Abstract
Background and Purpose
The sympathetic nervous system regulates bone remodelling, in part, through ß2‐adrenoceptor signalling. However, the physiological role of α1‐adrenoceptor signalling in bone in vivo remains unclear. Therefore, to obtain a deeper understanding of bone remodelling by the sympathetic nervous system, we investigated the role of α1B‐adrenoceptor signalling in bone metabolism.
Experimental Approach
Prazosin, a nonspecific α1‐adrenoceptor antagonist, was administered for 2 weeks in C57BL6 mice, and efficacy was evaluated by bone microarchitecture using microcomputed tomography and determination of bone formation by fluorescent labelling of bone. We also compared the bone phenotype of α1B‐adrenoceptor null mice (α1B −/−) with that of wild‐type littermates.
Key Results
We demonstrated that the systemic administration of prazosin decreased bone formation. In addition, α1B‐adrenoceptor‐deficient mice had a lower bone mass due to decreased bone formation but did not exhibit any changes in bone‐resorbing activity. Furthermore, stimulation with phenylephrine, a non‐specific α1‐adrenoceptor agonist, increased the expression of the transcriptional factor CCAAT/enhancer‐binding protein δ (Cebpd) in MC3T3‐E1 osteoblastic cells. The overexpression of Cebpd induced cellular proliferation in MC3T3‐E1 cells, whereas the silencing of Cebpd suppressed it.
Conclusions and Implications
Taken together, these results suggested that α1B‐adrenoceptor signalling is required for bone formation and regulated cellular proliferation through a mechanism relevant to the up‐regulation of Cebpd in osteoblasts and, thus, provide new evidence for the physiological importance of α1B‐adrenoceptor signalling in bone homeostasis.
Abbreviations
- BFR
bone formation rate
- BMSC
bone marrow stromal cells
- BV/TV
bone volume per total volume
- Cebpd
CCAAT/enhancer‐binding protein δ
- Ctsk
cathepsin K
- MAR
bone mineral apposition rate
- MS/BS
mineral surface per bone surface
- Nfatc1
nuclear factor of activated T‐cells, cytoplasmic‐1
- Ob.N/BS
osteoblast number per bone surface
- OC
osteocalcin
- Oc.N/BS
osteoclast number per bone surface
- Oc.S/BS
osteoclast surface per bone surface
- Osx
osterix
- RANKL
receptor activator of nuclear factor‐kB ligand
- Runx2
runt‐related transcription factor 2
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- Tb.Th
trabecular thickness
- TRAP
tartrate‐resistant acid phosphatase
- WT
wild type
- μCT
μ‐computed tomography
Tables of Links
| TARGETS | |
| GPCRs a | Catalytic receptors b |
| α1A‐adrenoceptor | Osteoprotegerin (OPG) |
| α1B‐adrenoceptor | Enzymes c |
| α1D‐adrenoceptor | Cathepsin K |
| ß2‐adrenoceptor |
| LIGANDS | |
| Noradrenaline | Prazosin |
| Phenylephrine | RANKL |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a, 2015b, 2015c).
Introduction
Throughout life, bone homeostasis is maintained by remodelling and balancing osteoclast‐mediated bone resorption and osteoblast‐mediated bone formation. Under normal conditions bone remodelling is regulated by systemic hormones and paracrine/autocrine factors. Recent studies have demonstrated that the sympathetic nervous system plays a role in bone metabolism (Takeda et al., 2002; Elefteriou et al., 2005; Togari et al., 2005). The actions of noradrenaline (NA) are known to be mediated through interactions with the membrane‐bound adrenoceptor, which has been categorized into three families: α1, α2 and ß, with at least nine subtypes being identified to date: α1A‐, α1B‐, α1D‐, α2A‐, α2B‐, α2C‐, ß1‐, ß2‐ and ß3‐adrenoceptors (Kobilka, 2011). All adrenoceptor families belong to the GPCR superfamily. We previously reported the expression of mRNA for α1B‐ and ß2‐adrenoceptors in human osteoblasts (Togari, 2002). Evidence has accumulated to show that the sympathetic nervous system regulates bone remodelling and tooth movement, in part, through ß2‐adrenoceptors (Takeda et al., 2002; Elefteriou et al., 2005; Togari et al., 2005; Kondo et al., 2013). Previous studies revealed that ß2‐adrenoceptor signalling in osteoblasts suppressed bone osteoblastgenesis and increased osteoclastogenesis, thereby enhancing bone resorption (Takeuchi et al., 2001; Takeda et al., 2002; Elefteriou et al., 2005; Togari et al., 2005). α1‐Adrenoceptors were previously shown to have an important role in regulating the functionality of osteoblasts (Suzuki et al., 1998, 1999; Huang et al., 2009; Hirai et al., 2014a, 2015a). We also reported direct nerve‐osteoblast communication in vitro and demonstrated that NA increased cell proliferation by suppressing K+ channels via Gi/o‐coupled α1B‐adrenoceptors in human osteoblasts (Obata et al., 2007; Kodama & Togari, 2013); however, the physiological role of α1‐adrenoceptor signalling in bone in vivo remains unclear. Thus, to obtain a deeper understanding of bone remodelling by the sympathetic nervous system, we investigated the role of α1‐adrenoceptor signalling in bone metabolism.
Transcription factor CCAAT/enhancer‐binding protein δ (C/EBPδ, Cebpd) is a member of the C/EBP family and plays a role in cellular functions. Six members of the C/EBP family have been characterized: α, ß, δ, γ, ε and ζ (Hanson, 1998; Yamanaka et al., 1998). Although all C/EBP family members share the same DNA‐binding specificity, they have highly diverse tissue‐specific functions (Ramji & Foka, 2002). C/EBPs act as regulators of gene expression by either direct DNA binding or interacting with other transcriptional activators (Gutierrez et al., 2002). Several studies have implicated Cebpd in the regulation of target genes of diverse biological functions that include growth arrest, apoptosis, differentiation, stem cell self‐renewal and tumour suppression (O'Rourke et al., 1999; Thangaraju et al., 2005; Gery et al., 2005; Barbaro et al., 2007; Sarkar et al., 2012; Balamurugan & Sterneck, 2013). In osteoblasts, Cebpd has been shown to activate the transcription of insulin‐like growth factor 1, which plays a key role in skeletal growth by stimulating bone cell replication and differentiation (Umayahara et al., 1999).
The findings of pharmacological studies using prazosin, an α1‐adrenoceptor antagonist, and genetic studies suggested that α1B‐adrenoceptor signalling regulates bone mass and cellular proliferation by regulating the expression of Cebpd in osteoblasts. The results obtained will, at least in part, elucidate the mechanism underlying the regulation of bone mass by the sympathetic nervous system.
Methods
Ethics statement
All mice were treated in accordance with the Guidelines for Animal Experiments at the School of Dentistry, Aichi Gakuin University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). A total of 217 mice were used in the experiments as detailed here. Food and water were available ad libitum. Animals were housed together in automatically controlled conditions of temperature (23 ± 1°C) and humidity (50 ± 10%) under a 12 h light : 12 h dark cycle.
Mice
α1B‐Adenoceptor‐deficient mice (α1B −/−) were provided by CARD (Center for Animal Resources and Development, Kumamoto University). The generation of α1B −/− was as described previously (Cavalli et al., 1997). The α1B −/− mice had been backcrossed onto the C57BL/6 J background for more than five generations. We used α1B −/− and their wild‐type (WT) littermates. The genotypes of the offspring were screened using PCR.
Drugs and treatment
C57BL/6 J mice were originally obtained from Japan SLC, Inc. (Hamamatsu, Japan). α1‐Adrenoceptor‐mediated pathways were stimulated using phenylephrine, a nonspecific α1‐adrenoceptor agonist (Sigma‐Aldrich, St. Louis, MO, USA). Prazosin, a non‐specific α1‐adrenoceptor antagonist (Sigma‐Aldrich), was dissolved in saline at a concentration of 10 mg·mL−1. Male, eight‐week‐old mice were randomized by wt., assigned to groups and acclimatized to their cages for 2 weeks prior to the experiment. Mice were treated with prazosin at 1, 3, 10 and 30 μg·kg−1 or saline i.p. every morning for 2 weeks. Body wt. was measured weekly, and the prazosin dose adjusted accordingly. Bone tissue samples were dissected and kept at −80°C for total RNA until assayed (Hirai et al., 2014b).
Bone densitometry and body composition analyses
The distal region of the right femur was subjected to a three‐dimensional μ‐computed tomography (μCT) analysis using an R‐mCT μCT scanner (RIGAKU, Tokyo, Japan). Scanning was initiated 1.0 mm above the distal femoral growth plate, and a total of 75 consecutive 20‐μm‐thick sections were analysed, encompassing a length of 1.5 mm of the secondary spongiosa. The measured volume of interest in the femur was obtained by selecting the cancellous bone (separate from the cortical shaft) using contour areas that were drawn semiautomatically (Tanaka et al., 2015). tri/3d‐bon (Ratoc, Tokyo, Japan) software was used to analyse the cancellous parameters: bone volume per total volume (BV/TV, %), trabecular number (Tb.N, 1 mm‐1), trabecular thickness (Tb.Th, μm) and trabecular separation (Tb.Sp, μm).
Bone histomorphometry
In the dynamic histomorphometric analysis, all mice were injected i.p. with calcein (10 μg g−1) at 4 and 2 days before death. At the end of the experiments, the right femur of each mouse was dissected and fixed in 70% ethanol. Five‐micrometre‐thick sagittal sections were made as undecalcified sections. To assess the bone formation rate (BFR), metaphyseal cancellous bone in the femur was used to obtain the bone fraction in a rectangular area of 0.34 mm2 (0.5 × 0.67 mm), with its closest and furthest edges being 0.3 and 0.8 mm distal to the growth plate respectively (Tanaka et al., 2015). Regarding decalcified sections, the left tibiae of mice were dissected, fixed in 4% paraformaldehyde and then decalcified in 20% EDTA for 2 weeks. Sagittal sections (5 μm thick) were made as decalcified sections and stained with tartrate‐resistant acid phosphatase (TRAP) for the osteoclast analysis. Measurements were made within an area of 0.8 mm2 (1.0 × 0.8 mm), with its closest and furthest edges being 2.0 and 3.0 mm distal to the growth plate of the proximal ends of the tibia respectively. Osteoclast number per bone surface (Oc.N/BS) and osteoclast surface per bone surface (Oc.S/BS) were evaluated by scoring TRAP‐positive multinucleated cells attached to the bone surface (Hirai et al., 2015b).
Cell cultures
MC3T3‐E1 cells were purchased from the RIKEN Cell Bank. MC3T3‐E1 cells were cultured in α‐MEM (Invitrogen, Carlsbad, CA, USA) containing 10% FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere. To induce differentiation, the culture medium was replaced with α‐MEM containing 50 μg·mL‐1 ascorbic acid and 5 mM ß‐glycerophosphate. The culture medium was changed every 2–3 days (Hirai et al., 2014a).
Construction of expression plasmids and transfection
The entire coding sequence of mouse Cebpd cDNA was amplified by KOD‐plus Neo DNA polymerase (Toyobo, Japan) and inserted into the mammalian expression vector pcDNA3. The primers used were as follows: forward 5′‐CGCGGA TCCCCAACTTGGACGCCAGGTC‐3′ and reverse 5′‐TGCTCT AGACAGAGTCTCAAAGGCCCACG‐3′. Amplified DNA was cloned into the pcDNA3 vector at the BamHI and XbaI restriction enzyme sites (underlined). The correct sequences of the subcloned cDNA fragments were confirmed by complete nucleotide sequencing (Hirai et al., 2014b). MC3T3‐E1 cells were plated at a density of 1.0 × 105 cells cm−2. Cells were transfected after 24 h with pcDNA‐Cebpd, or the empty vector using FuGENE HD reagent (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Generation of MC3T3‐E1 cell lines with the stable expression of Cebpd
MC3T3‐E1 cells were maintained in α‐MEM supplemented with 10% FBS, 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere. Two micrograms of pcDNA3‐negative and full‐length Cebpd were stably transfected into MC3T3‐E1 cells by FuGENE HD, followed by drug selection with 800 μg·mL−1 of the neomycin analogue G418. Resistant colonies were selected and expanded. Twenty stable lines (Cebpd series) were established. All subsequent experiments were conducted with three of these lines, Cebpd −1, −2 and −3, which expressed high levels of Cebpd.
siRNA nucleofection
MC3T3‐E1 cells were grown in α‐MEM supplemented with 10% FBS and 1% penicillin/streptomycin to ~70% confluency, followed by transient transfection with either siRNA targeting Cebpd or non‐silencing RNA diluted in Opti‐MEM using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. Silencer select siRNAs were used (Ambion/Applied Biosystems, Foster City, CA, USA). Cebpd siRNAs and non‐silencing RNA were both used at final concentrations of 10 nM. The medium was then replaced with fresh medium. Cells were harvested for total RNA extraction at the indicated time points.
5‐Bromo‐2′‐deoxyuridine (BrdU) incorporation assay
Cell proliferation activity was assessed by BrdU incorporation using the Cell Proliferation ELISA BrdU kit (Roche Applied Science, Penzberg, Germany) according to the manufacturer's instructions (Kodama & Togari, 2013). MC3T3‐E1 cells were grown in α‐MEM supplemented with 10% FBS and 1% penicillin/streptomycin to ~70% confluency, followed by transfection with either siRNA targeting Cebpd or non‐silencing RNA diluted in Opti‐MEM using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. After 6 h, culture media were replaced with fresh α‐MEM containing 10% FBS. BrdU‐labelling solution (100 μM) was added at 20 μL per well, and the cells were incubated for an additional 4 h at 37°C. After this, the labelling media were removed, cells were fixed and DNA was denatured with FixDenat solution. The cells were incubated with a peroxidase‐conjugated anti‐BrdU antibody for 1.5 h at room temperature. The cells were then washed three times with PBS, followed by the addition of substrate solution (tetraethyl‐benzidine) at 100 μL per well. After a 15 min incubation, 1 M H2SO4 was added at 25 μL per well to stop the peroxidase reaction, and the absorbance of wells was measured at 450 nm using Multiskan FC (Thermo Fisher Scientific, Waltham, MA, USA).
RNA extraction and real‐time PCR
Total RNA was isolated with an RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the protocol of the manufacturer. One microgram of RNA was reverse transcribed into cDNA using the QuantiTect Reverse Transcription Kit according to the protocol of the manufacturer (Qiagen). Gene expression was analysed with the Step‐One‐Plus real‐time PCR system with stepone software v2.0 (Applied Biosystems). Reactions were performed in 20 μL volumes using a QuantiTect SYBR Green PCR Kit (Qiagen). Cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative quantity for each sample was normalized to the average level of the constitutively expressed housekeeping gene Gapdh. The primers used are listed in Supporting Information Table S1. As needed, the amplified fragment by PCR was cloned and subjected to automated sequencing (Hirai et al., 2009).
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All data are expressed as mean ± SEM. The two‐tailed t‐test combined with Bonferroni's correction following one‐way anova was used for multiple comparisons. Differences with P values < 0.05 were considered significant.
Results
Decrease in bone mass by the systemic administration of prazosin to mice
To evaluate the functionality of α1‐adrenoceptor signalling in bone, we investigated the effects of the pharmacological blockade of α1‐adrenoceptor signalling on bone mass by performing μCT‐based bone densitometry in bones after the systemic administrations of prazosin, an α1‐adrenoceptor antagonist, at 10 and 30 μg·kg−1 for 2 weeks. As shown in Figure 1A, the bone volume per trabecular volume (BV/TV) of the distal end of the femur of 12‐week‐old males was significantly lower in prazosin‐treated mice at 30 μg·kg−1 than in saline‐treated mice. No significant differences were observed in the Tb.Th or Tb.N between mice injected i.p. with prazosin in the range of 1–30 μg·kg−1 and saline‐treated mice (Figure 1B, C). Moreover, Tb.Sp of the femur was significantly greater in prazosin‐treated mice in the range of 3–30 μg·kg−1 than in saline‐treated mice (Figure 1D). The administration of prazosin in the range of 1–1000 μg·kg−1 had no effect on body wt. These results suggest that the dose of prazosin used in this study did not have adverse effects. To examine the mechanisms responsible for bone loss due to the prazosin treatment, we performed a bone histomorphometric analysis on bone formation parameters by assessing calcein double labelling in the distal femur after the systemic administration of prazosin for 2 weeks. This analysis provided an in vivo estimate of osteoblastic activity with respect to the accumulation of bone mass. Bone formation parameters such as mineral surface per bone surface (MS/BS), the bone mineral apposition rate (MAR) and BFRs were significantly lower in prazosin‐treated mice than in saline‐treated mice (Figure 2A). We then examined the expression of gene‐related bone formation using a quantitative real‐time PCR analysis. We extracted total RNA from the distal end of the femur and analysed the mRNA expression of Runt‐related transcription factor 2 (Runx2), osterix (Osx) and osteocalcin (OC). Consistent with the decrease observed in bone formation, the mRNA expression of Runx2, Osx and OC in bone was significantly decreased in prazosin‐treated mice (Figure 2B), indicating that mice administered the α1‐adrenoceptor antagonist had a lower bone mass concomitant with decreased bone formation.
Figure 1.
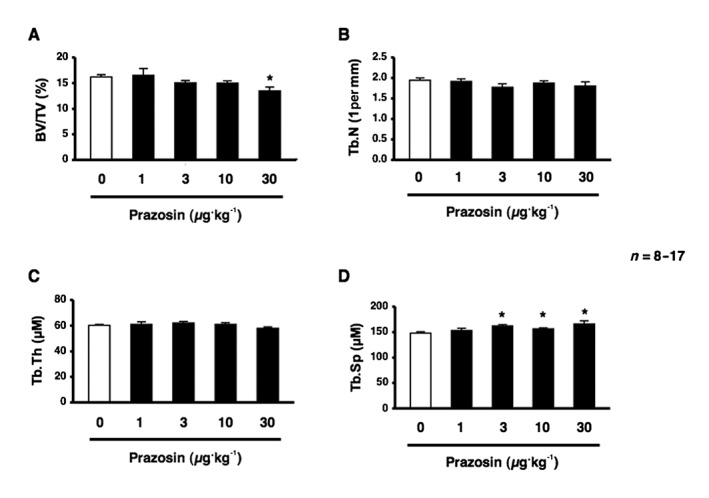
Blockade of α1‐adrenoceptor signalling impaired cancellous bone mass in mice. (A–D) μCT‐based bone densitometry of the distal region of the femur in 12‐week‐old male mice administered saline (white bar) or prazosin at 1, 3, 10 and 30 μg·kg−1 body wt. day−1 for 2 weeks. (A) BV/TV. (B) Tb.N. (C) Tb.Th. (D) Tb.Sp. n = 8 or 17 mice per group. Similar results were obtained in three independent experiments. Values are expressed as means ± SEM. * P < 0.05 significantly different from control mice.
Figure 2.
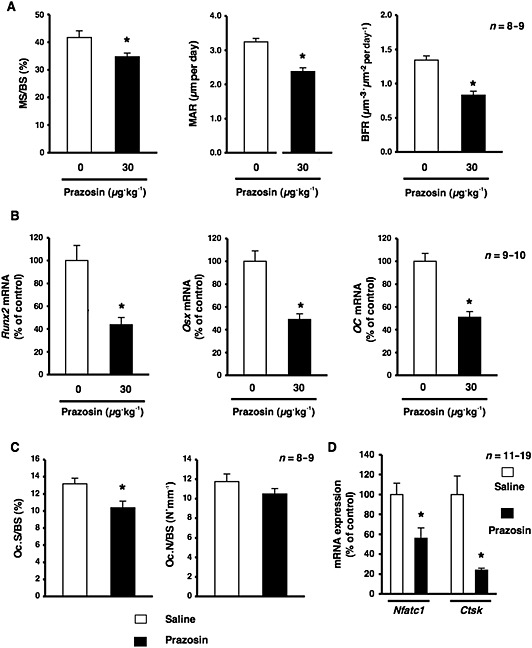
Effects of the systemic administration of prazosin on osteoblastic bone formation and resorption in mice. (A) An analysis of the MS/BS, MAR and BFR in the cancellous bone compartment of the distal femur metaphysis of mice administered saline and prazosin; n = 8 or 9 mice per group. Similar results were obtained in three independent experiments. Values are expressed as means ± SEM; * P < 0.05, significantly different from each control value. (B) Effects of the systemic administration of prazosin on Runx2, Osx and OC mRNA expression in cancellous bone. Total RNA was isolated from the distal region of the femur in 12‐week‐old male mice administered saline or prazosin, followed by the determination of Runx2, Osx and OC mRNA levels by real‐time qRT‐PCR using specific primers; n = 9 or 10 mice per group. Similar results were obtained in three independent experiments. Values are expressed as means ± SEM; * P < 0.05, significantly different from each control value. (C) Mice were treated with prazosin at 30 μg·kg−1 per day or saline for 2 weeks, followed by the staining of osteoclasts with TRAP. The amounts of Oc.S/BS and Oc.N/BS were measured (n = 8 or 9 mice per group). Values are expressed as means ± SEM. * P < 0.05 significantly different from the control. (D) Effects of the systemic administration of prazosin on Nfatc1 and Ctsk mRNA expression in bone. Total RNA was isolated from the distal region of the femur in 12‐week‐old male mice administered saline or prazosin, followed by the determination of Nfatc1 and Ctsk mRNA levels by real‐time qRT‐PCR using specific primers. n = 11 or 19 mice per group. Values are expressed as means ± SEM. * P < 0.05 significantly different from the control.
To confirm the effects of prazosin on bone resorption, we next performed a bone histomorphometric analysis on bone resorption parameters such as the bone surface covered by osteoclasts (Oc.S/BS) and Oc.N/BS with TRAP‐positive osteoclasts. The results obtained showed that Oc.S/BS of the femur was significantly lower in prazosin‐treated mice at 30 μg·kg−1 than in control mice. No significant differences were observed in Oc.N/BS between mice administered prazosin 30 μg·kg−1 i.p. and control mice (Figure 2C). In addition, the mRNA expression of osteoclast‐related genes such as cathepsin K (Ctsk) and nuclear factor of activated T‐cells, cytoplasmic‐1 (Nfatc1) was significantly decreased in prazosin‐treated mice, suggesting impaired bone resorption (Figure 2D).
Ablation of α1B ‐adrenoceptors decreased femoral cancellous bone mass
The pharmacological blockade of α1‐adrenoceptor signalling significantly decreased bone mass, as reflected by decreased bone formation, which suggested that α1‐adrenoceptor signalling, played an important role in modulating bone physiology. α1‐Adrenoceptors have been classified into three subtypes: α1A, α1B and α1D. In order to identify the α1‐adrenoceptor subtypes expressed by osteoblasts and osteoclasts, we performed real‐time PCR using primers specific for each receptor subtype. α1B‐ and α1D‐adrenoceptors were expressed in MC3T3‐E1 and differentiated MC3T3‐E1 cells cultured in α‐MEM containing 10% FBS, 50 μg·mL−1 ascorbic acid and 5 mM ß‐GP (Supporting Information Fig. S1). In addition, we showed the expression of mRNA for the receptors of α1B‐ and α1D‐adrenoceptors in bone marrow stromal cells (BMSCs) (Supporting Information Fig. S1). Therefore, to elucidate the mechanisms responsible for bone loss due to the prazosin treatment and the functional role of α1‐adrenoceptor signalling in bone metabolism, we next compared the bone phenotype of α1B‐adrenoceptor null mice (α1B −/−) with that of WT littermates. The results of μCT‐based bone densitometry showed that BV/TV of the distal end of the femurs of 10‐week‐old males was significantly lower in α1B −/− mice than in WT mice (Figure 3A). Moreover, the Tb.N of the femur was also significantly lower in α1B −/− mice than in WT mice (Figure 3B). No significant differences were observed in Tb.Th or Tb.Sp between WT and α1B −/− mice (Figure 3C, D). To elucidate further the mechanisms responsible for the α1B‐adrenoceptor deficiency leading to bone loss, we examined the parameters of bone formation. We observed a significant decrease in osteoblast number per bone surface (Ob.N/BS), and a reduced MS/BS, and MAR in α1B −/− mice (Figure 4A–C). BFR was also significantly decreased by approximately 40% in α1B −/− mice (Figure 4D). Furthermore, the mRNA expression of Runx2, Osx and OC in bone was significantly lower in α1B −/− mice than in WT littermates (Figure 4E). In contrast, no significant differences were observed in Oc.S/BS or Oc.N/BS between α1B −/− mice and WT mice (Figure 4F), indicating that their decreased bone mass was associated with a reduction in osteoblast number and activity, not changes in osteoclasts.
Figure 3.
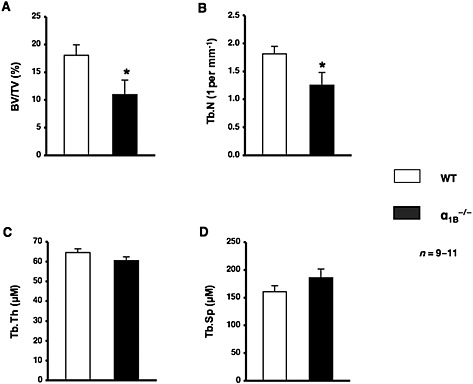
Ablation of α1B‐adrenoceptors led to a decreased bone mass. (A–D) μCT‐based bone densitometry of the distal region of the right femur from WT mice and α1B −/− mice. (A) BV/TV. (B) Tb.N. (C) Tb.Th. (D) Tb.Sp. n = 9 or 11 mice per group. Values are expressed as means ± SEM. * P < 0.05 significantly different from WT mice.
Figure 4.
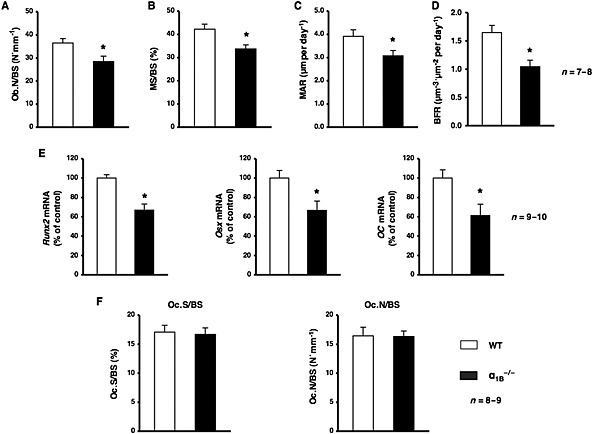
Ablation of α1B‐adrenoceptors led to a decrease in bone formation. (A–D) An analysis of the Ob.N/BS, MS/BS, MAR and BFR in the cancellous bone compartment of the distal femur metaphysis from WT mice and α1B −/− mice; n = 7 or 8 mice per group. (A) Ob.N/BS. (B) MS/BS. (C) MAR. (D) BFR. Values are expressed as means ± SEM. * P < 0.05, significantly different from WT mice. (E) Total RNA was isolated from the distal region of the femur from WT and α1B −/− mice, followed by the determination of Runx2, Osx and OC mRNA levels by real‐time qRT‐PCR using specific primers; n = 9 or 10 mice per group. Values are expressed as means ± SEM. * P < 0.05, significantly different from WT mice. (F) Sections of the primary trabecular regions of the femur from WT or α1B‐adrenoceptor‐deficient mice followed by the staining of osteoclasts with TRAP. The amount of Oc.S/BS and Oc.N/BS were measured (n = 8 or 9 mice per group).
α1 ‐Adrenoceptor signalling up‐regulated Cebpd gene expression in MC3T3‐E1 osteoblastic cells
To investigate the physiological function of α1‐adrenoceptor signalling in osteoblasts and identify the target molecules of α1‐adrenoceptor signalling, we examined the expression of genes in response to phenylephrine in MC3T3‐E1 cells. Total RNA was extracted from MC3T3‐E1 osteoblastic cells exposed to phenylephrine for 1, 2, 4 and 8 h and analysed by real‐time qRT‐PCR. As shown in Figure 5A, the expression of Cebpd mRNA was significantly increased by the exposure to phenylephrine for 1 and 2 h. In addition, pretreatment with the α1‐adrenoceptor antagonist prazosin completely inhibited phenylephrine ‐induced Cebpd expression, as determined using a real‐time qRT‐PCR analysis in MC3T3‐E1 osteoblastic cells, which suggested that the phenylephrine ‐induced expression of Cebpd was mediated by α1‐adrenoceptor signalling in MC3T3‐E1 osteoblastic cells (Figure 5B). Furthermore, we investigated the effects of the pharmacological blockade of α1‐adrenoceptor signalling on the expression of Cebpd in cancellous bone. We extracted total RNA from the distal end of the femur and analysed the expression of Cebpd mRNA after the systemic administration of prazosin for 2 weeks. As shown in Figure 5C, the expression of Cebpd mRNA in cancellous bone was significantly lower in prazosin‐treated mice than in saline‐treated mice. Furthermore, we compared the expression of Cebpd mRNA in α1B −/− mice and WT littermates. The expression of Cebpd mRNA in bone was significantly lower in α1B −/− mice than in WT littermates (Figure 5D), suggesting that α1B‐adrenoceptor signalling in osteoblasts plays a significant role in regulating Cebpd gene expression. Therefore, we expected Cebpd to be one of the target molecules of α1B‐adrenoceptor signalling in osteoblasts.
Figure 5.
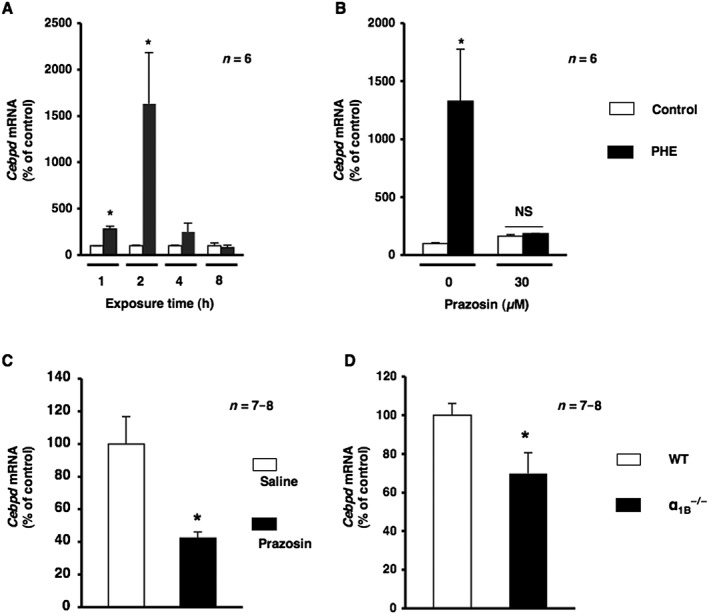
α1‐Adrenoceptor signalling mediated Cebpd expression. (A) Cells were treated with 10 μM phenylephrine (PHE) for 1, 2, 4 and 8 h, harvested and then processed for real‐time qRT‐PCR. Each value represents the mean ± SEM of six separate experiments. * P < 0.05, significantly different from each control value obtained in MC3T3‐E1 cells cultured in the absence of PHE. (B) α1‐Adrenoceptor signalling mediated Cebpd mRNA expression after the PHE stimulation in MC3T3‐E1 cells. Cells were incubated for 2 h in the presence of PHE with prazosin at a concentration of 10 μM, followed by the determination of Cebpd levels by real‐time qRT‐PCR. Each value represents the mean ± SEM of six separate experiments. * P < 0.05, significantly different from each control value. NS; not significant. (C) α1‐Adrenoceptor signalling regulated Cebpd in bone. Effects of the systemic administration of prazosin on Cebpd mRNA expression in cancellous bone. Total RNA was isolated from the distal region of the femur in 10‐week‐old male mice administered saline or prazosin, followed by the determination of Cebpd mRNA levels by real‐time qRT‐PCR using specific primers; n = 7 or 8 mice per group. Values are expressed as means ± SEM. * P < 0.05, significantly different from each control value. (D) Total RNA was isolated from the distal region of the femur from WT and α1B −/− mice, followed by the determination of Cebpd mRNA levels by real‐time qRT‐PCR using specific primers. n = 7 or 8 mice per group. Values are expressed as means ± SEM. * P < 0.05, significantly different from WT mice.
Cebpd was involved in cell proliferation in MC3T3‐E1 cells
To investigate the physiological function of α1B‐adrenoceptor signalling in osteoblasts, we determined whether Cebpd contributed to the regulation of cellular function in osteoblasts. Based on previous findings in which α1‐adrenoceptor signalling in osteoblasts mediated cellular proliferation (Suzuki et al., 1998; Kodama & Togari, 2013), we investigated whether Cebpd in osteoblasts contributed to cellular proliferation. Cebpd was silenced in MC3T3‐E1 cells, and cell proliferation activity was evaluated as DNA synthesis by the BrdU incorporation assay. Cells were cultured for 24 h, transfected with Cebpd siRNA (or non‐targeting siRNA) and then cultured for a further 24 h. As shown in Figure 6A, the incorporation of BrdU was significantly lower in these cells compared with those transfected with non‐targeting control siRNA. In addition, the expression of Ccne1, which encodes cyclin E1, was significantly decreased in Cebpd‐silenced cells (Figure 6B). The overexpression of Cebpd significantly increased cellular proliferation in MC3T3‐E1 cells (Figure 6C). These results indicated that Cebpd regulated cellular proliferation in MC3T3‐E1 cells. Furthermore, we observed that PHE‐induced cellular proliferation was significantly decreased in MC3T3‐E1 cells transfected with Cebpd siRNA 24 h after transfection (Figure 6D). Taken together, these results indicate that Cebpd promotes cellular proliferation in response to α1B‐adrenoceptor signalling in osteoblasts.
Figure 6.
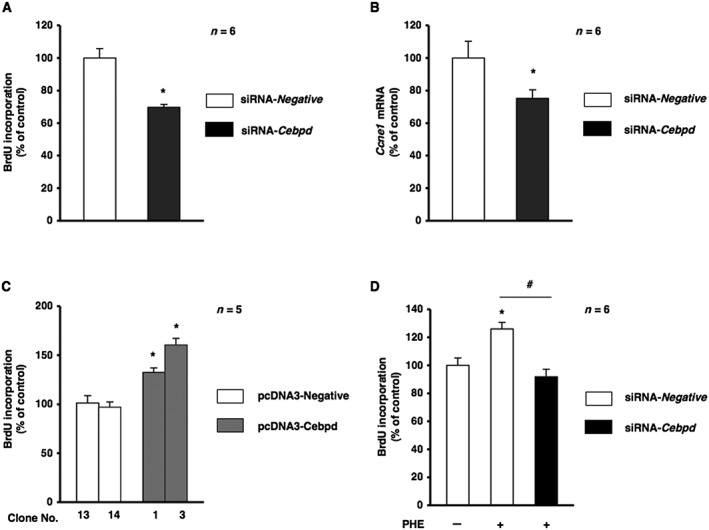
Cebpd was involved in cellular proliferation in MC3T3‐E1 cells.(A) Effects of silencing Cebpd on DNA synthesis as measured by the incorporation of BrdU. MC3T3‐E1 cells were treated with Cebpd siRNA (siRNA‐Cebpd) or non‐silencing RNA (siRNA‐Negative), followed by the determination of BrdU incorporation according to the indicated protocols (mean ± SEM, n = 6). * P < 0.05, significantly different from each control value. (B) MC3T3‐E1 cells were treated with Cebpd siRNA (siRNA‐Cebpd) or non‐silencing RNA (siRNA‐Negative) according to the indicated protocols. Real‐time qRT‐PCR analyses of transcription levels were performed using their specific primers for Ccne1. Relative mRNA expression was normalized to Gapdh (mean ± SEM, n = 6). * P < 0.05, significantly different from each control value. (C) Effects of the overexpression of Cebpd in MC3T3‐E1 cells. MC3T3‐E1 cells were stably transfected with expression vectors for Cebpd (pcDNA‐Cebpd) or control (pcDNA‐Negative) followed by the determination of BrdU incorporation according to the indicated protocols (mean ± SEM, n = 5). * P < 0.05, significantly different from the value obtained in cells transfected with the control vector. (D) Effects of Cebpd‐knockdown by siRNA on phenylephrine (PHE)‐regulated cellular proliferation in MC3T3‐E1 cells. MC3T3‐E1 cells were treated with Cebpd siRNA (siRNA‐Cebpd) or non‐silencing RNA (siRNA‐Negative), followed by further cultivation for 24 h in the absence or presence of PHE at a concentration of 0.3 μM, and subsequent determination of BrdU incorporation (mean ± SEM, n = 6). * P < 0.05, significantly different from value of siRNA‐Negative. # P < 0.05, significantly different from the value obtained in cells treated with siRNA‐Negative in the presence of PHE. Each figure is representative data from three independent experiments.
Discussion
In the present study, we presented in vitro and in vivo evidence for the importance of α1B‐adrenoceptor signalling in bone homeostasis. Both the pharmacological and genetic ablation of α1B‐adrenoceptor signalling impaired bone mass by decreasing bone formation, suggesting that α1B‐adrenoceptor signalling in osteoblasts plays an important role in bone remodelling under normal conditions. Mice with low activity in the sympathetic nervous system, such as leptin receptor‐deficient mice and dopamine ß‐hydroxylase‐deficient mice, both have a high bone mass due to increased bone formation and decreased bone resorption (Takeda et al., 2002; Elefteriou et al., 2005). Regardless of ß2‐adrenoceptor signalling being a key element in the regulation of bone remodelling, female mice with chronic sympathetic hyperactivity due to the double knockout of α2A‐ and α2C‐adrenoceptors, which are presynaptic autoreceptors that negatively regulate noradrenaline release, displayed a high bone mass with increased bone formation and decreased bone resorption (Fonseca et al., 2011). In contrast, the role of α1‐ARs signalling in bone remodelling had not yet been examined in detail. In the present study, we found that the systemic administration of prazosin decreased bone formation and resorption (Figure 2). Consistent with a previous study (Suga et al., 2010), the RANKL‐treated RAW264.7 cells contained mRNA encoding α1A‐, but not α1B‐ or α1D‐adrenoceptors (data not shown). These indicated that α1A‐adrenoceptor signalling in osteoclasts might participate in the reduced bone resorption induced by the blockade of α1‐adrenoceptor signalling. Further analyses are needed to clarify whether α1A‐adrenoceptor signalling in osteoclasts directly stimulates osteoclastogenesis. In addition, this study showed that the mRNAs of α1B‐, but not α1A‐adrenoceptor subtype, was expressed in BMSCs and differentiated BMSCs (Supporting Information Fig. S1). Taken together with the expression of α1‐adrenoceptor subtypes, these results prompted us to speculate that α1B‐adrenoceptor signalling in osteoblasts controls osteoclastogenesis through some osteoblast‐derived factors such as RANKL and osteoprotegerin. Osteoclastogenesis is regulated by RANKL, an osteoclast differentiation factor, and osteoprotegerin, a decoy receptor for RANKL, both of which are expressed by osteoblasts (Simonet et al., 1997). However, the results of the genetic experiments revealed that α1B‐adrenoceptor‐deficient mice had a lower bone mass due to decreased bone formation without affecting bone resorption (Figure 4). These results obtained in mice with global deletion of α1B‐adrenoceptors suggest that impaired bone formation was due, at least in part, to a cellular dysfunction in osteoblasts lacking α1B‐adrenoceptor signalling and that α1B‐adrenoceptor signalling functions in osteoblasts. These mice, however, have confounding systemic effects arising from other cell types and/or tissues. The identity of the cell type in which the sympathetic nervous system acts to regulate bone mass remains to be elucidated in future studies using mice with osteoblast‐specific α1B‐adrenoceptor ablation. To identify the localization of α1‐adrenoceptor subtypes in bone may be explained by the fact that α1‐adrenoceptor signalling in osteoblast plays an important role on bone formation. Because it was not technically feasible, we did not find any evidence of mRNA or protein of α1‐adrenoceptor subtypes located in bone. Therefore, the definitive role of α1B‐ adrenoceptors expressed in osteoblasts in the low bone mass observed in this study is still to be confirmed. Thus, these results indicate that the regulation of bone remodelling by the sympathetic nervous system is extremely complex. However, this study showed that not only ß2‐ but also α1B‐ adrenoceptor signalling in osteoblasts plays an important role in mediating bone remodelling through the sympathetic nervous system.
The physiological action of adrenaline (epinephrine) was previously demonstrated to be mediated by α1‐ as well as ß‐ adrenoceptor signalling in osteoblasts. Our previous studies also showed that cellular proliferation was facilitated via α1B‐ adrenoceptors and inhibited via ß‐ adrenoceptors in human osteoblastic SaM‐1 cells (Kodama & Togari, 2013). In the present study, phenylephrine stimulated chloroethylclonidine, cellular proliferation on BMSCs (Supporting Information Fig. S2). In addition, pretreatment with an α1B‐ adrenoceptor‐selective antagonist inhibited phenylephrine‐induced Cebpd expression in MC3T3‐E1 osteoblastic cells (data not shown). Furthermore, we observed that phenylephrine‐induced cellular proliferation was significantly decreased in MC3T3‐E1 cells transfected with Cebpd siRNA. Based on these results and our previous findings, we suggested that α1B‐ adrenoceptor signalling mediated cellular proliferation through a mechanism relevant to the up‐regulation of Cebpd in osteoblasts, and that the α1‐ adrenoceptor signalling by osteoblasts might participate in the mechanism underlying the regulation of bone formation by the sympathetic nervous system.
In conclusion, our results suggest that α1B‐ adrenoceptor signalling in osteoblasts is required for bone formation and regulates cellular proliferation through the up‐regulation of Cebpd and, thus, provide new evidence for the physiological importance of α1B‐ adrenoceptor signalling in bone homeostasis. In humans, a series of studies showed that an α‐blocker was associated with an increased risk of hip/femur fracture (Souverein et al., 2003; Song et al., 2012). A clearer understanding of the functions of the α1B‐ adrenoceptor and its interactions with ß2‐ adrenoceptors in bone remodelling will assist in elucidating the role of the sympathetic nervous system in bone metabolism, which will, in turn, facilitate the development of novel therapeutic strategies for the treatment of osteoporosis.
Author contributions
K. T., T. H., D. K., H. K., K. H. and A. T. designed the research study. K. T. and T. H. performed the experiments and analysed the data. T. H. wrote the paper.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Gene expression of α1‐AR subtypes in bone cells. In order to determine the α1‐AR subtypes expressed by osteoblasts, we performed real‐time PCR using primers specific for each receptor subtype in BMSCs and MC3T3‐E1 cells. Relative mRNA expression was normalized to Gapdh. Figures are representative of data from six independent determinations. α1A‐AR, α1B‐AR, and α1D‐AR transcripts were detected in C3H10T1/2 cells as a positive control. ND, not detected.
Figure S2 PHE stimulated cellular proliferation in BMSCs. Cells were treated with PHE at 0.03 to 0.3 µM for 24 h, followed by the determination of BrdU incorporation (mean ± SEM). Each value represents the means ± SEM of six independent determinations. *, P < 0.05, significantly different from control value.
Supporting Information
Acknowledgements
This work was supported in part by Grants‐in‐Aid for Scientific Research to D. K. (24791988), H. K. (15 K11061), and to A. T. (26462827) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Dr Akito Tanoue of the Department of Pharmacology, National Research Institute for Child Health and Development for providing us with α1B‐AR‐deficient mice.
Tanaka, K. , Hirai, T. , Kodama, D. , Kondo, H. , Hamamura, K. , and Togari, A. (2016) α1B‐Adrenoceptor signalling regulates bone formation through the up‐regulation of CCAAT/enhancer‐binding protein δ expression in osteoblasts. British Journal of Pharmacology, 173: 1058–1069. doi: 10.1111/bph.13418.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan K, Sterneck E (2013). The many faces of C/EBPdelta and their relevance for inflammation and cancer. Int J Biol Sci 9: 917–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M (2007). C/EBP delta regulates cell cycle and self‐renewal of human limbal stem cells. J Cell Biol 177: 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. (1997). Decreased blood pressure response in mice deficient of the alpha1b‐adrenergic receptor. Proc Natl Acad Sci USA 94: 11 589–11 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. (2005). Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514–520. [DOI] [PubMed] [Google Scholar]
- Fonseca TL, Jorgetti V, Costa CC, Capelo LP, Covarrubias AE, Moulatlet AC, et al. (2011). Double disruption of α2A‐ and α2C‐adrenoceptors results in sympathetic hyperactivity and high‐bone‐mass phenotype. J Bone Miner Res 26: 591–603. [DOI] [PubMed] [Google Scholar]
- Gery S, Tanosaki S, Hofmann WK, Koppel A, Koeffler HP (2005). C/EBP delta expression in a BCR‐ABL‐positive cell line induces growth arrest and myeloid differentiation. Oncogene 24: 1589–1597. [DOI] [PubMed] [Google Scholar]
- Gutierrez S, Javed A, Tennant DK, van Rees M, Montecino M, Stein GS, et al. (2002). CCAAT/enhancer‐binding proteins (C/EBP) ß and δ activate osteocalcin gene transcription and synergize with runx2 at the C/EBP element to regulate bone‐specific expression. J Biol Chem 277: 1316–1323. [DOI] [PubMed] [Google Scholar]
- Hanson RW (1998). Biological role of the isoforms of C/EBP minireview series. J Biol Chem 273: 28 543. [DOI] [PubMed] [Google Scholar]
- Hirai T, Tokumo K, Tsuchiya D, Nishio H (2009). Expression of mRNA for 5‐HT2 receptors and proteins related to inactivation of 5‐HT in mouse osteoblasts. J Pharmacol Sci 109: 319–323. [DOI] [PubMed] [Google Scholar]
- Hirai T, Tanaka K, Togari A (2014a). α1‐adrenergic receptor signaling in osteoblasts regulates clock genes and bone morphogenetic protein 4 expression through up‐regulation of the transcriptional factor nuclear factor IL‐3 (Nfil3)/E4 promoter‐binding protein 4 (E4BP4). J Biol Chem 289: 17 174–17 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Tanaka K, Togari A (2014b). ß‐adrenergic receptor signaling regulates Ptgs2 by driving circadian gene expression in osteoblasts. J Cell Sci 127: 3711–3719. [DOI] [PubMed] [Google Scholar]
- Hirai T, Tanaka K, Togari A (2015a). α1B‐adrenergic receptor signaling controls circadian expression of the Tnfrsf11b by regulating clock genes in osteoblasts. Biol Open 4: 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Kobayashi T, Nishimori S, Karaplis AC, Goltzman D, Kronenberg HM (2015b). Bone is a major target of PTH/PTHrP receptor signaling in regulation of fetal blood calcium homeostasis. Endocrinology 156: 2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HH, Brennan TC, Muir MM, Mason RS (2009). Functional alpha1‐ and beta2‐adrenergic receptors in human osteoblasts. J Cell Physiol 220: 267–275. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK (2011). Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci 32: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama D, Togari A (2013). Noradrenaline stimulates cell proliferation by suppressing potassium channels via G(i/o)‐protein‐coupled α1(1B)‐adrenoceptors in human osteoblasts. Br J Pharmacol 168: 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Kondo H, Miyazawa K, Goto S, Togari A (2013). Experimental tooth movement‐induced osteoclast activation is regulated by sympathetic signaling. Bone 52: 39–47. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Furuno T, Nakanishi M, Togari A (2007). Direct neurite‐osteoblastic cell communication, as demonstrated by use of an in vitro co‐culture system. FEBS Lett 581: 5917–5922. [DOI] [PubMed] [Google Scholar]
- O'Rourke JP, Newbound GC, Hutt JA, Dewille J (1999). CCAAT/enhancer‐binding protein delta regulates mammary epithelial cell G0 growth arrest and apoptosis. J Biol Chem 274: 16 582–16 589. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al., NC‐IUPHAR (2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Foka P (2002). CCAAT/enhancer‐binding proteins: structure, function and regulation. Biochem J 365: 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar TR, Sharan S, Wang J, Pawar SA, Cantwell CA, Johnson PF, et al. (2012). Identification of a Src tyrosine kinase/SIAH2 E3 ubiquitin ligase pathway that regulates C/EBP delta expression and contributes to transformation of breast tumor cells. Mol Cell Biol 32: 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. (1997). Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319. [DOI] [PubMed] [Google Scholar]
- Song HJ, Lee J, Kim YJ, Jung SY, Kim HJ, Choi NK, et al. (2012). ß1 selectivity of ß‐blockers and reduced risk of fractures in elderly hypertension patients. Bone 51: 1008–1015. [DOI] [PubMed] [Google Scholar]
- Souverein PC, Van Staa TP, Egberts AC, De la Rosette JJ, Cooper C, Leufkens HG (2003). Use of alpha‐blockers and the risk of hip/femur fractures. J Intern Med 254: 548–554. [DOI] [PubMed] [Google Scholar]
- Suga S, Goto S, Togari A (2010). Demonstration of direct neurite‐osteoclastic cell communication in vitro via the adrenergic receptor. J Pharmacol Sci 112: 184–191. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Palmer G, Bonjour JP, Caverzasio J (1998). Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3‐E1 osteoblast‐like cells. Bone 23: 197–203. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Palmer G, Bonjour JP, Caverzasio J (1999). Regulation of alkaline phosphatase activity by p38 MAP kinase in response to activation of Gi protein‐coupled receptors by epinephrine in osteoblast‐like cells. Endocrinology 140: 3177–3182. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. (2002). Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsuboi T, Arai M, Togari A (2001). Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3‐E1 osteoblast‐like cells. Biochem Pharmacol 61: 579–586. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hirai T, Ishibashi Y, Izumo N, Togari A (2015). Modulation of osteoblast differentiation and bone mass by 5‐HT2A receptor signaling in mice. Eur J Pharmacol 762: 150–157. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Rudelius M, Bierie B, Raffeld M, Sharan S, Hennighausen L, et al. (2005). C/EBP delta is a crucial regulator of pro‐apoptotic gene expression during mammary gland involution. Development 132: 4675–4685. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Kondo A (2005). The role of the sympathetic nervous system in controlling bone metabolism. Expert Opin Ther Targets 9: 931–940. [DOI] [PubMed] [Google Scholar]
- Togari A (2002). Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech 58: 77–84. [DOI] [PubMed] [Google Scholar]
- Umayahara Y, Billiard J, Ji C, Centrella M, McCarthy TL, Rotwein P (1999). CCAAT/enhancer‐binding protein delta is a critical regulator of insulin‐like growth factor‐I gene transcription in osteoblasts. J Biol Chem 274: 10 609–10 617. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Lekstrom‐Himes J, Barlow C, Wynshaw‐Boris A, Xanthopoulos KG (1998). CCAAT/enhancer binding proteins are critical components of the transcriptional regulation of hematopoiesis. Int J Mol Med 1: 213–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Gene expression of α1‐AR subtypes in bone cells. In order to determine the α1‐AR subtypes expressed by osteoblasts, we performed real‐time PCR using primers specific for each receptor subtype in BMSCs and MC3T3‐E1 cells. Relative mRNA expression was normalized to Gapdh. Figures are representative of data from six independent determinations. α1A‐AR, α1B‐AR, and α1D‐AR transcripts were detected in C3H10T1/2 cells as a positive control. ND, not detected.
Figure S2 PHE stimulated cellular proliferation in BMSCs. Cells were treated with PHE at 0.03 to 0.3 µM for 24 h, followed by the determination of BrdU incorporation (mean ± SEM). Each value represents the means ± SEM of six independent determinations. *, P < 0.05, significantly different from control value.
Supporting Information


