Abstract
Background
European hedgehogs (Erinaceus europaeus) are urban dwellers and host both Ixodes ricinus and Ixodes hexagonus. These ticks transmit several zoonotic pathogens like Borrelia burgdorferi (sensu lato), Anaplasma phagocytophilum, Rickettsia helvetica, Borrelia miyamotoi and “Candidatus Neoehrlichia mikurensis”. It is unclear to what extent hedgehogs in (sub) urban areas contribute to the presence of infected ticks in these areas, which subsequently pose a risk for acquiring a tick-borne disease. Therefore, it is important to investigate to what extent hedgehogs contribute to the enzootic cycle of these tick-borne pathogens, and to shed more light at the mechanisms of the transmission cycles involving hedgehogs and both ixodid tick species.
Methods
Engorged ticks from hedgehogs were collected from (sub) urban areas via rehabilitating centres in Belgium. Ticks were screened individually for presence of Borrelia burgdorferi (sensu lato), Borrelia miyamotoi, Anaplasma phagocytophilum, Rickettsia helvetica and “Candidatus Neoehrlichia mikurensis” using PCR-based methods. Infection rates of the different pathogens in ticks were calculated and compared to infection rates in questing ticks.
Results
Both Ixodes hexagonus (n = 1132) and Ixodes ricinus (n = 73) of all life stages were found on the 54 investigated hedgehogs. Only a few hedgehogs carried most of the ticks, with 6 of the 54 hedgehogs carrying more than half of all ticks (624/1205). Borrelia miyamotoi, A. phagocytophilum, R. helvetica and B. burgdorferi genospecies (Borrelia afzelii, Borrelia bavariensis and Borrelia spielmanii) were detected in both I. hexagonus and I. ricinus. Anaplasma phagocytophilum, R. helvetica, B. afzelii, B. bavariensis and B. spielmanii were found significantly more in engorged ticks in comparison to questing I. ricinus.
Conclusions
European hedgehogs seem to contribute to the spread and transmission of tick-borne pathogens in urban areas. The relatively high prevalence of B. bavariensis, B. spielmanii, B. afzelii, A. phagocytophilum and R. helvetica in engorged ticks suggests that hedgehogs contribute to their enzootic cycles in (sub) urban areas. The extent to which hedgehogs can independently maintain these agents in natural cycles, and the role of other hosts (rodents and birds) remain to be investigated.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-017-2065-0) contains supplementary material, which is available to authorized users.
Keywords: Anaplasma phagocytophilum, Borrelia burgdorferi (sensu lato), Borrelia miyamotoi, “Candidatus Neoehrlichia mikurensis”, Rickettsia helvetica, European hedgehog, Erinaceus europaeus, Lyme borreliosis, Ixodes hexagonus, Ixodes ricinus
Background
The incidence of tick-borne diseases has increased the last decades and poses important economic and medical consequences [1]. Lyme borreliosis is the most prevalent tick-borne disease in Europe and presents itself under a wide range of clinical manifestations. The most common and earliest manifestation is an expanding rash at the site of the tick bite (erythema migrans), and left untreated it can progress towards more severe disease manifestations. The disseminated infection can affect a patient’s nervous system, joints, lymph nodes, skin, and in rare cases the heart or eyes [2]. The causative agents of Lyme borreliosis are spirochetes belonging to the Borrelia burgdorferi (sensu lato) (s.l.) complex. They are generally transmitted by ticks of the Ixodes ricinus complex [3] and are maintained in enzootic cycles by different vertebrate hosts [4–6]. At least five genospecies of B. burgdorferi (s.l.) complex have been shown to be pathogenic to humans, namely B. burgdorferi (sensu stricto) (s.s.), B. afzelii, B. garinii, B. spielmanii and B. bavariensis [2, 7]. Each of these genospecies is maintained in nature through a distinct enzootic cycle involving ixodid ticks and vertebrates acting as reservoirs [8, 9]. The different Borrelia genospecies are generally associated with different clinical manifestations [7]. Current knowledge is that B. afzelii is predominantly involved in dermal infections (erythema migrans and acrodermatitis chronica atrophicans) [2] and is adapted to rodents and other small mammals [9, 10]. Borrelia garinii is associated with neurological manifestations and birds maintain most of the B. garinii strains [9, 11, 12]. Borrelia bavariensis is frequently found in rodents [13, 14] and hedgehogs [15] and is also associated with neuroborreliosis in humans [7]. Borrelia spielmanii infection in humans is rare, and only found in patients with erythema migrans. Its reservoir hosts are of the family Gliridae [16], but this Borrelia genospecies has also been detected in hedgehogs and their ticks [15, 17]. Besides the B. burgdorferi (s.l.) genospecies, other established pathogens circulate in enzootic cycles including the same ixodid ticks and vertebrate reservoirs, for example Anaplasma phagocytophilum, Rickettsia helvetica, Borrelia miyamotoi and “Candidatus Neoehrlichia mikurensis”. These pathogens can cause non-characteristic, viral-like symptoms in humans, and often confused with Lyme borreliosis [18]. Their infections are often self-limiting, but in immunocompromised patients, they can cause severe clinical manifestations [19–22]. In the framework of human health, therefore, it is important to identify the different components of the enzootic cycle of these tick-borne diseases, and to shed more light at the mechanisms of the transmission cycles.
The generalist tick species I. ricinus actively quests in the vegetation for hosts to feed on and may readily bite humans, thereby possibly transmitting pathogens. Ixodes hexagonus is a host specialist, feeding primarily on hedgehogs. It shows an endophilic behaviour preferring dark, humid places, and is usually found in the nests of its host species [23]. Besides hedgehogs, this tick species has been found to infest other host species as well [24]. Despite their nest dwelling behaviour, it is known to occasionally bite humans and companion animals, even though less frequently than I. ricinus does [24, 25]. Both ixodid species can be found on hedgehogs and are competent vectors for transmitting B. burgdorferi (s.l.) [26–28].
The European hedgehog (Erinaceus europaeus Linnaeus, 1758) is a nocturnal insectivorous mammal commonly found throughout Western Europe [29]. They seem to have adjusted to a wide variety of habitats and occur in rural, suburban, and urban areas but generally prefer grassland with sufficient edge habitats. Hedgehogs can reach up to nine times higher densities in urban areas with parks and garden, than in rural areas, with lowest densities in forests and open grassland fields and agricultural land without cover such as shrubs or dead wood [30–32]. Since they are one of the most successful urban adapters, hedgehogs and I. hexagonus could contribute to the spread and persistence of pathogens in a (sub) urban habitat via secondary enzootic cycles, even when the contact between I. hexagonus and humans is low [15, 33].
Only a few studies have been performed on the reservoir competence of the European hedgehog. These studies have shown that these mammals can be infected with different B. burgdorferi (s.l.) genospecies [15, 17, 23] as well as other tick-borne pathogens, such as A. phagocytophilum [34, 35], tick-borne encephalitis virus (TBEV) [28] and R. helvetica [36]. The role of the European hedgehog and both ixodid tick species feeding on it in the transmission cycle of many tick-borne pathogens like B. miyamotoi and “Candidatus Neoehrlichia mikurensis” is not completely illuminated, yet [37].
In this study, we aim to investigate the prevalence of B. burgdorferi (s.l.) genospecies, B. miyamotoi, A. phagocytophilum, “Ca. Neoehrlichia mikurensis” and R. helvetica in the different stages of the I. hexagonus and I. ricinus tick species sampled from European hedgehogs (E. europaeus) in Belgium. Furthermore, we aim to investigate the role of these tick species and that of the hedgehog in the enzootic cycle of the different disease pathogens. By using epidemiological analysis and comparing the infection prevalences of the different pathogens from engorged ticks collected from hedgehogs with questing nymphs from the vegetation, we aim to (i) determine the reservoir status of the European hedgehog for B. burgdorferi (s.l.) genospecies, B. miyamotoi, A. phagocytophilum, “Ca. Neoehrlichia mikurensis” and R. helvetica, and (ii) find indications for the vector competence of I. hexagonus for tick-borne pathogens.
Methods
Hedgehog and tick sampling
Since European hedgehogs are legally protected in Belgium, the current investigation was carried out on ticks sampled from hedgehogs that were brought to the rehabilitation centres of Herenthout and Heusen-Zolder in the Campine region, Belgium. In general, these hedgehogs were captured in gardens and urban areas by civilians. To grant the hedgehogs an easy and full recovery, removal of all ectoparasites upon arrival at the rehabilitation centre is a standard procedure. For this study, attached ticks of all life stages were collected by the centres’ volunteers in 2014 (both centres) and 2015 (only Herenthout) between the end of April and the end of October. Tick specimens were stored in 70% ethanol at room temperature until further investigation. Ticks were identified to species and life stage [38]. The number of attached ticks (tick burden) was recorded for each hedgehog. Since only hedgehogs that harboured ticks were used in this study, there is no data on the percentage of hedgehogs that were infested by ticks. Age (adult or juvenile) was determined based on weight [15] for all hedgehogs, except two. The questing I. ricinus ticks, that were caught by drag-sampling the vegetation in the same region as where the hedgehogs were collected, are part of a previously published study [39].
Sample preparation and molecular detection of tick-borne pathogens
All ticks were processed individually. Nucleic acids were extracted using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The extracted DNA was stored at -20 °C until further use. Ticks were tested individually for presence of B. burgdorferi (s.l.), B. miyamotoi, A. phagocytophilum, “Ca. Neoehrlichia mikurensis” and R. helvetica DNA using two separate multiplex real-time PCR assays as described before [39–42], followed by sequencing for species identification. For the identification of B. burgdorferi (s.l.) genospecies a conventional PCR assay targeting the 5S–23S intergenic region (IGS) was performed (see Additional file 1). Borrelia burgdorferi (s.l.) genospecies identification was determined by comparison of sequences to isolate in-house molecular databases [43]. As genospecies identification was successful for only 43.4% of the B. burgdorferi (s.l.) positive sequences, we proportionally assigned these unidentifiable sequences in each life stage per tick species to the different B. burgdorferi (s.l.) genospecies present in stage, using the proportion of each genospecies detected in that stage as a weighting factor (Hofmeester et al. submitted). We assumed that the probability to successfully identify a genospecies is equal for all B. burgdorferi (s.l.) genospecies. For confirmation of B. miyamotoi conventional PCR targeting glpQ gene was done [20]. The groEL gene of A. phagocytophilum was amplified and sequenced [44] (see Additional file 2). For all conventional PCR’s, both strands of PCR products were sequenced by BaseClear (Leiden, the Netherlands).
Statistical tests
All statistical tests were performed using R version 3.2.0 (R Core Team, 2016 [45]) and all graphs were made with the package ggplot2 [46]. To test the differences in distribution of tick species, tick burden and infection prevalence of the different pathogens in ticks on hedgehogs from different age classes, Kruskal-Wallis tests were employed. The number of mixed tick species infestations (both tick species on the same hedgehog) was compared to the number of single species infestations (only I. ricinus or I. hexagonus) with Pearson’s Chi-square test. With the prop.test function, we tested if the pathogens in the ticks occurred more frequently alone or co-existing with a different pathogen in the same tick. Afterwards we compared the infection prevalence of the pathogens in I. hexagonus with the prevalence in I. ricinus. Finally, to assess the transmission capabilities of the hedgehog for each pathogen, we compared the infection prevalence in the engorged ticks collected from hedgehogs with the infection prevalence in questing I. ricinus from the same region [39], and used this as a proxy to evaluate the reservoir status of the hedgehog. To evaluate the reservoir status of a host species, it is best to compare engorged I. ricinus larvae with questing I. ricinus larvae and nymphs. As the amount of engorged I. ricinus larvae in our study is too low to perform these analyses (n = 7), we decided to compare the infection prevalence of each pathogen in engorged I. ricinus larvae and nymphs collected from hedgehogs with the infection prevalence in host-seeking I. ricinus nymphs and adults. This way, we compare ticks that fed once (engorged larvae and questing nymphs) or twice (engorged nymphs and questing adults), and omit ticks that had the chance to feed three times (engorged adults). Engorged adults have a higher chance to be infected than a questing tick anyway, that has never fed more than twice. The difference between the pathogen communities in engorged and questing ticks is thus that the engorged ticks will have certainly fed, at least once, on hedgehogs, while the chance that the questing ticks will have fed on hedgehogs is rather low. Differences between the infection prevalence of the pathogens in engorged and questing ticks may then reflect the importance of the hedgehog in the transmission of pathogens. A higher infection prevalence of a certain pathogen in engorged larvae and nymphs will then suggest that the hedgehog has an important role in the transmission of that pathogen.
Results
Of the 54 hedgehogs investigated, 24 were adults and 28 were juveniles. For two hedgehogs, age was not determined. Both I. hexagonus and I. ricinus ticks of all life stages were found on the hedgehogs. The number of ticks per hedgehog ranged from one to 167. Most hedgehogs in our study carried only few ticks, while only few individuals harboured most the ticks. Six of the 54 hedgehogs carried more than half of all ticks (624/1205) and only 15 hedgehogs carried 25 or more ticks. Tick burden did not significantly differ between hedgehog age classes (χ 2 = 0.001, df = 1, P = 0.97). In total, we collected 1205 ticks and found significantly more I. hexagonus (n = 1132) than I. ricinus (n = 73) (χ 2 = 29.126, df = 1, P = 0.0001) The most common life stage of I. hexagonus retrieved from the hedgehogs were nymphs (n = 586) (χ 2 = 4.2656, df = 1, P = 0.04). I. ricinus, all life stages were equally common (χ 2 = 3.1908, df = 1, P = 0.07, Fig. 1). Some hedgehogs were found to harbour both species of ticks (n = 10), but infestations with only one tick species were more common (n = 44,, χ 2 = 21.407, df = 1, P = 0.0001)
Fig. 1.
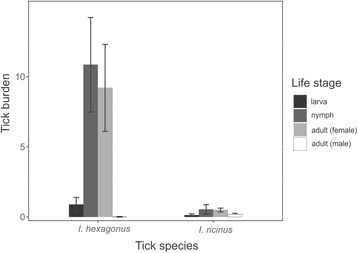
The distribution of the different life stages of Ixodes ricinus (IR) and Ixodes hexagonus (IH) collected from 54 hedgehogs in the Campine region, Belgium (mean ± standard error, SE)
Of the 1205 collected ticks, two got lost during sample preparation, hence the molecular analyses were performed on 1203 ticks. A total of 859 (71.4%) ticks was infected with at least one of the tested pathogens. Of these infected ticks, 524 (61%) had a single infection, and 335 ticks (39%) were infected with more than one pathogen of another genus. The number and percentage of infected I. ricinus and I. hexagonus ticks per life stage are provided in Table 1. A more detailed overview, including the different Borrelia burgdorferi (s.l.) genospecies, is provided in Additional file 3. Anaplasma phagocytophilum and R. helvetica were the two most common pathogens and occurred in 466 ticks (38.7% of all analysed ticks or 54% of all infected ticks) coming from 34 hedgehogs and in 481 ticks (40% of all analysed ticks or 56% of all infected ticks) coming from 37 hedgehogs, respectively. An infection with Borrelia burgdorferi (s.l.) occurred in 297 ticks (24.7% of all analysed ticks or 34.6% of all infected ticks) from 28 hedgehogs. We could identify the B. burgdorferi (s.l.) genospecies in 129 (43.4%) of these infected ticks, of which B. afzelii (n = 80), B. spielmanii (n = 28) and B. bavariensis (n = 17) were the most common. Borrelia turdi occurred once in each tick species and B. garinii and B. valaisiana each in one I. ricinus tick. An infection with B. miyamotoi occurred in 20 ticks from five hedgehogs. Only three (one I. hexagonus and two I. ricinus) from two hedgehogs were infected with “Ca. Neoehrlichia mikurensis”. The pathogen prevalence per tick species is depicted in Fig. 2. All pathogens except for R. helvetica (χ 2 = 15.983, df = 1, P = 0.0001) were found more often co-existing with another pathogen in a tick, than as the single pathogen infecting the tick. Ixodes ricinus seems to be more likely infected with at least one pathogen (59/72, 81.9%) than I. hexagonus (800/1131, 70.7%) but the difference between the two tick species was only marginally significant (χ 2 = 3.6355, df = 1, P = 0.06). More specifically, the infection prevalence of A. phagocytophilum, “Ca. Neoehrlichia mikurensis”, B. afzelii, B. garinii, B. valaisiana and B. turdi was highest in I. ricinus while infection with R. helvetica was highest in I. hexagonus (χ 2 = 16.333, df = 1, P = 0.0001). For the infection prevalence of B. miyamotoi, B. spielmanii and B. bavariensis, no difference between the tick species could be observed. There was no difference in infection prevalence between adult and juvenile hedgehogs for any of the detected pathogens (P > 0.05) (Table 1).
Table 1.
The number (#) of Ixodes ricinus and Ixodes hexagonus ticks infected with a certain pathogen, for all life stages together or for larvae (L), nymphs (N) or adults (A) separately, and the percentage (%) of infected ticks of the two species on all analyzed ticks from that species per life stage
| B. burgdorferi (s.l.) | B. miyamotoi | R. helvetica | A. phagocytophilum | “Ca. N. mikurensis” | |||
|---|---|---|---|---|---|---|---|
| I. hexagonus | L | No. | 3 | 2 | 10 | 2 | 0 |
| % | 6.3 | 4.2 | 20.8 | 4.2 | 0 | ||
| N | No. | 166 | 8 | 192 | 279 | 1.0 | |
| % | 28.4 | 1.4 | 32.8 | 47.7 | 0.2 | ||
| A | No. | 91 | 7 | 267 | 137 | 0 | |
| % | 18.3 | 1.4 | 53.6 | 27.5 | 0 | ||
| All | No. | 260 | 17 | 469 | 418 | 1 | |
| % | 23.0 | 1.5 | 41.5 | 37.0 | 0.09 | ||
| I. ricinus | L | No. | 1 | 0 | 0 | 3 | 0 |
| % | 14.3 | 0 | 0 | 42.9 | 0 | ||
| N | No. | 20 | 0 | 3 | 23 | 2 | |
| % | 71.4 | 0 | 10.7 | 82.1 | 7.1 | ||
| A | No. | 16 | 3 | 9 | 22 | 0 | |
| % | 43.2 | 8.1 | 24.3 | 59.5 | 0 | ||
| All | No. | 37 | 3 | 12 | 48 | 2 | |
| % | 51.4 | 4.2 | 16.7 | 66.7 | 2.8 |
Fig. 2.
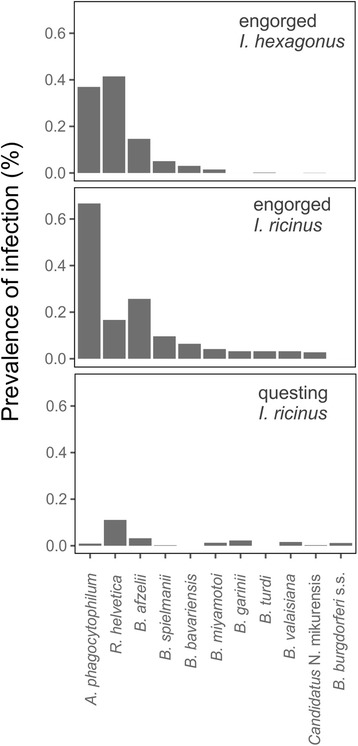
The prevalence of the distinct pathogens in Ixodes ricinus and Ixodes hexagonus ticks collected from hedgehogs (mean ± standard error, SE)
Co-infections of other pathogens with B. burgdorferi (s.l.) were investigated. For I. ricinus, 37 of the 59 infected ticks (62.7%) carried two (n = 31) or three (n = 6) pathogens. The most common co-infection in I. ricinus (24/37) was with B. burgdorferi (s.l.) and A. phagocytophilum. Of the 800 infected I. hexagonus ticks, 298 (37.3%) had a co-infection composed of two (n = 232) or three (n = 65) pathogens. Co-infections of A. phagocytophilum and R. helvetica (102/298), A. phagocytophilum and B. burgdorferi (s.l.) (86/298) and A. phagocytophilum, R. helvetica and B. burgdorferi (s.l.) (64/298) occurred most often. One I. hexagonus tick was infected with four pathogens: A. phagocytophilum, R. helvetica, B. burgdorferi (s.l.) and B. miyamotoi.
Ixodes ricinus larvae and nymphs from hedgehogs were infected more often (28/35) than questing I. ricinus nymphs and adults (367/1874) (χ 2 = 72.786, df = 1, P = 0.0001). We could not detect any difference in prevalence of R. helvetica, B. miyamotoi, B. garinii and B. valaisiana. For A. phagocytophilum (χ 2 = 807.24, df = 1, P = 0.0001), “Ca. Neoehrlichia mikurensis” (χ 2 = 14.989, df = 1, P = 0.0001), B. afzelii (χ 2 = 82.545, df = 1, P = 0.0001), B. spielmanii (χ 2 = 44.81, df = 1, P = 0.0001), B. bavariensis (χ 2 = 110.89, df = 1, P = 0.0001) and B. turdi (χ 2 = 110.89, df = 1, P = 0.0001), infection prevalence was significantly higher in the engorged ticks from the hedgehogs.
Afterwards we repeated these analyses for I. hexagonus collected from hedgehogs and compared the larvae and nymphs of this tick species with the questing I. ricinus nymphs and adults collected from the vegetation. This enables us to interpret more comprehensively the reservoir role of the hedgehog for the different pathogens, and the vector competence of I. hexagonus. We observed that B. garinii and B. valaisiana were more prevalent in the questing I. ricinus ticks. No significant difference in infection prevalence between questing or engorged ticks could be detected for B. turdi, B. miyamotoi and “Ca. Neoehrlichia mikurensis”. The prevalence of all other pathogens, including R. helvetica, is higher in the engorged than the questing ticks. Furthermore, when comparing just the ticks collected from hedgehogs that carried 25 or more ticks, we obtained the same outcome.
For A. phagocytophilum, R. helvetica, B. bavariensis and B. miyamotoi, the distribution of the infections was clustered in some hedgehogs, with most hedgehogs harbouring no, or only few infected ticks, while only few hedgehogs were responsible for most of the infected ticks. This is visualized in Fig. 3. Twelve of the 17 ticks infected with Borrelia bavariensis and 16 of the 20 ticks infected with B. miyamotoi came from one individual hedgehog (hedgehog #18). Hedgehog #33 harboured a total of 125 ticks of which 118 were infected with A. phagocytophilum (25.3% of all A. phagocytophilum infections). Still, there are hedgehogs that harbour many ticks, while no or few or these ticks are infected with one of these pathogens (Fig. 3).
Fig. 3.

The tick burden per hedgehog with the number of ticks per hedgehog harbouring infection with Anaplasma phagocytophilum, Rickettsia helvetica, Borrelia bavariensis and Borrelia miyamotoi
Of the A. phagocytophilum positive ticks, 43 were sequenced of which 33 I. hexagonus and 10 I. ricinus from 18 different individual hedgehogs. All the groEL sequences of the A. phagocytophilum isolates clustered with the zoonotic ecotype, ecotype I (not shown [44]).
Discussion
Our results confirm that hedgehogs are a host of all three stages of I. hexagonus and I. ricinus. Still, more I. hexagonus were found feeding on hedgehogs than I. ricinus ticks (Fig. 1). The aggregation of ticks on hedgehogs varied vastly between the individual hedgehogs, as only a few hedgehogs were recorded to carry most of the ticks (Fig. 3). This means that just a few hedgehogs contribute to tick maintenance, similar to what is seen on rodents [47]. This seems to be especially the case for I. hexagonus, and to a lesser extend for I. ricinus, since the burdens of I. ricinus on hedgehogs appears to be relatively low. Moreover, it is less likely that hedgehogs can maintain the I. ricinus life-cycle as the sole host species because, even though it can feed all life stages of this generalist tick species, hedgehog densities in forested areas, the preferred habitat of I. ricinus, are too low [30, 31]. Namely, if all I. ricinus stages should rely only on the hedgehog to feed on, many ticks would starve and perish since the number of encounters with this host would be low. We believe, rather, that a host community without large mammals but composed only of small or medium sized hosts such as rodents, birds and hedgehogs (like in (sub) urban area’s and parks), can already be sufficient to complete the life-cycle of I. ricinus. This because, as we show, large mammals are not the only hosts adult I. ricinus ticks feed on. More research is needed, however, to elucidate the role of hedgehogs in the life-cycle of this generalist tick species.
Since 71.4% of the ticks retrieved from hedgehogs were infected by at least one pathogen, hedgehogs can be considered as amplifying hosts and epidemiologically important wildlife species. Moreover, 39% of all infected ticks carried more than one pathogen of another genus. High prevalence of tick-borne pathogens B. bavariensis, B. spielmanii, B. afzelii, A. phagocytophilum and R. helvetica in engorged I. hexagonus and I. ricinus ticks obtained from E. europaeus, indicates that hedgehogs contribute to pathogen maintenance in natural cycles in urban and suburban areas. For B. bavariensis, B. spielmanii, B. afzelii, A. phagocytophilum and R. helvetica, the infection prevalence was higher in the engorged ticks of both species, in comparison to the infection rates in questing ticks from the same region (Fig. 2). This indicates that the hedgehog is a possible reservoir host of these pathogens and contributes to their enzootic cycle. On the other hand, “Ca. Neoehrlichia mikurensis” infection rate was not significantly higher in questing I. ricinus ticks than in engorged hedgehog ticks, indicating that hedgehogs do not play a main role in the maintenance of the enzootic cycle of this pathogen.
Engorged I. ricinus ticks tend to be more infected with any pathogen in comparison to engorged I. hexagonus, except for R. helvetica which was significantly more prevalent in I. hexagonus ticks. Perhaps this observation can be subscribed to transmission pathway of R. helvetica, which occurs transovarially as well as transstadially. Therefore, ticks in nature are usually thought to be the main reservoir and vectors of R. helvetica [48]. However, since transovarial transmission rates are less than 100%, vertebrate hosts like the hedgehog can act as an amplifier of this pathogen, playing a vital role in transmission cycles. The pathogens that are present in engorged I. ricinus ticks can originate from a previous blood meal from another host species, while the pathogens I. hexagonus carries are most probably coming from the hedgehog, since hedgehogs are their preferred host species. This way infection prevalence in engorged I. ricinus can be higher than engorged I. hexagonus, when they fed in a previous stage on a host species that functions as an efficient reservoir species for some of the investigated pathogens, such as small rodents or birds.
Remarkably, the infection of some pathogens such as B. bavariensis, B. miyamotoi, R. helvetica and A. phagocytophilum seem to be clustered per individual hedgehog, meaning that only a few hedgehogs contribute to the gross of the infected ticks. Borrelia miyamotoi is known to give short-term systemic infection in rodents, therefore making rodents excellent but transitory amplifying hosts of this bacterium [49]. Vertebrates other than rodents may also become infected: B. miyamotoi DNA was also found in the tissue of a European greenfinch and a great tit [50]. The clustering of infected fed ticks on only one hedgehog in this study indicate that B. miyamotoi might result in a short-term systemic infection in hedgehogs as well. The role of these animals in the transmission cycle is not clear; they could be transitory hosts. Another possible explanation for the fact that many ticks were infected with the same pathogen on the same hedgehog could be co-feeding transmission [51, 52]. With this route of transmission, no systemic infection of the vertebrate host is necessary. The host is only a transient bridge, bringing together infected and uninfected ticks in both space and time, thereby facilitating pathogen exchange. The host does not necessarily have to be infected himself [51, 52]. The bird associated borreliae, B. garinii and B. valaisiana, were each detected in one I. ricinus adult tick, and B. turdi occurred in one I. hexagonus female and one I. ricinus nymph. We can thus confirm the indication that hedgehogs are no reservoir hosts for the bird associated, only for the rodent-associated, B. burgdorferi (s.l.) genospecies [15].
Hedgehogs and their host-specific parasite I. hexagonus seem to play a role in maintaining some pathogens, like B. bavariensis, B. spielmanii, and A. phagocytophilum in cryptic cycles. The generalist feeding behaviour of I. ricinus and the low prevalence of these pathogens in questing I. ricinus suggest that they do not play a main role in the maintenance of the enzootic cycle of these pathogens. However, when feeding on hedgehogs I. ricinus may still be infected by I. hexagonus-associated pathogens and transmit them to humans. Borrelia bavariensis can cause neurological disease in humans [7], and B. spielmanii has been linked to EM in humans. Both pathogens have already been linked to hedgehogs [15, 17]. Moreover, co-infection of R. helvetica and B. burgdorferi (s.l.) has been shown in neuroborreliosis patients [53]. Also, co-infections are thought to affect the severity of disease and influence clinical outcomes in some cases [54]. Since hedgehogs seem to be large contributors to co-infection rates in ticks, this poses an increased health risk. The variant of A. phagocytophilum detected in these samples were all linked to human cases of anaplasmosis (ecotype I) [44].
Conclusions
From these findings, we conclude that hedgehogs are important components in the enzootic cycle of a diverse set of human pathogens, thereby contributing to the maintenance of various tick-borne diseases in (sub) urban areas. Humans are likely to encounter ticks infected with one or several of these pathogens while gardening or recreating in parks [55]. This poses a potential human health risk. Most hedgehogs, however, carry only few ticks and hedgehog densities are relatively low, thus hedgehogs will probably infect only few ticks with a certain pathogen. Further research is necessary to elucidate the interaction between hedgehog densities, tick burden and tick infection prevalence and to assess the precise impact of hedgehogs on the enzootic cycle of the various tick borne human pathogens, and the associated human health risk.
Acknowledgements
The authors would like to thank the animal shelters VOC Heusden-Zolder and VOC Neteland and their volunteers. We thank Arieke Docters van Leeuwen for her excellent technical assistance.
Funding
This investigation received financial support from IWT-Flanders, the Institute for the Promotion of Innovation through Science and technology in Flanders (to SR).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Authors’ contributions
SCR, SJ, KV and HS designed the study, SJ, EFM and RJ performed the lab work and molecular analyses, SCR computed and analysed the data, SCR and SJ wrote the manuscript and KV and HS provided valuable insights, helped to improve the manuscript with valued comments and supervised the study. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional files
IGS sequences obtained in this study. (XLSX 13 kb)
groEL sequences obtained in this study. (XLSX 10 kb)
The number of Ixodes ricinus and Ixodes hexagonus ticks infected with a certain pathogen, for all life stages together or for larvae (L), nymphs (N) or adults (A) separately. (CSV 719 bytes)
Contributor Information
Setareh Jahfari, Email: seta.jahfari@rivm.nl.
Sanne C. Ruyts, Email: sanne.ruyts@ugent.be
Ewa Frazer-Mendelewska, Email: ewa.frazer@rivm.nl.
Ryanne Jaarsma, Email: ryanne.jaarsma@rivm.nl.
Kris Verheyen, Email: kris.verheyen@ugent.be.
Hein Sprong, Email: hein.sprong@rivm.nl.
References
- 1.Randolph SE. Evidence that climate change has caused ‘emergence’of tick-borne diseases in Europe? Int J Med Microbiol. 2004;293:5–15. doi: 10.1016/s1433-1128(04)80004-4. [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease- a tick-borne spirochetosis? Science. 1982;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 4.Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4(9):660–669. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 5.Mannelli A, Bertolotti L, Gern L, Gray J. Ecology of Borrelia burgdorferi sensu lato in Europe: transmission dynamics in multi-host systems, influence of molecular processes and effects of climate change. FEMS Microbiol Rev. 2012;36(4):837–861. doi: 10.1111/j.1574-6976.2011.00312.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofmeester T, Coipan E, van Wieren S, Prins H, Takken W, Sprong H. Few vertebrate species dominate the Borrelia burgdorferi s.l. life cycle. Environ Res Lett. 2016;11(4):043001. doi: 10.1088/1748-9326/11/4/043001. [DOI] [Google Scholar]
- 7.Coipan EC, Jahfari S, Fonville M, Oei GA, Spanjaard L, Takumi K, et al. Imbalanced presence of Borrelia burgdorferi s.l. multilocus sequence types in clinical manifestations of Lyme borreliosis. Infect Genet Evol. 2016;42:66–76. [DOI] [PubMed]
- 8.Gray J. Review the ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22(5):249–258. doi: 10.1023/A:1006070416135. [DOI] [Google Scholar]
- 9.Gern L. Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: life in the wilds. Parasite. 2008;15(3):244–7. [DOI] [PubMed]
- 10.Humair P, Rais O, Gern L. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology. 1999;118(01):33–42. doi: 10.1017/S0031182098003564. [DOI] [PubMed] [Google Scholar]
- 11.Hanincová K, Taragelová V, Koci J, Schäfer SM, Hails R, Ullmann AJ, et al. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol. 2003;69(5):2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heylen D, Tijsse E, Fonville M, Matthysen E, Sprong H. Transmission dynamics of Borrelia burgdorferi s.l. in a bird tick community. Environ Microbiol. 2013;15(2):663–673. doi: 10.1111/1462-2920.12059. [DOI] [PubMed] [Google Scholar]
- 13.Huegli D, Hu C, Humair P-F, Wilske B, Gern L. Apodemus species mice are reservoir hosts of Borrelia garinii OspA serotype 4 in Switzerland. J Clin Microbiol. 2002;40(12):4735–4737. doi: 10.1128/JCM.40.12.4735-4737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu C, Wilske B, Fingerle V, Lobet Y, Gern L. Transmission of Borrelia garinii OspA serotype 4 to BALB/c mice by Ixodes ricinus ticks collected in the field. J Clin Microbiol. 2001;39(3):1169–71. [DOI] [PMC free article] [PubMed]
- 15.Skuballa J, Petney T, Pfäffle M, Oehme R, Hartelt K, Fingerle V, et al. Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis. 2012;3(1):8–13. doi: 10.1016/j.ttbdis.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Richter D, Schlee DB, Allgöwer R, Matuschka F-R. Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in Central Europe. Appl Environ Microbiol. 2004;70(11):6414–6419. doi: 10.1128/AEM.70.11.6414-6419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skuballa J, Oehme R, Hartelt K, Petney T, Bücher T, Kimmig P, Taraschewski H. European hedgehogs as hosts for Borrelia spp., Germany. Emerg Infect Dis. 2007;13(6):952–953. doi: 10.3201/eid1306.070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. Plos Negl Trop Dis. 2016;10(10):e0005042. [DOI] [PMC free article] [PubMed]
- 19.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wennerås C. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol. 2010;48(5):1956–1959. doi: 10.1128/JCM.02423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382(9892):658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31(6):260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Silaghi C, Beck R, Oteo JA, Pfeffer M, Sprong H. Neoehrlichiosis. an emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp Appl Acarol. 2016;68(3):279–297. doi: 10.1007/s10493-015-9935-y. [DOI] [PubMed] [Google Scholar]
- 23.Gern L, Rouvinez E, Toutoungi LN, Godfroid E. Transmission cycles of Borrelia burgdorferi sensu lato involving Ixodes ricinus and/or I. hexagonus ticks and the European hedgehog, Erinaceus europaeus, in suburban and urban areas in Switzerland. Folia Parasitol. 1997;44(4):309–314. [PubMed] [Google Scholar]
- 24.Ogden N, Cripps P, Davison C, Owen G, Parry J, Timms B, Forbes A. The ixodid tick species attaching to domestic dogs and cats in Great Britain and Ireland. Med Vet Entomol. 2000;14(3):332–338. doi: 10.1046/j.1365-2915.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 25.Wormser GP, Wormser V. Did Garin and Bujadoux actually report a case of Lyme radiculoneuritis? Open Forum Infect Dis. 2016;3:2. Oxford University Press. [DOI] [PMC free article] [PubMed]
- 26.Gern L, Toutoungi LN, Hu CM, Aeschlimann A. Ixodes (Pholeoixodes) hexagonus, an efficient vector of Borrelia burgdorferi in the laboratory. Med Vet Entomol. 1991;5(4):431–435. doi: 10.1111/j.1365-2915.1991.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 27.Toutoungi L, Gern L. Ability of transovarially and subsequent transstadially infected Ixodes hexagonus ticks to maintain and transmit Borrelia burgdorferi in the laboratory. Exp Appl Acarol. 1993;17(8):581–586. doi: 10.1007/BF00053487. [DOI] [PubMed] [Google Scholar]
- 28.Labuda M, Randolph SE. Survival strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zent Bakt. 1999;289(5):513–524. doi: 10.1016/S0934-8840(99)80005-X. [DOI] [PubMed] [Google Scholar]
- 29.Reeve N. Hedgehogs. London: T & AD Poyser; 1994. [Google Scholar]
- 30.Young R, Davison J, Trewby I, Wilson G, Delahay R, Doncaster C. Abundance of hedgehogs (Erinaceus europaeus) in relation to the density and distribution of badgers (Meles meles) J Zool. 2006;269(3):349–356. doi: 10.1111/j.1469-7998.2006.00078.x. [DOI] [Google Scholar]
- 31.Huijser M. Human impact oil populations of hedgehogs Erinaceus europaeus through traffic and changes in the landscape. Lutra. 1999;42:39–56. [Google Scholar]
- 32.Hubert P, Julliard R, Biagianti S, Poulle ML. Ecological factors driving the higher hedgehog (Erinaceus europeaus) density in an urban area compared to the adjacent rural area. Landsc Urban Plan. 2011;103(1):34–43. doi: 10.1016/j.landurbplan.2011.05.010. [DOI] [Google Scholar]
- 33.Pfäffle M, Petney T, Skuballa J, Taraschewski H. Comparative population dynamics of a generalist (Ixodes ricinus) and specialist tick (I. hexagonus) species from European hedgehogs. Exp Appl Acarol. 2011;54(2):151–164. doi: 10.1007/s10493-011-9432-x. [DOI] [PubMed] [Google Scholar]
- 34.Silaghi C, Skuballa J, Thiel C, Pfister K, Petney T, Pfäffle M, et al. The European hedgehog (Erinaceus europaeus) - a suitable reservoir for variants of Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2012;3(1):49–54. [DOI] [PubMed]
- 35.Skuballa J, Petney T, Pfäffle M, Taraschewski H. Molecular detection of Anaplasma phagocytophilum in the European hedgehog (Erinaceus europaeus) and its ticks. Vector Borne Zoonotic Dis. 2010;10(10):1055–1057. doi: 10.1089/vbz.2009.0150. [DOI] [PubMed] [Google Scholar]
- 36.Speck S, Perseke L, Petney T, Skuballa J, Pfäffle M, Taraschewski H, et al. Detection of Rickettsia helvetica in ticks collected from European hedgehogs (Erinaceus europaeus, Linnaeus, 1758) Ticks Tick Borne Dis. 2013;4(3):222–226. doi: 10.1016/j.ttbdis.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Krawczyk AI, van Leeuwen AD, Jacobs-Reitsma W, Wijnands LM, Bouw E, Jahfari S, et al. Presence of zoonotic agents in engorged ticks and hedgehog faeces from Erinaceus europaeus in (sub) urban areas. Parasit Vectors. 2015;8(1):210. doi: 10.1186/s13071-015-0814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur DR. British ticks. London: Butterworths; 1963. p. 213.
- 39.Heylen D, Fonville M, Leeuwen AD, Sprong H. Co‐infections and transmission dynamics in a tick‐borne bacterium community exposed to songbirds. Environ Microbiol. 2016;18(3):988–996. doi: 10.1111/1462-2920.13164. [DOI] [PubMed] [Google Scholar]
- 40.Heylen D, Adriaensen F, Van Dongen S, Sprong H, Matthysen E. Ecological factors that determine Ixodes ricinus tick burdens in the great tit (Parus major), an avian reservoir of Borrelia burgdorferi s.l. Int J Parasitol. 2013;43(8):603–611. doi: 10.1016/j.ijpara.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Jahfari S, Fonville M, Hengeveld P, Reusken C, Scholte EJ, Takken W, et al. Prevalence of Neoehrlichia mikurensis in ticks and rodents from north-west Europe. Parasit Vectors. 2012;5:74. [DOI] [PMC free article] [PubMed]
- 42.Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group rickettsiae. Am J Trop Med Hyg. 2005;73(6):1083–1085. [PubMed] [Google Scholar]
- 43.Coipan EC, Fonville M, Tijsse-Klasen E, van der Giessen JW, Takken W, Sprong H, Takumi K. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S–23S rDNA spacer region. Infect Genet Evol. 2013;17:216–222. doi: 10.1016/j.meegid.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, et al. Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vectors. 2014;7:365. doi: 10.1186/1756-3305-7-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Team. RC . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 46.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag; 2009.
- 47.van Duijvendijk G, Gort G, Takken W. Rodents as hosts for Ixodes ricinus and Borrelia afzelii. In: Braks MAH, van Wieren SE, Takken W, Sprong H, editors. Ecology and prevention of Lyme borreliosis. Wageningen Academic Publishers; 2016: 268–276.
- 48.Socolovschi C, Mediannikov O, Raoult D, Parola P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet Res. 2009;40(2):1–20. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burri C, Schumann O, Schumann C, Gern L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum? Ticks Tick Borne Dis. 2014;5(3):245–251. doi: 10.1016/j.ttbdis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Wagemakers A, Jahfari S, de Wever B, Spanjaard L, Starink MV, de Vries HJ, Sprong H, Hovius JW. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick Borne Dis. 2017;8(3):370–74. doi:10.1016/j.ttbdis.2016.12.012. [DOI] [PubMed]
- 51.Voordouw MJ. Co-feeding transmission in Lyme disease pathogens. Parasitology. 2015;142(02):290–302. doi: 10.1017/S0031182014001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randolph SE. Transmission of tick-borne pathogens between co-feeding ticks: Milan Labuda’s enduring paradigm. Ticks Tick Borne Dis. 2011;2(4):179–182. doi: 10.1016/j.ttbdis.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Koetsveld J, Tijsse-Klasen E, Herremans T, Hovius JW, Sprong H. Serological and molecular evidence for spotted fever group Rickettsia and Borrelia burgdorferi sensu lato co-infections in The Netherlands. Ticks Tick Borne Dis. 2016;7(2):371–7. [DOI] [PubMed]
- 54.Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19(4):708–727. doi: 10.1128/CMR.00011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulder S, van Vliet AJ, Bron WA, Gassner F, Takken W. High risk of tick bites in dutch gardens. Vector Borne Zoonotic Dis. 2013;13(12):865–871. doi: 10.1089/vbz.2012.1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.


