Abstract
Choristoderes are a group of extinct freshwater reptiles that were distributed throughout Laurasia from the Middle Jurassic to the Miocene. They are inferred to have had a lifestyle similar to that of extant gavialid crocodiles, but they differed from crocodiles in retaining an extensive palatal dentition. All choristoderes had teeth on the vomers, palatines and pterygoids, and teeth are rarely present on the parasphenoid. Palatal teeth are conical, as in the marginal dentition, and form longitudinal and transverse rows. Detailed examination of different genera shows that the orientation of the palatal tooth crowns changes with their position on the palate, supporting the view that they are involved in intra‐oral food transportation, presumably in combination with a fleshy tongue. Moreover, observed variation in palatal tooth shape and the width of palatal tooth batteries may provide additional clues about diet. The European Simoedosaurus lemoinei has sharper palatal teeth than its North American counterpart, S. dakotensis, suggesting a preference for softer prey – a conclusion consistent with the more gracile teeth and narrower snout.
Keywords: Champsosaurus, function, morphology, palatal dentition, Simoedosaurus, subthecodont
Introduction
Choristodera is an extinct (Middle Jurassic to Miocene) group of aquatic diapsid reptiles that typically occurred as part of a mesic Laurasian vertebrate assemblage (including fish, frogs, salamanders, turtles and crocodiles) in relatively warm, temperate climates (occasionally sub‐tropical areas: Matsumoto & Evans, 2010). The group is characterized by a unique combination of characters, including a dorsoventrally depressed cordiform skull and conical subthecodont teeth.
Choristoderes are represented by three morphotypes (short‐necked longirostrine, short‐necked brevirostrine and long‐necked brevirostrine). The short‐necked longirostrine taxa form a monophyletic clade named Neochoristodera (sensu Evans & Hecht, 1993). The other morphotypes (short‐necked brevirostrine and long‐necked brevirostrine) fall into a paraphyletic set, informally named non‐neochoristoderes, the earliest of which is Cteniogenys, a small lizard‐like reptile known from the Middle–Late Jurassic of Euramerica (Britain, Portugal, USA; Evans, 1989, 1990, 1991; Chure & Evans, 1998). The long‐necked taxa are all from the Early Cretaceous of Asia: Hyphalosaurus (H. lingyuanensis; H. bitaigouensis, China; Gao et al. 1999; Ji et al. 2004), Shokawa ikoi (Japan; Evans & Manabe, 1999) and probably Khurendukhosaurus orlovi (Mongolia and Russia; Sigogneau‐Russell & Efimov, 1984; Efimov & Storrs, 2000; Skutschas, 2008). Hyphalosaurus is represented by nearly complete skulls, but many of these are dorsoventrally compressed and are rarely preserved in ventral view (Gao & Ksepka, 2008). The Japanese long‐necked Shokawa is known from an articulated postcranial specimen and a few attributed jaw elements (Evans & Manabe, 1999). Khurendukhosaurus is known only from disarticulated postcranial elements and a few skull bones (basioccipital, exoccipital, maxilla, dentary; Skutschas, 2008). The short‐necked brevirostrine morphotype has a wider geographical and chronological range: Monjurosuchus splendens (Gao et al. 2000; Gao & Li, 2007; Matsumoto et al. 2007) and Philydrosaurus proseilus (Gao & Fox, 2005; Gao et al. 2007, 2013) are from the Early Cretaceous of Asia (China and Japan), whereas the European Lazarussuchus (L. inexpectatus; Hecht, 1992; L. dvoraki; Evans & Klembara, 2005) is recorded from the Late Paleocene (France; Matsumoto et al. 2013) to Early Miocene (France, Czech Republic, Germany; Hecht, 1992; Evans & Klembara, 2005; Böhme, 2008). Neochoristodera (short‐necked longirostrine type) is comprised of four genera of similar morphology – the Early Cretaceous Tchoiria (T. namsarai; T. klauseni; Efimov, 1975; Ksepka et al. 2005) and Ikechosaurus (I. magnus; I. sunailinae; I. gaoi; I. pijiagouensis; Efimov, 1979; Sigogneau‐Russell, 1981; Brinkman & Dong, 1993; Lü et al. 1999; Liu, 2004) from Asia (China, Mongolia), and the Late Cretaceous to Paleogene Champsosaurus (C. laramiensis, C. ambulator, C. natator, C. lindoei, C. albertensis, C. dolloi, C. gigas, C. tenuis; Erickson, 1972; Gao & Fox, 1998; Matsumoto, 2011) and Simoedosaurus (S. lemoinei and S. dakotensis; Gervais, 1877; Sigogneau‐Russell, 1985; Erickson, 1987) from Europe and North America (Brown, 1905; Sigogneau‐Russell & Russell, 1978).
The phylogenetic position of Choristodera within Reptilia remains problematic (Matsumoto et al. 2007, 2013), although many authors have placed the group somewhere on the diapsid stem (Evans, 1988; Gao & Fox, 1998; Rieppel & Reisz, 1999; Modesto & Sues, 2004). As with many phylogenetically problematic groups, they show a mixture of specialized and primitive traits. Among the latter is the presence of an extensive palatal dentition (Sigogneau‐Russell, 1979, 1985), a feature generally considered plesiomorphic within amniotes where all major lineages show a crownward trend toward reduction or loss. Gao et al. (2007) recently presented a brief overview of palatal morphology in Choristodera, prompted by new specimens from the Early Cretaceous of China. They concluded that the choristoderan palate was uniquely modified from the basal diapsid condition in features such as the elongated vomers, posteriorly relocated choana and reduction of the interpterygoid vacuity, features probably linked to the aquatic lifestyle. However, details of palatal tooth morphology, replacement and function remain poorly understood, despite the recovery of many new specimens of both neochoristoderes and non‐neochoristoderes. The aim in this review is to provide a detailed comparative study of the choristoderan palatal dentition and to relate it, where possible, to diet and/or feeding strategy.
Materials and methods
Of the 11 known choristodere genera, palatal elements are preserved in 9. Representatives of each were examined (Table 1) by stereomicroscopy, digital photographs and/or scanning electron microscopy (SEM), with some supplementary data taken from the literature. Champsosaurus lindoei (NMC 8920) was subjected to micro‐computed tomography (CT) at the National Museum of Nature and Science, Tokyo, Japan, using TESCO, Microfocus CT TXS 320‐ACTIS. The software Avizo 8.0 was used to visualize 3D images of the CT data. Palatal tooth patterns of choristoderes were plotted on a phylogenetic tree (Fig. 1) based on the bootstrap consensus tree of Matsumoto et al. (2013).
Table 1.
List of specimens examined
| Genus | Species | Specimen number | Element | References |
|---|---|---|---|---|
| Cteniogenys | sp. | BMNH R11756–11758 uncataloged UCL specimens | Isolated palatine | Evans (1990), personal observation (RM) |
| sp. | BMNH R11759 | Isolated pterygoid | ||
| Lazarussuchus | inexpectatus | Re 437 | Preserves posterior palatine and anterior pterygoid tooth rows | Hecht (1992) |
| Monjurosuchus | sp. | IVPP V14261 | Juvenile, complete palate | Personal observation (RM), Wang et al. (2005) |
| splendens | IVPP V13761 | Adult, partial pterygoid | ||
| Philydrosaurus | proseilus | LPMC 021 | Nearly complete palate pterygoid flange tooth row missing | Gao et al. (2007) |
| Hyphalosaurus | lingyuanensis | IVPP V11075 | Mostly covered by matrix | Gao et al. (1999), personal observation (RM) |
| baitaigouensis | LPMC no number | Nearly complete palatine and pterygoid | Gao & Ksepka (2008) | |
| Ikechosaurus | sunailinae | IVPP V2774 | Palatine | Sigogneau‐Russell (1981) |
| sunailinae | IVPP V9611‐3 | Complete palate | Brinkman & Dong (1993), personal observation (RM) | |
| sp. | IVPP V10596.1; IVPP V9611‐2 | Isolated parasphenoid; partial palatine and vomer | ||
| Tchoiria | namsarai | PIN3386/1 | Complete palate | Efimov (1975), Efimov & Storrs (2000) |
| namsarai | HMNS 96720 | Pterygoid flange missing | Personal observation (RM) | |
| klauseni | IGM 1/8 | Pterygoid flange missing | Ksepka et al. (2005) | |
| Champsosaurus | lindoei | RTMP 87.36.41; RTMP 94.163.01; NMC 8920 | Complete palate, juvenile and subadult | Gao & Fox (1998), personal observation (RM) |
| albertensis | RTMP 86.12.11 | Pterygoid flange missing | Gao & Fox (1998), personal observation (RM) | |
| laramiensis | AMNH 982; 981 | Complete palate | Brown (1905), personal observation (RM) | |
| ambulator | AMNH 983 | Complete palate | Brown (1905) | |
| natator | NMC 8919 | Pterygoid flange tooth row missing | Russell (1956), personal observation (RM) | |
| dolloi |
IRSNB R21; IRSNB R 1568; IRSNB R 3662 MNHN BR 2020 |
Pterygoid flange missing Isolated vomer |
Sigogneau‐Russell (1979), personal observation | |
| gigas | SMMP 77.33.34 | Complete palate | Erickson (1985), personal observation (RM) | |
| tenuis | SMM P79.14.1 | Vomer, partial palatine | Erickson (1981), personal observation (RM) | |
| sp. | MNHN BR‐9‐P; RTMP 92.36.270; RTMP 91.50.104; RTMP 89.36.332 | Complete palate; isolated palatine; isolated palatine; isolated vomer | Personal observation (RM) | |
| Simoedosaurus | lemoinei | MNHN BR1935; MNHN BR728; MNHN BR9947; MNHN BR1‐Gi‐13 | Pterygoid flange missing; Isolated palatine; isolated vomer; isolated pterygoid | Sigogneau‐Russell & Russell (1978), personal observation (RM) |
| dakotensis | SMM P76.10.1 | Complete palate | Erickson (1987), personal observation (RM) | |
| sp. | SMNS 59026 | Complete palate | Personal observation (RM) |
Figure 1.
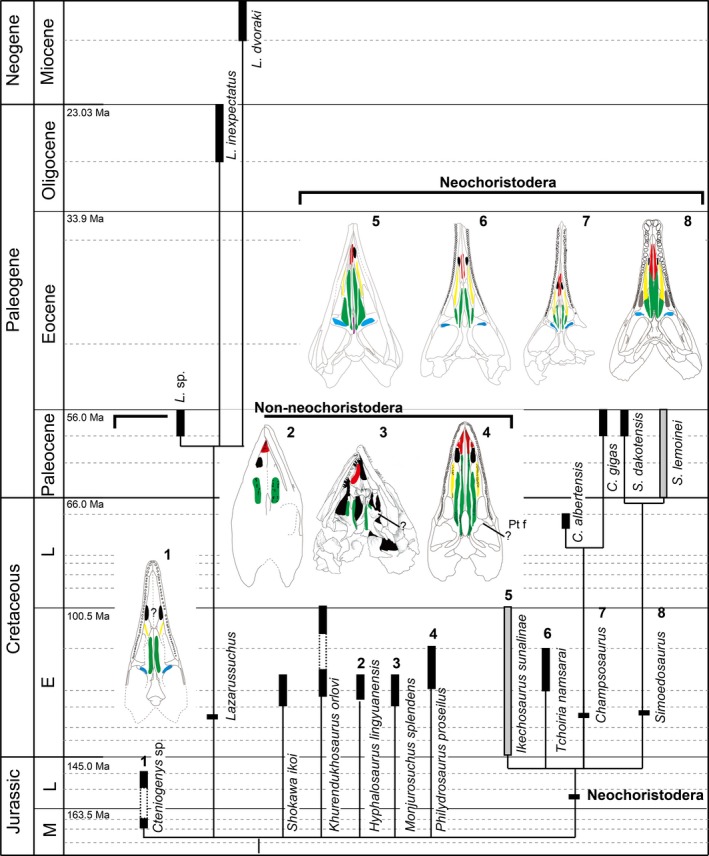
Choristoderan phylogenetic tree with palatal tooth arrangement; phylogenetic tree based on Matsumoto et al. (2013). Dentition on each palatal element marked in a different color; red, vomerine teeth; yellow, palatine teeth; green, longitudinal pterygoid tooth row; blue, pterygoid flange tooth row.
Abbreviations
Institutional
AMNH, American Museum Natural History, New York, USA; BMNH, The Natural History Museum, London, UK; IGM, Geological Institute of the Mongolian Academy of Sciences, Ulan Bataar, Mongolia; IRSNB, Institut Royal des Sciences naturelles de Belgique, Bruxelles, Belgium; IVPP V, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; LPMC, Liaoning Paleontological Museum of China; MNHN, Muséum National d'Histoire Naturelle, Paris, France; NMC, Canadian Museum of Nature, Ottawa, Canada; PIN, Paleontological Institute, Russian Academy of Sciences, Moscow, Russia; RTMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Canada; SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany; SMM, The Science Museum Minnesota, St Paul, Minnesota, USA.
Anatomical
ch, choana; D, dentary; Ept, ectopterygoid; F, frontal; Hy, hyoid; ip‐v, interpterygoid vacuity; J, jugal; Mx, maxilla; P, parietal; Pal, palatine; Psh, parasphenoid; Pt, pterygoid; pt f, pterygoid foramen; pl f, palatal foramen; Po, postorbital; Prf, prefrontal; rtp, replacement tooth pit; sbt f, subtemporal fenestra; Sp, splenial; Vo, vomer.
Description
Structure of the marginal dentition in choristoderes
The marginal teeth of choristoderes are essentially homodont in both upper and lower jaws, and the implantation is subthecodont. Teeth are replaced by the erosion of a pit in the lingual surface of the old tooth base. However, there are minor differences between non‐neochoristoderes and neochoristoderes in marginal tooth morphology. Teeth of non‐neochoristoderes are relatively simple, and striated enamel covers the tooth crown but not the base (Fig. 2a,b). Furthermore, the crown is straight, allowing the teeth to be closely packed along the tooth row. In contrast, teeth of neochoristoderes are completely covered by striated enamel and there is enamel infolding at the base. In some neochoristoderes, such as Simoedosaurus and Champsosaurus, the anterior teeth are sharper and more slender than the posterior ones (Fig. 2d,e). The crown is labiolingually compressed and there are keels on each side of the tooth. The sharp tooth apex is curved either posteriorly or medially depending on tooth position (anterior–posterior, upper–lower jaws) and taxon. Ikechosaurus is unique among neochoristoderes in having straight tooth crowns and a lack of enamel over the tooth base, as in non‐neochoristoderes, but it shows the basal dentine infolding of neochoristoderes (Matsumoto et al. 2014).
Figure 2.
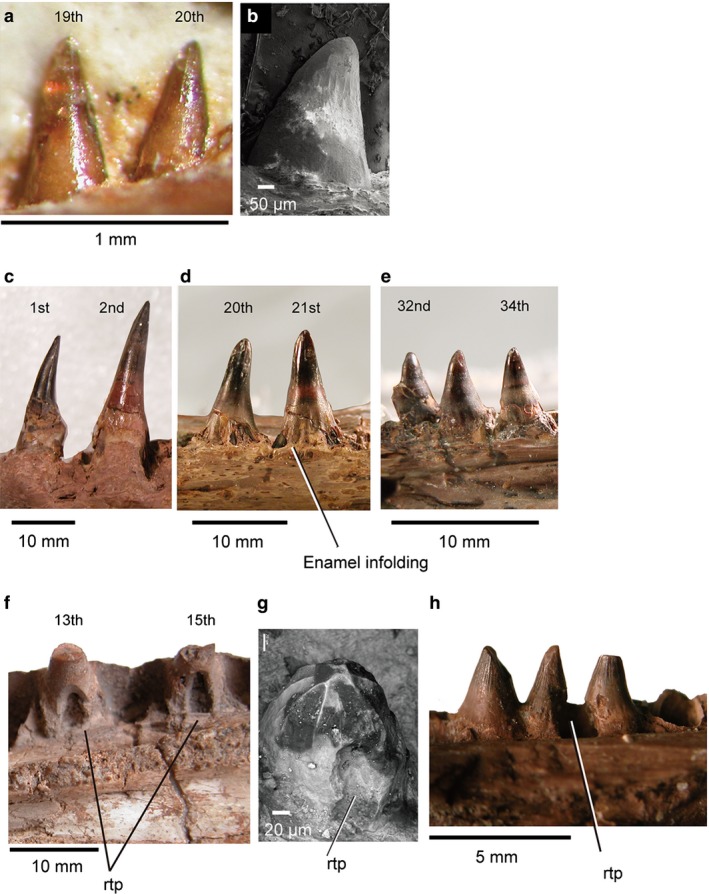
Marginal and palatal tooth morphology: (a) juvenile Monjurosuchus sp. (IVPP V14261) dentary teeth in medial view; (b) Cteniogenys sp. dentary (UCL uncataloged) in lateral view, SEM; (c) Champsosaurus gigas (SMM P77.33.24) left dentary teeth in lateral view; (d, e) Champsosaurus gigas (SMM P77.33.24) right maxillary teeth in lateral view, image reflected for ease of comparison; (f) Simoedosaurus lemoinei (MNHN BR 1935) replacement maxillary teeth in medial view; (g) Cteniogenys sp. (BMNH R11759), right pterygoid tooth; (h) Champsosaurus sp. isolated right palatine teeth (RTMP 92.36.270) in medial view. Tooth position is uncertain in (b), (g), (h) due to incompleteness of specimens.
General structure of the palatal dentition in choristoderes
Non‐neochoristoderes
The palatal dentition of neochoristoderes is relatively well known due to many specimens with nearly complete palatal elements, but this region is less known for non‐neochoristoderes, due to poor preservation or a lack of relevant elements (Table 1). In many cases, description of the palatal dentition is based on, at most, a few specimens (e.g. Hyphalosaurus). The teeth are arranged as a series of longitudinal rows on the vomer, palatine and pterygoid, a pattern inherited from that of basal amniotes (R. Matsumoto and S. E. Evans, unpublished data). Although choristoderes were all essentially aquatic, there is no obvious aquatic specialization in the palatal dentition of basal taxa. The presence of a pterygoid flange tooth row is confirmed in neochoristoderes and Cteniogenys (Figs 1 and 3), but it remains uncertain in most non‐neochoristoderes (e.g. Figs 1, 4 and 5: Monjurosuchus, Philydrosaurus, Hyphalosaurus and Lazarussuchus). In Monjurosuchus and Philydrosaurus, pterygoid flange teeth are obscured by poor preservation (Figs 1 and 5; Gao et al. 2007; RM personal observation). Gao et al. (2013) reported the pterygoid flange teeth to be absent in Philydrosaurus, based on a newly discovered juvenile specimen, but the flange is damaged. As in most diapsids, non‐neochoristoderes generally lack parasphenoid and ectopterygoid teeth.
Figure 3.
Pterygoid teeth in Cteniogenys sp. (BMNH R11759) from the Middle Jurassic of Kirtlington, Oxfordshire, UK; (a) reconstructed skull in palatal view (Evans, 1990); color coding of the different regions of the palatal dentition are the same as in Fig. 2; (b) anterior longitudinal tooth row; (c) posterior pterygoid teeth; (d) isolated pterygoid from UCL uncataloged specimens (magnification × 50); (e) enlarged image of (b), worn tooth crown from the longitudinal pterygoid tooth row in medial view; (f) enlarged image of (d), complete crown on the longitudinal pterygoid tooth row in medial view (magnification × 500); (g) enlarged image of (d), posterior pterygoid tooth (magnification × 500).
Figure 4.
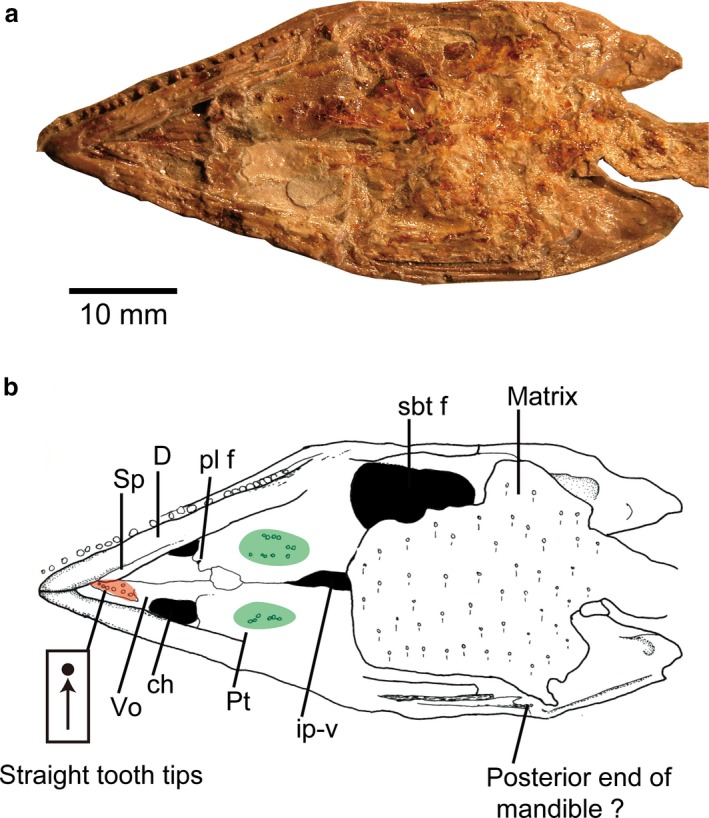
Hyphalosaurus lingyuanensis holotype from the Early Cretaceous of the Yixian Formation of China (IVPP V11075): (a) photograph of the skull in palatal view; (b) line drawing of (a); color coding of the different regions of the palatal dentition are the same as in Fig. 2.
Figure 5.
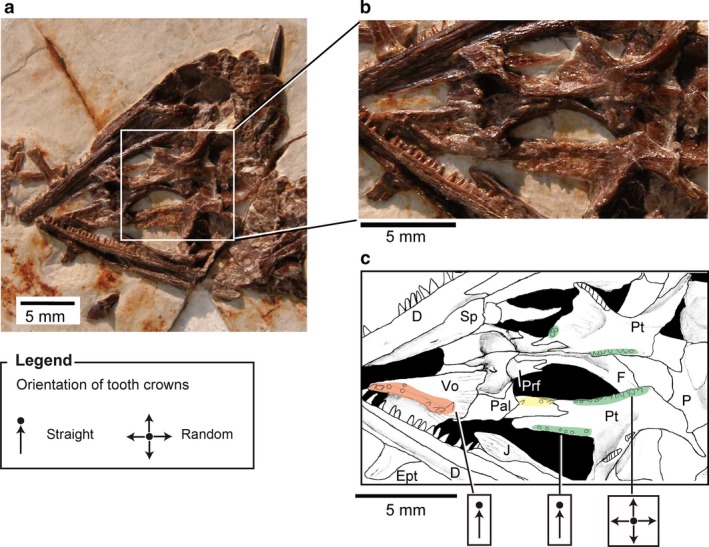
(a) Monjurosuchus sp. (IVPP V14261) from the Early Cretaceous of China, skull in palatal view: (b) photographs of the skull, enlarged view of palatal tooth area in (a); (c) line drawing of (b); color coding of the different regions of the palatal dentition are the same as in Fig. 2.
Neochoristoderes
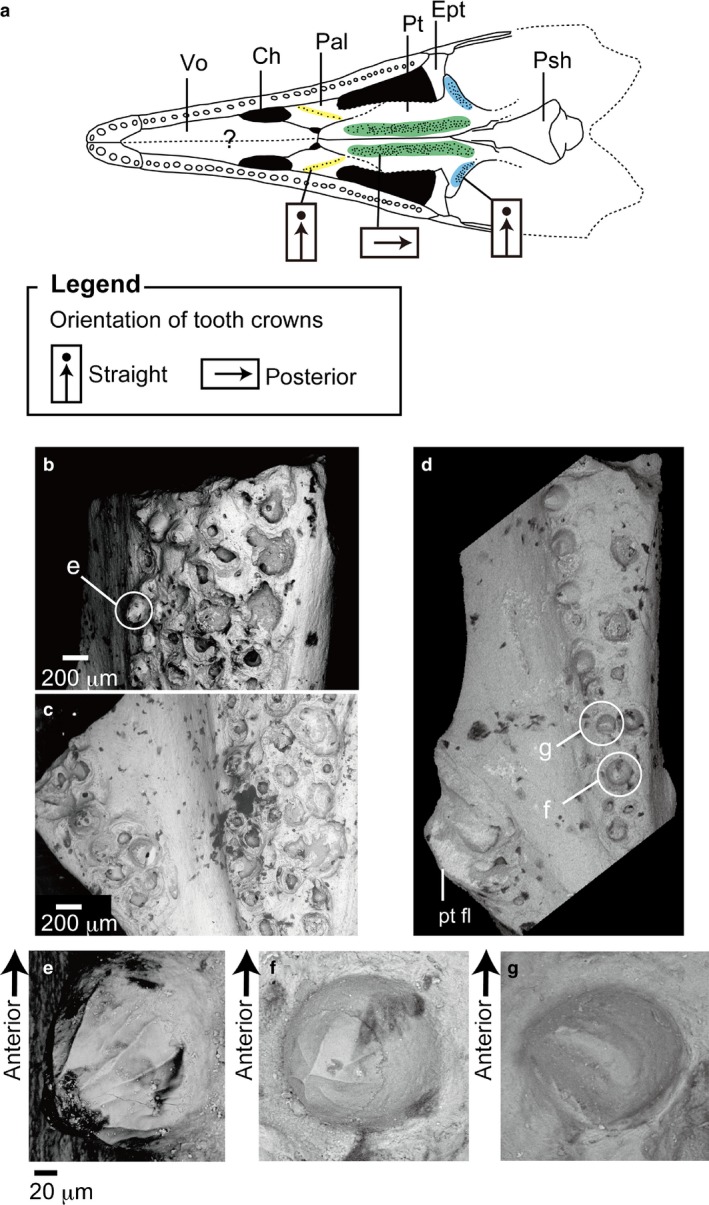
Some species are represented by multiple specimens and examination has shown that palatal features are consistent within species. As in non‐neochoristoderes, neochoristoderes generally lack parasphenoid and ectopterygoid teeth, the exception being the Early Cretaceous Ikechosaurus sunailinae (Fig. 6: IVPP V9611‐3; 10596.1), which has parasphenoid teeth. However, these were probably secondarily acquired as has been reported in Lepidosauromorpha (e.g. Kuehneosauridae; Evans, 2009). Lateral and medial tooth rows run longitudinally over the palate. The lateral row arises on the palatine, posterior to the choana, and runs onto the lateral side of the pterygoid, extending posterior to the end of the marginal tooth row. The row is generally continuous, but in some species it is interrupted by a gap at the palatine–pterygoid suture (e.g. Tchoiria klauseni; Ksepka et al. 2005). The medial row, on the other hand, begins on the vomer, anterior to the choana, runs along the medial side of the pterygoid, and terminates posterior to the interpterygoid vacuity. There is usually a toothless gap around the vomer–pterygoid suture. Both lateral and medial rows are generally single, except in a few Champsosaurus species (e.g. C. gigas and C. dolloi) where the pterygoid bears several rows (Fig. 7). However, the width of the tooth rows varies between species and between genera. In most species the longitudinal rows are fewer than 10 tooth positions across and the pterygoid flange tooth row has one–three tooth positions. However, in Simoedosaurus (Figs 8 and 9) and Ikechosaurus (Fig. 6) the longitudinal rows are generally more than 10 teeth across and the pterygoid flange tooth row may be 5–19 teeth across. These wide tooth batteries are a characteristic feature of Simoedosaurus and Ikechosaurus, so that the teeth cover most of the palatal surface (Figs 6, 8 and 9).
Figure 6.
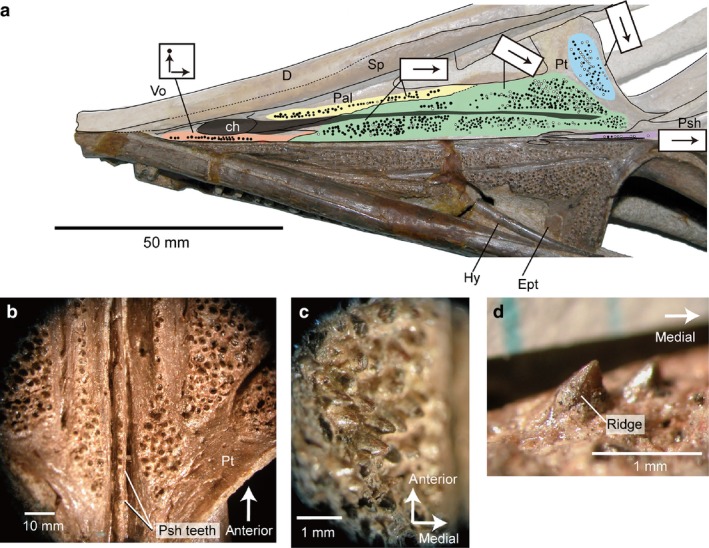
Ikechosaurus sunailinae from the Early Cretaceous Chabu‐Sumu locality, Inner Mongolia (IVPP V9611‐3), skull in palatal view: (a) photograph and line drawing of the skull in ventral view. Black circles indicate alveoli of palatal teeth; white circles showing presence of the teeth; color coding of the different regions of the palatal dentition are the same as in Fig. 2; gray zone indicating nasopalatal trough; arrows on tooth row showing orientation of tooth crowns. (b) Enlarged image of pterygoid and parasphenoid regions; (c) enlarged image of the longitudinal pterygoid tooth row (posterior); (d) pterygoid flange teeth in posterior view (image inverted for comparison).
Figure 7.
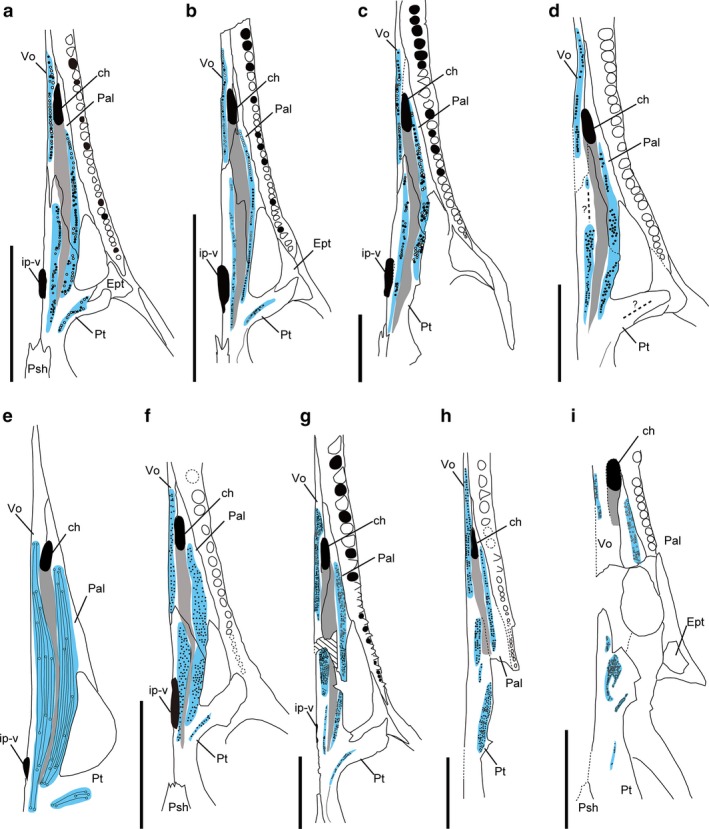
Comparison of the palatal dentition in Champsosaurus; (a–d) from the Late Cretaceous; (e, f) from the Late Cretaceous and the Paleocene; (g–i) from the Paleocene. (a) Semi‐adult C. lindoei (RTMP 87.36.41); (b) juvenile C. lindoei (RTMP 94.163.01); (c) C. albertensis (RTMP 86.12.11), pterygoid flange teeth incomplete; (d) C. natator (NMC8919) pterygoid flange teeth incomplete; (e) C. ambulator (modified from Sigogneau‐Russell, 1979); (f) C. laramiensis (redrawn based on Brown, 1905 and Sigogneau‐Russell, 1979); (g) C. gigas (SMM P77.33.24); (h) C. dolloi (IRSNB R21) pterygoid flange teeth incomplete; (i) C. tenuis (SMM P79.14.1) pterygoid flange teeth and pterygoid longitudinal tooth rows are incomplete. Scale bars: 50 mm, but scale is unknown in (e). Blue colored area marks the distribution of the palatal dentition, and gray colored area marks nasopalatal trough extending from the choana.
Figure 8.

Palatal dentition of Simoedosaurus sp. (SMNS 59026) (a–e) and S. lemoinei (f–i) from Mont Berru Reims, France: (a) photograph and line drawing of the skull (SMNS 59026) in palatal view; color coding of the different regions of the palatal dentition is the same as in Fig. 2; (b) lateral view of the snout (a); (c) enlarged image of anterior vomerine teeth of (b); (d) enlarged image of anterior palatine teeth of (b); (e) enlarged image of pterygoid teeth of (b) (reflected image); (f) isolated left vomer with teeth in lateral view, image inverted (MNHN BL9947); (g) isolated left palatine, image inverted (MNHN BR728); (h) posterior palatine dentition (MNHN BR 1935; neotype of S. lemoinei); (i) enlarged image of (h).
Figure 9.
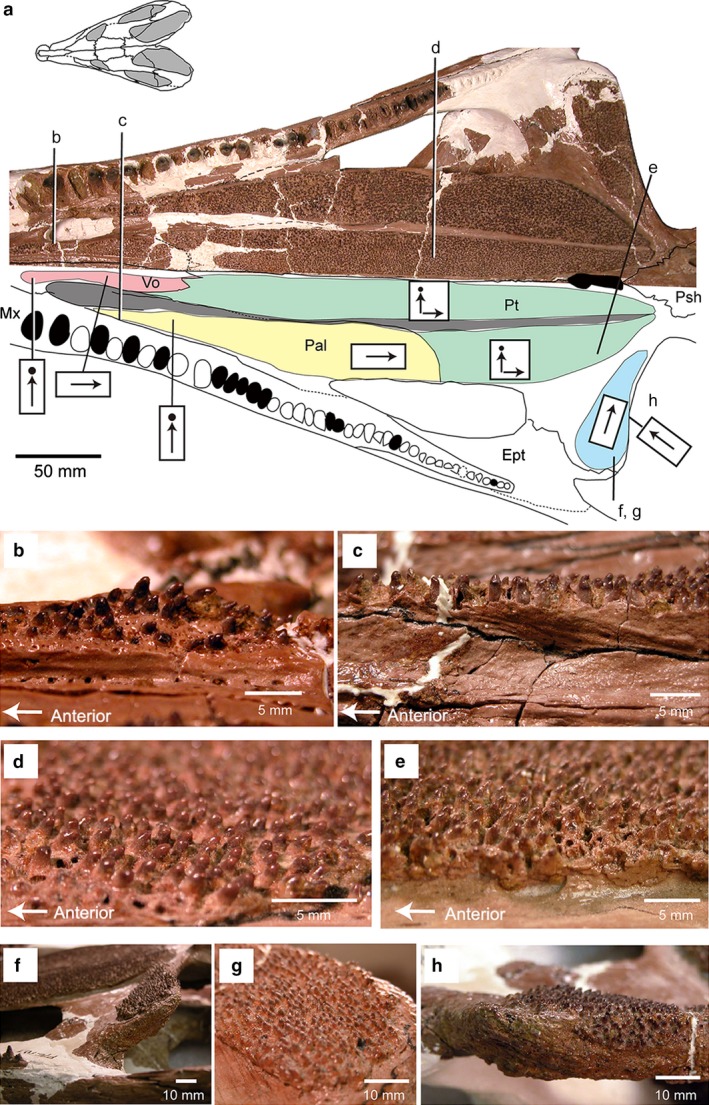
Simoedosaurus dakotensis (SMM P76.10.1) from the Paleocene of western North Dakota (near top of Slope Formation), USA: (a) skull in palatal view, digital image above and line drawing below (the letters b–h correspond to the following close‐up images below); color coding of the different regions of the palatal dentition is the same as in Fig. 2; (b) anterior vomerine teeth; (c) anterior palatine teeth; (d) posterior pterygoid, lateral tooth row; (e) posterior pterygoid, medial tooth row; (f) pterygoid flange in lateral view; (g) pterygoid flange in posterior view; (h) pterygoid flange teeth in lateral view.
Naso‐palatal trough
The medial and lateral longitudinal rows in neochoristoderes are separated by a distinct groove, the naso‐palatal trough. Erickson (1985) suggested that this might be the functional equivalent of the secondary palate (by extending the nasal groove posteriorly). This structure is less obvious in non‐neochoristoderes, but a newly discovered specimen of Philydrosaurus from the Early Cretaceous of China (Gao et al. 2007) appears to show some development of a trough extending posteriorly from the choana. However, due to the poor preservation of palatal elements in most non‐neochoristoderes, the distribution and evolutionary history of the nasopalatal trough among non‐neochoristoderes remains uncertain.
Palatal tooth replacement
The marginal tooth replacement pattern is almost mirrored in the palatal dentition. Marginal tooth replacement has been described in Cteniogenys (Evans, 1990). Initially, the new tooth grows at the lingual side of the old tooth base. The new tooth attaches weakly to the base and erodes a pit in it (Fig. 2f). A similar replacement pattern can be recognized on one of the pterygoid teeth in Cteniogenys. The tooth shows an erosion pit made by a new tooth on the lingual side of the old tooth base (Fig. 2g). In contrast, in Champsosaurus (RTMP 92.36.270) the eroding pit straddles two old teeth (Fig. 2h), suggesting two original teeth were being replaced by a single larger tooth. A matching replacement pattern for marginal and palatal teeth was also reported in living squamates (Mahler & Kearney, 2006), although there is some variation in the position of the replacement (e.g. Iguana shows both lingual and labial replacement in different tooth rows; Mahler & Kearney, 2006).
Palatal tooth platforms
Although there are differences of degree between species, the palatal tooth batteries of neochoristoderes sit on a bony platform that raises them above the level of the palatal surface. These platforms are particularly strongly developed anteriorly in Champsosaurus and Simoedosaurus, so that the palatine tooth bases lie almost level with, or even ventral to, the maxillary tooth bases (Fig. 10). Among non‐neochoristoderes, a tooth platform is, at most, weakly developed in Cteniogenys (clearly recognized only in the longitudinal pterygoid row) and Monjurosuchus.
Figure 10.
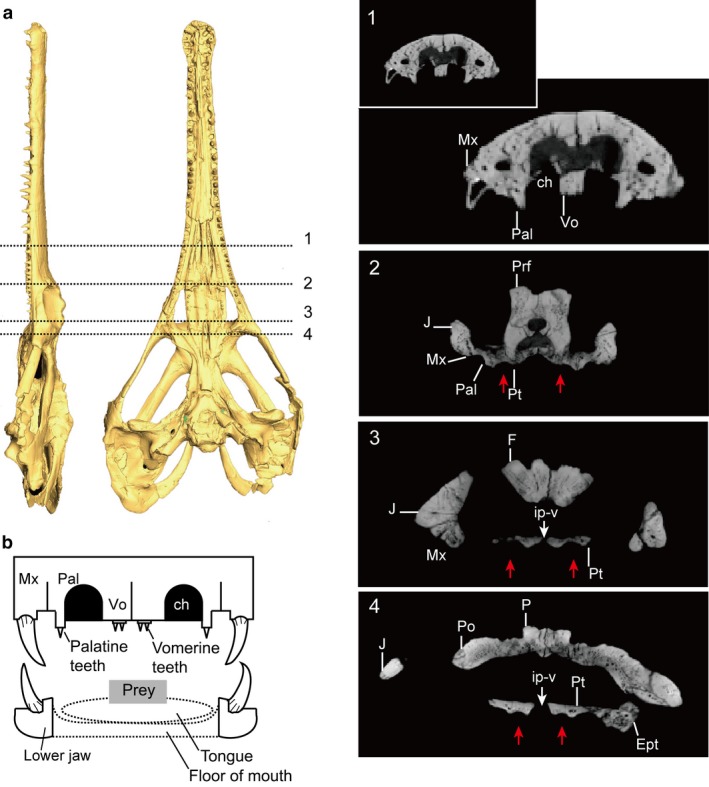
(a) CT image of Champsosaurus lindoei (NMC 8920) without scale; the numbers on the skull corresponding to slice images 1–4. Red arrows showing nasopalatal trough. (b) Diagram of skull and lower jaw in anterior section (Champsosaurus as the model), the palatine teeth lie on the ridge, soft tissues are drawn in dashed‐lines.
Tooth platforms may also be present along the posterior margin of the pterygoid flange but, in this case, the orientation of the tooth row also depends on that of the flange itself. The pterygoid flange is always angled forward in choristoderes, but the angulation is least in Neochoristodera (10–30 °; e.g. Ikechosaurus, IVPP V9611‐3) and greatest in Cteniogenys (45 °; e.g. BMNH 11759) and Monjurosuchus (40 °; e.g. IVPP 14261), with Philydrosaurus midway between the two extremes (35 °; Gao et al. 2007; Supporting Information). In Champsosaurus and Simoedosaurus, the pterygoid flange also extends ventrally, due to the ectopterygoid contribution, and leans slightly anteroventrally, so that the pterygoid flange tooth tips face anteriorly (Figs 9f–h and 11h). Despite the uncertain relationship of the ectopterygoid and pterygoid, Ikechosaurus has an anteriorly facing pterygoid flange tooth platform (uncertain in Tchoiria). This is absent in Cteniogenys and Monjurosuchus (Figs 1, 3c and 5b), but unknown in other non‐neochoristoderes.
Figure 11.
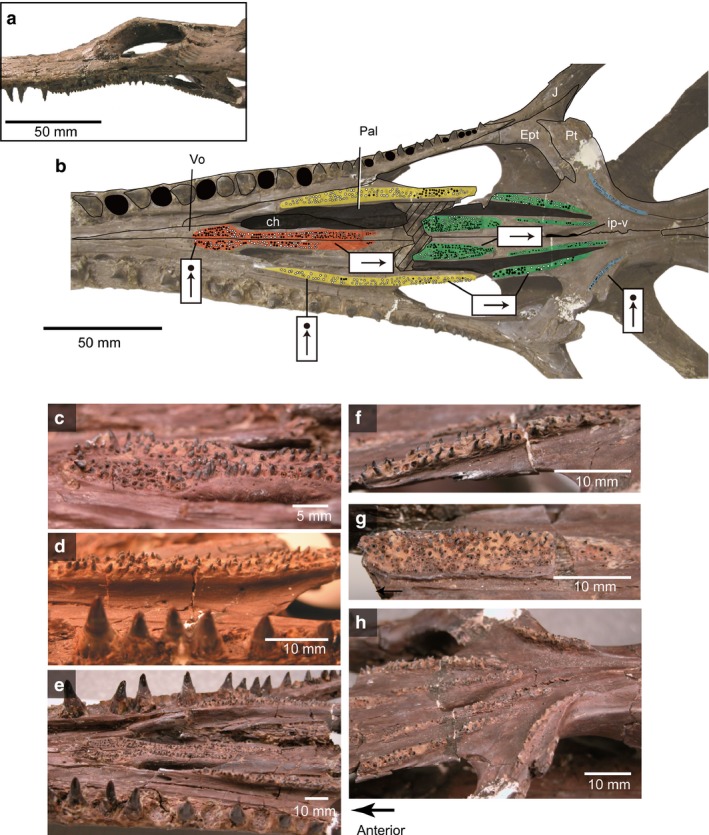
Champsosaurus gigas (SMM P77.33.24) from the Paleocene of western North Dakota: (a) skull in lateral view; (b) line drawing and photograph of palatal dentition; (c) anterior vomerine teeth (reflected image); (d) palatine tooth row (reflected image); (e) anterior palatal dentition; (f) pterygoid, lateral tooth row; (g) pterygoid, medial tooth row; (h) pterygoid flange tooth row in lateral view. Black circles indicate alveoli of palatal teeth; color coding of the different regions of the palatal dentition is the same as in Fig. 2.
Palatal tooth morphology, size and direction of tooth crowns
Individual palatal teeth (neochoristodere and non‐neochoristodere) show the same morphology as the marginal teeth of non‐neochoristoderes: they are conical, with enamel only partially covering the crown, and weakly developed ridges on the enamel surface (Fig. 2). All palatal teeth sit in shallow circular alveoli and lack basal enamel infolding.
In neochoristoderes the palatal teeth gradually become smaller from anterior to posterior ends of the longitudinal rows. In addition, the teeth on the lateral row are larger than those on the medial row. In the pterygoid flange row, teeth decrease in size from lateral to medial, but where the row is wide (Simoedosaurus and Ikechosaurus), the reduction is from anterolateral to posteromedial. This pattern differs in non‐neochoristoderes. Monjurosuchus sp. (IVPP V14261) shows a reverse pattern on the pterygoid (longitudinal row), with the largest teeth in the medial row, and at its posterior end. In Cteniogenys, there is no significant size difference along either the longitudinal pterygoid row or the pterygoid flange row.
Kordikova (2002) reviewed palatal tooth morphology in choristoderes based on Simoedosaurus and Champsosaurus, and stated that the tooth crowns point sharply backward. However, detailed examination in several species of neochoristoderes, mainly Champsosaurus and Simoedosaurus, shows that the orientation of palatal tooth crowns changes with the position on the palate. In Simoedosaurus, the European (Paleocene) S. lemoinei has anterior vomerine and palatine teeth that are blunt (Fig. 8c,f), with more posterior teeth becoming sharper and more backward pointed (Fig. 8e,g,i). In contrast, the shorter‐snouted North American S. dakotensis is characterized by blunt palatal tooth crowns throughout the tooth row, with the crowns oriented vertically on the vomer and palatine, but varying posteriorly from straight to recurved (Fig. 9). SMM P76.10.1 is the only specimen of S. dakotensis that preserves a complete skull and palatal dentition. However, the marginal teeth are preserved in position with no trace of wear of the tooth crowns. It is therefore unlikely that the shape of the palatal tooth crowns is due to wear. The pterygoid flange tooth crowns curve medially in both species, but in S. dakotensis the teeth along the edge of the subtemporal fenestra point anteriorly.
Among the eight valid species of Champsosaurus, there is variation in the number and width of the longitudinal palatal tooth rows, and in the orientation of the tooth crowns. For example, although the Late Cretaceous C. albertensis (Fig. 12; RTMP86.12.11) and the Paleocene C. gigas (Fig. 11; SMM P77.33.24) have similar sized skulls, the palatal dentition is different. C. albertensis has either straight or randomly oriented crowns anteroposteriorly (Fig. 12), whereas in C. gigas the crowns are straight on anterior teeth (vomer and palatine) but curve sharply posteriorly in the pterygoid part of the rows (Fig. 11). In addition, C. albertensis has a relatively small number of large teeth in the longitudinal pterygoid row, whereas C. gigas has a larger number of small teeth. This seems to be a general difference between the Late Cretaceous (e.g. C. lindoei; C. natotor) and Paleocene species (e.g. C. tenuis; C. dolloi). Minor ontogenetic variation is also recognized in Champsosaurus lindoei, in which the width of longitudinal palatal tooth row seems to increase slightly through ontogeny, from a single tooth wide in smaller individuals (Figs 7b and 12; RTMP 94.163.01: inner biquadrate width 50 mm) to double that in a larger individual (Fig. 7a; RTMP 87.36.41: inner biquadrate width 86 mm). However, Champsosaurus species are more consistent in the morphology of the pterygoid flange rows, which are usually one–two tooth positions wide with vertical tooth crowns (Fig. 7).
Figure 12.
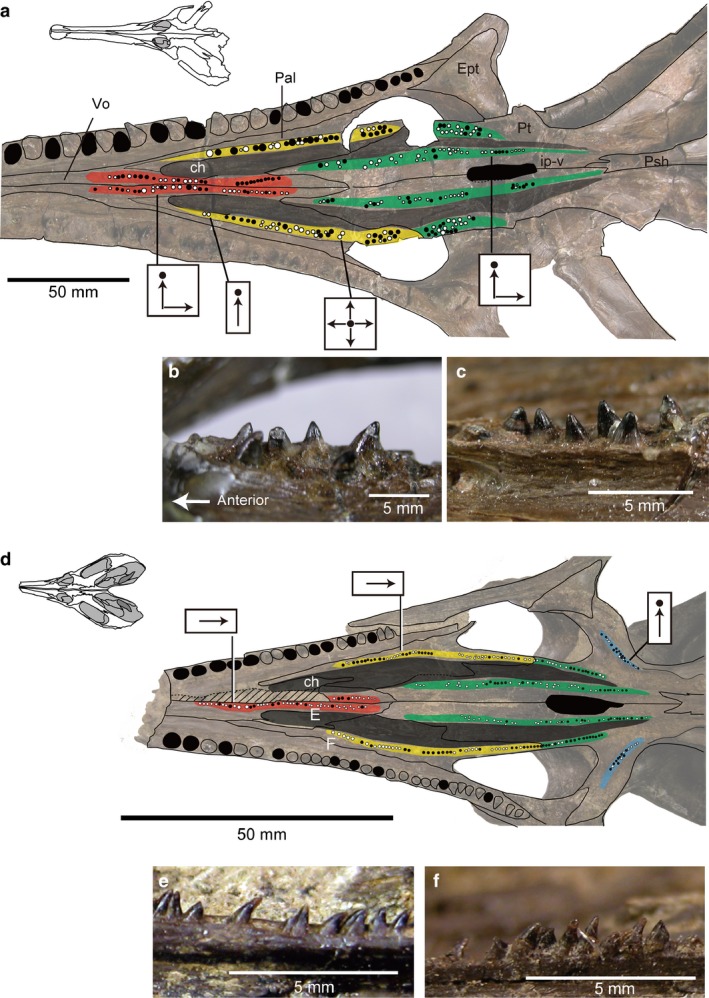
Late Cretaceous Champsosaurus; (a–c) C. albertensis (RTMP 86.12.11) from the Horseshoe Canyon Formation at Drumheller, Canada; (a) photo and line drawing of C. albertensis in palatal view; (b) right vomerine teeth in lateral view; (c) right palatine teeth in medial view, reflected image. (d–f) C. lindoei (RTMP 94.163.01) from the Oldman Formation Alberta, Canada; (d) photo and line drawing of C. lindoei in palatal view; (e) right vomerine teeth in lateral view; (f) right anterior palatine teeth in lateral view. Black circles indicate alveoli of palatal teeth; color coding of the different regions of the palatal dentition is the same as in Fig. 2.
In the wide longitudinal tooth rows of the Early Cretaceous Ikechosaurus, the anterior (vomer and palatine) crowns are straight or point backward, but those on the pterygoid are first directed posteriorly and then gradually turn medially (Fig. 6a). The expansion of the palatal tooth row is essentially similar to that of the Paleocene species Simoedosaurus lemoinei and S. dakotensis. However, unlike these Simoedosaurus, several well‐preserved palatal teeth of Ikechosaurus show distinctive ridges on the sides (Fig. 6d). Interestingly within the extensive pterygoid tooth and pterygoid flange tooth rows, the teeth form antero‐posteriorly and mediolaterally radiating lines, instead of having a random distribution as in Simoedosaurus. Furthermore, the parasphenoid tooth crowns incline posteriorly and, uniquely, the pterygoid flange teeth incline medially.
There are less available data on crown orientation in non‐neochoristoderes due to poor palatal preservation (e.g. Hyphalosaurus; Fig. 4). The teeth of the anterior longitudinal row (vomer, palatine) are generally straight [Hyphalosaurus, Monjurosuchus, Cteniogenys (vomer unknown in Cteniogenys)], but the posterior teeth show some variation. In Cteniogenys, some worn teeth are retained in the sockets, stripped of their enamel, and with the tips smooth and flat (Fig. 3g). Originally, they were presumably conical and covered by enamel as shown in Fig. 3e,f. The tooth tips incline in random directions in the posterior part of the tooth row (pterygoid), but some terminal teeth are inclined posteriorly. In Monjurosuchus, the conical tooth tips are randomly oriented, posterior, anterior or vertical (Fig. 5). This orientation is recognized in at least two specimens (IVPP V 13761, 14261), so is unlikely to be due simply to deformation.
Discussion
Evolutionary history of the palatal dentition in Choristodera
Tracing the evolutionary history of the choristoderan palatal dentition is complicated by a paucity of information on the palate of many non‐neochoristoderes (poor preservation, specimen orientation), the absence of Triassic representatives (ghost lineage) and a lack of consensus on the interrelationship of non‐neochoristoderes. However, a review of amniote palate morphology (R. Matsumoto and S. E. Evans, unpublished data) suggests that the primitive diapsid pattern consisted of longitudinal tooth rows on the vomer, palatine and pterygoid; a pterygoid flange row; and, probably, a tooth patch on the parasphenoid. This pattern would have been inherited by the common ancestor of all choristoderes and is broadly retained in the Jurassic Cteniogenys (Evans, 1990), albeit with an increase in width of the pterygoid tooth row and loss of the parasphenoid teeth. There was also a tendency to develop bony palatal ridges/platforms on the vomer, palatine and pterygoid to which the palatal teeth attached and which contribute to the formation of a nasopalatal trough. The latter feature is most obvious in Champsosaurus and Simoedosaurus (Figs 8, 9, 10, 11, 12) where it separates wide tooth batteries, and was thought to be a neochoristodere character. However, a weakly developed nasopalatal trough was recently reported in the non‐neochoristodere Philydrosaurus (Gao et al. 2007). It is not present in Cteniogenys nor is it evident in a juvenile Monjurosuchus sp. (IVPP 14261) for which the palate is known. Additional specimens are required to understand the evolutionary history of the nasopalatal trough in choristoderes.
The function of the palatal dentition
Although there is variation in the choristoderan palatal dentition, the basic pattern can be summarized as follows. Medial and lateral longitudinal tooth rows, of variable width, run from the vomers to the pterygoids, supplemented by transverse pterygoid flange rows. The palatal tooth crowns on the vomer and palatine point straight down, while the more posterior (pterygoid) teeth point backward and/or medially (e.g. Ikechosaurus), often with a reduction in size. The pterygoid flange teeth are straight or medially directed. This is especially clear in neochoristoderes Ikechosaurus, Simoedosaurus and Champsosaurus (C. gigas, juvenile C. lindoei). The anterior limit of the palatal dentition corresponds to the posterior limit of the lower jaw symphysis. Non‐neochorisotoderes have a shorter symphysis and their anterior palatal teeth extend close to the premaxilla (unknown in Cteniogenys), whereas in the long‐snouted neochoristoderes, with long symphyses, the palatal dentition begins further posteriorly. A mobile fleshy tongue, with both intrinsic and extrinsic musculature, is inferred to have been primitive for amniotes (Iwasaki, 2002), and seems to be correlated with the retention of a palatal dentition (lizards), or its replacement with analogous keratinized tubercles or ridges in the oral epithelium (crocodiles, turtles, mammals, birds). As suggested by Erickson (1985), therefore, a fleshy tongue probably worked with the palatal dentition in choristoderes during intra‐oral transport of prey. The positional changes of palatal tooth orientation may correspond to the feeding stages categorized by Schwenk (2000): (1) prey capture; (2) ingestion; (3) intraoral transport; (4) swallowing.
Prey capture: mainly involves the anterior marginal teeth, the crowns of which are directed medially in neochoristoderes, but are generally straight in non‐neochoristoderes.
- Ingestion: the straight anterior palatal teeth (on vomer and palatine) might have had a role in holding and reorienting the prey in combination with a fleshy tongue. In addition, in neochoristoderes, the ridges formed by the combination of raised palatal platforms and embedded teeth form a crest on the palatine medial to the marginal tooth row. This may help to hold prey where the larger marginal teeth are located (Fig. 13).
Figure 13.
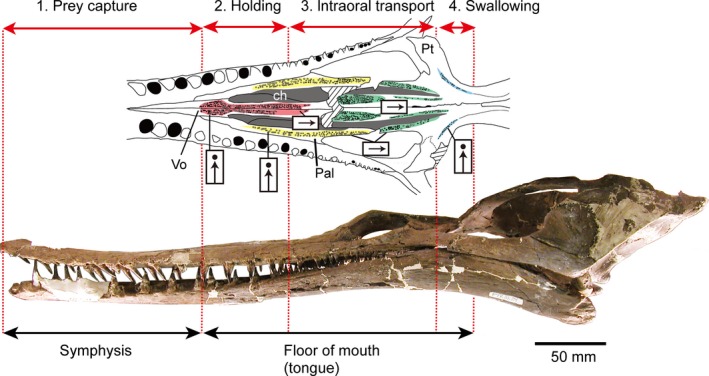 Summary of the morphological variation in the palatal dentition of neochoristoderes corresponding to feeding stages; color coding of the different regions of the palatal dentition is the same as in Fig. 2.
Summary of the morphological variation in the palatal dentition of neochoristoderes corresponding to feeding stages; color coding of the different regions of the palatal dentition is the same as in Fig. 2. Intraoral transport: the prey is manipulated by the tongue and pushed towards the back of the mouth with the posteriorly directed pterygoid teeth minimizing resistance against the tongue but preventing the prey from moving or sliding forward (Fig. 13).
Swallowing: at the back of the mouth, the tooth crowns of the transverse pterygoid tooth row point medially. This may have helped to hold prey and avoid forward slippage while the tongue pushes food into the pharynx (pharyngeal packing). These pterygoid teeth are also level with the point at which the marginal tooth size is reduced. However, the posterior palatal teeth in some Champsosaurus (e.g. C. albertensis) are oriented randomly, perhaps to resist moving prey.
In the absence of muscular pharyngeal walls in reptiles (Schwenk, 2000), the pharynx itself then needs to be compressed externally (by neck muscles or neck flexion) in order to finally push the food item into the esophagus to initiate swallowing. With external constriction, there is a danger of the prey being squeezed back into the mouth instead of into the esophagus (Schwenk, 2000), and the posterior pterygoid teeth may help to resist this. The parasphenoid teeth in Ikechosaurus may have a similar function as the parasphenoid lies in the roof of the pharyngeal region. Their inferred redevelopment suggests that something has changed with respect to feeding strategy – in prey size, oral soft tissues or neck muscles – so that a firmer grip is required here.
Observed variation in palatal tooth shape and the width of palatal tooth batteries may also provide clues about diet. A study of the extant fish (Cichlasoma) found a correspondence between pharyngeal tooth morphology and diet: fish with pointed pharyngeal teeth showed a greater preference for soft prey than those with more robust rounded teeth (Trapani, 2003; Trapani et al. 2005). Relating this to choristoderes, the European Simoedosaurus lemoinei has sharper palatal teeth than its North American counterpart, S. dakotensis, suggesting a preference for softer prey – a conclusion consistent with the more gracile marginal teeth and narrower snout. Moreover, some Champsosaurus species, before and after the K/Pg boundary, are differentiated by their palatal tooth morphology and arrangement. As noted above, the Cretaceous species C. albertensis, C. lindoei, C. natator tend to have fewer, larger pterygoid teeth than the Paleocene species C. gigas and C. dolloi. There are also differences in the snout length of these species. Paleocene species have slightly longer snouts and therefore higher marginal tooth count (59 in C. dolloi; 50 in C. gigas) than Cretaceous species (e.g. ~40 in C. laramiensis; 43 in C. albertensis). These differences could correlate with dietary change across the K/Pg boundary, reflecting the faunal and floral transformation associated with the major extinction event, as reported in mammals (Archibald & Bryant, 1990), birds (Longrich et al. 2011), insects (Labandeira et al. 2002), plants (Nichols & Johnson, 2008) and squamates (Longrich et al. 2012). Moreover, Champsosaurus and Simoedosaurus, having survived the extinction, seem to have co‐occurred at some Paleocene localities (e.g. Mont‐Berru, France; Fort Union Formation). They may have avoided competition by taking different prey, the long slender‐snouted Champsosaurus feeding on schools of fish, like the extant Gavialis, and the wider‐snouted Simoedosaurus taking single prey items as in broad‐snouted modern crocodiles (Evans & Hecht, 1993; R. Matsumoto and S. E. Evans, unpublished data on neck anatomy). The morphology of the marginal teeth and their size (diameter and height) relative to the skull width (inner biquadrate width) are similar in both genera (Matsumoto, 2011), but Simoedosaurus has wider longitudinal and transverse palatal tooth rows, corresponding to the greater snout width. These shagreen teeth may have provided a more efficient gripping surface to hold large prey. The Early Cretaceous species Ikechosaurus is unique in having an elongated snout as in Chamapsosaurus (Matsumoto & Evans, 2010; Fig. 7) with a broad palatal tooth row as in Simoedosaurus. Remarkably, the marginal dentition of Ikechosaurus resembles that of non‐neochoristoderes in that the exposed area of each tooth crown is short, probably due to soft‐tissue cover at the tooth base (Matsumoto et al. 2014). The wider palatal gripping surfaces in this taxon might be necessary to supplement marginal dentition.
Thus, differences in the palatal dentition suggest that the neochoristoderan diet may have varied interspecifically. However, the evolutionary history of the palatal dentition remains incomplete, because palatal morphology as a whole is known only in a limited number of non‐neochoristodere genera. Moreover, the question of diet and feeding strategy in this enigmatic group of reptiles needs to be approached from other perspectives, such as whole skull morphology and the structure of the marginal dentition.
Conclusions
Choristodera show a modification of the primitive diapsid tooth arrangement, with one or two longitudinal rows and a transverse pterygoid row.
Individual palatal teeth of neochoristoderes resemble the marginal teeth of non‐neochoristoderes in being conical with striations on the enamel and in having a similar mode of tooth replacement.
The width of the palatal tooth row, the sharpness of individual teeth and the orientation of the tooth tips show interspecific variation in Champsosaurus and Simoedosaurus.
The sharpness of the palatal teeth differs between species and may reflect prey preference: sharp teeth for soft prey; blunt teeth for harder prey.
Although the Late Cretaceous C. albertensis (RTMP86.12.11) and Paleocene C. gigas (SMM P77.33.24) have similar‐sized skulls, they differ in the pattern of their palatal dentition. C. albertensis has fewer, larger palatal teeth, an arrangement that may be correlated with prey size and preference.
The European Simoedosaurus lemoinei has sharper palatal teeth than its North American counterpart, S. dakotensis, a difference also reflected in the marginal dentition.
The orientation of the palatal tooth tips changes antero‐posteriorly across the palate in a manner consistent with the major stages of feeding: prey capture, ingestion, intraoral transport and swallowing.
Supporting information
Data S1. Summary of the palatal dentition in choristoderes.
Acknowledgements
Thanks are due to Mark Turmaine (UCL) for help with SEM: for access to specimens, Drs Don Brinkman and Don Henderson (Royal Tyrrell Museum, Canada); Dr Bruce Erickson and Ms Jackie Hoff (Science Museum Minnesota, USA); Drs Bernard Battail, Virginie Bouetel and Hervé Lelièvre (Muséum National d'Histoire Naturelle, Paris); Dr Denise Sigogneau‐Russell (Paris); Dr Rainer Schoch (Staatliches Museum für Naturkunde, Germany); Dr Annelise Folie (Institut Royal des Sciences Naturelles de Belgique); Dr Yuan Wang, Dr Zhijie Jack Tseng, Ms Fang Zheng, Mr Binghe Geng and Ms Shuqin Duan (Institute of Vertebrate Paleontology and Paleoanthropology, Beijing), Dr X.‐C. Wu and Ms Margaret Feuerstack (Canadian Museum of Nature, Ottawa, Canada), Dr Tamaki Sato (Tokyo Gakugei University); for access to μCT scanner, Dr Makoto Manabe and Ms Chisako Sakata (National Museum of Nature and Science). This research was supported by an Estes Memorial Award, Society of Vertebrate Paleontology, the Sasakawa Scientific Research Grant, Japan Society; and the Japan Society for the Promotion of Science Scholarship (JSPS) for the Postdoctoral Fellowship and the Scientific Research Grant for Young Scientists B.
References
- Archibald JD, Bryant L (1990) Differential Cretaceous‐Tertiary extinctions of nonmarine vertebrates: evidence from northeastern Montana In: Global Catastrophes in Earth History: An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality. (eds Sharpton VL, Ward PD.) Geol Soc Am Special Paper 247, 549–562. [Google Scholar]
- Böhme M (2008) Ectothermic vertebrates (Teleostei, Allocaudata, Urodela, Anura, Testudines, Choristodera, Crocodylia, Squamata) from the upper Oligocene of Oberleichtersbach (Northern Bavaria, Germany). Cour Forschungstinst Senckenb 260, 161–183. [Google Scholar]
- Brinkman DB, Dong ZM (1993) New material of Ikechosaurus sunailinae (Reptilia: Choristodera) from the Early Cretaceous Langhongdong formation, Ordos Basin, Inner Mongolia, and the interrelationships of the genus. Can J Earth Sci 30, 2153–2162. [Google Scholar]
- Brown B (1905) The osteology of Champsosaurus Cope. Am Mus Nat Hist Mem 9, 1–26. [Google Scholar]
- Chure DJ, Evans SE (1998) A new occurrence of Cteniogenys, with comments on its distribution and abundance. Mod Geol 23, 49–55. [Google Scholar]
- Efimov MB (1975) Khampsosaurid iz nizhnego mela Mongolii [a champsosaurid from the Lower Cretaceous of Mongolia]. Tr Sovmest Sov Mong Paleontol Eksped 2, 84–94. [Google Scholar]
- Efimov MB (1979) Tchoiria (Chamsosauridae) iz rannego mela Khamaryn‐Khurala, MNH [Tchoiria (Champsosauridae) from the Early Cretaceous of Khamaryn Khural, MNR]. Tr Sovmest Sov Mong Paleontol Eksped 8, 56–57. [Google Scholar]
- Efimov MB, Storrs GW (2000) Choristodera from the Lower Cretaceous of northern Asia In: The Age of Dinosaurs in Russia and Mongolia. (eds Benton MJ, Shishkin MA, Unwin DA, Kurochkin EN.), pp. 390–401. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Erickson BR (1972) The lepidosaurian reptile Champsosaurus in North America. Sci Mus Minn Monogr (Paleontol) 1, 1–91. [Google Scholar]
- Erickson BR (1981) Champsosaurus tenuis (Reptilia: Eosuchia), a new species from the late Paleocene of North America. Sci Pub Sci Mus Minn, N Ser 5, 1–14. [Google Scholar]
- Erickson BR (1985) Aspects of some anatomical structures of Champsosaurus Cope (Reptilia: Eosuchia). J Vertebr Paleontol 5, 111–127. [Google Scholar]
- Erickson BR (1987) Simoedosaurus dakotensis, new species, a diapsid reptile (Archosauromorpha: Choristodera) from the Paleocene of North America. J Vertebr Paleontol 7, 237–251. [Google Scholar]
- Evans SE (1988) The early history and relationships of the Diapsida In: The Phylogeny and Classification of the Tetrapoda. Volume 1: Amphibians, Reptiles, Birds. (ed. Benton MJ.), pp. 221–260, The Systematics Association, Special Volume 35A. Oxford: Clarendon Press. [Google Scholar]
- Evans SE (1989) New material of Cteniogenys (Reptilia: Diapsida) and a reassessment of the systematic position of the genus. Neues Jahrb Geol Paläontol Monatsh 1989, 577–589. [Google Scholar]
- Evans SE (1990) The skull of Cteniogenys, a choristodere (Reptilia: Archosauromorpha) from the Middle Jurassic of Oxfordshire. Zool J Linn Soc 99, 205–237. [Google Scholar]
- Evans SE (1991) The postcranial skeleton of the choristodere Cteniogenys (Reptilia: Diapsida) from the Middle Jurassic of England. Geobios 24, 187–199. [Google Scholar]
- Evans SE (2009) A new kuehneosaurid reptile from the Early Triassic of Poland. Palaeontol Pol 65, 149–184. [Google Scholar]
- Evans SE, Hecht MK (1993) A history of an extinct reptilian clade, the Choristodera: longevity, Lazarus‐taxa, and the fossil record. Evol Biol 27, 323–338. [Google Scholar]
- Evans SE, Klembara J (2005) A choristoderan reptile (Reptilia: Diapsida) from the Lower Miocene of northwest Bohemia (Czech Republic). J Vertebr Paleontol 25, 171–184. [Google Scholar]
- Evans SE, Manabe M (1999) A choristoderan reptile from the Lower Cretaceous of Japan. Spec Pap Palaeontol 60, 101–119. [Google Scholar]
- Gao K, Fox RC (1998) New choristoderes (Reptilia: Diapsida) from the Upper Cretaceous and Paleocene, Alberta and Saskatchewan, Canada, and phylogenetic relationships of the Choristodera. Zool J Linn Soc 124, 303–353. [Google Scholar]
- Gao KQ, Fox RC (2005) A new choristodere (Reptilia: Diapsida) from the Lower Cretaceous of western Liaoning Province, China, and phylogenetic relationships of Monjurosuchidae. Zool J Linn Soc 145, 427–444. [Google Scholar]
- Gao K‐Q, Ksepka DT (2008) Osteology and taxonomic revision of Hyphalosaurus (Diapsida: Choristodera) from the Lower Cretaceous of Liaoning, China. J Anat 212, 747–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K‐Q, Li Quanguo (2007) Osteology of Monjurosuchus splendens (Diapsida: Choristodera) based on a new specimen from the Lower Cretaceous of western Liaoning, China. Cretaceous Res 28, 261–271. [Google Scholar]
- Gao K‐Q, Tang ZL, Wang XL (1999) A long‐necked diapsid reptile from the Upper Jurassic/Lower Cretaceous of Liaoning Province, northeastern China. Vert Pal Asiat 37, 1–8. [Google Scholar]
- Gao K‐Q, Evans SE, Ji Q, et al. (2000) Exceptional fossil material of a semi‐aquatic reptile from China: the resolution of an enigma. J Vertebr Paleontol 20, 417–421. [Google Scholar]
- Gao K‐Q, Ksepka D, Hou LH, et al. (2007) Cranial morphology of an Early Cretaceous monjurosuchid (Reptilia: Diapsida) from Liaoning Province of China and evolution of the Choristoderan palate. Hist Biol 19, 215–224. [Google Scholar]
- Gao K‐Q, Zhou C‐F, Hou L, et al. (2013) Osteology and ontogeny of Early Cretaceous Philydrosaurus (Diapsida: Choristodera) based on new specimens from Liaoning Province, China. Cretaceous Res 45, 91–102. [Google Scholar]
- Gervais P (1877) Enumération de quelques ossements d'animaux vertébrés recueillis aux environs de Reims par M. Lemoine. J Zool 6, 74–79. [Google Scholar]
- Hecht MK (1992) A new choristodere (Reptilia, Diapsida) from the Oligocene of France: an example of the Lazarus effect. Geobios 25, 115–131. [Google Scholar]
- Iwasaki S (2002) Evolution of the structure and function of the vertebrate tongue. J Anat 201, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Chen W, Wang WL, et al. (2004) Mesozoic Jehol Biota of Western Liaoning, China, 375 pp. Beijing: Geological Publishing House; (in Chinese). [Google Scholar]
- Kordikova EG (2002) Comparative morphology of the palate dentition in Proganochelys quenstedti Baur 1887 from the Upper Triassic of Germany and Chelonian Ancestry. Neues Jahrb Geol Palaontol‐Abh 225, 195–249. [Google Scholar]
- Ksepka DT, Gao KQ, Norell MA (2005) A new choristodere from the Cretaceous of Mongolia. Am Mus Novit 3468, 1–22. [Google Scholar]
- Labandeira CC, Johnson KR, Wilf P (2002) Impact of the terminal Cretaceous event on plant–insect associations. Proc Natl Acad Sci USA 99, 2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J (2004) A nearly complete skeleton of Ikechosaurus pijiagouensis sp. nov. (Reptilia: Choristodera) from the Jiufotang Formation (Lower Cretaceous) of Liaoning, China. Vert Pal Asiat 42, 120–129 (in English with Chinese abstract). [Google Scholar]
- Longrich NR, Tokaryk TT, Field DJ (2011) Mass extinction of birds at the Cretaceous‐Paleogene (K‐Pg) boundary. Proc Natl Acad Sci USA 108, 15 253–15 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longrich NR, Bhullar BAS, Gauthier JA (2012) Mass extinction of lizards and snakes at the Cretaceous‐Paleogene boundary. Proc Natl Acad Sci USA 109, 21 396–21 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü J‐C, Kobayashi Y, Li Z‐G (1999) A new species of Ikechosaurus (Reptilia: Choristodera) from the Jiufutang Formation (Early Cretaceous) of Chifeng City, Inner Mongolia. Bull Inst R Sci Nat Belg Sci Terre 69(B Suppl.), 37–47. [Google Scholar]
- Mahler DL, Kearney M (2006) The palatal dentition in squamate reptiles: morphology, development, attachment, and replacement. Fieldiana Zool 108, 1–61. [Google Scholar]
- Matsumoto R (2011) The Palaeobiology of Choristodera (Reptilia: Diapsida). PhD Dissertation, University College London, UK, 437 pp. [Google Scholar]
- Matsumoto R, Evans SE (2010) Choristoderes and the freshwater assemblages of Laurasia. J Iberain Geol 36, 253–274. [Google Scholar]
- Matsumoto R, Evans SE, Manabe M (2007) The choristoderan reptile Monjurosuchus from the Early Cretaceous of Japan. Acta Palaeontol Pol 52, 329–350. [Google Scholar]
- Matsumoto R, Buffetaut E, Escuillie F, et al. (2013) New material of the choristodere Lazarussuchus (Diapsida, Choristodera) from the Paleocene of France. J Vertebr Paleontol 33, 319–339. [Google Scholar]
- Matsumoto R, Manabe M, Evans SE (2014) The first record of a long‐snouted choristodere (Reptilia, Diapsida) from the Early Cretaceous of Ishikawa Prefecture, Japan. Hist Biol 27, 583–594. [Google Scholar]
- Modesto SP, Sues H‐D (2004) The skull of the Early Triassic archosauromorph reptile Prolacerta broomi and its phylogenetic significance. Zool J Linn Soc 140, 335–351. [Google Scholar]
- Nichols DJ, Johnson KR (2008) Plants and the K‐T boundary, 280 pp. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Rieppel O, Reisz RR (1999) The origin and early evolution of turtles. Annu Rev Ecol Syst 30, 1–22. [Google Scholar]
- Russell LS (1956) The Cretaceous reptile Champsosaurus natator Parks. Bull Nat Mus Can 145, 1–51. [Google Scholar]
- Schwenk K (2000) Feeding in Lepidosaurs In: Feeding Form, Function and Evolution in Tetrapod Vertebrates. (ed. Schwenk K.), pp. 175–291. San Diego and London: Academic Press. [Google Scholar]
- Sigogneau‐Russell D (1979) Les Champsosaures europeens mise au point sur le Champsosaure d'Erquelinnes (landenien inferieur, Belgique). Ann Paléontol 65, 93–154. [Google Scholar]
- Sigogneau‐Russell D (1981) Presence d'un nouveau Champsosauride dans le Crétacé supérieur de Chine. C R Acad Sci Paris 292, 1–4. [Google Scholar]
- Sigogneau‐Russell D (1985) Definition of the type‐species of Simoedosaurus, S. lemoinei Gervais, 1877 (Choristodera, Reptilia). J Paleontol 59, 766–767. [Google Scholar]
- Sigogneau‐Russell D, Efimov MB (1984) Un Choristodera (Eosuchia?) insolite du Crétacé inférieur de Mongolie. Paläeontol Z 58, 279–294. [Google Scholar]
- Sigogneau‐Russell D, Russell DE (1978) Étude ostéologique du Reptile Simoedosaurus (Choristodera). Ann Paléontol (Vertébrés) 64, 1–84. [Google Scholar]
- Skutschas PP (2008) A choristoderan reptile from the Lower Cretaceous of Transbaikalia, Russia. Neues Jahrb Geol Palaontol‐Abh 247, 63–78. [Google Scholar]
- Trapani J (2003) Morphological variability in the Cuatro Cienegas cichlid, Cichlasoma minckleyi . J Fish Biol 62, 276–298. [Google Scholar]
- Trapani J, Yamamoto Y, Stock DW (2005) Ontogenetic transition from unicuspid to multicuspid oral dentition in a teleost fish: Astyanax mexicanus, the Mexican tetra (Ostariophysi: Characidae). Zool J Linn Soc 145, 523–538. [Google Scholar]
- Wang X, Miao D, Zhang Y (2005) Cannibalism in a semi‐aquatic reptile from the Early Cretaceous of China. Chiese Sci Bull 50, 281–283. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Summary of the palatal dentition in choristoderes.


