Abstract
OBJECTIVE
This study seeks to evaluate the efficacy and safety of intranasal (IN) dexmedetomidine as a sedative medication for non-invasive procedural sedation.
METHODS
Subjects 6 months to 18 years of age undergoing non-invasive elective procedures were included. Dexmedetomidine (3 mcg/kg) was administered IN 40 minutes before the scheduled procedure time. The IN dexmedetomidine cohort was matched and compared to a cohort of 690 subjects who underwent sedation for similar procedures without the use of dexmedetomidine to evaluate for observed events/interventions and procedural times.
RESULTS
One hundred (92%) of the 109 included subjects were successfully sedated with IN dexmedetomidine. There were no significant differences in the rate of observed events/interventions in comparison to the non-dexmedetomidine cohort. However, the IN dexmedetomidine group had a longer postprocedure sleep time when compared to the non-dexmedetomidine cohort (p < 0.001), which had a significant effect on recovery time (p = 0.024). Also, the dexmedetomidine cohort had longer procedure time and total admit time (p < 0.001 and p = 0.037, respectively).
CONCLUSIONS
IN dexmedetomidine may be used for non-invasive pediatric procedural sedation. Subjects receiving IN dexmedetomidine had a similar rate of observed events/interventions as the subjects receiving non-dexmedetomidine sedation, with the exception of sleeping time. Also, patients sedated with IN dexmedetomidine had longer time to discharge, procedure time, and total admit time in comparison to other forms of sedation.
Keywords: alpha 2-adrenoreceptor agonist, dexmedetomidine, drugs, hypnotic, imidazole, intranasal, Precedex
Introduction
The importance of procedural sedation and analgesia (PSA) for children undergoing procedures and imaging studies has led to an increase in demand for sedation outside of the operating room. Due to this demand, the role of pediatric critical care providers, hospitalists, and emergency physicians has also expanded to include PSA.1 For sedation providers outside of the operating room, dexmedetomidine (DEX) offers a safety profile similar to traditional sedatives (i.e., ketamine and midazolam) and has been reported to be efficacious when administered intranasally (IN).2–7
DEX is a highly selective and potent agonist at the α2-adrenoreceptor, with sedative, anxiolytic, and analgesic effects.8 The sedative properties of DEX are largely due to effects on the locus ceruleus, producing a level of consciousness mimicking natural sleep.9 DEX administered intravenously for sedation in the intensive care unit and non-invasive procedures is well established.10–15 IN administration of DEX is advantageous, as it is less invasive and anxiety provoking for the pediatric population. Additional data suggest that when compared to intravenously administrated DEX, IN DEX has a significantly lower risk of respiratory depression and hemodynamic changes.16 Moreover, IN DEX has the same success rate when compared to IN midazolam and ketamine.4
In this study, we hypothesized that IN DEX is effective in providing adequate sedation for non-invasive procedures. Our primary objective was to evaluate the efficacy of IN DEX as a sedative medication. Our secondary objective was to evaluate the safety profile of IN DEX compared to well-established intravenous sedative medications (i.e., midazolam, propofol, pentobarbital, and ketamine).
Methods
This was a prospective observational study, performed in our procedural suite, located at a tertiary medical center. Two pediatric critical care physicians who are experienced in providing PSA outside of the operating room provided all PSA. Prior to investigation, our institutional review board reviewed the study and approved the investigation. Consent for entry into the study was not required as no patient identifiers were collected and we did not experiment with new therapeutic methods outside of our regular practice. All subjects were informed about the database and were given the opportunity to opt-out from being included before PSA initiation. If a subject opted-out, the encounter information was not documented by the study nurses.
Subjects were contacted the night before the procedure and were given relevant instructions regarding when and where to arrive and fasting time. On the day of the non-invasive procedure, the physician providing the sedation evaluated each subject. The plan of care was then formulated and discussed with the family.
We created a database capturing all the relevant demographics and events for children undergoing sedation for elective, non-invasive outpatient procedures. The database was not limited to subjects undergoing IN DEX sedation, but rather included all planned PSA in our procedural suite. Sedation nurses collected the data immediately before discharging the patient and documented the following: the procedure type, age, sex, fasting time, weight, arrival time procedure start/end time, and discharge time. Sedation nurses also documented sedative medications used for the procedure, level of sedation (Table 1), and observed events. The level of sedation and vital signs were documented every 3 to 5 minutes. A successful sedation with IN DEX was determined by completion of the non-invasive study without any additional sedative medications required to maintain subject's sedation. This database was intended to track and study the observed events of sedative medications (Table 2) and our current practice.
Table 1.
University of Michigan Sedation Scale

Table 2.
Observed Events/Interventions *

IN DEX at a dose of 3 mcg/kg, with a maximum dose of 200 mcg, was divided into 2 equal doses, and administer using an IN mucosal atomization device (LMA MAD Nasal, Teleflex, NC) 40 minutes before the scheduled procedure time. Subjects were included if they were 6 months to 18 years of age, undergoing non-invasive procedures (i.e., radiologic imaging, echocardiography, electroencephalography, and auditory brainstem response) with an American Society of Anesthesiologists physical status score (ASA score) of I and II. We excluded subjects who were younger than 6 months of age, had a history of cardiac disease, had an ASA score of III to IV, and subjects who had to undergo procedures that would be invasive or painful.
A cohort of subjects undergoing non-invasive procedural sedation without DEX were compared to those subjects successfully sedated with IN DEX using a χ2 analysis run with IBM SPSS 22.0 (IBM, Armonk, NY) and had a 2-sided significance level of 0.05. Both cohorts were obtained from the same database, using the same recruitment process and similar inclusion criteria. Unless otherwise noted, data are presented as mean ± SDs.
Results
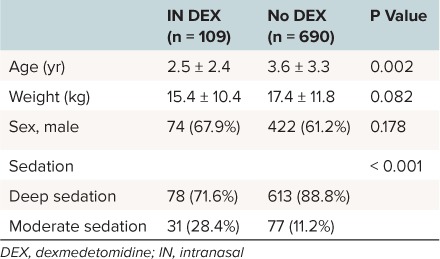
One hundred nine subjects underwent non-invasive procedural sedation with IN DEX, which satisfied an 80% power calculation requirement of 107 subjects (74 males and 35 females). The mean age and weight were 2.5 years old and 15.4 kg, respectively. The level of sedation was noted as either moderate or deep in 31 and 78 patients, respectively. The total number of subjects undergoing sedation without DEX for similar procedures was 690 (422 males and 268 females). The mean age and weight of the non-DEX cohort was 3.6 years and 17.4 kg, respectively. There was no difference in sex or weight between the IN DEX and non-DEX cohorts. The non-DEX cohort had more subjects in the deep sedation cohort, 607 subjects (84.3%), which was significantly different from the DEX cohort (Table 3).
Table 3.
Demographics of Patients Undergoing Non-Invasive Procedural Sedation With IN DEX and Without
One hundred subjects (92%) were successfully sedated using IN DEX. Thirty-five of these subjects (39%) also received IN midazolam, which is standard practice at our institution for subjects requiring sedation longer than 45 minutes. A subgroup analysis of procedure times found no statistical difference in subjects who had received additional IN midazolam compared to those given IN DEX alone; however, the sample size lacked adequate power. Nine subjects (8%) failed sedation with IN DEX and were given additional intravenous sedative medications. The failed IN DEX cohort was omitted from the χ2 analysis comparing observed events/interventions to the non-DEX cohort.
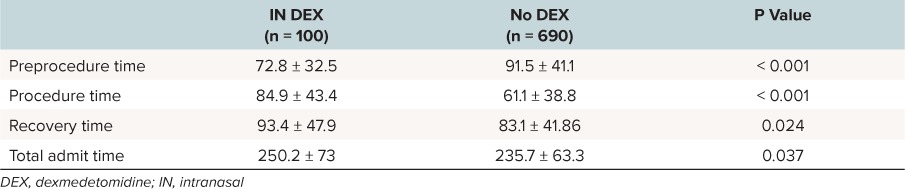
The group that received IN DEX had an increase duration of sleep time, defined as more than 1 hour after the end of the procedure (p < 0.001). There were no other statistical differences between the 2 cohorts when comparing observed events/interventions (Table 4). When comparing procedure times, IN DEX was associated with all of the following: longer duration of procedure, recovery time, and total admit time. However, the non-DEX cohort had a longer preprocedure time (Table 5).
Table 4.
Observed Events/Interventions of Subjects Undergoing Non-Invasive Procedural Sedation With IN DEX and Without
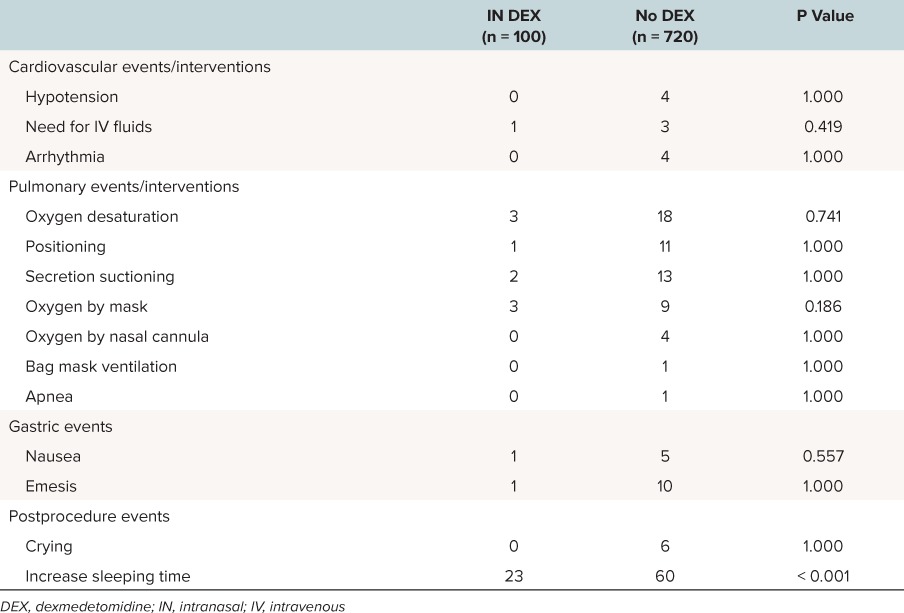
Table 5.
Procedure Times of Patients Undergoing Non-Invasive Procedural Sedation With IN DEX and Without (Minutes)
Discussion
There are an increasing number of published reports describing the use of DEX; however, there is little agreement regarding the dose and route of administration. Reported effective doses of IN DEX have ranged from 1 to 4 mcg/kg and doses have ranged from 81% to 87.5%.3,4 Our study demonstrates that IN DEX at 3 mcg/kg, as either a sole agent or in combination with IN midazolam, had a success rate of 92%.
IN DEX solves the problem of obtaining intravenous access for pediatric patients undergoing various diagnostic studies. This practice is gaining more popularity amongst sedation providers outside of the operating room due to a decrease in emotional stress in children that is related to the IN administration route.
Several studies note that DEX may cause bradycardia and hypotension, and may decrease cardiac output due to effects on the α2-adrenoreceptor, similar to clonidine.8,17 However, when comparing those effects to traditional agents used for pediatric sedation (i.e., midazolam and propofol) there has been no significant clinical difference.2,5,7 Similarly, our study compared IN DEX to non-DEX sedation cohort, which included midazolam, propofol, pentobarbital, and ketamine, and found no statistical difference in terms of cardiovascular, pulmonary, or gastric events. We did not compare side effects between the IN DEX cohort and each of the sedation medications individually due to inadequate sample size, but we believe that this should be addressed in future studies.
To our knowledge, sleep time and the use of DEX in any formulation has not been previously observed. When comparing the IN DEX cohort to the non-DEX cohort, we noted that the use of IN DEX was associated with increase rate of postprocedure sleep time, increase in duration of the procedure, recovery, and total admit time. This can present a problem for very busy sedation services that require rapid patient turnover time. The non-DEX cohort had a longer preprocedure time, which may have been due to variations related to the of preprocedure protocols.
Limitations of this study include: 1) This is a single institution study and the medication was used by 2 of 4 pediatric critical care physicians who routinely provided sedation for outpatient procedures. 2) This is a prospective observational trial and not a randomized controlled trial. 3) Subjects in the successful IN DEX cohort also received IN midazolam. An inadequate sample size did not allow assessment of additional IN midazolam in the 35 patients. 4) Although IN DEX was used in a variety of different procedures, a small sample size precluded assessment of the effect of procedure type on outcome. Further research using IN DEX would benefit from a large multicenter, which could include investigation of the effect of procedure type on success.
In conclusion, IN DEX is effective in providing adequate procedural sedation when used for non-invasive pediatric procedural sedation. Except for a longer sleep time, IN DEX was not associated with a higher incidence of adverse events when compared to a cohort that did not receive DEX sedation. It is important to emphasize that this approach should be adopted only in a setting in which the child is monitored and medications administered by a trained skilled provider.
Acknowledgments
Abstract presented at Society of Critical Care Medicine 44th Critical Care Congress, Phoenix, Arizona on January 19, 2015, and at Pediatric Academic Society 2015 Annual Meeting, San Diego, California on April 28, 2015.
Abbreviations
- ASA score
American Society of Anesthesiologists physical status score
- DEX
dexmedetomidine
- IN
intranasal
- PSA
procedural sedation and analgesia
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. Tobias J. Sedation of infants and children outside of the operating room. Curr Opin Anaesthesiol. 2015; 28( 4): 478– 485. [DOI] [PubMed] [Google Scholar]
- 2. Dere K, Sucullu I, Budak ET, . et al. A comparison of dexmedetomidine versus midazolam for sedation, pain and hemodynamic control, during colonoscopy under conscious sedation. Eur J Anaesthesiol. 2010; 27( 7): 648– 652. [DOI] [PubMed] [Google Scholar]
- 3. Li BL, Ni J, Huang JX, . et al. Intranasal dexmedetomidine for sedation in children undergoing transthoracic echocardiography study: a prospective observational study. Paediatr Anaesth. 2015; 25( 9): 891– 896. [DOI] [PubMed] [Google Scholar]
- 4. Surendar M, Pandey R, Saksena A, . et al. A comparative evaluation of intranasal dexmedetomidine, midazolam and ketamin for their sedative and analgesic properties: a triple blind randomized study. J Clin Pediatr Dent. 2014; 38( 3): 255– 261. [DOI] [PubMed] [Google Scholar]
- 5. Tsai CJ, Chu KS, Chen TI, . et al. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010; 65( 3): 254– 259. [DOI] [PubMed] [Google Scholar]
- 6. Tug A, Hanci A, Turk H, . et al. Comparison of two different intranasal doses of dexmedetomidine in children for magnetic resonance imaging sedation. Pediatr Drugs. 2015; 17( 6): 479– 485. [DOI] [PubMed] [Google Scholar]
- 7. Wu W, Chen Q, Zhang LC, Chen WH.. Dexmedetomidine versus midazolam for sedation in upper gastrointestinal endoscopy. J Int Med Res. 2014; 42( 2): 516– 522. [DOI] [PubMed] [Google Scholar]
- 8. Phan H, Nahata M.. Clinical uses of dexmedetomidine in pediatric populations. Pediatr Drugs. 2008; 10( 1): 49– 69. [DOI] [PubMed] [Google Scholar]
- 9. Yuen VM, Hui TW, Irwin MG, . et al. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia. 2010; 65( 9): 922– 929. [DOI] [PubMed] [Google Scholar]
- 10. Easley B. Sedation challenges in the pediatric ICU: is dexmedetomidine the solution. Indian Pediatr. 2009; 46( 9): 761– 763. [PubMed] [Google Scholar]
- 11. Carroll CL, Krieger D, Campbell M, . et al. Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J Hosp Med. 2008; 3( 2): 142– 147. [DOI] [PubMed] [Google Scholar]
- 12. Adams R, Brown GT, Davidson M, . et al. Efficacy of dexmedetomidine compared with midazolam for sedation in adult intensive care patients: a systematic review. Br J Anaesth. 2013; 111( 5): 703– 710. [DOI] [PubMed] [Google Scholar]
- 13. Lubisch N, Roskos R, Berkenbosch JW.. Dexmedetomidine for procedural sedation in children with autism and other behavior disorders. Pediatr Neurol. 2009; 41( 2): 88– 94. [DOI] [PubMed] [Google Scholar]
- 14. Mason KP, Zgleszewski SE, Dearden JL, . et al. Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg. 2006; 103( 1): 57– 62, table of contents. [DOI] [PubMed] [Google Scholar]
- 15. Mason KP, Zurakowski D, Zgleszewski SE, . et al. High dose dexmedetomidine as the sole sedative for pediatric mri. Paediatr Anaesth. 2008; 18( 5): 403– 411. [DOI] [PubMed] [Google Scholar]
- 16. Han G, Yu WW, Zhao P.. A randomized study of intranasal vs. Intravenous infusion of dexmedetomidine in gastroscopy. Int J Clin Pharmacol Ther. 2014; 52( 9): 756– 761. [DOI] [PubMed] [Google Scholar]
- 17. Wong J, Steil GM, Curtis M, . et al. Cardiovascular effects of dexmedetomidine sedation in children. Anesth Analg. 2012; 114( 1): 193– 199. [DOI] [PubMed] [Google Scholar]


