Abstract
Background and Purpose
There is currently no medication approved specifically to treat cocaine addiction. Behavioural interventions such as cue exposure therapy (CET) rely heavily on new learning. Antagonism of the metabotropic glutamate 5 (mGlu5) receptor has emerged as a potential treatment, by reducing the reinforcing properties of cocaine. However, mGlu5 receptor activity is necessary for learning; therefore, such agents could interfere with behavioural treatments. We used a novel rodent model of CET to test the effects of mGlu5 negative and positive allosteric modulators (NAM and PAM) on behavioural therapy.
Experimental Approach
Rats were trained to press a lever for cocaine in the presence of a discrete cue [conditioned stimulus (CS)] and then extinguished in the absence of the CS. Following lever extinction, half the rats received CS extinction in the same chambers but with the levers withdrawn; the remaining rats received no CS extinction. Before this session, rats received a systemic administration of either vehicle or a mGlu5 NAM (MTEP, experiment 1) or PAM (CDPPB, experiment 2). Cue‐induced reinstatement was tested in a drug‐free session the following day.
Key Results
At reinstatement, rats that had received CS extinction showed reduced responding. This effect was attenuated by MTEP treatment before CS extinction. In contrast, administration of CDPPB (PAM) led to decreased reinstatement the following day, regardless of extinction condition.
Conclusion and Implications
These results suggest that mGlu5 receptor activity is both necessary and sufficient for efficient extinction of a cocaine‐associated CS. Therefore, mGlu5 PAMs could enhance the efficacy of CET.
Abbreviations
- CDPPB
3‐cyano‐N‐(1,3‐diphenyl‐1H‐pyrazol‐5‐yl)benzamide
- CET
cue exposure therapy
- CS
conditioned stimulus
- mGlu5
metabotropic glutamate receptor (subtype 5)
- MPEP
2‐methyl‐6‐(phenylethynyl)pyridine
- MTEP
3‐((2‐methyl‐4‐thiazolyl)ethynyl)pyridine
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
Tables of Links
| TARGETS |
|---|
| GPCRs a |
| mGlu5 receptor |
| Ligand‐gated ion channels b |
| NMDA receptor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
Like most substance abuse disorders, cocaine addiction is a chronic relapsing condition, and much research has been dedicated towards mitigating the impact of relapse episodes. There is currently no medication approved specifically for treating cocaine addiction; however, emerging evidence shows that metabotropic glutamate 5 (mGlu5) receptors are necessary for drug seeking during relapse to cocaine (Backstrom and Hyytia, 2006; Backstrom and Hyytia, 2007; Kumaresan et al., 2009; Keck et al., 2013; 2014) or other drugs of abuse (Backstrom et al., 2004; Bespalov et al., 2005; Adams et al., 2008; Watterson et al., 2013). Negative allosteric modulators (NAM) of mGlu5 receptor signalling, such as the anxiolytic drug fenobam, are already either approved or in clinical trials for a number of different disorders (Olive, 2010; Nickols and Conn, 2014). Consequently, these compounds have been presented as potential agents for treating cocaine abuse.
The mGlu5 receptors are functionally linked to NMDA receptors via scaffold proteins such as Homer and Shank (Niswender and Conn, 2010; Gao et al., 2013). Via this mechanism, they are necessary for normal learning and memory (Lu et al., 1997). This is relevant to addiction treatment because the purpose of behavioural therapy is to change maladaptive behaviour by learning new responses to drug‐associated stimuli. For example, cue exposure therapy (CET) employs extinction learning, where previously drug‐associated stimuli are presented repeatedly in the absence of further drug reinforcement. Thus, the subject learns that the cue [or conditioned stimulus (CS)] is no longer associated with the drug, and this affords protection against relapse (Conklin and Tiffany, 2002). Despite their acute effect on the reinforcing effects of cocaine, if taken in conjunction with behavioural therapy like CET, mGlu5 NAMs may actually disrupt the learning process (Chesworth et al., 2013; Bird et al., 2014; Kim et al., 2015); hence, they represent a double‐edged sword.
A more useful strategy might be to administer a pharmacological adjunct that will enhance the learning that occurs during CET. Currently, behavioural therapies alone show at best only marginal long‐term protection against relapse (Conklin and Tiffany, 2002). This is probably because following extinction, the drug–cue associations are not erased. Instead, the extinction learning forms an inhibitory mask over the original associations (Bouton, 2002). This inhibitory learning is not as robust as the original associations and is highly context‐dependent, making relapse a common occurrence. Therefore, cognitive aids that facilitate and strengthen what is learned during extinction may improve prognosis for behavioural therapy and lessen the subsequent incidence of relapse. Interestingly, mGlu5 positive allosteric modulators (PAM) facilitate Pavlovian extinction of a fearful CS (Ganella et al., 2016); therefore, these agents may be more effective in creating long‐term resistance to relapse.
Preclinical research into substance abuse frequently makes use of the extinction‐ reinstatement model to investigate relapse‐like behaviour. Here, animals that have been trained to self‐administer a drug undergo extinction where the drug‐seeking response is no longer reinforced with drug delivery. This extinction learning reduces drug‐seeking responding; however, responding can be readily retrieved via a number of manipulations, such as stress or exposure to drug‐associated CS. These manipulations are analogous to factors that are known to trigger relapse in substance abuse clients, and therefore, this procedure has face validity as a model for relapse (Bossert et al., 2013). However, it is important to note that the extinction phase of this model does not provide a good imitation of CET or other behavioural therapies because it is not the CS, but the drug‐seeking response itself that is extinguished in animal models (i.e. instrumental extinction). This is in contrast to CET, where only drug‐associated CS are extinguished; and in a clinical situation, extinction of the response would be difficult to implement (Perry et al., 2014).
More recently, however, it has been shown in rats that Pavlovian extinction of a drug‐associated CS following instrumental extinction decreased responding during subsequent CS‐induced reinstatement (Torregrossa et al., 2010; 2013). This model reflects more directly the theory behind CET because the ‘treatment’ is Pavlovian extinction while the ‘outcome’ is operant drug‐seeking responding. Within this model, the partial NMDA receptor agonist (d‐cycloserine) facilitated CS extinction. Because mGlu5 receptors are closely linked with NMDA receptor function, and mGlu5 PAMs facilitated extinction of operant cocaine seeking (Cleva et al., 2011) and of a Pavlovian conditioned fear CS (Ganella et al., 2016), it seems likely that the mGlu5 receptor is also involved in the extinction of a cocaine‐associated CS. Here, we tested this possibility by examining the effect of an mGlu5 NAM and a PAM on the extinction of a cocaine‐associated CS.
Methods
Animals
Adult male Sprague–Dawley rats, weighing between 250 and 300 g at the commencement of procedures, were obtained from a commercial supplier (Animal Resources Centre, Perth, Australia) or were bred in an in‐house facility. Animals were maintained on a reverse 12/12 light–dark cycle (lights off at 0700 h), within a specific pathogen‐free facility. Rats were housed in open‐top cages with aspen bedding. They were housed in pairs until surgery, after which time they were single housed. All animals were acclimatized to the reverse‐cycle conditions for 7 days and were handled at least three times prior to surgery. All procedures were approved by the local Animal Ethics Committee, followed the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council 2004) and are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Group sizes
Experiment 1
N = 55. Final group sizes (after exclusions): Handle vehicle: n = 10, Handle MTEP: n = 11, CS extinction vehicle: n = 11, CS extinction MTEP: n = 11.
Experiment 2
N = 32. Final group sizes (after exclusions):‐ Handle vehicle: n = 7, Handle MTEP: n = 7, CS extinction vehicle: n = 8, CS extinction MTEP: n = 8.
Surgery
All animals were implanted with custom‐made catheters into the jugular vein as described previously (Kim et al., 2015). Briefly, rats were anaesthetized with oxygen mixed with isoflurane and injected with meloxicam (3 mg kg−1 i.p.), and 3.25 cm of Silastic tubing (inner diameter 0.51 mm, outer diameter 0.94 mm; Instech Solomon, Plymouth Meeting, PA, USA) was inserted into the left jugular vein. Animals were allowed to recover for at least 48 h post‐surgery before operant training began. During this time, they were weighed and monitored daily. The catheter was flushed daily with 0.05 mL heparin‐treated saline (50 U mL‐1) containing 10% neomycin antibiotic (CEVA, Glenorie, Australia) to maintain catheter patency. Throughout intravenous self‐administration (IVSA), animals were allowed 15 g day‐1 standard chow, and during this time, they were weighed at least three times per week.
Patency was checked regularly, and before commencement of extinction training. Rats were flushed with 0.04 mL of ketamine (100 mg mL‐1) followed by 0.05 mL heparin‐treated saline (10 U mL‐1). Patency was indicated by loss of muscle tone within 10 s. Any rat that was not patent was removed from the study (experiment 1: 3; experiment 2: 0).
Apparatus
All training sessions were carried out in standard operant chambers (29.5 × 32.5 × 23.5 cm; Med Associates, St. Albans, VT, USA) that were housed within sound‐ and light‐attenuating boxes equipped with ventilation fans as described previously (Farid et al., 2012). A discriminative vanilla cue was present underneath the active lever to provide spatial information. This cue was absent only when the lever was absent and was never explicitly paired with cocaine.
Procedure
After recovery from surgery, IVSA training began. Rats were initially placed in the operant chambers overnight to shape the response. Before this session, active levers were baited to encourage approach. Reponses on this lever (FR1) resulted in activation of the infusion pump, so that 0.03 mg kg−1 per infusion of cocaine was infused in a volume of 0.05 mL over 2.7 s. Responses on the active lever also illuminated a cue light (CS) located above the active lever. This was illuminated for 2.7 s. If no responses were made within a 30 min period, subjects received a cocaine prime, paired with the CS. Following each reinforcement, there was a timeout period of 20 s, during which time any further active responding was recorded but had no programmed consequences. Outside of the timeout period, active responses were reinforced on an FR1 schedule. Responses on the inactive lever were recorded but had no programmed consequences. The maximum number of responses was set at 300, and sessions terminated after 14 h, or when the maximum number of cocaine rewards had been obtained. All subsequent sessions were conducted in the dark phase and were of no more than 2 h in duration. Following overnight training, rats were given a minimum of eight IVSA sessions where they responded for cocaine on an FR1 schedule (with timeout). Once the response had been acquired (>10 rewards earned per session for two consecutive days), rats received 5 days of IVSA on an FR3 schedule. Any rat that did not acquire the response was excluded from the study (experiment 1: n = 9, experiment 2: n = 2). All IVSA sessions were 2 h. After 5 days of IVSA on FR3, the instrumental responding (lever press) was extinguished in seven daily 1 h sessions. During these sessions, both levers were extended, and responses on the previously active lever were recorded but had no programmed consequences. Neither the light CS nor the vanilla cue was present during these sessions.
On the day following the final extinction session, half of the rats were placed in the chambers for CS extinction. This session lasted 1 h, during which time the cue light was presented 120 times at 30 s time intervals. Importantly, both levers remained retracted throughout this session so that no cocaine‐seeking responses were possible. The remaining rats were handled on this day, but were not placed into the operant chambers. Twenty minutes before CS extinction or handling, all rats received an i.p. injection of either 3% DMSO (1 mL kg−1) or MTEP (2 mg kg−1 dissolved in 3% DMSO) for experiment 1. In experiment 2, rats received an i.p. injection of either 10% Tween 80 (4 mL kg−1) or CDPPB (60 mg kg−1) suspended in 10% Tween 80. Both experiments were between subjects, and groups were balanced for active lever responses on the last day of IVSA, and first and last day of lever extinction. The day following CS extinction, all rats were replaced in the chambers for a 1 h CS‐induced reinstatement session, where the active and inactive levers were available. Responses on the active lever resulted in illumination of the CS; however, no cocaine was infused. No pharmacological agents were administered on this day: reinstatement testing was completely drug‐free. Responses on the active and inactive levers were recorded over the session.
Randomization and blinding
Following the final day of lever extinction, rats were assigned to one of four groups on a pseudo‐random basis, equating response scores on the first and last day of extinction and the last day of IVSA. This was performed to ensure that there were no pre‐existing differences between the groups. All data were collected automatically, therefore, blinding was not necessary.
Normalization
Data were not normalized, except when comparing between experiment 1 and experiment 2. In the latter case, this was performed by taking the difference between the last day of lever extinction and reinstatement to provide a reinstatement score. Normalization was necessary because the groups were not equated between experiments (only within experiments).
Statistical comparison
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).Responding on active and inactive levers was recorded during all sessions. Responding across IVSA and lever extinction was analysed using mixed ANOVAs to ensure that there were no systematic pre‐existing differences between groups. For both experiments 1 and 2, responding on active and inactive levers during test was also subjected to a mixed ANOVA: drug × extinction × (lever). In the case where a significant interaction was found, we conducted follow‐up tests using the Bonferroni adjustment to control the family‐wise error rate at 0.05. In these cases, the per‐comparison error rate is 0.05 per k, where k is the number of contrasts tested. Therefore, where Bonferroni adjustment was used, the adjusted P‐value threshold is reported. All statistical analyses were conducted using spss v 20 (IBM, Armonk, NY, USA) or psy (UNSW: Bird, 2004).
Interpretation
The study has implications for refinement of treatment for addiction, and reduction of the incidence of relapse in recovering cocaine addicts.
Drugs
Cocaine hydrochloride (Johnson Matthey Macfarlan Smith, Edinburgh, UK) was dissolved in sterile saline at concentrations of 0.3 mg kg−1 per infusion. MTEP (Ascent, Bristol, UK) was dissolved in sterile saline containing 3% DMSO at a concentration of 2 mg mL. 3‐Cyano‐N‐(1,3‐diphenyl‐1H‐pyrazol‐5‐yl)benzamide (CDPPB, synthesized and purified by Professor Patrick Perlmutter and colleagues, Chemistry Department, Monash University) was suspended at a concentration of 15 mg mL‐1 in a solution of 10% Tween 80 in PBS.
Results
In both experiments, rats reliably acquired the cocaine‐seeking response such that responding increased on the active lever only. In experiments 1 and 2, three‐way mixed ANOVA revealed significant main effects for day and lever, as well as lever × day interaction with significant linear trend (all P‐values are <0.05). Similarly, across extinction sessions, responding decreased on the active lever only, with three‐way mixed ANOVA again showing significant effects for day and lever, as well as a significant day × lever interaction with linear trend (all P‐values are <0.05). There were no significant differences in responding between groups across acquisition or extinction for either experiment 1 or experiment 2. (all Fs < 1.)
MTEP interferes with CS extinction
In experiment 1, MTEP (2 mg kg−1) or vehicle was injected i.p. 20 min prior to CS extinction or handling. At cue‐induced reinstatement the following day, which was conducted drug‐free, rats that had received CS extinction emitted fewer drug‐seeking responses when the cue was re‐paired with lever press, as compared with rats that had been handled but not received CS extinction the previous day. Mixed ANOVA revealed no main effect for extinction (P = 0.276) nor a two‐way interaction with lever (P = 0.164). Importantly, however, there was a three‐way extinction × drug × lever interaction [F(1, 39) = 5.306, P < 0.05], indicating that when MTEP had been administered prior to CS extinction, the protective effect of CS extinction to attenuate subsequent relapse was reduced (Figure 1). This was confirmed by analysis of simple effects, which revealed that for the vehicle‐treated animals, there was a significant decrease on the active lever after CS extinction when compared with handled animals (P < 0.025), while this was not present in MTEP‐treated animals (P = 0.53). Therefore, antagonism of mGlu5 receptors hinders CS extinction in rats.
Figure 1.
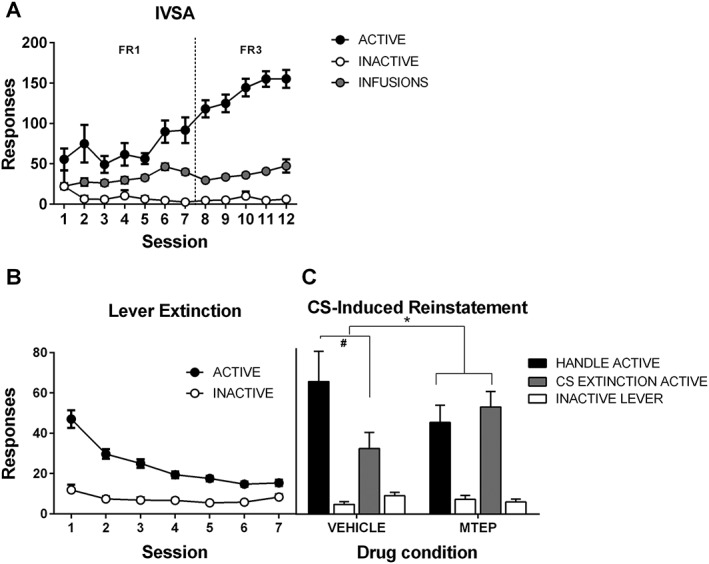
The mGlu5 receptor is necessary for CS extinction. (A) Rats learned to self‐administer cocaine in the presence of a light CS, such that responding on the active lever increased across days. (B) Responding on the active lever decreased over repeated lever extinction sessions. In both (A) and (B), responding is collapsed across groups. (C) CS extinction resulted in decreased CS‐induced reinstatement the following day (drug‐free test), and systemic administration of MTEP prior to CS extinction prevented this effect. * Indicates a significant interaction, P < 0.05, arising from a difference in active lever presses in Handle v CS extinction groups within vehicle condition (#P < 0.025), while no difference existed between these two groups in the MTEP condition. Final group sizes: Handle vehicle: n = 10, Handle MTEP: n = 11, CS extinction vehicle: n = 11, CS extinction MTEP: n = 11.
CDPPB reduces responding at relapse the following day
In experiment 2, CDPPB (60 mg kg−1) or vehicle was injected i.p. 20 min prior to CS extinction or handling. At cue‐induced reinstatement the following day, a significant drug × lever interaction revealed that rats that had received CDPPB the previous day showed fewer responses on the active lever when compared with rats that had been administered vehicle (P < 0.05; Figure 2). This was regardless of extinction condition; the three‐way interaction was not significant (P = 0.57).
Figure 2.
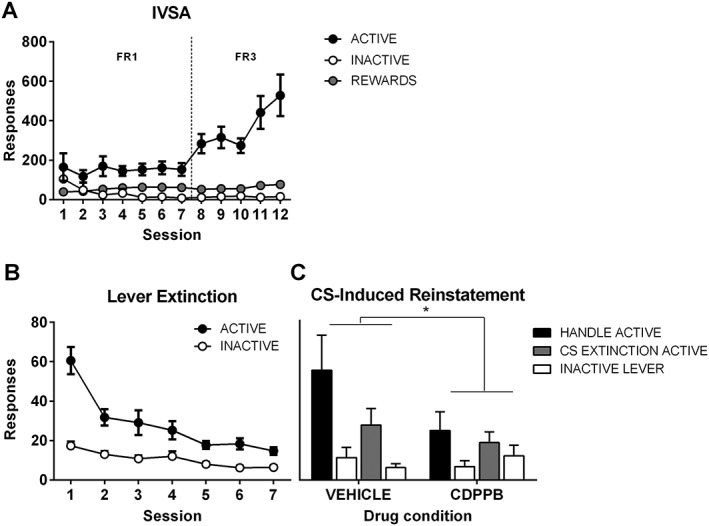
The mGlu5 PAM decreases cue‐induced reinstatement the following day. (A) Rats learned to self‐administer cocaine in the presence of a light CS, such that responding on the active lever increased across days. (B) Responding on the active lever decreased over repeated lever extinction sessions. In both (A) and (B), responding is collapsed across groups. (C) Systemic injection of CDPPB decreased responding at CS‐induced reinstatement carried out drug‐free 24 h later (*drug × lever interaction, P < 0.05). Final group sizes: Handle vehicle: n = 7, Handle MTEP: n = 7, CS extinction vehicle: n = 8, CS extinction MTEP: n = 8.
mGlu5 PAM results in more effective resistance to relapse than an mGlu5 NAM
In order to clarify the effect of the mGlu5 allosteric modulators, data were collapsed across the two experiments. We used the last day of extinction as a baseline control and analysed reinstatement scores (difference between responding on last day of extinction and at cue‐induced reinstatement, Figure 3). ANOVA revealed significant lever × drug interactions (P < 0.05), as well as a significant three‐way interaction (P < 0.05), indicating that the effect of the drug was dependent on the extinction experience. Follow‐up tests using the Bonferroni adjustment confirmed the previous findings that MTEP reduced the beneficial effect of CS extinction (P < 0.0167) while CDPPB decreased subsequent drug‐seeking overall (P < 0.0167).
Figure 3.
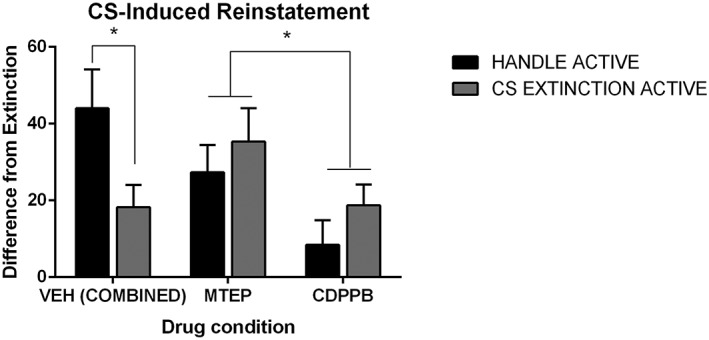
Differential active lever responding for experiments 1 and 2. Difference in responding between cue‐induced reinstatement and last day of extinction. For clarity, the vehicle (Veh) groups have been collapsed across the two experiments. CS extinction resulted in decreased responding at CS‐induced reinstatement 24 h later in Veh‐treated animals only. CS‐elicited drug seeking at reinstatement was decreased in rats administered a PAM on the previous day, when compared with animals that had received a NAM. *P < 0.05.
Importantly, follow‐up tests also revealed that responding was lower at reinstatement when rats had received CDPPB the previous day, as compared with MTEP [F(1, 67) = 11.584, P < 0.0167]. Thus, overall the data reveal that while the protection afforded by CS extinction is absent in both drug conditions, CDPPB afforded a better protection against relapse, because responding was lower in this group compared with vehicle or MTEP (P for both contrasts <0.0167).
Discussion
Our experiments show that Pavlovian extinction of a cocaine‐associated CS reduced reinstatement of an instrumental drug‐seeking response. We further showed that mGlu5 receptors are necessary for effective CS extinction, because systemic administration of a mGlu5 NAM eliminated this effect. In addition, activation of mGlu5 receptors produced lasting resistance to cue‐induced reinstatement, because administration of a selective mGlu5 PAM reduced CS‐induced cocaine seeking during a drug‐free test 24 h later, regardless of extinction experience.
The primary behavioural finding that extinction of a cocaine‐associated CS diminished its capacity to reinstate instrumental cocaine seeking is important because it provides preclinical support for the use of CET in the treatment of cocaine abuse. It also describes a more valid model for researching the neurochemistry subserving behavioural therapy. Our finding supports previous reports that CS extinction attenuated CS‐induced reinstatement (Torregrossa et al., 2010; Torregrossa et al., 2013). Other researchers have also shown that extinction of the CS, in the absence of lever extinction, also reduces CS‐elicited cocaine seeking (Buffalari et al., 2013). Together, these findings confirm that discrete drug‐associated cues are critical for supporting drug seeking (LeBlanc et al., 2012). These are a more appropriate model for behavioural therapy than the prototypical extinction‐reinstatement paradigm (Bossert et al., 2013), because they examine the effect of Pavlovian CS extinction on instrumental drug seeking, as opposed to using instrumental extinction where the response itself is not reinforced. Instrumental extinction is difficult to implement clinically, because in practical (human) terms, it is difficult to uncouple actions that lead to drug delivery from the drug itself. Furthermore, Pavlovian and instrumental extinction apparently recruit distinct, though overlapping, circuitries (Peters et al., 2009). Therefore, pharmacological adjuncts to behavioural therapy derived using the extinction‐reinstatement model may not necessarily be appropriate for use in combination with CET.
Over the past decade, there has been an increasing interest in the mGlu5 receptor as a potential target for treatment of cocaine abuse. For example, acute administration of an mGlu5 NAM such as MTEP or 2‐methyl‐6‐(phenylethynyl)pyridine (MPEP) decreased cocaine self‐administration in rodents (Kenny et al., 2005; Martin‐Fardon et al., 2009; Hao et al., 2010). This decrease in cocaine consumption appears to be due to a decrease in motivation to seek the drug following mGlu5 receptor antagonism (Paterson and Markou, 2005; Hao et al., 2010), and acute administration of mGlu5 receptor NAMs also decreased the magnitude of ‘relapse’ elicited by a cocaine prime or by a cocaine‐associated cue (Backstrom and Hyytia, 2006; Backstrom and Hyytia, 2007). These findings are promising because, although psychotomimetic effects have been reported in some trials (Friedmann et al., 1980; Pecknold et al., 1982), mGlu5 NAMs such as fenobam are generally well‐tolerated compounds that are already approved or in clinical trial to treat a range of disorders (Pecknold et al., 1982; Olive, 2010; Nickols and Conn, 2014).
However, the current findings suggest that therapeutically, antagonism of mGlu5 receptor signalling may be problematic in the longer term because it could inhibit the beneficial effects of cue (CS) extinction, which forms the basis of CET. Relapse is such a pervasive problem for addicts, in part because cues associated with drug‐taking activities elicit craving and strong motivation to seek the drug, even after prolonged drug withdrawal (Childress et al., 1986). CET aims to decrease the potency of these cues via extinction – presenting the cue repeatedly without the cocaine so the subject learns new, non‐drug associations. We showed that when a NAM was administered prior to CS extinction, any benefit in terms of decreased CS‐induced drug seeking at reinstatement the following day was lost. Therefore, although acute administration of a mGlu5 NAM may decrease the motivational properties of cocaine and cocaine‐associated cues, they also interfere with the learning process involved in effecting a behavioural change, hence ultimately increasing the likelihood of future relapse. Successful clinical application of mGlu5 NAMs would require taking the pharmaceutical immediately prior to, or during, a relapse episode, a strategy that in real terms would be difficult to implement. A more effective strategy would be to effect a change in behaviour by learning new responses to drug‐associated cues, such as occurs in CET, and to enhance this new learning with short‐term application of a cognitive aid. This should' theoretically at least, produce a greater resistance to relapse in the long term.
mGlu5 PAMs represent a desirable candidate for this latter strategy. mGlu5 receptors are critical for cognitive function and learning (Lu et al., 1997; Tan et al., 2015). Notably, a mGlu5 PAM can enhance extinction of Pavlovian fear cues (Ganella et al., 2016), and instrumental cocaine seeking (Cleva et al., 2011). Together with our finding that mGlu5 receptors are necessary for extinction of the conditioned properties of a cocaine‐associated cue, it seemed likely that administration of a PAM prior to cue extinction would facilitate extinction of the cocaine‐associated cue and hence produce greater protection against cue‐induced relapse.
Our finding that CDPPB decreased responding during a subsequent CS‐induced reinstatement test regardless of extinction condition is not straightforward to interpret. As expected, CS‐induced reinstatement was decreased when compared with rats administered vehicle, but this was not specific to the extinction condition. In other words, the mGlu5 PAM resulted in decreased drug seeking the following day regardless of whether it was administered in conjunction with CS extinction or not. Careful examination of the reinstatement data (Figure 2) reveals that the main effect is driven primarily by a decrease in responding in the Handle/CDPPB group. This is probably due in part to a floor effect, whereby reinstatement levels observed are already extremely low following CS extinction. We chose a priori to set a fixed number of extinction sessions between the two behavioural experiments. This choice was based on the fact that we were exploring the potential for a novel behavioural procedure to model therapy and relapse, and the outcome of the behavioural procedure itself was not assured. Clearly, there is potential to make numerous procedural variations during ongoing refinements. For example, future experiments with fewer CS exposures may provide a more sensitive measure against which to gauge potential facilitatory effects of CDPPB on CS extinction.
Nevertheless, CDPPB does produce a decrease in CS‐elicited drug seeking. It is important to reiterate that the CS‐induced reinstatement session was drug‐free, and because CDPPB has a plasma half‐life of 4.4 h (Kinney et al., 2005), it is most unlikely that the decrease in drug seeking >24 h later was due to residual acute pharmacological effects of the drug. Therefore, the systemic administration of CDPPB does appear to effect a change in motivation to seek the drug that is still apparent the next day. However, this cannot be solely due to facilitated extinction of the cocaine‐associated cue, because the rats that were handled on this day (but not subjected to CS extinction) also showed decreased cue‐elicited drug seeking at reinstatement. The most parsimonious explanation for this effect is that there was an enhancement of learning that may occur during the handling process, which led to a decrease in responding the following day. The handling process involved movement of the racks on which the home cages were held to an experimental area, followed by removal of each rat, one by one, i.p. injection then being held by the experimenter for a period of 1–2 min before being returned to their home cage and replaced on the racks. Throughout the experimental procedures, this sequence of events (excluding the injection days) was followed by the rat being placed in the operant chambers for either IVSA or lever extinction. Therefore, it is likely that this handling process creates an expectation that the rats will be subsequently placed in the operant chamber. In other words, it ‘reactivates’ either the self‐administration or the lever extinction memory, rendering it labile and subject to manipulation (Nader et al., 2000; Monfils et al., 2009).
There is now a sizeable quantity of literature on the extinction‐reconsolidation effect for use in addiction (Taylor et al., 2009; Xue et al., 2012; Torregrossa and Taylor, 2013, but see Hutton‐Bedbrook and McNally, 2013; Millan et al., 2013). Essentially, deficits in performance following manipulations at the time of retrieval can be interpreted either as attenuated reconsolidation of the drug‐seeking memory or enhanced reconsolidation of the extinction memory (Torregrossa and Taylor, 2013). Presumably, therefore, handling reactivates the memory of the extinction session, which is the more recent experience of the rats. Under these conditions, CDPPB would enhance reconsolidation of the extinction memory, hence amplifying this memory such that it is retrieved during a reinstatement test despite the presence of the cocaine‐associated CS. This interpretation is consistent with the mechanism of a mGlu5 PAM, which can enhance consolidation (Cleva et al., 2011) and in line with a facilitatory role of mGlu5 signalling in learning and memory processes (Manahan‐Vaughan and Braunewell, 2005). The alternative explanation that CDPPB is acting by disrupting reconsolidation of the self‐administration memory, resulting in decreased drug seeking at reinstatement, is counter‐intuitive, given that the mGlu5 receptors are functionally bound to the NMDA receptor and have been universally shown to enhance, rather than disrupt, learning processes (Lu et al., 1997; Manahan‐Vaughan and Braunewell, 2005; Olive, 2010; Cleva et al., 2011; Ganella et al., 2016). Future studies will undoubtedly investigate this issue; nevertheless, our current findings provide clear evidence that mGlu5 receptor signalling regulates the efficacy of CS extinction.
Our ultimate goal was to investigate the therapeutic potential for mGlu5 NAMs and PAMs to be used in conjunction with behavioural therapy for cocaine abuse. In this context, a systemic administration is more relevant because it provides information that can be translated more readily to a clinical situation. However, a shortcoming of such experiments is the inability to elucidate the neural mechanisms and circuitry that subserve these effects, which is of academic, if not clinical, interest. Thus, our findings lay the foundation for future experiments into the neuroanatomical loci activated following CS extinction, and where, within this circuitry, mGlu5 receptors actively produce behavioural change. In this regard, mGlu5 receptors are widely distributed throughout pertinent circuitry, particularly in the hippocampus, prefrontal cortex, nucleus accumbens and striatum (Romano et al., 1995).
The behavioural protocol employed in these experiments – examining the effect of extinction of a cocaine CS on instrumental responding for cocaine – is somewhat underexplored, and as such, the circuitry involved has not been fully uncovered. It was recently reported, using a similar procedure, that NMDA receptors in the anterior cingulate cortex and nucleus accumbens core were involved in encoding contextual and discrete aspects of CS extinction learning, respectively (Torregrossa et al., 2013). Given the interaction between mGlu5 and NMDA receptors, these structures would be an obvious starting point for a future investigation into the circuitry underlying the effects we observed. In addition, it is worth noting that systemic administration of high doses of MPEP activates neurons within the central amygdala (CeA), the bed nucleus of the stria terminalis (BNST) and the paraventricular nucleus of the hypothalamus, mimicking other anxiolytic and antidepressant drugs (Inta et al., 2012). The CeA and BNST are implicated in stress‐induced relapse following extinction of drug seeking (for a review, see Mantsch et al. 2016) and also project to the ventral tegmental area (Vranjkovic et al., 2014), which is crucial for encoding incentive salience of reward‐associated cues (Shultz, 1998) and therefore is likely to be implicated in CS extinction, and CS – elicited cocaine seeking.
To minimize the number of animals used, we decided to examine established doses of the mGlu5 NAM/PAM. For example, in rats, MTEP (2 mg kg−1) prevents extinction of a cocaine‐associated context (Kim et al., 2015) and a Pavlovian fear CS (Ganella et al., 2016). Notably, MTEP (3 mg kg−1) is sufficient to produce full occupancy of mGlu5 receptors in the rat brain following systemic administration (Anderson et al., 2003); hence, our dose is appropriate while avoiding potential off targets effects of a suprathreshold dose. CDPPB (60 mg kg−1) was chosen as it decreased instrumental extinction of cocaine seeking in rats (Cleva et al., 2011). Although lower doses of CDPPB (30 mg kg−1) may facilitate lever extinction of methamphetamine (Kufahl et al., 2012) and alcohol seeking (Gass et al., 2014), and extinction of a cocaine‐associated context (Gass and Olive, 2009), this dose failed to influence lever extinction of cocaine seeking, while the higher dose (60 mg kg−1) did (Cleva et al., 2011).
There are currently no pharmacological treatments approved for the treatment of cocaine addiction in humans (Kim and Lawrence, 2014); hence, cocaine was of particular interest to us. It is possible that the current findings may generalize to other drugs of abuse, especially as the deletion of mGlu5 receptors causes extinction deficits for methamphetamine (Chesworth et al., 2013) and cocaine (Bird et al., 2014), while CDPPB facilitates extinction of methamphetamine (Kufahl et al. 2012) and alcohol seeking (Gass et al., 2014). However, it is also true that the effects of mGlu5 NAMs on reward seeking can be reinforce‐specific (Bespalov et al., 2005); therefore, future studies will be required to assess whether the effects observed here are specific to cocaine seeking and extinction of cocaine‐associated CSs, or alternatively a more general effect on cognitive processing.
Conclusion
We found that extinction of a cocaine‐associated cue reduces the impact of subsequent cue‐induced reinstatement and that mGlu5 receptors are necessary for this effect. We also found that the mGlu5 PAM, CDPPB, was able to reduce cue‐elicited reinstatement the day after administration, even when applied without any specific CS extinction procedure. This is a promising finding for the application of mGlu5 PAMs in treatment of cocaine addiction, because it produced a behavioural change that persisted outside of the acute effects of the drug itself. This makes it more desirable as a treatment because, rather than acutely decreasing drug seeking, it provides the promise to strengthen the ability of behavioural therapies to protect against subsequent relapse. In other words, we suggest that short‐term treatment with cognitive aids that facilitate extinction learning could provide long‐term benefit in the form of increased protection against relapse.
Author contributions
C.J.P. contributed to experimental design and execution, interpreted data and prepared the manuscript. F.R. executed the majority of behavioural procedures. I.C.Z. provided technical assistance. J.H.K. contributed to conception and experimental design. A.J.L. contributed to conception, assisted with data interpretation and manuscript preparation and was project leader.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organizations engaged with supporting research.
Acknowledgements
Thanks to the Florey Core Animal Services for assistance with animal housing and maintenance. This research was funded by the National Health and Medical Research Council project grant 1022201 and the Victorian State Government Operational Infrastructure Scheme. Equipment was supported by a philanthropic grant from the Eric Ormond Baker Foundation.
Perry, C. J. , Reed, F. , Zbukvic, I. C. , Kim, J. H. , and Lawrence, A. J. (2016) The metabotropic glutamate 5 receptor is necessary for extinction of cocaine‐associated cues. British Journal of Pharmacology, 173: 1085–1094. doi: 10.1111/bph.13437.
References
- Adams CL, Cowen MS, Short JL, Lawrence AJ (2008). Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol‐seeking in rats. Int J Neuropsychopharmacol 11: 229–241. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E, et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, et al. (2003). In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3‐methoxy‐5‐(pyridin‐2‐ylethynyl)pyridine). Eur J Pharmacol 473: 35–40. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagal R (2004). mGluR5 antagonist MPEP reduces ethanol‐seeking and relapse behavior. Neuropsychopharmacology 29: 921–928. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P (2006). Ionotropic and metabotropic glutamate receptor antagonism attenuates cue‐induced cocaine seeking. Neuropsychopharmacology 31: 778–786. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P (2007). Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue‐induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 192: 571–580. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, et al. (2005). Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue‐ and schedule‐induced reinstatement of nicotine self‐administration behavior in rats. Neuropharmacology 49 (Suppl 1): 167–178. [DOI] [PubMed] [Google Scholar]
- Bird KD. 2004. Analysis of Variance via Confidence Intervals. Sage Publications: London, ISBN: 0 7619 6357. [Google Scholar]
- Bird MK, Lohmann P, West B, Brown RM, Kirchhoff J, Raymond CR, et al. (2014). The mGlu5 receptor regulates extinction of cocaine‐driven behaviours. Drug Alcohol Depend 137: 83–89. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013). The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229: 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52: 976–986. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Feltenstein MW, See RE (2013). The effects of varied extinction procedures on contingent cue‐induced reinstatement in Sprague–Dawley rats. Psychopharmacology (Berl) 230: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Lawrence AJ (2013). The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine‐seeking in mice. PLoS One 8, e68371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP (1986). Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81: 655–660. [DOI] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, et al. (2011). mGluR5 positive allosteric modulation enhances extinction learning following cocaine self‐administration. Behav Neurosci 125: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002). Applying extinction research and theory to cue‐exposure addiction treatments. Addiction 97: 155–167. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid WO, Lawrence AJ, Krstew EV, Tait RJ, Hulse GK, Dunlop SA (2012). Maternally administered sustained‐release naltrexone in rats affects offspring neurochemistry and behaviour in adulthood. PLoS One 7, e52812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann CTH, Davis LJ, Ciccone PE, Rubin RT (1980). Phase‐II double‐blind controlled‐study of a new anxiolytic, fenobam (Mcn‐3377) vs placebo. Curr Ther Res Clin Exp 27: 144–151. [Google Scholar]
- Ganella DE, Thangaraju P, Lawrence AJ, Kim JH (2016). Fear extinction in 17 day old rats is dependent on metabotropic glutamate receptor 5 signaling. Behav Brain Res 298: 32–36 [PMID: 25497704]. [DOI] [PubMed] [Google Scholar]
- Gao C, Tronson NC, Radulovic J (2013). Modulation of behavior by scaffolding proteins of the post‐synaptic density. Neurobiol Learn Mem 105: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF (2009). Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry 65: 717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Trantham‐Davidson H, Kassab AS, Glen WB Jr, Olive MF, Chandler LJ (2014). Enhancement of extinction learning attenuates ethanol‐seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci 34: 7562–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin‐Fardon R, Weiss F (2010). Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine‐escalated rats: factor in the transition to dependence. Biol Psychiatry 68: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton‐Bedbrook K, McNally GP (2013). The promises and pitfalls of retrieval‐extinction procedures in preventing relapse to drug seeking. Front Psychiatr 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Filipovic D, Lima‐Ojeda JM, Dormann C, Pfeiffer N, Gaspaini F, et al. (2012). The mGlu5 receptor antagonist MPEP activates specific stress‐related brain regions and lacks neurotoxic effects of the NMDA receptor antagonist MK‐801: significance for the use as anxiolytic/antidepressant drug. Neuropsychopharmacology 62: 2034–2039. [DOI] [PubMed] [Google Scholar]
- Keck TM, Yang HJ, Bi GH, Huang Y, Zhang HY, Srivastava R, et al. (2013). Fenobam sulfate inhibits cocaine‐taking and cocaine‐seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology (Berl) 229: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, Zou MF, Bi GH, Zhang HY, Wang XF, Yang HJ, et al. (2014). A novel mGluR5 antagonist, MFZ 10‐7, inhibits cocaine‐taking and cocaine‐seeking behavior in rats. Addict Biol 19: 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A (2005). Metabotropic glutamate 5 receptor blockade may attenuate cocaine self‐administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 179: 247–254. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). NC3Rs Reporting Guidelines Working Group. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lawrence AJ (2014). Drugs currently in Phase II clinical trials for cocaine addiction. Expert Opin Investig Drugs 23: 1105–1122. [DOI] [PubMed] [Google Scholar]
- Kim JH, Perry C, Luikinga S, Zbukvic I, Brown RM, Lawrence AJ (2015). Extinction of a cocaine‐taking context that protects against drug‐primed reinstatement is dependent on the metabotropic glutamate 5 receptor. Addict Biol 20: 482–489. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, LeMaire W, Burno M, Bickel DJ, Clements MK, et al. (2005). A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic‐like effects in rat behavioral models. J Pharmacol Exp Ther 313: 199–206. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Hood LE, Nemirovsky NE, Barabas P, Halstengard C, Villa A, et al. (2012). Positive allosteric modulation of mGluR5 accelerates extinction learning but not relearning following methamphetamine self‐administration. Front Pharmacol 3: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, et al. (2009). Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming‐ and cue‐induced reinstatement of cocaine seeking. Behav Brain Res 202: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT (2012). Pavlovian‐to‐instrumental transfer in cocaine seeking rats. Behav Neurosci 126: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, et al. (1997). Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long‐term potentiation (LTP) but normal CA3 LTP. J Neurosci 17: 5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan‐Vaughan D, Braunewell KH (2005). The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex 15: 1703–1713. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y (2016). Stress‐induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology 41: 335–356 [PMID: 25976297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Fardon R, Baptista MA, Dayas CV, Weiss F (2009). Dissociation of the effects of MTEP [3‐[(2‐methyl‐1,3‐thiazol‐4‐yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther 329: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Milligan‐Saville J, McNally GP (2013). Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol Learn Mem 101: 26–32. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009). Extinction‐reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324: 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE (2000). The labile nature of consolidation theory. Nat Rev Neurosci 1: 216–219. [DOI] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ (2014). Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 61: 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF (2010). Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol 639: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A (2005). The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 179: 255–261. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. (2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42: D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T (1982). Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double‐blind standard (diazepam) placebo‐controlled study. J Clin Psychopharmacol 2: 129–133. [PubMed] [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ (2014). Role of cues and contexts on drug‐seeking behaviour. Br J Pharmacol 171: 4636–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009). Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW (1995). Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol 355: 455–469. [DOI] [PubMed] [Google Scholar]
- Shultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Tan SZ, Ganella DE, Dick AL, Duncan JR, Ong‐Palsson E, Bathgate RA, et al. (2015). Spatial learning requires mGlu5 signalling in the dorsal hippocampus. Neurochem Res 40: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM (2009). Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56 (Suppl 1): 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Gordon J, Taylor JR (2013). Double dissociation between the anterior cingulate cortex and nucleus accumbens core in encoding the context versus the content of Pavlovian cocaine cue extinction. J Neurosci 33: 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Sanchez H, Taylor JR (2010). d‐cycloserine reduces the context specificity of Pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci 30: 10526–10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR (2013). Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 226: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR (2014). Stress‐induced cocaine seeking requires a beta‐2 adrenergic receptor‐regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci 34: 12504–12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF (2013). Attenuation of reinstatement of methamphetamine‐, sucrose‐, and food‐seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology (Berl) 225: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, et al. (2012). A memory retrieval‐extinction procedure to prevent drug craving and relapse. Science 336: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]


