Abstract
Recent years have witnessed growing interest in the role of peptides in animal nutrition. Chemical, enzymatic, or microbial hydrolysis of proteins in animal by-products or plant-source feedstuffs before feeding is an attractive means of generating high-quality small or large peptides that have both nutritional and physiological or regulatory functions in livestock, poultry and fish. These peptides may also be formed from ingested proteins in the gastrointestinal tract, but the types of resultant peptides can vary greatly with the physiological conditions of the animals and the composition of the diets. In the small intestine, large peptides are hydrolyzed to small peptides, which are absorbed into enterocytes faster than free amino acids (AAs) to provide a more balanced pattern of AAs in the blood circulation. Some peptides of plant or animal sources also have antimicrobial, antioxidant, antihypertensive, and immunomodulatory activities. Those peptides which confer biological functions beyond their nutritional value are called bioactive peptides. They are usually 2–20 AA residues in length but may consist of >20 AA residues. Inclusion of some (e.g. 2–8%) animal-protein hydrolysates (e.g., porcine intestine, porcine mucosa, salmon viscera, or poultry tissue hydrolysates) or soybean protein hydrolysates in practical corn- and soybean meal-based diets can ensure desirable rates of growth performance and feed efficiency in weanling pigs, young calves, post-hatching poultry, and fish. Thus, protein hydrolysates hold promise in optimizing the nutrition of domestic and companion animals, as well as their health (particularly gut health) and well-being.
Keywords: Animals, Nutrition, Peptides, Protein hydrolysates, Sustainability
Background
A protein is a macromolecule usually consisting of twenty different amino acids (AAs) linked via peptide bonds. Selenoproteins contain selenocysteine as a rare AA, but no free selenocysteine is present in animal cells. Protein is a major component of animal tissues (e.g., skeletal muscle, mammary glands, liver, and the small intestine) and products (e.g., meat, milk, egg, and wool). For example, the protein content in the skeletal muscle of growing beef cattle or pigs is approximately 70% on a dry-matter basis [1]. Thus, adequate intake of dietary protein is essential for maximum growth, production performance, and feed efficiency in livestock, poultry and fish. After being consumed in a meal by animals, the proteins in feed ingredients (e.g., blood meal, meat & bone meal, intestine-mucosa powder, fish meal, soybean meal, peanut meal, and cottonseed meal) are hydrolyzed into small peptides (di- and tri-peptides) and free AAs by proteases and oligopeptidases in the small intestine [2]; however, the types of resultant peptides can vary greatly with the physiological conditions of the animals and the composition of their diets. To consistently manufacture peptides from the proteins of animal and plant sources, robust chemical, enzymatic or microbial methods have been used before feeding to improve their nutritional quality and reduce any associated anti-nutritional factors [3, 4]. The last two methods can also improve the solubility, viscosity, emulsification, and gelation of peptides.
In animal production, high-quality protein is not hydrolyzed as feed additives. Only animal byproducts, brewer’s byproducts, and plant ingredients containing anti-nutritional factors are hydrolyzed to produce peptides for animal feeds. Proteases isolated from various sources (including bacteria, plants, and yeast) are used for the enzymatic method, whereas intact microorganisms are employed for culture in the microbial approach. To date, protein hydrolysates have been applied to such diverse fields as medicine, nutrition (including animal nutrition), and biotechnology [5]. The major objectives of this article are to highlight enzyme- and fermentation-based techniques for the industrial preparation of protein hydrolysates and to discuss the nutritional and functional significance of their bioactive peptides in animal feeding.
Definitions of amino acids, peptides, and protein
Amino acids are organic substances that contain both amino and acid groups. All proteinogenic AAs have an α-amino group and, except for glycine, occur as L-isomers in animals and feedstuffs. A peptide is defined as an organic molecule consisting of two or more AA residues linked by peptide bonds [2]. The formation of one peptide bond results in the removal of one water molecule. In most peptides, the typical peptide bonds are formed from the α-amino and α-carboxyl groups of adjacent AAs. Peptides can be classified according to the number of AA residues. An oligopeptide is comprised of 2 to 20 AA residues. Those oligopeptides containing ≤ 10 AA residues are called small oligopeptides (or small peptides), whereas those oligopeptides containing 10 to 20 AA residues are called large oligopeptides (or large peptides). A peptide, which contains ≥ 21 AA residues and does not have a 3-dimensional structure, is termed a polypeptide [6]. A protein consists of one or more high-molecular-weight polypeptides.
The dividing line between proteins and polypeptides is usually their molecular weight. Generally speaking, polypeptides with a molecular weight of ≥ 8,000 Daltons (i.e., ≥ 72 AA residues) are referred to as proteins [6]. For example, ubiquitin (a single chain of 72 AA residues) and casein α-S1 (200 AA residues) are proteins, but glucagon (29 AA residues) and oxytocin (9 AA residues) are peptides. However, the division between proteins and peptides simply on the basis of their molecular weights is not absolute. For example, insulin [51 AA residues (20 in chain A and 31 in chain B)] is well recognized as a protein because it has the defined 3-dimensional structure exhibited by proteins. In contrast, PEC-60 (a single chain of 60 AA residues) [7] and dopuin (a single chain of 62 AA residues) [8], which are isolated from the pig small-intestinal mucosae, are called polypeptides. Figure 1 illustrates the four orders of protein structures (1): primary structure (the sequence of AAs along the polypeptide chain; (2) secondary structure (the conformation of the polypeptide backbone); (3) tertiary structure (the three-dimentional arrangement of protein); and (4) quaternary structure (the spatial arrangement of polypeptide subunits). The primary sequence of AAs in a protein determines its secondary, tertiary, and quaternary structures, as well as its biological functions. The forces stabilizing polypeptide aggregates are hydrogen and electrostatic bonds between AA residues.
Fig. 1.
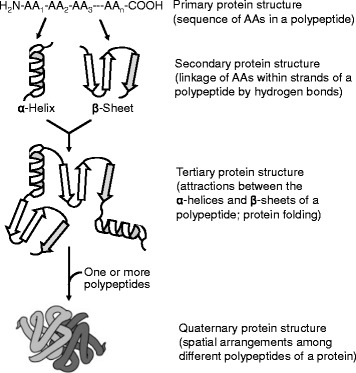
The four orders of protein structures. A protein has (1): a primary structure (the sequence of AAs along the polypeptide chain; (2) a secondary structure (the conformation of the polypeptide backbone); (3) a tertiary structure (the three-dimensional arrangement of protein); and (4) a quaternary structure (the spatial arrangement of polypeptide subunits). The primary sequence of AAs in a protein determines its secondary, tertiary, and quaternary structures, as well as its biological functions
Trichloroacetic acid (TCA; the final concentration of 5%) or perchloric acid (PCA; the final concentration of 0.2 mol/L) can fully precipitate proteins, but not peptides, from animal tissues, cells, plasma, and other physiological fluids (e.g., rumen, allantoic, amniotic, intestinal-lumen fluids, and digesta) [9, 10]. Ethanol (the final concentration of 80%) can effectively precipitate both proteins and nucleic acids from aqueous solutions [11]. This method may be useful to remove water-soluble inorganic compounds (e.g., aluminum) from protein hydrolysates. Of note, 1% tungstic acid can precipitate both proteins and peptides with ≥ 4 AA residues [10]. Thus, PCA or TCA can be used along with tungstic acid to distinguish small and large peptides.
Industrial production of protein hydrolysates
General considerations of protein hydrolysis
The method of choice for the hydrolysis of proteins depends on their sources. For example, proteins from feathers, bristles, horns, beaks or wool contain the keratin structure and, therefore, are usually hydrolyzed by acidic or alkaline treatment, or by bacterial keratinases [3]. In contrast, animal products (e.g., casein, whey, intestine, and meat) and plant ingredients (e.g., soy, wheat, rice, pea, and cottonseed proteins) are often subject to general enzymatic or microbial hydrolysis [4, 5]. The hydrolysis of proteins by cell-free proteases, microorganisms, acids, or bases results in the production of protein hydrolysates. The general procedures are outlined in Fig. 2. Depending on the method used, the hydrolysis times may range from 4 to 48 h. In cases where bacteriostatic or bactericidal preservatives (e.g., benzoic acid) are used in the prolonged hydrolysis of proteins by enzymes or microorganisms, the hydrolysis is usually terminated by heating to deactivate the enzyme or enzyme systems. After hydrolysis, the insoluble fractions are separated from the protein hydrolysates with the use of a centrifuge, a filter (e.g., with a 10,000 Dalton molecular weight cut-off), or a micro filtration system [3]. The filtration process is often repeated several times to obtain a desirable color and clarity of the solution. Charcoal powder is commonly used to decolorize and remove haze-forming components. If very low concentrations of salts are desired, the filtrate may be subjected to exchange chromatography to remove the salts. After filtration, the protein hydrolysate product is heat-treated (pasteurized) to kill or reduce the microorganisms. Finally, the product is dried and packaged.
Fig. 2.
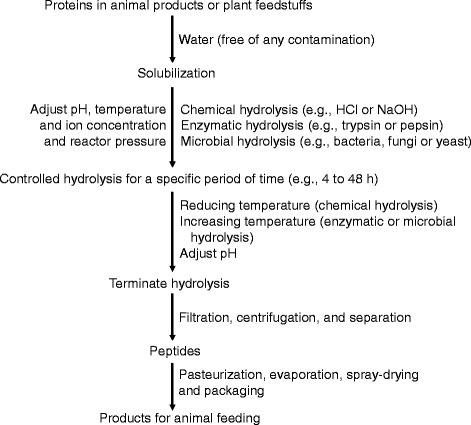
General procedures for the production of peptides from animal and plant proteins. Peptides (including bioactive peptides) can be produced from proteins present in animal products (including by-products) or plant-source feedstuffs material (e.g., soybeans and wheat) through chemical, enzymatic, or microbial hydrolysis. These general procedures may need to be modified for peptide production, depending on protein sources and product specifications
Degree of hydrolysis
The protein hydrolysates include free AAs, small peptides, and large peptides. The proportions of these products vary with the sources of proteins, the quality of water, the type of proteases, and the species of microbes. The degree of hydrolysis, i.e., the extent to which the protein is hydrolyzed, is measured by the number of peptide bonds cleaved, divided by the total number of peptide bonds in a protein and multiplied by 100 [3]. The number of peptide bonds cleaved is measured by the moles of free AAs plus the moles of TCA- or PCA-soluble peptides. Due to the lack of standards for all the peptides generated from protein hydrolysis, it is technically challenging to quantify peptides released from animal-, plant- or microbial-sources of proteins. The percentage of AAs in the free form or the peptide form is calculated as follows:
When the catabolism of AAs is limited (as in enzymatic hydrolysis), the percentage of AAs in peptides is calculated as (total AAs in protein – free AAs)/total AAs in protein x 100%. High-performance liquid chromatography (HPLC) is widely used to determine free AAs [12]. HPLC and other analytical techniques (e.g., nuclear magnetic resonance spectroscopy, matrix assisted laser desorption ionization-time of flight mass spectrometry, peptide mapping, and ion-exchange chromatography) are often employed to characterize peptides in protein hydrolysates [13, 14]. When standards are available, HPLC can be used to analyze peptides.
Methods for protein hydrolysis
Acid hydrolysis of proteins
Acid hydrolysis of a protein (gelatin) at a high temperature was first reported by the French chemist H. Braconnot in 1920. It is now established that the complete hydrolysis of protein in 6 mol/L HCl occurs at 110 °C for 24 h [12]. A much shorter period of time (e.g., 2 to 6 h) is used to produce peptides [3]. After the hydrolysis, the product is evaporated, pasteurized, and spray dried. The majority of acid protein hydrolysates are used as flavor enhancers (e.g., flavoring products such as hydrolyzed vegetable protein) [5]. The method of acid hydrolysis of a protein offers the advantage of low cost. However, this process results in the complete destruction of tryptophan, a partial loss of methionine, and the conversion of glutamine into glutamate and of asparagine into aspartate [5].
Alkaline hydrolysis of proteins
Alkaline agents, such as calcium, sodium, or potassium hydroxide (e.g., 4 mol/L), can be used at a high temperature (e.g., 105 °C) for 20 h to completely hydrolyze protein [12, 15]. Lower temperatures (e.g., 27 to 55 °C) and a shorter period of the hydrolysis time (e.g., 4 to 8 h) are often desirable for the generation of peptides in the food industry [5]. After the hydrolysis, the product is evaporated, pasteurized, and spray dried. Like acid hydrolysis of proteins, alkaline hydrolysis of proteins offers the advantage of low cost and can have a 100% recovery rate of tryptophan [12]. However, this process results in the complete destruction of most AAs (e.g., 100% loss). Thus, although alkaline hydrolysis is often used for the production of foaming agents (e.g., substitutes for egg proteins) and fire extinguisher foams, it is not widely used in the food industry.
Cell-free proteases
The peptide bonds of proteins can be broken down by many different kinds of proteases, which can be classified as exopeptidases and endopeptidases based on the type of reaction, namely hydrolysis of a peptide bond in the terminal region (an exopeptidase) or within an internal region (an endopeptidase) of a protein [2]. Some proteases hydrolyze dipeptides (dipeptidases), whereas others remove terminal AA residues that are substituted, cyclized, or linked by isopeptide bonds (namely peptide linkages other than those of α-carboxyl to α-amino groups; e.g., ω-peptidases). When a protease exhibits a marked preference for a peptide bond formed from a particular AA residue, the name of this AA is used to form a qualifier (e.g., “leucine” aminopeptidase and “proline” endopeptidase). In contrast, for enzymes with very complex or broad specificity, alphabetical or numerical serial names (e.g., peptidyl-dipeptidase A, peptidyl-dipeptidase B, dipeptidyl-peptidase I, and dipeptidyl-peptidase II) are employed for protein hydrolysis. Some proteases may have both exopeptidase and endopeptidase properties (e.g., cathepsins B and H). Enzymatic hydrolysis takes place under mild conditions (e.g., pH 6–8 and 30 - 60 °C) and minimizes side reactions.
Most of the cell-free enzymes for producing protein hydrolysates are obtained from animal, plant and microbial sources (Table 1). Enzymes of animal sources (particularly pigs) for protein hydrolysis are pancreatin, trypsin, pepsin, carboxylpeptidases and aminopeptidases; enzymes of plant sources are papain and bromelain; and enzymes of bacterial and fungal sources are many kinds of proteases with a broad spectrum of optimal temperatures, pH, and ion concentrations [16, 17]. The enzymes from commercial sources may be purified, semi-purified, or crude from the biological sources. The hydrolysis of proteins can be achieved by a single enzyme (e.g., trypsin) or multiple enzymes (e.g., a mixture of proteases known as Pronase, pepsin and prolidase). The choice of enzymes depends on the protein source and the degree of hydrolysis. For example, if the protein has a high content of hydrophobic AAs, the enzyme of choice would be the one that preferentially breaks downs the peptide bonds formed from these AAs. Fractionation of protein hydrolysates is often performed to isolate specific peptides or remove undesired peptides. It is noteworthy that the hydrolysis of some proteins (e.g., soy proteins and casein with papain for 18 h) can generate hydrophobic peptides and AAs with bitterness [18]. The addition of porcine kidney cortex homogenate or activated carbon to protein hydrolysates can reduce the bitterness of the peptide product [3]. Compared to acid and alkaline hydrolysis of proteins, the main advantages of enzyme hydrolysis of proteins are that: (a) the hydrolysis conditions (e.g., like temperature and pH) are mild and do not result in any loss of AAs; (b) proteases are more specific and precise to control the degree of peptide-bond hydrolysis; and (c) the small amounts of enzymes can be easily deactivated after the hydrolysis (e.g., 85 °C for 3 min) to facilitate the isolation of the protein hydrolysates. The disadvantages of enzymatic hydrolysis of protein include the relatively high cost and the potential presence of enzyme inhibitors in the raw protein materials.
Table 1.
Proteases commonly used for protein hydrolysis
| Class of enzyme | Name of enzyme | EC number | Specific cleavage |
|---|---|---|---|
| Endopeptidases | |||
| Aspartate protease | Chymosin (rennin; pH 1.8–2) | 3.4.23.4 | the Phe-Met bond, clotting of milk |
| Pepsin A (pH 1.8–2) | 3.4.23.1 | Aromatic AAs, hydrophobic AAs | |
| Cysteine protease | Bromelain (from pieapples) | 3.4.22.4 | Ala, Gly, Lys, Phe, Tyr |
| Cathepsin B | 3.4.22.1 | Arg, Lys, Phe | |
| Ficain (ficin; from fig tree) | 3.4.22.3 | Ala, Asn, Gly, Leu, Lys, Tyr, Val | |
| Papain (from papaya) | 3.4.22.2 | Arg, Lys, Phe | |
| Metallo protease | Bacillolysin (Bacillus bacteria) | 3.4.24.28 | Aromatic AAs, Ile, Leu, Val |
| Thermolysin (Bacillus bacteria) | 3.4.24.27 | Aromatic AAs, Ile, Leu, Val | |
| Serine protease | Chymotrypsin (pH 8–9) | 3.4.21.1 | Aromatic AAs, Leu |
| Subtilisin (from Bacillus bacteria) | 3.4.21.14 | Mainly hydrophobic AAs | |
| Trypsin (pH 8–9) | 3.4.21.4 | Arg, Lys | |
| Exopeptidases | |||
| Aminopeptidases | Aminopeptidasea | 3.4.11.1 | AA at the N-terminus of protein/peptide |
| Aminopeptidase Yb | 3.4.11.15 | Lys at the N-terminus of protein/peptide | |
| Carboxypeptidase | Carboxypeptidasec | 3.4.16.1 | Acidic, neutral, and basic AAs |
| Glycine carboxypeptidased | 3.4.17.4 | Gly at the C-terminus of protein/peptide | |
| Alanine carboxypeptidasee | 3.4.17.8 | D-Ala at the C-terminus of peptide | |
| Carboxypeptidase Sf | 3.4.17.9 | Gly at the C-terminus of protein/peptide | |
| Dipeptidase | Dipeptidase 1f | 3.4.13.11 | A wide range of dipeptides |
| Proline dipeptidase (prolidase)a | 3.4.13.9 | AA-Pro or -hydroxyproline at the C-terminus (not Pro-Pro) | |
| Prolyl dipeptidasea | 3.3.13.8 | Pro-AA or Hydroxyproline-AA | |
| Endo- and exo-peptidases | |||
| Pronase | A mixture of proteasesa (from Streptomyces griseus) | 3.4.24 | Acidic, neutral, and basic AAs |
| Other peptidases | Dipeptidyl-peptide IIIf | 3.4.14.4 | Release of an N-terminal dipeptide from a peptide comprising four or more AA residues, with broad specificity |
| Dipeptidyl-peptidase IVg | 3.4.14.5 | Release of an N-terminal dipeptide from a peptide consisting of prolineh | |
Adapted from Kunst [16] and Dixon and Webb [17]. AA amino acid
aMetallopeptidase (requiring Mn2+, Mg2+ or Zn2+ for activation)
bMetallopeptidase (requiring Co2+ for activation; inhibited by Zn2+ and Mn2+)
cSerine carboxypeptidase
dStrongly inhibited by Ag+ and Cu2+
eMetallopeptidase (requiring Mn2+, Mg2+, Zn2+, Ca2+ or Co2+ for activation)
fMetallopeptidase (requiring Zn2+ for activation)
gSerie protease
hAA1-Pro-AA2, where AA2 is neither proline nor hydroxyproline
The efficiency and specificity of protein hydrolysis differ between microbial- and animal-source proteases [19], as reported for their lipase activity [20, 21]. For example, the hydrolysis of 18 mg casein by 40 μg pancreatin (pancreatic enzymes from the porcine pancreas) for 1–2 h in a buffer solution (43 mmol/L NaCl, 7.3 mmol/L disodium tetraborate, 171 mmol/L boric acid and 1 mmol/L CaCl2, pH 7.4) yields the numbers and sequences of peptides differently than NS 4 proteases (from Nocardiopsis prasina) and NS 5 proteases (from Bacillus subtilis) [19]. The pancreatin exhibited the activities of trypsin (cleavage of peptide bonds from Arg and Lys sites), chymotrypsin (cleavage of peptide bonds from Phe, Trp, Tyr, and Leu), and elastase (cleavage of peptide bonds from (Ala and other aliphatic AAs). In contrast, the microbial proteases were characterized by relatively low trypsin, carboxypeptidase and elastase activities, but high chymotrypsin activity. The time course of hydrolysis is similar among the microbial and pancreatic enzymes when caseins are the substrates. However, the rates of peptide generation are higher for pancreatin than the microbial enzymes when soya protein is the substrate. The efficiency of production of peptides with a molecular weight less than 3 kDa is higher for pancreatin than the microbial enzymes when caseins are substrates, but is similar among the microbial and pancreatic enzymes when soya protein is the substrate. In contrast, the efficiency of production of peptides with a molecular weight between 3 and 10 kDa is similar among the microbial and pancreatic enzymes when caseins are substrates or when soya protein is the substrate within 1 h incubation, but is higher for the microbial enzymes than the animal-source pancreatin.
Microbial hydrolysis of protein
Microorganisms release proteases to hydrolyze extracellular proteins into large peptides, small peptides and free AAs. Small peptides can be taken up by the microbes to undergo intracellular hydrolysis, yielding free AAs. Microorganisms also produce enzymes other than proteases to degrade complex carbohydrates and lipids [22]. Protein fermentation is classified into a liquid- or solid-state type. Liquid-state fermentation is performed with protein substrates under high-moisture fermentation conditions, whereas the solid-state fermentation is carried out under low-moisture fermentation conditions. The low moisture level of the solid-state fermentation can help to reduce the drying time for protein hydrolysates.
Soy sauce (also called soya sauce), which originated in China in the 2nd century AD, was perhaps the earliest product of protein fermentation by microorganisms [3]. The raw materials were boiled soybeans, roasted grain, brine, and Aspergillus oryzae or Aspergillus sojae (a genus of fungus). In Koji culturing, an equal amount of boiled soybeans and roasted wheat is cultured with Aspergillus oryzae, A. sojae, and A. tamari; Saccharomyces cerevisiae (yeasts), and bacteria, such as Bacillus and Lactobacillus species. Over the past two decades, various microorganisms have been used to hydrolyze plant-source proteins, such as Lactobacillus rhamnosus BGT10 and Lactobacillus zeae LMG17315 for pea proteins, Bacillus natto or B. subtilis for soybean, and fungi A. oryzae or R. oryzae for soybean [23–25]. Lactic acid bacteria, such as Lactobacillus and Lactococcus species, are commonly used to ferment milk products. The major advantages of fermentation are that the appropriately used microorganisms can not only break down proteins into peptides and free AAs, but can also remove hyper-allergic or anti-nutritional factors present in the matrix of the ingredients (e.g., trypsin inhibitors, glycinin, β-conglycinin, phytate, oligosaccharides raffinose and stachyose, saponins in soybeans). The disadvantages of the microbial hydrolysis of protein are relatively high costs, as well as changes in microbial activity under various conditions and, therefore, inconsistency in the production of peptides and free AAs.
Bioactive peptides in protein hydrolysates
Definition
Bioactive peptides are defined as the fragments of AA sequences in a protein that confer biological functions beyond their nutritional value [25]. They have antimicrobial, antioxidant, antihypertensive, and immunomodulatory activities. These bioactive peptides are usually 2–20 AA residues in length, but some may consist of >20 AA residues [23]. Many of them exhibit common structural properties, such as a relatively small number of AAs, a high abundance of hydrophobic AA residues, and the presence of Arg, Lys, and Pro residues [24]. In animals, endogenous peptides fulfil crucial physiological or regulatory functions. For example, PEC-60 activates Na/K ATPase in the small intestine and other tissues [26]. Additionally, many intestinal peptides (secreted by Paneth cells) have an anti-microbial function [27]. Furthermore, the brain releases numerous peptides to regulate endocrine status, food intake, and behavior in animals [28].
Transport of small peptides in the small intestine
In the small intestine, peptide transporter 1 (PepT1) is responsible for the proton-driven transport of extracellular di- and tri-peptides through the apical membrane of the enterocyte into the cell [29]. However, due to the high activity of intracellular peptidases in the small intestine [2], it is unlikely that a nutritionally significant quantity of peptides in the lumen of the gut can enter the portal vein or the lymphatic circulation. It is possible that a limited, but physiologically significant, amount of peptides (particularly those containing an imino acid) may be absorbed intact from the luminal content to the bloodstream through M cells, exosomes, and enterocytes via transepithelial cell transport [30, 31]. Diet-derived peptides can exert their bioactive (e.g., physiological and regulatory) actions at the level of the small intestine, and the intestinally-generated signals can be transmitted to the brain, the endocrine system, and the immune system of the body to beneficially impact the whole body.
ACE-inhibitory peptides
The first food-derived bioactive peptide, which enhanced vitamin D-independent bone calcification in rachitic infants, was produced from casein [32]. To date, many angiotensin-I converting enzyme (ACE)-inhibitory peptides have been generated from milk or meat (Table 2). ACE removes the C-terminal dipeptide His-Leu in angiotensin I (Ang I) to form Ang II (a potent vasoconstrictory peptide), thereby conferring their anti-hypertensive effects [33]. The best examples for ACE-inhibitory peptides are Ile-Pro-Pro (IPP) and Val-Pro-Pro (VPP), both of which are derived from milk protein through the hydrolysis of neutral protease, alkaline protease or papain [34]. There is evidence that these two proline-rich peptides may partially escape gastrointestinal hydrolysis and be transported across the intestinal epithelium into the blood circulation [35]. Similarly, the hydrolysis of proteins from meat [36] and egg yolk [37] also generates potent ACE inhibitors.
Table 2.
Antihypertensive peptides generated from the hydrolysis of animal products
| Source | Protease(s) | Amino acid sequence | IC50, μmol/La |
|---|---|---|---|
| Pig muscle myosin | Thermolysin | Ile-Thr-Thr-Asn-Pro | 549 |
| Pig muscle myosin | Pepsin | Lys-Arg-Val-Ile-Thr-Tyr | 6.1 |
| Pig muscle actin | Pepsin | Val-Lys-Arg-Gly-Phe | 20.3 |
| Pig muscle troponin | Pepsin | Lys-Arg-Gln-Lys-Tyr-Asp-Ile | 26.2 |
| Pig muscle | Pepsin + Pancreatin | Lys-Leu-Pro | 500 |
| Pig muscle | Pepsin + Pancreatin | Arg-Pro-Arg | 382 |
| Chicken muscle | Thermolysin | Leu-Ala-Pro | 3.2 |
| Chicken muscle myosin | Thermolysin | Phe-Gln-Lys-Pro-Lys-Arg | 14 |
| Chicken muscle | Thermolysin | Ile-Lys-Trp | 0.21 |
| Chicken collagen | Aspergillus proteases + Proteases FP, A, G and N | Gly-Ala-X-Gly-Leu-X-Gly-Pro | 29.4 |
| Cow muscle | Thermolysin + Proteinase A | Val-Leu-Ala-Gln-Tyr-Lys | 32.1 |
| Cow muscle | Thermolysin + Proteinase A | Phe-His-Gly | 52.9 |
| Cow muscle | Proteinase K | Gly-Phe-His-Ile | 64.3 |
| Cow skin gelatin | Alcalase + Pronase E + Collagenase | Gly-Pro-Val | 4.67 |
| Cow skin gelatin | Alcalase + Pronase E + Collagenase | Gly-Pro-Leu | 2.55 |
| Bonito (fish) muscle | Thermolysin | Leu-Lys-Pro-Asn-Met | 2.4 |
| Bonito (fish) muscle | Thermolysin | Leu-Lys-Pro | 0.32 |
| Bonito (fish) muscle | Thermolysin | Ile-Lys-Pro | 6.9 |
| Salmon muscle | Thermolysin | Val-Trp | 2.5 |
| Salmon muscle | Thermolysin | Met-Trp | 9.9 |
| Salmon muscle | Thermolysin | Ile-Trp | 4.7 |
| Sardine muscle | Alcalase | Ile-Tyr | 10.5 |
| Sardine muscle | Alcalase | Ala-Lys-Lys | 3.13 |
| Sardine muscle | Alcalase | Gly-Trp-Ala-Pro | 3.86 |
| Sardine muscle | Alcalase | Lys-Tyr | 1.63 |
| Alaska pollack skin | Alcalase + Pronase + Collagenase | Gly-Pro-Leu | 2.65 |
| Alaska pollack skin | Alcalase + Pronase + Collagenase | Gly-Pro-Met | 17.1 |
| Shark muscle | Protease SM98011 | Glu-Tyr | 1.98 |
| Shark muscle | Protease SM98012 | Phe-Glu | 2.68 |
| Shark muscle | Protease SM98013 | Cys-Phe | 1.45 |
| Egg yolk | Pepsin | Tyr-Ile-Glu-Ala-Val-Asn-Lys-Val-Ser-Pro-Arg-Ala-Gly-Gln-Phe | 9.4b |
| Egg yolk | Pepsin | Tyr-Ile-Asn-Gln-Met-Pro-Gln-Lys-Ser-Arg-Glu | 10.1b |
Antioxidative and antimicrobial peptides
Many small peptides from animal products (e.g., fish and meat) (Table 3) and plant-source feedstuffs [25] have anti-oxidative functions by scavenging free radicals and/or inhibiting the production of oxidants and pro-inflammatory cytokines [38–41]. These small peptides can reduce the production of oxidants by the small intestine, while enhancing the removal of the oxidants, resulting in a decrease in their intracellular concentrations and alleviating oxidative stress (Fig. 3). Many of the bioactive peptides have both ACE-inhibitory and anti-oxidative effects [36, 37]. Additionally, some peptides from animal (Table 4) and plant protein-hydrolysates [25] also have antimicrobial effects, as reported for certain endogenous peptides in the small intestine [27]. These antimicrobial peptides exert their actions by damaging the cell membrane of bacteria, interfering with the functions of their intracellular proteins, inducing the aggregation of cytoplasmic proteins, and affecting the metabolism of bacteria [42–44], but the underlying mechanisms remain largely unknown [27].
Table 3.
Antioxidative peptides generated from the hydrolysis of animal proteins
| Source | Protease(s) | Amino acid sequence |
|---|---|---|
| Pig muscle actin | Papain + Actinase E | Asp-Ser-Gly-Val-Thr |
| Pig muscle | Papain + Actinase E | Ile-Glu-Ala-Glu-Gly-Glu |
| Pig muscle tropomyosin | Papain + Actinase E | Asp-Ala-Gln-Glu-Lys-Leu-Glu |
| Pig muscle tropomyosin | Papain + Actinase E | Glu-Glu-Leu-Asp-Asn-Ala-Leu-Asn |
| Pig muscle myosin | Papain + Actinase E | Val-Pro-Ser-Ile-Asp-Asp-Gln-Glu-Glu-Leu-Met |
| Pig collagen | Pepsin + Papain + othersa | Gln-Gly-Ala-Arg |
| Pig blood plasma | Alcalase | His-Asn-Gly-Asn |
| Chicken muscle | --- | His-Val-Thr-Glu-Glu |
| Chicken muscle | --- | Pro-Val-Pro-Val-Glu-Gly-Val |
| Deer muscle | Papain | Met-Gln-Ile-Phe-Val-Lys-Thr-Leu-Thr-Gly |
| Deer muscle | Papain | Asp-Leu-Ser-Asp-Gly-Glu-Gln-Gly-Val-Leu |
| Bovine milk casein | Pepsin, pH 2, 24 h | Tyr-Phe-Tyr-Pro-Glu-Leu |
| Bovine milk casein | Pepsin, pH 2, 24 h | Phe-Tyr-Pro-Glu-Leu |
| Bovine milk casein | Pepsin, pH 2, 24 h | Tyr-Pro-Glu-Leu |
| Bovine milk casein | Pepsin, pH 2, 24 h | Pro-Glu-Leu |
| Bovine milk casein | Pepsin, pH 2, 24 h | Glu-Leu |
| Bovine milk casein | Trypsin, pH 7.8, 24–28 h | Val-Lys-Glu-Ala-Met-Pro-Lys |
| Bovine milk casein | Trypsin, pH 7.8, 24–28 h | Ala-Val-Pro-Tyr-Pro-Gln-Arg |
| Bovine milk casein | Trypsin, pH 7.8, 24–28 h | Lys-Val-Leu-Pro-Val-Pro-Glu-Lys |
| Bovine milk casein | Trypsin, pH 7.8, 24–28 h | Val-Leu-Pro-Val-Pro-Glu-Lys |
| Bovine whey protein | Thermolysin, 80 °C, 8 h | Leu-Gln-Lys-Trp |
| Bovine whey protein | Thermolysin, 80 °C, 8 h | Leu-Asp-Thr-Asp-Tyr-Lys-Lys |
| Bovine β-Lactoglobulin | Corolase PP, 37 °C, 24 h | Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile |
| Bovine β-Lactoglobulin | Corolase PP, 37 °C, 24 h | Met-His-Ile-Arg-Leu |
| Bovine β-Lactoglobulin | Corolase PP, 37 °C, 24 h | Try-Val-Glu-Glu-Leu |
| Egg yolk | Pepsin | Tyr-Ile-Glu-Ala-Val-Asn-Lys-Val-Ser-Pro-Arg-Ala-Gly-Gln-Phe |
| Egg yolk | Pepsin | Tyr-Ile-Asn-Gln-Met-Pro-Gln-Lys-Ser-Arg-Glu |
Fig. 3.

Inhibition of cellular oxidative stress by dietary small peptides in the small intestine. The small peptides, which are supplemented to the diets of animals (particularly young animals), can reduce the production of oxidants by the small intestine and enhance the removal of the oxidants, leading to a decrease in their intracellular concentrations and alleviating oxidative stress. (−), inhibition; (+), activation; ↓, decrease
Table 4.
Antimicrobial peptides generated from the hydrolysis of animal proteins or synthesized by intestinal mucosal cells
| Source | Amino acid sequence | Gram-positive bacteria | Gram-negative bacteria |
|---|---|---|---|
| Bovine meat | Gly-Leu-Ser-Asp-Gly-Glu-Trp-Gln | Bacillus cereus Listeria monocytogenes | Salmonella typhimurium Escherichia coli |
| Gly-Phe-His-Ile | No effect | Pseudomonas aeruginosa | |
| Phe-His-Gly | No effect | Pseudomonas aeruginosa | |
| Bovine collagen | Peptides < 2 kDa (by collagenase)a | Staphylococcus aureus | Escherichia coli |
| Goat whey | GWH (730 Da) and SEC-F3 (1,183 Da) (hydrolysis by Alcalase) | Bacillus cereus Staphylococcus aureus | Salmonella typhimurium Escherichia coli |
| Red blood cells | Various peptides (24-h hydrolysis by fugal proteases) | Staphylococcus aureus | Escherichia coli Pseudomonas aeruginosa |
| Hen egg white lysozyme | Asn-Thr-Asp-Gly-Ser-Thr-Asp-Tyr-Gly-Ile-Leu-Gln-Ile-Asn-Ser-Arg (hydrolysis by papain and trypsin)b | Leuconostoc-mesenteroides | Escherichia coli |
| Trout by-products | Various peptides (20–30% of hydrolysis) (hydrolysis by trout pepsin) | Renibacterium-salmoninarum | Flavobacterium psychrophilum |
| Small intestine (Paneth cells) | α-Defensins, lysozyme C, angiogenin-4 and cryptdin-related sequence peptides | Gram-positive bacteria broad-spectrum) | Gram-negative bacteria (broad-spectrum) |
| Phospholipid-sn-2 esterase and C-type lectin | Gram-positive bacteria (broad-spectrum) | No effect |
Opioid peptides
The hydrolysis of certain proteins [e.g., casein, gluten (present in wheat, rye and barley), and soybeans] in the gastrointestinal tract can generate opioid peptides [45]. This can be performed in vitro by using digestive enzymes from the small intestine of mammals (e.g., pigs). Opioid peptides are oligopeptides (typically 4–8 AA residues in length) that bind to opioid receptors in the brain to affect the gut function [46, 47], as well as the behavior and food intake of animals (Table 5). Furthermore, the protein hydrolysates containing opioid-like peptides may be used as feed additives to alleviate stress, control pain and sleep, and modulate satiety in animals.
Table 5.
Opioid peptides generated from the enzymatic hydrolysis of animal and plant proteins in the gastrointestinal tract
| Source | Name of opioid peptide | Amino acid sequence |
|---|---|---|
| Milk casein | Bovine β-casomorphin 1–3 | Tyr-Pro-Phe-OH |
| Bovine β-casomorphin 1–4 | Tyr-Pro-Phe-Pro-OH | |
| Bovine β-casomorphin 1–4, amide | Tyr-Pro-Phe-Pro-NH2 | |
| Bovine β-casomorphin 5 | Tyr-Pro-Phe-Pro-Gly-OH | |
| Bovine β-casomorphin 7 | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-OH | |
| Bovine β-casomorphin 8 | Tyr-Pro-Phe-Pro-Gly-Pro-Ile-Pro-OHa | |
| Gluten protein | Gluten exorphin A5 | Gly-Tyr-Tyr-Pro-Thr-OH |
| Gluten exorphin B4 | Tyr-Gly-Gly-Trp-OH | |
| Gluten exorphin C | Tyr-Pro-Ile-Ser-Leu-OH | |
| Gliadorphin | Tyr-Pro-Gln-Pro-Gln-Pro-Phe-OH | |
| Soybean protein | Soymorphin-5b | Tyr-Pro-Phe-Val-Val-OH |
| Soymorphin-5, amide | Tyr-Pro-Phe-Val-Val-NH2 | |
| Soymorphin-6 | Tyr-Pro-Phe-Val-Val-Asn-OH | |
| Soymorphin-7 | Tyr-Pro-Phe-Val-Val-Asn-Ala-OH | |
| Spinach protein | Rubiscolin-5 | Gly-Tyr-Tyr-Pro-OH |
| Rubiscolin-6 | Gly-Tyr-Tyr-Pro-Thr-OH |
Adapted from Li-Chan [21], López-Barrios et al. [22], Shimizu and Son [35], Bah et al. [36], and Froetschel [42]
aAnother form of bovine β-casomorphin 8 has histidine instead of proline in position 8, depending on whether the peptide is derived from A1 or A2 beta-casein
bDerived from β-conglycinin β-subunit
Applications of plant- and animal-protein hydrolysates in animal nutrition
General consideration
A major goal for animal agriculture is to enhance the efficiency of feed utilization for milk, meat and egg production [48]. This approach requires optimal nutrition to support the function of the small intestine as the terminal site for the digestion and absorption of dietary nutrients [49]. To date, peptides generated from the hydrolysis of plant and animal proteins are included in the diets for feeding pigs, poultry, fish, and companion animals. The outcomes are positive and cost-effective for the improvement of intestinal health, growth and production performance [50]. The underlying mechanisms may be that: (a) the rate of absorption of small peptides is greater than that of an equivalent amount of free AAs; (b) the rate of catabolism of small peptides by the bacteria of the small intestine is lower than that of an equivalent amount of free AAs; (c) the composition of AAs entering the portal vein is more balanced with the intestinal transport of small peptides than that of individual AAs; (e) provision of functional AAs (e.g., glycine, arginine, glutamine, glutamate, proline, and taurine) to enhance anti-oxidative reactions and muscle protein synthesis [51, 52]; and (e) specific peptides can improve the morphology, motility and function of the gastrointestinal tract (e.g., secretion, motility, and anti-inflammatory reactions), endocrine status in favor of anabolism, and feed intake, compared with an equivalent amount of free AAs. In swine nutrition research, most of the studies involving the addition of peptides to diets have been conducted with post-weaning pigs to improve palatability, growth, health, and feed efficiency [53–58]. This is primarily because young animals have immature digestive and immune systems and weanling pigs suffer from reduced feed intake, gut atrophy, diarrhea, and impaired growth. Moreover, peptide products have been supplemented to the diets of calves [59], poultry [60, 61], fish [62, 63], and companion animals [64] to improve their nutrition status, gut function, and abilities to resist infectious diseases.
Plant peptides
As noted previously, plant-source protein ingredients often contain allergenic proteins and other anti-nutritional factors which can limit their practical use, particularly in the diets of young animals [50] and companion animals [64]. For example, soybeans can be processed to manufacture soybean meal and soybean protein concentrates for the elimination of some anti-nutritional substances. However, the soy products still contain considerable amounts of protein-type allergens (e.g., glycinin and β-conglycinin) and significant quantities of trypsin inhibitors, lectins (hemagglutinins), phytic acid, soy oligosaccharides (raffinose and stachyose), and steroid glycosides (soy saponins) [18, 24, 25]. Fermentation of soybeans by the commonly used microorganism (e.g., Aspergillus species, Bacillus species, and Lactobacillus species) has been reported to improve growth performance and feed efficiency in weanling pigs [50]. Thus, 3- to-7-week-old pigs fed a corn- and soybean meal-based diet containing 3% or 6% fermented soybean meal grew at a rate comparable to that of the same percentage of dried skim milk [54]. Likewise, 4.9% fermented soybean meal could replace 3.7% spray-dried plasma protein in the diets of 3- to-7-week-old pigs fed a corn- and soybean meal-based diet without affecting growth performance or feed efficiency [54]. Similar results were obtained for the Atlantic salmon fed a diet containing 40% protein from fermented soy white flakes [60]. Of interest, 50% of fish meal in the diet of juvenile red sea bream can be replaced by the same percentage of soybean protein hydrolysate [63]. The inclusion of plant-protein hydrolysate in diets is important in aquaculture because fish meal is becoming scarce worldwide. Furthermore, as a replacement of the expensive skim milk powder, the hydrolysate of soy protein isolate (19.7% in diet) can be used to sustain high growth-performance in calves [59]. Finally, acidic hydrolysates of plant proteins (e.g., wheat gluten which contains a high amount of glutamine plus glutamate), often called hydrolyzed vegetable proteins, can be included at a 1 to 2% level in the diets of companion animals to provide savory flavors due to the high abundance of glutamate in the products [64].
Animal peptides
Postweaning piglets fed a diet containing 6% spray-dried porcine intestine hydrolysate (SDPI; the co-product of heparin production) for 2 wk had better growth performance than those fed the control diet, the basal diet containing spray-dried plasma, or the basal diet containing dried whey [55, 56]. There was a carry-over effect on enhancing growth performance during weeks 3–5 postweaning in piglets that were previously fed the SDPI [56], which was likely due to an increased area of the intestinal villus as well as improved digestion and absorption of dietary nutrients [57]. Similarly, Stein (2002) reported that piglets (weaned at 20 days of age) fed a weanling diet containing 1.5, 3 or 4.5% SDPI had better growth performance and greater feed efficiency in comparison to piglets consuming the same amount of a fish meal-supplemented diet. Of note, these effects of the SDPI supplementation were dose-dependent. In addition, postweaning piglets fed a corn-, soybean meal-, and dried whey-based diet containing 6% enzymatically hydrolyzed proteins (from blend of swine blood and selected poultry tissues) exhibited a growth rate and a feed efficiency that were comparable to those for piglets fed a diet containing the same percentage of spray-dried blood cells [53]. Likewise, the inclusion of 2.5 5 or 7.5% hydrolyzed porcine mucosa in a corn- and soybean meal-based diet enhanced daily weight gain and nutrient retention in growing chicks [61]. Furthermore, broilers fed a diet containing 5% Atlantic salmon protein hydrolysates (from the viscera) had better growth performance than those fed a diet with or without 4% fish meal [60]. Finally, addition of the protein hydrolysate of fish by-products to the diet (at a 10% inclusion level) improved intestinal development, growth, immunological status, and survival in European sea bass larvae challenged with Vibrio anguillarum (a Gram-negative bacterium) [65]. Thus, SDPI or other hydrolysates of animal proteins hold promise for animal production.
Potential scale and economic value for the global use of animal and plant protein hydrolysates in animal feeding
Industrial processing of domestic farm animals generates large amounts of tissues (30–40% of body weight) not consumed by humans, including viscera, carcass-trimmings, bone (20–30% of body weight), fat, skin, feet, small-intestinal tissue (2% of body weight), feather (up to 10% of body weight), and collectible blood (5% body weight), with the global human-inedible livestock and poultry byproducts being ~54 billion kg/yr [66–68]. Likewise, fish processing industries produce large amounts of wastes (up to 55% of body weight), such as muscle-trimmings (15–20%), skin and fins (1–3%), bones (9–15%), heads (9–12%), viscera (12–18%), and scales, with the global human-inedible fish byproducts being ~6 billion kg/yr [66–69]. Thus, the global annual volume of total animal by-products generated by the processing industries is approximately 60 billion kg annually. Assuming that only 5% of the animal by-products and plant products for feed are used for protein hydrolysis, and based on the current average prices of animal, soybean, and wheat protein hydrolysates [70], their yields are 3, 6.75 and 12.75 billion kg/yr, respectively, and their economic values are 4.5, 3.88 and 20.02 billion US $/yr (Table 6). Thus, protein hydrolysates from the by-products of pigs or poultry and from plant ingredients hold great promise in sustaining the animal agriculture and managing companion animals worldwide.
Table 6.
Potential scale and economic values for the global use of animal and plant protein hydrolysates (PH) in animal feeding
| Type | Annual global productiona | Annual use for animal feedinga | Amount used for production of PHb | Current pricec | Total value for PH |
|---|---|---|---|---|---|
| Billion kg/yr | Billion kg/yr | Billion kg/yr | US $/kg | Billion US $/yr | |
| ABP | 172 | 60 | 3 | 1.5 | 4.50 |
| Soybean | 180 | 135 | 6.75 | 0.575 | 3.88 |
| Wheat | 750 | 255 | 12.75 | 1.57 | 20.02 |
ABP, animal byproducts (including livestock, poultry and fish)
aFood and Agriculture Organization [69]
bAssuming that 5% of the ABP or plant products for animal feeds are used to produce protein hydrolysates
cThe prices for peptone (a representative of animal protein hydrolysates), fermented soybean, and hydrolyzed wheat protein [70].
Future research directions
The nutritional value of protein hydrolysates as flavor enhancers, functional ingredients, and precursors for protein synthesis depends on the composition of free AAs, small peptides and large peptides in the products, as well as their batch-to-batch consistence. At present, such data are not available for the commercially available products of animal or plant hydrolysates and should be obtained with the use of HPLC and mass spectrometry. Only when the composition of protein hydrolysates is known, can we fully understand their functionally active components and the mechanisms of their actions. In addition, the net rates of the transport of small peptides across the small intestine are not known for all the protein hydrolysates currently used in animal feeding. This issue can be readily addressed with the use of Ussing chambers [71]. There is also concern that some animal protein hydrolysates, which contain a high proportion of oligopeptides with a high abundance of basic AAs, have a low palatability for animals (particularly weanling piglets), and, therefore, the inclusion of the protein hydrolysates in animal feeds may be limited. Such a potential problem may be substantially alleviated through: (a) the addition of exopeptidases and a longer period of hydrolysis to remove basic and aliphatic AAs from the C- and N-terminals of the polypeptides; and (b) appropriate supplementation with glycine, monosodium glutamate and inosine. Furthermore, the role of animal and plant protein hydrolysates in the signaling of intestinal epithelial cells and bacteria and metabolic regulation in these cells should be investigated to better understand how these beneficial products improve gut integrity, immunity, and health. Finally, the potential of protein hydrolysates as alternatives to dietary antibiotics should be explored along with studies to elucidate the underlying mechanisms. All these new lines of research will be particularly important for animals with compromised intestinal structure and function (e.g., neonates with intrauterine growth restriction and early-weaned mammals) and raised under adverse environmental conditions (e.g., high or low ambient temperatures).
Conclusion
Plant- and animal-protein hydrolysates provide highly digestible peptides and bioactive peptides, as well as specific AAs (e.g., glutamate) to confer nutritional and physiological or regulatory functions in animals. The industrial production of these protein hydrolysates involves: (a) strong acidic or alkaline conditions, (b) mild enzymatic methods, or (c) fermentation by microorganisms. The degree of hydrolysis is assessed by the number of peptide bonds cleaved divided by the total number of peptide bonds in a protein. Chemical hydrolysis is often employed to generate savory flavors, whereas microbial fermentation not only produces peptides but also removes anti-nutritional factors in protein ingredients. In addition to their nutritional value to supply AAs, bioactive peptides (usually 2–20 AA residues in length) have antimicrobial, antioxidant, antihypertensive, and immunomodulatory roles. These peptides exert beneficial effects on improving intestinal morphology, function, and resistance to infectious diseases in animals (including pigs, calves, chickens, companion animals, and fish), thereby enhancing their health and well-being, as well as growth performance and feed efficiency. This provides a cost-effective approach to converting animal by-products, brewer’s byproducts, or plant feedstuffs into high-quality protein-hydrolysate ingredients to feed livestock, poultry, fish, and companion animals.
Acknowledgments
We thank our colleagues for collaboration on animal nutrition research.
Funding
Work in our laboratories was supported by the National Natural Science Foundation of China (31572416, 31372319, 31330075 and 31110103909), Hubei Provincial Key Project for Scientific and Technical Innovation (2014ABA022), Hubei Hundred Talent program, Natural Science Foundation of Hubei Province (2013CFA097), Agriculture and Food Research Initiative Competitive Grants (2014-67015-21770 and 2015-67015-23276) from the USDA National Institute of Food and Agriculture, and Texas A&M AgriLife Research (H-8200).
Availability of data and materials
Not applicable.
Authors’ contributions
GW conceived this project. YQH and GW wrote the manuscript. ZLW, ZLD, and GHW contributed to the discussion and revision of the article. GW had the primary responsibility for the content of the paper. All authors read and approved this manuscript.
Competing interests
None of the authors have any competing interests in the manuscript.
Consent for publication
All authors read and approved the final manuscript.
Ethics approval and consent to participate
This article reviews published studies and does not require the approval of animal use or consent to participate.
Abbreviations
- AA
Amino acid
- ACE
Angiotensin-I converting enzyme
- HPLC
High-performance liquid chromatography
- PCA
Perchloric acid
- PH
Protein hydrolysates
- SDPI
Spray-dried porcine intestine hydrolysate
- TCA
Trichloroacetic acid
Contributor Information
Yongqing Hou, Email: houyq@aliyun.com.
Zhenlong Wu, Email: cauwzl@hotmail.com.
Zhaolai Dai, Email: daizhaolai@163.com.
Genhu Wang, Email: wanggenhu@gentechchina.com.
Guoyao Wu, Phone: 979-845-1817, Email: g-wu@tamu.edu.
References
- 1.Wu G, Cross HR, Gehring KB, Savell JW, Arnold AN, McNeill SH. Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts. J Anim Sci. 2016;94:2603–13. doi: 10.2527/jas.2016-0478. [DOI] [PubMed] [Google Scholar]
- 2.Wu G. Amino acids: biochemistry and nutrition. Boca Raton: CRC Press; 2013. [Google Scholar]
- 3.Pasupuleki VK, Braun S. State of the art manufacturing of protein hydrolysates. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York: Springer Science; 2010. pp. 11–32. [Google Scholar]
- 4.Dieterich F, Rogerio W, Bertoldo MT, da Silva VSN, Gonçalves GS, Vidotti RM. Development and characterization of protein hydrolysates originated from animal agro industrial byproducts. J Dairy Vet Anim Res. 2014;1:00012. doi: 10.15406/jdvar.2014.01.00012. [DOI] [Google Scholar]
- 5.Pasupuleki VK, Holmes C, Demain AL. Applications of protein hydrolysates in biotechnology. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York: Springer Science; 2010. pp. 1–9. [Google Scholar]
- 6.Kyte J. Structure in protein chemistry. 2. New York: Garland Science; 2006. p. 832. [Google Scholar]
- 7.Agerberth B, Söderling-Barros J, Jörnvall H, Chen ZW, Ostenson CG, Efendić S, et al. Isolation and characterization of a 60-residue intestinal peptide structurally related to the pancreatic secretory type of trypsin inhibitor: influence on insulin secretion. Proc Natl Acad Sci U S A. 1989;86:8590–4. doi: 10.1073/pnas.86.21.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZW, Bergman T, Ostenson CG, Efendic S, Mutt V, Jörnvall H. Characterization of dopuin, a polypeptide with special residue distributions. Eur J Biochem. 1997;249:518–22. doi: 10.1111/j.1432-1033.1997.t01-2-00518.x. [DOI] [PubMed] [Google Scholar]
- 9.Rajalingam D, Loftis C, Xu JJ, Kumar TKS. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci. 2009;18:980–93. doi: 10.1002/pro.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moughan PJ, Darragh AJ, Smith WC, Butts CA. Perchloric and trichloroacetic acids as precipitants of protein in endogenous ileal digesta from the rat. J Sci Food Agric. 1990;52:13–21. doi: 10.1002/jsfa.2740520103. [DOI] [Google Scholar]
- 11.Wilcockson J. The differential precipitation of nucleic acids and proteins from aqueous solutions by ethanol. Anal Biochem. 1975;66:64–8. doi: 10.1016/0003-2697(75)90724-1. [DOI] [PubMed] [Google Scholar]
- 12.Dai ZL, Wu ZL, Jia SC, Wu G. Analysis of amino acid composition in proteins of animal tissues and foods as pre-column o-phthaldialdehyde derivatives by HPLC with fluorescence detection. J Chromatogr B. 2014;964:116–27. doi: 10.1016/j.jchromb.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Sapan CV, Lundblad RL. Review of methods for determination of total protein and peptide concentration in biological samples. Proteomics Clin Appl. 2015;9:268–76. doi: 10.1002/prca.201400088. [DOI] [PubMed] [Google Scholar]
- 14.Larive CK, Lunte SM, Zhong M, Perkins MD, Wilson GS, Gokulrangan G, et al. Separation and analysis of peptides and proteins. Anal Chem. 1999;71:389R–423R. doi: 10.1021/a1990013o. [DOI] [PubMed] [Google Scholar]
- 15.McGrath R. Protein measurement by ninhydrin determination of amino acids released by alkaline hydrolysis. Anal Biochem. 1972;49:95–102. doi: 10.1016/0003-2697(72)90245-X. [DOI] [PubMed] [Google Scholar]
- 16.Kunst T. Protein modification in optimize functionality: protein hydrolysates. In: Whitaker J, Voragen A, Wong D, editor. Handbook of food enzymology. New York: Marcel Dekker; 2003. pp. 222–36. [Google Scholar]
- 17.Dixon MM, Webb EC. Enzymes. 3. New York: Academic; 1979. [Google Scholar]
- 18.Kim MR, Kawamura Y, Lee CH. Isolation and identification of bitter peptides of tryptic hydrolysate of soybean 11S glycinin by reverse-phase high-performance liquid chromatography. J Food Sci. 2003;68:2416–22. doi: 10.1111/j.1365-2621.2003.tb07039.x. [DOI] [Google Scholar]
- 19.Andriamihaja M, Guillot A, Svendsen A, Hagedorn J, Rakotondratohanina S, Tome’ D, et al. Comparative efficiency of microbial enzyme preparations versus pancreatin for in vitro alimentary protein digestion. Amino Acids. 2013;44:563–72. doi: 10.1007/s00726-012-1373-0. [DOI] [PubMed] [Google Scholar]
- 20.Layer P, Keller J. Lipase supplementation therapy: standards, alternatives, and perspectives. Pancreas. 2003;26:1–7. doi: 10.1097/00006676-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Sikkens EC, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:337–47. doi: 10.1016/j.bpg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Smid EJ, Lacroix C. Microbe-microbe interactions in mixed culture food fermentations. Curr Opin Biotechnol. 2013;24:148–54. doi: 10.1016/j.copbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Bah CS, Carne A, McConnell MA, Mros S, Bekhit A-D. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016;202:458–66. doi: 10.1016/j.foodchem.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Li-Chan ECY. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr Opin Food Sci. 2015;1:28–37. doi: 10.1016/j.cofs.2014.09.005. [DOI] [Google Scholar]
- 25.López-Barrios L, Gutiérrez-Uribe JA, Serna-Saldívar SO. Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J Food Sci. 2014;79:R273–83. doi: 10.1111/1750-3841.12365. [DOI] [PubMed] [Google Scholar]
- 26.Kairane C, Zilmer M, Mutt V, Sillard R. Activation of Na, K-ATPase by an endogenous peptide, PEC-60. FEBS Lett. 1994;345:1–4. doi: 10.1016/0014-5793(94)00407-2. [DOI] [PubMed] [Google Scholar]
- 27.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nature Rev Microbiol. 2011;9:356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 28.Engel JA, Jerlhag E. Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs. 2014;28:875–86. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhanghi BM, Matthews JC. Physiological importance and mechanisms of protein hydrolysate absorption. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York: Springer Science; 2010. pp. 135–77. [Google Scholar]
- 30.Gardner ML. Absorption of intact peptides: studies on transport of protein digests and dipeptides across rat small intestine in vitro. Q J Exp Physiol. 1982;67:629–37. doi: 10.1113/expphysiol.1982.sp002682. [DOI] [PubMed] [Google Scholar]
- 31.Gardner ML, Wood D. Transport of peptides across the gastrointestinal tract. Biochem Soc Trans. 1989;17:934–7. doi: 10.1042/bst0170934. [DOI] [PubMed] [Google Scholar]
- 32.Mellander O. The physiological importance of the casein phosphopeptide calcium salts. II. Peroral calcium dosage of infants. Acta Soc Med Ups. 1950;55:247–55. [PubMed] [Google Scholar]
- 33.Ryan JT, Ross RP, Bolton D, Fitzgerald GF, Stanton C. Bioactive peptides from muscle sources: meat and fish. Nutrients. 2011;3:765–91. doi: 10.3390/nu3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Power O, Jakeman P, FitzGerald RJ. Antioxidative peptides: enzymatic production, in4vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids. 2013;44:797–820. doi: 10.1007/s00726-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Augustin O, Rivero-Gutiérrez B, Mascaraque C, de Medina FS. Food-derived bioactive peptides and intestinal barrier function. Int J Mol Sci. 2014;15:22857–73. doi: 10.3390/ijms151222857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryder K, Ael-D B, McConnell M, Carne A. Towards generation of bioactive peptides from meat industry waste proteins: Generation of peptides using commercial microbial proteases. Food Chem. 2016;208:42–50. doi: 10.1016/j.foodchem.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 37.Zambrowicz A, Pokora M, Setner B, Dąbrowska A, Szołtysik M, Babij K, et al. Multifunctional peptides derived from an egg yolk protein hydrolysate: isolation and characterization. Amino Acids. 2015;47:369–80. doi: 10.1007/s00726-014-1869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu M, Son DO. Food-derived peptides and intestinal functions. Curr Pharm Des. 2007;13:885–95. doi: 10.2174/138161207780414287. [DOI] [PubMed] [Google Scholar]
- 39.Bah CS, Bekhit A-D, McConnell MA, Carne A. Generation of bioactive peptide hydrolysates from cattle plasma using plant and fungal proteases. Food Chem. 2016;213:98–107. doi: 10.1016/j.foodchem.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 40.Memarpoor-Yazdia M, Asoodehb A, Chamania JK. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J Funct Foods. 2012;4:278–86. doi: 10.1016/j.jff.2011.12.004. [DOI] [Google Scholar]
- 41.Power O, Jakeman P, FitzGerald RJ. Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids. 2013;44:797–820. doi: 10.1007/s00726-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 42.Lima CA, Campos JF, Filho JLM, Converti A, da Cunha MGC, Porto ALF. Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. J Food Sci Technol. 2015;52:4459–66. doi: 10.1007/s13197-014-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osman A, Goda HA, Abdel-Hamid M, Badran SM, Otte J. Antibacterial peptides generated by Alcalse hydrolysis of goat whey. LWT-Food Sci Technol. 2016;65:480–86. doi: 10.1016/j.lwt.2015.08.043. [DOI] [Google Scholar]
- 44.Wald M, Schwarz K, Rehbein H, Bußmann B, Beermann C. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 2016;205:221–28. [DOI] [PubMed]
- 45.Froetschel MA. Bioactive peptides in digesta that regulate gastrointestinal function and intake. J Anim Sci. 1996;74:2500–8. doi: 10.2527/1996.74102500x. [DOI] [PubMed] [Google Scholar]
- 46.San Gabriel A, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids. 2013;45:451–61. doi: 10.1007/s00726-012-1371-2. [DOI] [PubMed] [Google Scholar]
- 47.Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419–30. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 48.Wu G, Fanzo J, Miller DD, Pingali P, Post M, Steiner JJ, et al. Production and supply of high-quality food protein for human consumption: sustainability, challenges and innovations. Ann NY Acad Sci. 2014;1321:1–19. doi: 10.1111/nyas.12500. [DOI] [PubMed] [Google Scholar]
- 49.Wu G, Bazer FW, Cross HR. Land-based production of animal protein: impacts, efficiency, and sustainability. Ann NY Acad Sci. 2014;1328:18–28. doi: 10.1111/nyas.12566. [DOI] [PubMed] [Google Scholar]
- 50.McCalla J, Waugh T, Lohry E. Protein hydrolysates/peptides in animal nutrition. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York: Springer Science; 2010. pp. 179–90. [Google Scholar]
- 51.Hou YQ, Yin YL, Wu G. Dietary essentiality of “nutritionally nonessential amino acids” for animals and humans. Exp Biol Med. 2015;240:997–1007. doi: 10.1177/1535370215587913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou YQ, Yao K, Yin YL, Wu G. Endogenous synthesis of amino acids limits growth, lactation and reproduction of animals. Adv Nutr. 2016;7:331–42. doi: 10.3945/an.115.010850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindemann MD, Cromwell GL, Monegue HJ, Cook H, Soltwedel KT, Thomas S, et al. Feeding value of an enzymatically digested protein for early-weaned pigs. J Anim Sci. 2000;78:318–27. doi: 10.2527/2000.782318x. [DOI] [PubMed] [Google Scholar]
- 54.Kim SW, van Heugten E, Ji F, Lee CH, Mateo RD. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J Anim Sci. 2010;88:214–24. doi: 10.2527/jas.2009-1993. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman D. Interaction of intestinal hydrolysate and spray-dried plasma fed to weanling pigs, Iowa State University, Ames, IA, Experiment 9615.1996.
- 56.Zimmerman D. The duration of carry-over growth response to intestinal hydrolysate fed to weanling pigs, Iowa State University, Ames, IA, Experiment 9612, 1996.
- 57.Kim JH, Chae BJ, Kim YG. Effects of replacing spray dried plasma protein with spray dried porcine intestine hydrolysate on ileal digestibility of amino acids and growth performance in early-weaned pigs. Asian-Aust J Anim Sci. 2000;13:1738–1742. doi: 10.5713/ajas.2000.1738. [DOI] [Google Scholar]
- 58.Stein H. The effect of including DPS 50RD and DPS EX in the phase 2 diets for weanling pigs. Brookings: South Dakota State University; 2002. [Google Scholar]
- 59.Lalles JP, Toullec R, Pardal PB, Sissons JW. Hydrolyzed soy protein isolate sustains high nutritional performance in veal calves. J Dairy Sci. 1995;78:194–204. doi: 10.3168/jds.S0022-0302(95)76629-2. [DOI] [PubMed] [Google Scholar]
- 60.Opheim M, Sterten H, Øverland M, Kjos NP. Atlantic salmon (Salmo salar) protein hydrolysate – Effect on growth performance and intestinal morphometry in broiler chickens. Livest Sci. 2016;187:138–45. doi: 10.1016/j.livsci.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Frikha M, Mohiti-Asli M, Chetrit C, Mateos GG. Hydrolyzed porcine mucosa in broiler diets: effects on growth performance, nutrient retention, and histomorphology of the small intestine. Poult Sci. 2014;93:400–11. doi: 10.3382/ps.2013-03376. [DOI] [PubMed] [Google Scholar]
- 62.Refstie S, Sahlström S, Bråthen E, Baeverfjord G, Krogedal P. Lactic acid fermentation eliminates indigestible carbohydrates and antinutritional factors in soybean meal for Atlantic salmon (Salmo salar) Aquaculture. 2005;246:331–45. doi: 10.1016/j.aquaculture.2005.01.001. [DOI] [Google Scholar]
- 63.Khosravi S, Rahimnejad S, Herault M, Fournier V, Lee CR, Dio Bui HT, et al. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 2015;45:858–68. doi: 10.1016/j.fsi.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 64.Nagodawithana TW, Nelles L, Trivedi NB. Protein hydrolysates as hypoallergenic, flavors and palatants for companion animals. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York: Springer Science; 2010. pp. 191–207. [Google Scholar]
- 65.Kotzamanis YP, Gisbert E, Gatesoupe FJ, Zambonino Infante J, Cahu C. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A. 2007;147:205–14. doi: 10.1016/j.cbpa.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 66.Martínez-Alvarez O, Chamorro S, Brenes A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res Int. 2015. doi: 10.1016/j.foodres.2015.04.005.
- 67.Ghosh PR, Fawcett D, Sharma SB, Poinern DEJ. Progress towards sustainable utilisation and management of food wastes in the global economy. Int J Food Sci. Volume 2016, Article ID 3563478. [DOI] [PMC free article] [PubMed]
- 68.Irshad A, Sureshkumar S, Shalima Shukoor A, Sutha M. Slaughter house by-product utilization for sustainable meat industry-a review. Int J Res Dev. 2015;5:4725–734. [Google Scholar]
- 69.Food and Agriculture Organization [67]. http://www.fao.org/worldfoodsituation/csdb/en/. Accessed on 8 Dec 2016.
- 70.Animal feed prices. https://www.alibaba.com/product. Accessed on 8 Dec 2016.
- 71.Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, etal. Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids. 2014;46:2037–45. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


