Abstract
The distribution and regulation of the cohesin complexes have been extensively studied during mitosis. However, the dynamics of their different regulators in vertebrate meiosis is largely unknown. In this work, we have analyzed the distribution of the regulatory factor Sororin during male mouse meiosis. Sororin is detected at the central region of the synaptonemal complex during prophase I, in contrast with the previously reported localization of other cohesin components in the lateral elements. This localization of Sororin depends on the transverse filaments protein SYCP1, but not on meiosis‐specific cohesin subunits REC8 and SMC1β. By late prophase I, Sororin accumulates at centromeres and remains there up to anaphase II. The phosphatase activity of PP2A seems to be required for this accumulation. We hypothesize that Sororin function at the central region of the synaptonemal complex could be independent on meiotic cohesin complexes. In addition, we suggest that Sororin participates in the regulation of centromeric cohesion during meiosis in collaboration with SGO2‐PP2A.
Keywords: centromere, cohesin complex, meiosis, Sororin, synaptonemal complex
Subject Categories: Cell Cycle; Chromatin, Epigenetics, Genomics & Functional Genomics
Introduction
Faithful chromosome segregation during mitosis and meiosis requires that sister chromatids are intimately connected with each other from their replication at the S phase until their disjunction at the onset of anaphase(s). This association, called sister chromatid cohesion, is mediated by cohesin, an evolutionarily conserved four‐subunit complex that entraps sister chromatin fibers within its ring‐shaped structure 1, 2. In vertebrate somatic cells, the cohesin complex consists of two SMCs (Structural Maintenance of Chromosomes) proteins, named SMC1α and SMC3, the kleisin RAD21/Scc1/Mcd1, and SA1 or SA2 1. The dynamics of cohesin throughout the cell cycle is controlled by a set of regulatory proteins that facilitate its loading, the establishment of cohesion between sister chromatids, its maintenance, and its release from chromosomes. In vertebrate cells, cohesin is loaded onto the chromatin by telophase/G1 with the participation of a heterodimer consisting of the proteins Scc2/NIPBL and Scc4/MAU2 3. The establishment of cohesion during DNA replication in S phase depends on SMC3 acetylation by the acetyltransferases ESCO1/ESCO2 4, 5, 6 and on the subsequent recruitment of Sororin, which stabilizes cohesin on DNA.
Sororin was first identified in a screen for substrates of the anaphase promoting complex in Xenopus egg extracts, and shown to interact with the cohesin subunits 7. It has been proposed that binding of Sororin to PDS5, another regulator that binds to the cohesin subunits SA1/2 8, prevents the activity of WAPL, a cohesin releasing factor 9. In this way, Sororin maintains cohesion during G2 and mitosis. In vertebrate cells, cohesin is released from chromosomes in two steps in mitosis 10. During prophase and prometaphase, most cohesin dissociates from chromosome arms by the so‐called prophase pathway. The phosphorylation of Sororin by the kinases CDK1 and Aurora B promotes its dissociation from cohesin at chromosome arms, allowing the interaction of WAPL with PDS5 9, 11, 12, 13. The releasing activity of WAPL, together with the phosphorylation of cohesin by the kinase PLK1, leads to the dissociation of cohesin from chromosome arms. During this “prophase pathway”, cohesin at centromeres is protected by the complex shugoshin SGO1/PP2A 14, which maintains Sororin and SA2 in a dephosphorylated state 12, 15. During prometaphase, the tension generated at centromeres/kinetochores induces the redistribution of SGO1/PP2A so that centromeric cohesin becomes deprotected and can be cleaved by the protease Separase at the onset of anaphase, once the spindle‐assembly checkpoint is satisfied 15, 16, 17. All these data indicate that Sororin is a crucial cohesin regulator needed for the establishment, maintenance, and dissociation of cohesin during the cell cycle 18. However, the relevance of Sororin during meiosis is unknown.
Meiosis is a specialized cell division characterized by two rounds of chromosome segregation after a single round of DNA replication to yield haploid gametes from diploid germ cells. During prophase of the first meiotic division, the homologous chromosomes must correctly achieve the processes of pairing, synapsis, and recombination to allow their successful segregation during the first meiotic division 19. These processes are mediated by the formation of a meiosis‐specific zipper‐like proteinaceous structure called the synaptonemal complex (SC) 20. During the leptotene stage of prophase I, the so‐called axial elements form along each chromosome and are then named lateral elements once the homologs begin to pair during the zygotene stage. During this stage, the interaction of transverse filaments onto the lateral elements of both homologs allows the formation of the central element at SC central regions. During pachytene, the homologs are synapsed and a fully formed tripartite SC is found along the entire length of each autosomal bivalent. Once recombination is completed, the homologs and their lateral elements desynapse by diplotene due to the disassembly of central element proteins. Mammalian axial/lateral elements are composed by the SC proteins SYCP2 and SYCP3 21, 22, different cohesin complexes 23, 24, the cohesin regulatory proteins NIPBL and MAU2 25, condensin complexes 25, and the HORMA‐domain proteins HORMAD1 and HORMAD2 26. The central element is formed by the proteins SYCE1‐3 and TEX12, whereas the transverse filaments are formed by SYCP1 20.
Meiotic sister chromatid cohesion is also mediated by cohesin complexes 23, 24. In mammalian meiosis, in addition to the canonical mitotic cohesin subunits, several meiosis‐specific cohesin subunits have been described. Thus, the meiotic paralogs of SMC1α and SA1/SA2 are SMC1β 27 and STAG3 28, respectively, while the kleisin RAD21 has two meiosis‐specific paralogs, REC8 29 and RAD21L 30, 31, 32, 33. Similar to vertebrate mitosis, sister chromatid cohesion is released in two steps during mammalian meiosis. During the first meiotic division, cohesin at chromosome arms is cleaved by Separase during the metaphase I/anaphase I transition to allow the segregation of recombined homologous chromosomes. At meiosis I, centromeric cohesin is protected against Separase cleavage by the complex shugoshin SGO2/PP2A. During the second meiotic division, the SGO2/PP2A complex that was protecting centromeric cohesin redistributes depending on tension, during prometaphase II, and then, centromeric cohesin is cleaved by Separase during the metaphase II/anaphase II transition to permit chromatid segregation 34, 35, 36. However, the functions of the different cohesin complexes and their regulators during mammalian meiosis remain unknown.
In the present study, we have analyzed the distribution of Sororin during the meiotic divisions in male mouse. We also studied its distribution in mutant spermatocytes for the SC central region proteins SYCE3 and SYCP1, the meiosis‐specific cohesin subunits REC8 and SMC1β, and the cohesin protector SGO2. Our results indicate that during prophase I Sororin is present at the SC central region, while cohesin subunits are located along the axial/lateral elements of the SC and that Sororin loading is dependent on SYCP1, but not on REC8 and SMC1β‐containing cohesin complexes. We also found that Sororin is present at the centromeres during both meiotic divisions and that its loading is independent on SGO2.
Results
Sororin is present at the central region of the synaptonemal complex
To determine the pattern of localization of Sororin in male mouse meiosis, we have employed two distinct antibodies (see Materials and Methods). The specificity of both antibodies was validated by knocking down and overexpression experiments in somatic cells (Figs EV1 and EV2). We used immunofluorescence to determine the distribution of Sororin on surface‐spread spermatocytes. To identify the different meiotic stages, we performed a double‐immunolabeling of Sororin with SYCP3, a structural protein component of the axial/lateral elements of the SCs 37. During prophase I, Sororin was first detected at zygotene, as short lines that colocalized with regions where axial elements, as detected by SYCP3, appeared thickened and thus paired (Fig 1A). With progression of homologous pairing during zygotene, Sororin signals grew longer and colocalized with the SYCP3‐labeled lateral elements involved in SC assembly. By pachytene, when synapsis between homologs is complete, Sororin colocalized with SYCP3 at fully assembled autosomal SCs (Fig 1B). At this stage, Sororin also appeared at the pseudoautosomal region of homology between the sex chromosomes, where their axial elements appeared synapsed, and also very faintly at the unsynapsed axial elements (Fig 1B). Additionally, Sororin appeared at a large and round signal corresponding to the round body, an electron‐dense nuclear structure within the granular component of the nucleolus 38 (Fig 1B). By diplotene, Sororin was observed as lines at the still synapsed chromosomal regions, while desynapsed lateral elements were only labeled with SYCP3 (Fig 1C). These Sororin‐labeled lines became progressively shorter and fainter with ongoing desynapsis. This pattern of localization of Sororin at the SC central region was consistent with both antibodies used for this study (Fig EV3A and B). By late diplotene, Sororin appeared at the centromeres (Fig 1D). This centromere labeling persisted in diakinesis and prometaphase I spermatocytes (Fig 1E).
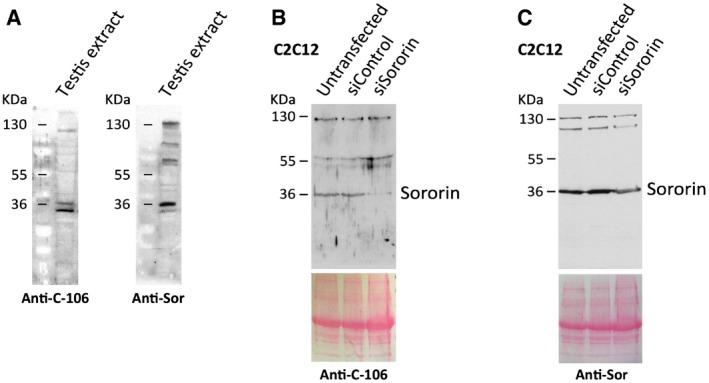
Figure EV1. Analysis of the specificity of the Sororin antibodies in testis and in C2C12 cell extracts.
-
AWestern blot analysis of extracts prepared from adult testes with the Sororin antibodies used in this study, anti‐Sor and C‐106.
-
B, CC2C12 cells were transfected with Sororin siRNA or siRNA control, and the whole extracts were analyzed by Western blot with antibodies against Sororin: C‐106 (B) and anti‐Sor (C). Equal protein loading was ascertained by Ponceau S staining of blotted membranes.
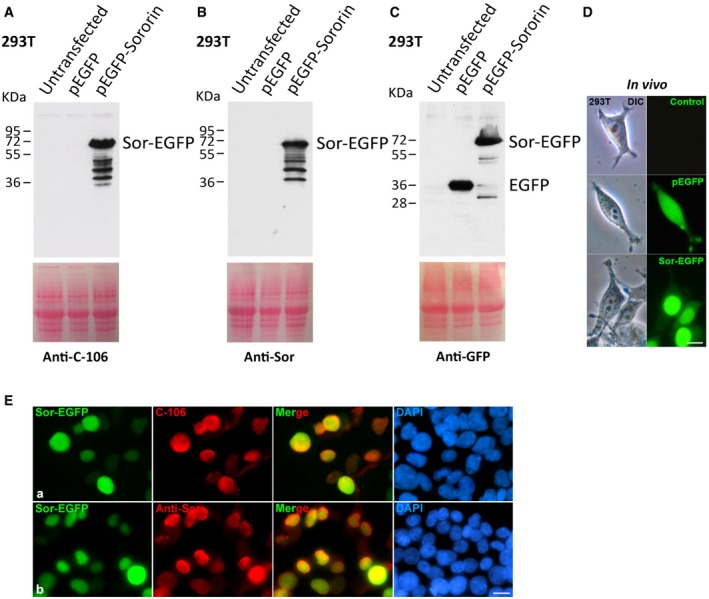
Figure EV2. Analysis of the specificity of the Sororin antibodies in overexpression experiments.
-
A–CHEK 293T cells were transfected with Sororin‐EGFP or EGFP, and the whole extracts were analyzed by Western blot with antibodies against Sororin C‐106 (A), anti‐Sor (B), and against EGFP (C). Equal protein loading was ascertained by Ponceau S staining of blotted membranes.
-
DGFP‐positive transfection was determined by in vivo observation of the cells. Scale bar: 10 μm.
-
ESororin‐EGFP (green) overexpression and immunolabeling of Sororin (red) with antibodies C‐106 (a) and Anti‐Sor (b) in HEK 293T cells. Scale bar: 10 μm.
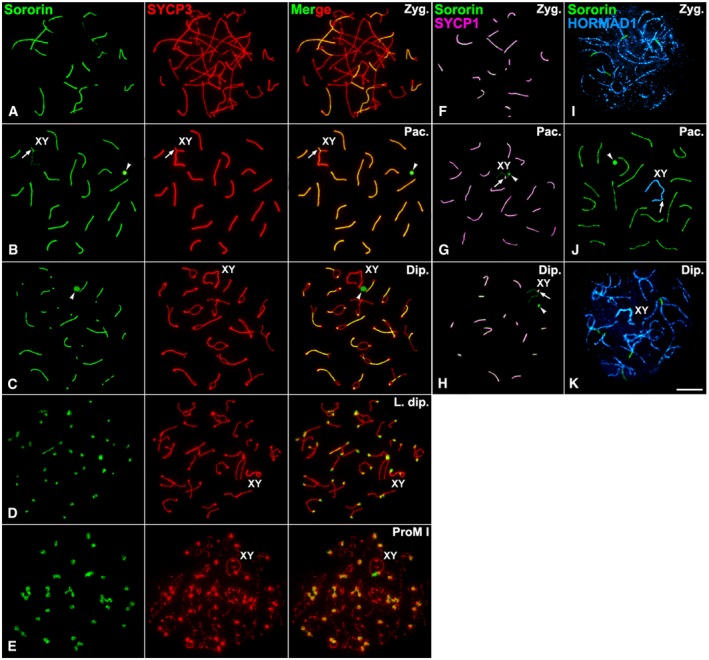
Figure 1. Sororin distribution during prophase I.
-
A–EDouble‐immunolabeling of Sororin (green) and SYCP3 (red) on spread spermatocytes at (A) zygotene (Zyg.), (B) pachytene (Pac.), (C) diplotene (Dip.), (D) late diplotene (L. dip.), and (E) prometaphase I (ProM I).
-
F–HDouble‐immunolabeling of Sororin (green) and SYCP1 (pseudocolored in pink) on spread spermatocytes at (F) zygotene, (G) pachytene, and (H) diplotene.
-
I–KDouble‐immunolabeling of Sororin (green) and HORMAD1 (pseudocolored in blue) on spread spermatocytes at (I) zygotene, (J) pachytene, and (K) diplotene.
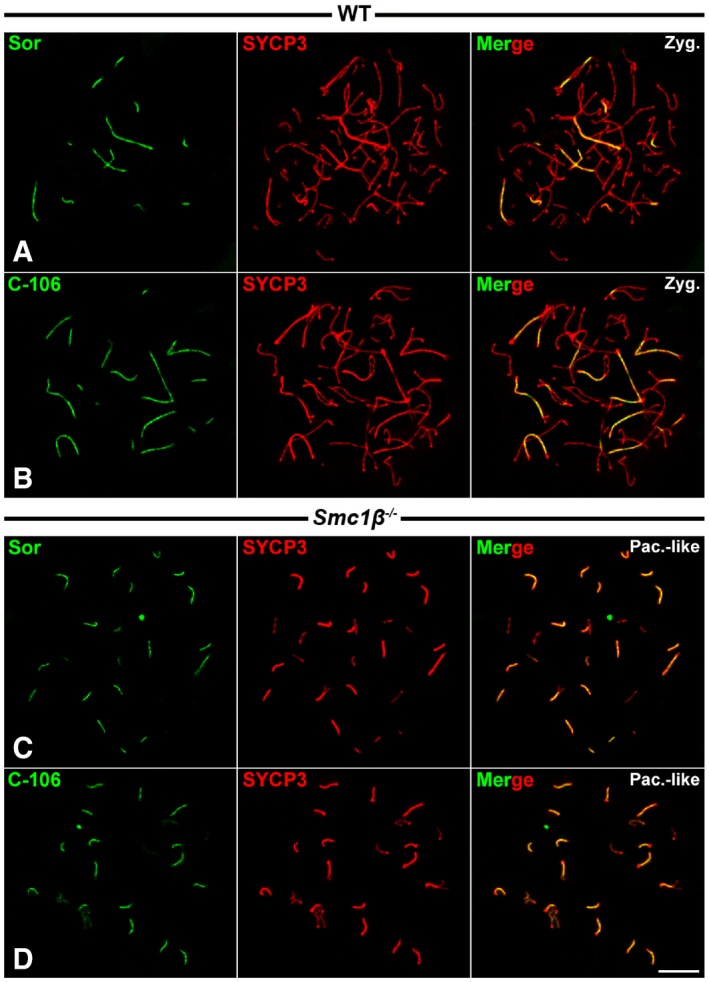
Figure EV3. Immunofluorescence of anti‐Sororin antibodies in spermatocytes.
-
A–DDouble‐immunolabeling of Sororin (green) with anti‐Sor (A, C) and antibody C‐106 (B, D) and SYCP3 (red) on WT and Smc1β −/− spread spermatocytes at zygotene (Zyg.) and pachytene‐like (Pac.‐like), respectively.
Data information: Scale bar: 10 μm.
In order to corroborate the presence of Sororin at the SC central region, we performed a double‐immunolabeling with SYCP1, a transverse filaments protein present at the SC central region 22. Indeed, Sororin and SYCP1 colocalized at the SC central region from zygotene up to diplotene (Fig 1F–H). When performing a double‐immunolabeling of Sororin and HORMAD1, a protein associated with unsynapsed and desynapsed axial/lateral elements throughout prophase I 26, 39, Sororin was detected at synapsed regions, while HORMAD1 was localized both at the unsynapsed and desynapsed axial/lateral elements (Fig 1I–K).
Given that our results surprisingly showed that Sororin was located at the SC central region, whereas all the cohesin subunits described so far are located along the axial/lateral elements, we compared the pattern of distribution of Sororin with SMC3, a subunit which is common to all cohesin complexes, and REC8, a meiosis‐specific subunit. First, we compared the distribution of Sororin and SMC3. As both Sororin and SMC3 antibodies were produced in rabbit, a double‐immunolocalization could be risky to interpret. For this reason, we decided to compare Sororin and SYCP3, vs SMC3 and SYCP3 independently. Our results clearly showed that in early and late zygotene Sororin only detected the SC central region where synapsis had been already established (Fig 2A and C), while SMC3 colocalized with SYCP3 at the axial/lateral elements during these stages (Fig 2B and D). Then, we compared the distribution of Sororin and REC8 by detecting the latter with an anti‐myc primary antibody in transgenic REC8‐myc mice 40. In zygotene spermatocytes, Sororin was clearly visible at the SC central region, while REC8 appeared along the axial/lateral elements (Fig 2E). These results support that cohesins are detected at the axial/lateral elements, but Sororin is located at the SC central region.
Figure 2. Sororin distribution in comparison with cohesins during prophase I.
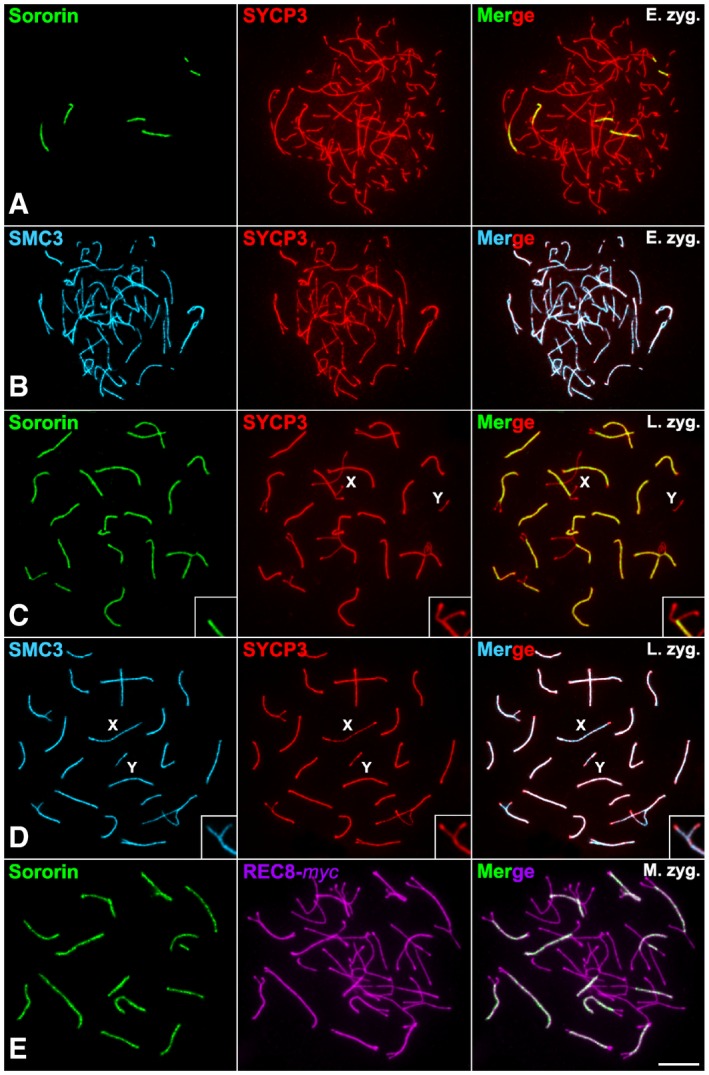
-
A–DDouble‐immunolabeling of SYCP3 (red) and Sororin (green) (A, C) or SMC3 (pseudocolored in cyan) (B, D) on spread spermatocytes at (A, B) early zygotene (E. zyg.) and (C, D) late zygotene (L. zyg.).
-
EDouble‐immunolabeling of Sororin (green) and REC8‐myc (pseudocolored in purple) on a spread mid‐zygotene spermatocyte.
The loading of Sororin at the central region of the synaptonemal complex depends on SYCP1
The presence of Sororin at the SC central regions during prophase I stages prompted us to test whether its localization was dependent on the presence of the central element or the transverse filaments at the SC central region. We first analyzed the distribution of Sororin in spermatocytes from mice knockout for the central element protein SYCE3 41. In these Syce3 −/− mutants, spermatocytes arrest at a pachytene‐like stage with normally assembled axial/lateral elements that appeared aligned homologously along their entire lengths, but never synapsed. The homologous axial/lateral elements may appear clearly separated or converging at sites called axial associations, which are not connected by transverse filaments, and do not show a central element between them 41. We found that Sororin appeared as foci along the aligned axial/lateral elements in pachytene‐like spermatocytes (Fig 3A). These data indicate that the presence of Sororin foci along the axial/lateral elements is not dependent on the presence of the SC central element, since in the absence of SYCE3, other central element proteins required downstream for its assembly such as SYCE1, SYCE2, and TEX12 are not present and do not allow the formation of the central element 20.
Figure 3. Sororin distribution in Syce3 −/−, Sycp1 −/−, Rec8 −/−, Smc1β −/−, and Spo11 −/− spermatocytes.
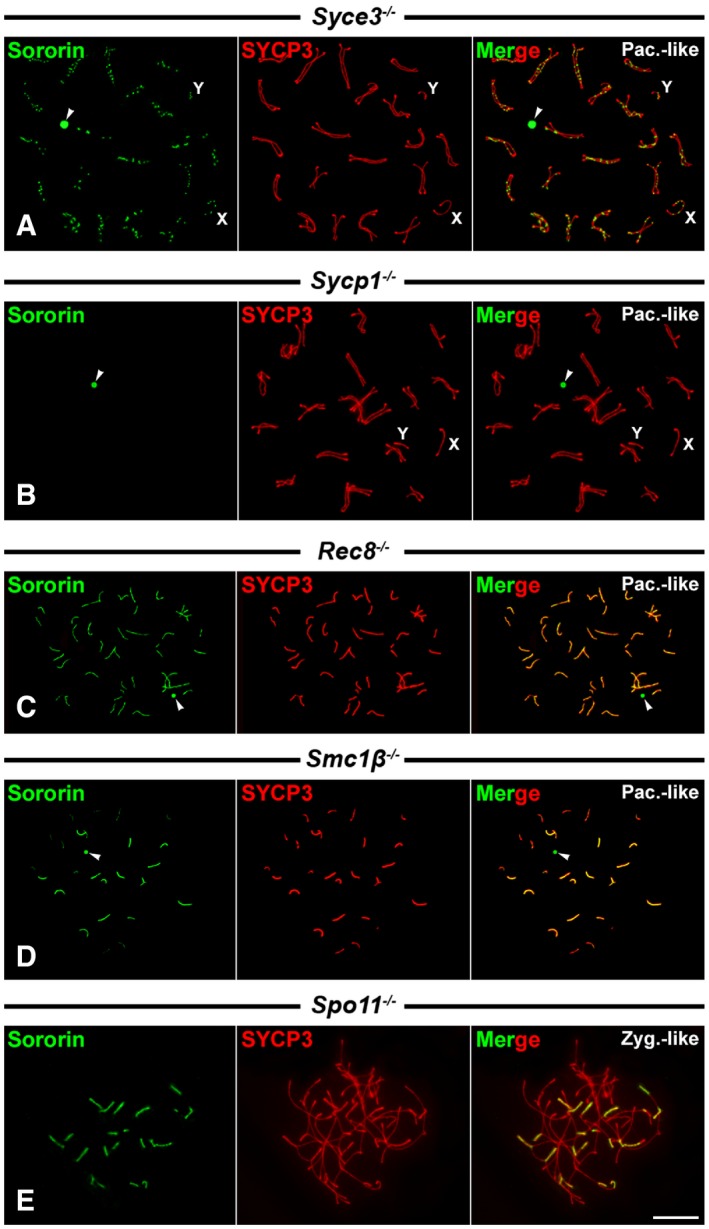
-
A–EDouble‐immunolabeling of Sororin (green) and SYCP3 (red) on spread pachytene‐like (Pac.‐like) and zygotene‐like (Zyg.‐like) spermatocytes of different genotypes. Unsynapsed axial elements of sex chromosomes (X,Y) are indicated.
We next tested whether the presence of Sororin at the SC central region was dependent on SYCP1, by analyzing the distribution of Sororin in Sycp1 −/− knockout spermatocytes 42. These mutant Sycp1 −/− spermatocytes show, like Syce3 −/− ones, an arrest at a pachytene‐like stage with normally assembled axial/lateral elements that also appear paired and aligned homologously along their entire lengths. In these Sycp1 −/− pachytene‐like spermatocytes, we were not able to detect the presence of Sororin signals along the aligned axial elements (Fig 3B). However, Sororin labeling at the round body persisted (Fig 3B). Consequently, the loading of Sororin at the SC central region depends on SYCP1.
The loading of Sororin at the central region of the synaptonemal complex is independent on REC8 and SMC1β‐containing cohesin complexes
Next we tested whether the presence of Sororin at the SC central region was dependent on the presence of meiotic cohesin complexes. For this purpose, we analyzed the distribution of Sororin in mutants for the meiosis‐specific cohesin subunits REC8 43 and SMC1β 27.
Rec8 −/− mutant spermatocytes arrest at a pachytene‐like stage with chromosome cores that are not homologously synapsed and are shorter than the lateral elements in wild‐type pachytene spermatocytes. In Rec8 −/− pachytene‐like spermatocytes, 40 short chromosome cores corresponding to univalents are found, composed of two closely associated axes, one per chromatid, with SYCP1 detected between them 44, 45. Accordingly, although homologous synapsis does not occur, it has been proposed that in the absence of REC8, the formation of a SC‐like structure between sister cores occurs 45. We found that Sororin appeared as continuous lines that colocalized with SYCP3 along the chromosome cores (Fig 3C). Thus, these data also support that the loading of Sororin at the SC central region can be directed by SYCP1, even between sister chromatid cores, but is independent on REC8‐containing cohesin complexes.
Smc1β −/− mutant spermatocytes also arrest at a pachytene‐like stage. In these spermatocytes, there is a high degree of homologous synapsis and most bivalents show SCs that are approximately half as long as those found in wild‐type pachytene spermatocytes 27, 46. However, in these mutant spermatocytes homologous synapsis is rarely complete, and some partially synapsed bivalents and unsynapsed univalents are also found. Our results showed that Sororin colocalized with SYCP3‐labeled SCs (Fig 3D). This pattern of localization of Sororin at the SC central region in Smc1β −/− spermatocytes was consistently found when employing both antibodies used for this study (Fig EV3C and D). Altogether, our results on the distribution of Sororin on Rec8 −/− and Smc1β −/− mutant spermatocytes indicate that the loading of Sororin onto the SC central region is independent on meiosis‐specific cohesin complexes containing REC8 and/or SMC1β subunits.
The loading of Sororin at the central region of the synaptonemal complex is independent on double‐strand breaks and homologous pairing
In order to assess the potential dependence of Sororin localization on the formation of double‐strand breaks during meiotic recombination, we performed a double‐immunolabeling of Sororin and SYCP3 in Spo11 −/− zygotene‐like spermatocytes where double‐strand breaks are not formed 47. Our results showed that Sororin was located at the SC central regions formed between heterologous chromosomes (Fig 3E). This result suggests that Sororin loading to the SC central region does not depend on homologous pairing or the formation of double‐strand breaks.
Sororin is detected at the centromeres in metaphase I and metaphase II
Since Sororin relocalized from the SC central region to the centromeres by late diplotene (Fig 1), we tested whether Sororin was maintained at centromeres during both meiotic divisions. In metaphase I spermatocytes, Sororin mostly colocalized with SYCP3 at the inner centromere domain of all autosomes, as previously defined 34, 37 (Fig 4A–F). In this regard, the Sororin signal at the centromere of the Y chromosome was larger than that found for SYCP3 (Fig 4E and F). On the contrary, Sororin was not present along the SYCP3‐labeled interchromatid domain of autosomal (Fig 4B–D) or sex (Fig 4E and F) bivalents. Interestingly, Sororin also appeared at the centriolar area at cell poles (Fig 4A). This pattern of distribution of Sororin was maintained in anaphase I (Fig 4G) but disappeared in interkinesis nuclei (Fig 4H). Sororin centromeric signals reappeared in prophase II spermatocytes (Fig 4I). By metaphase II, Sororin was observed as two signals at each centromere, and also at cell poles (Fig 4J–L). Given that Sororin was located at the centromeres, we next double‐immunolocalized Sororin and kinetochores (labeled by an anti‐centromere autoantibody ACA serum) in order to study their relative distribution. We observed that in metaphase I bivalents the Sororin centromeric signals were either below the closely associated sister kinetochores when centromeres were side‐viewed, or surrounding sister kinetochores when centromeres were top‐viewed (Fig 5A). By metaphase II, Sororin signals appeared either as pairs of dots just below each kinetochore, or as rings surrounding them (Fig 5B). The same Sororin centromeric labeling was observed during anaphase II (Fig 5C). It is interesting to mention that the Sororin signal at the centromere of the Y chromosome was always brighter and larger than those found in autosomes.
Figure 4. Sororin distribution during the meiotic divisions.
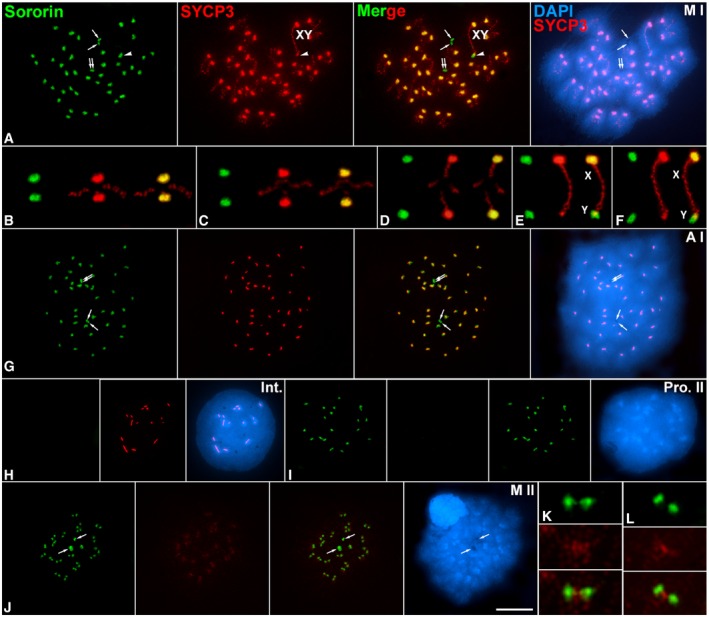
-
A–JDouble‐immunolabeling of Sororin (green) and SYCP3 (red), and counterstaining of the chromatin with DAPI (blue) on spread spermatocytes at (A) metaphase I (M I), (G) anaphase I (A I), (H) interkinesis (Int.), (I) prophase II (Pro. II), and (J) metaphase II (M II). The sex bivalent (XY) is indicated. Arrows indicate the centriolar area at cell poles. Selected metaphase I autosomal (B, C) and sex (E, F) bivalents.
-
K, LEnlarged metaphase II centromeres.
Figure 5. Relative distribution of Sororin and kinetochores during the meiotic divisions.
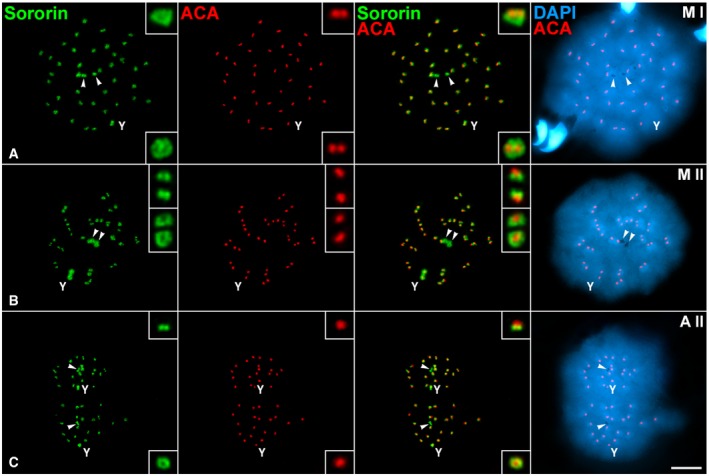
-
A–CDouble‐immunolabeling of Sororin (green) and kinetochores (ACA serum, red), and counterstaining of the chromatin with DAPI (blue) on spread spermatocytes at (A) metaphase I (M I), (B) metaphase II (M II), and (C) anaphase II (A II).
The centromere loading of Sororin is independent on SGO2
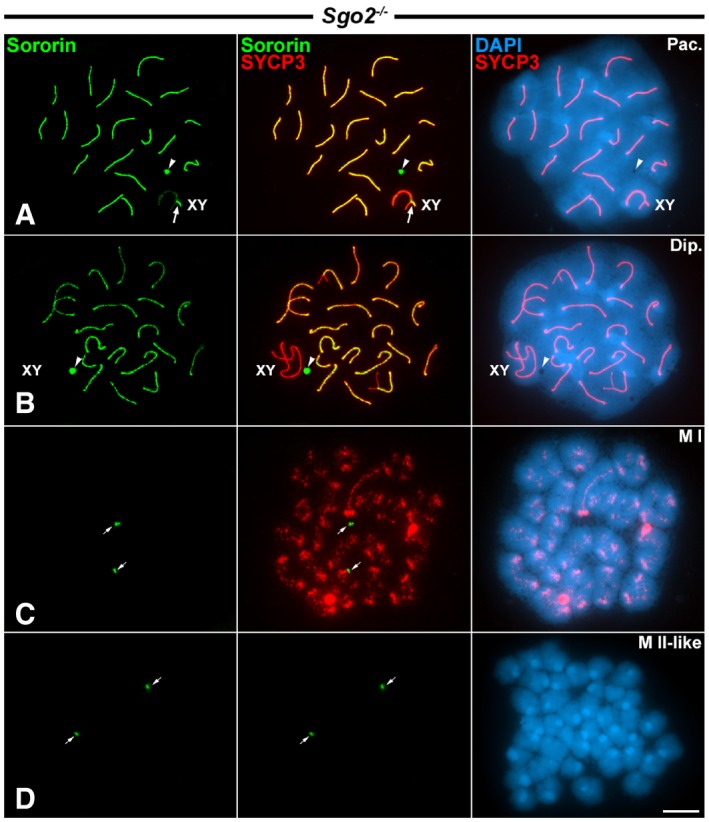
Since Sororin redistributed to the centromeres by late diplotene and showed a distribution at the inner centromere domain similar to that of shugoshin SGO2 34, we tested whether the loading of Sororin to the centromeres was dependent on SGO2. For this purpose, we double‐immunolabeled Sororin and SYCP3 on Sgo2 −/− spermatocytes 36. Our results showed that in Sgo2 −/− pachytene and diplotene spermatocytes, Sororin had a distribution pattern similar to that observed in wild‐type spermatocytes, that is, at the SC central regions, and also at the round body (Fig 6A and B). However, in Sgo2 −/− metaphase I spermatocytes no centromeric Sororin signals were detected (Fig 6C). Similarly, no Sororin was detected at the centromeres in metaphase II‐like spermatocytes, which showed all chromatids individualized (Fig 6D). Interestingly, Sororin signals were evident at cell poles in metaphase I and metaphase II‐like spermatocytes (Fig 6C and D). These results indicate that the centromere loading of Sororin during late diplotene is apparently dependent on the presence of SGO2. In this context, it is worth noting that cohesin complexes are still present at the centromeres of metaphase I chromosomes in Sgo2 −/− spermatocytes 36. Thus, Sororin is absent from metaphase I centromeres lacking SGO2 despite the presence of cohesins.
Figure 6. Distribution of Sororin in Sgo2 −/− knockout spermatocytes.
-
A–DDouble‐immunolabeling of Sororin (green) and SYCP3 (red), and counterstaining of the chromatin with DAPI (blue) on spread Sgo2 −/− spermatocytes at (A) pachytene (Pac.), (B) diplotene (Dip.), (C) metaphase I (M I), and (D) metaphase II‐like (M II‐like).
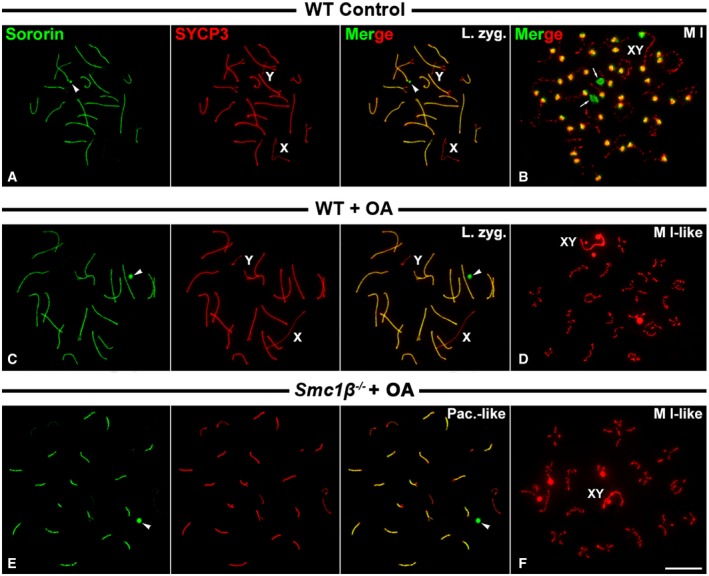
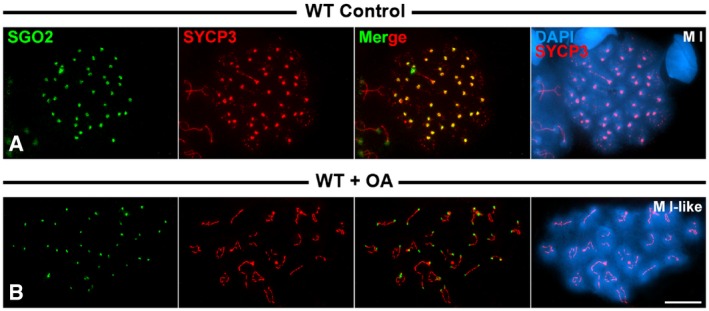
Since in the absence of SGO2 its partner, the phosphatase PP2A, is not loaded to metaphase I centromeres 48, we tested whether the phosphatase activity of PP2A was important for the recruitment of Sororin. For this purpose, we treated cultured spermatocytes with okadaic acid, a potent phosphatase inhibitor. Consistent with our previous results, Sororin was detected at the SC central region during prophase I stages, and at the centromeres of metaphase I chromosomes in wild‐type‐cultured spermatocytes (Fig 7A and B). However, Sororin was not detected at the centromeres of metaphase I chromosomes in okadaic acid‐treated cultured wild‐type spermatocytes, although it was clearly seen at the SC central regions of prophase I spermatocytes (Fig 7C and D). This result suggests that the phosphatase activity of PP2A could be necessary for the recruitment of Sororin. However, since okadaic acid induces the entry into metaphase I of mid‐pachytene spermatocytes in only 6 h 49, we cannot disregard the possibility that the okadaic acid treatment per se avoids the accurate maturation of the inner centromere in such short period of time. In fact, in those okadaic acid‐induced metaphases I SYCP3 was not accumulated at the inner centromeres, but persisted at the interchromatid domain along the chromosome arms (compare Fig 7B and D). Thus, we then tested whether SGO2 was present at the inner centromeres of metaphases I induced after the okadaic acid treatment. Our results showed that SGO2 was present at metaphase I centromeres in those spermatocytes, although the signals were smaller than those found in control‐cultured spermatocytes (Fig EV4A and B). Thus, in okadaic acid‐induced metaphases I Sororin is not loaded to the centromeres even in the presence of SGO2. Our results thus suggest that the recruitment or maintenance of Sororin to the centromeres is independent on the presence of SGO2.
Figure 7. Distribution of Sororin in okadaic acid‐treated spermatocytes.
-
A, BWT control‐cultured spermatocytes in (A) late zygotene (L. zyg.) and (B) metaphase I (M I). Arrows indicate the cell poles.
-
C, DWT‐cultured spermatocytes treated with okadaic acid in (C) late zygotene (L. zyg.) and (D) metaphase I‐like (M I‐like).
-
E, FSmc1β −/− cultured spermatocytes treated with okadaic acid in (E) pachytene‐like (Pac.‐like) and (F) metaphase I‐like (M I‐like).
Figure EV4. Distribution of SGO2 in okadaic acid‐treated spermatocytes.
-
A, BDouble‐immunolabeling of SGO2 (green) and SYCP3 (red) on spread cultured wild‐type control (WT Control) metaphase I spermatocyte (A), and okadaic acid‐treated wild‐type (WT + OA) metaphase I‐like spermatocyte (B).
Data information: Scale bar: 10 μm.
Finally, and in order to know whether the presence of meiotic cohesins at the centromeres was necessary for the recruitment of Sororin, we treated Smc1β −/− spermatocytes, which normally arrest at a pachytene‐like stage, with okadaic acid to induce their entry into metaphase I. We found that Sororin was detected at paired regions in pachytene‐like spermatocytes, as previously shown in untreated spermatocytes (compare Figs 7E and 3D), but was not present at the centromeres of induced metaphases I (Fig 7F). In these Smc1β −/− metaphase I‐like bivalents, SYCP3 showed the same distribution as in okadaic acid‐treated metaphase I bivalents from wild‐type individuals (compare Fig 7F and D). Since in okadaic acid‐treated wild‐type spermatocytes Sororin was likewise not present at metaphase I centromeres, we could not ascertain whether the loading of Sororin at metaphase I centromeres was dependent on the presence of meiotic cohesins.
Discussion
In order to infer Sororin function(s) during both meiotic divisions, we have analyzed by immunofluorescence its distribution in wild‐type and knockout male mice for different proteins participating in the SC assembly, cohesin subunits, the formation of DNA double‐strand breaks, and the protection of centromere cohesion.
Sororin at the central region of the synaptonemal complex
Our results on the distribution and dynamics of Sororin during prophase I stages indicate that this cohesin regulator is present at the SC central region in a SYCP1‐dependent manner (Fig 8). The presence of Sororin at SC central regions is an unexpected result since the cohesin subunits SMC1α, SMC1β, SMC3, RAD21, RAD21L, REC8, and STAG3 23, as well as the cohesin regulators PDS5B 50, WAPL 51, NIPBL, and MAU2 25, all appear along the axial/lateral elements, but not at the SC central region. These data suggest that Sororin function at the SC central region could be independent on cohesin complexes. Consistent with this possibility, our results show that Sororin is present at chromosome cores in pachytene‐like Rec8 −/− and Smc1β −/− mutant spermatocytes. Thus, Sororin localization at the SC central region is independent on the major meiotic cohesin complexes containing these subunits. However, and since there are other cohesin complexes during mouse meiosis 23, we cannot exclude that cohesin complexes with RAD21, RAD21L, or SMC1α could be interacting with Sororin. Conversely, Sororin is absent in Sycp1 −/− knockout spermatocytes unlike cohesin. This behavior of Sororin is very similar to that of SMC5 and SMC6, which also appear at the SC central region in male mice in a SYCP1‐dependent manner, and independently of meiotic cohesin complexes containing REC8 and SMC1β 52. Moreover, SMC6, as Sororin, redistributes from SC central regions to the centromeres during late diplotene. It is tempting to speculate that Sororin could regulate SMC5/6 complexes at SC central regions. The localization of Sororin at SC central regions suggests that, by regulating or cooperating with SMC5/6 complexes, it could be involved in distinct non‐mutually exclusive processes such as chromosome pairing, recruitment of SC central element proteins that facilitate the formation and/or stabilization of the SC, or the interaction with chromatin fibers at sites of recombination. Our results showing the presence of Sororin at regions where non‐homologous chromosomes are paired in zygotene‐like mutant Spo11 −/− spermatocytes, where no double‐strand breaks are formed 47, indicate that the loading of Sororin to the SC central region is independent on homologous pairing. These results also indicate that the loading of Sororin is independent on the formation of double‐strand breaks, which is consistent with the fact that SYCP1 is also detected at the SC central region in Spo11 −/− spermatocytes 47.
Figure 8. Representative scheme of the distribution of Sororin during male mouse meiotic divisions.

Sororin is indicated in green, SYCP3 in red, and their colocalization in yellow. The kinetochores, and the microtubules and centrioles are indicated in blue and light blue, respectively. Nuclei and condensed chromosomes, as well as spermatocyte cytoplasms, appear in gray and white, respectively. To simplify, the scheme only represents one autosomal bivalent and the sex pair XY. Chromosomes are telocentric, and the metaphase I autosomal and sex bivalents show a single interstitial and distal chiasma, respectively. Amplified details of the centromeric areas of an autosome in metaphase I and metaphase II, and of a segregating chromatid in anaphase II, are also shown.
Sororin at the centromeres
Sororin has been detected at the inner centromere domain in prometaphase human 9, 53 and mouse chromosomes 54. We have found that Sororin appears at the centromeres from late diplotene onwards, after its disappearance from the SC central region during desynapsis (Fig 8). This result suggests that either the Sororin pool that was previously located at the SC central regions redistributes to the centromeres, or that a new Sororin pool is specifically loaded to the centromeres at this stage. A similar pattern of dynamic redistribution from the SC central region to the centromeres during mammalian meiosis has been reported for the chromosomal passenger protein INCENP 55, 56 and more recently for the SMC5/6 complex 52. Thus, our results indicate that Sororin is another protein that shows a dynamic relocalization from the SC central regions to the centromeres.
In metaphase I, Sororin is detected at the inner centromere domain (Fig 8) with a similar distribution to SGO2 and MCAK 34, 55, 57, SYCP3, and the cohesins RAD21 37 and RAD21L 31. Since shugoshin SGO2 also loads to the centromeres during late diplotene 34, and it is known that during mammalian meiosis it serves as a platform for the recruitment of MCAK 36, 48, 55, 58 and PP2A 48, 58, we studied whether SGO2 is also involved in the recruitment of Sororin to the centromeres. Our results showed that in mutant Sgo2 −/− metaphase I spermatocytes Sororin was not present at the centromeres, thus firstly suggesting that the recruitment of Sororin could be dependent on the presence of SGO2. However, the absence of Sororin at the centromeres of okadaic acid‐induced metaphase I wild‐type spermatocytes, where SGO2 was present, argues against that possibility. Thus, the present results lead us to suggest that the loading of Sororin at metaphase I centromeres may depend on the phosphatase activity of PP2A that should be inhibited by okadaic acid. This means that the loading of Sororin would depend more likely on the functional activity of the complex SGO2‐PP2A, and therefore on the phosphatase activity of PP2A, rather than on the physical presence of SGO2 itself. Alternatively, the recruitment or maintenance of Sororin to the centromeres could be dependent on other proteins or mechanisms that act at the inner centromere after the loading of SGO2, and that are inhibited with okadaic acid.
During meiosis II, we have observed that Sororin shows the same pattern of centromere localization as SGO2 34, 55 and MCAK 55, 57, appearing the three proteins as rings encircling each sister kinetochore during metaphase II and anaphase II (Fig 8). It has been proposed that in mitosis the complex between phosphorylated SGO1 and PP2A maintains Sororin in a hypophosphorylated state to protect centromeric cohesin from premature removal by WAPL until the metaphase/anaphase transition 15. On the other hand, it is known that the complex SGO2‐PP2A protects centromeric cohesion during mouse meiosis 34, 35, 36, 48, 58. We speculate that in male mouse meiosis the tension‐dependent redistribution of SGO2, and of PP2A, and its dissociation from Sororin could allow the phosphorylation of REC8‐containing cohesin complexes, a requisite needed for their efficient cleavage by Separase during meiosis II 59, 60.
Altogether, our results show that Sororin, a regulatory factor of the cohesin complexes, has a dynamic relocalization from the SC central region to the centromeres suggesting novel and specific meiotic functions. These intriguing findings indicate that Sororin could be involved in specific functions at the SC central regions during prophase I stages by regulating/reinforcing the SC structure, or serving as a docking platform for other undetermined proteins. We demonstrate that the loading of Sororin during meiotic prophase I is dependent on SYCP1, independent on meiotic cohesin complexes containing REC8 and SMC1β, and independent on double‐strand breaks and homologous pairing. We also suggest that the centromere loading of Sororin is independent on SGO2 and that Sororin could participate in the regulation of centromeric cohesion during both meiotic divisions together with the SGO2/PP2A complex.
Materials and Methods
Animals
Mice used in this study were as follows: wild type (C57BL/6), and knockouts for Syce3 −/− 41, Sycp1 −/− 42, Rec8 −/− 44, Smc1β −/− 27, Spo11 −/− 47, Sgo2 −/− 36, and REC8‐myc transgenic mice 40. Animals were handled according to the regulatory standards, and experiments were approved by the UAM Ethics Committee.
Spreading of spermatocyte chromatin and immunocytology
Testes from adult males were removed, detunicated, and seminiferous tubules then processed for spermatocyte spreading by the drying‐down technique previously described 61. Spread spermatocyte preparations were rinsed three times for 5–10 min in PBS and incubated in a humid chamber for 2 h at 37°C or overnight at 4°C with the corresponding primary antibodies diluted in PBS. In double‐immunolabeling experiments, primary antibodies from different host species were incubated simultaneously. Following three washes in PBS for 5–10 min, the slides were incubated for 45 min at room temperature with the corresponding secondary antibodies. The slides were subsequently rinsed in PBS and counterstained for 3 min with 10 μg/ml DAPI (4′,6‐diamidino‐2‐phenylindole). After a final rinse in PBS, the slides were mounted with Vectashield (Vector Laboratories) and sealed with nail polish.
Immunofluorescence images were collected on an Olympus BX61 microscope equipped with epifluorescence optics, a motorized z‐drive, and an Olympus DP71 digital camera controlled by analySIS software (Soft Imaging System). Final images were processed with Adobe Photoshop 7 or CS5.
Antibodies for immunocytology
We used two rabbit polyclonal antibodies raised against mouse Sororin: serum C‐106 was generated by José Luis Barbero using a full‐length recombinant mouse protein expressed in E. coli following previous protocols 62; the other antibody, anti‐Sor, has been described previously 54, 63. Both antibodies were purified. By Western blot, both antibodies recognize a doublet with an apparent molecular weight of 36 kDa in extracts prepared from adult mouse testes (Fig EV1), as previously reported for Sororin in Xenopus 7 and human cell extracts 11, 53. The two antibodies used in this study (anti‐Sor and C‐106) offered highly similar results in immunofluorescence and therefore present comparable staining patterns of Sororin during prophase I (Fig EV3). As the serum C‐106 provided slightly cleaner results, it is the one used for the images presented in this work at a 1:10 dilution.
Other primary antibodies were used as follows: a mouse monoclonal antibody against mouse SYCP3 (Santa Cruz, sc‐74569) at 1:100; rabbit polyclonal K919, K987, and K1059 34 antibodies against rat SYCP1, human SMC3, and mouse SGO2, respectively, developed by José Luis Barbero at 1:10; purified guinea pig antibody against mouse HORMAD1 at 1:200, kindly provided by Attila Toth 26; and human anti‐centromere autoantibody (ACA serum) labeling kinetochores (Antibodies Incorporated, 435‐2RG‐7) at 1:50. REC8‐myc was revealed with a mouse monoclonal antibody against the myc epitope tag (GeneTex, GTX 628259) at a 1:10 dilution. The following secondary antibodies were employed at the indicated dilution: Alexa 488‐ and Alexa 594‐conjugated donkey anti‐rabbit IgG (Molecular Probes) at 1:100; Alexa 594‐conjugated donkey anti‐mouse IgG (Molecular Probes) at 1:100; Alexa 594‐conjugated goat anti‐guinea pig IgG (Molecular Probes) at 1:100; Alexa 594‐conjugated donkey anti‐human IgG (Molecular Probes) at 1:100.
Sororin overexpression and knockdown
HEK 293T cells were transiently transfected with pEGFP‐N1‐Sororin and empty pEGFP expression vectors and whole‐cell extracts were prepared 24 h after the transfection. C2C12 cells were transiently transfected with Sororin siRNA (Thermo Scientific Dharmacon, cat# M‐048366‐01) at a final concentration of 100 nM, or siRNA control (Scramble), and whole‐cell extracts were prepared 48 h after the transfection. About 20 μg of protein were loaded on reducing 10% polyacrylamide–SDS gels, and proteins were detected by Western blotting. Sororin was detected with two polyclonal rabbit antibodies (C‐106 and anti‐Sor). Antibodies were detected by using ImmobilonTM Western Chemiluminescent HRP Substrate from Millipore according to the manufacturer's recommendations.
Culture of seminiferous tubules and okadaic acid treatment
Culture of seminiferous tubules was performed as previously described 64. In brief, testes were removed and detunicated and fragments of seminiferous tubules were cultured for 1 h in agarose gel half‐soaked in MEMα culture medium (Gibco A10490‐01) supplemented with KnockOut Serum Replacement (KRS) (Gibco 10828‐010) and antibiotics (Penicillin/Streptomycin; Biochrom AG, A2213) at 34°C in an atmosphere with 5% CO2. Cultured seminiferous tubules were treated with 5 μM okadaic acid (Calbiochem, cat. 495604) for 6 h or kept in culture medium without okadaic acid as control. After treatment, the seminiferous tubules were subjected to the spreading technique as previously described.
Author contributions
RG, NF‐M, MR‐T, IB, AV, SP, and JAS performed all the experiments. JLB and AL developed the Sororin antibodies. EL, TF, and MA contributed with their KO mice and in the discussion of the results. AL and AMP designed the Western blot, overexpression, and RNAi experiments and corrected the manuscript. RG and JAS supervised the project, analyzed results, and wrote the paper. All authors discussed the results and contributed to the correction of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Review Process File
Acknowledgements
We express our sincere thanks to Christer Höög, Rolf Jessberger, John C. Schimenti, and Kerry Schimenti, and to Scott Keeney and Ignasi Roig for providing mice and slides from the knockouts of SYCP1, SMC1β, REC8, and SPO11, respectively, and to Kim Nasmyth for providing the REC8‐myc transgenic mice. We also thank Attila Toth for the HORMAD1 antibody, and Lorena Barreras for her technical assistance. This work was supported by grants SAF2011‐28842‐C02‐01 and BFU2014‐53681‐P (to JAS), SAF2011‐25252, BFU2014‐59307‐R and Junta de CyLeon (to AMP), grant MEIONet BFU2015‐71786‐REDT (to JAS and AMP), BFU2013‐48481‐R (to AL) from Ministerio de Economía y Competitividad (Spain) BFU2013‐48481‐R (to AL) from Ministerio de Economía y Competitividad (Spain) and FEDER, grant AP98712012 (to JLB) from Fundación Mutua Madrileña, grant AI 1090/2‐1 (to MA) from the Priority Program SPP 1384 of the German Science Foundation, and grant 25891015 (to TF) from the Japan Society for the Promotion of Science.
EMBO Reports (2016) 17: 695–707
References
- 1. Nasmyth K (2011) Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol 13: 1170–1177 [DOI] [PubMed] [Google Scholar]
- 2. Remeseiro S, Losada A (2013) Cohesin, a chromatin engagement ring. Curr Opin Cell Biol 25: 63–71 [DOI] [PubMed] [Google Scholar]
- 3. Gillespie PJ, Hirano T (2004) Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr Biol 14: 1598–1603 [DOI] [PubMed] [Google Scholar]
- 4. Rolef Ben‐Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, Uhlmann F (2008) Eco1‐dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321: 563–566 [DOI] [PubMed] [Google Scholar]
- 5. Unal E, Heidinger‐Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE (2008) A molecular determinant for the establishment of sister chromatid cohesion. Science 321: 566–569 [DOI] [PubMed] [Google Scholar]
- 6. Zhang J, Shi X, Li Y, Kim BJ, Jia J, Huang Z, Yang T, Fu X, Jung SY, Wang Y et al (2008) Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 31: 143–151 [DOI] [PubMed] [Google Scholar]
- 7. Rankin S, Ayad NG, Kirschner MW (2005) Sororin, a substrate of the anaphase‐promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell 18: 185–200 [DOI] [PubMed] [Google Scholar]
- 8. Losada A, Yokochi T, Hirano T (2005) Functional contribution of Pds5 to cohesin‐mediated cohesion in human cells and Xenopus egg extracts. J Cell Sci 118: 2133–2141 [DOI] [PubMed] [Google Scholar]
- 9. Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V, Bando M, Shirahige K, Hyman AA, Mechtler K et al (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143: 737–749 [DOI] [PubMed] [Google Scholar]
- 10. Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- 11. Dreier MR, Bekier ME 2nd, Taylor WR (2011) Regulation of sororin by Cdk1‐mediated phosphorylation. J Cell Sci 124: 2976–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishiyama T, Sykora MM, Huis in ‘t Veld PJ, Mechtler K, Peters JM (2013) Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci USA 110: 13404–13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shintomi K, Hirano T (2009) Releasing cohesin from chromosome arms in early mitosis: opposing actions of Wapl‐Pds5 and Sgo1. Genes Dev 23: 2224–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52 [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Rankin S, Yu H (2013) Phosphorylation‐enabled binding of SGO1‐PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol 15: 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323 [DOI] [PubMed] [Google Scholar]
- 17. Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister‐chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400: 37–42 [DOI] [PubMed] [Google Scholar]
- 18. Zhang N, Pati D (2012) Sororin is a master regulator of sister chromatid cohesion and separation. Cell Cycle 11: 2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Handel MA, Schimenti JC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11: 124–136 [DOI] [PubMed] [Google Scholar]
- 20. Fraune J, Schramm S, Alsheimer M, Benavente R (2012) The mammalian synaptonemal complex: protein components, assembly and role in meiotic recombination. Exp Cell Res 318: 1340–1346 [DOI] [PubMed] [Google Scholar]
- 21. Moens PB, Heyting C, Dietrich AJ, van Raamsdonk W, Chen Q (1987) Synaptonemal complex antigen location and conservation. J Cell Biol 105: 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schalk JA, Dietrich AJ, Vink AC, Offenberg HH, van Aalderen M, Heyting C (1998) Localization of SCP2 and SCP3 protein molecules within synaptonemal complexes of the rat. Chromosoma 107: 540–548 [DOI] [PubMed] [Google Scholar]
- 23. McNicoll F, Stevense M, Jessberger R (2013) Cohesin in gametogenesis. Curr Top Dev Biol 102: 1–34 [DOI] [PubMed] [Google Scholar]
- 24. Suja JA, Barbero JL (2009) Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn 5: 94–116 [DOI] [PubMed] [Google Scholar]
- 25. Visnes T, Giordano F, Kuznetsova A, Suja JA, Lander AD, Calof AL, Strom L (2014) Localisation of the SMC loading complex Nipbl/Mau2 during mammalian meiotic prophase I. Chromosoma 123: 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wojtasz L, Daniel K, Roig I, Bolcun‐Filas E, Xu H, Boonsanay V, Eckmann CR, Cooke HJ, Jasin M, Keeney S et al (2009) Mouse HORMAD1 and HORMAD2, two conserved meiotic chromosomal proteins, are depleted from synapsed chromosome axes with the help of TRIP13 AAA‐ATPase. PLoS Genet 5: e1000702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Revenkova E, Eijpe M, Heyting C, Hodges CA, Hunt PA, Liebe B, Scherthan H, Jessberger R (2004) Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6: 555–562 [DOI] [PubMed] [Google Scholar]
- 28. Prieto I, Suja JA, Pezzi N, Kremer L, Martinez AC, Rufas JS, Barbero JL (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 3: 761–766 [DOI] [PubMed] [Google Scholar]
- 29. Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C (2003) Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol 160: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gutierrez‐Caballero C, Herran Y, Sanchez‐Martin M, Suja JA, Barbero JL, Llano E, Pendas AM (2011) Identification and molecular characterization of the mammalian alpha‐kleisin RAD21L. Cell Cycle 10: 1477–1487 [DOI] [PubMed] [Google Scholar]
- 31. Herran Y, Gutierrez‐Caballero C, Sanchez‐Martin M, Hernandez T, Viera A, Barbero JL, de Alava E, de Rooij DG, Suja JA, Llano E et al (2011) The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 30: 3091–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishiguro K, Kim J, Fujiyama‐Nakamura S, Kato S, Watanabe Y (2011) A new meiosis‐specific cohesin complex implicated in the cohesin code for homologous pairing. EMBO Rep 12: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J, Hirano T (2011) RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol 192: 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gomez R, Valdeolmillos A, Parra MT, Viera A, Carreiro C, Roncal F, Rufas JS, Barbero JL, Suja JA (2007) Mammalian SGO2 appears at the inner centromere domain and redistributes depending on tension across centromeres during meiosis II and mitosis. EMBO Rep 8: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10: 42–52 [DOI] [PubMed] [Google Scholar]
- 36. Llano E, Gomez R, Gutierrez‐Caballero C, Herran Y, Sanchez‐Martin M, Vazquez‐Quinones L, Hernandez T, de Alava E, Cuadrado A, Barbero JL et al (2008) Shugoshin‐2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev 22: 2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parra MT, Viera A, Gomez R, Page J, Benavente R, Santos JL, Rufas JS, Suja JA (2004) Involvement of the cohesin Rad21 and SCP3 in monopolar attachment of sister kinetochores during mouse meiosis I. J Cell Sci 117: 1221–1234 [DOI] [PubMed] [Google Scholar]
- 38. Schultz MC (1990) Three structures associated with the nucleolus in male rat germinal cells: round body, coiled body, and “nubecula” and general presence of round body at male meiosis. Am J Anat 189(189): 11–23 [DOI] [PubMed] [Google Scholar]
- 39. Fukuda T, Daniel K, Wojtasz L, Toth A, Hoog C (2010) A novel mammalian HORMA domain‐containing protein, HORMAD1, preferentially associates with unsynapsed meiotic chromosomes. Exp Cell Res 316: 158–171 [DOI] [PubMed] [Google Scholar]
- 40. Kudo NR, Wassmann K, Anger M, Schuh M, Wirth KG, Xu H, Helmhart W, Kudo H, McKay M, Maro B et al (2006) Resolution of chiasmata in oocytes requires separase‐mediated proteolysis. Cell 126: 135–146 [DOI] [PubMed] [Google Scholar]
- 41. Schramm S, Fraune J, Naumann R, Hernandez‐Hernandez A, Hoog C, Cooke HJ, Alsheimer M, Benavente R (2011) A novel mouse synaptonemal complex protein is essential for loading of central element proteins, recombination, and fertility. PLoS Genet 7: e1002088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Vries FA, de Boer E, van den Bosch M, Baarends WM, Ooms M, Yuan L, Liu JG, van Zeeland AA, Heyting C, Pastink A (2005) Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev 19: 1376–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21: 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC (2004) Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. Genesis 40: 184–194 [DOI] [PubMed] [Google Scholar]
- 45. Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8: 949–961 [DOI] [PubMed] [Google Scholar]
- 46. Novak I, Wang H, Revenkova E, Jessberger R, Scherthan H, Hoog C (2008) Cohesin Smc1beta determines meiotic chromatin axis loop organization. J Cell Biol 180: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baudat F, Manova K, Yuen JP, Jasin M, Keeney S (2000) Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell 6: 989–998 [DOI] [PubMed] [Google Scholar]
- 48. Rattani A, Wolna M, Ploquin M, Helmhart W, Morrone S, Mayer B, Godwin J, Xu W, Stemmann O, Pendas A et al (2013) Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. Elife 2: e01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiltshire T, Park C, Caldwell KA, Handel MA (1995) Induced premature G2/M‐phase transition in pachytene spermatocytes includes events unique to meiosis. Dev Biol 169: 557–567 [DOI] [PubMed] [Google Scholar]
- 50. Fukuda T, Hoog C (2010) The mouse cohesin‐associated protein PDS5B Is expressed in testicular cells and is associated with the meiotic chromosome axes. Genes (Basel) 1: 484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang J, Hakansson H, Kuroda M, Yuan L (2008) Wapl localization on the synaptonemal complex, a meiosis‐specific proteinaceous structure that binds homologous chromosomes, in the female mouse. Reprod Domest Anim 43: 124–126 [DOI] [PubMed] [Google Scholar]
- 52. Gomez R, Jordan PW, Viera A, Alsheimer M, Fukuda T, Jessberger R, Llano E, Pendas AM, Handel MA, Suja JA (2013) Dynamic localization of SMC5/6 complex proteins during mammalian meiosis and mitosis suggests functions in distinct chromosome processes. J Cell Sci 126: 4239–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang N, Panigrahi AK, Mao Q, Pati D (2011) Interaction of Sororin protein with polo‐like kinase 1 mediates resolution of chromosomal arm cohesion. J Biol Chem 286: 41826–41837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carretero M, Ruiz‐Torres M, Rodriguez‐Corsino M, Barthelemy I, Losada A (2013) Pds5B is required for cohesion establishment and Aurora B accumulation at centromeres. EMBO J 32: 2938–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parra MT, Gomez R, Viera A, Llano E, Pendas AM, Rufas JS, Suja JA (2009) Sequential assembly of centromeric proteins in male mouse meiosis. PLoS Genet 5: e1000417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parra MT, Viera A, Gomez R, Page J, Carmena M, Earnshaw WC, Rufas JS, Suja JA (2003) Dynamic relocalization of the chromosomal passenger complex proteins inner centromere protein (INCENP) and aurora‐B kinase during male mouse meiosis. J Cell Sci 116: 961–974 [DOI] [PubMed] [Google Scholar]
- 57. Parra MT, Gomez R, Viera A, Page J, Calvente A, Wordeman L, Rufas JS, Suja JA (2006) A perikinetochoric ring defined by MCAK and Aurora‐B as a novel centromere domain. PLoS Genet 2: e84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tanno Y, Kitajima TS, Honda T, Ando Y, Ishiguro K, Watanabe Y (2010) Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev 24: 2169–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brar GA, Kiburz BM, Zhang Y, Kim JE, White F, Amon A (2006) Rec8 phosphorylation and recombination promote the step‐wise loss of cohesins in meiosis. Nature 441: 532–536 [DOI] [PubMed] [Google Scholar]
- 60. Katis VL, Lipp JJ, Imre R, Bogdanova A, Okaz E, Habermann B, Mechtler K, Nasmyth K, Zachariae W (2010) Rec8 phosphorylation by casein kinase 1 and Cdc7‐Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev Cell 18: 397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peters AH, Plug AW, van Vugt MJ, de Boer P (1997) A drying‐down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res 5: 66–68 [DOI] [PubMed] [Google Scholar]
- 62. Pezzi N, Prieto I, Kremer L, Perez Jurado LA, Valero C, Del Mazo J, Martinez AC, Barbero JL (2000) STAG3, a novel gene encoding a protein involved in meiotic chromosome pairing and location of STAG3‐related genes flanking the Williams‐Beuren syndrome deletion. Faseb J 14: 581–592 [DOI] [PubMed] [Google Scholar]
- 63. Remeseiro S, Cuadrado A, Carretero M, Martinez P, Drosopoulos WC, Canamero M, Schildkraut CL, Blasco MA, Losada A (2012) Cohesin‐SA1 deficiency drives aneuploidy and tumourigenesis in mice due to impaired replication of telomeres. EMBO J 31: 2076–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T (2011) In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471: 504–507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Review Process File


