Abstract
Background:
A randomized, prospective clinical, radiographical, and histological study was conducted to evaluate healing after alveolar ridge preservation technique using two different graft materials, namely, a novel autogenous graft material i. e., autogenous tooth graft (ATG) and beta-tricalcium phosphate (β-TCP) alloplast.
Materials and Methods:
Fifteen patients undergoing extraction of at least three teeth were selected. Atraumatic extractions were performed. Of the three extraction sockets, one was grafted with ATG, other with β-TCP, and the third was left ungrafted. Cone-beam computed tomography scans were taken immediately after grafting and 4 months postoperatively to check the changes in alveolar crest height and width at all the sites. Three patients in whom implant placement was done after complete healing; bone samples were harvested using a 3 mm diameter trephine during osteotomy preparation from both the ridge preserved sites and studied histologically.
Results:
There was a statistically significant difference when the changes in width and height of alveolar crest were compared within all the three groups (P < 0.05). Among three sites, ATG-grafted sites showed the most superior results with a minimal reduction in alveolar crest height and width. Histological analysis also showed the same trend with more new bone formation at ATG-grafted sites as compared to β-TCP-grafted sites.
Conclusion:
Postextraction, ridge preservation leads to more predictable maintenance of alveolar ridge height and width. ATG as compared to β-TCP provided superior results. Based on this, we conclude that ATG material can serve as a better alternative to conventional bone graft materials.
Key words: Bone graft(s), bone regeneration, imaging, ridge preservation
INTRODUCTION
In the current times, where implant placement is the most favored option for replacement of a missing tooth, preservation of alveolar ridge is of vital importance. Following tooth extraction, dimensional changes of the residual alveolar ridge are inevitable. At the time of tooth extraction, preexisting pathologies such as periodontal disease and periapical lesions hastens the process of bone resorption.[1] Within first 6 months of tooth extraction, alveolar ridge loses its height and width by ≥50%.[2,3] Thus, to maintain the bone volume necessary for optimal functional and esthetic outcome of a dental implant, ridge preservation is the need of hour. Placement of various graft materials inside freshly extracted socket is advocated by multiple studies as a “ridge preservation technique.”[3,4,5,6,7,8]
Literature has reported alveolar ridge preservation using autogenous, allogeneic, xenograft, and alloplast graft materials.[4,5,6] For most favorable results, an ideal bone graft should have the properties of osteoconduction, osteoinduction, and osteoproliferation.[9,10] Out of all the available options, allografts, xenografts, and alloplasts demonstrate only osteoconduction (except demineralized freeze-dried bone allograft which is osteoinductive). Whereas autogenous bone possesses all three properties; thus, it is still considered as the gold standard. However, the risk of infection at the donor side, limited availability, and marked resorption are few shortcomings of autogenous bone graft.
Autogenous dentin graft was developed and clinically applied in Korea from 2008 onward. It has shown good clinical and histological results.[10,11] However, there is a paucity of data supporting the use of autogenous tooth graft (ATG) in clinical applications.
The primary objective of the current study is to clinically and radiographically evaluate the efficacy of ATG in preserving alveolar ridge dimensions in comparison to beta-tricalcium phosphate (β-TCP) alloplast. The secondary objective is to histologically determine bone formation potential of ATG.
MATERIALS AND METHODS
The present study was approved by the Institutional Ethical Committee (MGVKBHDC/15-16/1634). The current trial is registered with Clinical Trials Registry–India with registration number-CTRI/2015/10/006304. All clinical procedures were performed in accordance with the Declaration of Helsinki.
Study design
A randomized, controlled, prospective, clinical study was designed to investigate the efficacy of ATG in alveolar ridge preservation as compared to β-TCP alloplast against ungrafted sites. Patients having at least three teeth indicated for extraction were selected. The three extraction sockets were allocated to one of the following groups using random number table:
Socket grafted with ATG
Socket grafted with β-TCP alloplast (SyboGraf™ – T Plug, Eucare Pharmaceuticals Pvt. Ltd., Chennai, Tamil Nadu, India)
Socket left ungrafted (control).
Participants
Patients who reported to the Department of Oral Surgery in the month of January and February 2015 were screened. A total of fifteen patients (9 males and 6 females, aged 28–45 years, mean age: 35.6 ± 5.7 years) who met the following inclusion criteria were recruited [Figure 1]:
Figure 1.
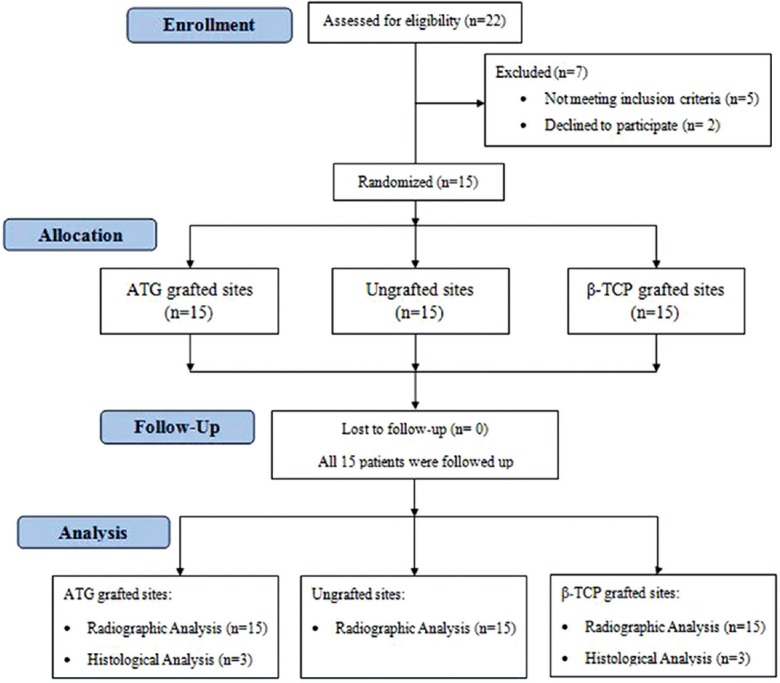
Study flowchart
Subjects having at least three nonadjacent teeth indicated for extraction
Patients who were overall healthy
The alveolar socket which was free of any preexisting periapical pathology based on intraoral periapical radiographs
Alveolar sockets with intact four wall architecture and depth ≥5 mm based on assessment using UNC-15 periodontal probe (GDC, Hoshiarpur, Punjab, India).
Patients were excluded based on following criteria:
Pregnant or lactating
History of any surgical or nonsurgical therapy or antibiotic therapy in the last 6 months
Subjects with habits such as smoking, tobacco chewing, or alcohol
History of radiation therapy.
Surgical protocol
Written informed consent was obtained from all the participants. Flapless, atraumatic extraction of the teeth was performed using periotomes and forceps. After extraction, to confirm the eligibility of surgical site, the alveolar wall integrity was checked using UNC-15 periodontal probe (GDC, Hoshiarpur, Punjab, India).
For the control site, the extraction socket was left to heal by its natural course. All the grafted and ungrafted sites were covered with a chorion membrane (Tissue Bank, Tata Memorial Hospital, Mumbai, India). One criss-cross suture was given to stabilize the membrane. Cone-beam computed tomography (CBCT) scan for the area of interest was taken. Postoperative instructions given and amoxicillin (500 mg TDS) was prescribed for 5 days. Patients were recalled for suture removal after 7 days. Then, after 4 months, a second CBCT scan was taken.
Autogenous tooth graft material preparation
After the extracted teeth were harvested, they were scaled and caries was removed using a round carbide bur. The teeth were powdered using a conventional grinder having motor rating 1500 W and speed of 700 rpm. The crushed granules were passed through two autoclaved stainless steel sieves in a sequential manner to obtain graft with particle size measuring between 300 and 500 μm.[12] [Figure 2] The graft particles were clinically sterilized by a procedure whose efficacy was established by a separate in vitro study. To achieve graft sterilization, the graft particles were immersed in 1 N lactic acid for 15–20 min which also partially decalcified them. Later using sterile normal saline, graft particles were thoroughly washed for 60 s to remove any residual traces of lactic acid.
Figure 2.
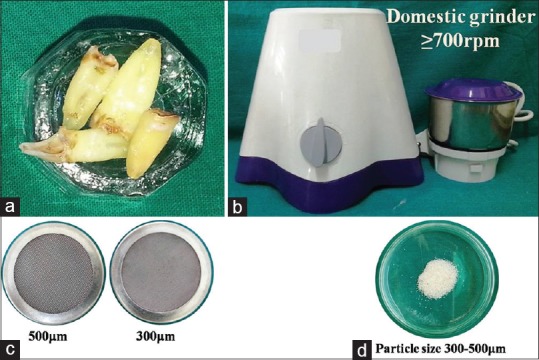
Preparatory steps of autogenous tooth graft. (a) Cleaned extracted teeth; (b) Conventional grinder; (c) Two autoclavable stainless steel sieves; (d) Prepared graft
Assessment
The study period of 4 months was divided into three time intervals, i.e., 1st day (immediately after grafting procedure), 7th day posttherapy, and 4 months posttherapy. Clinical evaluation was done at all visits and CBCT scans were obtained at the 1st day and at the end of 4 months. Three dimensional radiographic crestal bone changes in height and width after 4 months were selected as the primary outcome and histological new bone formation as secondary outcome.
Cone-beam computed tomography analysis
Frame/slice of each CBCT scan was adjusted in an axial view and center aligned over the area of interest. For the buccal and lingual cortical height, the measurements were done using linear measurement tool in a cross-sectional slice. For mesial and distal cortical height, measurements were done in a tangential slice. All the vertical measurements were done from the most coronal aspect of cortical bone to the fixed anatomical point apically. Buccolingual width was measured in a cross-sectional slice and mesiodistal in the tangential slice. The amount of crestal bone loss is the difference between independently measured postoperative and preoperative values by above protocol.
Histological evaluation
Subjects in which implant placement was performed 4 months after the ridge preservation, an additional informed written consent for bone biopsy was obtained. Bone tissues were harvested using a trephine bur (3 mm in diameter) at the time osteotomy preparation. The harvested specimens were sent to Department of Oral Pathology for histological evaluation of new bone formation.
Statistical analyses
For all treatment groups, comparison of change in ridge mean width and height dimensions was done using Student's unpaired t-test. The Student's t-test was performed at the degree of freedom “9” and confidence interval of 95%. For all tests, P < 0.05 was considered significant. The obtained results were described and plotted. For statistical analysis, “SPSS-9” (IBM India Pvt LTD, Bangalore, India) software was used.
RESULTS
Subject-based analysis
Of the 45 selected sites in 15 patients, 30 sites were grafted with ridge preservation technique (15 grafted with ATG and 15 grafted with β-TCP) and remaining 15 were left ungrafted as control sites. Among 30 grafted sites, 18 were in the maxilla (10-ATG and 8-β-TCP) and 12 were in mandible (5-ATG and 7-β-TCP). Eight ungrafted sites were in maxilla and remaining seven were in mandible.
On the 7th day follow-up visit, uneventful healing was observed in all the patients at all the sites. No patient reported with any sign of infection or graft rejection. At the end of 4 months, a satisfactory clinical healing was observed in all the patients. Visually, ATG sites showed minimum alveolar ridge width shrinkage compared to sites grafted with β-TCP and ungrafted sites [Figure 3]. In the process of harvesting bone biopsy, ATG-grafted sites were found to be comparatively harder in consistency than β-TCP-grafted sites.
Figure 3.
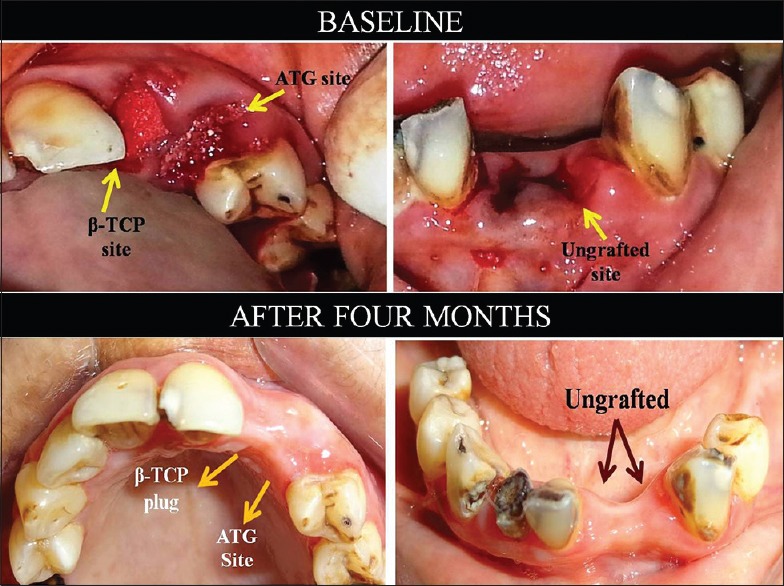
Clinical postoperative comparison of alveolar ridge width shrinkage for grafted and ungrafted sites in a representative case
Dimensional changes
The dimensional changes were calculated by comparing the CBCT scans taken immediately after grafting procedure (1st day) and 4 months postoperatively [Figure 4].
Figure 4.
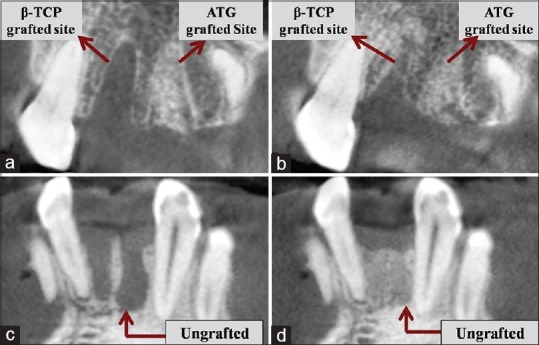
Four months postoperative radiographic images (cone-beam computed tomography images) comparing with baseline images. (a and c) Baseline radiographs; (b and d) Four months postoperative radiographs
Vertical ridge height changes
Mean and standard deviation values based on a comparison of CBCT scans for changes in ridge height related to the baseline buccal, lingual, mesial, and distal socket walls are shown graphically in Figure 5. Statistical significant differences were obtained when the mean (buccal, lingual, mesial, and distal) vertical resorption for all the experimental groups was compared. ATG-grafted sites consistently showed least reduction in ridge height, i.e., 0.28 ± 0.13 mm which was significantly lower as compared to β-TCP-grafted sites with 1.72 ± 0.56 mm reduction and ungrafted sites with 2.60 ± 0.88 mm reduction (P < 0.05).
Figure 5.
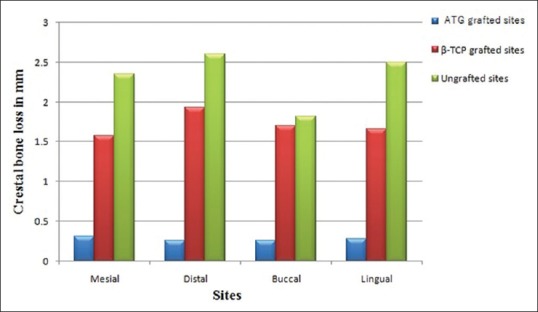
Mean height change in millimeter
Horizontal ridge width changes
Mean and standard deviation values based on a comparison of the CBCT scans for changes in ridge width related to the baseline buccolingual and mesiodistal width of extraction socket are shown graphically in Figure 6. The mean width of the socket was calculated as a mean of buccolingual and mesiodistal widths. The mean width change was greatest for ungrafted sites (2.29 ± 0.40 mm) followed by β-TCP-grafted sites (1.45 ± 0.40 mm) and least for autogenous tooth-grafted sites (0.15 ± 0.08 mm). A mean width change for autogenous tooth-grafted sites was significantly lesser when compared to β-TCP-grafted sites and ungrafted sites (P < 0.05).
Figure 6.
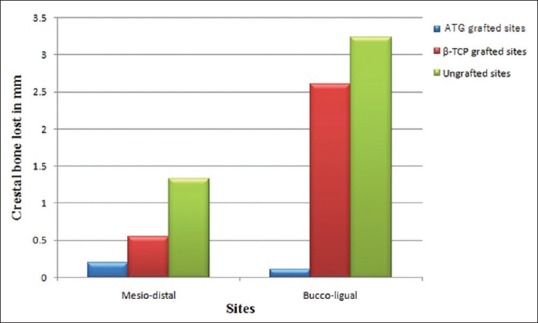
Mean width change in millimeter
Histological observations
Bone was harvested from the grafted sites of three patients who chose to opt for the implant placement postoperatively. Of the six grafted sites from these patients, four sites were in maxilla and two were in mandible.
Histology specimens from ATG-grafted sites showed newly formed bone associated with connective tissue stroma rich in angiogenesis. ATG particles had started to resorb and were surrounded by osteoid indicating new bone formation. Minimum fibrous tissue or no inflammatory cellular infiltration was observed [Figure 7].
Figure 7.
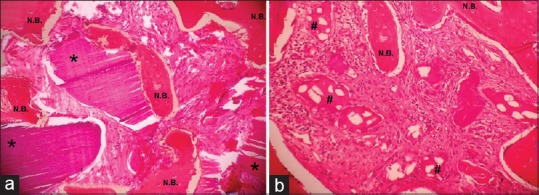
Hematoxylin and eosin staining of biopsy tissue taken from (a) autogenous tooth grafted site after 4 months (×40); (b) Beta-tricalcium phosphate grafted site after 4 months (×40). *Indicates residual autogenous tooth graft particles; #Indicates residual beta-tricalcium phosphate particles; N.B. – New bone formation
In compassion, β-TCP-grafted sites showed less osteoid formation. Graft particles were poorly integrated with newly forming bone. The connective tissue stroma surrounding β-TCP graft particles showed minimal evidence of angiogenesis and presence of scattered inflammatory infiltrate at some isolated sites [Figure 7].
DISCUSSION
This is a randomized, controlled, clinical pilot study comparing ridge preservation with conventional β-TCP alloplast and ATG. It is a prospective study having 4 months follow-up period. Radiographically and statistically significant differences were found in both the experimental groups. This was assessed based on the primary outcome variables such as a change in mean width and height of alveolar ridge, as well as histological healing pattern.
A separate in vitro study was conducted prior to clinical trial to establish the efficacy of sterilization protocol for ATG. Available data have used various inorganic acids such as nitric acid or HCl with varying concentrations for chemical sterilization.[13] Since its residues come in contact with human tissues, we preferred to use an organic acid, i.e., lactic acid. After rigorous experimental trials of various concentrations and exposure periods, 1N lactic acid for an exposure period of 15–20 min was found to be efficacious. The graft particles were thoroughly washed for 60 s to remove traces of residual lactic acid prior to incubation. Even after 48 and 72 hours of incubation in nutrient broth (Hi-Media Laboratories Pvt. Ltd., Mumbai, India), the absence of turbidity confirmed optimal microbial inhibition.
Significant resorption and healing of β-TCP particles are expected in 3–6 months after its placement.[14] Most of the β-TCP gets biodegraded by both osteoclastic activities subsequent to the particle disaggregation and/or chemical dissolution of the molecule in the calcium and phosphate components followed by replacement with healthy bone.[15,16,17] ATG resorbs within 4–6 months after grafting. The remodeling process with new bone formation continues up to 1–2 years.[11,18] Based on these references, a 4 months follow-up period was selected for the current study.
CBCT scans were taken on the day of extraction immediately after grafting procedure and after 4 months posttreatment. CBCT scan being three dimensional in nature has helped us to evaluate changes in alveolar bone in all possible dimensions and measure them precisely. After 4 months posttreatment, the mean width change was highest for ungrafted sites followed by β-TCP sites, and it was least for ATG sites. The change in mean width was statistically significant when ATG-grafted sites were compared with other grafted sites (P < 0.05). When vertical bone resorption was compared, at ATG sites mean height showed consistently least reduction with respect to β-TCP and ungrafted sites. This change in alveolar height was statistically significant for ATG sites (P < 0.05).
Literature has provided evidence in support to the fact that ridge preservation procedures reduce the bone dimensional changes compared with extraction without ridge preservation procedures.[7] However, systematic review demonstrates, in spite of employing evidence-based ridge preservation techniques, a complete prevention of vertical and horizontal bone resorption is an unpredictable event.[8] The results of this study are in accordance with these findings as there was some loss of ridge width and height in both the experimental groups despite performing ridge preservation technique. Thus, ridge preservation procedures do not result in complete dimensional stability but are designed to reduce the loss of ridge dimension compared to sites left to heal naturally after tooth extraction. In our study, within the ridge preservation groups, ATG sites showed consistently minimum vertical, as well as horizontal bone resorption, as evidenced by least change in alveolar width and height 4 months after therapy.
From a histological standpoint, ATG particles were surrounded by vital bone with areas of resorption. This is consistent with a study done by Kim et al.[19] In the β-TCP group, the degree of new bone formation was generally moderate and newly formed bone was immature in nature. It was surrounded by varying degree of osteoid material and in one case, mild inflammatory infiltrate was observed.
The possible explanation for better results with ATG is validated by various in vitro and animal studies which have demonstrated its biocompatibility, osteoinductive, and osteoconductive potential.[10,20,21] In addition, clinical studies have shown its use in reconstructive periodontal surgery with superior results.[9,16]
Murata reported the first clinical case of sinus augmentation using auto-dentin as a bone graft material. Interestingly, dentin and bone are almost similar with respect to composition. They consist of body fluid (10%), collagen (18%), noncollagenous proteins (2%), and hydroxyapatite (HA) (70%) in weight volume. According to Urist in 1965, demineralized dentin matrix and demineralized bone matrix contain mainly type-I collagen with growth factors such as bone morphogenetic proteins 2 and fibroblast growth factors. These bioactive molecules are thought to contribute to osteoinduction and osteoconductive property of human tooth as a graft material.[10]
Mineral content wise, tooth consists of low-crystalline HA and possibly other calcium phosphate minerals such as β-TCP, amorphous calcium phosphate, and octacalcium phosphate which is quite similar to human bone tissues.[22] Kim et al. indicated that the content of autogenous tooth is quite comparable to that of autogenous cortical bone.[23] Hence, properties like these might explain the better results imparted by ATG.
Limitations
The study is pilot in nature. Additional studies are needed to validate these findings using larger sample size. Additional research needs to be conducted to differentiate osseo-remodeling properties of dentin, cementum, and enamel. Longer duration of evaluation will give more predictable results and would confirm its stability. In addition, more studies are needed to evaluate the effect on implants placed in ATG-grafted alveolar ridge preservation sites in terms of their osseointegration and stability.
CONCLUSIONS
The clinical outcome of alveolar ridge preservation procedure is satisfactory with no reported complications. The study is able to validate the need of alveolar ridge preservation technique in maintaining height and width of residual alveolar ridge after extraction. ATG has shown more promising results as compared to β-TCP in achieving minimum volumetric alveolar bone loss when it is grafted immediately in postextraction socket. Histologically, it has shown to facilitate new bone formation. This resorbable material allows most predictable, consistent, and reproducible bone regeneration. Derived from an extracted human tooth, it is the most easily available and cost effective material. Thus, rather than disposing extracted teeth as biomedical waste, they can be used as an autogenous graft material and serve as a better alternative to most of the conventional graft materials. Further randomized clinical trials are needed to establish its regenerative potential in various periodontal defects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to thank Mr. Pradeep K. Joshi for his assistance.
REFERENCES
- 1.Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32:212–8. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23(Suppl 5):1–21. doi: 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 3.Van der Weijden F, Dell'Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J Clin Periodontol. 2009;36:1048–58. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 4.Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J Periodontol. 2003;74:990–9. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 5.Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69:1044–9. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- 6.Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin Oral Implants Res. 2011;22:779–88. doi: 10.1111/j.1600-0501.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 7.Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J Periodontol. 2003;74:990–9. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 8.Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin Oral Implants Res. 2011;22:779–88. doi: 10.1111/j.1600-0501.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 9.Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontol 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 10.Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81:1264–72. doi: 10.1902/jop.2010.100016. [DOI] [PubMed] [Google Scholar]
- 11.Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20:471–5. doi: 10.1097/ID.0b013e3182386d74. [DOI] [PubMed] [Google Scholar]
- 12.Position paper. Tissue banking of bone allografts used in periodontal regeneration*. J Periodontol. 2001;72:834–8. doi: 10.1902/jop.2001.72.6.834. [DOI] [PubMed] [Google Scholar]
- 13.Murata M, Akazawa T, Takahata M, Ito M, Tazaki J, Hino J, et al. Bone induction of human tooth and bone crushed by newly developed automatic mill. J Ceram Soc Jpn. 2010;118:434–7. [Google Scholar]
- 14.Artzi Z, Weinreb M, Givol N, Rohrer MD, Nemcovsky CE, Prasad HS, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: A 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004;19:357–68. [PubMed] [Google Scholar]
- 15.Lu JX, Gallur A, Flautre B, Anselme K, Descamps M, Thierry B, et al. Comparative study of tissue reactions to calcium phosphate ceramics among cancellous, cortical, and medullar bone sites in rabbits. J Biomed Mater Res. 1998;42:357–67. doi: 10.1002/(sici)1097-4636(19981205)42:3<357::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Trisi P, Rao W, Rebaudi A, Fiore P. Histologic effect of pure-phase beta-tricalcium phosphate on bone regeneration in human artificial jawbone defects. Int J Periodontics Restorative Dent. 2003;23:69–77. [PubMed] [Google Scholar]
- 17.Kwong CH, Burns WB, Cheung HS. Solubilization of hydroxyapatite crystals by murine bone cells, macrophages and fibroblasts. Biomaterials. 1989;10:579–84. doi: 10.1016/0142-9612(89)90110-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim YK, Lee HJ, Kim KW, Kim SG, Um IW. Guide bone regeneration using autogenous teeth: Case reports. J Korean Assoc Oral Maxillofac Surg. 2011;37:142–7. [Google Scholar]
- 19.Kim YK, Yun PY, Um IW, Lee HJ, Yi YJ, Bae JH, et al. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J Adv Prosthodont. 2014;6:521–7. doi: 10.4047/jap.2014.6.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011;37:375–9. [Google Scholar]
- 21.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–5. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 23.Kim GW, Yeo IS, Kim SG, Um IW, Kim YK. Analysis of crystalline structure of autogenous tooth bone graft material: X-ray diffraction analysis. J Korean Assoc Oral Maxillofac Surg. 2011;37:225–8. [Google Scholar]


