Abstract
OBJECTIVE
Compare efficacy and safety of 10 to 15 mg/kg with 20 to 30 mg/kg acetaminophen in febrile children 6 months to ≤ 11 years from 3 double-blind, randomized, single or multiple dose studies.
METHODS
Doses were compared on sum of the temperature differences (SUMDIFF), maximum temperature difference (MAXDIFF), temperature differences at each time point, and dose by time interactions. Alanine aminotransferase (ALT) was evaluated in the 72-hour duration study.
RESULTS
A single dose of acetaminophen 20 to 30 mg/kg produced a greater effect on temperature decrement and duration of antipyretic effect over 8 hours than a single dose of 10 to 15 mg/kg. When equivalent total doses (i.e., 2 doses of 10 to 15 mg/kg given at 4-hour intervals and 1 dose of 20 to 30 mg/kg) were given over the initial 8-hour period, there were no significant temperature differences. Over a 72-hour period, 10 to 15 mg/kg acetaminophen administered every 4 hours maintained a more consistent temperature decrement than 20 to 30 mg/kg acetaminophen administered every 8 hours. Following doses of 60 to 90 mg/kg/day for up to 72 hours, no child had a clinically important increase in ALT from baseline. The number of children with reported adverse events was similar between doses.
CONCLUSIONS
Data demonstrate the antipyretic effect of acetaminophen is dependent on total dose over a given time interval. These 3 studies provide clinical evidence that the recommended standard acetaminophen dose of 10 to 15 mg/kg is a safe and effective dose for treating fever in pediatric patients when administered as a single dose or as multiple doses for up to 72 hours.
Keywords: acetaminophen, alanine aminotransferase, dosing, fever, pediatric, randomized controlled trial
Introduction
Acetaminophen (N-acetyl-p-aminophenol) has been used widely as both an antipyretic and analgesic for the treatment of fever and pain in children because of its well-established efficacy and favorable safety pro-file.1–3 Pharmacokinetic data and efficacy studies have indicated that single doses of 10 to 15 mg/kg at 4-hour intervals provide effective antipyretic activity.4,5 Nonstandard doses of acetaminophen, such as a single dose of 25 to 30 mg/kg, occasionally have been reported to treat fever and pain.6–8 Data supporting the direct relationship between increasing doses of acetaminophen and increased antipyretic effect have been evaluated for doses in the range of 5 to 30 mg/kg.1,5,9 Moreover, linear pharmacokinetics occur for oral doses of up to 30 mg/kg (double the standard dose).2 However, limited data directly compare the efficacy of 10 to 15 mg/kg to 20 to 30 mg/kg doses of acetaminophen in children.
Three legacy studies were conducted between 1981 and 1990. They were designed to explore the efficacy and safety of single and multiple doses of acetaminophen at 10 to 15 and 20 to 30 mg/kg.10–12 The earliest study (Study I) compared the standard single acetaminophen doses of 10 to 15 mg/kg given every 4 hours (6 doses in 24 hours) to double standard doses, consisting of 20 to 30 mg/kg every 8 hours (3 doses in 24 hours) during a 3-day period, totaling 60 to 90 mg/kg per day.10 These 2 dosing regimens exceeded the then approved and currently approved total daily dose. Acetaminophen is currently approved for use in children as an antipyretic and analgesic at the dose of 10 to 15 mg/kg every 4 hours (not to exceed 5 doses in 24 hours). The 2 subsequent studies (Studies II and III) compared a single acetaminophen dose of 10 to 15 mg/kg with a single dose of 20 to 30 mg/kg over an 8-hour period.11,12 The temperature decrement data following the initial dose in each of these studies have been reported previously in a review of pediatric antipyretic dosing.5 Some pharmacokinetic data, but none of the efficacy data, from a small subset of Study I were reported by one of the investigators,13–16 and summary mean and median alanine aminotransferase (ALT) data from Study I were presented to the Food and Drug Administration (FDA).17
The paper herein offers full details about the comparative effect of standard and double-standard doses and results of the safety evaluation of higher than standard dosing over a 72-hour period.
Materials and Methods
Study Design
Three double-blind, randomized clinical studies that compared 2 dosing regimens of acetaminophen in febrile infants and children were identified in the sponsor's database (Table 1 summarizes study parameters and subject demographics for each of these 3 studies).
Table 1.
Comparison of Study Parameters
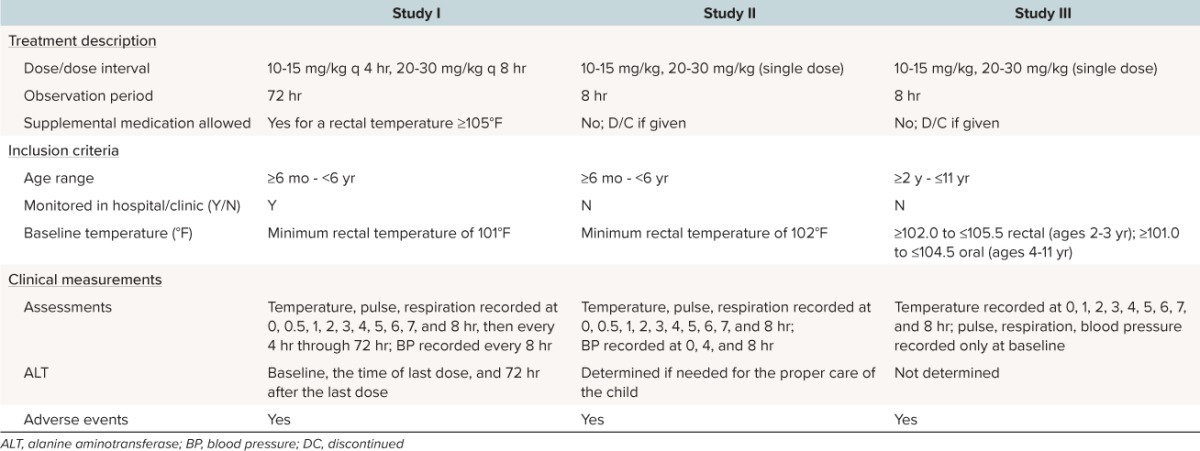
Study I, conducted between 1981 and 1982, was a multiple dose double-blind study comparing the antipyretic efficacy and safety of 10 to 15 mg/kg acetaminophen given every 4 hours to 20 to 30 mg acetaminophen given every 8 hours for up to 72 hours with placebo given at 4 hours and then every 8 hours to maintain blinding. All patients were hospitalized and between 6 months and < 6 years of age with a minimum rectal temperature of 101°F.10 Study II, conducted between 1982 and 1983, was a single dose double-blind study comparing the antipyretic efficacy and safety of 10 to 15 mg/kg and 20 to 30 mg/kg of acetaminophen throughout an 8-hour observation period.11 All participating children were between 6 months and < 6 years of age with a minimum rectal temperature of 102°F. Study III, conducted between 1989 and 1990, was an investigator and parent blinded, parallel, single dose study comparing the efficacy and side effect profile of acetaminophen 10 to 15 mg/kg to 20 to 30 mg/kg and ibuprofen suspension 5 mg/kg and 10 mg/kg throughout an 8-hour observation period.12 Children were between 2 and 11 years of age, with an acute febrile illness, and prestratified on the basis of their baseline temperature (≥ 102.0°F to ≤ 105.5°F in children 2 years through 3 years of age and ≥ 101.0°F to ≤ 104.5°F in children 4 years through 11 years of age) and then randomized to 1 of the 4 treatment groups.
Study I was conducted at 4 sites in the United States, Study II at 3 US sites, and Study III at 10 US sites. For each study and site, a local or regional independent institutional review board reviewed and approved the informed consent documents and protocol. Parents or legal guardians provided informed consent for children participating in each study; participants could withdraw from the study at any time without consequence to their care or treatment.
Inclusion and Exclusion Criteria
Inclusion criteria for the 3 studies are presented in Table 1. Children were excluded from all studies if they were unable to take oral medication, were dehydrated or malnourished, received antipyretic medication within 6 hours prior to the dosing of study medication, or had a known sensitivity to acetaminophen. For Study III, which included ibuprofen, additional exclusion criteria were used. Children were excluded if they were below the fifth percentile or above the 95th percentile of their appropriate weight for age, had a blood coagulation defect, anemia secondary to blood loss, hepatic, metabolic, endocrine, neoplastic or peptic ulcer disease, a diagnosis of any chronic renal disease, were under 6 years old with a history of febrile seizures, had received antibiotics or systemic steroidal medication within 72 hours prior to the study, or would require antibiotics during the 8 hours of the study.
Treatments
Dosing in Study I was based on the weight of the child using a schedule provided to each investigator. Scheduled doses for the 10 to 15 mg/kg group ranged from 10.8 to 15 mg/kg and scheduled doses for the 20 to 30 mg/kg group ranged from 21.6 to 30 mg/kg. All doses were administered by study personnel. All temperature measurements were made by study personnel. In Study I, study medication was provided as 30-mL unit dose vials containing acetaminophen elixir (McNeil Consumer Healthcare, Fort Washington, PA) at a concentration of 16 mg/mL for the 10 to 15 mg/kg dose or 32 mg/mL (marketed concentration) for the 20 to 30 mg/kg dose. For blinding, both strengths were identical in appearance.
In Study I, children were randomized in blocks of 10 to receive acetaminophen 10 to 15 mg/kg every 4 hours or 20 to 30 mg/kg every 8 hours. Children in the 20 to 30 mg/kg dose group also received a matching placebo dose at 4 hours and then every 8 hours to maintain double blinding. At 1 site, however, double blinding was not maintained after the first 13 children. At this site, the investigator went to an open-label study and eliminated the placebo dose in an attempt to reduce diarrhea; 13 patients were entered into the open study. Investigators at the other 3 sites maintained double-blind conditions for all their patients. The drug was originally formulated in January 1981 and was reformulated in January 1982 to contain one half the sorbitol concentration due to side effects (diarrhea) in children at the 1 site. Across the 4 study sites, 14 of 48 children enrolled received the original formulation and 34 received the reduced sorbitol formulation. Febrile children were treated for up to 72 hours; treatment could be discontinued at the next 8-hour interval for children who became afebrile.
Dosing in Study II was based on the weight of the child using a schedule provided to each investigator; the dosing schedules provided to the investigators in Studies I and II were identical. In Study II, scheduled doses for the 10 to 15 mg/kg group ranged from 10.8 to 15 mg/kg and scheduled doses for the 20 to 30 mg/kg group ranged from 21.6 to 30 mg/kg. All doses were administered by study personnel. All temperature measurements were made by study personnel. Study medication was provided as 30-mL unit dose vials containing acetaminophen elixir (McNeil Consumer Healthcare) at a concentration of 16 mg/mL for the 10 to 15 mg/kg dose or 32 mg/mL (marketed concentration) for the 20 to 30 mg/kg dose. For blinding, both strengths were identical in appearance. In Study II, children were randomized in blocks of 10 to receive a single dose of acetaminophen 10 to 15 mg/kg or a single dose of 20 to 30 mg/kg.
Dosing in Study III was based on the weight of the child using a schedule provided to each investigator. The doses were recorded and the actual dose calculated for each individual subject. The scheduled doses for the 10 to 15 mg/kg group ranged from 11.4 to 13.8 mg/kg. The actual individual doses administered ranged from 11.4 to 13.5 mg/kg and the mean and median dose administered was 12.4 mg/kg. The scheduled dose for the 20 to 30 mg/kg group ranged from 22.8 to 27.6 mg/kg. The actual doses administered ranged from 22.8 to 26.9 mg/kg and the mean and median dose administered was 24.8 mg/kg. Temperature measurements were recorded by the parent/guardian hourly for 8 hours with the same electronic study thermometer. In Study III, acetaminophen elixir (McNeil Consumer Healthcare) was provided as 60-mL unit dose vials at a concentration of 32 mg/mL for both acetaminophen doses.
In Study III, the 2 acetaminophen doses also were compared to 2 ibuprofen doses (5 mg/kg and 10 mg/kg). Children were stratified by baseline temperature into 2 groups: oral temperature ≤ 102.5°F or rectal temperature = 103.5°F and oral temperature > 102.5°F or rectal temperatures > 103.5°F. Within these groups, children were randomized in blocks of 8 to receive a single dose of either acetaminophen 12.5 mg/kg, acetaminophen 25 mg/kg, ibuprofen 5 mg/kg, or ibuprofen 10 mg/kg. Since the acetaminophen and ibuprofen treatments administered in this study were not identical in appearance, actions were taken to maintain blinding of the investigator and parent/guardian: doses were administered by a separate member of the study personnel in a separate room at the principal investigator's facility. Only results of the acetaminophen treatment arms in this study are reported herein.
In Studies I and II, the liquid product contained 7% alcohol in the solvent system. At the time of the conduct of Study III, the alcohol was replaced by glycols. Since the conduct of these trials and up until the present, although there have been minor changes in flavoring or other inert ingredients, there has been no change made in the active ingredient or its concentration. The concentration remains 160 mg acetaminophen/5 mL (32 mg/mL).
In Study I, rescue medication was allowed if a child's rectal temperature was ≥ 105°F. In Studies II and III, use of rescue medication resulted in the child being discontinued from the study (Table 1).
Assessments
The times at which temperature and vital signs (e.g., pulse, respiration rate, and blood pressure) were measured are listed in Table 1 by study, along with the conditions and timing for the assessment of ALT levels. Adverse events (AEs) were recorded in all studies.
Statistical Analyses
Temperature differences from baseline were calculated as baseline temperature minus temperature at each time point. The sum of the temperature differences (SUMDIFF) over the initial 8 hours was calculated as the sum of each child's temperature difference; measurements at 0.5 and 1 hour postdosing received half as much weight as the other measurements. The maximum temperature difference (MAXDIFF) over the initial 8 hours was defined for each subject as the MAXDIFF at any time point of measurement.
In Study I, if a child received supplemental treatment for fever, the remaining temperature readings were considered to be either the initial temperature or the last recorded temperature, whichever was higher (worst observation carried forward). Adjustment for missing measurements was not specified in Study I. In Study II, missing temperatures or temperatures >15 minutes from the scheduled time of measurement were replaced with the temperature from the previous time point and in Study III they were estimated by interpolation.
In Studies I and II, the 2 doses were compared on SUMDIFF and MAXDIFF using an analysis of covariance (ANCOVA) with initial temperature as the covariate. In Study III, the temperature differences were evaluated in a repeated measures analysis with dose, initial fever group, time and all interactions in the model. MAXDIFF was analyzed using ANCOVA with dose as a factor and baseline temperature as a covariate (Studies I and II) or using a 2-way (dose, initial fever group, dose by initial fever group interaction) analysis of variance (ANOVA; Study III). Number of hours a temperature reduction of at least 1°F was achieved was analyzed in Study III using a 2-way (dose, initial fever group, dose by initial fever group interaction) ANOVA. Temperature differences at each time point were analyzed with a 1-way ANOVA (Study I), ANCOVA with initial fever as the covariate (Study II), or in a repeated measures analysis (Study III). Dose by time interactions were evaluated in each study with a repeated measures analysis.
In Studies I and II, pulse and respiratory rates were assessed within each treatment group during the first 8 hours. A 2-way ANOVA (patient, time) was used to assess changes over time. If the effect of time was significant, the least squares means at each time were compared to baseline; a Bonferroni procedure was used in Study II. Pulse and respiratory rate were collected only at baseline in Study III.
Results
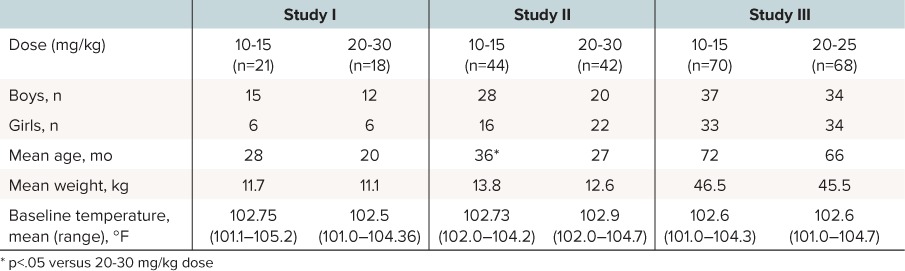
In total, there were 281 children enrolled in the acetaminophen treatment groups in these 3 studies, with 263 included in the efficacy analysis (Table 2). In Study I, 48 children enrolled and 39 were included in the efficacy analysis. Reasons for ineligibility included children whose ages were not within range (n = 2), whose initial temperatures were too low (n = 2), and who experienced other problems: vomited after 2 doses, inadequate heparin lock, mother discontinued, surgery after 6 hours, or wrong drug received (n = 1 each). In Study II, 88 children enrolled, and 86 completed and were included in the efficacy analysis. Two children discontinued due to vomiting and were not included in analyses. In Study III, 145 children enrolled, and 138 children completed and were included in the efficacy analysis. Reasons for ineligibility included wrong dose (n=2), age outside study limit (n = 2), vomited study medication, insufficient temperatures recorded, and wrong randomization (n = 1 each).
Table 2.
Comparison of Subject Demographics and Baseline Temperature
Patient Demographics and Identifiers
Baseline characteristics and temperatures of children in Studies I, II, and III are summarized in Table 2. In Studies I and III, no significant differences between dose groups with respect to age, weight, and gender were observed. In Study II, both dose groups were similar with respect to weight and gender; however, mean age was significantly higher for the 10 to 15 mg/kg versus the 20 to 30 mg/kg dose group (36 months versus 27 months; p = 0.0215). In all studies, there were no significant differences in initial temperature between 10 to 15 mg/kg and 20 to 30 mg/kg dose groups.
Efficacy
The SUMDIFF and MAXDIFF results are presented in Table 3. The mean temperature differences from baseline results are presented in Figures 1 to 4. In Study I, where equivalent total doses were given over the initial 8-hour period, there were no significant differences with respect to SUMDIFF or MAXDIFF between the 10 to 15 mg/kg and 20 to 30 mg/kg doses. In Study II, the difference between a single 10 to 15 mg/kg dose and a single 20 to 30 mg/kg dose approached statistical significance (p = 0.0536) for SUMDIFF while a single 20 to 30 mg/kg dose produced a significantly greater (p = 0.0028) MAXDIFF compared to a single 10 to 15 mg/kg dose. In Study III, a single 20 to 30 mg/kg dose produced a significantly (p < 0.0001) greater temperature decrement than the 10 to 15 mg/kg dose with respect to SUMDIFF and MAXDIFF. Children with higher initial temperatures had greater decreases from baseline on average. The effect of initial temperature as a covariate or a factor on SUMDIFF and MAXDIFF was significant in Study I (SUMDIFF, p = 0.0010; MAXDIFF, p = 0.0002), Study II (SUMDIFF, p = 0.0063; MAXDIFF, p = 0.0001), and Study III (SUMDIFF, p = 0.0001; MAX-DIFF, p = 0.0001).
Table 3.
Least Squares Mean Values for SUMDIFF and MAXDIFF During Initial 8-Hour Treatment Period
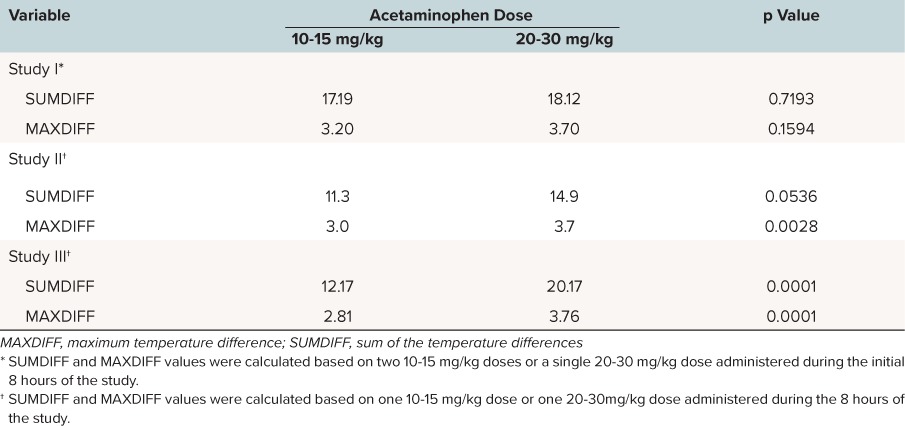
Figure 1.
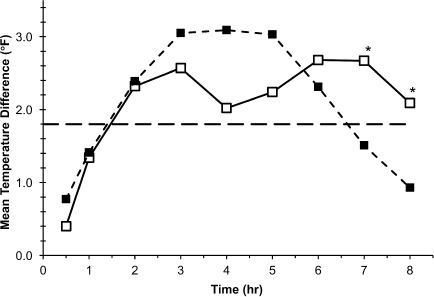
Mean temperature differences from baseline to each time point in Study I, 0 to 8 hours.
*p < 0.05.
□ Study I, 10–15 mg/kg (n = 21); ▪ Study I, 20–30 mg/kg (n = 18); ⁃⁃ 1.8°F (1°C) temperature change.
In Study I, temperature differences from baseline were not significantly different between the 10 to 15 mg/kg and 20 to 30 m/kg doses for the initial 6 hours of the study. Mean temperature differences from baseline were greater in children treated with the 20 to 30 mg/kg dose versus the first 10 to 15 mg/kg dose at the 3-, 4-, and 5-hour time points and approached statistical significance at 4 hours (2.02 versus 3.09, respectively; p = 0.0579). Mean temperature differences were statistically significantly greater following the second 10 to 15 mg/kg dose versus the 20 to 30 mg/kg dose at the later time points: 7 hours (2.67°F versus 1.51°F, respectively; p = 0.0291) and 8 hours (2.09°F versus 0.93°F, respectively; p = 0.0294; Figure 1). Analysis of mean change from baseline in temperature over the subsequent 12- to 72-hour dosing period showed that differences between temperatures measured at 4-hour intervals were not significant between the smaller and larger dose groups, except at 28 hours where the 20 to 30 mg/kg dose decrement versus the 10 to 15 mg/kg dose (4.21°F versus 2.73°F, respectively; p = 0.0221) was statistically significant (Figure 2). In Study II, temperature differences from baseline were not significantly different between the 10 to 15 mg/kg and 20 to 30 m/kg doses during the study with the exception of the 4- and 5-hour time points. Adjusted differences in mean temperature differences were greater in children treated with the 20 to 30 mg/kg dose at all time points and were significantly greater for the 20 to 30 mg/kg dose group than the 10 to 15 mg/kg dose group at 4 hours (3.19°F versus 2.28°F, respectively; p = 0.0328) and 5 hours (2.41°F versus 1.51°F; p = 0.0203; Figure 3). In Study III, mean temperature differences from baseline were greater in children treated with the 20 to 30 mg/kg dose versus the 10 to 15 mg/kg dose at all times points and were statistically significant beginning 2 hours after dosing and continuing through 8 hours after dosing (p < 0.014; Figure 4).
Figure 2.
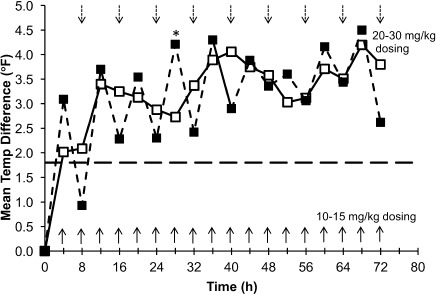
Mean temperature differences from baseline to each time point in Study I, 0 to 72 hours.
*p < 0.05.
□Study I, 10–15 mg/kg (n = 21); ▪ Study I, 20–30 mg/kg (n = 18); ⁃⁃1.8°F (1°C) temperature change.
Figure 3.
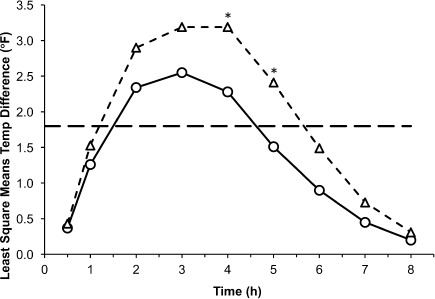
Mean temperature differences from baseline to each time point in Study II, 0 to 8 hours.
*p < 0.05.
◯ Study II, 10–15 mg/kg (n = 44); △ Study II, 20–30 mg/kg (n = 42); ⁃⁃ 1.8°F (1°C) temperature change.
Figure 4.
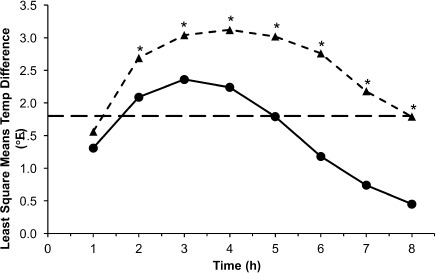
Mean temperature differences from baseline to each time point in Study III, 0 to 8 hours.
*p < 0.05.
• Study III, 10–15 mg/kg (n = 77); ▴ Study III, 20–30 mg/kg (n = 68); ⁃⁃ 1.8°F (1°C) temperature change.
In Study III, an additional analysis was conducted: the number of hours a temperature reduction of at least 1 degree was achieved. The duration of temperature reduction of at least 1°F was longer in children treated with the 20 to 30 mg/kg dose versus the 10 to 15 mg/kg dose (6.87 hours versus 5.40 hours, p < 0.0001).
Vital Signs
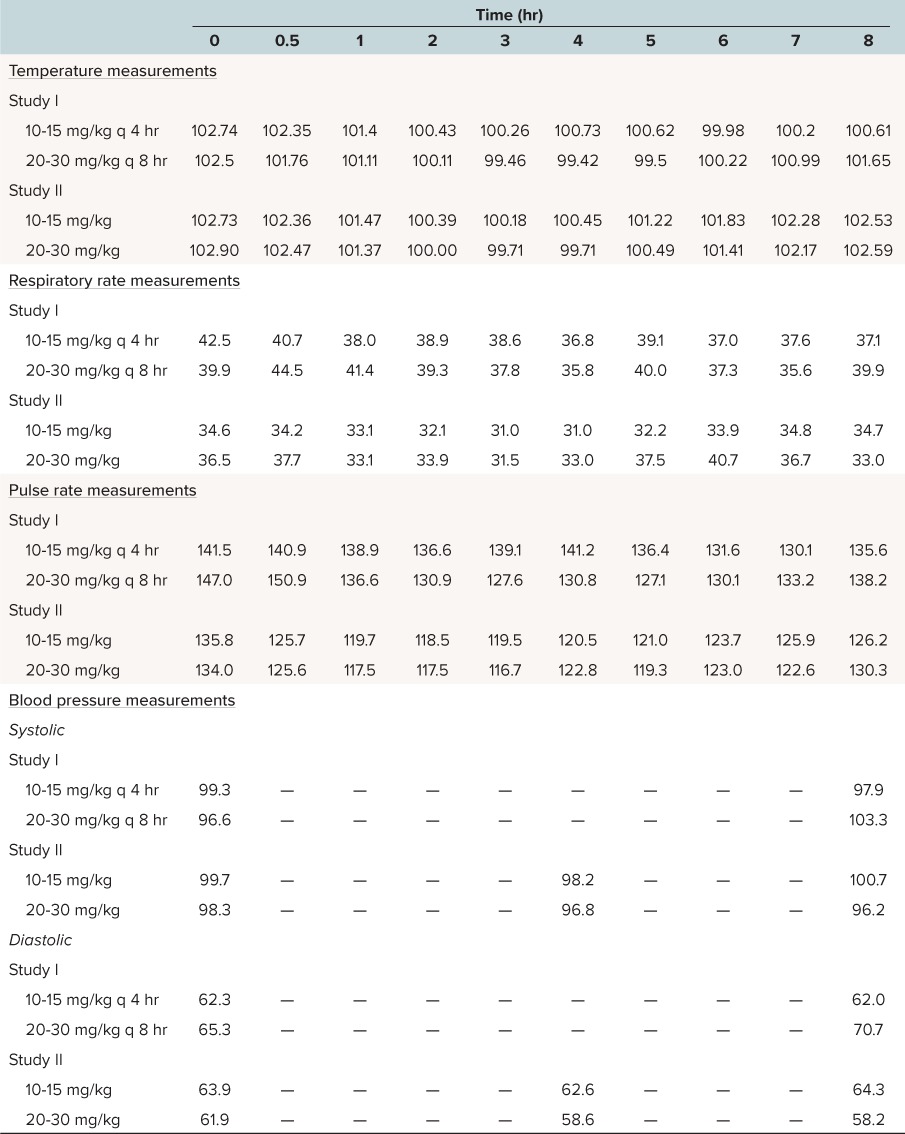
In addition to temperature, pulse and respiratory rates and blood pressure were monitored in Studies I and II. Review of these data show that as temperature falls, there were modest decreases in pulse and respiratory rates. Changes in blood pressure were noted for some children in these studies (Table 4).
Table 4.
Mean Vital Sign Measurements
Tolerability
In each of the 3 studies there were no significant differences between doses with respect to the number of children with reported AEs. In Study I (n = 48), AEs of mild to moderate intensity included diarrhea (9 AEs in the 10 to 15 mg/kg dose group [n = 27] and 6 AEs in the 20 to 30 mg/kg dose group [n = 21]), and vomiting (1 AE for each dose). One child in the 10 to 15 mg/kg dose group experienced a severe, uncontrolled fever that was considered unrelated to the treatment; this child was excluded from the efficacy analysis because of a low initial temperature. In Study II (n = 86), 2 children in the 20 to 30 mg/kg dose group (n = 42) reported an episode of vomiting; no AEs were reported in the 10 to 15 mg/kg dose group (n = 44). In Study III (n = 142), no significant differences were noted among treatments in the number of children with reported AEs. Six children in the 10 to 15 mg/kg dose group (n = 70) reported 7 AEs, 4 children in the 20 to 30 mg/kg dose group (n = 72) reported 4 AEs. In the 10 to 15 mg/kg dose group, AEs of mild to moderate intensity included rash (n = 1), pain, abdomen (n = 2), appetite decreased (n = 1), somnolence (n = 1), headache (n = 1), and nausea (n = 1). In the 20 to 30 mg/kg dose group, AEs of mild to moderate severity included vomiting (n = 2), headache (n = 1), and diarrhea (n = 1).
Study I: ALT Activity
While all of the original laboratory reference ranges for ALT in this study are no longer available, for the purpose of this discussion, we defined the upper limit of reference range (ULRR) to be 40 U/L (Figures 5 and 6). For the combined dosing groups, 34 had baseline ALT values ≤ 40 U/L. Of these, only 1 subsequent ALT value was > 40 U/L (41 U/L at 72 hours after last dose). Two children in the 10 to 15 mg/kg dose group and 3 in the 20 to 30 mg/kg dose group had ALT values >40 U/L at baseline. For 1 child in the 10 to 15 mg/kg group, ALT levels remained above ULRR at the end of the study period and at 72 hours after last dose (baseline, 186 U/L; at last dose, 194 U/L; 72 hours after last dose, 90 U/L). The other child (baseline, 47 U/L) had values within the reference range at the time of last dose (36 U/L) and 72 hours after last dose (23 U/L; Figure 5). In 2 children in the 20 to 30 mg/kg dose group with elevated baseline ALT values (58 U/L and 82 U/L), each had values within the reference range at the time of last dose (37 U/L and 26 U/L, respectively) and at 72 hours after last dose (22 U/L and 18 U/L, respectively). In the third child with an elevated baseline ALT value (74 U/L), the levels remained above ULRR at the end of the study period (44 U/L) but were within the reference range at 72 hours after last dose (20 U/L; Figure 6).
Figure 5.
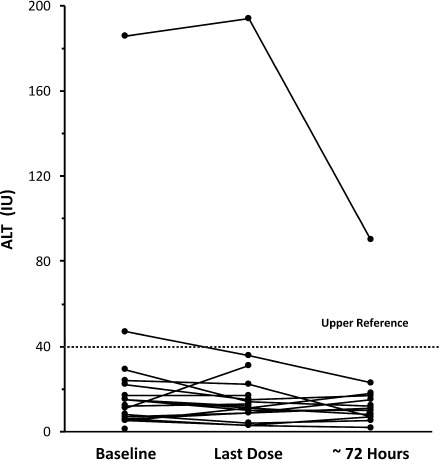
Alanine aminotransferase values for 10 to 15 mg/kg dose group
⋯⋯⋯defined upper limit of reference range for analysis purposes.
Figure 6.
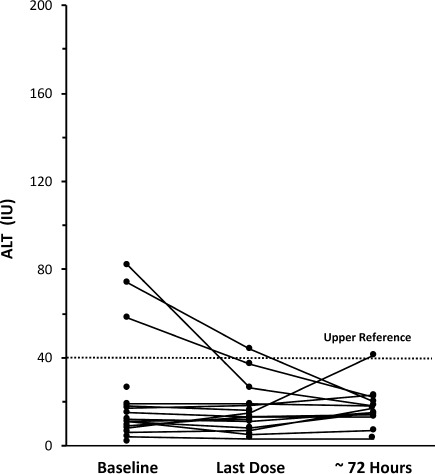
Alanine aminotransferase values for 20 to 30 mg/kg dose group
⋯⋯⋯defined upper limit of reference range for analysis purposes.
Discussion
Results from these 3 studies confirm that the recommended standard acetaminophen dose of 10 to 15 mg/kg18 is a safe and effective dose for use in pediatric patients when administered as a single dose or as multiple doses for up to 72 hours. Although a 20 to 30 mg/kg dose produced a greater maximum temperature decrement during an 8-hour period compared to a single 10 to 15 mg/kg dose over that same 8-hour period, when repeated 10 to 15 mg/kg doses are given at 4-hour intervals, there was no substantive difference in maximum temperature decrement or the SUMDIFF. Notably, throughout the remainder of the 72-hour study period, while the patterns of temperature reduction were more variable with the higher dose, there was no statistically significant difference between the doses at any time point except 28 hours. These studies demonstrate that the use of a dose of at least 10 mg/kg produces an adequate antipyretic effect4; and the use of doses of 10 to 15 mg/kg given at 4-hour intervals, as found in the labeled dosing schedules, is an appropriate dose for children. Data from dosing 20 to 30 mg/kg at 8-hour intervals, which is not current recommended dosing, provide insight into the nature and pattern of the temperature decrement achieved with that higher dose given at an 8-hour dosing interval. However, given that these higher doses are not labeled doses and that the lower dose provides sufficient antipyretic effect, we see no need to change current dosing level and intervals.
Studies have demonstrated that acetaminophen antipyretic activity is dose dependent.1,6,19,20 Tréluyer and colleagues6 showed a significantly greater maximum temperature decrease and a significantly longer duration of antipyretic activity for single initial doses of oral acetaminophen 30 mg/kg versus 15 mg/kg. The time to reach a temperature lower than 101.3°F was approximately 30 minutes shorter for the 30 mg/kg dose versus the 15 mg/kg dose. Windorfer and Vogel20 showed that doses of 20 mg/kg produced greater temperature decrements than 10 mg/kg and both of which were superior to 5 mg/kg, which produced little response. A more recent analysis of data from 13 previously unpublished pediatric antipyretic studies showed an onset of temperature decrement at the first temperature measurement 30 minutes after administration of 10 to 15 mg/kg or 20 to 30 mg/kg acetaminophen.5 The 20 to 30 mg/kg dose achieved greater maximum temperature decrements 4 and 8 hours after acetaminophen administration (1.74°C and 0.67°C, respectively) than the 10 to 15 mg/kg dose (1.33°C and 0.35°C, respectively).5
In 2011, an FDA Advisory Panel recommended that liquid forms be made in just 1 strength (160 mg acetaminophen/5 mL) and that dosing instructions to reduce fever should be added for children as young as 6 months.21 The FDA Panel also recommended that dosing instructions be based on weight, not just age.21 Since that time, the more concentrated infants' drops formula (80 mg acetaminophen/0.8 mL) has been replaced with a product that is the same concentration as the children's liquid (160 mg/5 mL). Dosing for children under age 2 has not been added to the label. Although an evidence-based dosing schedule has been widely used by healthcare professionals to meet the needs of their pediatric patients for nearly 3 decades, FDA has yet to approve dosing instructions for children under age 2 years for product labels. The studies cited herein include children from ages 6 months to under 2 years (the group for which FDA has not yet agreed to provide labeled dosing). They were given the 160 mg acetaminophen/5 mL concentration. Importantly, the dosing was administered in these trials by weight. Weight-based dosing schedules have been available on labels for children 2 years and over and in health-care professional resources for infants since 1983, as is widely known among practitioners.22
Acetaminophen has a long-established and favorable safety profile and is well tolerated in children when administered at the recommended dose of 10 to 15 mg/kg every 4 hours (not to exceed 5 doses, 50 to 75 mg/kg, in 24 hours).5,9,23–27 However, in reference to treatment of potential acetaminophen overdose, poison center guidelines recommend that asymptomatic children < 6 years old be referred for healthcare evaluation following an acute ingestion of ≥ 200 mg/kg or repeated supratherapeutic ingestion (≥ 200 mg/kg within the previous 24 hours, ≥ 150 mg/kg/day for the preceding 2 days, or ≥ 100 mg/kg/day for the preceding 3 days or more).27 In Study I, where doses of up to 90 mg/kg/day were given over a 3-day period, no child with a baseline ALT level lower than 40 U/L had an increase in ALT activity from baseline that reached even 42 U/L, and there was no evidence indicating hepatic injury over the 72-hour study period. These findings are similar to other studies using recommended doses.2,16,25,27
Our article has several limitations. One of the limitations is the duration of time that has passed since the studies were originally conducted. As a result, the study designs were developed within the limits of knowledge and methodology available at that time. The 3 studies do not have identical protocols, including differences in dosing duration, inclusion/exclusion criteria, and age ranges studied. However, they all had the very well defined end point of a temperature measurement at a specified point in time, which made it possible to provide a common element across all 3. All 3 studies included children ages 2 to under 6 years of age. Two of the 3 studies also included children ages 6 months to under 2 years of age, and the third study also included children ages 6 years to 11 years. This allowed us to evaluate the entire pediatric age range from 6 months to 11 years of age. The product formulations used were not administered in identical ways. In order to accommodate a double dose amount of acetaminophen and yet maintain blinding, in 2 studies the marketed product of 160 mg/5 mL was given in twice the usual volumes to provide for a double standard dose, while a formulation that was half the concentration was given in the same double volume amounts to provide a standard dose of acetaminophen. However, these limitations do not affect the integrity of the data, and the conclusions drawn from these analyses are relevant to current clinical practice.
Conclusion
Data from 3 pediatric clinical trials demonstrate that the antipyretic effect of acetaminophen is dose dependent. In these studies, when equivalent doses are given over that same 8-hour period, either 10 to 15 mg/kg every 4 hours or 20 to 30 mg/kg given once, no clinically relevant differences in antipyretic efficacy are observed. However, a single dose of acetaminophen 20 to 30 mg/kg produced a greater effect on temperature decrement and duration of antipyretic effect than a single dose of acetaminophen 10 to 15 mg/kg when measured over an 8-hour period. Over a 72-hour period, 10 to 15 mg/kg of acetaminophen administered every 4 hours maintained a more consistent temperature decrement than 20 to 30 mg/kg acetaminophen administered every 8 hours. These studies provide clinical evidence that the standard acetaminophen dose of 10 to 15 mg/kg given at 4-hour intervals is a safe, effective, and appropriate dose for treating fever.
Acknowledgment
The authors acknowledge Kathleen Boyle, PhD, CMPP from KE Boyle Consultants, LLC (Exton, PA) who, under the direction of the authors, supported manuscript preparation from the study reports and managed the review process.
Abbreviations
- AE
adverse event
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- FDA
Food and Drug Administration
- MAXDIFF
maximum temperature difference
- SUMDIFF
sum of the temperature differences
- ULRR
upper limit of reference range
Footnotes
Disclosure The authors were employees of or consultants to Johnson & Johnson Consumer Inc. throughout the time the article was prepared for publication. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org.
REFERENCES
- 1. Temple AR. Review of comparative antipyretic activity in children. Am J Med. 1983; 75( 5A): 38– 46. [DOI] [PubMed] [Google Scholar]
- 2. Cranswick N, Coghlan D.. Paracetamol efficacy and safety in children: the first 40 years. Am J Ther. 2000; 7( 2): 135– 141. [DOI] [PubMed] [Google Scholar]
- 3. Shepherd M, Aickin R.. Paracetamol versus ibuprofen: a randomized controlled trial of outpatient analgesia efficacy for paediatric acute limb fractures. Emerg Med Australas. 2009; 21( 6): 484– 490. [DOI] [PubMed] [Google Scholar]
- 4. Temple AR. Pediatric dosing of acetaminophen. Pediatr Pharmacol. 1983; 3( 3–4): 321– 327. [PubMed] [Google Scholar]
- 5. Temple AR, Temple BR, Kuffner EK.. Dosing and antipyretic efficacy of oral acetaminophen in children. Clin Ther. 2013; 35( 9): 1361– 1375. [DOI] [PubMed] [Google Scholar]
- 6. Tréluyer JM, Tonnelier S, d'Athis P, . et al. Antipyretic efficacy of an initial 30-mg/kg loading dose of acetaminophen versus a 15-mg/kg maintenance dose. Pediatrics. 2001; 108( 4): e73. [DOI] [PubMed] [Google Scholar]
- 7. Bhananker SM, Azavedo L, MacCormick J.. Topical lidocaine and oral acetaminophen provide similar analgesia for myringotomy and tube placement for children. Can J Anesth. 2006; 53( 11): 1111– 1116. [DOI] [PubMed] [Google Scholar]
- 8. Bolton P, Bridge HS, Montgomery CJ, Merrick PM.. The analgesic efficacy of preoperative high dose (40 mg/kg-1) oral acetaminophen after bilateral myringotomy and tube insertion in children. Pediatr Anaesthesia. 2002; 12( 1): 29– 35. [DOI] [PubMed] [Google Scholar]
- 9. Ji P, Wang Y, Li Z, . et al. Regulatory review of acetaminophen clinical pharmacology in young pediatric patients. J Pharma Sciences. 2012; 101( 12): 4383– 4389. [DOI] [PubMed] [Google Scholar]
- 10. EMA 24570. . A double-blind multiple dose study of the comparative antipyretic effectiveness and safety of standard and double standard doses of acetaminophen in febrile children. McNeil Clinical Study 80-220 Report Synopsis; 1986. http://art45-paediatric-studies.ema.europa.eu/clinicaltrials/details.php?PkID=24570. Accessed December 19, 2016
- 11. EMA 24571. . A phase III double-blind, single dose study of the comparative antipyretic effectiveness and safety of standard and double standard doses of acetaminophen in febrile children. McNeil Clinical Study 82-222 Report Synopsis; 1984. http://art45-paediatric-studies.ema.europa.eu/clinicaltrials/details.php?PkID=24571. Accessed December 19, 2016
- 12. EMA 47402. . A single dose study to compare the efficacy of acetaminophen elixir dosed at 12.5 mg/kg and 25 mg/kg and ibuprofen suspension dosed at 5 mg/kg and 10 mg/kg in febrile children. McNeil Clinical Study 89-932 Report Synopsis; 1991. http://art45-paediatric-studies.ema.europa.eu/clinicaltrials/details.php?PkID=47402. Accessed December 19, 2016
- 13. Powell DC, Nahata MC.. Kinetics of Acetaminophen (Ac) Following Single Strength (SS-Ac) vs Double Strength (DS-AC) Administration to Febrile Children. Washington, DC: Society for Pediatric Research; 1982. [Google Scholar]
- 14. Nahata MC, Powell DA.. Kinetics of acetaminophen (Ac) following single strength (SS-Ac) vs double strength (DS-Ac) administration to febrile children. Clin Research. 1982; 30: 634A. [Google Scholar]
- 15. Nahata MC, Powell DA, Durrell DE, Miller MA.. Acetaminophen (APAP) kinetics in infants and children after single and repeated doses. Clin Pharmacol Ther. 1984; 35: 262. [Google Scholar]
- 16. Nahata MC, Powell DA, Durrell DE, Miller MA.. Acetaminophen accumulation in pediatric patients after repeated therapeutic doses. Eur J Clin Pharmacol. 1984; 27( 1): 57– 59. [PubMed] [Google Scholar]
- 17. Temple AR. Labeling improvements for pediatric acetaminophen products. US FDA Advisory Committee Meeting Materials. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM171602.pdf. Accessed December 19, 2016.
- 18. Children's Tylenol (acetaminophen) suspension. Daily Med. National Institutes of Health. US National Library of Medicine. http://dailymed.nlm.nih.gov/dailymed/search.cfm?labeltype=all&query=CHILDRENS+TYLENOL. Accessed December 19, 2016.
- 19. Wilson JT, Brown RD, Bocchini JA Jr, Kearns GL.. Efficacy, disposition and pharmacodynamics of aspirin, acetaminophen and choline salicylate in young febrile children. Ther Drug Monit. 1982; 4( 2): 147– 180. [DOI] [PubMed] [Google Scholar]
- 20. Windorfer A, Vogel C.. Investigations concerning serum concentration and temperature following oral application of a new paracetamol preparation. Klinische Padiatrie. 1976; 188( 5): 430– 434. [PubMed] [Google Scholar]
- 21. US Food and Drug Administration. . Consumer updates. Reducing fever in children: safe use of acetaminophen. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm263989.htm. Accessed December 19, 2016.
- 22. Children's Tylenol acetaminophen chewable tablets, elixir, drops. Physicians' Desk Reference. 1189–1190. Ed 37. Oradell, NJ: Medical Economics Company, Inc; 1983. [Google Scholar]
- 23. Lesko SM, Mitchell AA.. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999; 104( 4): e39. [DOI] [PubMed] [Google Scholar]
- 24. Autret-Leca E, Gibb IA, Goulder MA.. Ibuprofen versus paracetamol in pediatric fever: objective and subjective findings from a randomized, blinded study. Curr Med Res Opin. 2007; 23( 9): 2205– 2211. [DOI] [PubMed] [Google Scholar]
- 25. McIntyre J, Hull D.. Comparing efficacy and tolerability of ibuprofen and paracetamol in fever. Arch Dis Child. 1996; 74( 2): 164– 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dart RC, Erdman AR, Olson KR, . et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol. 2006; 44( 1): 1– 18. [DOI] [PubMed] [Google Scholar]
- 27. Wilson JT, Helms R, Pickering BD, . et al. Acetaminophen controlled-release sprinkles versus acetaminophen immediate-release elixir in febrile children. J Clin Pharmacol. 2000; 40( 4): 360– 369. [DOI] [PubMed] [Google Scholar]


