Abstract
The metabotropic glutamate receptor subtype 5 (mGlu5) is a family C GPCR that has been implicated in various neuronal processes and, consequently, in several CNS disorders. Over the past few decades, GPCR‐based drug discovery, including that for mGlu5 receptors, has turned considerable attention to targeting allosteric binding sites. Modulation of endogenous agonists by allosteric ligands offers the advantages of spatial and temporal fine‐tuning of receptor activity, increased selectivity and reduced adverse effects with the potential to elicit improved clinical outcomes. Further, with greater appreciation of the multifaceted nature of the transduction of mGlu5 receptor signalling, it is increasingly apparent that drug discovery must take into consideration unique receptor conformations and the potential for stimulus‐bias. This novel paradigm proposes that different ligands may differentially modulate distinct signalling pathways arising from the same receptor. We review our current understanding of the complexities of mGlu5 receptor signalling and regulation, and how these relate to allosteric ligands. Ultimately, a deeper appreciation of these relationships will provide the foundation for targeted drug design of compounds with increased selectivity, not only for the desired receptor but also for the desired signalling outcome from the receptor.
Linked Articles
This article is part of a themed section on Molecular Pharmacology of G Protein‐Coupled Receptors. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v173.20/issuetoc
Tables of Links
| Targets | |
|---|---|
| GPCRsa | Ligand‐gated ion channelsc |
| Adenosine A2A receptors | AMPA receptors |
| Calcium‐sensing receptors | NMDA receptors |
| Dopamine D2 receptors | Enzymes d |
| mGlu1 receptors | Akt |
| mGlu5 receptors | ERK1/2 |
| μ‐opioid receptors | GRK2, G protein‐coupled receptor kinase 2 |
| Ion channels b | p38 MAPK |
| TRPV1 channels | |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a b c dAlexander et al., 2013a, 2013b, 2013c, 2013d).
Abbreviations
- 7TMs
seven transmembrane‐spanning domains
- CPPHA
N‐(4‐chloro‐2‐[(1,3‐dioxo‐1,3‐dihydro‐2H‐isoindol‐2‐yl)methyl]phenyl)‐2‐hydroxybenzamide
- DFB
((3‐Fluorophenyl)methylene)hydrazone‐3‐fluorobenzaldehyde
- DHPG
(S)‐3,5‐DHPG, (S)‐3,5‐dihydroxyphenylglycine
- iCa2+
intracellular calcium
- LTD
long‐term depression
- MPEP
2‐methyl‐6‐(phenylethynyl)pyridine
- mTOR
mammalian target of rapamycin
- NAL
neutral allosteric ligand
- NAM
negative allosteric modulator
- NCFP
N‐(4‐chloro‐2‐[(1,3‐dioxoisoindolin‐2‐yl)methyl)phenyl)picolinamide]
- PAM
positive allosteric modulator
- VFT
Venus flytrap
Introduction
Glutamate is the main excitatory neurotransmitter in the brain, providing the balance to the major inhibitory neurotransmitter GABA in neuronal synaptic interplay. Until the mid‐1980s, glutamate was thought to act solely via a family of ligand‐gated ion channels, the ionotropic glutamate receptors comprised three subtypes: NMDA, AMPA and kainate. It was not until this neurotransmitter was shown to stimulate inositol phosphate that the first G protein‐coupled metabotropic glutamate (mGlu) receptor was cloned (Houamed et al., 1991; Masu et al., 1991). Eight mGlu receptor subtypes have since been characterized and are subdivided based on signal transduction mechanisms, pharmacology and sequence homology (Niswender and Conn, 2010). Group I consists of mGlu1 and mGlu5 receptors that preferentially activate phospholipase C via Gq/11 coupling, whereas Group II (mGlu2 and mGlu3 receptors) and group III (mGlu4, mGlu6, mGlu7 and mGlu8 receptors) couple to Gi/o and adenylate cyclase inhibition (Niswender and Conn, 2010).
The mGlu receptors are family C GPCRs – a group that also includes GABAB receptors, the calcium‐sensing receptor and multiple olfactory, taste and pheromone receptors, distinguished by a large extracellular N‐terminal, Venus flytrap (VFT) domain. This N‐terminal domain comprises two distinct lobes that close around glutamate upon binding, with the closure of one lobe sufficient for receptor activation, and closure of both lobes required for maximal activity (O'Hara et al., 1993; Costantino and Pellicciari, 1996; Kunishima et al., 2000; Kniazeff et al., 2004; Muto et al., 2007). Constitutive receptor dimerization occurs via a disulfide bond in an as yet unsolved loop region linking the ‘top’ of the two VFT domains (Romano et al., 1996; Kunishima et al., 2000; Romano et al., 2001; Muto et al., 2007). Linking the N‐terminal VFT to the seven transmembrane‐spanning domains (7TMs) is a cysteine rich domain. The cysteine rich domain is crucial for the transmission of conformational changes induced by glutamate binding in the VFT to the 7TMs that mediate coupling with intracellular effectors (Rondard et al., 2006).
Widely expressed and mainly postsynaptic throughout the cortex, striatum, hippocampus, caudate nucleus and nucleus accumbens, and in astrocytes, glia and peripheral sensory neurons (Shigemoto et al., 1993; Crawford et al., 2000; Walker et al., 2001), mGlu5 receptors are crucial for synaptic plasticity and neuronal development (Jia et al., 1998; Fendt and Schmid, 2002; Bortolotto et al., 2005; Galik et al., 2008; Wijetunge et al., 2008; She et al., 2009; Xu et al., 2009; Ballester‐Rosado et al., 2010; Black et al., 2010; Chen et al., 2012; Xiao et al., 2013; Hamilton et al., 2014). mGlu5 receptors have been implicated in the pathology or as a therapeutic target for numerous CNS disorders, including psychosis and schizophrenia (Kinney et al., 2003; Brody et al., 2004; Chen et al., 2010), motor control (Ribeiro et al., 2014), anxiety and depression (Li et al., 2006; Inta et al., 2013), reward and addiction (Bird et al., 2010; Olsen et al., 2010; Eiler et al., 2011; Stoker et al., 2012; Chesworth et al., 2013), appetite and energy homeostasis (Bradbury et al., 2005) and pain (Galik et al., 2008; Kolber et al., 2010). The immense therapeutic potential of targeting mGlu5 receptors has been extensively summarized (Noetzel et al., 2012a; Gregory et al., 2013a; Lindsley and Stauffer, 2013; Palucha‐Poniewiera et al., 2013; Pilc et al., 2013), with candidates entering clinical trials for anxiety, depression and Fragile X syndrome with varying successes and failures (Pecknold et al., 1982; Porter et al., 2005; Levenga et al., 2011; Wieronska and Pilc, 2013; Jaeschke et al., 2015; Lindemann et al., 2015). As discussed in detail in the following, mGlu5 receptors promiscuously couple to various G proteins and interact with complex yet fluid protein scaffolding complexes, such that our understanding of signal transduction mechanisms remains convoluted. Here we attempt to delineate the complex nature of mGlu5 receptor‐mediated cellular responses and discuss new therapeutic modalities to target mGlu5 receptors, providing increased receptor specificity to avoid adverse effects and improve clinical outcomes.
Signal transduction of mGlu5 receptors
Preferentially coupled to Gαq/11, mGlu5 receptor activation leads to activation of phospholipase C and production of inositol‐1,4,5‐triphosphate (IP3) and DAG, and subsequently, mobilization of intracellular calcium (iCa2+) (Abe et al., 1992). Calcium, in combination with DAG, leads to the activation of PKC, PLA2, MAPK and the modulation of ion channels (Hermans and Challiss, 2001; Conn et al., 2008; Ribeiro et al., 2010) (Figure 1). Activation of this canonical signalling pathway is linked to various cell processes including synaptic plasticity (Borgdorff and Choquet, 2002; Gerrow and Triller, 2010; Kato et al., 2012). In addition to Gq coupling, mGlu5 receptors also couple to Gs in HEK293 cells (Francesconi and Duvoisin, 1998) and are linked to cAMP formation in LLC‐PK1 cells and oocytes (Joly et al., 1995). However, when expressed in CHO cells or astrocytes, no agonist stimulated increases in cAMP accumulation were observed (Abe et al., 1992; Balazs et al., 1997; Ribeiro et al., 2010), suggesting that mGlu5 receptor promiscuous G protein coupling is cell type‐dependent.
Figure 1.
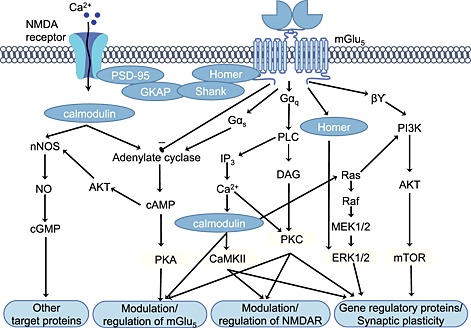
Activation of mGlu5 receptors triggers diverse distinct and converging signalling pathways. The group I mGlu receptor family preferentially couples to Gq, leading to activation of the canonical PLC–IP3–DAG–Ca2+ pathway. As reviewed in the text, mGlu5 receptors promiscuously couple to other G proteins as well as alternate second messengers and kinases via mechanisms that can be independent of Ca2+ mobilization. Protein–protein interactions with scaffolding and regulatory proteins (blue ovals) add further diversity: integrating different signalling pathways, directly mediating interactions between mGlu5 receptors and ion channels such as the NMDA receptor and regulating the activity of the mGlu5 receptor itself. As an additional level of complexity, the signalling pathways and effectors stimulated by mGlu5 receptor activation also show considerable cell type dependence.
Downstream of G protein coupling, agonist stimulation of mGlu5 receptors leads to phosphorylation of ERK1/2 (Thandi et al., 2002; Hu et al., 2007) and p38 MAPK (Peavy and Conn, 1998; Rush et al., 2002). Interestingly, mGlu5 receptor‐mediated phosphorylation of ERK1/2 has been reported to be independent of PKC, PI3K and calcium (Peavy and Conn, 1998; Thandi et al., 2002). Additional evidence has implicated Homer 1b/c scaffolding protein (Mao et al., 2005), epidermal growth factor receptor tyrosine kinase, proline‐rich tyrosine kinase 2 and Src activation in coupling mGlu5 receptors to ERK1/2 phosphorylation (Peavy et al., 2001; Thandi et al., 2002; Wang et al., 2007; Nicodemo et al., 2010). mGlu5 receptor‐mediated ERK1/2 phosphorylation leads to activation of downstream transcription factors including Elk‐1, cAMP response element binding‐protein and c‐Jun, which regulate gene expression involved in long‐term depression (LTD) (Rush et al., 2002; Gallagher et al., 2004; Wang et al., 2007). Group I mGlu receptor activation of PI3K, Akt and mammalian target of rapamycin (mTOR) has also been implicated in LTD (Chan et al., 1999; Hou and Klann, 2004). The mechanism driving group I mGlu receptor‐mediated synaptic plasticity is the interplay between ERK‐MAPK and PI3K–mTOR pathways resulting in de novo protein synthesis and late‐phase LTD (Waung and Huber, 2009). Moreover, in disease states, the balance of mGlu5 receptor signal transduction may be perturbed. For example, in a mouse model of Huntington's disease, mGlu5 receptor coupling to Akt, ERK1/2 and intracellular Ca2+ release is enhanced, while IP3 formation is decreased (Ribeiro et al., 2010). In addition to complex acute signalling outcomes, regulatory processes triggered by mGlu5 receptor activation show a similar level of intricacy.
Regulation of mGlu5 receptor signalling
Signal transduction arising from mGlu5 receptors is tightly controlled by the phosphorylation status of the receptor. The frequency of glutamate‐induced iCa2+ oscillations are governed by a ‘dynamic uncoupling’ mechanism where the receptor undergoes rapid cycles of phosphorylation and dephosphorylation (Kawabata et al., 1996; Nash et al., 2002; Bradley and Challiss, 2011a). The major mediator of mGlu5 receptor phosphorylation and subsequent desensitisation of mGlu5 receptor signalling is PKC, although other kinases also play a role such as G protein‐coupled receptor kinases (GRK), tyrosine kinases and Ca2+/calmodulin‐dependent protein kinase II (see Mao et al., 2008). The phosphorylation status of mGlu5 receptors regulates interactions with other proteins. Trafficking and signalling of mGlu5 receptors are regulated by calmodulin that interacts with the C terminus of the receptor (Choi et al., 2011). PKC phosphorylation of mGlu5 receptors inhibits calmodulin binding (Lee et al., 2008) and facilitates seven in absentia homolog 1A binding, promoting endocytosis and receptor down‐regulation via ubiquitination‐dependent mechanisms (Kammermeier and Ikeda, 2001; Moriyoshi et al., 2004; Ko et al., 2012). Calmodulin binding sites also partially overlap with that of Norbin, a neuronal protein that binds mGlu5 receptors to promote cell surface expression and signalling (Wang et al., 2009). Another key adaptor protein that regulates mGlu5 receptor function and expression is the Homer family (Brakeman et al., 1997; Xiao et al., 1998; Tu et al., 1999; Ango et al., 2002; Kammermeier and Worley, 2007; Lv et al., 2014). Of note, Homer interactions with mGlu5 receptors are enhanced by receptor phosphorylation mediated by cyclin‐dependent kinase 5 (Orlando et al., 2009). Recently, mGlu5 receptors were also shown to be a target of PKA phosphorylation, with mGlu5 receptor phosphorylation levels being linked to elevated intracellular cAMP levels (Uematsu et al., 2015). Collectively, these data highlight the critical role mGlu5 receptor phosphorylation plays in regulating receptor function; however, not all mechanisms rely on phosphorylation. For example, GRK2 mediates mGlu5 receptor desensitization and internalization (Sorensen and Conn, 2003) via a mechanism that can be phosphorylation independent (Ribeiro et al., 2009). Moreover, in disease states, abnormal interactions can occur, for example, disruption of Homer scaffolds in Fragile X syndrome results in abnormal mGlu5 receptor function (Ronesi et al., 2012). Thus, there is much diversity in both the cellular responses and regulatory mechanisms triggered by mGlu5 receptor activation. This diversity extends further to mGlu5 receptor‐mediated modulation of the response to other glutamate receptors.
Activation of mGlu5 receptors modulates function of other glutamatergic receptors
Activation of group I mGlu receptors modulates the activity of various ion channels, in particular, the NMDA and AMPA ionotropic glutamate receptors (Homayoun et al., 2004; Nakamoto et al., 2007; Kato et al., 2012). Mechanisms of modulation include activation of downstream second messengers, kinases and direct protein–protein interactions (Benquet et al., 2002; Collett and Collingridge, 2004; Homayoun et al., 2004; Ribeiro et al., 2010; Moutin et al., 2012; Gao et al., 2013). The overall response of an individual cell to glutamate is highly integrated. For example, NMDA receptor activation is involved in early phase synaptic plasticity, with rapid calcium influx leading to modulation of AMPA receptor trafficking (Borgdorff and Choquet, 2002). The modulation of ionotropic receptors by mGlu5 receptors and vice versa is complex. For example, mGlu5 receptor activation positively modulates the NMDA receptor through increasing open channel probability, with PKC‐dependent Src signalling, and stabilization of a Homer–Shank protein anchor implicated (Lu et al., 1999; Tu et al., 1999; Benquet et al., 2002; Kotecha et al., 2003). In contrast, mGlu5 receptor activation inhibits NMDA receptor‐mediated activation of adenylate cyclase, resulting in reduced cAMP and neuronal nitric oxide synthase activity, signal transduction mechanisms that are involved in NMDA receptor‐mediated excitotoxicity (Llansola and Felipo, 2010). The relationship between ionotropic receptors and mGlu5 receptors has resulted in extensive efforts to design therapies targeting mGlu5 receptors in particular for the treatment of CNS disorders related to NMDA receptor dysfunction (Conn et al., 2008; Matosin and Newell, 2013; Veerman et al., 2014). Indeed, targeting mGlu5 receptors represents a promising approach to overcome NMDA receptor dysfunction while avoiding excitotoxicity associated with direct stimulation of NMDA receptors.
In addition to modulating ionotropic glutamate receptors, mGlu5 receptors are known to partner with the mGlu1 receptor. In the hippocampus, chemically induced LTD is partially blocked by antagonists of either receptor, and a combination of both mGlu1 and mGlu5 receptor antagonists is required to completely block the response (Volk et al., 2006). This is in spite of the fact that genetic deletion of mGlu5 receptors alone is sufficient to prevent (S)‐3,5‐dihydroxyphenylglycine (DHPG)‐induced LTD (Huber et al., 2001), supporting the hypothesis that heterodimerization plays a role. The mechanisms underlying this remain to be fully elucidated, with both mGlu1/ mGlu5 receptor heterodimerization and synergistic signalling (independent of heterodimerization) proposed (Romano et al., 1996; Goudet et al., 2005; Doumazane et al., 2011; Sevastyanova and Kammermeier, 2014). Collectively, it is clear that, where expressed, mGlu5 receptors are integral to the interplay of receptors involved in the overall cellular response to glutamate.
Activation of mGlu5 receptors affects other ion channels and GPCRs
In addition to modulating glutamatergic receptors, mGlu5 receptor activation influences the function of other ion channels and GPCRs. In HEK293 cells and isolated cortical neurons, mGlu5 receptor activation inhibits N‐type and P/Q‐type calcium channels in a voltage‐dependent manner, with both βγ subunits of Gi/o and Homer scaffolding proteins involved (McCool et al., 1998; Kammermeier et al., 2000). In hippocampal neurons, mGlu5 receptor activation facilitates L‐type voltage‐dependent calcium channels, two mechanisms have been reported, one indirect via mGlu5 receptor‐stimulated Ca2+ and PKC and the other via direct receptor–channel interactions (Topolnik et al., 2009; Kato et al., 2012). Within the periphery, mGlu5 receptors located in the dorsal root ganglia are implicated in both inflammatory and neuropathic pain, with injury to nerves, spinal cord or peripheral tissues resulting in the release of glutamate from sensory neurons (Valerio et al., 1997; Walker et al., 2001). In these tissues, mGlu5 receptors are co‐localized with the non‐selective cation channel TRPV1, enhancing the activity of these channels via DAG formation (Varney and Gereau, 2002; Kim et al., 2009). Recent work suggests that mGlu5 receptor‐mediated potentiation of TRPV1 is biphasic, transiently potentiating TRPV1‐mediated inward currents but inhibiting voltage‐gated calcium channels (Masuoka et al., 2015). The mechanism mediating these two different responses remains to be elucidated.
Co‐localization of mGlu5 receptors with several different GPCRs has functional consequences on the signalling and/or pharmacology of the individual GPCR (Schroder et al., 2009; Brown et al., 2012). For example, co‐stimulation or blockade of mGlu5 and adenosine A2A receptors results in synergistic effects; however, the mechanisms mediating the cross‐talk between these receptors appear to be dependent on the brain region studied (Ferre et al., 2002; Nishi et al., 2003; Domenici et al., 2004; Tebano et al., 2005; Tebano et al., 2006). Intriguingly, this synergy between mGlu5 and adenosine A2A receptors has been proposed to involve the formation of heteromers (Ferre et al., 2002; Rodrigues et al., 2005). In fact, in addition to heteromers with mGlu1 receptors mentioned earlier, mGlu5 receptors heterodimerize with numerous GPCRs including calcium‐sensing receptors (Gama et al., 2001) and μ‐opioid receptors (Schroder et al., 2009). Furthermore, mGlu5, adenosine A2A and D2 dopamine receptors are thought to form higher‐order oligomers (Cabello et al., 2009), capable of changing the pharmacological properties of a heteromer ‘subunit’ (Ferre et al., 1999; Popoli et al., 2001). It is clear that the signalling pathways triggered and interacting proteins modulated by mGlu5 receptor activation are complex and highly dependent on the cellular and tissue context. Therefore, it is perhaps not surprising that ligand pharmacology can differ depending on the system under exploration.
Localization of mGlu5 receptor signalling
Increasing evidence suggests that in addition to different signalling profiles in distinct cell types or brain regions, within a single cell mGlu5 receptors show sub‐cellular compartmentalization that can impact signal transduction and regulatory mechanisms. On the plasma membrane, mGlu5 receptors are associated with caveolae and lipid rafts resulting in constitutive internalization via interactions with caveolin‐1 (Francesconi et al., 2009). Importantly, mGlu5 receptors are mostly (>50–80%) found on intracellular membranes including the inner and outer nuclear membranes and the endoplasmic reticulum (Hubert et al., 2001; Kumar et al., 2008). Because intracellular mGlu5 receptors are oriented with the N‐terminus in the lumen of the organelle, and a cytoplasmic C‐terminus, the same complement of effector proteins is available, unless effector proteins are similarly subject to sub‐cellular localization (Jong et al., 2014). This compartmentalization of mGlu5 receptors results in differential pharmacology by virtue of accessibility of ligands. Intracellular receptors are only able to be activated by agonists that are able to diffuse, or be actively transported across membranes (Jong et al., 2005). While activation of mGlu5 receptors on the plasma membrane is associated with rapid transient calcium mobilization, activation of intracellular mGlu5 receptors is associated with sustained responses arising from ERK1/2 stimulated Elk‐1 phosphorylation (Jong et al., 2009), induction of LTD in rat hippocampal slices (Purgert et al., 2014) and activation of mediators of neuronal growth, differentiation and sustained synaptic transmission (Jong et al., 2009; Kumar et al., 2012). Thus, in targeting mGlu5 receptors, drug discovery programmes may also need to consider not only pharmacokinetics within the body, but also pharmacokinetics within the individual cell.
Targeting mGlu5 receptors: orthosteric ligands
As highlighted earlier, mGlu5 receptors are implicated in various neuronal processes, and selective mGlu5 receptor drugs are attractive putative therapies for numerous CNS disorders. Traditional drug discovery strategies have targeted the endogenous ligand binding (orthosteric) site to activate or block receptor activity. Several group I mGlu selective ligands have been discovered (Table 1), with DHPG prevailing as the most commonly utilized group I mGlu selective pharmacological tool (reviewed in Brauner‐Osborne et al., 2007). However, high affinity/potency and mGlu5 receptor selective orthosteric ligands have remained elusive, likely due to the fact that the orthosteric site is highly conserved across the mGlu receptor family. The complete activation or inhibition of receptor responses achieved by orthosteric ligands also presents an additional liability, as such compounds lack the delicate balance on neurotransmission, which may lead to undesirable or adverse effects. Thus, many research groups have turned their focus to targeting allosteric binding sites that are topographically distinct from the orthosteric site (Christopoulos and Kenakin, 2002; Leach et al., 2007).
Table 1.
Molecular pharmacology of selected mGlu5 receptor orthosteric ligands
| Compound | mGlu5 receptor pharmacology | Subtype selectivity | References |
|---|---|---|---|
| Glutamate | Agonist, affinity: 700 nM | 1 > 3 ≥ 5 ≥ 2 = 4 = 8 > 6 >>7 | (Brabet et al., 1998; Niswender and Conn, 2010) |
| Quisqualate | Agonist, affinity: 29 nM | 5 = 1 > 2 ≥ 3 > group III | (Porter et al., 1992; Mutel et al., 2000) |
| ACPD | Agonist, affinity: 2.1 μM | 2 > 5 = 3> 1 = 6 = 8> 4 >>7 | (Tückmantel et al., 1997; Cartmell et al., 1998; Wu et al., 1998) |
| (S)‐CHPG | Agonist, affinity: 380 μM | 5 ≥ 1 > group II and III | (Doherty et al., 1997; Mutel et al., 2000) |
| (S)‐DHPG | Agonist, affinity: 3.9 μM | 5 >1 > group II and III | (Wisniewski et al., 2002; Hathaway et al., 2015) |
| (S)‐4CPG | Antagonist, affinity: 26 μM | 1 > 5 > group II and III | (Hayashi et al., 1994; Thomsen et al., 1994; Doherty et al., 1999; Mutel et al., 2000) |
| (S)‐MCPG | Antagonist, affinity: 153–316 μM | 5 = 1 > group II and III | (Brabet et al., 1995; Kingston et al., 1995; Doherty et al., 1999; Mutel et al., 2000) |
CHPG: 2‐Chloro‐5‐hydroxyphenylglycine; 4CPG: 4‐carboxyphenylglycine; MCPG: α‐methyl‐4‐carboxylphenylglycine.
Targeting mGlu5 receptors: allosteric ligands
GPCRs readily lend themselves to allosteric modulation due to their dynamic receptor conformations and diversity in potential allosteric binding pockets (Lagerstrom and Schioth, 2008). Binding of a ligand to an allosteric site may modulate the receptor through changes in orthosteric agonist affinity and/or efficacy, a property referred to as cooperativity (Christopoulos and Kenakin, 2002). Allosteric ligands that enhance orthosteric agonist pharmacology are referred to as positive allosteric modulators (PAMs); those that inhibit are negative allosteric modulators (NAMs). Neutral allosteric ligands (NALs) represent a third class that bind to allosteric sites yet have no discernible effect on the pharmacology of orthosteric ligands (Christopoulos et al., 2014). Advantages of allosteric modulator focussed discovery are three‐fold: first, binding to a less conserved allosteric site allows for greater selectivity. Second, as pure allosteric modulators require the presence of orthosteric agonist for activation, they possess the ability to fine‐tune temporal and spatial receptor responses. This is a key difference between CNS drugs currently available on the market, which are largely orthosteric agonists or antagonists, because with allosteric modulators, receptor physiology is maintained, rather than silenced or completely switched on (Christopoulos and Kenakin, 2002; Leach et al., 2007; Melancon et al., 2012). Third, cooperativity between allosteric and orthosteric ligands is both reciprocal and saturable; therefore, allosteric modulators have a ceiling level to their effect and are theoretically safe in the case of overdose.
The concept of allosteric modulation has evolved with increasing understanding of GPCRs, from the simple two‐state model, which dictates that the receptor exists in equilibrium between the active and inactive state, to that of the multi‐state model that highlights the ability of ligands to stabilize unique receptor conformations to induce distinct downstream responses (Vauquelin and Van Liefde, 2005). Numerous models have been applied to quantify allosteric interactions at the mGlu receptor family (Hall, 2000; Rovira et al., 2008); however, the approach that has gained the most traction is the operational model of allosterism. The operational model of allosterism combines the simple allosteric ternary complex model with the operational model of agonism (Black and Leff, 1983; Leach et al., 2007). The advantage to this approach is the potential to delineate the contribution of affinity and efficacy modulation to the overall cooperativity observed. Moreover, an increasing number of allosteric ligands for many GPCRs including mGlu5 receptors (Kinney et al., 2005; Noetzel et al., 2012b; Gregory et al., 2013c; Rook et al., 2013) have been identified that have intrinsic efficacy in their own right (PAM‐agonists), an aspect that is also captured and quantifiable in this model (Keov et al., 2011; Christopoulos et al., 2014). Allosteric modulator discovery for mGlu5 receptors has been particularly fruitful, yielding diverse chemotypes covering the full spectrum of allosteric pharmacology, including PAMs, PAM‐agonists, NALs, weak (or partial) NAMs and full NAMs (Table 2). Notably, mGlu5 receptor PAMs have efficacy in models of psychosis and cognition, while mGlu5 receptor NAMs have demonstrated efficacy in preclinical models of anxiety, depression, autism, addiction and movement disorders. Despite this success, there are now multiple reports of adverse effect liability associated with both mGlu5 receptor NAMs and PAMs (Table 2). Moreover, mGlu5 receptor allosteric modulator structure–activity relationships are notoriously difficult to interpret (Wood et al., 2011). This is likely attributable to three major contributing factors. First, a lack of quantitative analyses delineating how chemical modifications alter ligand affinity versus cooperativity. Moreover, the nature, direction and magnitude of cooperativity are dependent upon the two ligands under investigation. This phenomenon is referred to as ‘probe‐dependence’, with the most physiologically relevant effect being the pharmacology observed with the endogenous agonist glutamate. However, in many experimental paradigms, in particular native systems, it is not feasible to use glutamate due to the presence of other glutamate receptors and transporters such that surrogate selective orthosteric agonists are used. Second, an under‐appreciation of the full scope of drug action, with the majority of allosteric ligand‐based discovery programmes relying on glutamate‐mediated iCa2+ mobilization to classify ligand pharmacology. As detailed earlier, mGlu5 receptor‐calcium mobilization represents only the tip of the iceberg with respect to the myriad of possible cellular responses that may be triggered by mGlu5 receptor activation. Furthermore, the production and release of glutamate by cells, receptor expression levels and constitutive activity have the potential to influence the degree of agonist efficacy of allosteric ligands (Noetzel et al., 2012b). Third, a paucity in our understanding of the specific ligand–receptor interactions that govern allosteric modulation.
Table 2.
Molecular pharmacology and therapeutic potential of selected mGlu5 receptor allosteric ligands
| Ligand | Pharmacology | Therapeutic potential | References |
|---|---|---|---|
| PAMs and PAM‐agonists | |||
|
VU0360172 VU0403602 VU0424465 DPFE CDPPB |
PAM: glu‐Ca2+ mobilization (iCa2+); PAM‐agonist: glu‐pERK1/2. Affinity: 269–282 nM PAM‐agonist: glu‐iCa2+. Affinity: 5 nM PAM‐agonist: glu‐iCa2+ Affinity: 12 nM PAM‐agonist: glu‐iCa2+ and pERK1/2. Affinity: 8 μM PAM: quisqualate‐PI hydrolysis; PAM‐agonist: glu‐iCa2+, pERK1/2 and p‐Akt. Affinity: 224–1700 nM |
Psychosis, absence seizures, cognition enhancement, traumatic brain injury, post‐traumatic stress disorder, addiction, Huntington's disease, seizures and neurotoxicity | (Rodriguez et al., 2010b; Gregory et al., 2012; D'Amore et al., 2013; Loane et al., 2014; Bridges et al., 2013; Rook et al., 2013; Gregory et al., 2013b; Gregory et al., 2013c; Kinney et al., 2005; Uslaner et al., 2009; Cleva et al., 2011; Reichel et al., 2011; Fowler et al., 2013; Horio et al., 2013; Ganella et al., 2014; Gass et al., 2014b; Gass et al., 2014a; Parmentier‐Batteur et al., 2014; Sethna and Wang, 2014; Doria et al., 2015) |
| NAMs | |||
| MPEPFenobam | NAM: glu‐iCa2+, DHPG and quisqualate‐PI hydrolysis; Inverse agonist: PI hydrolysis; Affinity: 3–20 nMNAM: quisqualate‐iCa2+ and PI hydrolysis; Inverse agonist: PI hydrolysis. Affinity: 31–53 nM | Analgesia, anxiety, depression, addiction, neuroprotection, movement disorders e.g. levodopa‐induced dyskinesia, Fragile X Syndrome, memory impairments, psychostimulant | (Gasparini et al., 1999; Salt et al., 1999; Bruno et al., 2000; Spooren et al., 2000a; Spooren et al., 2000b; Schulz et al., 2001; Walker et al., 2001; Balschun and Wetzel, 2002; Campbell et al., 2004; Li et al., 2006; Belozertseva et al., 2007; Gannon and Millan, 2011; Sun et al., 2012; Yeganeh et al., 2013; Pecknold et al., 1982; Porter et al., 2005; Berry‐Kravis et al., 2009; Jacob et al., 2009; Rylander et al., 2010; Crock et al., 2012; Vinueza Veloz et al., 2012; Watterson et al., 2013; Wieronska and Pilc, 2013; Huang et al., 2014; Ko et al., 2014) |
| NALs | |||
| 5MPEP | NAL: glu‐iCa2+ and PI hydrolysis; DHPG‐Ca2+ mobilization. Affinity: 388‐875 nM | Pharmacological tools | (Rodriguez et al., 2005; Chen et al., 2007; Chen et al., 2008; Ayala et al., 2009; Gregory et al., 2013c; Gregory and Conn, 2015) |
| VU0478006 (ML353) | NAL: glu‐iCa2+ Affinity: 18 nM. | ||
Italicized text in the ‘Therapeutic potential’ column denotes adverse effect liability associated with these allosteric ligand classes.
Location of mGLu5 allosteric binding sites
Promisingly, the recent X‐ray crystal structures of both human mGlu1 and mGlu5 receptor 7TMs, complexed with NAMs (Dore et al., 2014; Wu et al., 2014), have paved the way for an improved understanding of the complexities of allosteric interactions within the 7TMs. In both crystal structures, the allosteric ligand‐binding pocket is extracellularly accessible and resides between TM2, TM3, TM5, TM6 and TM7 (Dore et al., 2014; Wu et al., 2014). This pocket is consistent with extensive previous mutagenesis studies of the ‘2‐methyl‐6‐(phenylethynyl)pyridine (MPEP) binding site’ or the common allosteric site that is shared across multiple mGlu5 receptor allosteric modulator chemotypes, including NAMs, PAMs and NALs (Gregory and Conn, 2015). Indeed, the crystal structures are also consistent with structure‐function studies of allosteric pockets across multiple family C GPCRs (Bennett et al., 2015; Gregory and Conn, 2015), suggesting a common location for binding cavities in family C GPCRs that can be selectively targeted with small molecule allosteric ligands. Commensurate with this concept is the fact that some mGlu5 receptor allosteric ligands have activity at other mGlu subtypes (Mathiesen et al., 2003; O'Brien et al., 2004; Sheffler et al., 2010). Comparison of the allosteric binding pockets in the two structures reveals that the mGlu1 receptor pocket is located higher within the 7TMs (Bennett et al., 2015; Gregory and Conn, 2015). This suggests that although there is a common allosteric site for family C GPCRs, the overall geography of the pocket can be dramatically different for individual receptors enabling selective ligand discovery.
In addition to this well‐characterized allosteric site, at least one other allosteric site has proposed for mGlu5 receptors. The mGlu5 receptor PAMs of glutamate‐stimulated iCa2+ mobilization, N‐(4‐chloro‐2‐[(1,3‐dioxo‐1,3‐dihydro‐2H‐isoindol‐2‐yl)methyl]phenyl)‐2‐hydroxybenzamide (CPPHA), and its derivative, N‐(4‐chloro‐2‐[(1,3‐dioxoisoindolin‐2‐yl)methyl)phenyl)picolinamide] (NCFP), show non‐competitive behaviour with the MPEP‐site radioligand, [3H]methoxyPEPy (O'Brien et al., 2004; Noetzel et al., 2013). Mutagenesis studies identified Phe585 in TM1 as a possible contributor to this secondary allosteric site, and perhaps more importantly, showed no effects of mutations of this residue on the binding of MPEP allosteric site ligands, and vice versa (Chen et al., 2008). Additional chemotypes have been suggested to bind to secondary allosteric sites on mGlu5 receptors (Hammond et al., 2010; Rodriguez et al., 2010a); however, it remains to be established whether or not these chemotypes recognize the same binding pocket as CPPHA and NCFP or if there are additional allosteric sites. To date, no ‘second site’ mGlu5 receptor allosteric modulators have been developed that are active in vivo. Despite this, the discovery of ligands that interact with secondary allosteric binding pockets coupled with our appreciation of the complex physiology of mGlu5 receptor activation offers the potential for the development of allosteric ligands that have differential pharmacology and in vivo profiles.
New paradigms: biased allosteric modulation and agonism
In the past two decades, it has become apparent that the consequences of GPCR activation are not linearly linked to receptor occupancy and that different agonists binding to the same receptor can activate distinct cellular responses. Referred to as stimulus‐bias, this phenomenon arises from ligands stabilizing different subsets of receptor activation states (Kenakin et al., 2012; Kenakin and Christopoulos, 2013). Stimulus‐bias has been described for different orthosteric agonists of mGlu1 and group III mGlu receptors (Emery et al., 2012; Jalan‐Sakrikar et al., 2014). Of note, DHPG and quisqualate, two orthosteric agonists commonly used as surrogates for glutamate in native preparations, are biased compared with glutamate in recombinant cells and neurons expressing mGlu1 receptors when comparing phosphoinositide hydrolysis, ERK1/2 phosphorylation and cytoprotection (evidenced by differential relative potencies and/or efficacies) (Emery et al., 2012; Hathaway et al., 2015). It remains to be determined whether or not different orthosteric agonists will also be biased for different outcomes of mGlu5 receptor activation. However, comparison of relative efficacies of mGlu5 receptor PAM‐agonists with that of glutamate revealed that allosteric agonists were biased towards ERK1/2 phosphorylation over intracellular Ca2+ mobilization, the opposite profile to glutamate (Gregory et al., 2012) (Table 2). This is not necessarily surprising given that mGlu5 receptor‐induced ERK1/2 phosphorylation can occur independently of canonical Ca2+ signalling (Thandi et al., 2002). Biased agonists provide the opportunity to study how individual transducer pathways relate to the overall effect of a ligand in complex systems such as tissue slices or in behavioural models; however, it also add an additional layer of complexity to understanding allosteric interactions.
The simultaneous binding of an allosteric modulator to an agonist bound receptor stabilizes a different complement of receptor activation states than can be achieved by the endogenous ligand alone, giving rise to cooperativity. In light of evidence that mGlu5 receptor allosteric agonists exhibit stimulus‐bias, it is perhaps not surprising that there is also evidence that mGlu5 receptor allosteric modulators can differentially modulate the functional outcomes of endogenous agonist stimulation. An early study comparing the mGlu5 receptor PAMs for iCa2+ mobilization, CPPHA and ((3‐fluorophenyl)methylene)hydrazone‐3‐fluorobenzaldehyde (DFB) found that while DFB was a PAM for ERK1/2 phosphorylation, CPPHA was a NAM‐agonist (Zhang et al., 2005). These differences were attributed to the fact that these two allosteric ligands interact with distinct allosteric sites. Subsequently, comparison of the cooperativities of diverse mGlu5 receptor PAM scaffolds including CPPHA revealed that these PAMs have lower cooperativity with glutamate for ERK1/2 phosphorylation compared with iCa2+ mobilization (Gregory et al., 2012; Gregory et al., 2013c). These data suggest that ligands binding within the same pocket can have differential cooperativity with endogenous agonist depending upon the measure of receptor activity. Furthermore, point mutations in the common allosteric site can engender molecular switches in allosteric modulator cooperativity; however, differential effects were observed for different NAM and PAM scaffolds (Muhlemann et al., 2006; Gregory et al., 2013b; Turlington et al., 2013; Gregory et al., 2014). Therefore, it is evident that the structural determinants and receptor conformations that mediate cooperativity are subtly different for individual allosteric ligands and that these subtleties in ligand–receptor interactions may give rise to biased modulation. It should be noted, however, that iCa2+ mobilization and ERK1/2 phosphorylation are measured at different time points, <60 s for iCa2+ mobilization versus 5–10 min for ERK1/2 phosphorylation. Differences in assay timing, as well as compound kinetics, may contribute to the reported biased agonism and modulation. To date, there have been no detailed studies examining multiple proximal and distal signalling endpoints for mGlu5 receptors to rigorously assess the prevalence of allosteric ligand bias. Further research that incorporates the appropriate controls, analysis and quantification (Kenakin and Christopoulos, 2013) to probe mGlu5 receptor biased agonism and modulation is required to better understand the significance, prevalence and therapeutic potential of these phenomena.
Despite the preliminary evidence, recent studies suggest that biased pharmacology of mGlu5 receptor allosteric ligands may result in distinct effects at the synaptic level. NCFP was unable to potentiate induction of LTD and LTP at the hippocampal‐SCA1 synapse – a property distinct from other mGlu5 receptor PAMs (Noetzel et al., 2013). Despite the fact that NCFP potentiates mGlu5 receptor activation in astrocytes and midbrain slices, similar to other mGlu5 receptor PAMs (O'Brien et al., 2004; Rodriguez et al., 2010b; Noetzel et al., 2012b). More recently, a novel mGlu5 receptor PAM, VU0409551, was disclosed that has efficacy in preclinical models of anti‐psychotic activity and cognition enhancement, but does not potentiate mGlu5 receptor modulation of NMDA receptor currents in hippocampal neurons and, similar to NCFP, does not potentiate NMDA receptor mediated LTP at the hippocampal‐SCA1 synapse (Rook et al., 2015). These data suggest that modulating the interaction between mGlu5 receptors and NMDA receptors is not necessarily required to drive in vivo efficacy of mGlu5 receptor PAMs and that additional, yet to be determined, mGlu5 receptor‐linked mechanisms are involved. If we consider the myriad of possible cellular responses that can be triggered by mGlu5 receptor activation, it is perhaps not surprising that biased agonism and modulation is operative. Two recent reports described adverse effect liability (seizures and neurotoxicity) associated with on‐target activity of the mGlu5 receptor PAMs, VU0424465 and 5PAM523, with both intrinsic efficacy and the magnitude of cooperativity implicated (Rook et al., 2013; Parmentier‐Batteur et al., 2014). However, it remains to be fully elucidated why these compounds had adverse effects, while other related compounds do not. Therefore, biased allosteric modulators offer two advantages: as tool compounds to dissect the physiology of therapeutic versus adverse effects and as an avenue for improved therapeutic effects. Encouragingly, biased ligands for other GPCRs have reached clinical trials, with angiotensin AT1 (Felker et al., 2015) and μ opioid receptor biased ligands (Soergel et al., 2014) in early trials for the treatment of acute heart failure and moderate to severe pain respectively.
In the field of drug discovery, stimulus‐bias raises additional challenges and potential pitfalls. The use of biased orthosteric agonists as pharmacological tools in native preparations may confound interpretation of allosteric modulator pharmacology. For example, with quisqualate, a number of mGlu5 receptor PAMs displayed affinity modulation (Bradley et al., 2011b); however, with glutamate as the agonist, there was no evidence for affinity modulation with these same PAMs and others, and only modulation of glutamate efficacy was observed (Gregory et al., 2012). Thus, in assessing allosteric modulation, and ligand pharmacology, it is essential to consider probe dependence when interpreting results from recombinant and native systems and translating this to in vivo effects. Furthermore, as noted earlier, in addition to coupling to a wide variety of effectors, mGlu5 receptors are also subject to subcellular compartmentalization. This leads to the interesting notion of stimulus‐bias based not only on chemical pharmacophore but also location of the receptor within the cell and accessibility to different ligands. Thus, in characterizing allosteric modulator pharmacology, it is important to understand the complete pharmacological profile, as well as the impact of different orthosteric agonists to the individual signalling fingerprint.
Concluding remarks
The past decade has seen a large focus on developing mGlu5 receptor‐targeted therapies for the treatment of often refractory CNS disorders. In appreciating the breadth of physiological possibilities, it is clear that mGlu5 receptors indeed play a significant role in the symphony of neuronal responses, ranging from the sensation of pain to the formation of memory. The richness of possible cellular responses associated with mGlu5 receptor activation offers a large variety of options in reaching therapeutic endpoints. While several mGlu5 receptor allosteric modulators have moved into early phase clinical trials, several have also failed due to adverse effects and inability to meet expected clinical endpoints (Pecknold et al., 1982; Jacquemont et al., 2014; Scharf et al., 2015). Both the adverse effects and lack of efficacy can likely be attributed to an under‐appreciation of the full scope of ligand pharmacology, whether this be due to off‐target activity, a paucity in our understanding of on‐target stimulus‐bias or a lack of selectivity due to unappreciated stimulus‐bias at other subtypes not detected in traditional counter‐screening paradigms. Thus, a greater understanding of the full scope of mGlu5 receptor allosteric ligand activity, as well as receptor function, may aid in developing therapies with minimal adverse effects and desired clinical outcomes.
Conflict of interest
None.
Sengmany, K. , and Gregory, K. J. (2016) Metabotropic glutamate receptor subtype 5: molecular pharmacology, allosteric modulation and stimulus bias. British Journal of Pharmacology, 173: 3001–3017. doi: 10.1111/bph.13281.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S (1992). Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem 267: 13361–13368. [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al. (2013a). The concise guide to PHARMACOLOGY 2013/14: G protein‐coupled receptors. Br J Pharmacol 170: 1459–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA et al (2013b). The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol 170: 1607–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA et al (2013c). The Concise Guide to PHARMACOLOGY 2013/14: Ligand‐Gated Ion Channels. Br J Pharmacol 170: 1582–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M et al (2013d). The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol 170: 1797–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin JP et al (2002). Homer‐dependent cell surface expression of metabotropic glutamate receptor type 5 in neurons. Mol Cell Neurosci 20: 323–329. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN et al. (2009). mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology 34: 2057–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs R, Miller S, Romano C, de Vries A, Chun Y, Cotman CW (1997). Metabotropic glutamate receptor mGluR5 in astrocytes: pharmacological properties and agonist regulation. J Neurochem 69: 151–163. [DOI] [PubMed] [Google Scholar]
- Ballester‐Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J et al. (2010). mGluR5 in cortical excitatory neurons exerts both cell‐autonomous and ‐nonautonomous influences on cortical somatosensory circuit formation. J Neurosci 30: 16896–16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W (2002). Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav 73: 375–380. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY (2007). Antidepressant‐like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol 17: 172–179. [DOI] [PubMed] [Google Scholar]
- Bennett KA, Dore AS, Christopher JA, Weiss DR, Marshall FH (2015). Structures of mGluRs shed light on the challenges of drug development of allosteric modulators. Curr Opin Pharmacol 20: 1–7. [DOI] [PubMed] [Google Scholar]
- Benquet P, Gee C, Gerber U (2002). Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci 22: 9679–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry‐Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J et al. (2009). A pilot open label, single dose trial of fenobam in adults with Fragile X syndrome. J Med Genet 46: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Reid CA, Chen F, Tan HO, Petrou S, Lawrence AJ (2010). Cocaine‐mediated synaptic potentiation is absent in VTA neurons from mGlu5‐deficient mice. Int J Neuropsychopharmacol 13: 133–141. [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P (1983). Operational models of pharmacological agonism. Proc R Soc London B Biol Sci 220: 141–162. [DOI] [PubMed] [Google Scholar]
- Black YD, Xiao D, Pellegrino D, Kachroo A, Brownell AL, Schwarzschild MA (2010). Protective effect of metabotropic glutamate mGluR5 receptor elimination in a 6‐hydroxydopamine model of Parkinson's disease. Neurosci Lett 486: 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D (2002). Regulation of AMPA receptor lateral movements. Nature 417: 649–653. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL (2005). The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology 49: 13–25. [DOI] [PubMed] [Google Scholar]
- Brabet I, Mary S, Bockaert J, Pin JP (1995). Phenylglycine derivatives discriminate between mGluR1‐ and mGlu5‐mediated responses. Neuropharmacology 34: 895–903. [DOI] [PubMed] [Google Scholar]
- Brabet I, Parmentier ML, De Colle C, Bockaert J, Acher F, Pin JP (1998). Comparative effect of L‐CCG‐I, DCG‐IV and gamma‐carboxy‐L‐glutamate on all cloned metabotropic glutamate receptor subtypes. Neuropharmacology 37: 1043–1051. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Campbell U, Giracello D, Chapman D, King C, Tehrani L et al. (2005). Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice. J Pharmacol Exp Ther 313: 395–402. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Challiss RA (2011a). Defining protein kinase/phosphatase isoenzymic regulation of mGlu(5) receptor‐stimulated phospholipase C and Ca(2)(+) responses in astrocytes. Br J Pharmacol 164: 755–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Langmead CJ, Watson JM, Challiss RA (2011b). Quantitative analysis reveals multiple mechanisms of allosteric modulation of the mGlu5 receptor in rat astroglia. Mol Pharmacol 79: 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL et al. (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386: 284–288. [DOI] [PubMed] [Google Scholar]
- Brauner‐Osborne H, Wellendorph P, Jensen AA (2007). Structure, pharmacology and therapeutic prospects of family C G‐protein coupled receptors. Curr Drug Targets 8: 169–184. [DOI] [PubMed] [Google Scholar]
- Bridges TM, Rook JM, Noetzel MJ, Morrison RD, Zhou Y, Gogliotti RD et al. (2013). Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure‐like adverse events in rats involving a receptor agonism‐dependent mechanism. Drug Metab Dispos 41: 1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA (2004). Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry 9: 35–41. [DOI] [PubMed] [Google Scholar]
- Brown RM, Mustafa S, Ayoub MA, Dodd PR, Pfleger KD, Lawrence AJ (2012). mGlu5 receptor functional interactions and addiction. Front Pharmacol 3: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, Ksiazek I, Battaglia G, Lukic S, Leonhardt T, Sauer D et al. (2000). Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 39: 2223–2230. [DOI] [PubMed] [Google Scholar]
- Cabello N, Gandia J, Bertarelli DC, Watanabe M, Lluis C, Franco R et al. (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher‐order oligomers in living cells. J Neurochem 109: 1497–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ (2004). The mGluR5 antagonist 2‐methyl‐6‐(phenylethynyl)‐pyridine (MPEP) potentiates PCP‐induced cognitive deficits in rats. Psychopharmacology (Berl) 175: 310–318. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Adam G, Chaboz S, Henningsen R, Kemp JA, Klingelschmidt A et al. (1998). Characterization of [3H]‐(2S,2'R,3'R)‐2‐(2',3'‐dicarboxy‐cyclopropyl)glycine ([3H]‐DCG IV) binding to metabotropic mGlu2 receptor‐transfected cell membranes. Br J Pharmacol 123: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN (1999). AKT/PKB and other D3 phosphoinositide‐regulated kinases: kinase activation by phosphoinositide‐dependent phosphorylation. Annu Rev Biochem 68: 965–1014. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lu HC, Brumberg JC (2012). mGluR5 knockout mice display increased dendritic spine densities. Neurosci Lett 524: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Stoker A, Markou A (2010). The glutamatergic compounds sarcosine and N‐acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 209: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Goudet C, Pin JP, Conn PJ (2008). N‐{4‐Chloro‐2‐[(1,3‐dioxo‐1,3‐dihydro‐2H‐isoindol‐2‐yl)methyl]phenyl}‐2‐hydroxybe nzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73: 909–918. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP et al (2007). Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71: 1389–1398. [DOI] [PubMed] [Google Scholar]
- Chesworth R, Brown RM, Kim JH, Lawrence AJ (2013). The metabotropic glutamate 5 receptor modulates extinction and reinstatement of methamphetamine‐seeking in mice. PLoS One 8: e68371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Chung S, Roche KW (2011). Differential binding of calmodulin to group I metabotropic glutamate receptors regulates receptor trafficking and signaling. J Neurosci 31: 5921–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T (2002). G protein‐coupled receptor allosterism and complexing. Pharmacol Rev 54: 323–374. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Changeux JP, Catterall WA, Fabbro D, Burris TP, Cidlowski JA et al. (2014). International union of basic and clinical pharmacology. XC. multisite pharmacology: recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacol Rev 66: 918–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ et al. (2011). mGluR5 positive allosteric modulation enhances extinction learning following cocaine self‐administration. Behav Neurosci 125: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett VJ, Collingridge GL (2004). Interactions between NMDA receptors and mGlu5 receptors expressed in HEK293 cells. Br J Pharmacol 142: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CK (2008). Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino G, Pellicciari R (1996). Homology modeling of metabotropic glutamate receptors. (mGluRs) structural motifs affecting binding modes and pharmacological profile of mGluR1 agonists and competitive antagonists. J Med Chem 39: 3998–4006. [DOI] [PubMed] [Google Scholar]
- Crawford JH, Wainwright A, Heavens R, Pollock J, Martin DJ, Scott RH et al. (2000). Mobilisation of intracellular Ca2+ by mGluR5 metabotropic glutamate receptor activation in neonatal rat cultured dorsal root ganglia neurones. Neuropharmacology 39: 621–630. [DOI] [PubMed] [Google Scholar]
- Crock LW, Stemler KM, Song DG, Abbosh P, Vogt SK, Qiu CS et al. (2012). Metabotropic glutamate receptor 5 (mGluR5) regulates bladder nociception. Mol Pain 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore V, Santolini I, van Rijn CM, Biagioni F, Molinaro G, Prete A et al. (2013). Potentiation of mGlu5 receptors with the novel enhancer, VU0360172, reduces spontaneous absence seizures in WAG/Rij rats. Neuropharmacology 66: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE (1997). (RS)‐2‐chloro‐5‐hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1, receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology 36: 265–267. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Collingridge GL, Jane DE (1999). Antagonist activity of alpha‐substituted 4‐carboxyphenylglycine analogues at group I metabotropic glutamate receptors expressed in CHO cells. Br J Pharmacol 126: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenici MR, Pepponi R, Martire A, Tebano MT, Potenza RL, Popoli P (2004). Permissive role of adenosine A2A receptors on metabotropic glutamate receptor 5 (mGluR5)‐mediated effects in the striatum. J Neurochem 90: 1276–1279. [DOI] [PubMed] [Google Scholar]
- Dore AS, Okrasa K, Patel JC, Serrano‐Vega M, Bennett K, Cooke RM et al. (2014). Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature 511: 557–562. [DOI] [PubMed] [Google Scholar]
- Doria JG, de Souza JM, Andrade JN, Rodrigues HA, Guimaraes IM, Carvalho TG et al. (2015). The mGluR5 positive allosteric modulator, CDPPB, ameliorates pathology and phenotypic signs of a mouse model of Huntington's disease. Neurobiol Dis 73: 163–173. [DOI] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 25: 66–77. [DOI] [PubMed] [Google Scholar]
- Eiler WJ 2nd, Baez M, Yu J, Witkin JM (2011). mGlu5 receptor deletion reduces relapse to food‐seeking and prevents the anti‐relapse effects of mGlu5 receptor blockade in mice. Life Sci 89: 862–867. [DOI] [PubMed] [Google Scholar]
- Emery AC, DiRaddo JO, Miller E, Hathaway HA, Pshenichkin S, Takoudjou GR et al. (2012). Ligand bias at metabotropic glutamate 1a receptors: molecular determinants that distinguish beta‐arrestin‐mediated from G protein‐mediated signaling. Mol Pharmacol 82: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker GM, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA et al. (2015). Heart failure therapeutics on the basis of a biased ligand of the angiotensin‐2 type 1 receptor: rationale and design of the BLAST‐AHF Study (biased ligand of the angiotensin receptor study in acute heart failure). JACC Heart Fail 3: 193–201. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schmid S (2002). Metabotropic glutamate receptors are involved in amygdaloid plasticity. Eur J Neurosci 15: 1535–1541. [DOI] [PubMed] [Google Scholar]
- Ferre S, Popoli P, Rimondini R, Reggio R, Kehr J, Fuxe K (1999). Adenosine A2A and group I metabotropic glutamate receptors synergistically modulate the binding characteristics of dopamine D2 receptors in the rat striatum. Neuropharmacology 38: 129–140. [DOI] [PubMed] [Google Scholar]
- Ferre S, Karcz‐Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA et al. (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A 99: 11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SW, Walker JM, Klakotskaia D, Will MJ, Serfozo P, Simonyi A et al. (2013). Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiol Learn Mem 99: 25–31. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM (1998). Role of the second and third intracellular loops of metabotropic glutamate receptors in mediating dual signal transduction activation. J Biol Chem 273: 5615–5624. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS (2009). Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci 29: 3590–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galik J, Youn DH, Kolaj M, Randic M (2008). Involvement of group I metabotropic glutamate receptors and glutamate transporters in the slow excitatory synaptic transmission in the spinal cord dorsal horn. Neuroscience 154: 1372–1387. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM (2004). Extracellular signal‐regulated protein kinase activation is required for metabotropic glutamate receptor‐dependent long‐term depression in hippocampal area CA1. J Neurosci 24: 4859–4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama L, Wilt SG, Breitwieser GE (2001). Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem 276: 39053–39059. [DOI] [PubMed] [Google Scholar]
- Ganella DE, Thangaraju P, Lawrence AJ, Kim JH (2014). Fear extinction in 17 day old rats is dependent on metabotropic glutamate receptor 5 signaling. Behav Brain Res. doi: 10.1016/j.bbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Millan MJ (2011). Positive and negative modulation of circadian activity rhythms by mGluR5 and mGluR2/3 metabotropic glutamate receptors. Neuropharmacology 60: 209–215. [DOI] [PubMed] [Google Scholar]
- Gao C, Tronson NC, Radulovic J (2013). Modulation of behavior by scaffolding proteins of the post‐synaptic density. Neurobiol Learn Mem 105: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I et al. (1999). 2‐Methyl‐6‐(phenylethynyl)‐pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38: 1493–1503. [DOI] [PubMed] [Google Scholar]
- Gass JT, Trantham‐Davidson H, Kassab AS, Glen WB Jr, Olive MF, Chandler LJ (2014a). Enhancement of extinction learning attenuates ethanol‐seeking behavior and alters plasticity in the prefrontal cortex. J Neurosci 34: 7562–7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr, McGonigal JT, Trantham‐Davidson H, Lopez MF, Randall PK et al. (2014b). Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self‐administration in adulthood. Neuropsychopharmacology 39: 2570–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrow K, Triller A (2010). Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol 20: 631–639. [DOI] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F et al. (2005). Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem 280: 24380–24385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Conn PJ (2015). Molecular insights into metabotropic glutamate receptor allosteric modulation. Mol Pharmacol 88: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Herman EJ, Ramsey AJ, Hammond AS, Byun NE, Stauffer SR et al. (2013c). N‐aryl piperazine metabotropic glutamate receptor 5 positive allosteric modulators possess efficacy in preclinical models of NMDA hypofunction and cognitive enhancement. J Pharmacol Exp Ther 347: 438–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Nguyen ED, Malosh C, Mendenhall JL, Zic JZ, Bates BS et al. (2014). Identification of specific ligand–receptor interactions that govern binding and cooperativity of diverse modulators to a common metabotropic glutamate receptor 5 allosteric site. ACS Chem Neurosci 5: 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Nguyen ED, Reiff SD, Squire EF, Stauffer SR, Lindsley CW et al. (2013b). Probing the metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulator (PAM) binding pocket: discovery of point mutations that engender a “molecular switch” in PAM pharmacology. Mol Pharmacol 83: 991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Niswender CM (2013a). Pharmacology of metabotropic glutamate receptor allosteric modulators: structural basis and therapeutic potential for CNS disorders. Prog Mol Biol Transl Sci 115: 61–121. [DOI] [PubMed] [Google Scholar]
- Gregory KJ, Noetzel MJ, Rook JM, Vinson PN, Stauffer SR, Rodriguez AL et al. (2012). Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure‐function studies and structure–activity relationships. Mol Pharmacol 82: 860–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA (2000). Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation. Mol Pharmacol 58: 1412–1423. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Esseltine JL, DeVries RA, Cregan SP, Ferguson SS (2014). Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer's disease. Mol Brain 7: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AS, Rodriguez AL, Townsend SD, Niswender CM, Gregory KJ, Lindsley CW et al. (2010). Discovery of a novel chemical class of mGlu(5) allosteric ligands with distinct modes of pharmacology. ACS Chem Neurosci 1: 702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway HA, Pshenichkin S, Grajkowska E, Gelb T, Emery AC, Wolfe BB et al. (2015). Pharmacological characterization of mGlu1 receptors in cerebellar granule cells reveals biased agonism. Neuropharmacology 93: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Sekiyama N, Nakanishi S, Jane DE, Sunter DC, Birse EF et al. (1994). Analysis of agonist and antagonist activities of phenylglycine derivatives for different cloned metabotropic glutamate receptor subtypes. J Neurosci 14: 3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Challiss RA (2001). Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G‐protein‐coupled receptors. Biochem J 359: 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B (2004). Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology 29: 1259–1269. [DOI] [PubMed] [Google Scholar]
- Horio M, Fujita Y, Hashimoto K (2013). Therapeutic effects of metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on phencyclidine‐induced cognitive deficits in mice. Fundam Clin Pharmacol 27: 483–488. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E (2004). Activation of the phosphoinositide 3‐kinase‐Akt‐mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor‐dependent long‐term depression. J Neurosci 24: 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O'Hara PJ, Mulvihill ER et al. (1991). Cloning, expression, and gene structure of a G protein‐coupled glutamate receptor from rat brain. Science 252: 1318–1321. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW (2007). Metabotropic glutamate receptor 5 modulates nociceptive plasticity via extracellular signal‐regulated kinase‐Kv4.2 signaling in spinal cord dorsal horn neurons. J Neurosci 27: 13181–13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C‐C, Liang Y‐C, Lee C‐C, Hsu K‐S (2014). Cocaine Withdrawal Impairs mGluR5‐Dependent Long‐Term Depression in Nucleus Accumbens Shell Neurons of Both Direct and Indirect Pathways. Mol Neurobiol 1–11. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF (2001). Chemical induction of mGluR5‐ and protein synthesis – dependent long‐term depression in hippocampal area CA1. J Neurophysiol 86: 321–325. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y (2001). Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. J Neurosci 21: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Vogt MA, Luoni A, Filipovic D, Lima‐Ojeda JM, Pfeiffer N et al. (2013). Significant increase in anxiety during aging in mGlu5 receptor knockout mice. Behav Brain Res 241: 27–31. [DOI] [PubMed] [Google Scholar]
- Jacob W, Gravius A, Pietraszek M, Nagel J, Belozertseva I, Shekunova E et al. (2009). The anxiolytic and analgesic properties of fenobam, a potent mGlu5 receptor antagonist, in relation to the impairment of learning. Neuropharmacology 57: 97–108. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Berry‐Kravis E, Hagerman R, von Raison F, Gasparini F, Apostol G et al. (2014). The challenges of clinical trials in Fragile X syndrome. Psychopharmacology (Berl) 231: 1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke G, Kolczewski S, Spooren W, Vieira E, Bitter‐Stoll N, Boissin P et al. (2015). Metabotropic glutamate receptor 5 negative allosteric modulators: discovery of 2‐chloro‐4‐[1‐(4‐fluorophenyl)‐2,5‐dimethyl‐1H‐imidazol‐4‐ylethynyl]pyridine (basimglurant, RO4917523), a promising novel medicine for psychiatric diseases. J Med Chem 58: 1358–1371. [DOI] [PubMed] [Google Scholar]
- Jalan‐Sakrikar N, Field JR, Klar R, Mattmann ME, Gregory KJ, Zamorano R et al. (2014). Identification of positive allosteric modulators VU0155094 (ML397) and VU0422288 (ML396) reveals new insights into the biology of metabotropic glutamate receptor 7. ACS Chem Neurosci 5: 1221–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow‐Newerly W et al. (1998). Selective abolition of the NMDA component of long‐term potentiation in mice lacking mGluR5. Learn Mem 5: 331–343. [PMC free article] [PubMed] [Google Scholar]
- Joly C, Gomeza J, Brabet I, Curry K, Bockaert J, Pin JP (1995). Molecular, functional, and pharmacological characterization of the metabotropic glutamate receptor type 5 splice variants: comparison with mGluR1. J Neurosci 15: 3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, O'Malley KL (2009). Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem 284: 35827–35838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Sergin I, Purgert CA, O'Malley KL (2014). Location‐dependent signaling of the group 1 metabotropic glutamate receptor mGlu5 . Mol Pharmacol 86: 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, Kingston AE, Romano C, O'Malley KL (2005). Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem 280: 30469–30480. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR (2001). A role for seven in absentia homolog (Siah1a) in metabotropic glutamate receptor signaling. BMC Neurosci 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF (2007). Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci U S A 104: 6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR (2000). Homer proteins regulate coupling of group I metabotropic glutamate receptors to N‐type calcium and M‐type potassium channels. J Neurosci 20: 7238–7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Kassai H, Watabe AM, Aiba A, Manabe T (2012). Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L‐type Ca2+ channel in mouse CA1 pyramidal cells. J Physiol 590: 3019–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S, Tsutsumi R, Kohara A, Yamaguchi T, Nakanishi S, Okada M (1996). Control of calcium oscillations by phosphorylation of metabotropic glutamate receptors. Nature 383: 89–92. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A (2013). Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov 12: 205–216. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Watson C, Muniz‐Medina V, Christopoulos A, Novick S (2012). A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci 3: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A (2011). Allosteric modulation of G protein‐coupled receptors: a pharmacological perspective. Neuropharmacology 60: 24–35. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC et al. (2009). Membrane‐delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci 29: 10000–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston AE, Burnett JP, Mayne NG, Lodge D (1995). Pharmacological analysis of 4‐carboxyphenylglycine derivatives: comparison of effects on mGluR1 alpha and mGluR5a subtypes. Neuropharmacology 34: 887–894. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ et al. (2003). Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther 306: 116–123. [DOI] [PubMed] [Google Scholar]
- Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK et al. (2005). A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic‐like effects in rat behavioral models. J Pharmacol Exp Ther 313: 199–206. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP (2004). Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol 11: 706–713. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Isozaki K, Kim I, Lee JH, Cho HJ, Sohn SY et al. (2012). PKC phosphorylation regulates mGluR5 trafficking by enhancing binding of Siah‐1A. J Neurosci 32: 16391–16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko WK, Pioli E, Li Q, McGuire S, Dufour A, Sherer TB et al. (2014). Combined fenobam and amantadine treatment promotes robust antidyskinetic effects in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP)‐lesioned primate model of Parkinson's disease. Mov Disord 29: 772–779. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ et al. (2010). Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain‐like behavior. J Neurosci 30: 8203–8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al‐Mahrouki A, Roder JC, Orser BA, MacDonald JF (2003). Co‐stimulation of mGluR5 and N‐methyl‐D‐aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem 278: 27742–27749. [DOI] [PubMed] [Google Scholar]
- Kumar V, Jong YJ, O'Malley KL (2008). Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5‐trisphosphate‐mediated nuclear Ca2+ release. J Biol Chem 283: 14072–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Fahey PG, Jong YJ, Ramanan N, O'Malley KL (2012). Activation of intracellular metabotropic glutamate receptor 5 in striatal neurons leads to up‐regulation of genes associated with sustained synaptic transmission including Arc/Arg3.1 protein. J Biol Chem 287: 5412–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T et al. (2000). Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407: 971–977. [DOI] [PubMed] [Google Scholar]
- Lagerstrom MC, Schioth HB (2008). Structural diversity of G protein‐coupled receptors and significance for drug discovery. Nat Rev Drug Discov 7: 339–357. [DOI] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A (2007). Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28: 382–389. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee J, Choi KY, Hepp R, Lee JY, Lim MK et al. (2008). Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc Natl Acad Sci U S A 105: 12575–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenga J, Hayashi S, de Vrij FM, Koekkoek SK, van der Linde HC, Nieuwenhuizen I et al. (2011). AFQ056, a new mGluR5 antagonist for treatment of fragile X syndrome. Neurobiol Dis 42: 311–317. [DOI] [PubMed] [Google Scholar]
- Li X, Need AB, Baez M, Witkin JM (2006). Metabotropic glutamate 5 receptor antagonism is associated with antidepressant‐like effects in mice. J Pharmacol Exp Ther 319: 254–259. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Porter RH, Scharf SH, Kuennecke B, Bruns A, von Kienlin M et al. (2015). Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J Pharmacol Exp Ther 353: 213–233. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Stauffer SR (2013). Metabotropic glutamate receptor 5‐positive allosteric modulators for the treatment of schizophrenia (2004–2012). Pharm pat anal 2: 93–108. [DOI] [PubMed] [Google Scholar]
- Llansola M, Felipo V (2010). Metabotropic glutamate receptor 5, but not 1, modulates NMDA receptor‐mediated activation of neuronal nitric oxide synthase. Neurochem Int 56: 535–545. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Tchantchou F, Kumar A, Barrett JP, Akintola T et al. (2014). Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration, and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics 11: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD et al. (1999). G‐protein‐coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 2: 331–338. [DOI] [PubMed] [Google Scholar]
- Lv MM, Cheng YC, Xiao ZB, Sun MY, Ren PC, Sun XD (2014). Down‐regulation of Homer1b/c attenuates group I metabotropic glutamate receptors dependent Ca(2)(+) signaling through regulating endoplasmic reticulum Ca(2)(+) release in PC12 cells. Biochem Biophys Res Commun 450: 1568–1574. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ (2005). The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal‐regulated protein kinase cascades in neurons. J Neurosci 25: 2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu XP, Fibuch EE, Wang LS et al. (2008). Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo . Neuropharmacology 55: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S (1991). Sequence and expression of a metabotropic glutamate receptor. Nature 349: 760–765. [DOI] [PubMed] [Google Scholar]
- Masuoka T, Nakamura T, Kudo M, Yoshida J, Takaoka Y, Kato N et al. (2015). Biphasic modulation of TRPV1‐mediated intracellular calcium elevation by mGluR5 in sensory neurons contributes to heat sensitivity. Br J Pharmacol 172: 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen JM, Svendsen N, Brauner‐Osborne H, Thomsen C, Ramirez MT (2003). Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB‐1893 and MPEP. Br J Pharmacol 138: 1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin N, Newell KA (2013). Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia. Neurosci Biobehav Rev 37: 256–268. [DOI] [PubMed] [Google Scholar]
- McCool BA, Pin JP, Harpold MM, Brust PF, Stauderman KA, Lovinger DM (1998). Rat group I metabotropic glutamate receptors inhibit neuronal Ca2+ channels via multiple signal transduction pathways in HEK 293 cells. J Neurophysiol 79: 379–391. [DOI] [PubMed] [Google Scholar]
- Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, Christopoulos A et al. (2012). Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem 55: 1445–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K, Iijima K, Fujii H, Ito H, Cho Y, Nakanishi S (2004). Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proc Natl Acad Sci U S A 101: 8614–8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F et al. (2012). Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J Cell Biol 198: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlemann A, Ward NA, Kratochwil N, Diener C, Fischer C, Stucki A et al. (2006). Determination of key amino acids implicated in the actions of allosteric modulation by 3,3'‐difluorobenzaldazine on rat mGlu5 receptors. Eur J Pharmacol 529: 95–104. [DOI] [PubMed] [Google Scholar]
- Mutel V, Ellis GJ, Adam G, Chaboz S, Nilly A, Messer J et al. (2000). Characterization of [3H]Quisqualate binding to recombinant rat metabotropic glutamate 1a and 5a receptors and to rat and human brain sections. J Neurochem 75: 2590–2601. [DOI] [PubMed] [Google Scholar]
- Muto T, Tsuchiya D, Morikawa K, Jingami H (2007). Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc Natl Acad Sci U S A 104: 3759–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST (2007). Fragile X mental retardation protein deficiency leads to excessive mGluR5‐dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A 104: 15537–15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RA (2002). Determinants of metabotropic glutamate receptor‐5‐mediated Ca2+ and inositol 1,4,5‐trisphosphate oscillation frequency. Receptor density versus agonist concentration. J Biol Chem 277: 35947–35960. [DOI] [PubMed] [Google Scholar]
- Nicodemo AA, Pampillo M, Ferreira LT, Dale LB, Cregan T, Ribeiro FM et al. (2010). Pyk2 uncouples metabotropic glutamate receptor G protein signaling but facilitates ERK1/2 activation. Mol Brain 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Liu F, Matsuyama S, Hamada M, Higashi H, Nairn AC et al. (2003). Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci U S A 100: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, Lindsley CW et al. (2013). A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. [Erratum appears in Mol Pharmacol. 2013 Oct; 84(4): 654]. Mol Pharmacol 83: 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Jones CK, Conn PJ (2012a). Emerging approaches for treatment of schizophrenia: modulation of glutamatergic signaling. Discov Med 14: 335–343. [PMC free article] [PubMed] [Google Scholar]
- Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, Zhou Y et al. (2012b). Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol 81: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JA, Lemaire W, Wittmann M, Jacobson MA, Ha SN, Wisnoski DD et al. (2004). A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain. J Pharmacol Exp Ther 309: 568–577. [DOI] [PubMed] [Google Scholar]
- O'Hara PJ, Sheppard PO, Thogersen H, Venezia D, Haldeman BA, McGrane V et al. (1993). The ligand‐binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11: 41–52. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Childs DS, Stanwood GD, Winder DG (2010). Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5). PLoS One 5: e15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando LR, Ayala R, Kett LR, Curley AA, Duffner J, Bragg DC et al. (2009). Phosphorylation of the homer‐binding domain of group I metabotropic glutamate receptors by cyclin‐dependent kinase 5. J Neurochem 110: 557–569. [DOI] [PubMed] [Google Scholar]
- Palucha‐Poniewiera A, Wieronska JM, Branski P, Burnat G, Chruscicka B, Pilc A (2013). Is the mGlu5 receptor a possible target for new antidepressant drugs? Pharmacol Rep 65: 1506–1511. [DOI] [PubMed] [Google Scholar]
- Parmentier‐Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O'Brien JA et al. (2014). Mechanism based neurotoxicity of mGlu5 positive allosteric modulators – development challenges for a promising novel antipsychotic target. Neuropharmacology 82: 161–173. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP et al. (2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy RD, Conn PJ (1998). Phosphorylation of mitogen‐activated protein kinase in cultured rat cortical glia by stimulation of metabotropic glutamate receptors. J Neurochem 71: 603–612. [DOI] [PubMed] [Google Scholar]
- Peavy RD, Chang MS, Sanders‐Bush E, Conn PJ (2001). Metabotropic glutamate receptor 5‐induced phosphorylation of extracellular signal‐regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J Neurosci 21: 9619–9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T (1982). Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double‐blind standard (diazepam) placebo‐controlled study. J Clin Psychopharmacol 2: 129–133. [PubMed] [Google Scholar]
- Pilc A, Wieronska JM, Skolnick P (2013). Glutamate‐based antidepressants: preclinical psychopharmacology. Biol Psychiatry 73: 1125–1132. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L et al. (2001). The selective mGlu(5) receptor agonist CHPG inhibits quinpirole‐induced turning in 6‐hydroxydopamine‐lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2a) receptors. Neuropsychopharmacology 25: 505–513. [DOI] [PubMed] [Google Scholar]
- Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S et al. (2005). Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J Pharmacol Exp Ther 315: 711–721. [DOI] [PubMed] [Google Scholar]
- Porter RH, Roberts PJ, Jane DE, Watkins JC (1992). (S)‐homoquisqualate: a potent agonist at the glutamate metabotropic receptor. Br J Pharmacol 106: 509–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgert CA, Izumi Y, Jong YJ, Kumar V, Zorumski CF, O'Malley KL (2014). Intracellular mGluR5 can mediate synaptic plasticity in the hippocampus. J Neurosci 34: 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011). Loss of object recognition memory produced by extended access to methamphetamine self‐administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36: 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, Devries RA, Hamilton A, Guimaraes IM, Cregan SP, Pires RG et al. (2014). Metabotropic glutamate receptor 5 knockout promotes motor and biochemical alterations in a mouse model of Huntington's disease. Hum Mol Genet 23: 2030–2042. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Ferreira LT, Paquet M, Cregan T, Ding Q, Gros R et al. (2009). Phosphorylation‐independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein‐coupled receptor kinase 2 in neurons. J Biol Chem 284: 23444–23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Cregan SP, Ferguson SS (2010). Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets 9: 574–595. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA (2005). Co‐localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92: 433–441. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Tarr JC, Zhou Y, Williams R, Gregory KJ, Bridges TM et al. (2010a). Identification of a glycine sulfonamide based non‐MPEP site positive allosteric potentiator (PAM) of mGlu5. in Probe Reports from the NIH Molecular Libraries Program, Bethesda (MD): National Center for Biotechnology Information (US). [PubMed]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL et al. (2010b). Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol 78: 1105–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ (2005). A close structural analog of 2‐methyl‐6‐(phenylethynyl)‐pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol Pharmacol 68: 1793–1802. [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL (1996). Metabotropic glutamate receptor 5 is a disulfide‐linked dimer. J Biol Chem 271: 28612–28616. [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y et al. (2001). Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol Pharmacol 59: 46–53. [PubMed] [Google Scholar]
- Rondard P, Liu J, Huang S, Malhaire F, Vol C, Pinault A et al. (2006). Coupling of agonist binding to effector domain activation in metabotropic glutamate‐like receptors. J Biol Chem 281: 24653–24661. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Collins KA, Hays SA, Tsai NP, Guo W, Birnbaum SG et al. (2012). Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci 15 (431–440): s431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridge TM et al. (2015). Biased mGlu5‐positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP et al. (2013). Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol Psychiatry 73: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira X, Roche D, Serra J, Kniazeff J, Pin JP, Giraldo J (2008). Modeling the binding and function of metabotropic glutamate receptors. J Pharmacol Exp Ther 325: 443–456. [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R (2002). Group I metabotropic glutamate receptor (mGluR)‐dependent long‐term depression mediated via p38 mitogen‐activated protein kinase is inhibited by previous high‐frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro . J Neurosci 22: 6121–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H et al. (2010). A mGluR5 antagonist under clinical development improves L‐DOPA‐induced dyskinesia in Parkinsonian rats and monkeys. Neurobiol Dis 39: 352–361. [DOI] [PubMed] [Google Scholar]
- Salt TE, Binns KE, Turner JP, Gasparini F, Kuhn R (1999). Antagonism of the mGlu5 agonist 2‐chloro‐5‐hydroxyphenylglycine by the novel selective mGlu5 antagonist 6‐methyl‐2‐(phenylethynyl)‐pyridine (MPEP) in the thalamus. Br J Pharmacol 127: 1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SH, Jaeschke G, Wettstein JG, Lindemann L (2015). Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr Opin Pharmacol 20:124–134. [DOI] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Gasparini F, Lingenhohl K, Kuhn R, Koch M (2001). The metabotropic glutamate receptor antagonist 2‐methyl‐6‐(phenylethynyl)‐pyridine (MPEP) blocks fear conditioning in rats. Neuropharmacology 41: 1–7. [DOI] [PubMed] [Google Scholar]
- Schroder H, Wu DF, Seifert A, Rankovic M, Schulz S, Hollt V et al. (2009). Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro‐opioid receptor. Neuropharmacology 56: 768–778. [DOI] [PubMed] [Google Scholar]
- Sethna F, Wang H (2014). Pharmacological enhancement of mGluR5 facilitates contextual fear memory extinction. Learn Mem 21: 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevastyanova TN, Kammermeier PJ (2014). Cooperative signaling between homodimers of metabotropic glutamate receptors 1 and 5. Mol Pharmacol 86: 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She WC, Quairiaux C, Albright MJ, Wang YC, Sanchez DE, Chang PS et al. (2009). Roles of mGluR5 in synaptic function and plasticity of the mouse thalamocortical pathway. Eur J Neurosci 29: 1379–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Wenthur CJ, Brunner JA, Daniels JS, Morrison RD, Blobaum AL et al. (2010). Development of the first selective mGlu3 NAM from an mGlu5 PAM hit. in Probe Reports from the NIH Molecular Libraries Program, Bethesda (MD): National Center for Biotechnology Information (US). [PubMed]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N (1993). Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163: 53–57. [DOI] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Sadler B, Connell J, Marion AS, Cowan CL et al. (2014). First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol 54: 351–357. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Conn PJ (2003). G protein‐coupled receptor kinases regulate metabotropic glutamate receptor 5 function and expression. Neuropharmacology 44: 699–706. [DOI] [PubMed] [Google Scholar]
- Spooren W, Gasparini F, Bergmann R, Kuhn R (2000a). Effect of the prototypical mGlu5 receptor antagonist 2‐methyl‐6‐(phenylethyl)‐pyridine (MPEP) on motor behaviour: rotarod, locomotor activity and rotational responses in the unilateral 6‐OHDA‐lesioned rat. Eur J Pharmacol 406: 403–410. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S et al. (2000b). Anxiolytic‐like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2‐methyl‐6‐(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther 295: 1267–1275. [PubMed] [Google Scholar]
- Stoker AK, Olivier B, Markou A (2012). Involvement of metabotropic glutamate receptor 5 in brain reward deficits associated with cocaine and nicotine withdrawal and somatic signs of nicotine withdrawal. Psychopharmacology (Berl) 221: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XR, Chen L, Chen WF, Yung WH (2012). Electrophysiological and behavioral effects of group I metabotropic glutamate receptors on pallidal neurons in rats. Brain Res 1477: 1–9. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Pepponi R, Domenici MR, Popoli P (2006). Is the functional interaction between adenosine A(2A) receptors and metabotropic glutamate 5 receptors a general mechanism in the brain? Differences and similarities between the striatum and the hippocampus. Purinergic Signal 2: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC et al. (2005). Adenosine A2A receptors and metabotropic glutamate 5 receptors are co‐localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N‐methyl‐d‐aspartate effects. J Neurochem 95: 1188–1200. [DOI] [PubMed] [Google Scholar]
- Thandi S, Blank JL, Challiss RA (2002). Group‐I metabotropic glutamate receptors, mGlu1a and mGlu5, couple to extracellular signal‐regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. J Neurochem 83: 1139–1153. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Boel E, Suzdak PD (1994). Actions of phenylglycine analogs at subtypes of the metabotropic glutamate receptor family. Eur J Pharmacol 267: 77–84. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Chamberland S, Pelletier JG, Ran I, Lacaille JC (2009). Activity‐dependent compartmentalized regulation of dendritic Ca2+ signaling in hippocampal interneurons. J Neurosci 29: 4658–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P et al. (1999). Coupling of mGluR/Homer and PSD‐95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583–592. [DOI] [PubMed] [Google Scholar]
- Tückmantel W, Kozikowski AP, Shaomeng W, Pshenichkin S, Wroblewski JT (1997). Synthesis, molecular modeling, and biology of the 1‐benzyl derivative of APDC – an apparent mGluR6 selective ligand. Bioorg Med Chem Lett 7: 601–606. [Google Scholar]
- Turlington M, Noetzel MJ, Chun A, Zhou Y, Gogliotti RD, Nguyen ED et al. (2013). Exploration of allosteric agonism structure–activity relationships within an acetylene series of metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulators (PAMs): discovery of 5‐((3‐fluorophenyl)ethynyl)‐N‐(3‐methyloxetan‐3‐yl)picolinamide (ML254). J Med Chem 56: 7976–7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier‐Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH et al (2009). Dose‐dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology 57:531–538. [DOI] [PubMed] [Google Scholar]
- Uematsu K, Heiman M, Zelenina M, Padovan J, Chait BT, Aperia A et al. (2015). Protein kinase A directly phosphorylates metabotropic glutamate receptor 5 to modulate its function. J Neurochem 132: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, Rizzonelli P, Paterlini M, Moretto G, Knopfel T, Kuhn R et al. (1997). mGluR5 metabotropic glutamate receptor distribution in rat and human spinal cord: a developmental study. Neurosci Res 28: 49–57. [DOI] [PubMed] [Google Scholar]
- Varney MA, Gereau RW 4th (2002). Metabotropic glutamate receptor involvement in models of acute and persistent pain: prospects for the development of novel analgesics. Curr Drug Targets CNS Neurol Disord 1: 283–296. [DOI] [PubMed] [Google Scholar]
- Vauquelin G, Van Liefde I (2005). G protein‐coupled receptors: a count of 1001 conformations. Fundam Clin Pharmacol 19: 45–56. [DOI] [PubMed] [Google Scholar]
- Veerman SR, Schulte PF, de Haan L (2014). The glutamate hypothesis: a pathogenic pathway from which pharmacological interventions have emerged. Pharmacopsychiatry 47: 121–130. [DOI] [PubMed] [Google Scholar]
- Vinueza Veloz MF, Buijsen RA, Willemsen R, Cupido A, Bosman LW, Koekkoek SK et al. (2012). The effect of an mGluR5 inhibitor on procedural memory and avoidance discrimination impairments in Fmr1 KO mice. Genes Brain Behav 11: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM (2006). Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long‐term depression. J Neurophysiol 95: 2427–2438. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A et al. (2001). mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology 40: 10–19. [DOI] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H et al. (2009). Norbin is an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science 326: 1554–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L (2007). Regulation of mitogen‐activated protein kinases by glutamate receptors. J Neurochem 100: 1–11. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF (2013). Attenuation of reinstatement of methamphetamine‐, sucrose‐, and food‐seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology (Berl) 225: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Huber KM (2009). Protein translation in synaptic plasticity: mGluR‐LTD, Fragile X. Curr Opin Neurobiol 19: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieronska JM, Pilc A (2013). Glutamate‐based anxiolytic ligands in clinical trials. Expert Opin Investig Drugs 22: 1007–1022. [DOI] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC (2008). mGluR5 regulates glutamate‐dependent development of the mouse somatosensory cortex. J Neurosci 28: 13028–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski K, Car H. (2002). (S)‐3,5‐DHPG: a review. CNS drug reviews 8:101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MR, Hopkins CR, Brogan JT, Conn PJ, Lindsley CW (2011). “Molecular switches” on mGluR allosteric ligands that modulate modes of pharmacology. Biochemistry 50: 2403–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y et al. (2014). Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Wright RA, Rockey PK, Burgett SG, Arnold JS, Rosteck PR Jr et al. (1998). Group III human metabotropic glutamate receptors 4, 7 and 8: molecular cloning, functional expression, and comparison of pharmacological properties in RGT cells. Brain Res Mol Brain Res 53: 88–97. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD et al. (1998). Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer‐related, synaptic proteins. Neuron 21: 707–716. [DOI] [PubMed] [Google Scholar]
- Xiao XL, Ma DL, Wu J, Tang FR (2013). Metabotropic glutamate receptor 5 (mGluR5) regulates proliferation and differentiation of neuronal progenitors in the developmental hippocampus. Brain Res 1493: 1–12. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF (2009). mGluR5 has a critical role in inhibitory learning. J Neurosci 29: 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh F, Nikbakht F, Bahmanpour S, Rastegar K, Namavar R (2013). Neuroprotective effects of NMDA and group I metabotropic glutamate receptor antagonists against neurodegeneration induced by homocysteine in rat hippocampus: in vivo study. J Mol Neurosci 50: 551–557. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ (2005). Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther 315: 1212–1219. [DOI] [PubMed] [Google Scholar]


