Abstract
A DNA synthesizer was successfully employed for preparation of well-defined polymers by atom-transfer radical polymerization (ATRP), in a technique termed AutoATRP. This method provides well-defined homopolymers, diblock copolymers, and biohybrids under automated photomediated ATRP conditions. PhotoATRP was selected over other ATRP methods because of mild reaction conditions, ambient temperature, tolerance to oxygen, and no need to introduce reducing agents or radical initiators. Both acrylate and methacrylate monomers were successfully polymerized with excellent control in the DNA synthesizer. Diblock copolymers were synthesized with different targeted degrees of polymerization and with high retention of chain-end functionality. Both hydrophobic and hydrophilic monomers were grafted from DNA. The DNA-polymer hybrids were characterized by SEC and DLS. The AutoATRP method provides an efficient route to prepare a range of different polymeric materials, especially polymer-biohybrids.
Keywords: DNA, photochemistry, polymerization, structure determination, synthetic methods
Autorad
Photoinduced atom-transfer radical polymerization is conducted in an automated DNA synthesizer to prepare well-defined homopolymers, diblock copolymers, and biohybrids. This technique provides a clean polymerization system under ambient temperature with oxygen tolerance.
Atom-transfer radical polymerization (ATRP) has transformed polymer synthesis by providing a robust route for the preparation of well-defined polymers with precisely controlled molecular weight (MW), narrow molecular weight distribution (Mw/Mn), and designed architectures.[1] ATRP has also been successfully applied to the preparation of a diverse range of biological conjugates, including DNA-polymer hybrids,[2] protein-polymer hybrids,[3] and polysaccharide polymer conjugates.[4] These bioconjugates show properties which could potentially be used as biological sensors or drug delivery agents.[5] However, discovery, utilization, and tuning the functionality of these materials to prepare a broad spectrum of biohybrids for evaluation in a specific application has been limited by tedious synthetic efforts and tremendous labor cost. Automated synthesizers overcame this obstacle in the synthesis of peptides,[6] oligonucleotides,[7] and oligosaccharides.[8] A synthetic strategy based on general building blocks and protection/deprotection/coupling cycles using an automated synthesis platform was also expanded to the synthesis of small organic molecules[9] and sequence-coded polymers.[10]
Given the widespread success of these approaches to automated synthesis, we considered the possibility of using a DNA/RNA or peptide synthesizer technique to obtain well-defined polymers and biohybrids. Considering the limitation that biological molecules are sensitive to temperature and environmental conditions, photochemically mediated ATRP (photoATRP) was employed.[11] PhotoATRP operates with only ppm levels of a copper catalyst at ambient temperature with some degree of oxygen tolerance. Compared to initiators for continuous activator regeneration (ICAR),[12] activators regenerated by electron transfer (ARGET),[13] or supplemental activator and reducing agent atom-transfer radical polymerization (SARA) ATRP,[14] photoATRP operates without additional reactants such as reducing agents or radical initiators.[11g,i,15] This ■aspect■ is crucial for preparation of stable biohybrids without denaturation. As a proof of concept, we employed photoATRP in a modified commercial DNA/RNA synthesizer to prepare well-defined homopolymers, block copolymers, and DNA-polymer hybrids. This system can be easily extended to other reversible deactivation radical polymerization (RDRP) techniques such as photo-induced electron/energy-transfer-reversible addition-fragmentation chain transfer (PET-RAFT)[16] and to different types of automated synthesizers.
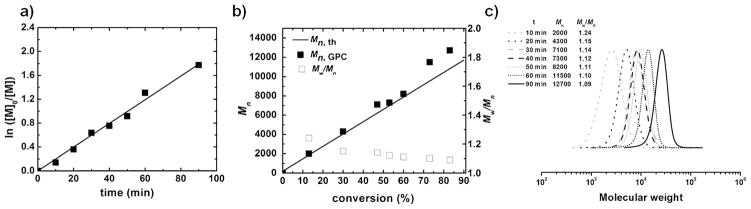
We fitted a MerMade 4 DNA synthesizer (Bioautomation) with a magnetic stirrer and a UV light (λ =365 nm) source through optical fiber cables. The computer was programmed to inject different reaction components under designed reaction conditions (see the Supporting Information for more details). Polymerization of n-butyl acrylate (BA) was conducted using ethyl α-bromoisobutyrate (EBiB) as an ATRP initiator with CuBr2 and tris[2-(dimethylamino)ethyl]-amine (Me6TREN) as the ligand in MeCN under irradiation by three optical fibers (50 mWcm−2). Under photoATRP conditions, the ATRP deactivator, copper(II), was photo-reduced in the presence of excess electron-donor compounds (in this case alkyl amine ligands). All the solutions were degassed and protected under an argon atmosphere. After 1.5 hours of irradiation, the conversion of BA reached 83%, thus providing a polymer with Mn = 12,700 and Mw/Mn = 1.09 (entry 1, Table 1). To investigate the reaction kinetics and evolution of MW with conversion, the polymerization was conducted under the same reaction conditions but stopped after different time periods to analyze the samples and examine the progress of the polymerization. The results are summarized in Figure 1. A linear semilogarithmic kinetic plot versus monomer conversion was observed. The experimental molecular weights closely matched the theoretical values, and the dispersity decreased from 1.24 to 1.09 with conversion. These preliminary results indicated that conducting photo-ATRP within an automated DNA synthesizer provides a highly efficient polymer synthesis protocol.
Table 1.
Preparation of homopolymers at different targeted DP values by AutoATRP using the synthesizer.[a]
| Entry | Monomer | DP | t [h] | Conv. [%][b] | Mn,th [c] | Mn,SEC[d] | Mw/Mn[d] |
|---|---|---|---|---|---|---|---|
| 1[e] | n-butyl acrylate | 100 | 1.5 | 83 | 10 800 | 12700 | 1.09 |
| 2 | n-butyl acrylate | 100 | 2 | 78 | 10 200 | 10600 | 1.10 |
| 3[f] | n-butyl acrylate | 62 | 8 | 75 | 6150 | 5780 | 1.09 |
| 4 | methyl acrylate | 150 | 2 | 94 | 12 300 | 11200 | 1.10 |
| 5 | tert-butyl acrylate | 100 | 2 | 93 | 12 100 | 11500 | 1.15 |
| 6 | ethyl acrylate | 135 | 2 | 62 | 8570 | 7600 | 1.16 |
| 7[g] | OEOA480 | 50 | 4 | 71 | 16 000 | 15000 | 1.13 |
| 8 | methyl methacrylate | 135 | 2 | 21 | 3040 | 4700 | 1.46 |
| 9[h] | methyl methacrylate | 135 | 2 | 16 | 2400 | 2900 | 1.19 |
Reaction conditions: [monomer]0/[EBiB]0/[CuBr2]0/[Me6TREN]0 = DP:1:0.03:0.18, 50% MeCN (v%), irradiation with three optical fibers (3 × 50 mWcm−2) at ambient temperature.
Determined by 1H NMR spectroscopy.
Calculated on the basis of conversion (i.e. Mn,th = MEBiB + DP × conversion × Mmonomer).
Determined by SEC in THF, based on linear PMMA calibration standards.
Solutions used in this entry were degassed.
Irradiation with one optical fiber.
In DMSO with UV irradiation.
EBPA used instead of EBiB.
Figure 1.
Results for polymerization of BA by AutoATRP in the synthesizer (entry 1, Table 1) under the following reaction conditions: [BA]0/[EBiB]0/[CuBr2]0/[Me6TREN]0 = 100:1:0.03:0.18, 50% MeCN (v%), irradiation by three optical fibers (3 × 50 mWcm−2, 365 nm) at ambient temperature. a) Semilogarithmic kinetic plot. b) Evolution of molecular weight and molecular weight distribution. c) SEC traces evolution.
As photoATRP can tolerate oxygen in the presence of a limited amount of air,[17] polymerization of BAunder similar conditions but without precautionary degassing was attempted. The polymerization was slightly slower than the reaction in the absence of oxygen, thus reaching 78% conversion after 2 hours and still providing a well-defined polymer (Mn = 10600 and Mw/Mn = 1.10; entry 2, Table 1). To make this protocol more convenient and broadly applicable, none of the solutions used in the following experiments were subjected to treatments of removing oxygen. The polymerization with only one optical fiber source (50 mWcm−2) provided a slower reaction and reached 75% conversion after 8 hours (entry 3), thus indicating that the rate of polymerization could be controlled by light sources and intensities. Other acrylates including, methyl, tert-butyl, ethyl, and poly(ethylene glycol) methyl ether acrylate (OEOA; Mn = 480) were successfully polymerized in a controlled manner (entries 4–7). Polymerization of methyl methacrylate using EBiB as an ATRP initiator resulted in a polymer with a broader MW distribution (Mw/Mn = 1.46; entry 8) because the reactivity of the initiator is lower than that of the growing chain, a result of a penultimate unit effect.[18] The reaction with ethyl α-bromophenylacetate (EBPA) as an initiator gave a polymer with better control (Mw/Mn = 1.19; entry 9). Instead of optical fibers, the polymerizations with UV irradiation from a lamp were also successful (entry 7; see the Supporting Information), and the synthesis of block copolymers and DNA-polymer conjugates in the following was performed with UV irradiation.
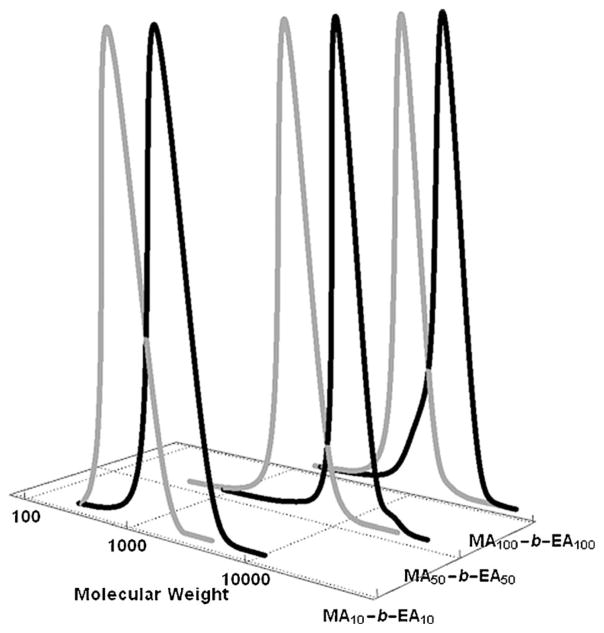
To show the versatility of this protocol, block copolymers with different targeted degrees of polymerization were prepared. After the conversion of the first monomer, methyl acrylate, reached greater than 90%, typically after 3 or 4 hours, the second monomer, ethyl acrylate, was injected by the automated synthesizer. The results are summarized in Figure 2 and Tables S3–S5 (see the Supporting Information). Samples were taken out at timed intervals and characterized by 1H NMR spectroscopy and size-exclusion chromatography (SEC). As shown in Figure S2, the resonances of the double bond of methyl acrylate gradually diminished with the appearance of the methyl peak of poly(methyl acrylate). The peak of the initiator EBiB (CH3CH2CO2−) could be easily detected by NMR spectroscopy for the polymer with a DP =10 ■(DP = degrees of polymerization)■, and was used to calculate the precise number average molecular weight (Mn,NMR) of the synthesized polymer. For polymers with DP =50 and 100, the molecular weights (Mn,SEC) obtained from SEC were directly compared to the values calculated based on conversion from 1H NMR data. Three MA-b-EA block copolymers, with DP = 10, 50, and 100, were obtained with low dispersity (ca. 1.10) and predetermined molecular weights (Tables S3–S5).
Figure 2.
SEC traces of block copolymers of MA-b-EA at different targeted degrees of polymerization in DMSO with UV irradiation. Gray line: SEC trace of PMA, black line: SEC trace of PMA-b-EA.
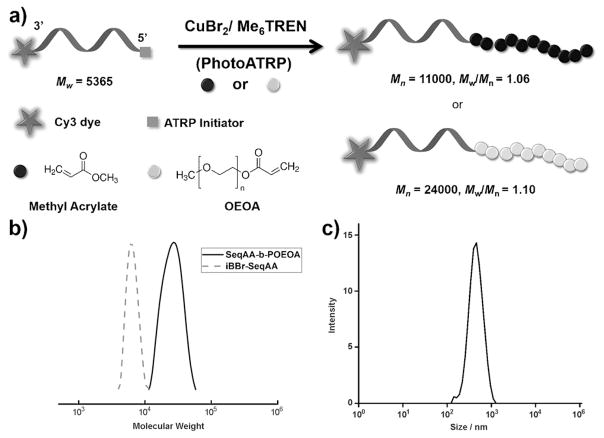
The preparation of a series of DNA-polymer hybrids was investigated to assess the direct compatibility of this method with DNA synthesis. To prepare a biohybrid polymer, a DNA oligonucleotide (SeqAA, 5′-ATC TGA GAC TCA CTG-3′, Mw = 5365 based on MALDI) was synthesized with a Cy3 dye at the 3′-end to facilitate the visualization of the product and confirm that the polymer synthesis conditions did not lead to cleavage of the DNA strand. The 5′-end included an ATRP initiator moiety we described previously to prepare the DNA macroinitiator.[2a] The ATRP-initiator phosphoramidite with an α-bromoisobutyrate (iBBr) group was readily coupled to the 5′-hydroxy group of a DNA sequence on a solid support in almost quantitative yields. Treatment with ammonium hydroxide released SeqAA-iBBr from the CPG support, and could be used to synthesize DNA-polymer bioconjugate in solution phase.
The reaction conditions of photoATRP were slightly modified for block copolymer synthesis using a very low concentration of SeqAA-iBBr (Table S7). Both a hydrophilic monomer, OEOA (Mn = 480), and a hydrophobic monomer, MA, were selected and polymerized in DMSO as a solvent. The results are summarized in Figure 3. SeqAA-b-PMA and SeqAA-b-POEOA480 were obtained in a well-controlled manner, thus yielding polymers with Mn,SEC = 11000 and 24000, and with low dispersities Mw/Mn = 1.06 and 1.10, respectively. An SEC trace of SeqAA-b-POEOA480 indicated that the molecular weight of the block copolymer cleanly shifted to a higher range from the original iBBr-SeqAA (Figure 3b). The SeqAA-b-PMA was also characterized in water using dynamic light scattering and zeta potential analysis, which showed well-defined particles with a diameter of (0.45 ± 0.09) μm and a zeta potential of −25.4 ± 1 mV (Figure 3c). The automated and straightforward synthesis of a DNA-polymer hybrid thus exemplifies the simplicity of linking different monomers to a DNA sequence through AutoATRP.
Figure 3.
a) Synthetic scheme for of DNA-polymer hybrids in the automated synthesizer under photoATRP conditions: [Monomer]0/[iBBr-SeqAA]0/[CuBr2]0/[Me6TREN]0 = 1000:1:0.5:3, in DMSO with UV irradiation. b) GPC traces for SeqAA-b-POEOA from iBBr-SeqAA. c) DLS data of SeqAA-b-PMA.
In summary, a DNA synthesizer was used for photoATRP to synthesize a series of well-controlled homopolymers and block copolymers. Photomediated ATRP does not require any additional photoinitiators or photosensitizers, and therefore provides a very clean polymerization system under mild reaction conditions. It was readily extended to the synthesis of DNA biohybrids. Both a hydrophobic monomer (MA) and a hydrophilic monomer (OEOA480) were employed in a chain extension from a DNA sequence. This protocol thus provides a convenient alternative option for non-experts in synthetic polymer chemistry to obtain highly controlled bioconjugates without any degassing procedures. This synthetic approach was conducted in the solution phase and requires high conversion of the first block (>90%). Otherwise, either the gradient copolymer can be formed or the remaining first monomer should be removed by dialysis or washed out. This trivial obstacle may be overcome by using a solid-phase support, and is part of an ongoing investigation, which will be reported in due course.
Supplementary Material
Acknowledgments
We acknowledge support from the NSF (CHE-1400052) and National Institutes of Health (R01DE020843) for this work.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under http://dx.doi.org/10.1002/anie.201611567.
Contributor Information
Dr. Xiangcheng Pan, Department of Chemistry, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA).
Sushil Lathwal, Department of Chemistry and Center for Nucleic Acids Science & Technology, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA).
Stephanie Mack, Department of Chemistry and Center for Nucleic Acids Science & Technology, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA).
Jiajun Yan, Department of Chemistry, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA).
Prof. Subha R. Das, Department of Chemistry and Center for Nucleic Acids Science & Technology, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA)
Prof. Krzysztof Matyjaszewski, Department of Chemistry, Carnegie Mellon University 4400 Fifth Avenue, Pittsburgh, PA 15213 (USA)
References
- 1.a) Wang JS, Matyjaszewski K. J Am Chem Soc. 1995;117:5614–5615. [Google Scholar]; b) Kato M, Kamigaito M, Sawamoto M, Higashimura T. Macromolecules. 1995;28:1721–1723. [Google Scholar]; c) Patten TE, Xia J, Abernathy T, Matyjaszewski K. Science. 1996;272:866–868. doi: 10.1126/science.272.5263.866. [DOI] [PubMed] [Google Scholar]; d) Matyjaszewski K, Tsarevsky NV. J Am Chem Soc. 2014;136:6513–6533. doi: 10.1021/ja408069v. [DOI] [PubMed] [Google Scholar]; e) Matyjaszewski K. Macromolecules. 2012;45:4015–4039. [Google Scholar]; f) Matyjaszewski K, Xia J. Chem Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 2.a) Averick SE, Dey SK, Grahacharya D, Matyjaszewski K, Das SR. Angew Chem Int Ed. 2014;53:2739–2744. doi: 10.1002/anie.201308686. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2014;126:2777–2782. [Google Scholar]; b) Troiber C, Wagner E. Bioconjugate Chem. 2011;22:1737–1752. doi: 10.1021/bc200251r. [DOI] [PubMed] [Google Scholar]; c) Averick S, Paredes E, Li W, Matyjaszewski K, Das SR. Bioconjugate Chem. 2011;22:2030–2037. doi: 10.1021/bc200240q. [DOI] [PubMed] [Google Scholar]; d) Lou X, Lewis MS, Gorman CB, He L. Anal Chem. 2005;77:4698–4705. doi: 10.1021/ac050706h. [DOI] [PubMed] [Google Scholar]; e) Pan P, Fujita M, Ooi WY, Sudesh K, Takarada T, Goto A, Maeda M. Polymer. 2011;52:895–900. [Google Scholar]; f) Kwak M, Herrmann A. Chem Soc Rev. 2011;40:5745–5755. doi: 10.1039/c1cs15138j. [DOI] [PubMed] [Google Scholar]; g) Kwak M, Herrmann A. Angew Chem Int Ed. 2010;49:8574–8587. doi: 10.1002/anie.200906820. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2010;122:8754–8768. [Google Scholar]; h) Peng L, Wu CS, You M, Han D, Chen Y, Fu T, Ye M, Tan W. Chem Sci. 2013;4:1928–1938. doi: 10.1039/C2SC21198J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Averick SE, Paredes E, Grahacharya D, Woodman BF, Miyake-Stoner SJ, Mehl RA, Matyjaszewski K, Das SR. Langmuir. 2012;28:1954–1958. doi: 10.1021/la204077v. [DOI] [PubMed] [Google Scholar]; b) Lele BS, Murata H, Matyjaszewski K, Russell AJ. Biomacromolecules. 2005;6:3380–3387. doi: 10.1021/bm050428w. [DOI] [PubMed] [Google Scholar]; c) Averick S, Simakova A, Park S, Konkolewicz D, Magenau AJD, Mehl RA, Matyjaszewski K. ACS Macro Lett. 2012;1:6–10. doi: 10.1021/mz200020c. [DOI] [PubMed] [Google Scholar]; d) Peeler JC, Woodman BF, Averick S, Miyake-Stoner SJ, Stokes AL, Hess KR, Matyjaszewski K, Mehl RA. J Am Chem Soc. 2010;132:13575–13577. doi: 10.1021/ja104493d. [DOI] [PubMed] [Google Scholar]; e) Klok HA. Macromolecules. 2009;42:7990–8000. [Google Scholar]; f) Pelegri-O’Day EM, Lin E-W, Maynard HD. J Am Chem Soc. 2014;136:14323–14332. doi: 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]; g) Heredia KL, Maynard HD. Org Biomol Chem. 2007;5:45–53. doi: 10.1039/b612355d. [DOI] [PubMed] [Google Scholar]; h) Gong Y, Leroux JC, Gauthier MA. Bioconjugate Chem. 2015;26:1172–1181. doi: 10.1021/bc500611k. [DOI] [PubMed] [Google Scholar]; i) Zhang P, Sun F, Liu S, Jiang S. J Controlled Release. 0000 doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Keefe AJ, Jiang S. Nat Chem. 2012;4:59–63. doi: 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Murata H, Cummings CS, Koepsel RR, Russell AJ. Biomacromolecules. 2014;15:2817–2823. doi: 10.1021/bm5008629. [DOI] [PubMed] [Google Scholar]; l) Cummings C, Murata H, Koepsel R, Russell AJ. Biomaterials. 2013;34:7437–7443. doi: 10.1016/j.biomaterials.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Miura Y, Hoshino Y, Seto H. Chem Rev. 2016;116:1673–1692. doi: 10.1021/acs.chemrev.5b00247. [DOI] [PubMed] [Google Scholar]
- 5.a) Lutz JF, Bçrner HG. Prog Polym Sci. 2008;33:1–39. [Google Scholar]; b) Shi J, Votruba AR, Farokhzad OC, Langer R. Nano Lett. 2010;10:3223–3230. doi: 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Alemdaroglu FE, Ding K, Berger R, Herrmann A. Angew Chem Int Ed. 2006;45:4206–4210. doi: 10.1002/anie.200600524. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2006;118:4313–4317. [Google Scholar]; d) Rodríguez-Pulido A, Kondrachuk AI, Prusty DK, Gao J, Loi MA, Herrmann A. Angew Chem Int Ed. 2013;52:1008–1012. doi: 10.1002/anie.201206783. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2013;125:1042–1046. [Google Scholar]; e) de Vries JW, Zhang F, Herrmann A. J Controlled Release. 2013;172:467–483. doi: 10.1016/j.jconrel.2013.05.022. [DOI] [PubMed] [Google Scholar]; f) Schnitzler T, Herrmann A. Acc Chem Res. 2012;45:1419–1430. doi: 10.1021/ar200211a. [DOI] [PubMed] [Google Scholar]; g) Cobo I, Li M, Sumerlin BS, Perrier S. Nat Mater. 2015;14:143–159. doi: 10.1038/nmat4106. [DOI] [PubMed] [Google Scholar]; h) Duncan R. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]; i) Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. J Controlled Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]; Vintiloiu A, Leroux J-C. J Controlled Release. 2008;125:179–192. doi: 10.1016/j.jconrel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Merrifield RB. Science. 1965;150:178–185. doi: 10.1126/science.150.3693.178. [DOI] [PubMed] [Google Scholar]
- 7.Caruthers M. Science. 1985;230:281–285. doi: 10.1126/science.3863253. [DOI] [PubMed] [Google Scholar]
- 8.Plante OJ, Palmacci ER, Seeberger PH. Science. 2001;291:1523–1527. doi: 10.1126/science.1057324. [DOI] [PubMed] [Google Scholar]
- 9.a) Li J, Ballmer SG, Gillis EP, Fujii S, Schmidt MJ, Palazzolo AME, Lehmann JW, Morehouse GF, Burke MD. Science. 2015;347:1221–1226. doi: 10.1126/science.aaa5414. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Adamo A, Beingessner RL, Behnam M, Chen J, Jamison TF, Jensen KF, Monbaliu JCM, Myerson AS, Revalor EM, Snead DR, Stelzer T, Weeranoppanant N, Wong SY, Zhang P. Science. 2016;352:61–67. doi: 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- 10.a) Roy RK, Meszynska A, Laure C, Charles L, Verchin C, Lutz JF. Nat Commun. 2015;6:7237. doi: 10.1038/ncomms8237. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ouahabi AA, Kotera M, Charles L, Lutz JF. ACS Macro Lett. 2015;4:1077–1080. doi: 10.1021/acsmacrolett.5b00606. [DOI] [PubMed] [Google Scholar]; c) Lutz JF. Macromolecules. 2015;48:4759–4767. [Google Scholar]; d) Gunay US, Petit BE, Karamessini D, Al Ouahabi A, Amalian J-A, Chendo C, Bouquey M, Gigmes D, Charles L, Lutz J-F. ChemistrySelect. 2016;1:114–126. ■■or Chem. Eur. J.?■ ■. [Google Scholar]
- 11.a) Dadashi-Silab S, Atilla Tasdelen M, Yagci Y. J Polym Sci Part A. 2014;52:2878–2888. [Google Scholar]; b) Ciftci M, Tasdelen MA, Li W, Matyjaszewski K, Yagci Y. Macromolecules. 2013;46:9537–9543. [Google Scholar]; c) Dadashi-Silab S, Doran S, Yagci Y. Chem Rev. 2016;116:10212–10275. doi: 10.1021/acs.chemrev.5b00586. [DOI] [PubMed] [Google Scholar]; d) Mosnáček J, Ilčíková M. Macromolecules. 2012;45:5859–5865. [Google Scholar]; e) Ribelli TG, Konkolewicz D, Bernhard S, Matyjaszewski K. J Am Chem Soc. 2014;136:13303–13312. doi: 10.1021/ja506379s. [DOI] [PubMed] [Google Scholar]; f) Ribelli TG, Konkolewicz D, Pan X, Matyjaszewski K. Macromolecules. 2014;47:6316–6321. [Google Scholar]; g) Konkolewicz D, Schroder K, Buback J, Bernhard S, Matyjaszewski K. ACS Macro Lett. 2012;1:1219–1223. doi: 10.1021/mz300457e. [DOI] [PubMed] [Google Scholar]; h) Frick E, Anastasaki A, Haddleton DM, Barner-Kowollik C. J Am Chem Soc. 2015;137:6889–6896. doi: 10.1021/jacs.5b03048. [DOI] [PubMed] [Google Scholar]; i) Pan X, Malhotra N, Simakova A, Wang Z, Konkolewicz D, Matyjaszewski K. J Am Chem Soc. 2015;137:15430–15433. doi: 10.1021/jacs.5b11599. [DOI] [PubMed] [Google Scholar]; j) Pan X, Tasdelen MA, Laun J, Junkers T, Yagci Y, Matyjaszewski K. Prog Polym Sci. 2016;62:73–125. [Google Scholar]; k) Chen M, Zhong M, Johnson JA. Chem Rev. 2016;116:10167–10211. doi: 10.1021/acs.chemrev.5b00671. [DOI] [PubMed] [Google Scholar]; l) Corrigan N, Shanmugam S, Xu J, Boyer C. Chem Soc Rev. 2016;45:6165–6212. doi: 10.1039/c6cs00185h. [DOI] [PubMed] [Google Scholar]
- 12.Matyjaszewski K, Jakubowski W, Min K, Tang W, Huang J, Braunecker WA, Tsarevsky NV. Proc Natl Acad Sci USA. 2006;103:15309–15314. doi: 10.1073/pnas.0602675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Jakubowski W, Matyjaszewski K. Angew Chem Int Ed. 2006;45:4482–4486. doi: 10.1002/anie.200600272. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2006;118:4594–4598. [Google Scholar]; b) Min K, Gao H, Matyjaszewski K. Macromolecules. 2007;40:1789–1791. [Google Scholar]
- 14.a) Konkolewicz D, Wang Y, Zhong M, Krys P, Isse AA, Gennaro A, Matyjaszewski K. Macromolecules. 2013;46:8749–8772. [Google Scholar]; b) Konkolewicz D, Wang Y, Krys P, Zhong M, Isse AA, Gennaro A, Matyjaszewski K. Polym Chem. 2014;5:4396–4417. [Google Scholar]; c) Konkolewicz D, Krys P, Gois JR, Mendonca PV, Zhong M, Wang Y, Gennaro A, Isse AA, Fantin M, Matyjaszewski K. Macromolecules. 2014;47:560–570. [Google Scholar]; d) Anastasaki A, Nikolaou V, Nurumbetov G, Wilson P, Kempe K, Quinn JF, Davis TP, Whittaker MR, Haddleton DM. Chem Rev. 2016;116:835–877. doi: 10.1021/acs.chemrev.5b00191. [DOI] [PubMed] [Google Scholar]; e) Boyer C, Corrigan NA, Jung K, Nguyen D, Nguyen TK, Adnan NNM, Oliver S, Shanmugam S, Yeow J. Chem Rev. 2016;116:1803–1949. doi: 10.1021/acs.chemrev.5b00396. [DOI] [PubMed] [Google Scholar]
- 15.a) Pan X, Malhotra N, Zhang J, Matyjaszewski K. Macromolecules. 2015;48:6948–6954. [Google Scholar]; b) Pan X, Malhotra N, Dadashi-Silab S, Matyjaszewski K. Macromol Rapid Commun. 2017 doi: 10.1002/marc.201600651. [DOI] [PubMed] [Google Scholar]
- 16.a) Xu J, Jung K, Atme A, Shanmugam S, Boyer C. J Am Chem Soc. 2014;136:5508–5519. doi: 10.1021/ja501745g. [DOI] [PubMed] [Google Scholar]; b) Shanmugam S, Boyer C. J Am Chem Soc. 2015;137:9988–9999. doi: 10.1021/jacs.5b05903. [DOI] [PubMed] [Google Scholar]; c) Xu J, Shanmugam S, Duong HT, Boyer C. Polym Chem. 2015;6:5615–5624. [Google Scholar]
- 17.a) Mosnáček J, Eckstein-Andicsova A, Borska K. Polym Chem. 2015;6:2523–2530. [Google Scholar]; b) Yang Q, Lalevée J, Poly J. Macromolecules. 2016;49:7653–7666. [Google Scholar]
- 18.a) Lin CY, Coote ML, Petit A, Richard P, Poli R, Matyjaszewski K. Macromolecules. 2007;40:5985–5994. [Google Scholar]; b) Nanda AK, Matyjaszewski K. Macromolecules. 2003;36:8222–8224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.