‐Okereke AM, Coss D, et al. (2014). Neurokinin B induces c‐fos transcription via protein kinase C and activation of serum response factor and Elk‐1 in immortalized GnRH neurons. Endocrinology 155: 3909–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Sauerbruch S, Schulz G, Harteneck C (2003). Molecular and functional characterization of the melastatin‐related cation channel TRPM3. J Biol Chem 278: 21493–21501. [DOI] [PubMed] [Google Scholar]
- Hall DP, Cost NG, Hegde S, Kellner E, Mikhaylova O, Stratton Y, et al. (2014). TRPM3 and miR‐204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell 26: 738–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Treisman R (1995). Differential activation of c‐fos promoter elements by serum, lysophosphatidic acid, G proteins and polypeptide growth factors. EMBO J 14: 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Egami K, Rao L, Shin M, Kasim S, Hess EJ (2003). Expression of c‐Fos in the brain after activation of L‐type calcium channels. Dev Neurosci 25: 403–411. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Hill CS, Chawla S, Treisman R, Bading H (1997). Calcium controls gene expression via three distinct pathways that can function independently of the Ras/mitogen‐activated protein kinases (ERKs) signaling cascade. J Neurosci 15: 6189–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaionnou V (1992). Pleiotropic effects of a null mutation in the c‐fos proto‐oncogene. Cell 71: 577–586. [DOI] [PubMed] [Google Scholar]
- Kaufmann A, Keim A, Thiel G (2013). Regulation of immediate‐early gene transcription following activation of Gαq‐coupled designer receptors. J Cell Biochem 114: 681–696. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Thiel G (2002). Epidermal growth factor and thrombin induced proliferation of immortalized human keratinocytes is coupled to the synthesis of Egr‐1, a zinc finger transcriptional regulator. J Cell Biochem 85: 381–391. [DOI] [PubMed] [Google Scholar]
- Keim A, Müller I, Thiel G (2012). Efficient genetic manipulation of 1321 N1 astrocytoma cells using lentiviral gene transfer. J Neurosci Methods 206: 138–142. [DOI] [PubMed] [Google Scholar]
- Koga K, Takaesu G, Yoshida R, Nakaya M, Kobayashi T, Kinjyo I, et al. (2009). Cyclic adenosine monophosphate suppresses the transcription of proinflammatory cytokines via the phosphorylated c‐Fos protein. Immunity 30: 372–383. [DOI] [PubMed] [Google Scholar]
- Lee G, Gilman M (1994). Dual modes of control of c‐fos mRNA induction by intracellular calcium in T cells. Mol Cell Biol 14: 4579–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Chen J, Sun L, Wu S, Gray KR, Rich A, et al. (2003). Expression and characterization of human transient receptor potential melastatin 3 (hTRPM3). J Biol Chem 278: 20890–20897. [DOI] [PubMed] [Google Scholar]
- Lesch A, Rubil S, Thiel G (2014). Activation and inhibition of transient receptor potential TRPM3‐induced gene transcription. Br J Pharmacol 171: 2645–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch A, Hui X, Lipp P, Thiel G (2015). Transient receptor potential melastatin‐3 (TRPM3)‐induced activation of AP‐1 requires Ca2+ ions and the transcription factors c‐Jun, ATF2, and ternary complex factor. Mol Pharmacol 87: 617–628. [DOI] [PubMed] [Google Scholar]
- Majeed Y, Agarwal AK, Naylor J, Seymour VAL, Jiang S, Muraki K, et al. (2010). Cis‐isomerism and other chemical requirements of steroid agonists and partial agonists acting at TRPM3 channels. Br J Pharmacol 161: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SI, Müller I, Mannebach S, Endo T, Thiel G (2011). Signal transduction of pregnenolone sulfate in insulinoma cells. Activation of Egr‐1 expression involving TRPM3, voltage‐gated calcium channels, ERK, and ternary complex factors. J Biol Chem 286: 10084–10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SI, Rössler OG, Endo T, Charnay P, Thiel G (2009). Epidermal growth factor‐induced proliferation of astrocytes requires Egr transcription factors. J Cell Sci 122: 3340–3350. [DOI] [PubMed] [Google Scholar]
- Mayer SI, Thiel G (2009). Calcium influx into MIN6 insulinoma cells induces expression of Egr‐1 involving extracellular signal‐regulated protein kinase and the transcription factors Elk‐1 and CREB. Eur J Cell Biol 88: 19–33. [DOI] [PubMed] [Google Scholar]
- Mayer SI, Willars GB, Nishida E, Thiel G (2008). Elk‐1, CREB, and MKP‐1 regulate Egr‐1 expression in gonadotropin‐releasing hormone stimulated gonadotrophs. J Cell Biochem 105: 1267–1278. [DOI] [PubMed] [Google Scholar]
- Misra RP, Bonni A, Miranti CK, Rivera VM, Sheng M, Greenberg ME (1994). L‐type voltage‐sensitive calcium channel activation stimulates gene expression by a serum response factor‐dependent pathway. J Biol Chem 269: 25483–25493. [PubMed] [Google Scholar]
- Monje P, Marinissen MJ, Gutkind JS (2003). Phosphorylation of the carboxy‐terminal transactivation domain of c‐Fos by extracellular signal‐regulated protein kinase mediates the transcriptional activation of AP‐1 and cellular transformation induced by platelet‐derived growth factor. Mol Cell Biol 23: 7030–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I, Endo T, Thiel G (2010). Regulation of AP‐1 activity in glucose‐stimulated insulinoma cells. J Cell Biochem 110: 1481–1494. [DOI] [PubMed] [Google Scholar]
- Müller I, Rössler OG, Thiel G (2011). Pregnenolone sulfate activates basic region leucine zipper transcription factors in insulinoma cells: role of voltage‐gated Ca2+ channels and transient receptor potential melastatin 3 channels. Mol Pharmacol 80: 1179–1189. [DOI] [PubMed] [Google Scholar]
- Naylor J, Li J, Milligan CJ, Zeng F, Sukumar P, Hou B, et al. (2010). Pregnenolone sulphate‐ and cholesterol‐regulated channels coupled to vascular smooth muscle secretion and contraction. Circ Res 106: 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ (2008). Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol 155: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler J, Lis A, Giehl KM, Flockerzi V, Philipp SE (2005). Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J Biol Chem 280: 22899–22906. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledgebase of drug targets and their ligands. Nucleic Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C (1999). L‐type Ca2+ channels are essential for glutamate‐mediated CREB phosphorylation and c‐fos gene expression in striatal neurons. J Neurosci 19: 6348–6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler OG, Henß I, Thiel G (2008). Transcriptional response to muscarinic acetylcholine receptor stimulation: regulation of Egr‐1 biosynthesis by Elk‐1, ERK, MKP‐1 and calcineurin in carbachol stimulated human neuroblastoma cells. Arch Biochem Biophys 470: 93–102. [DOI] [PubMed] [Google Scholar]
- Rössler OG, Thiel G (2009). Thrombin induces Egr‐1 expression in fibroblasts involving elevation of the intracellular Ca2+ concentration, phosphorylation of ERK and activation of ternary complex factor. BMC Mol Biol 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro PS, Ahn NG (1998). Feedback regulation of Raf‐1 and mitogen‐activated protein kinase (MAP) kinase kinase 1 and 2 by MAP kinase phosphatase‐1 (MKP‐1). J Biol Chem 273: 1788–1793. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2002). AP‐1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136. [DOI] [PubMed] [Google Scholar]
- Sheng M, McFadden G, Greenberg ME (1990). Membrane depolarization and calcium induce c‐fos transcription via phosphorylation of transcription factor CREB. Neuron 4: 571–582. [DOI] [PubMed] [Google Scholar]
- Slack DN, Seternes O‐M, Gabrielsen M, Keyse SM (2001). Distinct binding determinants for ERK2/p38α and JNK MAP kinases mediate catalytic activation and substrate selectivity of MAP kinase phosphatase‐1. J Biol Chem 276: 16491–16500. [DOI] [PubMed] [Google Scholar]
- Spohn D, Rössler OG, Philipp SE, Raubuch M, Kitajima S, Griesemer D, et al. (2010). Thapsigargin induces expression of activating transcription factor 3 in human keratinocytes involving Ca2+ ions and c‐Jun N‐terminal protein kinase. Mol Pharmacol 78: 865–876. [DOI] [PubMed] [Google Scholar]
- Thiel G, Kaufmann K, Magin A, Lietz M, Bach K, Cramer M (2000). The human transcriptional repressor protein NAB1: expression and biological activity. Biochim Biophys Acta 1493: 289–301. [DOI] [PubMed] [Google Scholar]
- Thiel G, Lesch A, Keim A (2012). Transcriptional response to calcium‐sensing receptor stimulation. Endocrinology 153: 4716–4728. [DOI] [PubMed] [Google Scholar]
- Thiel G, Müller I, Rössler OG (2013). Signal transduction via TRPM3 in pancreatic β‐cells. J Mol Endocrinol 50: R75–R83. [DOI] [PubMed] [Google Scholar]
- Thiel G, Rössler OG (2011). Immediate‐early transcriptional response to angiotensin II in human adrenocortical cells. Endocrinology 152: 4211–4223. [DOI] [PubMed] [Google Scholar]
- Thiel G, Rössler OG (2014). Resveratrol stimulates AP‐1‐regulated gene transcription. Mol Nutr Food Res 58: 1402–1413. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ginty DD, Bonni A, Greenberg ME (1995). L‐type voltage‐sensitive Ca2+ channel activation regulates c‐fos transcription at multiple levels. J Biol Chem 270: 4224–4235. [DOI] [PubMed] [Google Scholar]
- Treisman R (1995). Journey to the surface of the cell: Fos regulation and the SRE. EMBO J 14: 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Held K, Janssens A, Tóth BI, Kerselaers S, Nilius B, et al. (2014). Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nat Chem Biol 10: 188–195. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. (2011). TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70: 482–494. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Drews A, Loch S, Mohr F, Philipp SE, Lambert S, et al. (2010). TRPM3 channels provide a regulated influx pathway for zinc in pancreatic beta cells. Pflugers Arch 460: 755–765. [DOI] [PubMed] [Google Scholar]
- Wagner TFJ, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. (2008). Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat. Cell Biol. 10: 1421–1430. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME (1996). Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK‐dependent mechanism. J Neurosci 16: 5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio‐Bulcock PA, Everett J, Harteneck C, Valenzuela CF (2011). Activation of steroid‐sensitive TRPM3 channels potentiates glutamaterigic transmission of cerebellar Purkinje neurons from developing rats. J Neurochem 119: 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M (2002). c‐fos regulates neuronal excitability and survival. Nat Genet 30: 416–420. [DOI] [PubMed] [Google Scholar]
- Zhao R, Liu L, Rittenhouse AR (2007). Ca2+ influx though both L‐ and N‐type Ca2+ channels increases c‐fos expression by electrical stimulation of synpathetic neurons. Eur J Neurosci 25: 1127–1135. [DOI] [PubMed] [Google Scholar]


