Summary
Regulatory T (Treg) cells are a suppressive CD4+ T‐cell subset. We generated induced Treg (iTreg) cells and explored their therapeutic potential in a murine model of rapidly progressive glomerulonephritis. Polyclonal naive CD4+ T cells were cultured in vitro with interleukin‐2 (IL‐2), transforming growth factor‐β1, all‐trans‐retinoic acid and monoclonal antibodies against interferon‐γ and IL‐4, generating Foxp3+ iTreg cells. To enhance their suppressive phenotype, iTreg cultures were modified with the addition of a monoclonal antibody against IL‐12p40 or by using ROR γt–/– CD4+ T cells. Induced Treg cells were transferred into models of delayed‐type hypersensitivity and experimental glomerulonephritis. The iTreg cells exhibited comparable surface receptor expression and in vitro suppressive ability to natural Treg cells, but did not regulate antigen‐specific delayed‐type hypersensitivity or systemic inflammatory immune responses, losing Foxp3 expression in vivo. In glomerulonephritis, transferred iTreg cells did not prevent renal injury or modulate systemic T helper type 1 immune responses. Induced Treg cells cultured with anti‐IL‐12p40 had an enhanced suppressive phenotype in vitro and regulated dermal delayed‐type hypersensitivity in vivo, but were not protective against renal injury, losing Foxp3 expression, especially in the transferred cells recruited to the kidney. Use of ROR γt–/– CD4+ T cells or iTreg cells generated from sensitized CD4+ Foxp3– cells did not regulate renal or systemic inflammatory responses in vivo. In conclusion, iTreg cells suppress T‐cell proliferation in vitro, but do not regulate experimental glomerulonephritis, being unstable in this inflammatory milieu in vivo.
Keywords: all‐trans‐retinoic acid, Foxp3, induced regulatory T cell, rapidly progressive glomerulonephritis, retoinic acid receptor‐related orphan receptor γt
Abbreviations
- ATRA
all‐trans‐retinoic acid
- CCR
C–C chemokine receptor
- cDNA
complementary deoxyribonucleic acid
- CTV
cell trace violet
- DTH
delayed‐type hypersensitivity
- FACS
fluorescence‐activated cell sorting
- FCA
Freund's complete adjuvant
- Foxp3
forkhead box P3
- Foxp3‐GFP
forkhead box P3‐green fluorescent protein
- GBM
glomerular basement membrane
- GCS
glomerular cross‐section
- GFP
green fluorescent protein
- GITR
glucocorticoid‐induced tumour necrosis factor receptor
- GN
glomerulonephritis
- HPF
high‐power field
- ICOS
inducible T‐cell co‐stimulator
- IFN‐γ
interferon‐γ
- IgG
immunoglobulin G
- IL
interleukin
- iTreg cells
induced regulatory T cells
- mAb
monoclonal antibody
- mRNA
messenger ribonucleic acid
- nTreg cells
natural regulatory T cells
- PAS
periodic acid Schiff
- PMA
phorbol‐12‐myristate‐13‐acetate
- RORγt
retoinic acid receptor‐related orphan receptor γt
- RPGN
rapidly progressive glomerulonephritis
- RT‐PCR
real time polymerase chain reaction
- SG
sheep globulin
- Teff
effector T cell
- TGF‐β1
transforming growth factor β1
- Th
T helper
- Tr1
regulatory type 1
- Treg cells
regulatory T cells
Introduction
Most forms of rapidly progressive glomerulonephritis (RPGN) are mediated by abnormal adaptive immune responses, where CD4+ T‐cell subsets not only promote pathological antibody deposition, but also act as cellular effectors in the kidney. Regulatory T (Treg) cells, a suppressive T‐cell subset, could limit injury in these conditions. Treg cells are reduced in number or function in human diseases causing RPGN, such as systemic lupus erythematosus1 and anti‐neutrophil cytoplasmic antibody‐associated vasculitis,2, 3, 4, 5 and play a role in restoring tolerance in anti‐glomerular basement membrane (GBM) disease.6, 7 Natural Treg cells are generated in the thymus (referred to as nTreg or tTreg cells), express the transcription factor Foxp3,8 and mediate central and peripheral tolerance, preventing autoimmune effector T (Teff) cell responses and regulating inflammation. Treg cells are also generated in the periphery from naive CD4+ T cells – known as induced Treg cells (iTreg) cells. Transforming growth factor‐β1 (TGF‐β1) up‐regulates Foxp3 expression in CD4+ T cells.9 All‐trans‐retinoic acid (ATRA), a vitamin A derivative produced by dendritic cells, is an important co‐factor with TGF‐β1 for the generation of Foxp3+ Treg cells from naive CD4+ T cells.10, 11 It antagonises retoinic acid receptor‐related orphan receptor‐γt (RORγt) expression and interleukin‐17A (IL‐17A) production in cultured CD4+ T cells and increases the expression of suppressive surface receptors on human nTreg cells, making them resistant to T helper type 1 (Th1) and Th17 conversion.11, 12, 13, 14
In experimental RPGN, endogenous Treg cells limit immunity and tissue injury,15, 16 and nTreg transfer constrains disease.17 As nTreg cells comprise a small population of all circulating CD4+ T cells, generating iTreg cells in vitro allows the therapeutic potential of Treg cells to be more easily investigated. We generated polyclonal iTreg cells from naive CD4+ T cells using ATRA, TGF‐β1 and IL‐2, and aimed to stabilize the Treg phenotype by supplementing medium with anti‐interferon‐γ (IFN‐γ), anti‐IL‐4 and anti‐IL‐12p40 monoclonal antibodies (mAb). Despite a regulatory phenotype in vitro, these cells did not induce beneficial immunomodulation in vivo in models of delayed‐type hypersensitivity (DTH) and RPGN, losing Foxp3 expression, demonstrating an unstable phenotype in an inflammatory environment.
Materials and methods
Animals were housed in specific pathogen‐free facilities at Monash Medical Centre Animal Facility (Melbourne, Australia). Foxp3‐GFP and RORγt–/– mice15, 18 were bred‐in house. Ly5.1 congenic mice were from the Walter and Eliza Hall Institute (Melbourne, Australia). Experiments were performed according to the National Health and Medical Research Council code for the care and use of animals for scientific purposes and were approved by Monash University Animal Ethics Committee B (MMCB13/35). Male mice (aged 6–10 weeks) were used, and killed at the completion of experiments or if showing signs of lethargy, persistent recumbency, hunched posture, rough coat or loss of body condition. Data are presented as mean (± SEM), using Student's t‐test (two groups) or analysis of variance (three or more groups). Differences in survival were assessed with a log‐rank test. Significance was defined as P < 0·05.
In vitro culture and induction of iTreg, nTreg, Treg and Teff cells from naive and sensitized mice
CD4+ T cells from spleens and lymph nodes of naive Foxp3‐GFP or RORγt–/– mice were purified using L3T4 microbeads (Miltenyi Biotec Australia, Macquarie Park, NSW, Australia). The iTreg cells were induced using methods adapted from published protocols.19, 20 Twenty‐four‐well plates were coated with 1 ml anti‐CD3 (BioXcell, West Lebanon, NH, 17A2; 10 μg/ml; overnight 4°C, washed twice with PBS before use). CD4+ T cells (0·5 × 106/well) were cultured in 1 ml of RPMI‐complete (RPMI with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l‐glutamine, 50 μm 2‐mercaptoethanol) with anti‐CD28 (BioXcell, 37.51; 2 μg/ml), ATRA (Sigma‐Aldrich, Sydney, Australia; 1pmol/l), recombinant human (rh) TGF‐β1 (Biolegend, San Diego, CA; 20 ng/ml), IL‐2 (eBioscience, San Diego, CA; 7·29 ng/ml), anti‐IFN‐γ (BioXcell, R4‐6A2; 10 μg/ml) and anti‐IL‐4 (11B11, in‐house; 500 ng/ml). A neutralizing anti‐IL‐12p40 mAb (C17.8; in‐house; 20 μg/ml21) was added to some cultures (iTreg cells +anti‐IL‐12p40). Cells were incubated at 37°C with 5% CO2 for 3 days, then cell supernatants were replaced with 1 ml of RPMI‐complete with IL‐2. Cells were harvested on day 5. Cell supernatants on day 5 were aspirated and stored at −80°C.
To obtain nTreg cells, isolated CD4+ cells from naive Foxp3‐GFP mice were sorted on GFP using a Mo‐Flo XDP cell sorter (> 97% cells CD4+ Foxp3+). To generate Treg and iTreg cells from mice sensitized to the nephritogenic antigen, naive Foxp3‐GFP mice were sensitized with sheep globulin (SG) [0·5 mg in Freund's complete adjuvant (FCA)] subcutaneously to the tailbase and neck. Spleens and lymph nodes were harvested 10 days later. CD4+ T cells were isolated as above, and populations of Foxp3– and Foxp3+ cells were obtained by cell sorting. Treg cells were cultured from sensitized CD4+ Foxp3+ cells in anti‐CD3 coated plates, with medium, IL‐2 and anti‐CD28; iTreg cells +anti‐IL‐12p40 from sensitized mice were generated from CD4+ Foxp3– cells as described above; Teff cells were generated from sensitized CD4+ Foxp3– cells in anti‐CD3‐coated plates, with medium, IL‐2, anti‐CD28 and anti‐IL‐4. Cell supernatants were replaced with 1 ml of RPMI‐complete with IL‐2 after 3 days of culture. Cells were harvested on day 5.
Treg cell suppressive assay, cytokine production and mRNA expression
T effector cells were naive CD4+ T cells from the spleens of Ly5.1 mice, labeled with Cell Trace Violet (CTV) cell proliferation kit (Life Technologies, Victoria, Australia; 10 μm). Co‐cultures of Teff cells (1 × 105) with serial dilutions of nTreg cells, iTreg cells or iTreg cells +anti‐IL‐12p40 were stimulated with plate‐bound anti‐CD3 (10 μg/ml), soluble anti‐CD28 (0·4 μg/ml) and RPMI‐complete (72 hr, 37°C, 5% CO2), to compare suppression of Teff proliferation by FACS.22 Supernatants from cultured iTreg cells were assayed using a Mouse Th1/Th2/Th17 cytometric bead array (BD Biosciences, North Ryde, NSW, Australia) and DuoSet ELISA for mouse TGF‐β1 (R&D Systems, Minneapolis, USA). Messenger RNA was extracted from 1 × 106 cells (Qiagen RNeasy Mini kits, Victoria, Australia), and cDNA was generated using an Applied Biosystems (Foster City, CA) high‐capacity cDNA reverse transcription kit. RT‐PCR was performed using Taqman or Power SYBR Green mastermix and probes (sequences available upon request; Life Technologies) in a Rotor‐Gene RG3000 RT thermal cycler (Corbett Life Science, Qiagen).
Assessment of immune responses to sheep globulin immunization
Ly5.1 congenic mice were sensitized to SG (0·5 mg in FCA) to the tailbase subcutaneously. Either iTreg cells or medium (control) were injected into the tail vein on the same day. After 9 days, 0·5 mg SG or horse globulin (control) was injected into the right and left footpads, respectively. On day 10, mice were killed and footpad swelling was measured, assessing dermal DTH. EliSpot assays for IFN‐γ (BD Biosciences) and IL‐17A (eBioscience) were performed using 1 or 2 × 106 splenocytes/well (in duplicate), stimulated with SG.23 Spots were enumerated by EliSpot platereader (Autoimmun Diagnostika GmbH, Strassberg, Germany). Serum mouse anti‐sheep IgG antibody levels were measured by ELISA.24
Experimental accelerated anti‐GBM disease model and adoptive transfer studies
‘Accelerated autologous phase anti‐GBM GN’ as a model of RPGN, was induced in mice sensitized subcutaneously with 0·5 mg of SG/FCA to the tailbase at day −4, then sheep anti‐mouse GBM globulin (as a nephritogenic antigen) was injected intravenously on day 0 (5 or 10 mg for mild or severe RPGN, respectively). Experiments ended on day 10. Adoptive transfer of iTreg cells was performed in a model of severe RPGN; mice received 10 × 106 cells iTreg cells, iTreg cells +anti‐IL‐12p40 or an equivalent volume of medium on day −4 and day 0 (for experiments with RORγt–/– iTreg cells, cell doses were 10 × 106 and 1·25 × 106/mouse on day −4 and 0, respectively). In the model of milder RPGN, 10 × 106 iTreg cells, iTreg cells +anti‐IL‐12p40 (generated from naive or sensitized mice), or medium were transferred on day −4 only.
Assessment of renal injury and renal leucocytes
Urine was collected from mice to assess proteinuria, by Bradford's assay, and urinary creatinine. Paraffin‐embedded Periodic acid Schiff 3‐μm sections were assessed for glomerular segmental necrosis and crescents (≥ 40 glomeruli/mouse) and interstitial injury (10 high‐power fields [hpf] at 200×).15 Renal leucocyte accumulation was assessed in periodate lysine paraformaldehyde fixed 6‐μm‐thick sections (≥ 20 glomeruli and 10 interstitial hpf/mouse).25 Interstitial macrophage accumulation was graded based on the percentage of the interstitial hpf containing macrophages (0, no macrophages; 1, 0–25%; 2, 25–50%; 3, 50–75%; or 4, 75–100%). Primary mAb were: CD4+ T cells (anti‐CD4, GK1.5), macrophages (anti‐CD68, FA/11), neutrophils (anti‐Gr‐1, RB6‐8C5) with isotype control mAb. Kidneys were digested with 1 ml Hanks’ buffered saline solution, 4 mg/ml collagenase D and 100 μg/ml DNase I (30 min at 37°C), red blood cells lysed and CD45+ cells isolated, using CD45 microbeads (Miltenyi) and cell depletion columns.26
Flow cytometry
Cells were stained with mAb from eBioscience: CD45.1 (A20), CD45.2 (104), CD4 (RM4‐5), inducible T‐cell co‐stimulator (ICOS; 7E.17G9), glucocorticoid‐induced tumour necrosis factor receptor (GITR; DTA‐1), IL‐17A (17B7); BD Biosciences: CD25 (PC61), CTLA‐4 (CD152, UC10‐4F10‐11), CD44 (IM7), CD69 (H1.2F3), IFN‐γ (XMG1.2); and Biolegend: Helios (22F6), CD45 (30‐F11) and propidium iodide. Intracellular cytokine staining was performed using an eBioscience Foxp3 fixation/permeabilization kit for IL‐17A and BD Biosciences Cytofix/Cytoperm for IFN‐γ. Cells were stimulated with 50 ng/ml PMA and 1 μg/ml ionomycin (1 hr), then 10 μg/ml brefeldin A (4 hr) before staining and fixation/permeabilization. FACS was performed on a FACSCanto II instrument (BD Biosciences). Data were assessed using flowjo software (Treestar, Ashland, OR).
Results
iTreg phenotype following in vitro culture
Culture of naive CD4+ T cells in Treg polarizing conditions (ATRA, TGF‐β1, IL‐2, anti‐IFN‐γ and anti‐IL‐4 mAb) induced Foxp3 expression in > 90% of CD4+ T cells (referred to as ‘iTregs’; Fig. 1a). After 5 days of culture, iTreg cells produced IL‐10, IFN‐γ and tumour necrosis factor (TNF), with minimal IL‐6 and IL‐17A production (Fig. 1b). The medium and cytokine mix was supplemented with a neutralizing anti‐IL‐12p40 mAb, to limit IL‐12 and IL‐23 signalling, required for IFN‐γ and IL‐17A production by the cultured cells (referred to as ‘iTregs+anti‐IL‐12p40’), resulting in reduced IFN‐γ, TNF and IL‐10 production, with comparable Foxp3 expression to iTregs (Fig. 1a,b). The iTregs and iTregs+anti‐IL‐12p40 expressed surface markers consistent with Treg cells, including GITR and ICOS,27, 28, 29, 30 with low expression of the nuclear transcription factor Helios, (denoting nTreg cells31, 32; Fig. 1c). The iTregs+anti‐IL‐12p40 produced more TGF‐β1 than iTregs (Fig. 1d). Both types of iTreg cells suppressed Teff cell proliferation in vitro more effectively than nTreg cells, with anti‐IL‐12p40 mAb induction further improving the suppressive capacity of iTregs (Fig. 1e,f).
Figure 1.
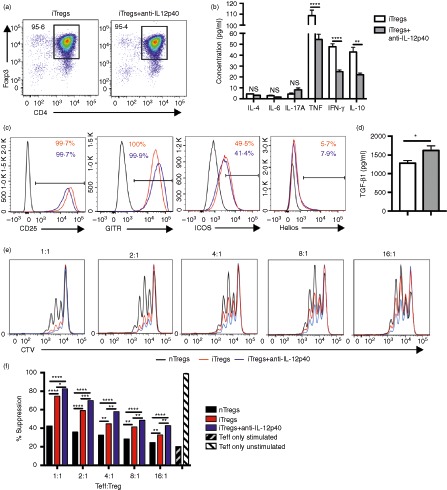
In vitro cultured induced regulatory T cells (iTregs) and iTreg cells treated with anti‐interleukin‐12p40 (iTregs+IL‐12p40) monoclonal antibody have a regulatory T‐cell phenotype. (a) Representative FACS plot showing CD4+ Foxp3+ expression on day 5 of in vitro culture for iTregs and iTregs+anti‐IL‐12p40. (b) Assessment of supernatant at day 5 from cultured iTregs and iTregs+anti‐IL‐12p40 for expression of IL‐4, IL‐6, IL‐17A, tumour necrosis factor (TNF), interferon‐γ) (IFN‐γ) and IL‐10 (cells harvested from different wells, n = 6 per group). (c) Representative histograms showing the expression of the surface markers CD25, GITR, ICOS and the nuclear transcription factor Helios, by iTregs and iTregs+anti‐IL‐12p40, gated on CD4+ Foxp3+ cell populations, following 5 days of culture. Black line represents fluorescence minus one control, red line represents iTregs, blue line represents iTregs+anti‐IL‐12p40. (d) Assessment of supernatant at day 5 from cultured iTregs and iTregs+anti‐IL‐12p40 for expression of transforming growth factor‐β1 (TGF‐β1) (cells harvested from different wells, n = 6 per group). (e) Representative histograms from the Treg suppressive assay, demonstrating effector T cells (Teff) (labelled with CTV) proliferation following 72 hr of in vitro co‐culture with Treg cells. Teff : Treg ratio is displayed above each FACS plot. Co‐cultures were plated in triplicate and Teff proliferation was analysed by FACS. Black, red and blue lines indicate nTreg cells (sorted from naive Foxp3‐GFP + mice), cultured iTregs and iTregs+anti‐IL‐12p40, respectively. (f) Percentage of suppression of Teff cell proliferation at varying Teff : Treg ratios. Results represent the mean of co‐cultures performed in triplicate, on two separate occasions. *P < 0·05, **P < 0·01, ****P < 0·0001. CTV, Cell Trace Violet; NS, not significant.
Evaluation of chemokine receptor, surface marker and transcription factor mRNA expression (Fig. 2a–m) found that iTregs and iTregs+anti‐IL‐12p40 had reduced CCR5 and CCR6 expression (Fig. 2b,c), but increased CCR8 expression compared with nTreg cells (Fig. 2e). The iTregs+anti‐IL‐12p40 had enhanced CD103 (Itgae) expression (Fig. 2i). Both types of iTreg cells had similar CTLA‐4 expression to nTreg cells (Fig. 2j). Although there was no difference in Tbet mRNA expression, iTregs and iTregs+anti‐IL‐12p40 had less GATA3, but greater Rorc mRNA expression (encoding RORγt) than nTreg cells (Fig. 2k–m).
Figure 2.
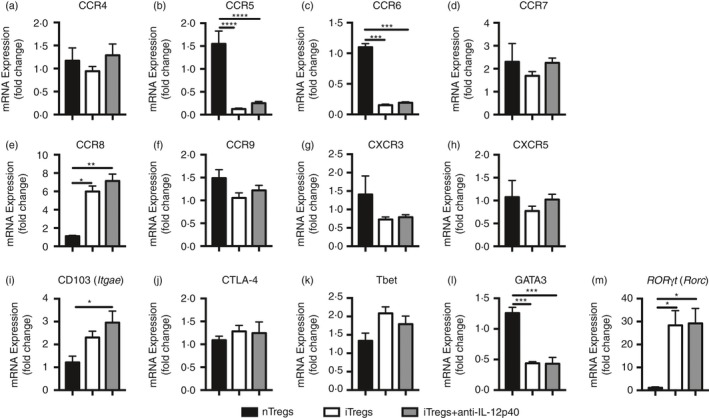
RT PCR expression of chemokine receptors, regulatory molecules and T‐cell nuclear transcription factors highlights some differences between natural regulatory T cells (nTregs) (from sorted GFP + cells from naive Foxp3‐GFP mice), induced regulatory T cells (iTregs) and iTregs+anti‐interleukin‐12p40 (IL‐12p40). mRNA was extracted from 1 × 106 cells collected on different occasions (sorted nTregs, n = 4; cultured iTregs and iTregs+anti‐IL12p40, n = 6). Gene of interest has been compared to 18S expression and presented as relative expression to nTregs (control group). (a) CCR4, (b) CCR5, (c) CCR6, (d) CCR7, (e) CCR8, (f) CCR9, (g) CXCR3, (h) CXCR5, (i) CD103 (Itgae), (j) CTLA‐4, (k) Tbet, (l) GATA3 and (m) ROR γt (Rorc) between groups. *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Effect of iTregs and iTregs+anti‐IL‐12p40 in modulating immune responses to SG in vivo
Different doses of iTregs or iTregs+anti‐IL‐12p40 were transferred into CD45.1 (Ly5.1) congenic mice that were then sensitized to SG, the nephritogenic antigen used in the RPGN model. Dermal DTH responses were assessed 10 days later. At transfer, > 90% of the cultured iTreg cells were Foxp3+ (data not shown). DTH responses were not reduced in mice treated with iTregs (between 1 × 106 and 10 × 106 cells/mouse), but transfer of 10 × 106 iTregs+anti‐IL‐12p40 into mice suppressed dermal DTH (Fig. 3a, g).
Figure 3.
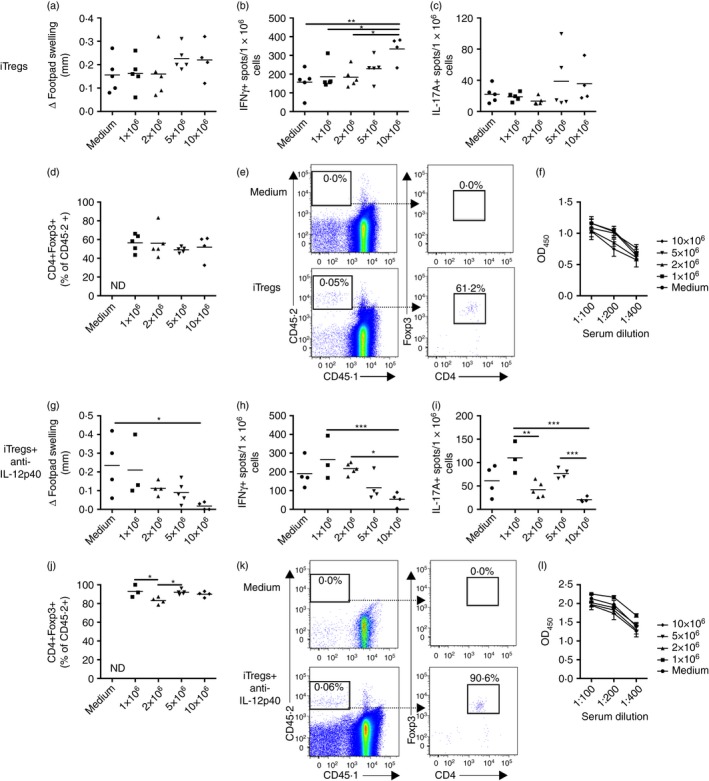
At a dose of 10 × 106 cells/mouse, induced regulatory T cells (iTregs) enhanced, but iTregs+anti‐interleukin‐12p40 (IL‐12p40) attenuated, T helper type 1 (Th1) immune responses in mice sensitized to sheep globulin (SG) for 10 days. Ly5.1 congenic mice received different doses of iTregs or iTregs+anti‐IL12p40 at the time of sensitization to SG. Results for iTreg transfers: (a) Measurement of dermal delayed‐type hypersensitivity (DTH) by measurement of footpad swelling 24 hr after re‐challenge with SG into footpad (assessed 10 days after sensitization to SG). EliSpot measurement of (b) IFN γ + and (c) IL‐17A+ spots/1 × 106 stimulated splenocytes. (d) Proportion of transferred cells (CD45.2+) retaining CD4 and Foxp3 expression, recovered from the spleen of recipient (Ly5.1 congenic) mice (3 × 106 splenocytes were stained for flow cytometry, gating on live single cells, with ≥ 1 million events collected per mouse). (e) Representative FACS plots of splenocytes from mice receiving medium (control) or 10 × 106 iTregs. (f) Serum mouse anti‐sheep IgG titres. Results for iTregs+anti‐IL‐12p40 transfers: (g) Measurement of dermal DTH. EliSpot measurement of (h) IFN‐γ + and (i) IL‐17A+ spots/1 × 106 stimulated splenocytes. (j) Proportion of transferred cells (CD45.2+) retaining CD4 and Foxp3 expression, recovered from the spleens of recipient (Ly5.1 congenic) mice. (k) Representative FACS plots of splenocytes from mice receiving medium (control) or 10 × 106 iTregs+anti‐IL‐12p40. (l) Serum mouse anti‐sheep IgG titres. *P < 0·05, **P < 0·01, ***P < 0·001.
Systemic Th1 responses (IFN‐γ) were increased in mice receiving 10 × 106 iTregs, but reduced in mice given 10 × 106 iTregs+anti‐IL‐12p40 (Fig. 3b,h). Although similar systemic Th17 responses (IL‐17A) were seen between controls and mice treated with iTregs, IL‐17A was reduced in mice receiving 10 × 106 iTregs+anti‐IL‐12p40 compared with lower doses (Fig. 3c,i). A population of transferred cells (CD45.2+) could be identified in spleens of recipient Ly5.1 mice, demonstrating successful transfer and survival of the cultured cells. Compared with > 90% Foxp3 expression by iTreg cells at transfer, Foxp3 expression was reduced (to ~49–56%) in the recovered iTregs (Fig. 3d,e), suggesting that the regulatory phenotype of these cells is unstable in vivo. Transferred iTregs+anti‐IL‐12p40 were more stable, with ~83–93% being Foxp3+ (Fig. 3j,k). Humoral immunity, measured by serum anti‐SG IgG titres, was not altered in mice receiving iTregs or iTregs+anti‐IL‐12p40 (Fig. 3f,l). Therefore, iTreg cells cultured without anti‐IL‐12p40 were more unstable and promoted Th1 responses in vivo whereas mice receiving 10 × 106 iTregs+anti‐IL‐12p40 had reduced cellular immunity and better maintained their regulatory phenotype.
Transfer of iTreg cells in experimental RPGN
To determine if iTreg transfer would protect mice from GN, mice were sensitized to SG and injected with 10 mg of anti‐GBM globulin 4 days later.33 Then, 10 × 106 iTregs, iTregs+anti‐IL‐12p40 or medium (control) was transferred into mice on the days of sensitization and anti‐GBM globulin administration. The experiment was terminated 8 days after anti‐GBM globulin because the mice developed severe GN, with a tendency for reduced survival in the medium‐treated and iTreg‐treated groups (see Supplementary material, Fig S1a; P = 0·08). In mice killed 8 days after anti‐GBM globulin, severe GN with widespread segmental necrosis was observed (see Supplementary material, Fig. S1b).
A lower dose (5 mg) of anti‐GBM globulin was administered to sensitized mice, for milder injury, to allow better assessment of the iTreg cells’ immunomodulatory capacity. 10 × 106 iTregs, iTregs+anti‐IL‐12p40 or medium were transferred into mice on the day of sensitization. There were no differences in functional or histological renal injury between groups (Fig. 4a–e). While CD4+ T‐cell and neutrophil recruitment to glomeruli were unchanged, glomerular macrophage infiltration was increased in mice treated with iTregs+anti‐IL‐12p40 (Fig. 4f–h). Interstitial CD4+ T‐cell, macrophage and neutrophil recruitment was similar between groups (Fig. 4i–k). Systemic immune responses were increased in mice receiving iTregs+anti‐IL‐12p40, with increased IFN‐γ production and a trend towards greater IL‐17A production (Fig. 5a,b). Assessment of the transferred (CD45.2+) cells recovered from the spleen showed that only 62% and 64% of the iTregs and iTregs+anti‐IL‐12p40, respectively, remained Foxp3+ (Fig. 5c). A small population of CD45.2+ cells were identified among renal CD4+ cells in both groups receiving cells, but the proportion of CD45.2+ cells that maintained Foxp3 expression was low (Fig. 5d,e). Serum antigen‐specific antibodies were reduced in iTregs+anti‐IL‐12p40‐treated mice compared with medium treated mice at a serum dilution of 1 : 400 (Fig. 5f). Therefore, iTregs, induced with or without anti‐IL‐12p40, do not protect against the development of RPGN, but enhance systemic Th1 immunity and on migration to the kidney lose Foxp3 expression and regulatory phenotype.
Figure 4.
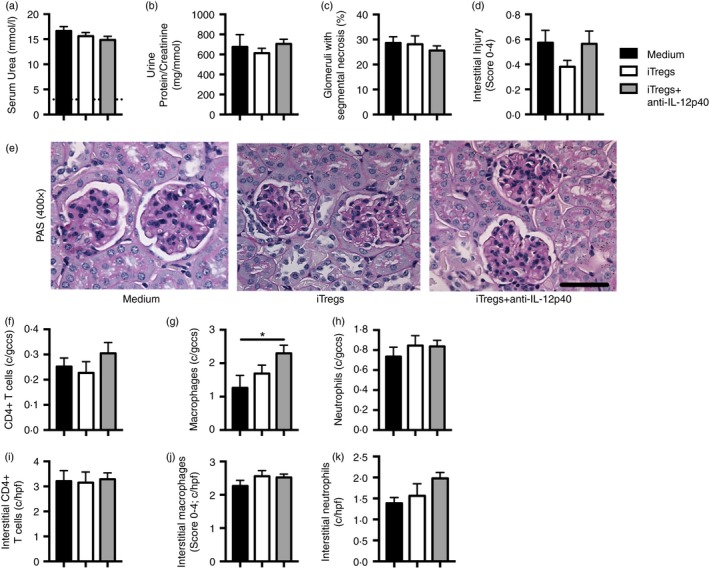
Induced regulatory T cells (iTregs) and iTregs+anti‐interleukin‐12p40 (IL‐12p40) ‐treated mice were not protected from anti‐glomerular basement membrane (GBM) disease in a model of milder rapidly progressive glomerular nephritis (RPGN). Ly5.1 congenic mice were sensitized subcutaneously to sheep globulin/Freund's complete adjuvant (SG/FCA) and given medium (n = 7), 10 × 106 iTregs (n = 8) or 10 × 106 iTregs+anti‐IL‐12p40 (n = 9). Intravenous sheep anti‐mouse GBM globulin (5 mg) was administered 4 days later, before mice were killed after a further 10 days. Renal injury was assessed by (a) serum urea (dotted line represents non‐nephritic WT mice; n = 4), (b) urine protein/creatinine ratio, (c) percentage of glomeruli with segmental necrosis and (d) interstitial injury score. (e) Representative images of PAS‐stained glomeruli (400×; scale bar = 50 μm). Immunohistochemical staining on periodate lysine paraformaldehyde‐fixed frozen kidney sections was performed to quantify (f) CD4+ cells, (g) macrophages and (h) neutrophils within glomeruli and (i) CD4+ cells, (j) macrophages and (k) neutrophils within the cortical interstitium. *P < 0·05, c/gcs, cells per glomerular cross‐section; c/hpf, cells per high‐power field.
Figure 5.
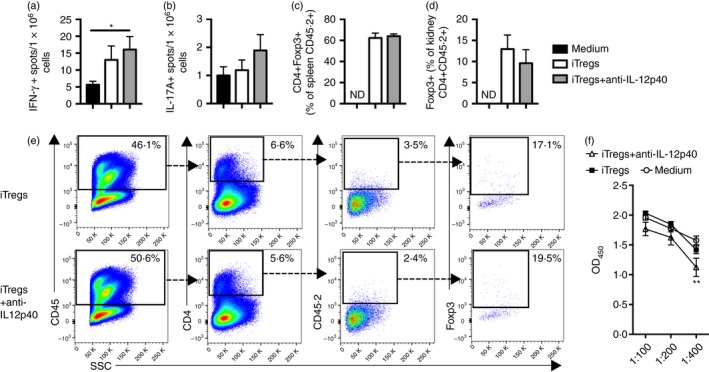
Induced regulatory T cells (iTregs) +anti‐interleukin‐12p40 (IL‐12p40) ‐treated mice, with milder rapidly progressive glomerular nephritis (RPGN), had enhanced T helper type 1 (Th1) cellular immune responses, with iTregs and iTregs+anti‐IL‐12p40 demonstrating an unstable phenotype and restricted renal recruitment. EliSpot measurement of (a) IFN‐γ + and (b) IL‐17A+ spots/1 × 106 stimulated splenocytes, respectively, in mice treated with either medium (n = 7), 10 × 106 iTregs (n = 8) or 10 × 106 iTregs+anti‐IL‐12p40 (n = 9). (c) Proportion of splenocytes expressing CD4 and Foxp3 on CD45.2+ cells from recipient (Ly5.1 congenic) mice, highlighting a change in phenotype of the transferred iTregs and iTregs+anti‐IL‐12p40 (3 × 106 splenocytes were stained for flow cytometry, gating on live single cells, with ≥ 1 million events collected per mouse). (d) Proportion of renal CD45.2+ cells expressing Foxp3 in mice receiving iTregs or iTregs+ani‐IL‐12p40, assessed by flow cytometry. (e) Concatenated FACS plots of renal leucocytes, demonstrating the presence of a small population of transferred iTregs and iTregs+anti‐IL‐12p40, with diminished Foxp3 expression, within the kidney. (f) Serum mouse anti‐sheep IgG titres (** result for medium versus iTreg+anti‐IL‐12p40 group). *P < 0·05, **P < 0·01.
A suppressive iTreg cell phenotype could not be promoted through the inhibition of RORγt expression
Recent reports have suggested that Treg cells are a heterogeneous population, with some Treg cells acquiring a Th1 and Th17 phenotype.34, 35, 36, 37 To explore whether deleting RORγt stabilized their phenotype, iTreg cells were generated from naive CD4+ RORγt–/– T cells. After 5 days of culture, RORγt–/– iTreg cells had comparable Foxp3 expression to iTreg cells (data not shown), and produced significantly less IL‐17A, TNF and TGF‐β1, comparable IFN‐γ and increased IL‐10 (see Supplementary material, Fig. S2a,b). However, transfer of RORγt–/– iTreg cells into mice subjected to experimental RPGN did not limit renal injury (see Supplementary material, Fig. S2c–e).
iTreg and iTreg+anti‐IL‐12p40 phenotype changes upon transfer into sensitized mice
We hypothesized that more iTregs and iTregs+anti‐IL‐12p40 express IFN‐γ and IL‐17A after transfer into mice with antigen‐driven inflammation. Ly5.1 congenic mice sensitized to SG had 10 × 106 iTregs, iTregs+anti‐IL‐12p40 or medium transferred into them, and the mice were killed 6 days later, so the proportion of transferred cells (CD45.2+) expressing IFN‐γ and IL‐17A could be determined. Both iTregs and iTregs+anti‐IL‐12p40 recovered from the spleens of sensitized mice showed loss of Foxp3 expression (72·2 ± 2·4% and 69·3 ± 2·4% of recovered CD45.2+ CD4+ cells, respectively, were Foxp3+) and demonstrated a significant increase in IFN‐γ expression compared with before transfer (Fig. 6a,b). Although very few iTregs or iTregs+anti‐IL‐12p40 produced IL‐17A during in vitro culture, there was a trend towards increased IL‐17A expression by these cells after transfer into sensitized mice (Fig. 6c). These data confirm that the iTreg and iTregs+anti‐IL‐12p40 phenotype is unstable, and that they produce Teff cytokines after transfer into mice that are sensitized to the nephritogenic antigen.
Figure 6.
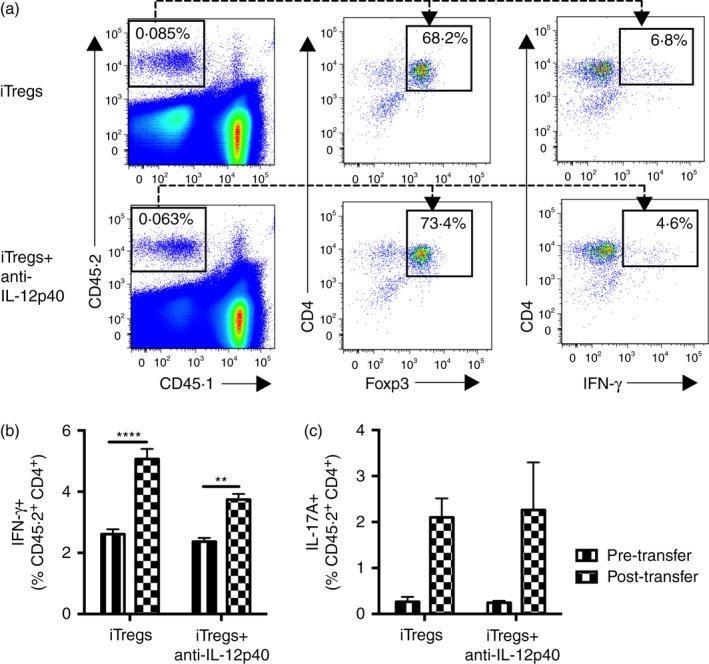
Induced regulatory T cells (iTregs) and iTregs+anti‐interleukin‐12p40 (IL‐12p40) change phenotype 6 days after transfer into mice sensitized to sheep globulin (SG), with increased IFN‐γ expression. Ly5.1 congenic mice were sensitized to 0.5mg SG in Freund's complete adjuvant subcutaneously and received 10 × 106 iTregs (n = 7) or iTregs+anti‐IL‐12p40 (n = 9) intravenously, before euthanasia 6 days later. Immediately pre‐transfer iTregs and iTregs+anti‐IL‐12p40 were assessed by FACS (n = 3, taken from different culture wells). (a) Concatenated FACS plots of splenocytes from recipient mice, assessing the proportion of transferred iTregs and iTregs+anti‐IL‐12p40 (CD45.2+) that were CD4+ Foxp3+ and CD4+ IFN‐γ +. (b) Comparison of the proportion of iTregs and iTregs+anti‐IL‐12p40, recovered from the spleens of recipient mice, expressing interferon‐γ (IFN‐γ) pre‐ and post‐transfer. iTregs and iTregs+anti‐IL‐12p40 were identified by flow cytometry as CD45.2+ CD4+ cells. (c) Comparison of the proportion of iTregs and iTregs+anti‐IL‐12p40, recovered from the spleens of recipient mice, expressing IL‐17A pre‐ and post‐transfer. **P < 0·01, ****P < 0·0001.
iTreg+anti‐IL‐12p40 induced from effector CD4+ Foxp3– T cells do not protect mice from glomerulonephritis
To establish if iTreg cells derived from effector CD4+ T cells would be more suppressive in vitro and in vivo than iTreg cells generated from naive CD4+ cells, naive Foxp3‐GFP mice were sensitized to SG 10 days before isolation of CD4+ Foxp3– and CD4+ Foxp3+ cells. Using CD4+ Foxp3– cells from sensitized mice, iTreg cells were generated (including the addition of anti‐IL‐12p40 in cultures). Two populations of control cells were generated: sorted CD4+ Foxp3+ Treg cells (representing a mixed population of nTreg cells and endogenously induced Treg cells in the sensitized donor mice), cultured with IL‐2, anti‐CD3 and anti‐CD28, and Teff cells from sorted CD4+ Foxp3– cells cultured with IL‐2, anti‐CD3, anti‐CD28 and anti‐IL‐4. Higher proportions of iTregs+anti‐IL‐12p40 (from either naive CD4+ and sensitized CD4+ Foxp3– cells) expressed Foxp3 than control Treg cells, whereas Foxp3 expression in Teff cells was minimal (Fig. 7a). Treg cells, both types of iTregs+anti‐IL‐12p40 and Teff cells had high CD25 and GITR expression, but only Treg cells from sorted CD4+ Foxp3+ cells exhibited a population of cells with high Helios expression (representing a mixed population of thymically derived Treg cells and peripherally induced Treg cells from sensitized mice; Fig. 7b). Both types of iTregs+anti‐IL‐12p40 had comparable production of IL‐4, IL‐6, IL‐17A, IFN‐γ and TNF to Treg cells, but reduced IL‐10. Teff cells produced more IL‐4, TNF, IFN‐γ and IL‐10 than Treg cells or iTregs+anti‐IL‐12p40 from naive or sensitized cells (Fig. 7c). TGF‐β1 levels were highest in iTregs+anti‐IL‐12p40 from sensitized CD4+ Foxp3– cells compared with the other groups (Fig. 7d).
Figure 7.
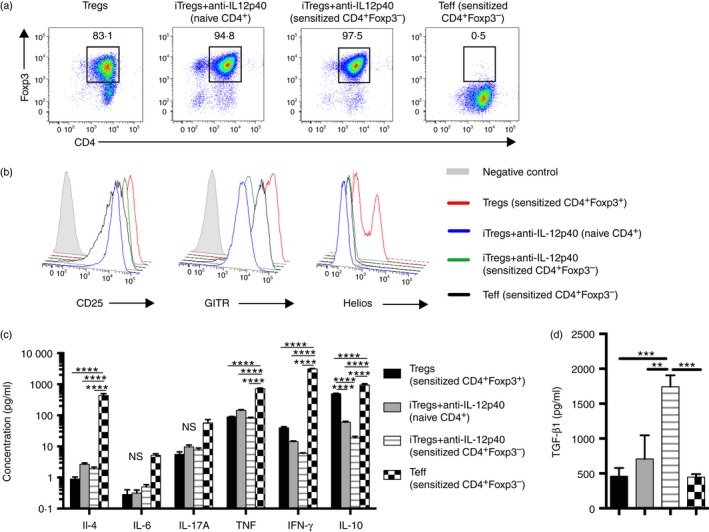
In vitro‐generated induced regulatory T cells (iTregs) +anti‐interleukin‐12p40 (IL‐12p40) from CD4+ Foxp3– cells from mice immunized to sheep globulin (SG) produce more transforming growth factor‐β1 (TGF‐β1) than iTregs+anti‐IL‐12p40 from naive CD4+ cells. To assess whether the phenotype of iTreg cells generated from CD4+ Foxp3– cells from sensitized mice would be more regulatory than those from naive CD4+ cells, naive Foxp3‐GFP mice were sensitized with 0.5mg SG/Freund's complete adjuvant for 10 days, before cell sorting for CD4+ Foxp3– cells, and cells were cultured for 5 days, with the cytokine cocktail including anti‐IL‐12p40. Control groups were Treg cells, using the CD4+ Foxp3+ cells from the immunized mice (cultured with anti‐CD3, anti‐CD28 and IL‐2), and effector T cells (Teffs) from the CD4+ Foxp3– cells (cultured with anti‐CD3, anti‐CD28, anti‐IL‐4 and IL‐2). All groups were cultured with their respective cytokine cocktail for 3 days, then medium and IL‐2 were replaced. Cells and supernatant were harvested at day 5. (a) Representative FACS plot showing CD4+ Foxp3+ expression on day 5 of in vitro culture for Treg cells, iTregs+anti‐IL‐12p40 from naive CD4+ or sensitized CD4+ Foxp3– cells and Teff cells. (b) Representative surface markers CD25, GITR, and the nuclear transcription factor Helios, gated on CD4+ Foxp3+ cell populations, following 5 days of culture. (c) Assessment of supernatant at day 5 for expression of IL‐4, IL‐6, IL‐17A, tumour necrosis factor (TNF), interferon‐γ (IFN‐γ) and IL‐10 and (d) TGF‐β1 (cells harvested from different wells; n = 5 for Treg cells and n = 6 per other groups). **P < 0·01, *** P < 0·001 ****P < 0·0001.
Comparing expression of chemokine receptor expression on the four types of cells cultured ex vivo for 5 days revealed both similarities and differences (Fig. 8a–m). The iTregs+anti‐IL‐12‐p40 derived from naive and immunized mice were similar in expression of most chemokine receptors, but iTregs+anti‐IL‐12p40 from immunized mice expressed more CCR9, CXCR5 and CD103. Compared with iTregs+anti‐IL‐12p40 from naive CD4+ T cells, Treg cells expressed more CTLA4. Teff cells expressed more CCR4, CCR6, CCR7 and CCR9 than both types of iTregs+anti‐IL‐12p40. They also expressed less CD103 and RORγt but more Tbet and GATA3. Taken together, these in vitro data presented in Figs 7 and 8 indicate that iTregs+anti‐IL‐12p40 from sensitized CD4+ Foxp3– cells have a suppressive phenotype.
Figure 8.
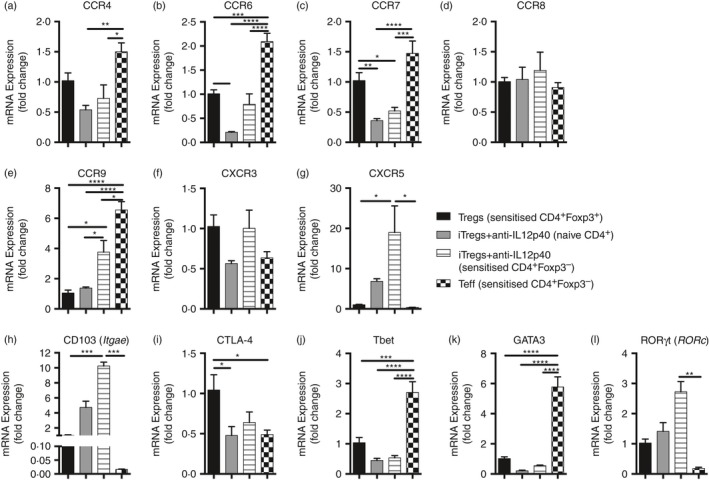
RT PCR expression of chemokine receptors, regulatory molecules and T‐cell nuclear transcription factors highlights some differences between induced regulatory T cells (iTregs) +anti‐interleukin‐12p40 (IL‐12p40) (generated from naive CD4+ T cells and CD4+ Foxp3– cells from mice sensitized to sheep globulin), Tregs and effector T (Teff) cells. Naive Foxp3‐GFP mice were sensitized to sheep globulin for 10 days, spleens and lymph nodes were harvested, and CD4+ Foxp3– and CD4+ Foxp3+ cells were sorted. Sensitized CD4+ Foxp3– cells were used to generate iTregs+anti‐IL‐12p40 and Teff. Sensitized CD4+Foxp3+ cells were used to generate Treg cells. The iTregs+anti‐IL‐12p40 were also cultured from naive CD4+ T cells. mRNA was extracted from 1 × 106 cells (Treg cells n = 5; other groups n = 6). Gene of interest has been compared to 18S expression and presented as relative expression to Treg cells. (a) CCR4, (b) CCR6, (c) CCR7, (d) CCR8, (e) CCR9, (f) CXCR3, (g) CXCR5, (h) CD103 (Itgae) (i) CTLA‐4, (j) Tbet, (k) GATA3 and (l) ROR γt (Rorc) between groups. *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
However, transfer of iTregs+anti‐IL‐12p40 derived from these sorted CD4+ Foxp3– cells from sensitized mice did not attenuate accelerated anti‐GBM glomerulonephritis. Cells (10 × 106 iTregs+anti‐IL‐12p40 induced from CD4+ Foxp3– cells from sensitized mice; 98% CD4+ Foxp3+ at transfer) or medium were transferred into mice on the day of sensitization. Serum urea levels were similar between groups, but mice receiving iTregs+anti‐IL‐12p40 (from sensitized CD4+ Foxp3– cells) had worse proteinuria and glomerular segmental necrosis 10 days after receiving sheep anti‐mouse GBM antibody (Fig. 9a–c). Tubulointerstitial injury was similar between groups (Fig. 9d). More macrophages were identified within the glomeruli of mice receiving iTregs+anti‐IL‐12p40 (from sensitized CD4+ Foxp3– cells), but glomerular CD4+ T cells and neutrophils and interstitial CD4+ T cells, macrophages and neutrophils were comparable between groups (Fig. 9e–j). More stimulated splenocytes made IFN‐γ, with a trend to increased IL‐17A production, whereas humoral immune responses did not differ between groups (Fig. 9k–m). Hence, similar to iTreg cells generated from naive CD4+ Foxp3– cells, iTreg cells derived from sensitized CD4+ Foxp3– cells did not attenuate experimental glomerulonephritis.
Figure 9.
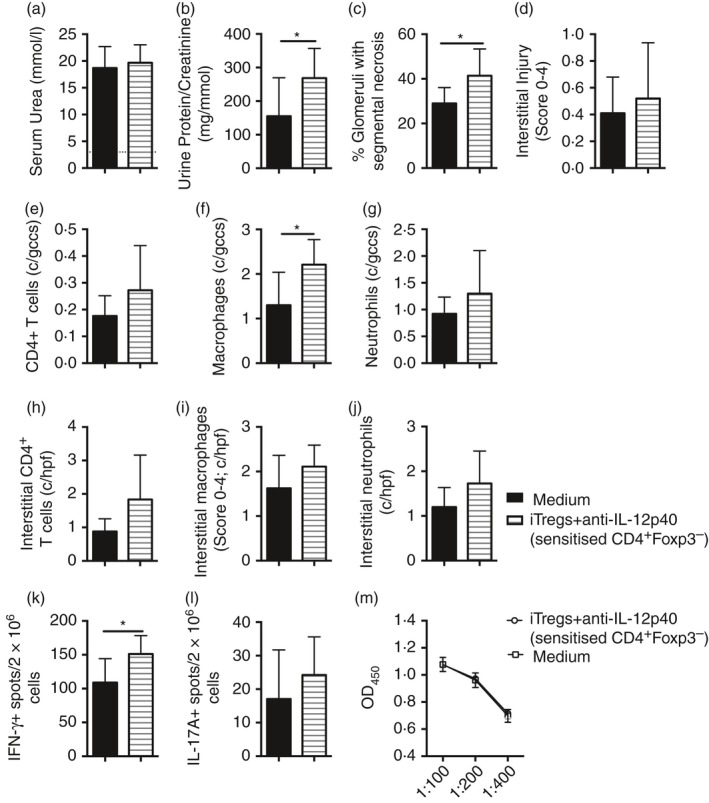
Induced regulatory T cells (iTregs) +anti‐interleukin‐12p40 (IL‐12p40), generated from CD4+ Foxp3– cells from mice sensitized to sheep globulin (SG), enhanced renal injury in a model of mild rapidly progressive glomerular nephritis (RPGN). Ly5.1 congenic mice were sensitized subcutaneously to 0.5mg SG/Freund's complete adjuvant and given medium (n = 8) or 10 × 106 iTregs+anti‐IL‐12p40 cultured from CD4+ Foxp3– cells from SG‐immunized Foxp3‐GFP mice (n = 9). Intravenous sheep anti‐mouse glomerular basement membrane (GBM) globulin (5 mg) was administered 4 days later, before mice were killed after a further 10 days. Renal injury was assessed by (a) serum urea (dotted line represents non‐nephritic wild‐type (WT) mice; n = 4), (b) urine protein/creatinine ratio, (c) percentage of glomeruli with segmental necrosis and (d) interstitial injury score. Immunohistochemical staining on periodate lysine paraformaldehyde‐fixed frozen kidney sections was performed to quantify (e) CD4+ cells, (f) macrophages and (g) neutrophils within glomeruli and (h) CD4+ cells, (i) macrophages and (j) neutrophils within the cortical interstitium. EliSpot measurement of (k) IFN‐γ + and (l) IL‐17A+ spots/1 × 106 stimulated splenocytes. (m) Serum mouse anti‐sheep IgG titres. *P < 0·05. c/gcs, cells per glomerular cross section; c/hpf, cells per high‐power field.
Discussion
Endogenous Treg cells comprise only a small proportion of the total CD4+ T‐cell population. Therefore, reliable methods of inducing Treg cells from naive CD4+ T cells or expanding nTreg cells ex vivo are desirable to explore their use as a cellular therapy for re‐establishing tolerance in autoimmune disease. We generated polyclonal iTreg cells ex vivo, and tested the hypothesis that these Foxp3+ iTreg cells could suppress disease and prevent nephritogenic immunity in experimental RPGN.
We generated polyclonal iTreg cells with > 90% Foxp3 expression from naive CD4+ T cells using ATRA and TGF‐β1, with a regulatory phenotype in vitro, consistent with published findings.12, 38, 39, 40, 41 As iTreg cells produced IFN‐γ and TNF, a neutralizing IL‐12p40 mAb was added to cultures, which further enhanced their suppressive ability in vitro. We assessed the regulatory capacity of iTregs and iTregs+anti‐IL‐12p40 in vivo using dermal DTH. Only iTregs+anti‐IL‐12p40 reduced DTH and pro‐inflammatory cytokine production. In the RPGN model, neither iTregs nor iTregs+anti‐IL‐12p40 protected against GN. Both demonstrated an unstable phenotype, with loss of Foxp3 expression evident in the transferred cells recovered from the spleen and kidney. Modifying the iTregs by using RORγt–/– CD4+ T cells, or by generating iTregs+anti‐IL‐12p40 from CD4+ Foxp3– T cells from SG‐sensitized mice, did not suppress inflammatory immune responses or RPGN. When polyclonal iTregs and iTregs+anti‐IL‐12p40 were transferred into SG‐sensitized mice, they not only lost Foxp3 expression, but a significantly greater proportion produced IFN‐γ compared with before transfer, demonstrating that they gained an effector phenotype in vivo.
Induced Treg cells have been shown to change phenotype in models of alloimmunity. Transfer of iTreg cells with mismatched bone marrow did not protect against experimental graft‐versus‐host disease; the majority of recovered iTreg cells (at 10 days) lost Foxp3 expression and produced IFN‐γ.42 Although ATRA enhanced the proportion of iTreg cells expressing Foxp3, it did not render the cells more phenotypically stable.42 Similarly, in murine xenograft‐versus‐host disease, ATRA expanded Treg cells from human CD4+ CD25+ cells produced IFN‐γ and IL‐17A after transfer.41 These data, together with our results showing both loss of Foxp3 and the induction of IFN‐γ in cells after transfer, show that the stability of iTreg cells in an inflammatory microenvironment is a significant issue.
Studies reporting successful modulation of autoimmunity with ex vivo generated iTreg cells use CD4+ T cells from transgenic mice with a T‐cell receptor specific for autoantigens,43, 44, 45, 46 suggesting that a further reason why iTreg cells did not suppress nephritogenic immunity may relate to their polyclonality. However, there are reports of polyclonal ex vivo iTreg cells regulating experimental inflammatory diseases and transplantation.9, 47, 48, 49, 50, 51
Using CD4+ T cells from SG‐sensitized mice (the nephritogenic antigen in the model of RPGN used), in vitro iTregs+anti‐IL‐12p40 derived from the CD4+ Foxp3– T cells produced more TGF‐β1, but less IL‐10 than CD4+ Foxp3+ Treg cells, with high Foxp3 expression, suggesting that they were not regulatory type 1 (Tr1) cells.52, 53 When transferred into the mild RPGN model, they had an injurious rather than a regulatory phenotype in vivo, worsening glomerular segmental necrosis and proteinuria, enhancing macrophage accumulation in glomeruli and IFN‐γ production from stimulated splenocytes, suggesting that these iTreg cells reverted to an effector phenotype.
Adoptive transfer studies of nTreg cells in experimental RPGN showed that the recipients had reduced systemic IFN‐γ and TNF production and fewer renal leucocytes, but in one study the transferred nTreg cells were found in secondary lymphoid organs, not within nephritic kidneys,17 suggesting that modulation of systemic immunity is an important mechanism of action by Treg cells. However, other studies have demonstrated that endogenous Treg cells home to the kidney in experimental RPGN, with Treg cell depletion altering both systemic immunity and renal Treg cell infiltrates,15, 16 suggesting that Treg cells can migrate to the kidney to suppress local inflammatory responses. Interactions between tissue chemokines and chemokine receptors on Treg cells are important for Treg migration to sites of inflammation, particularly in the dermis.54, 55, 56 Less is known about Treg cell localization to the kidney, but CCR6 is likely to be important.57, 58 Although there were a number of similarities in chemokine receptor expression in iTreg cells, both the Treg cells and the iTregs+anti‐IL‐12p40 from the sensitized CD4+ T cells had significantly less CCR6 expression than Teff cells. Differences in chemokine expression required for Teff and Treg cell homing to inflammatory sites might explain why iTreg cells were unable to suppress Teff cells in this model of RPGN, in contrast to other groups who have found iTreg cells (generated in a similar manner) to have a suppressive phenotype in models of colitis.20, 48
Despite high Foxp3 expression in our cultured iTregs and iTregs+anti‐IL‐12p40, RORγt expression was up‐regulated compared with nTreg cells. RORγt and Foxp3 can be co‐expressed in naive CD4+ T cells, and TGF‐β‐induced Foxp3 inhibits RORγt activity.59 Therefore, it is possible that during in vitro culture conditions, Foxp3 expression was sufficient to restrict RORγt‐mediated IL‐17A transcription, but the in vivo inflammatory milieu encountered by iTreg cells may have contributed to their loss of Foxp3 expression, removing antagonism of RORγt, promoting conversion to a Teff phenotype. However, transfer of RORγt–/– iTreg cells into experimental RPGN did not protect mice from injury, showing that excessive RORγt expression in itself was not the primary reason for the lack of effect of iTreg cells.
In conclusion, while Foxp3+ iTreg cells can be generated in vitro with TGF‐β1 and ATRA, and are able to suppress Teff cell proliferation in vitro, they were unstable in experimental RPGN, losing Foxp3 expression and promoting Th1 responses. These iTreg cells had a chemokine receptor expression profile that was different from that of nTreg cells, which may restrict their trafficking to the kidney. Hence, transfer of in vitro generated polyclonal iTreg cells is not yet a viable therapeutic strategy in GN and further work is required to stabilize their phenotype, to ensure that they will have suppressive, not detrimental pro‐inflammatory actions.
Disclosures
The authors declare no conflict of interest.
Supporting information
Figure S1. Induced regulatory T cells (iTregs) and iTregs+anti‐interleukin‐12p40 (IL‐12p40) did not protect mice from severe rapidly progressive glomerular nephritis (RPGN). (a) Survival between mice receiving medium (n = 5), 10 × 106 iTregs (n = 9) or iTregs+anti‐IL‐12p40 (n = 9) in a model of severe RPGN (using the accelerated anti‐glomerular basement membrane (GBM) disease model, with mice sensitized subcutaneously to sheep globulin/Freund's complete adjuvant, receiving 10 mg of sheep anti‐GBM globulin intravenously), terminated early at day 8 following sheep anti‐mouse GBM antibody administration (P = 0·08). (b) Representative PAS‐stained glomeruli from medium, iTreg‐treated and iTreg+anti‐IL‐12p40‐treated mice, showing severe glomerular segmental necrosis (400 ×; scale bar = 50 μm).
Figure S2. Assessment of the phenotype of induced regulatory T (iTreg) cells derived from RORγt–/– mice and the ability of these RORγt–/– iTreg cells to suppress renal injury and systemic immune responses in the model of severe rapidly progressive glomerular nephritis (RPGN). (a) Assessment of supernatant at day 5 from cultured iTreg cells and RORγt–/– iTreg cells for the expression of interleukin‐4 (IL‐4), IL‐6, IL‐17, tumour necrosis factor (TNF), interferon‐γ (IFN‐γ) and IL‐10 and (b) transforming growth factor‐β1 (TGF‐β1) (cells harvested from different wells, n = 6 per group). Ly5.1 congenic mice were sensitized subcutaneously to sheep globulin in Freund's complete adjuvant and given medium (n = 4), 10 × 106 iTreg cells (n = 6) or 10 × 106 iTregs+anti‐IL‐12p40 (n = 5). Intravenous sheep anti‐mouse glomerular basement membrane (GBM) globulin (10 mg) was administered 4 days later, and medium or 1·25 × 106 iTregs or iTregs+anti‐IL‐12p40 were transferred on the same day, before mice were killed after a further 10 days. Renal injury was assessed by (c) serum urea, (d) percentage of glomeruli with crescents and (e) percentage of glomeruli with segmental necrosis. *P < 0·05, **P < 0·01, ****P < 0·0001.
Acknowledgements
Sources of Support: National Health and Medical Research Council of Australia (NHMRC), grant numbers 1017559 (JRG) and 1048575 (ARK). JRG and ARK designed experiments, wrote and revised the manuscript. JRG performed all experiments and analysed the data. MAA provided assistance with renal digestions and FACS of the kidneys. SRH revised the manuscript.
Contributor Information
Joanna R. Ghali, Email: joanna.ghali@monash.edu, Email: joanna.ghali@monashhealth.org
A. Richard Kitching, Email: richard.kitching@monash.edu.
References
- 1. Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+ CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 2007; 178:2579–88. [DOI] [PubMed] [Google Scholar]
- 2. Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL et al Patients with antineutrophil cytoplasmic antibody‐associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression‐resistant effector cell population. Arthritis Rheum 2013; 65:1922–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdulahad WH, Stegeman CA, van der Geld YM, Doornbos‐van der Meer B, Limburg PC, Kallenberg CG. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener's granulomatosis in remission. Arthritis Rheum 2007; 56:20. [DOI] [PubMed] [Google Scholar]
- 4. Morgan MD, Day CJ, Piper KP, Khan N, Harper L, Moss PA et al Patients with Wegener's granulomatosis demonstrate a relative deficiency and functional impairment of T‐regulatory cells. Immunology 2010; 130:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rimbert M, Hamidou M, Braudeau C, Puechal X, Teixeira L, Caillon H et al Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody‐associated vasculitis. PLoS ONE 2011; 6:e18734. doi: 10.1371/journal.pone.0018734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cairns LS, Phelps RG, Bowie L, Hall AM, Saweirs WW, Rees AJ et al The fine specificity and cytokine profile of T‐helper cells responsive to the α3 chain of type IV collagen in Goodpasture's disease. J Am Soc Nephrol 2003; 14:2801–12. [DOI] [PubMed] [Google Scholar]
- 7. Salama AD, Chaudhry AN, Holthaus KA, Mosley K, Kalluri R, Sayegh MH et al Regulation by CD25+ lymphocytes of autoantigen‐specific T‐cell responses in Goodpasture's (anti‐GBM) disease. Kidney Int 2003; 64:1685–94. [DOI] [PubMed] [Google Scholar]
- 8. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 9. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N et al Conversion of peripheral CD4+ CD25– naive T cells to CD4+ CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coombes JL, Siddiqui KR, Arancibia‐Carcamo CV, Hall J, Sun CM, Belkaid Y et al A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF‐β and retinoic acid‐dependent mechanism. J Exp Med 2007; 204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M et al Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007; 317:256–60. [DOI] [PubMed] [Google Scholar]
- 12. Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor‐α favours regulatory T cell induction at the expense of IL‐17‐secreting T helper cell differentiation. Eur J Immunol 2007; 37:2396–9. [DOI] [PubMed] [Google Scholar]
- 13. Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM et al Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat‐3/Stat‐5 independent signaling pathway. Blood 2008; 111:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q et al Critical role of all‐trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A 2014; 111:E3432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ooi JD, Snelgrove SL, Engel DR, Hochheiser K, Ludwig‐Portugall I, Nozaki Y et al Endogenous foxp3+ T‐regulatory cells suppress anti‐glomerular basement membrane nephritis. Kidney Int 2011; 79:977–86. [DOI] [PubMed] [Google Scholar]
- 16. Paust HJ, Ostmann A, Erhardt A, Turner JE, Velden J, Mittrucker HW et al Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int 2011; 80:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H et al CD4+ CD25+ regulatory T cells inhibit experimental anti‐glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 2005; 16:1360–70. [DOI] [PubMed] [Google Scholar]
- 18. Steinmetz OM, Summers SA, Gan PY, Semple T, Holdsworth SR, Kitching AR. The Th17‐defining transcription factor RORγt promotes glomerulonephritis. J Am Soc Nephrol 2011; 22:472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maganto‐Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK et al Foxp3+‐inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol 2011; 187:3521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlsson F, Robinson‐Jackson SA, Gray L, Zhang S, Grisham MB. Ex vivo generation of regulatory T cells: characterization and therapeutic evaluation in a model of chronic colitis. Methods Mol Biol 2011; 677:47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kitching AR, Tipping PG, Holdsworth SR. IL‐12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol 1999; 29:1–10. [DOI] [PubMed] [Google Scholar]
- 22. Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol 2004; Chapter 3: 3–12. [DOI] [PubMed] [Google Scholar]
- 23. Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR et al The immunodominant myeloperoxidase T‐cell epitope induces local cell‐mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A 2012; 109:E2615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR. Immune modulation with interleukin‐4 and interleukin‐10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol 1997; 27:530–7. [DOI] [PubMed] [Google Scholar]
- 25. Kitching AR, Turner AL, Wilson GR, Semple T, Odobasic D, Timoshanko JR et al IL‐12p40 and IL‐18 in crescentic glomerulonephritis: IL‐12p40 is the key Th1‐defining cytokine chain, whereas IL‐18 promotes local inflammation and leukocyte recruitment. J Am Soc Nephrol 2005; 16:2023–33. [DOI] [PubMed] [Google Scholar]
- 26. Chan AJ, Alikhan MA, Odobasic D, Gan PY, Khouri MB, Steinmetz OM et al Innate IL‐17A‐producing leukocytes promote acute kidney injury via inflammasome and Toll‐like receptor activation. Am J Pathol 2014; 184:1411–8. [DOI] [PubMed] [Google Scholar]
- 27. Grant CR, Liberal R, Mieli‐Vergani G, Vergani D, Longhi MS. Regulatory T‐cells in autoimmune diseases: challenges, controversies and‐yet‐unanswered questions. Autoimmun Rev 2015; 14:105–16. [DOI] [PubMed] [Google Scholar]
- 28. Petrillo MG, Ronchetti S, Ricci E, Alunno A, Gerli R, Nocentini G et al GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev 2015; 14:117–26. [DOI] [PubMed] [Google Scholar]
- 29. Busse M, Krech M, Meyer‐Bahlburg A, Hennig C, Hansen G. ICOS mediates the generation and function of CD4+ CD25+ Foxp3+ regulatory T cells conveying respiratory tolerance. J Immunol 2012; 189:1975–82. [DOI] [PubMed] [Google Scholar]
- 30. Kornete M, Sgouroudis E, Piccirillo CA. ICOS‐dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol 2012; 188:1064–74. [DOI] [PubMed] [Google Scholar]
- 31. Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T et al Foxp3‐dependent and ‐independent molecules specific for CD25+ CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol 2006; 18:1197–209. [DOI] [PubMed] [Google Scholar]
- 32. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y et al Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010; 184:3433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Odobasic D, Ghali JR, O'Sullivan KM, Holdsworth SR, Kitching AR. Glomerulonephritis induced by heterologous anti‐GBM globulin as a planted foreign antigen. Curr Protoc Immunol 2014; 106:15.26.1–20. [DOI] [PubMed] [Google Scholar]
- 34. Zhou X, Bailey‐Bucktrout SL, Jeker LT, Penaranda C, Martinez‐Llordella M, Ashby M et al Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo . Nat Immunol 2009; 10:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komatsu N, Mariotti‐Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T‐cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A 2009; 106:1903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh‐hora M, Kodama T et al Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014; 20:62–8. [DOI] [PubMed] [Google Scholar]
- 37. Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K et al Identification of IL‐17‐producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 2009; 106:4793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benson MJ, Pino‐Lagos K, Rosemblatt M, Noelle RJ. All‐trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co‐stimulation. J Exp Med 2007; 204:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jhunjhunwala S, Chen LC, Nichols EE, Thomson AW, Raimondi G, Little SR. All‐trans retinoic acid and rapamycin synergize with transforming growth factor‐β1 to induce regulatory T cells but confer different migratory capacities. J Leukoc Biol 2013; 94:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma J, Liu Y, Li Y, Gu J, Liu J, Tang J et al Differential role of all‐trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J Leukoc Biol 2014; 95:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scotta C, Esposito M, Fazekasova H, Fanelli G, Edozie FC, Ali N et al Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4+CD25+FOXP3+ T regulatory cell subpopulations. Haematologica 2013; 98:1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beres A, Komorowski R, Mihara M, Drobyski WR. Instability of Foxp3 expression limits the ability of induced regulatory T cells to mitigate graft versus host disease. Clin Cancer Res 2011; 17:3969–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD et al Adaptive islet‐specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo . J Immunol 2006; 176:4730–9. [DOI] [PubMed] [Google Scholar]
- 44. DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen‐specific TGFβ‐induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol 2007; 179:4685–93. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen TL, Makhlouf NT, Anthony BA, Teague RM, DiPaolo RJ. In vitro induced regulatory T cells are unique from endogenous regulatory T cells and effective at suppressing late stages of ongoing autoimmunity. PLoS ONE 2014; 9:e104698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: antigen‐specific TGF β‐induced regulatory T cells suppress Th17‐mediated autoimmune disease. J Immunol 2008; 181:8209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF‐beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol 2008; 180:2830–8. [DOI] [PubMed] [Google Scholar]
- 48. Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P et al Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut 2006; 55:671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3+ regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol 2012; 4:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B et al Polyclonal CD4+ Foxp3+ Treg cells induce TGFβ‐dependent tolerogenic dendritic cells that suppress the murine lupus‐like syndrome. J Mol Cell Biol 2012; 4:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo X, Jie Y, Ren D, Zeng H, Zhang Y, He Y et al In vitro‐expanded CD4+CD25highFoxp3+ regulatory T cells controls corneal allograft rejection. Hum Immunol 2012; 73:1061–7. [DOI] [PubMed] [Google Scholar]
- 52. Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015; 12:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin‐10‐secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 2006; 212:28–50. [DOI] [PubMed] [Google Scholar]
- 54. Suga H, Sugaya M, Miyagaki T, Ohmatsu H, Okochi H, Sato S. CXCR3 deficiency prolongs Th1‐type contact hypersensitivity. J Immunol 2013; 190:6059–70. [DOI] [PubMed] [Google Scholar]
- 55. Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+ CD25high Foxp3+ regulatory T cells bear functional skin‐homing receptors. J Immunol 2006; 177:4488–94. [DOI] [PubMed] [Google Scholar]
- 56. Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C et al Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol 2001; 166:996–1002. [DOI] [PubMed] [Google Scholar]
- 57. Kluger MA, Luig M, Wegscheid C, Goerke B, Paust HJ, Brix SR et al Stat3 programs Th17‐specific regulatory T cells to control GN. J Am Soc Nephrol 2014; 25:1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A et al CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 2010; 21:974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD et al TGF‐β‐induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 2008; 453:236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Induced regulatory T cells (iTregs) and iTregs+anti‐interleukin‐12p40 (IL‐12p40) did not protect mice from severe rapidly progressive glomerular nephritis (RPGN). (a) Survival between mice receiving medium (n = 5), 10 × 106 iTregs (n = 9) or iTregs+anti‐IL‐12p40 (n = 9) in a model of severe RPGN (using the accelerated anti‐glomerular basement membrane (GBM) disease model, with mice sensitized subcutaneously to sheep globulin/Freund's complete adjuvant, receiving 10 mg of sheep anti‐GBM globulin intravenously), terminated early at day 8 following sheep anti‐mouse GBM antibody administration (P = 0·08). (b) Representative PAS‐stained glomeruli from medium, iTreg‐treated and iTreg+anti‐IL‐12p40‐treated mice, showing severe glomerular segmental necrosis (400 ×; scale bar = 50 μm).
Figure S2. Assessment of the phenotype of induced regulatory T (iTreg) cells derived from RORγt–/– mice and the ability of these RORγt–/– iTreg cells to suppress renal injury and systemic immune responses in the model of severe rapidly progressive glomerular nephritis (RPGN). (a) Assessment of supernatant at day 5 from cultured iTreg cells and RORγt–/– iTreg cells for the expression of interleukin‐4 (IL‐4), IL‐6, IL‐17, tumour necrosis factor (TNF), interferon‐γ (IFN‐γ) and IL‐10 and (b) transforming growth factor‐β1 (TGF‐β1) (cells harvested from different wells, n = 6 per group). Ly5.1 congenic mice were sensitized subcutaneously to sheep globulin in Freund's complete adjuvant and given medium (n = 4), 10 × 106 iTreg cells (n = 6) or 10 × 106 iTregs+anti‐IL‐12p40 (n = 5). Intravenous sheep anti‐mouse glomerular basement membrane (GBM) globulin (10 mg) was administered 4 days later, and medium or 1·25 × 106 iTregs or iTregs+anti‐IL‐12p40 were transferred on the same day, before mice were killed after a further 10 days. Renal injury was assessed by (c) serum urea, (d) percentage of glomeruli with crescents and (e) percentage of glomeruli with segmental necrosis. *P < 0·05, **P < 0·01, ****P < 0·0001.


