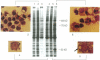Abstract
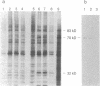
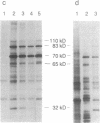
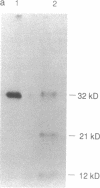
Exposure of cells to elevated temperatures and other environmental stresses results in the expression of specific genes encoding the so-called heat shock proteins (HSPs). Since exogenous H2O2 induces in human monocytes the synthesis of HSPs, and previous induction of HSPs protects these cells from oxidative injury, we investigated whether HSP synthesis was also induced during generation of reactive oxygen species by the phagocyte itself during phagocytosis. As a model system, we analyzed the effects of erythrophagocytosis on protein synthesis by the human premonocytic line U937, in which phagocytosis is induced during differentiation with 1,25-dihydroxyvitamin D3. Exposure to whole erythrocytes, but not to erythrocyte ghosts, induced in the phagocytic cells only the synthesis of the 70- and 83- to 90-kDa HSPs and a 32-kDa oxidation-related stress protein identical by partial peptide mapping to heme oxygenase. The radioprotective aminothiol N-(2'-mercaptoethyl)-1,3-propanediamine (WR-1065), which can substitute for glutathione as hydrogen donor, prevented this induction. These results suggest that oxygen free radicals generated in the presence of hemoglobin-derived iron and consecutive glutathione depletion are involved in induction of stress protein synthesis during erythrophagocytosis. HSPs synthesized during phagocytosis may play a role in the phagocyte's defense mechanisms and in protective immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Mechanisms of heat-shock gene activation in higher eukaryotes. Adv Genet. 1987;24:31–72. doi: 10.1016/s0065-2660(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Butler J. D., Gahl W. A., Tietze F. Cystine depletion by WR-1065 in cystinotic cells. Mechanism of action. Biochem Pharmacol. 1985 Jun 15;34(12):2179–2185. doi: 10.1016/0006-2952(85)90415-0. [DOI] [PubMed] [Google Scholar]
- Caltabiano M. M., Poste G., Greig R. G. Isolation and immunological characterization of the mammalian 32-/34-kDa stress protein. Exp Cell Res. 1988 Sep;178(1):31–40. doi: 10.1016/0014-4827(88)90375-8. [DOI] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin Exp Immunol. 1982 Mar;47(3):635–644. [PMC free article] [PubMed] [Google Scholar]
- Davies K. J., Goldberg A. L. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J Biol Chem. 1987 Jun 15;262(17):8220–8226. [PubMed] [Google Scholar]
- Dayer J. M., Sundström L., Polla B. S., Junod A. F. Cultured human alveolar macrophages from smokers with lung cancer: resolution of factors that stimulate fibroblast proliferation, production of collagenase, or prostaglandin E2. J Leukoc Biol. 1985 May;37(5):641–649. doi: 10.1002/jlb.37.5.641. [DOI] [PubMed] [Google Scholar]
- Dewald B., Thelen M., Baggiolini M. Two transduction sequences are necessary for neutrophil activation by receptor agonists. J Biol Chem. 1988 Nov 5;263(31):16179–16184. [PubMed] [Google Scholar]
- Durand R. E. Radioprotection by WR-2721 in vitro at low oxygen tensions: implications for its mechanisms of action. Br J Cancer. 1983 Mar;47(3):387–392. doi: 10.1038/bjc.1983.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa C., Woo C. H., Fudenberg H. H., Schmid R. Erythrocyte catabolism by macrophages in vitro. The effect of hydrocortisone on erythrophagocytosis and on the induction of heme oxygenase. J Clin Invest. 1973 Apr;52(4):812–822. doi: 10.1172/JCI107245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. E., Ralph P., Litcofsky P., Moore M. A. Distinct activities of interferon-gamma, lymphokine and cytokine differentiation-inducing factors acting on the human monoblastic leukemia cell line U937. Cancer Res. 1985 Jan;45(1):9–13. [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Both near ultraviolet radiation and the oxidizing agent hydrogen peroxide induce a 32-kDa stress protein in normal human skin fibroblasts. J Biol Chem. 1987 Oct 25;262(30):14821–14825. [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Maridonneau-Parini I., Clerc J., Polla B. S. Heat shock inhibits NADPH oxidase in human neutrophils. Biochem Biophys Res Commun. 1988 Jul 15;154(1):179–186. doi: 10.1016/0006-291x(88)90667-5. [DOI] [PubMed] [Google Scholar]
- Montesano R., Mossaz A., Vassalli P., Orci L. Specialization of the macrophage plasma membrane at sites of interaction with opsonized erythrocytes. J Cell Biol. 1983 May;96(5):1227–1233. doi: 10.1083/jcb.96.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Bonventre J. V., Krane S. M. 1,25-Dihydroxyvitamin D3 increases the toxicity of hydrogen peroxide in the human monocytic line U937: the role of calcium and heat shock. J Cell Biol. 1988 Jul;107(1):373–380. doi: 10.1083/jcb.107.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla B. S., Healy A. M., Wojno W. C., Krane S. M. Hormone 1 alpha,25-dihydroxyvitamin D3 modulates heat shock response in monocytes. Am J Physiol. 1987 Jun;252(6 Pt 1):C640–C649. doi: 10.1152/ajpcell.1987.252.6.C640. [DOI] [PubMed] [Google Scholar]
- Polla B. S., Werlen G., Clerget M., Pittet D., Rossier M. F., Capponi A. M. 1,25-dihydroxyvitamin D3 induces responsiveness to the chemotactic peptide f-Met-Leu-Phe in the human monocytic line U937: dissociation between calcium and oxidative metabolic responses. J Leukoc Biol. 1989 May;45(5):381–388. doi: 10.1002/jlb.45.5.381. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Yoshida T., Kikuchi G. Induction of heme oxygenase by hemin in cultured pig alveolar macrophages. Arch Biochem Biophys. 1978 Jun;188(2):243–250. doi: 10.1016/s0003-9861(78)80006-x. [DOI] [PubMed] [Google Scholar]
- Snyder L. M., Fortier N. L., Trainor J., Jacobs J., Leb L., Lubin B., Chiu D., Shohet S., Mohandas N. Effect of hydrogen peroxide exposure on normal human erythrocyte deformability, morphology, surface characteristics, and spectrin-hemoglobin cross-linking. J Clin Invest. 1985 Nov;76(5):1971–1977. doi: 10.1172/JCI112196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Suhan J. P. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986 Nov;103(5):2035–2052. doi: 10.1083/jcb.103.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Johnston R. B., Jr Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J Exp Med. 1984 Feb 1;159(2):405–416. doi: 10.1084/jem.159.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]