Abstract
Pioneering studies within the last few years have allowed the in vitro expansion of tissue‐specific adult stem cells from a variety of endoderm‐derived organs, including the stomach, small intestine, and colon. Expansion of these cells requires activation of the receptor Lgr5 by its ligand R‐spondin 1 and is likely facilitated by the fact that in healthy adults the stem cells in these organs are highly proliferative. In many other adult organs, such as the liver, proliferating cells are normally not abundant in adulthood. However, upon injury, the liver has a strong regenerative potential that is accompanied by the emergence of Lgr5‐positive stem cells; these cells can be isolated and expanded in vitro as organoids. In an effort to isolate stem cells from non‐regenerating mouse livers, we discovered that healthy gallbladders are a rich source of stem/progenitor cells that can be propagated in culture as organoids for more than a year. Growth of these organoids was stimulated by R‐spondin 1 and noggin, whereas in the absence of these growth factors, the organoids differentiated partially toward the hepatocyte fate. When transplanted under the liver capsule, gallbladder‐derived organoids maintained their architecture for 2 weeks. Furthermore, single cells prepared from dissociated organoids and injected into the mesenteric vein populated the liver parenchyma of carbon tetrachloride‐treated mice. Human gallbladders were also a source of organoid‐forming stem cells. Thus, under specific growth conditions, stem cells can be isolated from healthy gallbladders, expanded almost indefinitely in vitro, and induced to differentiate toward the hepatocyte lineage.
Keywords: gallbladder, noggin, organoids, R‐spondin 1, stem cells
Subject Categories: Methods & Resources, Stem Cells
Introduction
The identification and characterization of tissue‐specific adult stem cells is an important goal, not only in terms of understanding normal tissue biology and homeostasis, but also as a possible means toward the development of novel therapies. Among the many organs for which an enhanced understanding of stem cells could have clinical benefit, the liver ranks high, as there are many causes of impaired liver function and they are often associated with severe morbidity 1.
During embryogenesis, the liver originates from the foregut, which also gives rise to the stomach, small intestine, pancreas, and gallbladder 2. The stem cells of the small intestine have been characterized quite well. By lineage tracing experiments, these cells have been shown to reside at the base of the intestinal crypts and to express a number of stem cell markers, including the G protein‐coupled receptor Lgr5 3. Lgr5, a receptor for R‐spondin 1, potentiates Wnt signaling, which in turn stimulates cell division and helps maintain the stem cell phenotype. The small intestine stem cells can be isolated as Lgr5‐positive cells and, in the presence of R‐spondin 1, be propagated indefinitely in tissue culture, as organoids bearing crypt‐like structures 4, 5. It is likely that the high proliferative rate of Lgr5‐positive small intestine stem cells in vivo contributes to the ability of these cells to establish organoids in vitro.
Unlike the small intestine, proliferating cells are not abundant in adult healthy livers 6. However, when damaged, the liver exhibits regenerative capacity 7, 8. Partial hepatectomy leads to robust proliferation of hepatocytes and cholangiocytes, which are highly differentiated cells. However, if hepatocyte proliferation is impaired, for example, by chemical insult, then liver repair can be facilitated by the emergence of so‐called oval cells resident in the canals of Hering. Oval cells are bipotent and can differentiate into cholangiocytes (also known as biliary epithelial cells) or hepatocytes 6, 9, 10, 11, 12.
In mice, the contribution of oval cells and, more broadly, liver stem cells to acutely induced liver regeneration is limited 13, 14. Nevertheless, chemical damage induces the emergence of Lgr5‐positive cells in mouse livers, and in the presence of R‐spondin 1, these cells can be expanded in vitro as organoids that can differentiate along the cholangiocyte and hepatocyte lineages 15. Intrahepatic biliary duct (IHBD) cells from adult human livers can also be expanded as organoids in the presence of R‐spondin 1 16. Similar to mouse, these organoids express stem cell markers, such as Lgr5 and Prom1 (prominin‐1/CD133).
From a clinical perspective, obtaining an expandable liver cell population for transplantation would benefit patients with chronic liver diseases or with genetic defects. However, access to healthy livers, as a potential source of stem cells, is limited. Here, we propose an alternative, since we observed that stem/progenitor cells could be isolated easily from mouse gallbladders. These cells could be propagated for more than a year in tissue culture as organoids without the need for feeder layers and could be induced to partially differentiate toward the hepatocyte fate. Using a similar protocol, stem cells could also be isolated from human gallbladders.
Results
Establishment of liver and gallbladder 3D cell cultures
In an effort to isolate liver stem cells, non‐damaged livers together with their extrahepatic biliary ducts (EHBDs) and gallbladder were harvested from 2‐month‐old mice, minced, and incubated with PBS/EDTA for 2 h to generate small cell clusters. These cells were then embedded in Matrigel® and cultured in serum‐free media containing nicotinamide and a cocktail of growth factors (epidermal growth factor, EGF; fibroblast growth factor 10, FGF10; hepatocyte growth factor, HGF; R‐spondin 1; and noggin). Within a day, numerous spheroids (organoids) formed. These organoids could be propagated, by weekly passaging, for more than a year, suggesting that they contained stem cells (Fig EV1). Single cells isolated from these organoids were also capable of establishing organoids that, again, could be propagated for prolonged periods of time in tissue culture (data not shown).
Figure EV1. Organoid cultures prepared from liver/gallbladder tissues.
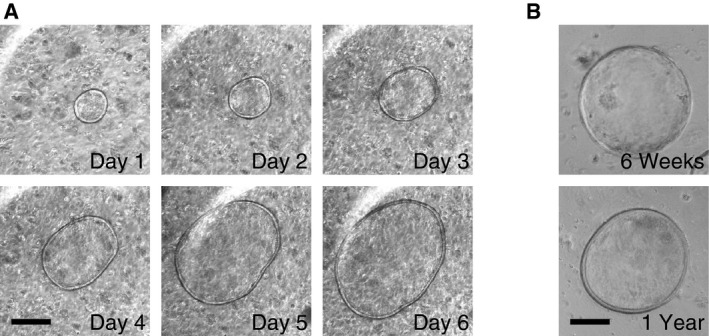
- The liver with the EHBDs and gallbladder were minced and cultured in Matrigel® in the presence of R‐spondin 1 and noggin. Phase‐contrast microscopy images of a representative organoid were captured every day over a six‐day period. Scale bar: 200 μm.
- Images of gallbladder organoids cultured for 6 weeks and 1 year. Scale bar: 200 μm.
The ability to obtain organoids from non‐damaged livers with such high efficiency was surprising to us, given that in previous studies, optimization of stem cell isolation was achieved either by carbon tetrachloride (CCl4)‐induced damage to the liver or by flow sorting for cells expressing specific stem cell markers 15. We, therefore, decided to perform a more careful dissection of the liver and associated biliary tree. Liver lobes were processed separately from the EHBDs and from the gallbladder. No spheroids were obtained from tissue fragments derived from the liver lobes, whereas the gallbladder was a rich source of spheroids. Some organoids were also derived from the EHBDs, although their number was much lower than the number of organoids obtained from the gallbladder (Fig 1A). This experiment was repeated several times using mice, whose age ranged from 7 days to 1 year, with identical results (Fig 1B). Thus, the organoids obtained in our initial experiments most likely originated from the gallbladder and EHBDs, rather than from the liver parenchyma itself. Overall, these data suggest that non‐damaged gallbladders are a source of stem/progenitor cells that can be propagated for extended time periods ex vivo.
Figure 1. Derivation of organoids from mouse gallbladder tissue.
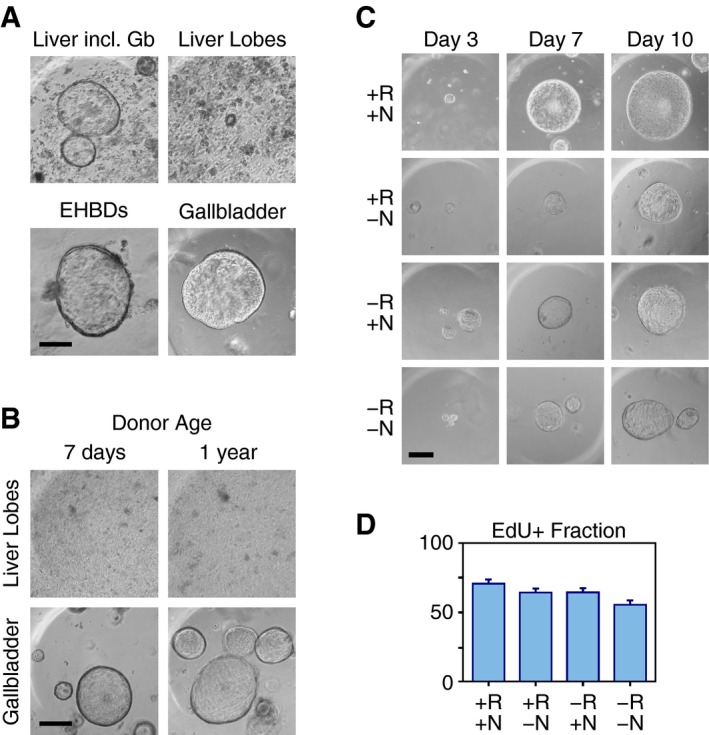
- Liver with the extrahepatic biliary ducts (EHBDs) and gallbladder (Liver incl. Gb), liver lobes, EHBDs, or gallbladder tissues were minced and cultured in Matrigel® in the presence of R‐spondin 1 and noggin. Phase‐contrast microscopy images were acquired 1 week later. Scale bar: 200 μm.
- Liver lobes or gallbladders harvested from 7‐day‐ or 1‐year‐old mice were minced and cultured in Matrigel® in the presence of R‐spondin 1 and noggin. Phase‐contrast microscopy images were acquired 1 week later. Scale bar: 200 μm.
- Phase‐contrast images of gallbladder organoids cultured for 3, 7, or 10 days in the presence (+) or absence (−) of R‐spondin 1 (R) and/or noggin (N), as indicated. The same organoid was photographed at each time point. Scale bar: 200 μm.
- Fraction of EdU‐positive (EdU+) cells in organoids cultured for 5 days in media containing R‐spondin 1 and/or noggin, as indicated. The results, presented as means and standard errors, are derived from three independent experiments, each examining five organoids per condition. Statistically significant differences: +R+N vs. −R−N (P < 0.001); +R−N vs. −R−N (P < 0.05); −R+N vs. −R−N (P < 0.05).
R‐spondin 1 and noggin facilitate growth of gallbladder organoids
The growth and survival of small intestine‐derived organoids is absolutely dependent on R‐spondin 1 and noggin 4, 5. Organoids derived from CCl4‐damaged livers also require R‐spondin 1 for survival beyond 1–2 weeks 15. To examine the dependency of gallbladder organoids on R‐spondin 1 and noggin, freshly isolated gallbladder cells were placed in Matrigel® with full media or full media lacking R‐spondin 1 or noggin or both. Organoids formed and could be propagated in all conditions tested. However, the organoids grew larger when both R‐spondin 1 and noggin were present (Fig 1C). The differences in organoid size were paralleled by differences in the fraction of cells in S phase, as determined by incorporation of the thymidine analog EdU (Figs 1D and EV2A). Furthermore, consistent with noggin being an inhibitor of TGFβ signaling 17, 18, a TGFβ receptor kinase inhibitor stimulated the formation of very large organoids in the absence of noggin, but not R‐spondin 1 (Fig EV2B).
Figure EV2. Promotion of gallbladder organoid growth by R‐spondin 1, noggin, and a TGFβ receptor kinase inhibitor.
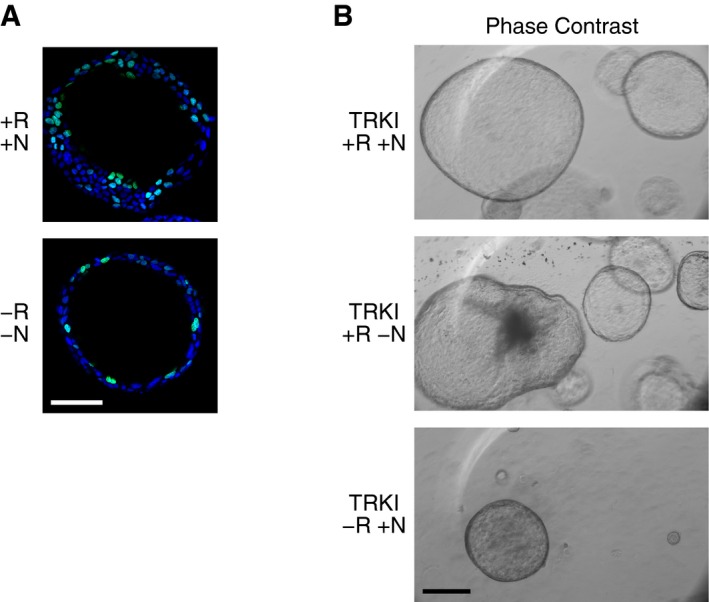
- Representative images of EdU‐stained gallbladder organoids cultured in media with or without R‐spondin 1 and noggin, as indicated (R, R‐spondin 1; N, noggin; +, presence; −, absence). The nuclei were counterstained with DAPI. Scale bar: 200 μm.
- Phase‐contrast images of gallbladder‐derived organoids cultured with a TGFβ receptor kinase inhibitor (TRKI) for 2 months in the presence (+) or absence (−) of R‐spondin 1 (R) and/or noggin (N), as indicated. Scale bar: 200 μm.
In conclusion, these results show a dependency of gallbladder‐derived organoids on R‐spondin 1 and noggin. However, this dependency was much lower than for small intestine organoids, since the latter do not survive in tissue culture even for a few days in the absence of R‐spondin 1 4.
Gene expression patterns in gallbladder organoids
As a first step toward characterizing the gallbladder‐derived organoids, we determined the expression levels of 21,258 annotated transcripts using gene expression arrays. For comparison, we also analyzed organoids derived from livers including the EHBDs and gallbladder (hereafter, liver organoids), organoids derived from the small intestine and freshly harvested gallbladder, liver, and small intestine tissue. For each organoid/tissue, two independent biological replicates were examined and, in all cases, the concordance between the replicates was very high (R 2 between 0.93 and 0.99; Fig EV3A and B). The quality of the array expression data was further examined by selecting 114 genes implicated in stem cell or gallbladder and liver biology and determining their expression levels in all our samples (organoids and tissues) using Nanostring®, a method that relies on single molecule counting. The concordance between the array and Nanostring® data was also very high (R 2 = 0.77) with discrepancies observed primarily in the context of genes expressed at very low levels, reflecting the different sensitivities of the two assays (Fig EV3C; Dataset EV1).
Figure EV3. Validation of gene expression array data and comparison of organoid gene expression profiles.
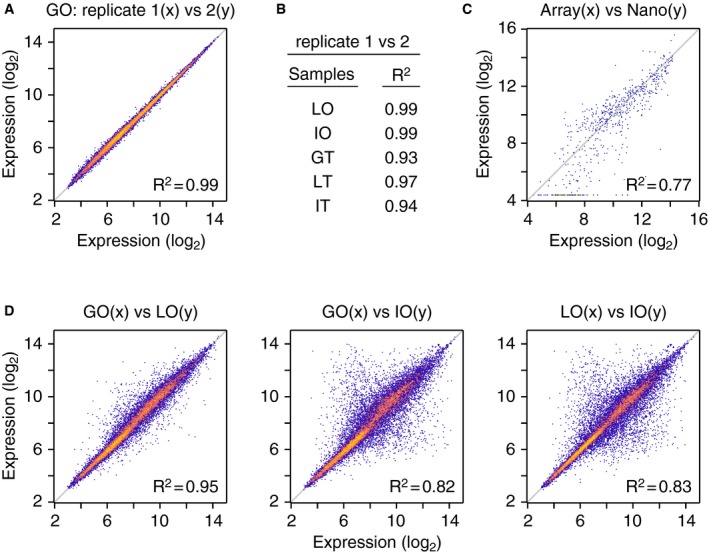
- Comparison of expression levels (log2 scale) of all transcripts from the two gallbladder organoid (GO) replicates. The value of the linear regression coefficient of determination (R 2) is indicated.
- Coefficient of determination values for comparisons between the two replicates of liver organoids (LO), intestinal organoids (IO), gallbladder tissue (GT), liver tissue (LT), and intestinal tissue (IT).
- Comparison of expression levels (log2 scale) of 114 genes, as determined by array and Nanostring® methods. Mean values of expression of the 114 genes were determined for GO, LO, IO, GT, LT, and IT, yielding a total of 684 comparisons. The value of the linear regression coefficient of determination (R 2) is indicated. Dataset EV1 contains the Nanostring® dataset.
- Comparison of mean expression levels (log2 scale) of all transcripts between gallbladder and liver, gallbladder and intestine, and liver and intestine organoids. The values of the linear regression coefficients of determination (R 2) are indicated.
To probe the relation between the gallbladder, liver, and small intestine organoids, we first compared the expression of all 21,258 transcripts in these samples. The gallbladder and liver organoids had very similar gene expression patterns (R 2 = 0.95), while the small intestine organoids had more divergent patterns (R 2 = 0.82; Fig EV3D). The high similarity between gallbladder and liver organoids is consistent with the liver organoids originating primarily from the gallbladder and EHBDs and reflects our inability to obtain organoids from the lobes of healthy livers (Fig 1B).
To further explore whether the organoids from the entire liver were primarily of gallbladder origin, we focused on expression of tissue‐specific genes. For the purposes of this study, tissue‐specific genes were defined as the genes, whose expression was at least twofold higher in one tissue, as compared to each of the other two tissues being analyzed. This criterion identified 413 gallbladder‐specific, 190 liver‐specific, and 572 small intestine‐specific genes (Fig 2A; Dataset EV2). Interestingly, the gallbladder and liver organoids expressed the gallbladder tissue‐specific, but not the liver‐ or small intestine tissue‐specific genes, whereas the small intestine organoids expressed the small intestine‐specific genes (Fig 2A). These results suggest that the organoids maintained the gene expression patterns of the tissue from which they were derived and are consistent with the entire liver organoids being primarily of gallbladder and EHBD origin. Interestingly, the list of gallbladder tissue‐specific genes included the stem cell marker Prom1 and the transcription factor Sox17 19 (Figs 2A and EV4).
Figure 2. Gene expression profiles of gallbladder‐derived organoids.
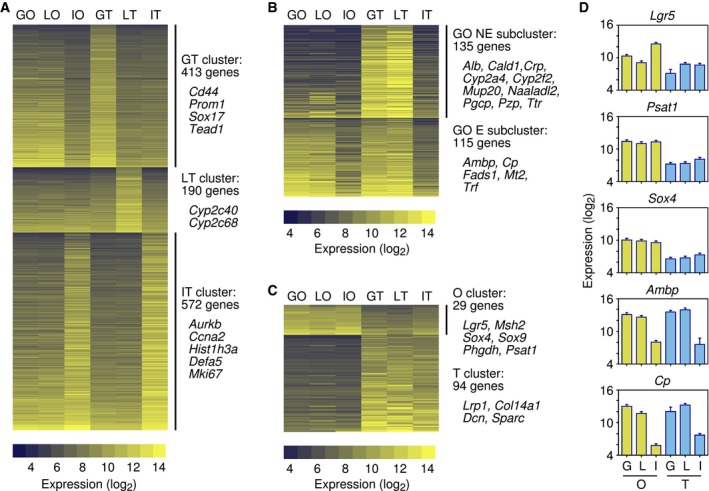
- Clusters of gallbladder tissue (GT)‐specific, liver tissue (LT)‐specific, and small intestine tissue (IT)‐specific genes (Dataset EV2). The number of genes and the names of selected genes in each cluster are indicated. Gene expression levels (log2 scale) are shown for gallbladder organoids (GO), liver organoids (LO), intestinal organoids (IO) and gallbladder (GT), liver (LT), and intestinal (IT) tissues.
- Genes expressed in gallbladder and liver tissues, but not in the intestine, divided into two subclusters, depending on whether the genes were expressed or not in gallbladder organoids (Dataset EV3). The number of genes and the names of selected genes in each subcluster are indicated.
- Clusters of organoid (O)‐specific and tissue (T)‐specific genes (Dataset EV4). The number of genes and the names of selected genes in each cluster are indicated.
- Expression levels of selected genes from the clusters shown in (B) and (C). G, gallbladder; L, liver; I, intestine; O, organoid; T, tissue. Error bars are square root of MSE from ANOVA 2F (see Materials and Methods) (n = 2).
Figure EV4. Gene expression profiles of gallbladder‐derived organoids.
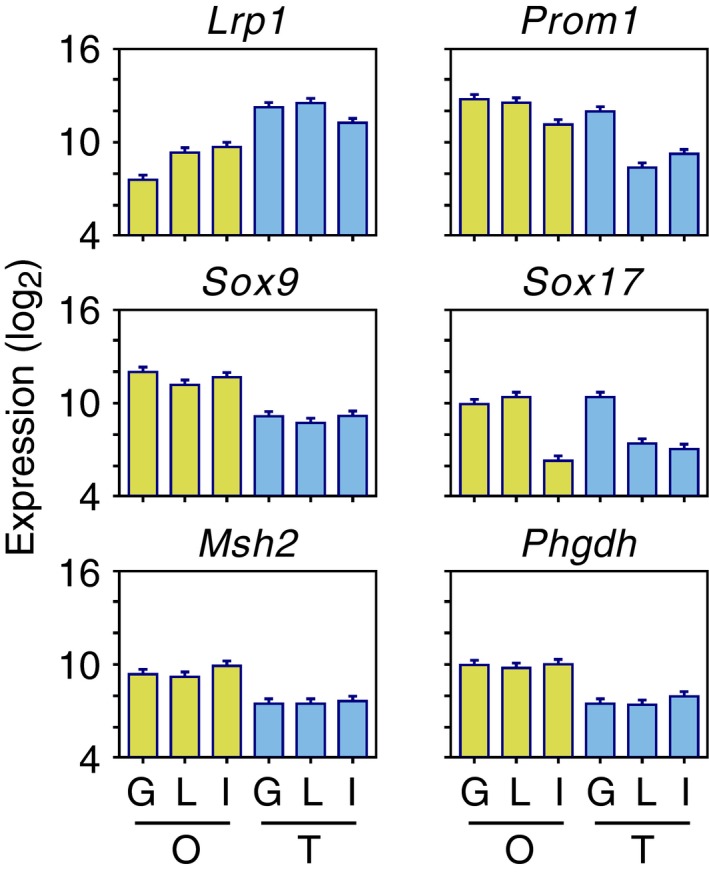
Expression levels (log2 scale) of selected genes from the clusters shown in Fig 2. G, gallbladder; L, liver; I, intestine; O, organoid; T, tissue. Error bars are square root of MSE from ANOVA 2F (see Materials and Methods) (n = 2).
As part of this analysis, we also identified genes that were expressed in the gallbladder and liver tissues, but not in the small intestine, again applying twofold differences in expression as the threshold. A total of 250 genes fit these criteria; of these, 115 were also expressed in the gallbladder organoids at levels comparable to those in gallbladder tissue (Fig 2B and D), whereas the remaining 135 genes were expressed at lower levels in the gallbladder organoids (Fig 2B; Dataset EV3).
We concluded the gene expression array analysis by identifying organoid‐specific genes. These were defined as the genes, whose expression in the gallbladder and small intestine organoids differed by more than twofold (higher or lower) from their expression in the tissues. Twenty‐nine genes were expressed at higher levels in the organoids, including the stem cell markers Lgr5, Msh2, Sox4, and Sox9 and two genes, Phgdh and Psat1, that function in the metabolic pathway that converts 3‐phosphoglycerate to phosphoserine 20, 21 (Fig 2C and D; Dataset EV4). The list of genes whose expression was lower in the organoids, included Lrp1, a gene that inhibits canonical Wnt signaling 22 (Figs 2C and EV4). However, most of the other genes in this category were genes expressed by connective tissue cells, simply reflecting the fact that the organoids, but not the tissues, consist entirely of epithelial cells.
Expression of stem and biliary cell markers in gallbladder‐derived organoids
The gallbladder has a common developmental origin with EHBDs. Indeed, several genes in the gallbladder‐specific cluster, such as Cd44, Prom1, and Sox17 (Fig 2A), are also expressed in extrahepatic biliary epithelial cells 23, 24. Thus, to further characterize the gallbladder‐derived organoids, we examined by immunofluorescence the expression of selected biliary cell markers using liver tissue as control. Prom1 (prominin‐1/CD133), Cldn3 (claudin‐3), Epcam (epithelial cell adhesion molecule), and Itga6 (integrin A6) were all highly expressed in the gallbladder organoids, whereas their expression in the liver was restricted to the bile ducts (Cldn3 and Itga6) or was not observed (Fig 3).
Figure 3. Expression of biliary cell markers in gallbladder organoids.
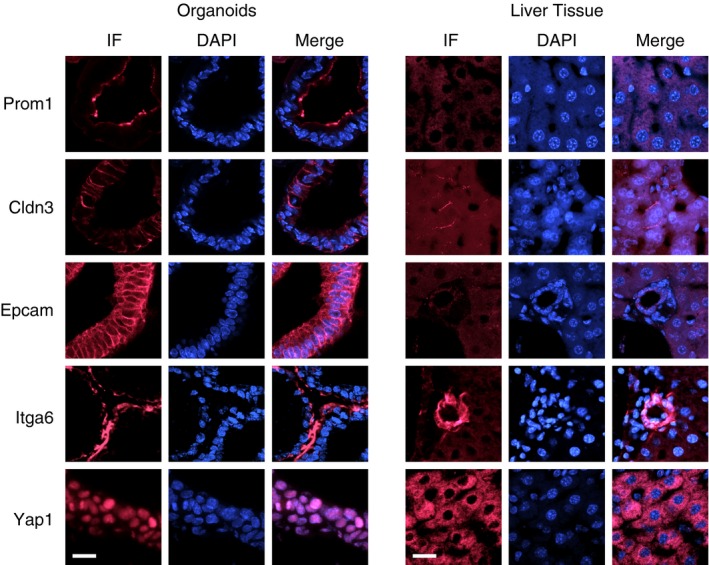
Expression of Prom1, Cldn3, Epcam, Itga6, and Yap1 in gallbladder‐derived organoids and liver tissue, as monitored by immunofluorescence (IF). The cell nuclei were counterstained with DAPI. Scale bars: 25 μm.
As expected, gallbladder and EHBD tissues also stained positive for biliary cell markers, including Krt19, Sox17, and A6 (Fig 4A). Interestingly, most A6‐positive cells of the gallbladder and EHBDs also expressed Hnf4α, which is considered to be a hepatocyte marker (Fig 4B). Co‐expression of biliary and hepatocyte markers is characteristic of embryonic hepatoblasts 25 and is also observed in periportal hepatocytes of regenerating livers 26, but generally not in healthy livers (Fig 4B). Apparently, in the gallbladder tissue and organoids, there is expression of both biliary cell markers and markers considered to be hepatocyte specific (Figs 2B, 3, 4 and EV4).
Figure 4. Expression of biliary cell and hepatocyte markers in gallbladder tissue, EHBDs, and liver lobes.
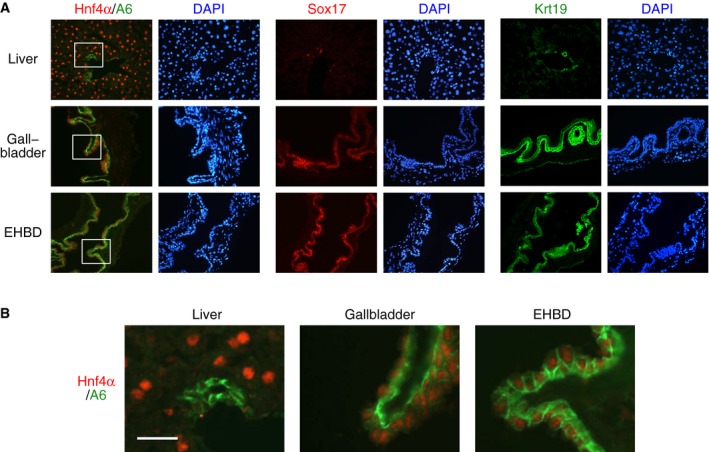
- Immunofluorescence analysis of expression of Hnf4α, A6, Sox17, and Krt19. The nuclei were counterstained with DAPI. EHBD, extrahepatic biliary duct.
- Higher magnification of the regions demarcated by the white rectangles in (A). Scale bar: 25 μm.
Finally, we also examined in gallbladder organoids, by immunofluorescence, the intracellular localization of Yap1, a downstream target of the Hippo pathway. Yap1 exhibited nuclear localization in the organoids, but was cytoplasmic in the liver (Fig 3), consistent with the intracellular localization of Yap1 being a key determinant of its pro‐proliferative activity 27.
Differentiation of gallbladder organoids in vitro
Stem cell cultures have the potential to serve as a replenishable source of differentiated cells. Given the common origin of the gallbladder and liver during embryonic development and the expression of hepatocyte markers in gallbladder epithelial cells (Figs 2B, 4 and EV4), we examined whether the gallbladder organoids could be induced to differentiate along a hepatocyte fate.
We tried a number of protocols to induce the differentiation of gallbladder organoids and in the end converged on simply removing R‐spondin 1, noggin, and nicotinamide from the media for a period of 2 weeks. At the end of this time period, we determined the expression levels of the same 21,258 annotated transcripts, as cited above, using gene expression arrays. Two independent biological replicates were examined, yielding highly reproducible results (R 2 = 0.99). The gene expression levels in the differentiated organoids were then compared to the levels in the non‐differentiated organoids and in gallbladder and liver tissues. Only a small number of genes showed changes in gene expression greater than twofold, indicating incomplete differentiation (Dataset EV5). Sixty‐seven genes were upregulated upon differentiation (Fig 5A); these included two liver‐specific genes, Cyp2c40 and Cyp2c68 (Figs 2A and 5A), and many liver‐/gallbladder‐specific genes that were not expressed in the undifferentiated organoids, including Cald1, Crp, Cyp2a4, Cyp2f2, Mup20, Naaladl2, Pgcp, Pzp, and Ttr (Figs 2B and 5A). The level of induction for many of these genes was remarkable (Fig 5B). Of the genes downregulated upon differentiation, Lgr5 is notable (Fig 5A and B).
Figure 5. Partial differentiation of gallbladder organoids toward the hepatocyte fate.
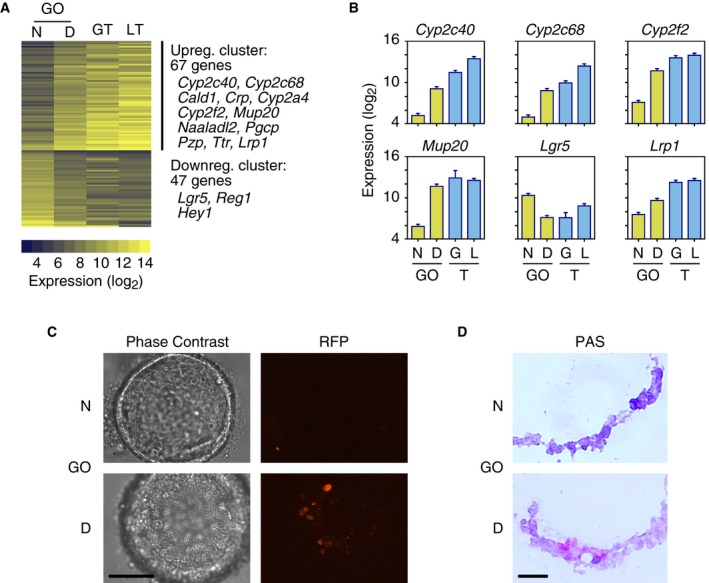
- Clusters of genes, whose expression changes toward the hepatocyte fate, upon the removal of R‐spondin 1 and noggin from the organoid culture media (Dataset EV5). The number of genes and the names of selected genes in each cluster are indicated. Gene expression levels (log2 scale) are shown for non‐differentiated (N) and differentiated (D) gallbladder‐derived organoids (GO), gallbladder tissue (GT), and liver tissue (LT). Upreg, upregulated; Downreg, downregulated.
- Expression levels of selected genes from the clusters of panel A. GO, gallbladder organoid; N, non‐differentiated; D, differentiated; G, gallbladder; L, liver; T, tissue. Error bars are square root of MSE from ANOVA 2F (see Materials and Methods) (n = 2).
- Removal of R‐spondin 1 and noggin from the media induces the expression of red fluorescent protein (RFP) in a subset of gallbladder organoid cells. A transgene expressing tamoxifen‐inducible Cre is driven by the endogenous Alb promoter; a second transgene expresses RFP after Cre‐mediated rearrangement. Scale bar: 200 μm.
- Glycogen accumulation upon organoid differentiation, as revealed by periodic acid–Schiff (PAS) staining (red). Scale bar: 25 μm.
As a second method to monitor differentiation, we prepared gallbladder organoids from mice in which a tamoxifen‐dependent Cre recombinase was inserted in the 3′ untranslated region of the Alb gene; these mice also contained a gene encoding a red fluorescent protein (RFP) that only becomes expressed after the induction of Cre recombinase activity 28, 29. Organoids grown in the presence of R‐spondin 1, noggin, and nicotinamide did not express RFP, whereas when these three factors were removed from the media, RFP was expressed (Fig 5C), suggesting that the Alb promoter had been activated.
Finally, we examined whether differentiation led to the accumulation of intracellular glycogen, which can be monitored by PAS staining. Indeed, a fraction of the cells in the differentiated gallbladder organoids were PAS positive, indicating differentiation toward a hepatocyte fate (Fig 5D).
Engraftment of gallbladder organoids and organoid cells in vivo
Having identified conditions for establishing gallbladder organoid cultures in vitro, we next ascertained their potential for engraftment. For this purpose, gallbladder organoids were established from mice harboring a GFP transgene under the control of the human ubiquitin C promoter. Organoids prepared from these mice expressed GFP (Fig EV5A), thereby allowing the engrafted cells to be distinguished from the host cells.
Figure EV5. Gallbladder organoids prepared from mice expressing constitutively GFP and engraftment in the liver parenchyma.
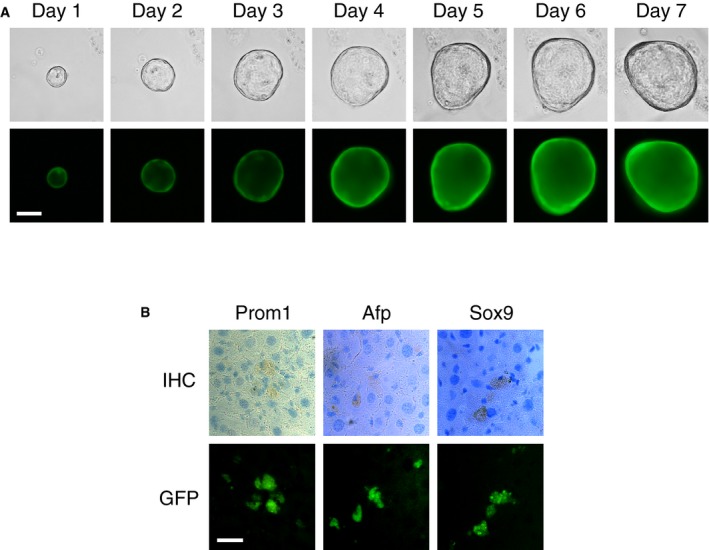
- Phase‐contrast (top panels) and epifluorescence (bottom panels) microscopy images of a representative organoid over a seven‐day period. Scale bar: 200 μm.
- Engraftment of cells isolated from gallbladder organoids in the liver parenchyma. Immunohistochemistry images (top) showing expression of Prom1, α‐fetoprotein (Afp), and Sox9 in cells that express GFP (bottom). Scale bar: 25 μm.
GFP‐positive gallbladder‐derived organoids suspended in Matrigel® were injected into the hepatic subcapsular space of immunocompromised B6.Rag2xγc mice and engraftment was monitored 2 weeks later by immunohistochemistry. In five out of six injected mice, organoids were successfully engrafted. These organoids maintained the same shape and tissue architecture, as observed in vitro, and continued to express biliary cell markers, such as Krt19, Cd44, and Cldn3 (Fig 6A and B). Interestingly, almost no cell in the engrafted organoids expressed Ki67, indicating that these cells were no longer actively proliferating (Fig 6B). We next examined whether the organoids persisted for longer periods of time in the engrafted mice; however, we could not detect any organoids 4 weeks after engraftment in a total of eight mice.
Figure 6. Engraftment of gallbladder organoids in the hepatic subcapsular space.
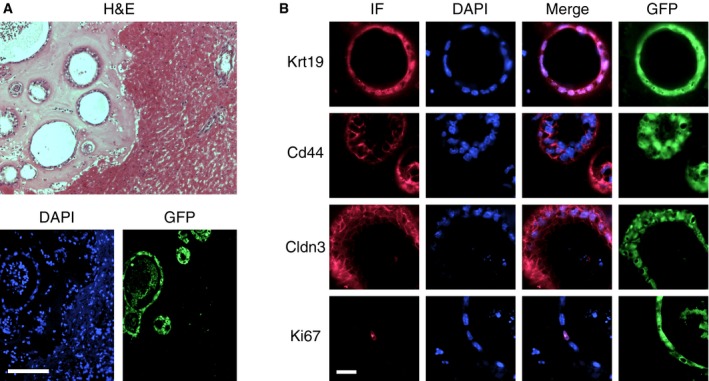
- (Top) Low‐magnification view showing organoids embedded in Matrigel® (left) next to the liver parenchyma (right) 2 weeks after engraftment. H&E, hematoxylin & eosin staining. (Bottom) Immunofluorescence staining for GFP expression to mark the engrafted organoids and DAPI counterstaining of the cell nuclei. The liver parenchyma corresponds to the right half of the images. Scale bar: 200 μm.
- Characterization of engrafted organoids by immunofluorescence (IF). Almost no organoid cell expressed Ki67 (the one positive cell shown in the image was an exception), in contrast to the high levels of cell proliferation and Ki67 expression observed in organoids cultured in vitro. Scale bar: 25 μm.
As a second approach to study engraftment, organoids were dissociated into single cell suspensions and injected into the mesenteric vein of B6.Rag2xγc mice for homing to the liver. Two days before cell injection, CCl4 had been administered intraperitoneally to the mice to induce liver damage, thereby possibly providing a niche that would allow incorporation of the injected stem cells into the liver parenchyma. Engraftment was monitored by immunohistochemistry 1 week after cell injection (Fig EV5B). Interestingly, small clusters of GFP‐positive cells were observed that expressed also Prom1, Sox9 (stem cell markers), and Afp (alpha‐fetoprotein; a hepatoblast marker). However, a similar analysis performed 4 weeks after cell injection failed to identify any GFP‐positive cells.
Establishment of human gallbladder organoids
To determine whether the conditions allowing the establishment and propagation of mouse gallbladder organoids were also suitable for humans, we obtained samples from patients undergoing removal of their gallbladder. We followed essentially the same protocol as the one described above for mice, except that human recombinant growth factors (EGF, HGF, and FGF10) were used. We obtained organoids that morphologically were indistinguishable from the mouse gallbladder organoids (Fig EV6A). The human organoids could be propagated in tissue culture for at least 16 weeks and expressed biliary cell markers, similar to the mouse organoids (Fig EV6B).
Figure EV6. Organoids prepared from human gallbladder tissue.
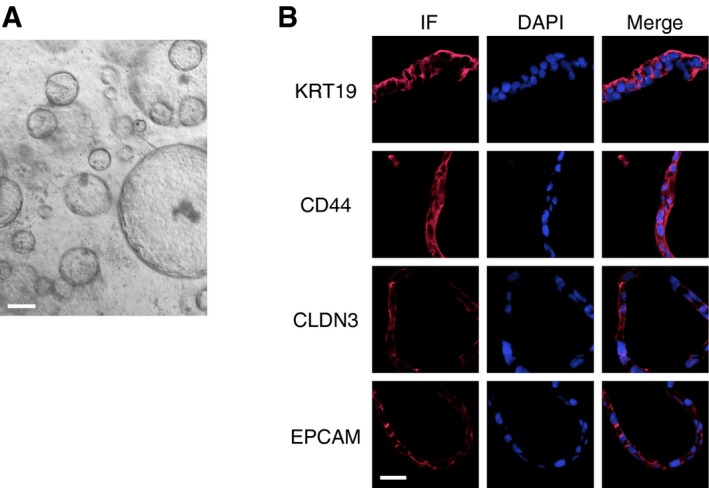
- Phase‐contrast image of organoids derived from human gallbladder tissue. Scale bar: 200 μm.
- Expression of biliary cell markers, as determined by immunofluorescence (IF), in human gallbladder organoids. Scale bar: 25 μm.
Discussion
The liver has long been recognized as unique among organs for its ability to regenerate 30. Regeneration is mediated by the proliferation of differentiated hepatocytes, as well as by precursor cells. In rats, the precursor cells express markers of both hepatocytes and biliary cells and were named oval cells 9. In mice and humans, the contribution of bipotent precursor cells, like oval cells, to liver regeneration appears to be more limited 13, 14. However, a recent study has identified in injured mouse livers periportal hepatocytes that express both biliary and hepatocyte markers; these hybrid hepatocytes can regenerate the liver parenchyma, suggesting that precursor cells with oval cell‐like properties are not limited to rats 26. Precursor cells probably represent a transit cell compartment derived from intrahepatic stem cells 31, 32. The precise niche of these stem cells has not been fully elucidated, but significant evidence points to the canals of Hering within the liver lobules, where the bile ducts originate 33, 34, 35.
Efforts to identify hepatic stem/precursor cells have relied heavily on models of liver damage. One property of stem cells is their capacity to self‐regenerate, and a recent study has indeed described the isolation of intrahepatic stem cells that retain high proliferative capacity for more than one year 15. Briefly, following hepatocyte injury with CCl4, putative stem cells expressing Lgr5 can be identified in close proximity to the IHBDs. These cells were isolated by FACS and could be expanded almost indefinitely in Matrigel® in the presence of R‐spondin 1, EGF, HGF, and FGF10. Furthermore, these putative liver stem cells could be differentiated in vitro to acquire hepatocyte functions and, following such differentiation, they could be transplanted into the livers of Fah −/− mice 15. Thus, Lgr5‐positive liver cells represent a source of stem cells with therapeutic potential 36. The only drawback relates to the method of preparing these cells, which involves either inducing liver damage or, alternatively, isolating IHBDs.
The EHBDs and gallbladder have also emerged as a source of stem/progenitor cells 37, 38, 39. In fact, stem cells can be isolated from these tissues without inducing tissue injury. In one study, stem cells residing in the mouse gallbladder were isolated by sorting Epcam+/Cd49f(Itga6)++ cells; these cells could self‐renew on a feeder layer for more than 20 passages and could be engrafted into the subcutaneous neck region space 39. Stem cells were also isolated from human gallbladders and again cultured on feeder layers 40, 41. The mouse and human gallbladder‐derived stem cells had gene expression profiles that distinguished them from stem cells derived from IHBDs; nevertheless, both the IHBD and gallbladder‐derived stem cells could be differentiated into hepatocytes in vitro 42.
Our study provides further evidence for the presence of stem/progenitor cells in the gallbladder. In our case, though, the method of isolating these cells was quite simple and did not involve a cell sorting step. Instead, non‐injured gallbladders were minced and the cells plated directly on Matrigel®. The gallbladder‐derived cells could be propagated for more than one year in culture and expressed stem cell markers, such as Lgr5, Prom1, Sox4, and Msh2 (Figs 2 and EV4). On the basis of expression of the markers cited above and the capacity to propagate these cells as organoids for more than one year in tissue culture, one may consider that the cells that we have isolated are indeed stem cells. Nevertheless, we have not demonstrated that these cells function as stem cells in vivo and, therefore, pending further characterization, we cannot formally rule out that we have isolated progenitor cells.
An important question is why the gallbladder is a more efficient source of stem/progenitor cells than the liver itself. Perhaps, the answer relates to the fact that the gallbladder epithelial cells express both biliary cell and hepatocyte markers and, in this way, resemble hepatoblasts and precursor cells of regenerating livers. Like the precursor cells in regenerating livers, hepatoblasts are bipotent cells and give rise to both hepatocytes and biliary epithelial cells during embryonic development 25. Hepatoblasts can be readily detected in E10.5 to E15.5 mouse livers, but their number decreases greatly at later‐stage embryos and they are apparently eliminated during postnatal life. Our study raises the possibility that, during development, cells segregated to the gallbladder epithelium maintain stem/progenitor‐like features by escaping differentiation toward the hepatocyte or biliary epithelial lineages.
The relationship of the stem/progenitor cells identified here with those obtained in other studies remains to be determined. It is likely that the IHBD‐derived stem cells are distinct from the gallbladder‐derived stem cells, since the former are much more dependent on R‐spondin 1 for growth and have higher levels of Lgr5 expression than the cells that we isolated 15. In contrast, the stem cells obtained from mouse and fetal human gallbladders by others may be similar to the ones that we isolated, simply because the tissue of origin is the same. However, this needs to be experimentally determined, because in the other studies the stem cells were cultured on feeder cell layers, whereas we cultured the cells in Matrigel® in the presence of R‐spondin 1 and noggin 38, 39, 40, 41. Even if the cells are similar or identical, the ability to bypass the need for feeder layers and the apparently higher capacity to expand organoids are clear advantages of the method that we described.
Stem cells isolated from the gallbladder could be differentiated toward a hepatocyte fate without the addition of chemicals or lentiviral transfection, by simply removing R‐spondin 1, noggin, and nicotinamide from the media. Furthermore, gallbladder‐derived stem cells survived well when engrafted into mice, at least in the short term. However, our experience matches that of others in that the differentiation protocol and engraftment into mice were inefficient 15. Thus, better methods are needed to induce efficient differentiation of gallbladder organoids into hepatocytes and for engraftment of these cells into patients.
In conclusion, the use of gallbladder‐derived stem/progenitor cells could have important implications for the study of liver and biliary tree diseases. Moreover, it is possible to foresee that in the future gallbladder‐derived stem cells could be differentiated into hepatocytes and transplanted into patients, allowing new therapies to be developed for liver‐related genetic diseases and chronic liver insufficiency 43. Along these lines, the gallbladder is not an essential organ and its removal is a routine medical procedure.
Materials and Methods
Mice
C57BL/6J and C57BL/6‐Tg(UBC‐GFP)30Scha/J mice were purchased from Jackson Labs; C57BL/6JRccHsd mice from Harlan Laboratories; and B6.Rag2 × common gamma chain (γc)‐double‐knockout mice from Taconic Biosciences. Alb‐Cre‐ERT2 mice 28 were obtained from Pierre Chambon, and Gt(ROSA)26Sortm1Hjf mice 29 from Ivan Rodriguez. The latter two strains were crossed to generate mice that contain both the Cre‐ERT2 and td‐RFP alleles in homozygous form.
Organoid preparation and culture
Entire livers (including the EHBDs and gallbladder) were isolated from C57BL/6J mice. The tissues were cut into 2‐ to 3‐mm‐diameter pieces, washed in cold PBS, and incubated in PBS–EDTA overnight at 4°C. The next day, the cells were filtered through 70‐μm cell strainers (BD Bioscience). After centrifugation, the cell pellets were mixed with cold Matrigel® (Corning) and plated in 24‐well plates. After the Matrigel® formed a gel, tissue culture media were added. The tissue culture media were based on AdDMEM/F12 (Life Technologies) supplemented with B27, N2 (Life Technologies), and 1.25 μM N‐acetylcysteine (Sigma‐Aldrich). The following growth factors were also added: 50 ng/ml murine recombinant EGF (Life Technologies), 50 ng/ml murine recombinant FGF10 (R&D Systems), 10 mM nicotinamide (Sigma‐Aldrich), 50 ng/ml murine recombinant HGF (Peprotech), and R‐spondin 1 and noggin home‐made conditioned media (prepared as in 4). TGFβ receptor kinase inhibitor (TRKI) (SB‐431542; Calbiochem) was added to the media at 2 mM final concentration for the experiment described in Fig EV2. Medium was changed every 3 days, and organoids were split every 4–5 days by mechanical dissociation. For EHBD‐ and gallbladder‐derived organoid cultures, EHBDs or gallbladder, respectively, were isolated from C57BL/6J mice, and the tissues were cut into pieces and processed, as described above, except that the initial incubation with PBS–EDTA was for 2 h, instead of overnight.
Immunohistochemistry and immunofluorescence
Liver tissues were embedded in OCT (Tissue‐Tek), and slices of 10 μm thickness were cut using a cryostat (Leica CM 1850). Organoids were removed from Matrigel® using Cell Recovery Solution (Corning) and then embedded in OCT and sliced, as described above. Tissue and organoid sections were fixed for 1 h in 4% paraformaldehyde at room temperature and stained using standard immunofluorescence techniques and commercially available antibodies (Yap1, Cell Signaling; Cldn3, GeneTex; Epcam, AbCAM; Prom1, E‐Bioscience; integrin A6, Millipore; Krt19, Dako; Hnf4α, Santa Cruz; Sox17, R&D; A6, gift from Valentina Factor, NIH). Nuclei were counterstained with DAPI, and images were acquired using a Zeiss 700 confocal microscope. Cell proliferation was assessed with the EdU Click‐it Kit (Life Technologies), and images were acquired with the organoids still embedded in Matrigel® using the confocal microscope. To assess glycogen storage, organoids were examined by the periodic acid–Schiff staining method (PAS, Sigma‐Aldrich).
Gene expression array and Nanostring® analysis
RNA was extracted from liver, gallbladder, or small intestine tissues and liver, gallbladder, or small intestine organoids using the RNeasy Mini RNA Extraction Kit (Qiagen). Transcript expression levels were determined using GeneChip® Mouse Gene 1.0 ST Arrays (Affymetrix) for 21,258 transcripts. Expression levels of 114 selected genes (Dataset EV4) were additionally determined by the Nanostring® method (Nanostring® Technologies), to validate the array data.
After scaling, the array and Nanostring® data were converted into log2 values. The data from all samples and replicates were processed together using a two‐factor (gene vs. sample type) ANOVA with multiple replicates, thus allowing a uniform mean variance (mean square of the error, MSE) to be calculated for all comparisons, except for specific comparisons, for which a Cochran's test indicated the need to use individual variances. The gene expression array data have been deposited at the GEO database with accession number GSE77461; the Nanostring® data are shown in Dataset EV1.
Organoid differentiation
To induce differentiation, we cultured the organoids for 2 weeks in the presence of the media described above, except for not adding R‐spondin 1, noggin, and nicotinamide. To monitor differentiation by RFP expression, organoids were prepared from mice containing homozygous Cre‐ERT2 and td‐RFP alleles and then switched to media lacking R‐spondin 1, noggin, and nicotinamide for 2 weeks. Then, 4‐hydroxytamoxifen (1.3 μM, Sigma‐Aldrich) was added, and 2 days later, the organoids, while still embedded in Matrigel®, were scored for fluorescence under a confocal microscope.
Engraftment assays
Gallbladder organoids were prepared from mice harboring a GFP transgene under the control of the human ubiquitin C promoter (C57BL/6‐Tg(UBC‐GFP)30Scha/J mice).
Liver subcapsular whole organoid injection
Dense organoid cultures were gently disrupted with a pipette to break the Matrigel® into small fragments, while preserving the organoids as whole spheres. The organoids were then gently centrifuged, resulting in intact organoids being collected at the bottom of the tube; most of the media and Matrigel® were then removed with a pipette. The organoid pellets were chilled on ice and resuspended in ice‐cold Matrigel®. The organoid and Matrigel® suspension was then loaded into cold 300‐μl syringes with 30‐G needles and stored on ice until use to prevent Matrigel® polymerization. B6.Rag2xγc mice were anaesthetized with isoflurane, and the abdominal cavity was opened by a short transverse laparotomy (~1 cm) 3–5 mm below the xyphoid/costal arch. The left liver lobe was lifted and fixed with the help of a sterile cotton swab, and then 50 μl of the organoid suspension was injected into the subcapsular space. After controlling hemorrhage, the abdominal wall was closed by suturing. The mice were euthanized 2 weeks later, and the site of organoid injection was examined by histology.
Mesenteric vein organoid cell injection
For mesenteric vein delivery, gallbladder organoid cultures were gently disrupted with a pipette and incubated with Dispase (Corning). The resultant cell suspension was washed three times in PBS and resuspended in PBS at a concentration of 3 × 106 cells/ml. Two days before the cell injection, B6.Rag2xγc mice were injected i.p. with CCl4 (1 ml/kg, Sigma‐Aldrich) dissolved in olive oil. Animals were anaesthetized with isoflurane; a 2‐cm midline laparotomy was performed, the mesentery was exposed and immobilized on a moist swab, and 50 μl of the cell suspension, containing approximately 1.5 × 105 cells, was injected into the mesenteric vein. The injection site was compressed to control hemorrhage and suturing closed the laparotomy.
Human organoid preparation
Gallbladder specimens were obtained from patients undergoing surgery at the Inselspital Bern. Informed consent was obtained prior to surgery in compliance with the local ethical committee. Immediately following the removal of the gallbladder, a small piece was transferred to the laboratory in cold PBS. The tissue was further washed in cold PBS and its luminal part was separated and used for organoid isolation (approximately 4 cm2 for each isolation). The tissue was minced, incubated in PBS containing EDTA at 4°C for 2 h, and then repeatedly passed up and down through a 5‐ml pipette in order to release the cells. The cell preparation was then filtered through a 70‐μm sterile mesh, centrifuged, the supernatant removed, and the pellet chilled on ice for 5 min. Depending on the size of the pellet, cells were resuspended in 200–600 μl of Matrigel® and plated. After the Matrigel® solidified by incubation at 37°C, it was covered with culture media, which had the same composition as the media used for mouse organoids, except that the mouse recombinant growth factors (FGF10, HGF, EGF) were substituted with human factors.
Author contributions
NL, IK, AK, TM, and MES performed experiments. OS and TDH performed the bioinformatic analysis. NL, IK, AK, IT, DC, DS, and TDH conceived the project, analyzed the data, interpreted results, and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Review Process File
Acknowledgements
The authors thank Erich Cerny for helpful discussions and the iGE3 Genomics Platform of the University of Geneva for performing the Gene Expression Array and Nanostring experiments. This study was supported by an EMBO long‐term postdoctoral fellowship to I.K., by funds from the Canton of Geneva to T.D.H. and from the Clinic of Visceral Surgery and Medicine to D.S., and by an Advanced ERC grant from the European Commission to I.T.
EMBO Reports (2016) 17: 769–779
References
- 1. Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W, Brulport M, Gabelein G, Schormann W, Schulze M et al (2006) Present status and perspectives of cell‐based therapies for liver diseases. J Hepatol 45: 144–159 [DOI] [PubMed] [Google Scholar]
- 2. Lemaigre FP (2009) Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology 137: 62–79 [DOI] [PubMed] [Google Scholar]
- 3. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- 4. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt‐villus structures in vitro without a mesenchymal niche. Nature 459: 262–265 [DOI] [PubMed] [Google Scholar]
- 5. Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duncan AW, Dorrell C, Grompe M (2009) Stem cells and liver regeneration. Gastroenterology 137: 466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michalopoulos GK (2007) Liver regeneration. J Cell Physiol 213: 286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michalopoulos GK (2010) Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176: 2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evarts RP, Nagy P, Marsden E, Thorgeirsson SS (1987) A precursor‐product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 8: 1737–1740 [DOI] [PubMed] [Google Scholar]
- 10. Fausto N (2004) Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 39: 1477–1487 [DOI] [PubMed] [Google Scholar]
- 11. Dipaola F, Shivakumar P, Pfister J, Walters S, Sabla G, Bezerra JA (2013) Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury in mice. Hepatology 58: 1486–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michalopoulos GK (2014) The liver is a peculiar organ when it comes to stem cells. Am J Pathol 184: 1263–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schaub JR, Malato Y, Gormond C, Willenbring H (2014) Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep 8: 933–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yanger K, Knigin D, Zong Y, Maggs L, Gu G, Akiyama H, Pikarsky E, Stanger BZ (2014) Adult hepatocytes are generated by self‐duplication rather than stem cell differentiation. Cell Stem Cell 15: 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ et al (2013) In vitro expansion of single Lgr5+ liver stem cells induced by Wnt‐driven regeneration. Nature 494: 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, van Wenum M, Fuchs SA, de Ligt J et al (2015) Long‐term culture of genome‐stable bipotent stem cells from adult human liver. Cell 160: 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith WC (1999) TGF beta inhibitors. New and unexpected requirements in vertebrate development. Trends Genet 15: 3–5 [DOI] [PubMed] [Google Scholar]
- 18. Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13: 616–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uemura M, Hara K, Shitara H, Ishii R, Tsunekawa N, Miura Y, Kurohmaru M, Taya C, Yonekawa H, Kanai‐Azuma M et al (2010) Expression and function of mouse Sox17 gene in the specification of gallbladder/bile‐duct progenitors during early foregut morphogenesis. Biochem Biophys Res Commun 391: 357–363 [DOI] [PubMed] [Google Scholar]
- 20. Zilberberg A, Yaniv A, Gazit A (2004) The low density lipoprotein receptor‐1, LRP1, interacts with the human frizzled‐1 (HFz1) and down‐regulates the canonical Wnt signaling pathway. J Biol Chem 279: 17535–17542 [DOI] [PubMed] [Google Scholar]
- 21. Jaeken J, Detheux M, Van Maldergem L, Foulon M, Carchon H, Van Schaftingen E (1996) 3‐Phosphoglycerate dehydrogenase deficiency: an inborn error of serine biosynthesis. Arch Dis Child 74: 542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hart CE, Race V, Achouri Y, Wiame E, Sharrard M, Olpin SE, Watkinson J, Bonham JR, Jaeken J, Matthijs G et al (2007) Phosphoserine aminotransferase deficiency: a novel disorder of the serine biosynthesis pathway. Am J Hum Genet 80: 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R et al (2012) Biliary tree stem/progenitor cells in glands of extrahepatic and intrahepatic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 220: 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM, Alvaro D (2012) The biliary tree–a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 9: 231–240 [DOI] [PubMed] [Google Scholar]
- 25. Si‐Tayeb K, Lemaigre FP, Duncan SA (2010) Organogenesis and development of the liver. Dev Cell 18: 175–189 [DOI] [PubMed] [Google Scholar]
- 26. Font‐Burgada J, Shalapour S, Ramaswamy S, Hsueh B, Rossell D, Umemura A, Taniguchi K, Nakagawa H, Valasek MA, Ye L et al (2015) Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162: 766–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piccolo S, Dupont S, Cordenonsi M (2014) The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94: 1287–1312 [DOI] [PubMed] [Google Scholar]
- 28. Schuler M, Dierich A, Chambon P, Metzger D (2004) Efficient temporally controlled targeted somatic mutagenesis in hepatocytes of the mouse. Genesis 39: 167–172 [DOI] [PubMed] [Google Scholar]
- 29. Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ (2007) Faithful activation of an extra‐bright red fluorescent protein in “knock‐in” Cre‐reporter mice ideally suited for lineage tracing studies. Eur J Immunol 37: 43–53 [DOI] [PubMed] [Google Scholar]
- 30. Higgins GM, Anderson RM (1931) Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 186–202 [Google Scholar]
- 31. Kamiya A, Inagaki Y (2015) Stem and progenitor cell systems in liver development and regeneration. Hepatol Res 45: 29–37 [DOI] [PubMed] [Google Scholar]
- 32. Fausto N, Campbell JS (2003) The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120: 117–130 [DOI] [PubMed] [Google Scholar]
- 33. Wilson JW, Leduc EH (1958) Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol 76: 441–449 [DOI] [PubMed] [Google Scholar]
- 34. Engelhardt NV, Factor VM, Medvinsky AL, Baranov VN, Lazareva MN, Poltoranina VS (1993) Common antigen of oval and biliary epithelial cells (A6) is a differentiation marker of epithelial and erythroid cell lineages in early development of the mouse. Differentiation 55: 19–26 [DOI] [PubMed] [Google Scholar]
- 35. De Alwis N, Hudson G, Burt AD, Day CP, Chinnery PF (2009) Human liver stem cells originate from the canals of Hering. Hepatology 50: 992–993 [DOI] [PubMed] [Google Scholar]
- 36. Huch M, Boj SF, Clevers H (2013) Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med 8: 385–387 [DOI] [PubMed] [Google Scholar]
- 37. Kuver R, Savard CE, Lee SK, Haigh WG, Lee SP (2007) Murine gallbladder epithelial cells can differentiate into hepatocyte‐like cells in vitro. Am J Physiol Gastrointest Liver Physiol 293: G944–G955 [DOI] [PubMed] [Google Scholar]
- 38. Lee SP, Savard CE, Kuver R (2009) Gallbladder epithelial cells that engraft in mouse liver can differentiate into hepatocyte‐like cells. Am J Pathol 174: 842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manohar R, Komori J, Guzik L, Stolz DB, Chandran UR, LaFramboise WA, Lagasse E (2011) Identification and expansion of a unique stem cell population from adult mouse gallbladder. Hepatology 54: 1830–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carpino G, Cardinale V, Gentile R, Onori P, Semeraro R, Franchitto A, Wang Y, Bosco D, Iossa A, Napoletano C et al (2014) Evidence for multipotent endodermal stem/progenitor cell populations in human gallbladder. J Hepatol 60: 1194–1202 [DOI] [PubMed] [Google Scholar]
- 41. Manohar R, Li Y, Fohrer H, Guzik L, Stolz DB, Chandran UR, LaFramboise WA, Lagasse E (2015) Identification of a candidate stem cell in human gallbladder. Stem Cell Res 14: 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME et al (2011) Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology 54: 2159–2172 [DOI] [PubMed] [Google Scholar]
- 43. Kadyk LC, Collins LR, Littman NJ, Millan MT (2015) Proceedings: moving toward cell‐based therapies for liver disease. Stem Cells Transl Med 4: 207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Dataset EV3
Dataset EV4
Dataset EV5
Review Process File


