Abstract
One adaptation crucial to the survival of mammalian lineages that secondarily transitioned from land to water environments was the ability to capture and consume prey underwater. Phocid seals have evolved diverse feeding strategies to feed in the marine environment, and the objectives of this study were to document the specialized feeding morphologies and identify feeding strategies used by extant phocids. This study used principal component analysis (PCA) to determine the major axes of diversification in the skull for all extant phocid taxa and the recently extinct Caribbean monk seal (n = 19). Prey data gathered from the literature and musculoskeletal data from dissections were included to provide a comprehensive description of each feeding strategy. Random Forest analysis was used to determine the morphological, ecological and phylogenetic variables that best described each feeding strategy. There is morphological evidence for four feeding strategies in phocids: filter; grip and tear; suction; and pierce feeding. These feeding strategies are supported by quantitative cranial and mandibular characters, dietary information, musculoskeletal data and, for some species, behavioral observations. Most phocid species are pierce feeders, using a combination of biting and suction to opportunistically catch prey. Grip and tear and filter feeding are specialized strategies with specific morphological adaptations. These unique adaptations have allowed leopard seals (Hydrurga leptonyx) and crabeater seals (Lobodon carcinophaga) to exploit novel ecological niches and prey types. This study provides the first cranial and mandibular morphological evidence for the use of specialized suction feeding in hooded seals (Cystophora cristata), northern elephant seals (Mirounga angustirostris) and southern elephant seals (Mirounga leonina). The most important variables in determining the feeding strategy of a given phocid species were cranial and mandibular shape, diet, and phylogeny. These results provide a framework for understanding the evolution and adaptability of feeding strategies employed by extant phocid species, and these findings can be applied to other pinniped lineages and extinct taxa.
Keywords: adaptation, diet, evolution, feeding, morphology, Phocidae, Pinnipedia, skull
Introduction
Multiple, independently evolving mammalian lineages have secondarily adapted aquatic lifestyles, including cetaceans (whales), sirenians (manatees and dugongs) and pinnipeds (seals, sea lions and walruses). During the transition from terrestrial to aquatic habitats, marine mammals encountered new physiological demands, and one adaptation crucial to the survival of these marine mammal lineages was the ability to capture and consume prey in the marine environment. Mammals foraging in water must cope with increases in the density, pressure and viscosity in the aquatic environment and, depending on the depth, circumvent the decrease in visibility. These environmental pressures have resulted in ecological and morphological specializations related to food processing, locomotion and prey capture (Taylor, 1987; Uhen, 2007).
Unlike cetaceans and sirenians, pinnipeds have retained a dual terrestrial–aquatic lifestyle, spending portions of their life cycle on land to thermoregulate, breed and molt. Foraging, however, occurs entirely underwater, and as a result pinnipeds have evolved a diverse suite of sensory system adaptations specialized for seeking out prey, including eyesight (Levenson & Schusterman, 1999; Hanke et al. 2009; Berg et al. 2012), hearing (Kastak & Schusterman, 1998; Reichmuth et al. 2013) and specialized vibrissae (Dehnhardt et al. 2001; Ginter et al. 2012). However, pinnipeds rely on many of these same sensory systems to function on land. Pinnipeds are therefore an interesting comparative system for investigating feeding adaptations at the land–sea interface.
Direct studies of pinniped feeding are logistically difficult to obtain because many species are protected, forage at extreme depths and/or live in remote habitats. Therefore, most of the knowledge about pinniped feeding strategies stems from indirect evidence, including morphological studies (Adam & Berta, 2002; Jones & Goswami, 2010; Jones et al. 2013; Churchill & Clementz, 2015) and experiments on captive animals (Marshall et al. 2008, 2014; Hocking et al. 2013, 2014). Morphological studies are ideal for broad comparative analyses of feeding. For example, Debey et al. (2013) demonstrated that pinnipeds have proportionally larger orbit sizes than their closest terrestrial relatives and, for most species, orbit size was positively correlated with diving depth. In addition, recent morphological studies have suggested that pinnipeds have evolved specialized morphologies for underwater feeding (Adam & Berta, 2002; Jones & Goswami, 2010; Jones et al. 2013; Churchill & Clementz, 2015).
Based on qualitative descriptions of skull and dental characters, Adam & Berta (2002) proposed four feeding strategies for pinnipeds: filter feeding; grip and tear feeding; pierce feeding; and suction feeding. Each of these strategies was associated with distinct cranial, mandibular and dental morphologies. Phocid seals were the only pinniped lineage found to use all four feeding strategies (Adam & Berta, 2002; Table 1). Phocids are also the most speciose pinniped lineage with high ecological and morphological diversity. For these reasons, phocids are a model group for studying interspecific variation in feeding morphologies within pinnipeds.
Table 1.
Diet, foraging strategy and species information for each species
| Species | Common name | Total | Male | Female | Feeding strategya | Dietb |
|---|---|---|---|---|---|---|
| n | N | n | ||||
| Cystophora cristata | Hooded seal | 16 | 9 | 7 | Pierce | Malacostracans |
| Erignathus barbatus | Bearded seal | 16 | 2 | 2 | Suction | Actinopterygians/malacostracans |
| Halichoerus grypus | Gray seal | 16 | 5 | 3 | Pierce | Actinopterygians |
| Histriophoca fasciata | Ribbon seal | 8 | – | – | Pierce | Actinopterygians |
| Hydrurga leptonyx | Leopard seal | 13 | 2 | 4 | Filter/grip and tear | Aves/malacostracans |
| Leptonychotes weddellii | Weddell seal | 12 | 2 | 4 | Pierce | Actinopterygians |
| Lobodon carcinophaga | Crabeater seal | 17 | 3 | 7 | Filter | Malacostracans |
| Mirounga angustirostris | Northern elephant seal | 28 | 14 | 11 | Pierce | Cephalopods |
| Mirounga leonina | Southern elephant seal | 2 | – | 1 | Pierce | Cephalopods |
| Monachus monachus | Mediterranean monk seal | 2 | 1 | – | Pierce | Actinopterygians |
| Neomonachus schauinslandi | Hawaiian monk seal | 15 | 6 | 3 | Pierce | Actinopterygians |
| Neomonachus tropicalis | Caribbean monk seal | 15 | 5 | 10 | Pierce | – |
| Ommatophoca rossii | Ross seal | 5 | 2 | 1 | Pierce | Cephalopods |
| Pagophilus groenlandicus | Harp seal | 15 | 4 | 5 | Pierce | Actinopterygians |
| Phoca largha | Spotted seal | 1 | 1 | – | Pierce | Actinopterygians |
| Phoca vitulina | Harbor seal | 14 | 8 | 4 | Pierce | Actinopterygians |
| Pusa caspica | Caspian seal | 3 | 3 | – | Pierce | Actinopterygians |
| Pusa hispida | Ringed seal | 15 | 5 | 4 | Pierce | Actinopterygians/malacostracans |
| Pusa sibirica | Baikal seal | 7 | – | 4 | Pierce | Actinopterygians |
The objectives of this study were to document the specialized feeding morphologies and identify the feeding strategies used by extant phocids. PCA was used to determine the major axes of diversification in skull morphology for all extant phocid taxa. Prey data gathered from the literature and musculoskeletal data from dissections were included to provide a comprehensive description of each feeding strategy. Random Forest analysis was then used to determine the morphological, ecological and phylogenetic variables that best described each feeding strategy.
Materials and methods
Specimens
Cranial and mandible data were collected from the skulls of 234 specimens representing all 18 extant phocid species. The recently extinct Caribbean monk seal (Neomonachus tropicalis) (Scheel et al. 2014; IUCN, 2015) was also included for a total of 19 species. Specimens were measured from the mammal collections at the Natural History Museum of Los Angeles County, Los Angeles, California (LACM), the National Museum of Natural History, Washington, DC (NMNH), and the San Diego Natural History Museum, San Diego, California (SDMNH). Adults were preferentially sampled whenever possible to avoid age‐dependent behavioral and morphological shifts in feeding strategy (Le Boeuf & Laws, 1994; Goodman‐Lowe, 1998). Approximate age was primarily identified based on the original age data determined during specimen collection. Younger specimens without original collection data were identified based on the presence of significantly open sutures, as open sutures in phocids close after weaning and before sexual maturity (Doutt, 1942; Sivertsen, 1954; Jones & Goswami, 2010). All animals without data available and closed cranial sutures were conservatively assigned as of unknown age (Jones & Goswami, 2010). Sex was known for most specimens, and every attempt was made to include equal numbers of males and females. The final sex distribution was 33% male (n = 72), 32% female (n = 70) and 35% unsexed (n = 78; Table 1). Museum accession numbers, collection localities, sex and age are listed in the supporting information (Table S1).
Data collection
Three‐dimensional (3D) measurements were taken using a Microscribe G2 digitizer with 0.2 mm accuracy. Qualitative characters from Adam & Berta (2002) were transformed into quantitative measurements when possible. Additional landmarks were selected based on homology across all specimens, repeatability, and potential role in feeding (Adam & Berta, 2002; Jones & Goswami, 2010; Jones et al. 2013). Forty‐six cranial landmarks were measured on each specimen (Table 2; Fig. 1), and 22 mandible landmarks were measured for each specimen (Table 3; Fig. 2). Measurements were repeated three times and averaged. Non‐shape information (e.g. size, translation and rotation) was removed from the landmark configurations using a full Procrustes fit implemented in morphoj v. 1.05d (Klingenberg, 2011).
Table 2.
Cranial landmarks measured on each specimen
| # | Description |
|---|---|
| 1 | Rostral‐most tip of rostrum |
| 2 | Caudal margin of occipital condyle |
| 3 | Caudal‐most point of palate midline |
| 4 | Rostral‐most point of pterygoid at suture of pterygoid and palatine |
| 5 | Caudal‐most suture of palatine, pterygoid and alisphenoid |
| 6 | Suture of maxilla and palatine (left) |
| 7 | Suture of maxilla and palatine (right) |
| 8 | Caudal edge of last postcanine tooth (left) |
| 9 | Caudal edge of last postcanine tooth (right) |
| 10 | Junction between L8 and L9 at palate midline |
| 11 | Caudal edge of second postcanine tooth (left) |
| 12 | Caudal edge of second postcanine tooth (right) |
| 13 | Junction between L11 and L12 at palate midline |
| 14 | Rostral edge of canine along lingual palatine border (left) |
| 15 | Rostral edge of canine along lingual palatine border (right) |
| 16 | Junction between L14 and L15 at palate midline |
| 17 | Caudal‐most edge of first postcanine (left) |
| 18 | Palate midline medial to L17 |
| 19 | Junction of nasal, frontal and maxillaa |
| 20 | Dorsal‐most junction of jugal and squamosal (right) |
| 21 | Rostral‐most point at parietal and squamosal suture |
| 22 | Dorsal‐ and caudal‐most suture of jugal and maxilla |
| 23 | Rostral suture of premaxilla and maxilla (left) |
| 24 | Rostral suture of premaxilla and maxilla (right) |
| 25 | Dorsal junction of jugal and maxilla (left) |
| 26 | Dorsal junction of jugal and maxilla (right) |
| 27 | Dorsal‐most junction of jugal and squamosal (left) |
| 28 | Lateral‐most point of mastoid process (left) |
| 29 | Lateral‐most point of mastoid process (right) |
| 30 | Maximum breadth on lateral margin of occipital condyle (left) |
| 31 | Maximum breadth on lateral margin of occipital condyle (right) |
| 32 | Lateral‐most tip of external auditory meatus |
| 33 | Medial‐most point of auditory bulla at suture of basioccipital and basisphenoid |
| 34 | Rostral‐most tip of auditory bulla |
| 35 | Caudal‐most tip of auditory bulla |
| 36 | Caudal‐most point of infraorbital foramena |
| 37 | Suture of premaxilla and maxilla rostral to caninea |
| 38 | Caudal‐most point of infraorbital foramena |
| 39 | Medial‐most point of infraorbital foramena |
| 40 | Lateral‐most point of infraorbital foramena |
| 41 | Rostral‐most suture of parietal and squamosal (left)a |
| 42 | Caudal‐most suture of parietal, squamosal and supraoccipital (left)a |
| 43 | Rostral‐most projection of parietal (left)a |
| 44 | Caudal‐most projection of parietal and supraoccipital (left)a |
| 45 | Rostral suture of parietal and frontal on dorsal midline (left)a |
| 46 | Caudal suture of parietal and supraoccipital on dorsal midline (left)a |
All measurements from the ventral side of the skull, except where marked with an asterisk.
Figure 1.

Three‐dimensional cranial landmarks (n = 46) measured on each specimen and included in the analyses, shown on a ringed seal skull. Numbers correspond with landmarks listed in Table 2. (A) Ventral view of cranial landmarks. (B) Lateral view of cranial landmarks.
Table 3.
Mandibular landmarks measured on each specimen
| # | Description |
|---|---|
| 1 | Rostral‐most tip of mandible |
| 2 | Caudal‐most point of mandibular condyle |
| 3 | Lateral edge of canine midpoint |
| 4 | Medial edge of mandibular symphysis caudal to the first incisor |
| 5 | Ventral edge of mandible underneath the canine |
| 6 | Lateral and caudal edge of last postcanine |
| 7 | Ventral edge of mandible underneath the last postcanine |
| 8 | Lateral point at middle of M1 |
| 9 | Lateral‐most point of mandibular condyle |
| 10 | Medial‐most point of mandibular condyle |
| 11 | Ventral edge of mandible underneath the tip of the coronoid process |
| 12 | Dorsal‐most point of coronoid process |
| 13 | Rostral‐most point of coronoid process |
| 14 | Caudal‐most point of coronoid process |
| 15 | Rostral‐most point on curving edge of coronoid process |
| 16 | Caudal‐most point on curving edge of coronoid process |
| 17 | Ventral and lateral edge of canine |
| 18 | Dorsal‐most tip of canine |
| 19 | Ventral edge of mandible underneath the start of the coronoid process |
| 20 | Caudal‐most point of angular process |
| 21 | Rostral edge of first incisor |
| 22 | Caudal edge of last postcanine |
Figure 2.
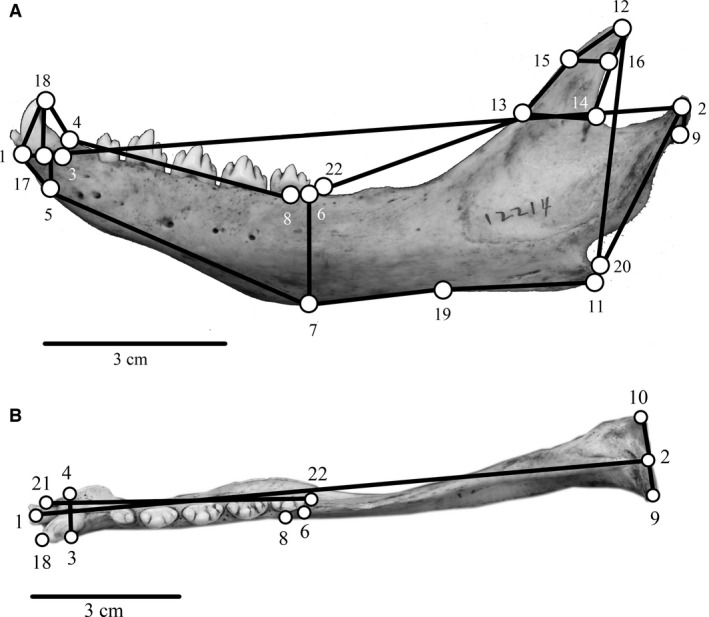
Three‐dimensional mandible landmarks (n = 22) measured on each specimen and included in the analyses, shown on a ringed seal mandible. Numbers correspond with landmarks listed in Table 3. (A) Lateral view of mandible landmarks. (B) Dorsal view of mandible landmarks.
Statistical analyses
An unsupervised Random Forest analysis conducted in r v. 2.51.1 (R Developmental Core Team, 2008) was used to determine the natural clustering of specimens in the dataset. Unsupervised Random Forest is an unbiased membership test because it does not include a priori information on groups (Breiman, 2001; Dinsdale et al. 2013). For each specimen, a combination of cranial and mandibular variables and phylogenetic affiliations was included (Table 1; Fig. S1). Based on these data, a normalized proximity measure for each specimen was generated to create a dissimilarity measure (1 − proximity) for partitioning around medoids (PAM), where a medoid is an object that minimizes the dissimilarity or distances between clusters (Dinsdale et al. 2013; Marden, 2015). PAM was then used to determine the appropriate number of clusters present in the dataset. The dissimilarity matrix was visualized using a multidimensional scaling plot, which allowed the determination of group membership for each specimen.
Because of the large morphological dataset, principal component analysis (PCA) was used to reduce the dimensionality of the dataset and describe the major axes of variation (Wiley, 1981; Weinberg et al. 2009; Klingenberg, 2011). PCA was first used to investigate intraspecific variation in the dataset related to sex, with the exception of species with small sample sizes (Table 1). As individuals within a species were more similar to each other in morphospace than to other species, all specimens, regardless of sex, were used for the remainder of the analyses. Two datasets were analyzed using PCA: (i) a cranial dataset including 19 species, 211 specimens and 46 landmarks; and (ii) a mandible dataset including 19 species, 220 specimens and 22 landmarks. All analyses were conducted in MorphoJ (Klingenberg, 2011).
Canonical variate analysis (CVA) and a supervised Random Forest analysis were used to examine the separation of species into feeding strategies based on the results of the unsupervised Random Forest analysis and PCA. CVA uses an ordination method to maximize the separation of user‐input groups defined a priori (Wiley, 1981). Each species was assigned to a feeding strategy based on the unsupervised Random Forest and PCA results, and CVA was performed separately on the cranial and mandibular 3D landmark data to test the feeding strategy groupings. A permutation test of pairwise distances between groups was performed with 10 000 iterations per comparison. These analyses were conducted in morphoj (Klingenberg, 2011).
A supervised Random Forest model implemented in r v. 2.51.1 (R Developmental Core Team, 2008) was used to determine the variables that best discriminated between feeding strategies. Like CVA, the supervised Random Forest requires the assignment of a priori groups, and these groups were determined from the results of the unsupervised Random Forest, PCAs and CVAs. Trees were then generated to classify the groups based on user‐input variables. The mean decreasing accuracy, which is a variable importance measure, was estimated and ranked how much each variable contributed to discriminating between the a priori groups (Dinsdale et al. 2013). The mean decreasing accuracy determines how much the accuracy of the Random Forest changes due to changes in each variable; the more the accuracy decreases by changing a given variable, the higher that variable is ranked in importance (Dinsdale et al. 2013). The variables included for each specimen in the supervised Random Forest model were cranial PC scores, mandible PC scores, feeding strategy assignment, dominant prey type and phylogenetic affiliations (e.g. genera, subfamily). The analysis produces an out‐of‐bag error for the model. The out‐of‐bag error is estimated by removing a third of the classifier data during each set of bootstraps to estimate error rates, which is repeated until the test set error converges. Out‐of‐bag error is useful as an unbiased estimate of test set error, as well as for assessing classifier strength and the dependency of the model (Breiman, 2001).
Phylogenetic signal
Closely related taxa are expected to be more similar to each other due to shared evolutionary history. Because species are related to each other and part of a ranked and structured phylogeny, they cannot be treated as if they are each independent samples drawn from the same distribution in statistical analyses (Felsenstein, 1985). Phylogenetic signal is a way to measure the statistical effect of phylogenetic relationships on trait values (Revell et al. 2008). To test the degree of phylogenetic signal present in the dataset, two distance matrices were constructed and statistically compared for the degree of correlation. The first matrix was a patristic distance matrix, where the patristic distance is the sum of branch lengths between two nodes in a phylogenetic tree, and was produced from a time‐calibrated molecular phylogeny modified from Fulton & Strobeck (2010) in mesquite v. 2.75 (Maddison & Maddison, 2011; Fig. S1). The second matrix was a shape distance matrix and was generated based on Euclidean distances between pairs of species for each significant PC (i.e. cranial PCs 1–6, mandible PCs 1–4) from MorphoJ (Klingenberg, 2011). The two matrices were compared using a Spearman's rank correlation analysis and a non‐parametric Mantel test.
Results
Unsupervised Random Forest analysis
The PAM matrix generated in the unsupervised Random Forest analysis predicted the existence of between four and eight naturally occurring groups based on the combination of cranial and mandibular morphologies and phylogenetic affiliations (Table S2; Fig. S2). The following species were each consistently clustered together across all unsupervised Random Forest analyses: (i) northern and southern elephant seals; (ii) Caribbean monk seals, Hawaiian monk seals and Mediterranean monk seals; (iii) Baikal seals, Caspian seals, harbor seals, harp seals, ribbon seals, ringed seals and Ross seals; and (iv) crabeater seals and leopard seals. Bearded seals and hooded seals were most often grouped with northern and southern elephant seals and/or each other or were assigned their own unique feeding categories. The rest of the species (i.e. grey seals, Weddell seals and spotted seals) were not consistently assigned to a single group, as they shared characteristics of multiple groups.
PCA: cranium
The cranial PCA generated 138 principal axes of variation, and PCs 1–6 combined explained 68.1% of the variation (Table S3). PC1 is often associated with size, indicated by nearly equal PC coefficients with the same sign (Wiley, 1981); however, that was not the case in this analysis as the PC coefficients were non‐equal with both positive and negative values (Table S3). PC1 represented the contrast between skull length (rostro‐caudally) and skull width (medio‐laterally; Table S3). Species at the positive end of PC1 (e.g. crabeater and leopard seals) had shorter (rostro‐caudally) caudal portions of the skull, wider (medio‐laterally) rostra, larger orbits and flatter (dorso‐ventrally) palates, while species at the negative end (e.g. hooded seals, northern and southern elephant seals) had longer skulls, pointed rostra and sloped palates. PC2 represented changes in skull thickness (dorso‐ventrally) and parietal bone shape (Table S3). Species with positive PC2 scores (e.g. crabeater and leopard seals) had wide and thick skulls, round rostra, enlarged auditory bullae, and larger parietal bones (rostro‐caudally and dorso‐ventrally), whereas species with negative PC2 scores (e.g. hooded and bearded seals) had narrower (medio‐laterally) caudal portions of the skull with smaller parietal bones, longer palates and pterygoid hamuli (rostro‐caudally), and smaller auditory bullae. PC3 represented the contrast between rostral elongation and skull thickness, and showed a significant relationship between phylogeny and shape (P = 0.02). Monachines (southern seals) had the most positive PC3 values, and phocines (northern seals) had the most negative PC3 values. Most monachines had longer (rostro‐caudally) rostra, orbits and palates, and a thicker (dorso‐ventrally) skull, while most phocines had shorter and more compressed skulls and palates. PC4, which represented changes in the zygomatic region, parietal bones and pterygoid hamuli, also showed a significant relationship between phylogeny and shape (P = 0.01). Most phocines, with the exception of hooded seals, had positive PC4 values, which were represented by a wider (medio‐laterally) zygomatic region, thicker (dorso‐ventrally) parietal bones and more prominent pterygoid hamuli (dorso‐ventrally). Monachines tended to have more negative PC4 scores (with the exception of the Caribbean and Hawaiian monk seals), which were characterized by longer (rostro‐caudally) parietal bones, less pronounced pterygoid hamuli and more rostrally placed orbits. Because of the confounding influence of phylogeny on PC3 and PC4, these PCs were not used as the main determinants of the morphological specializations for feeding. However, these PCs were useful in helping to discern patterns in intraspecific variation and interspecific overlap. PC5 was characterized by changes in the shapes of the palate and parietal bones (Table S3). Species with positive PC5 values (e.g. crabeater seals, Caribbean and Mediterranean monk seals) had short (rostro‐caudally) and wide (medio‐laterally) palates and smaller parietal bones, while species with negative PC5 values (e.g. ribbon, leopard and Ross seals) had long, narrow palates and large parietal bones. PC6 represented the contrast between skull width (medio‐laterally) and skull thickness (dorso‐ventrally; Table S3). Species with positive PC6 values (e.g. Caribbean and Hawaiian monk seals) had narrow (medio‐laterally) skulls in the orbit and zygomatic arch region, and had an overall thicker (dorso‐ventrally) skull, while species with negative PC6 values (e.g. bearded seals, northern and southern elephant seals) had wider and more compressed skulls.
Leopard seals and crabeater seals consistently occupied unique regions of cranial morphospace separate from most other phocids, and were most often found in close proximity to each other (Figs 3 and 4). The results of this cranial PCA were concordant with the unsupervised Random Forest analysis where leopard seals and crabeater seals were consistently placed into their own groups, either with each other or individually (Table S2; Fig. S2). Both leopard seals and crabeater seals shared similar cranial characters, including large orbits, flat (dorso‐ventrally) palates and wide (medio‐laterally) skulls. Leopard seals had the most extreme PC2 scores of all phocids and were characterized by having longer (rostro‐caudally) and wider (dorso‐ventrally) parietal bones and enlarged auditory bulla compared with all other phocids (Figs 3 and 4).
Figure 3.

Cranial PCA results comparing PC1 and PC2. Each shape represents a species, and each point represents a specimen. Ovals represent 90% confidence intervals for each feeding strategy: grip and tear feeders (dotted line); filter feeders (thin solid line); suction feeders (dashed line); pierce feeders (thick solid line). Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Figure 4.
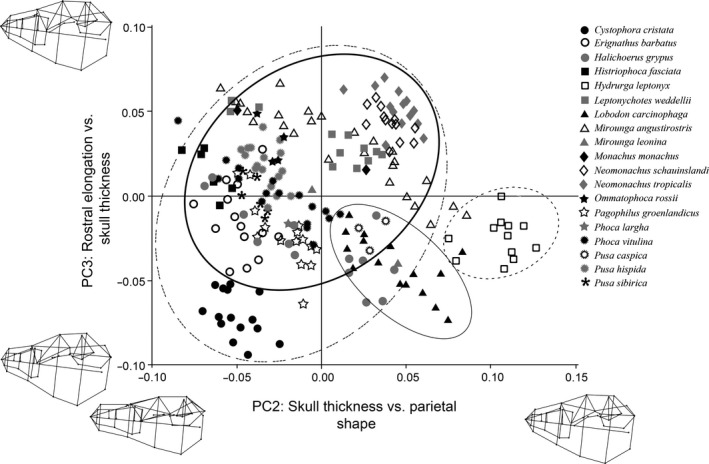
Cranial PCA results comparing PC2 and PC3. Each shape represents a species, and each point represents a specimen. Ovals represent 90% confidence intervals for each feeding strategy: grip and tear feeders (dotted line); filter feeders (thin solid line); suction feeders (dashed line); pierce feeders (thick solid line). Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Northern and southern elephant seals were most closely associated with each other (Figs 3 and 4), and often occupied overlapping regions of cranial morphospace with bearded seals and hooded seals (Fig. 3). This finding is similar to the results of the unsupervised Random Forest analyses; northern and southern elephant seals were always placed into the same group, and bearded seals and hooded seals were most often associated with elephant seals and/or with each other (Table S2; Fig. S2). Cranial characters shared among all four species were associated with negative PC1 scores, and included having long (rostro‐caudally) skulls, pointed rostra, sloped palates and long (rostro‐caudally) pterygoid hamuli. Northern and southern elephant seals showed a high degree of intraspecific variability (Figs 3 and 4) but, in general, showed increases in skull width (medio‐laterally), orbit size and palate width (medio‐laterally)/depth (dorso‐ventrally) that led to their separation from most other phocids. Bearded seals and hooded seals showed a high degree of overlap in cranial morphospace due to similar PC1 and PC2 scores (Figs 3 and 4). Cranial characters associated with their overlap included having wide (medio‐laterally), long (rostro‐caudally) and sloped palates, overall skull thickening (dorso‐ventrally) and long (rostro‐caudally) pterygoid hamuli.
Caribbean, Hawaiian and Mediterranean monk seals consistently grouped together in cranial morphospace (Figs 3 and 4), which matched the predictions of the unsupervised Random Forest analyses (Table S2; Fig. S2). Cranial characters associated with the clustering of monk were the increased width (medio‐laterally) of the zygomatic region, skull thickening (dorso‐ventrally) and an elongated (rostro‐caudally) rostrum. Unlike the aforementioned species, monk seals did not exhibit extremely positive or negative PC scores for any cranial characters, and were frequently found in close proximity to other phocid species (e.g. Caspian, elephant and Weddell seals) in cranial morphospace (Figs 3 and 4).
Most other phocids, specifically Baikal seals, Caspian seals, spotted seals, harbor seals, harp seals, ribbon seals, ringed seals and Ross seals, formed their own overlapping group in cranial morphospace (Figs 3 and 4). These results are consistent with the predictions of the unsupervised Random Forest analyses, which consistently placed these species into the same group across all iterations of the model (Table S2; Fig. S2). Cranial characters associated with this grouping were short (rostro‐caudally) and narrow (medio‐laterally) caudal portions of the skull, wide (medio‐laterally) rostra, thickened (dorso‐ventrally) palates, smaller parietal bones and smaller auditory bullae. Similar to the monk seals, most of these species did not exhibit extremely positive or negative PC scores for any cranial characters.
The two remaining phocids, Weddell seals and grey seals, were not consistently associated with any one species in cranial morphospace (Figs 3 and 4). This also matches the results of the unsupervised Random Forest, as these two species were not assigned to one particular group – rather they shared characteristics with multiple groups (Table S2). Weddell seals shared some cranial characteristics with crabeater seals (Fig. 3), elephant seals (Fig. 4) and Mediterranean monk seals (Fig. 4). Grey seals showed a high degree of intraspecific variability in cranial shape (Figs 3 and 4), resulting in their non‐specific overlap with most other species (Figs 3 and 4).
PCA: mandible
The mandible PCA generated 66 axes of variation, and the first four PCs combined explained 71.6% of the observed variation (Table S4). There were no significant correlations between phylogeny and mandible shape. Similar to the cranial analysis, PC1 was not associated with size (based on unequal PC coefficients with positive and negative values); rather PC1 represented the contrast between the shape of the mandible and mandibular symphysis/toothrow (Table S4). Species with positive PC1 values (e.g. Baikal seals, crabeater seals and Hawaiian monk seals) had thicker (dorso‐ventrally) mandibles with large coronoid processes and short (rostro‐caudally) toothrows, while species with negative PC1 values (e.g. hooded, grey and elephant seals) had thin (dorso‐ventrally) mandibles with small coronoid processes and long toothrows. PC2 represented changes in dorsal and ventral mandible shape (Table S4). Species with positive PC2 values (e.g. hooded and ribbon seals) had long (rostro‐caudally) coronoid processes and thick (dorso‐ventrally) toothrows, while species with negative PC2 values (e.g. leopard and monk seals) had thick (dorso‐ventrally) mandibles caudal of the toothrow and thin, narrow (rostro‐caudally) coronoid processes. PC3 represented the changes in coronoid process shape and size (Table S4). Species with positive PC3 values (e.g. grey and monk seals) had short (dorso‐ventrally) coronoid processes with thick (dorso‐ventrally) mandibles, while species with negative PC3 values (e.g. leopard and crabeater seals) had tall (dorso‐ventrally) and wide (rostro‐caudally) coronoid processes and thin (dorso‐ventrally) mandibles. PC4 was characterized by changes in the mandible thickness and mandible condyle size (Table S4). Species with positive PC4 values (e.g. Weddell and elephant seals) had thick caudal portions of the mandible (dorso‐ventrally) and wide mandibular condyles (medio‐laterally), while species with negative PC4 scores (e.g. hooded and bearded seals) had long coronoid processes (rostro‐caudally) and thin mandibular condyles (medio‐laterally).
Crabeater seals consistently occupied the most distinct region of mandibular morphospace, separate from all other species (Figs 5 and 6). Mandible characters associated with the separation of crabeater seals from other phocids included having an elongated (rostro‐caudally) toothrow, a wide (medio‐laterally) mandibular symphysis and a thick (dorso‐ventrally) mandible. Unlike the cranial PCA, there was no overlap between leopard seals and crabeater seals in mandibular morphospace. These results are similar to the predictions of the unsupervised Random Forest analyses, which either grouped crabeater seals with leopard seals or separated the two species into their own individual groups (Table S2; Fig. S2). Both leopard seals and crabeater seals had similar PC3 scores (Figs 5 and 6), which were related to having a tall (dorso‐ventrally) and wide (rostro‐caudally) coronoid process. Leopard seals did not occupy as distinct a region of mandibular morphospace as in the cranial PCA; rather, leopard seals were found overlapping with the Caribbean and Hawaiian monk seals (Fig. 5), grey seals (Fig. 5), and bearded seals (Fig. 6). Mandibular characters unique to leopard seals included a shortening (rostro‐caudally) of the mandible, an increase in mandible thickness (medio‐laterally) and a wide (rostro‐caudally) coronoid process.
Figure 5.

Mandibular PCA results comparing PC1 and PC2. Each shape represents a species, and each point represents a specimen. Ovals represent 90% confidence intervals for each feeding strategy: grip and tear feeders (dotted line); filter feeders (thin solid line); suction feeders (dashed line); pierce feeders (thick solid line). Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Figure 6.

Mandibular PCA results comparing PC1 and PC3. Each shape represents a species, and each point represents a specimen. Ovals represent 90% confidence intervals for each feeding strategy: grip and tear feeders (dotted line); filter feeders (thin solid line); suction feeders (dashed line); pierce feeders (thick solid line). Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Unlike the unsupervised Random Forest and cranial PCA, the bearded seal did not occupy a separate region of mandible morphospace; instead, the bearded seal showed a high degree of interspecific overlap and no distinguishing mandible characters (Figs 5 and 6). Bearded seals did not closely associate with any particular species. However, similar to the unsupervised Random Forest and cranial PCA, hooded seals and elephant seals occupied similar regions of mandibular morphospace (Fig. 6). Characters associated with the separation of hooded seals from other phocids included a tall (dorso‐ventrally) and curved coronoid process, a thin elongated (rostro‐caudally) mandible, a long, thin (rostro‐caudally) toothrow, and an arched caudal edge of the mandible. Northern and southern elephant seals were distinct in mandibular morphospace with a wide (medio‐laterally), short (rostro‐caudally) mandible, a wide and thick coronoid process, an enlarged mandibular condyle width (medio‐laterally), and close proximity of the condyles to the coronoid process. Compared with the cranial PCA, there was more overlap between hooded seals, bearded seals and elephant seals with other phocids (Figs 5 and 6).
Similar to the unsupervised Random Forest analyses and cranial PCA, Caribbean, Hawaiian and Mediterranean monk seals were often closely associated in mandibular morphospace (Figs 5 and 6; Table S2; Fig. S2). Compared with the cranial PCA, there was a much greater degree of overlap between monk seals and other phocids in mandibular morphospace (Figs 5 and 6). The only PC that separated monk seals from other phocids was PC2, and was related to having a mandible that was thicker (dorso‐ventrally) caudal of the toothrow and a thin, narrow (rostro‐caudally) coronoid process.
The majority of phocids (i.e. Baikal seals, Caspian seals, spotted seals, harbor seals, harp seals, ribbon seals, ringed seals and Ross seals) grouped together in mandibular morphospace. This grouping was predicted by the unsupervised Random Forest and was consistent with the cranial PCA results (Figs 5 and 6; Table S2; Fig. S2). These species showed a high degree of interspecific overlap and occupied a large portion of mandibular morphospace (Figs 5 and 6). Mandibular characteristics of these species include having shorter (dorso‐ventrally) coronoid process with a thick (dorso‐ventrally) mandible.
Similar to the unsupervised Random Forest analysis and cranial PCA, Weddell seals and grey seals were not consistently associated with any particular species in mandibular morphospace (Figs 5 and 6). Weddell seals were associated with multiple species, including monk seals (Fig. 5), leopard seals (Fig. 5), Baikal seals (Fig. 6) and ringed seals (Fig. 6). Similar to the cranial PCA, grey seals showed a high degree of intraspecific variation, overlapping with multiple species, including elephant seals (Fig. 5), harbor seals (Fig. 5), ribbon seals (Fig. 6) and ringed seals (Fig. 6).
CVA
For the cranial and mandibular CVA, phocids were conservatively classified into four feeding strategies based on the results of the unsupervised Random Forest analysis and cranial and mandibular PCAs. Leopard seals were classified as grip and tear feeders. Crabeater seals were categorized as filter feeders. Hooded seals, bearded seals, northern and southern elephant seals were categorized as suction feeders. All other phocids were classified as pierce feeders.
Three canonical variates explained 100% of the cranial variation, and the results generated from the permutation tests for the Mahalanobis distances among groups were all significant (P < 0.01; Table S5). CV1 explained 54.3% of the variation, and separated the suction feeders from the grip and tear and filter feeders (Fig. 7). Positive CV1 loadings were related to an increased palate width (medio‐laterally) and an arched palate, exemplified by suction feeding phocids (Table S6). Negative CV1 loadings were associated with an increased palate length (rostro‐caudally), a flat palate and increased orbit width, which were exemplified by filter and grip and tear feeders (Table S6). CV2 separated the pierce feeders from the filter feeders (33.5% of the variation; Figs 7 and 8). Pierce feeders had positive loadings for CV2, which were associated with an increased length of the orbit and pterygoid bones (Table S6). Filter feeders had the most negative CV2 loadings, which were related to a narrow rostrum anterior of the zygomatic arch (Table S6). CV3 separated filter feeders from grip and tear feeders, explaining 12.3% of the variation (Fig. 8). Filter feeders had positive CV3 loadings, which were characterized by having a flattened (dorso‐ventrally) and short (rostro‐caudally) palate. Grip and tear feeders had the most negative CV3 loadings, which were associated with having an elongated rostrum (Table S6). The four feeding strategies formed significant, non‐overlapping clusters in all of the cranial CVA plots (Figs 7 and 8).
Figure 7.

Cranial CVA results comparing CV1 and CV2. Each shape represents a feeding strategy, and each point represents a specimen. Ovals represent 95% confidence intervals for each feeding strategy. Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Figure 8.
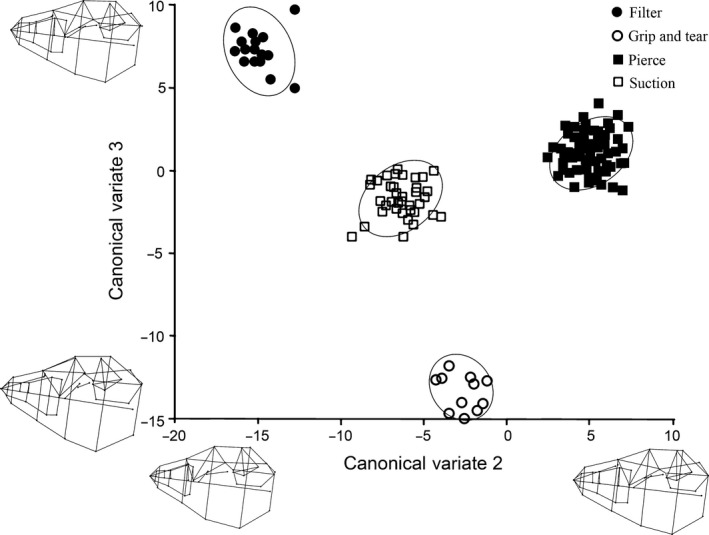
Cranial CVA results comparing CV2 and CV3. Each shape represents a feeding strategy, and each point represents a specimen. Ovals represent 95% confidence intervals for each feeding strategy. Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Three canonical variates explained 100% of the mandibular variation, and the results from the permutation tests for the Mahalanobis distances among groups were all significant (P < 0.01; Table S7). CV1 was associated with the separation of pierce feeders from grip and tear feeders (47% of the variation; Fig. 9). Positive CV1 loadings were associated with having a long (rostro‐caudally) toothrow, exemplified by generalist feeders, while negative CV1 loadings were associated with a short mandible, exemplified by grip and tear feeders (Table S8). CV2 was related to the separation of suction feeders from filter feeders (39.4% of the variation; Figs 9 and 10). Positive CV2 scores were related to elongate (rostro‐caudally) and thin mandibles, characterized by suction feeders, while negative CV2 loadings were associated with filter feeders, which had short, thick mandibles (Table S8). CV3 separated the grip and tear feeders from filter feeders (13.7% of the variation; Fig. 10). Grip and tear feeders had positive CV3 scores, which were related to an increased caudal portion of the mandible. Filter feeders had negative CV3 loadings, which were associated with a short (rostro‐caudally) and thick (dorso‐ventrally) mandibular symphysis (Table S8). The filter feeders and grip and tear feeders consistently separated out from the pierce and suction feeders (Figs 9 and 10). There was more overlap between the pierce and suction feeders in the mandible CVA than in the cranial CVA (Figs 9 and 10).
Figure 9.
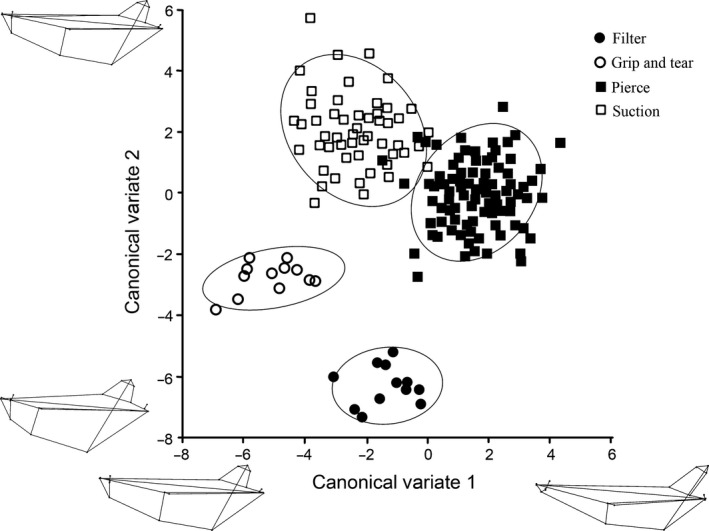
Mandibular CVA results comparing CV1 and CV2. Each shape represents a feeding strategy, and each point represents a specimen. Ovals represent 95% confidence intervals for each feeding strategy. Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Figure 10.
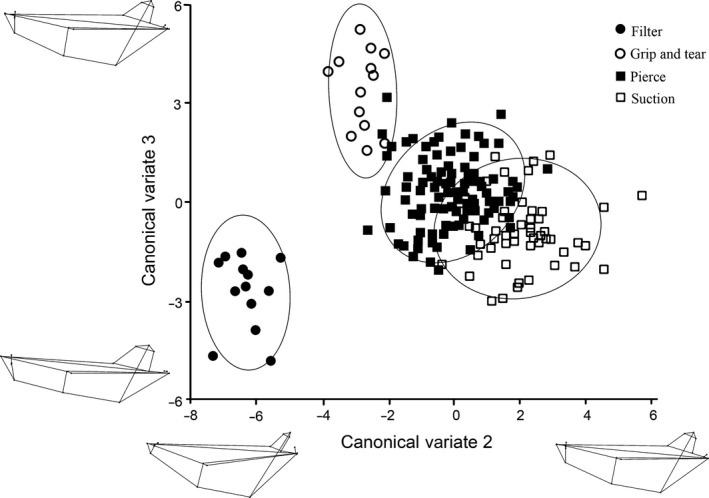
Mandibular CVA results comparing CV2 and CV3. Each shape represents a feeding strategy, and each point represents a specimen. Ovals represent 95% confidence intervals for each feeding strategy. Wireframe diagrams of the shape changes in the positive and negative direction are shown along each axis.
Supervised Random Forest analysis
The supervised Random Forest analysis produced an out‐of‐bag error of 0%, indicating that it successfully grouped each specimen into the correct feeding strategy during each iteration of the model. The primary predictors of feeding strategy in decreasing order of importance were prey type, genera, skull PC1, skull PC2 and mandible PC1 (Fig. S3). Other skull PCs, mandible PCs and phylogenetic affiliation variables were less important predictors of feeding strategy. The first primary predictor of feeding strategy was prey type, suggesting that species consuming the same prey types were more likely to have the same feeding strategy. The second most important predictor was related to phylogeny, as species within a genus were more likely to use the same feeding strategy. It is unsurprising that species within the same genera use similar feeding strategies, as these species share similar evolutionary trajectories, are often found in similar geographic areas and share the same most recent common ancestor (Fig. S1). However, it is interesting to note that other phylogenetic affiliations above genera – including the separation of monachines and phocines – were not important in predicting the use of a given feeding strategy. The three other most important determiners of feeding strategy were related to skull and mandible morphology. In particular, skull PC1, skull PC2 and mandible PC1 were found to be useful predictors of phocid feeding strategies. This finding supports the use of cranial and mandibular morphologies in determining feeding strategies of extant phocid species.
Discussion
Pinnipeds have evolved multiple feeding strategies to capture and consume prey underwater. Here, specific feeding strategies used by extant phocids were identified and documented based on cranial and mandibular morphologies. There was evidence for the use of three specialized feeding modes, grip and tear, filter, and suction feeding, as well as evidence for a more generalist feeding mode, pierce feeding, that combines both biting and suction. These feeding modes are additionally supported by dietary information, musculoskeletal data and, for some species, behavioral observations. The results of this study are largely concordant with previous studies, with some important distinctions (Adam & Berta, 2002; Jones & Goswami, 2010; Jones et al. 2013). Using 3D cranial morphometrics, Jones & Goswami (2010) were able to separate filter and grip and tear feeding phocids, but they were unable to readily distinguish suction and pierce feeders. In a later study of pinniped mandibles, Jones et al. (2013) found that filter feeding phocids grouped together, although other phocids were less clearly separated into feeding strategies based on mandibular morphology. In a recent morphometric study of tooth morphology, Churchill & Clementz (2015) found a strong correlation between tooth morphology and prey capture strategies. Similar to the present study, they identified filter, biting and suction feeding among phocids. Each of these feeding strategies will be discussed in a comparative context below.
It should be noted that, like most animals, phocids are opportunistic foragers and capture prey as efficiently as possible. Therefore, while species may have specialized morphologies associated with a particular feeding strategy, this does not preclude them from using other feeding modes. In fact, many species show intraspecific variation in foraging strategy that can be related to many different variables, including age (Villegas‐Amtmann et al. 2008), sex (Le Boeuf et al. 1994), prey type (Hocking et al. 2014) and season (Hall‐Aspland et al. 2005). The ability of animals, such as phocids, to utilize multiple feeding strategies throughout their life makes it difficult to place species into discrete and static feeding categories. The results of this study show that phocids have specialized morphological adaptations for particular feeding strategies, even if they are capable of using multiple feeding modes throughout their life. Understanding the morphological adaptations that particular taxa have for feeding provides insights into the evolutionary pressures that led to the transition of pinnipeds from terrestrial to aquatic habitats, and helps us to understand how morphology shapes foraging behaviors in these taxa. Additionally, for many phocids, little is known about how these species forage, so describing their feeding morphologies and adaptations to particular foraging modes provide the only clues to how these species successfully feed in the marine environment.
Grip and tear feeding is a specialist feeding strategy that is associated with specialized feeding characters. Grip and tear feeding is characterized by gripping prey with enlarged incisors and canines, shaking the prey to tear off flesh, and ingesting bite‐sized pieces of meat (Penney & Lowry, 1967; Hiruki et al. 1999; Adam & Berta, 2002). The leopard seal is the only extant phocid species to use grip and tear feeding, and its use of this strategy is supported by cranial and mandible characters (this study), as well as qualitative characters (Adam & Berta, 2002), behavior studies (Penney & Lowry, 1967; Hocking et al. 2013) and diet data (Pauly et al. 1998).
Based on the results of this study, grip and tear feeders (i.e. leopard seals) have large orbits, long parietal bones (rostro‐caudally) and a long skull with a wide base. Mandible characters associated with grip and tear feeding include a shortening of the mandibular body, an increase in mandible thickness and a wide coronoid process. In addition, the leopard seal has a longer jaw relative to body size than similarly sized phocid species (Jones et al. 2013), as well as enlarged mastication muscles (e.g. digastric, masseter and temporalis; Bryden & Felts, 1974). These longer jaw and enlarged coronoid processes provide increased surface area for these muscle attachments, which are instrumental in producing a strong bite force (Bryden & Felts, 1974). The leopard seal is also well known for its enlarged canines and canine‐like incisors that are used for gripping and tearing prey (Adam & Berta, 2002).
The leopard seal has a diverse diet that includes large marine vertebrates (e.g. penguins, crabeater seals, Antarctic fur seals; Penney & Lowry, 1967; Siniff & Bengtson, 1977; Pauly et al. 1998; Walker et al. 1998) and small marine invertebrates (e.g. krill, fish; Pauly et al. 1998; Walker et al. 1998; Hocking et al. 2013). One of the most extraordinary food items discovered in the stomach of a leopard seal was a full‐grown platypus (Troughton, 1947)! Grip and tear feeding is ideal for capturing large prey items like penguins and other pinnipeds; however, it is not well suited for capturing smaller prey items (e.g. krill). In a study of captive individuals, Hocking et al. (2013) showed that leopard seals were able to use filter and suction feeding when capturing small prey. These results are consistent with the findings of the present study. In this study, leopard seals and crabeater seals occupied similar regions of morphospace, which suggests similar cranial and mandible morphologies. Leopard seals and crabeater seals also share similar tooth morphologies, including having even spacing between teeth, proportionally long postcanines, and highly curved, multi‐cusped postcanines (Churchill & Clementz, 2015). Evidence for convergence between feeding strategies in the leopard seal and crabeater seal is also supported by diet composition studies, which show that both leopard seals and crabeater seals consume large quantities of Antarctic krill (Euphausia superb; Lowry et al. 1988; Pauly et al. 1998; Casaux et al. 2011). It therefore appears that while the leopard seal exhibits morphological specializations for grip and tear feeding, a specialized feeding mode for capturing large prey items, this species has adapted to use multiple feeding strategies (e.g. filter feeding), allowing them to alternate feeding modes and prey types as necessary.
Filter feeding is another specialized feeding strategy used by one extant pinniped species, the crabeater seal (Taylor, 1987; Adam & Berta, 2002; Jones & Goswami, 2010). Filter feeders engulf water with high concentrations of small prey, and water is then expelled from the sides of the mouth, trapping prey inside the oral cavity (Taylor, 1987). The crabeater seal uses its highly specialized, multi‐cusped teeth as a sieve to filter prey from the water, and prey become trapped by the teeth (King, 1961; Adam & Berta, 2002). Filter feeding in the crabeater seal is supported by cranial and mandible characters (this study), qualitative and quantitative data (Adam & Berta, 2002; Koretsky et al. 2014; Churchill & Clementz, 2015), behavioral observations (Klages & Cockcroft, 1990), and diet data (Pauly et al. 1998).
Compared with the skull of other phocids, the crabeater seal has a narrow palate, enlarged orbits and an elongated caudal portion of the skull. The crabeater seal mandible is thick (dorso‐ventrally), has an elongated toothrow and has a thick mandibular symphysis. In addition, the ramus caudal to the symphysis is curved inward in crabeater seals, which is unique among most phocid species (King, 1961). In addition, the crabeater seal is well known for its distinct multi‐cusped postcanine teeth. In occlusion, these teeth form a sieve‐like structure used for filtering prey items from the water column (King, 1961; Adam & Berta, 2002). There is also a pair of bony protuberances caudal to the cheek teeth on the upper and lower jaws (King, 1961). These bony protuberances were suggested by King (1961) to prevent prey from escaping from the back of the mouth. In addition, the muscles of the head (including facial, hyoid and mastication muscles) are much smaller in size and weight than in other Antarctic species (Bryden & Felts, 1974). This may be due to not needing large mastication muscles when filtering small prey items from the water (Bryden & Felts, 1974).
The crabeater seal is a prey specialist, with malacostracan species (namely Antarctic krill) comprising 96% of its diet (King, 1961; Oritsland, 1977; Green & Williams, 1986; Lowry et al. 1988; Klages & Cockcroft, 1990). Therefore, the crabeater seal shares both the same feeding strategy and prey specialization as the best‐known marine mammal filter feeders, mysticetes (baleen whales; Pauly et al. 1998).
Additionally, behavioral observations confirm the use of filter feeding in captive crabeater seals (Klages & Cockcroft, 1990). The crabeater seal can also use suction in prey capture. For example, a captive crabeater seal used suction to pull in fish up to 50 cm away from its mouth (Klages & Cockcroft, 1990). It should be noted that most phocids are capable of using some degree of suction, so it is therefore not surprising that both leopard seals and crabeater seals are able to use suction in addition to filtering to capture prey (Kastelein & Mosterd, 1989; Klages & Cockcroft, 1990; Kastelein et al. 1994; Adam & Berta, 2002; Born et al. 2003; Marshall et al. 2008). In these species, suction is likely used to pull prey into the oral cavity, and then filtering mechanisms (including the specialized dentition) are responsible for trapping prey inside the oral cavity and expelling water from the sides of the mouth.
Suction feeding is a strategy used by many aquatic species, including beaked whales (Heyning & Mead, 1996), most fishes (Muller & Osse, 1984; Holzman et al. 2008), gray whales (Johnston & Berta, 2011), long‐finned pilot whales (Werth, 2000) and nurse sharks (Motta et al. 2002). Suction feeding is characterized by the creation of negative pressure by oral and/or pharyngeal expansion to ingest prey (Adam & Berta, 2002; Werth, 2007; Marshall et al. 2008). Although most pinnipeds are hypothesized to use some degree of suction in prey capture (Kastelein & Mosterd, 1989; Klages & Cockcroft, 1990; Kastelein et al. 1994; Adam & Berta, 2002; Born et al. 2003; Marshall et al. 2008), suction feeding can also be a specialized strategy as exemplified by the extant walrus (Odobenus rosmarus; Kastelein & Mosterd, 1989; Kastelein et al. 1994; Born et al. 2003). The walrus uses oral suction to separate prey items, predominantly benthic invertebrates, from their shells (Kastelein & Mosterd, 1989; Kastelein et al. 1994; Born et al. 2003). The walrus generates large suction pressures by modulating the speed of the tongue, the tightness of its lips on prey items and the motion (e.g. retraction and depression) of its tongue (Kastelein et al. 1994). In this study, suction feeding was found to be both a specialized mode of prey capture, exemplified by the walrus, as well as a generalist mode of prey capture used by most phocids in some form.
Bearded seals appear to be similar to walruses, using a specialized form of suction feeding. This designation is supported by cranial characters (this study), as well as quantitative and qualitative data (Adam & Berta, 2002; Churchill & Clementz, 2015), behavioral observations (Marshall et al. 2008) and dietary data (Pauly et al. 1998). Cranial characters that distinguish bearded seals from other phocid species include a wide middle of the skull, a change in pterygoid hamuli position and a wide palate. In contrast to the skull, the bearded seal does not have a specialized mandible. It is possible that other evolutionary pressures (e.g. ecological, physiological, sexual) are constraining the shape of the mandible in this species. In addition to the cranial specializations, the bearded seal has highly reduced postcanine teeth that are rounded nubs with no cusps (Churchill & Clementz, 2015). This condition is also observed in the suction feeding walrus. Because grabbing and biting are not necessary in suction feeding, it is likely that the selective pressures are relaxed on tooth morphology in suction feeding species.
Dissections of the facial muscles show that bearded seals also have unique musculoskeletal elements. For example, the tip of the tongue is bifurcated, with small symmetrical left and right lobes (personal observation). A similar condition has been observed in juvenile and adult gray whales (Andrews, 1914; Kienle et al. 2015), and it has been suggested that cleft tongues are related to suckling in juveniles and have been co‐opted for suction feeding in adults (Kienle et al. 2015). Additionally, the styloglossus is better developed and larger than in other phocids (personal observation). In the bearded seal the styloglossus provides a strong attachment of the tongue to the cranium, potentially playing a role in creating traction for the tongue when suction feeding.
Like other specialized suction feeders, bearded seals primarily consume soft‐bodied benthic prey, including malacostracans, actinopterygians, bivalves, marine worms and gastropods; unsurprisingly, their diet is similar to the benthic suction feeding walrus (Wilke, 1954; Finley, 1983; Lowry et al. 1988; Antonelis, 1994; Hjelset, 1999; Dehn, 2007). These soft‐bodied prey are likely easier to consume as a suction feeder than fast‐moving, hard prey.
Additionally, captive feeding studies confirm the use of suction feeding in bearded seals. When feeding underwater, bearded seals used suction (as opposed to biting or hydraulic jet setting) 96.3% of the time. Suction feeding was characterized by pursing the lips with a small gape angle of the mouth, rapidly opening the lower jaw and depressing the hyoid apparatus (Marshall et al. 2008). Together these movements brought water and prey into the oral cavity (Marshall et al. 2008). Biting was never observed underwater in any of the trials (Marshall et al. 2008). Taken together, the results of this study with these other studies suggest that the bearded seal is a specialized suction feeding species with a morphology, diet and behavior most similar to the walrus.
In addition to bearded seals, the results of this study provide evidence that northern and southern elephant seals use specialized suction feeding as their primary mode of prey capture. Numerous qualitative and quantitative characters separate northern and southern elephant seals from other phocids (this study). In addition, tooth morphology (Churchill & Clementz, 2015), diet data (Pauly et al. 1998), feeding observations (personal observations) and recent bio‐logging data (Naito et al. 2013) suggest elephant seals use specialized suction feeding for prey capture.
Cranial characters associated with the separation of elephant seals from other phocids include an elongated skull, a narrow rostrum and an expanded base of the skull. Elephant seals also have an enlarged zygomatic region, increased orbit size and an increased skull width. The mandible is differentiated from other species by being wide and thick with a short coronoid process. Elephant seals also have an enlarged mandibular condyles width (medio‐laterally) and a close proximity of the condyles to the coronoid process. Northern and southern elephant seals were most closely associated with the bearded seal in cranial morphospace. Additionally, both bearded seals and elephant seals have highly reduced postcanine teeth, which often appear as rounded nubs with no cusps (Churchill & Clementz, 2015). This condition indicates that teeth are not necessary for prey capture in these species (Adam & Berta, 2002; Koretsky et al. 2014; Churchill & Clementz, 2015). The northern elephant seal also has well‐developed palatal musculature and a robust, muscular tongue, both of which are potentially used in generating suction forces (personal observation). The identification of bearded seals and elephant seals as suction feeders is consistent with morphological results from other studies (Jones & Goswami, 2010; Jones et al. 2013). Similar results were also obtained by Churchill & Clementz (2015), although they identified elephant seals as non‐biting feeders based on proportionally smaller postcanine dentition and a large diastema.
In addition, diet composition studies have demonstrated that the diet of northern and southern elephant seals is primarily composed of cephalopods and soft‐bodied fish (Green & Williams, 1986; Antonelis et al. 1987; Green & Burton, 1993; Le Boeuf & Laws, 1994; Naito et al. 2013). These soft‐bodied prey items are the prey of other known suction feeders, including beaked whales (Heyning & Mead, 1996), dwarf and pygmy sperm whales (Bloodworth & Marshall, 2005) and long‐finned pilot whales (Werth, 2000).
Suction feeding in elephant seals is further supported by observations of captive northern elephant seals. When fed, individuals oriented fish in a rostrocaudal direction toward their mouth and used suction to pull the prey into the oral cavity. Individuals were never observed to use their teeth or biting motions in prey consumption (personal observation). Furthermore, Naito et al. (2013) suggested that northern elephant seals used suction feeding to capture small prey, based on the rate and pattern of jaw motion events recorded by jaw accelerometers on wild, adult northern elephant seals. Therefore, anatomical, musculoskeletal, behavioral and dietary data provide convincing evidence for the use of suction feeding in northern and southern elephant seals.
Based on quantitative and qualitative data, the hooded seal is also a specialized suction feeder. Hooded seals have cranial and mandibular characters similar to bearded seals and elephant seals, which are likely specialized suction feeders (discussed above). Cranial characters that differentiate hooded seals from other feeding types include rostral shortening, enlarged orbits and zygomatic arch, an elongated palate, and an overall thickening of the skull. Mandibular characters that separate hooded seals from other phocids are a tall, curved coronoid process, a thin elongated mandible, a thin toothrow and an arched caudal edge of the mandible. Additionally, the diet of hooded seals is similar to the other suction feeding phocids and is comprised of soft‐bodied prey, mostly malacostracan species (Pauly et al. 1998; Haug et al. 2004, Tucker et al. 2009). Unfortunately no musculoskeletal data are currently available for the hooded seal, nor have any behavioral or biomechanical studies been performed on hooded seals, to date. The morphological and dietary data from this study provide preliminary evidence of suction feeding in hooded seals, which is supported by the tooth morphology results of Churchill & Clementz (2015).
Pierce feeding is a generalist feeding strategy used by many aquatic and terrestrial species. Aquatic tetrapods must overcome the inability to pin down struggling prey in an aqueous environment. Therefore, many aquatic species use a piercing bite to capture small prey items that can be easily swallowed (Taylor, 1987). Pierce feeders generally capture prey using small, sharp teeth and then swallow the prey whole (Klages & Cockcroft, 1990; Kastelein et al. 1991; Adam & Berta, 2002). Most pinnipeds are hypothesized to use pierce feeding in prey capture based on qualitative characters, including the rostral placement of the first molar to the midpoint on the dentary, enlarged orbits, and an enlarged infraorbital foramen (Taylor, 1987; Adam & Berta, 2002). Pierce feeding appears to be the dominant feeding mode for most phocids. However, despite the name, pierce feeding is not limited to biting prey. Pierce feeders also likely use suction feeding in prey capture, without the associated specialized feeding morphologies seen in other species (e.g. bearded seals, elephant seals, hooded seals).
Quantitative characters associated with pierce feeding include having elongated orbits, enlarged pterygoid bones and a long toothrow. Additionally most pierce feeders have homodont postcanine teeth with pointed, cusped teeth. Churchill & Clementz (2015) identified this group as biters based on having proportionally larger postcanines and unequal but limited tooth spacing. Pierce feeding species primarily consume ray‐finned fish (Pauly et al. 1998; Fig. S1). Therefore, it may be advantageous for these fish specialists to use multiple feeding strategies (e.g. biting and suction) in prey capture, as most species consume multiple species as a part of their typical diet. The only exception to this is the Ross seal, which is classified as a pierce feeder but primarily consumes cephalopods (King, 1983). King (1983) hypothesized that the Ross seal potentially used gripping and suction to capture prey. However, the small sample size and quantitative analyses did not provide enough evidence to warrant a change in feeding strategy for Ross seals.
It is expected that future behavioral, physiological and experimental work will result in the discovery of additional methods of prey capture. For example, monk seals consistently formed their own group in the unsupervised Random Forest models; however, while they were closely grouped together in cranial and mandibular morphospace, there were no distinguishing characters to suggest this group had unique feeding morphologies. In addition, little is known about the feeding behaviors and diet of monk seals in general, so no additional evidence can be used to tease apart their unique cranial morphology at this time.
In the present study, the best predictors of feeding strategies for phocid seals are prey type, phylogeny, and cranial and mandible shape. Therefore, together these different pieces of information on evolutionary history, diet and morphology can be used to determine the feeding strategy of unknown specimens. This would be most useful in the context of fossil pinnipedimorphs for whom little to no life history data are available.
In the broader context, crown pinnipeds include the Odobenidae (walruses) and Otariidae (fur seals, sea lions) in addition to phocids (Fig. S1). The extant walrus has long been recognized as a specialized suction feeder, primarily consuming mollusks (Pauly et al. 1998), although this was not the case for some fossil walruses that were more likely generalist fish eaters (Adam & Berta, 2002). Similar to phocids, most otariids were found to employ a generalist pierce feeding strategy (Adam & Berta, 2002). This is in general agreement with Churchill & Clementz (2015), although they found a broad overlap between the biting and non‐biting categories; for example, the Steller sea lion and Antarctic fur seal clustered with ribbon, grey and hooded seals. Ancestral pinnipeds (e.g. stem pinniped Enaliarctos) have been hypothesized as having employed a generalist pierce feeding strategy (Adam & Berta, 2002), although this hypothesis will require further examination in the context of a rigorous comprehensive phylogeny of phocids that incorporates new fossil discoveries (e.g. Amson & de Muizon, 2014), as well as morphological and molecular data.
Conclusions
This study found morphological evidence for four feeding strategies in phocids: filter; grip and tear; suction; and pierce feeding. Distinct cranial and mandible morphologies are associated with each feeding strategy. These feeding strategies are additionally supported by quantitative cranial and mandibular characters, dietary information, musculoskeletal data and, for some species, behavioral observations. However, despite having specialized feeding morphologies, many species are capable of using multiple feeding modes in prey capture. Most phocid species are pierce feeders, using a combination of biting and suction to opportunistically catch prey. Grip and tear and filter feeding are specialized strategies with specific morphological adaptations. These unique adaptations have allowed leopard seals and crabeater seals to exploit novel ecological niches and prey types. This study provides the first cranial and mandibular morphological evidence for the use of specialized suction feeding in hooded seals, northern elephant seals and southern elephant seals. The most important variables in determining the feeding strategy of a given species were cranial and mandibular shape, prey type, and phylogeny. These results provide a framework for understanding the evolution and adaptability of feeding strategies employed by extant phocids, and these findings can be applied to other pinniped lineages and extinct taxa.
Supporting information
Fig. S1. Time‐calibrated phylogeny of phocids modified from Scheel et al. (2014) and Fulton & Strobeck (2010).
Fig. S2. Plots showing PAM groupings (four and eight groups, respectively) from the unsupervised Random Forest analyses.
Fig. S3.Results of the mean decreasing accuracy measure of variable importance from the supervised Random Forest analysis.
Table S1. Sex, age, location and museum accession numbers for each specimen included in the study.
Table S2. Results of the PAM groupings for each specimen from the unsupervised Random Forest analyses.
Table S3. Positive and negative eigenvalues for the first six cranial principal components.
Table S4. Positive and negative eigenvalues for the first four mandible principal components.
Table S5. Mahalanobis distances among the feeding strategies in the cranial CVA.
Table S6. Positive and negative coefficients for the three cranial canonical variates.
Table S7. Mahalanobis distances among the feeding strategies in the mandible CVA.
Table S8. Positive and negative coefficients for the three mandible canonical variates.
Acknowledgements
The work described here was completed in partial fulfillment of a Master's of Science degree by the senior author in the Department of Biology at San Diego State University. This research was partially supported by the Lerner Grey Grant for Marine Research. The authors thank P. Unitt (SDMNH), D. Janiger (LACM), J. Dines (LACM), C. W. Potter (NMNH) and J. Ososky (NMNH) for their time and access to mammal collections. The authors would also like to thank W. Ary, E. Ekdale, R. Furbish, T. Luckau, J. Martin, R. Mehta, M. Smallcomb, S. Young and N. Zellmer for advice and invaluable help improving this study.
References
- Adam PJ, Berta A (2002) Evolution of prey capture strategies and diet in the Pinnipedimorpha (Mammalia, Carnivora). Oryctos 4, 83–107. [Google Scholar]
- Amson E, de Muizon C (2014) A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seals phylogeny. Syst Paleontol 12, 523–548. [Google Scholar]
- Antonelis GA, Lowry MS, DeMaster DP, Fiscus CH (1987) Assessing northern elephant seal feeding habits by stomach lavage. Mar Mam Sci 3, 308-322. [Google Scholar]
- Antonelis GA, Melin SR, Bukhtiyarov YA (1994) Early spring feeding habits of bearded seals (Erignathus barbatus) in the central Bering Sea, 1981. Arctic 47, 74–79. [Google Scholar]
- Andrews RC (1914) The California gray whale (Rhachianectes glaucus Cope). Mem Amer Mus Nat Hist 1, 227–287. [Google Scholar]
- Bloodworth B, Marshall CD (2005) Feeding kinematics of Kogia and Tursiops (Odontoceti: Cetacea): characterization of suction and ran feeding. J Exp Biol 208, 3721–3730. [DOI] [PubMed] [Google Scholar]
- Born EW, Rysgaard S, Ehlme G, et al. (2003) Underwater observations of foraging free‐living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26, 348–357. [Google Scholar]
- Breiman L (2001) Random forests. Mach Learn 45, 5–32. [Google Scholar]
- Bryden MM, Felts WJL (1974) Quantitative anatomical observations on the skeletal and muscular systems of four species of Antarctic seals. J Anat 118, 589–600. [PMC free article] [PubMed] [Google Scholar]
- Casaux R, Bertolin ML, Carlini A (2011) Feeding habits of three seal species at the Danco Coast, Antarctica: a re‐assessment. Polar Biol 34, 1615–1620. [Google Scholar]
- Churchill M, Clementz MT (2015) Functional implications of variation in tooth spacing and crown size in Pinnipedimorpha (Mammalia: Carnivora). Anat Rec 298, 878–902. [DOI] [PubMed] [Google Scholar]
- Debey LB, Pyenson ND (2013) Osteological correlates and phylogenetic analysis of deep diving in living and extinct pinnipeds: what good are big eyes? Mar Mamm Sci 29, 48–83. [Google Scholar]
- Dehn LA, Sheffield GG, Follmann EH, et al. (2007) Feeding ecology of phocid seals and some walrus in the Alaskan and Canadian Arctic as determined by stomach contents and stable isotope analysis. Polar Biol 30, 167–181. [Google Scholar]
- Dehnhardt G, Mauck B, Hanke W, et al. (2001) Hydrodynamic trail‐following in harbor seals (Phoca vitulina). Science 293, 102–104. [DOI] [PubMed] [Google Scholar]
- Dinsdale EA, Edwards RA, Bailey BA, et al. (2013) Multivariate analysis of functional metagenomes. Front Genet 4, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doutt KJ (1942) A review of the genus Phoca . Ann Carnegie Mus 29, 61–125. [Google Scholar]
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125, 1–15. [Google Scholar]
- Finley KJ, Evans CR (1983) Summer diet of the bearded seal (Erignathus barbatus) in the Canadian High Arctic. Arctic 36, 82–89. [Google Scholar]
- Fulton TL, Strobeck C (2010) Multiple markers and multiple individuals refine true seal phylogeny and bring molecules and morphology back in line. Proc R Soc Lond B 277, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginter CG, DeWitt TJ, Fish FE, et al. (2012) Fused traditional and geometric morphometrics demonstrate pinniped whisker diversity. PLoS ONE 7, e34481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman‐Lowe GD (1998) Diet of the Hawaiian monk seal (Monachus schauinslandi) from the Northwestern Hawaiian Islands during 1991 to 1994. Mar Biol 132, 535–546. [Google Scholar]
- Green K, Burton HR (1993) Comparison of the stomach contents of southern elephant seals, Mirounga leonina, at Macquarie and Heard Islands. Mar Mamm Sci 9, 10–22. [Google Scholar]
- Green K, Williams R (1986) Observations on food remains in faeces of elephant, leopard and crabeater seals. Polar Biol 6, 43–45. [Google Scholar]
- Hall‐Aspland SA, Rogers TL, Canfield RB (2005) Stable carbon and nitrogen isotope analysis reveals seasonal variation in the diet of leopard seals. Mar Ecol Prog Ser 305, 249–259. [Google Scholar]
- Hanke FD, Hanke W, Scholtyssek C, et al. (2009) Basic mechanisms in pinniped vision. Exp Brain Res 199, 299–311. [DOI] [PubMed] [Google Scholar]
- Haug T, Nilssen KT, Lindblom L (2004) Feeding habits of harp and hooded seals in drift ice waters along the east coast of Greenland in summer and winter. Polar Res 23, 35–42. [Google Scholar]
- Heyning JE, Mead JG (1996) Suction Feeding in Beaked Whales: Morphological and Observational Evidence. Contributions in Science, Natural History Museum of Los Angeles County. Lawrence: Allen Press. [Google Scholar]
- Hjelset AM, Andersen M, Gjertz I, et al. (1999) Feeding habits of bearded seals (Erignathus barbatus) from the Svalbard area, Norway. Polar Biol 21, 186–193. [Google Scholar]
- Hiruki LM, Schwartz MK, Boveng PL (1999) Hunting and social behavior of leopard seals (Hydrurga leptonyx) at Seal Island, South Shetland Islands, Antarctica. Zool Soc Lond 249, 97–109. [Google Scholar]
- Hocking DP, Evans AR, Fitzgerald EMG (2013) Leopard seals (Hydrurga leptonyx) use suction and filter feeding when hunting small prey underwater. Polar Biol 36, 211–222. [Google Scholar]
- Hocking DP, Salverson M, Fitzgerald EMG, et al. (2014) Australian fur seals (Arctocephalus pusillus doriferus) use raptorial biting and suction feeding when targeting prey in different foraging scenarios. PLoS ONE 9, e112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman R, Day SW, Mehta RS, et al. (2008) Integrating the determinants of suction feeding performance of centrarchid fishes. J Exp Biol 211, 3296–3305. [DOI] [PubMed] [Google Scholar]
- IUCN (2015) IUCN Red List of Threatened Species. Version 2012.2. www.iucnredlist.org.
- Johnston C, Berta A (2011) Comparative anatomy and evolutionary history of suction feeding in cetaceans. Mar Mamm Sci 27, 493–513. [Google Scholar]
- Jones KE, Goswami A (2010) Quantitative analysis of the influences of phylogeny and ecology on phocid and otariid pinniped (Mammalia; Carnivora) cranial morphology. J Zool 280, 297–308. [Google Scholar]
- Jones KE, Ruff CB, Goswami A (2013) Morphology and biomechanics of the pinniped jaw: mandibular evolution without mastication. Anat Rec 296, 1049–1063. [DOI] [PubMed] [Google Scholar]
- Kastak D, Schusterman RJ (1998) Low‐frequency amphibious hearing in pinnipeds: methods, measurements, noise, and ecology. J Acoust Soc Am 103, 2216–2228. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Mosterd P (1989) The excavation technique for molluscs of Pacific walruses (Odobenus rosmarus divergens) under controlled conditions. Aquat Mamm 15, 3–5. [Google Scholar]
- Kastelein RA, Gerrits NM, Dubbeldam JL (1991) The anatomy of the Walrus head (Odobenus rosmarus) Part 2: description of the muscles and of their role in feeding and haul‐out behaviour. Aquat Mamm 17, 156–180. [Google Scholar]
- Kastelein RA, Muller M, Terlouw A (1994) Oral suction of a Pacific walrus (Odobenus rosmarus divergens) in air and under water. Z Säugetierkd 59, 105–115. [Google Scholar]
- Kienle SS, Ekdale EG, Reidenberg JS, et al. (2015) Tongue musculature and functional morphology of a neonate gray whale (Cetacea, Mysticeti, Eschrichtius robustus). Anat Rec 298, 660–674. [DOI] [PubMed] [Google Scholar]
- King JE (1961) The feeding mechanism and jaws of the crabeater seal (Lobodon carcinophagus). Mammalia 25, 462–466. [Google Scholar]
- King JE (1983) Seals of the World. New York, NY: Comstock Publishing Associates. [Google Scholar]
- Klages NTW, Cockcroft VG (1990) Feeding behaviour of a captive crabeater seal. Polar Biol 10, 403–404. [Google Scholar]
- Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11, 353–357. [DOI] [PubMed] [Google Scholar]
- Koretsky IA, Rahmat SJ, Peters N (2014) Remarks on the correlation and implications of the mandibular structure and diet in some seals (Mammalia, Phocidae). Vestn Zool 48, 255–268. [Google Scholar]
- Levenson DH, Schusterman RJ (1999) Dark adaptation and visual sensitivity in shallow and deep‐diving pinnipeds. Mar Mamm Sci 15, 1303–1313. [Google Scholar]
- Le Boeuf BJ, Laws RM (1994) Elephant Seals: Population Ecology, Behavior, and Physiology. Los Angeles: University of California Press. [Google Scholar]
- Le Boeuf BJ, Crocker DE, Costa DP, et al. (2000) Foraging ecology of northern elephant seals. Ecol Monogr 70, 353–382. [Google Scholar]
- Lowry LF, Frost KJ, Burns JJ (1980) Feeding of bearded seals in the Bering and Chukchi Seas and trophic interaction with Pacific walruses. Arctic 33, 330–342. [Google Scholar]
- Lowry LF, Testa JW, Calvert W (1988) Notes on the winter feeding of crabeater and leopard seals near the Antarctic peninsula. Polar Biol 8, 475–478. [Google Scholar]
- Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org.
- Marden JI (2015) Multivariate Statistics Old School. Online. http://istics.net/pdfs/multivariate.pdf.
- Marshall CD, Kovacs KM, Lydersen C (2008) Feeding kinematics, suction and hydraulic jetting capabilities in bearded seals (Erignathus barbatus). J Exp Biol 211, 699–708. [DOI] [PubMed] [Google Scholar]
- Marshall CD, Wieskotten S, Hanke W, et al. (2014) Feeding kinematics, suction, and hydraulic jetting performance of harbor seals (Phoca vitulina). PLoS ONE 9, e86710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PJ, Hueter RE, Tricas TC, et al. (2002) Kinematic analysis of suction feeding in the Nurse Shark, Ginglymostoma cirratum (Orectolobiformes, Ginglymostomatidae). Copeia 1, 24–38. [Google Scholar]
- Muller M, Osse JWM (1984) Hydrodynamics of suction feeding in fish. Trans Zool Soc Lond 37, 51–135. [Google Scholar]
- Naito Y, Costa DP, Adachi T, et al. (2013) Unravelling the mysteries of a mesopelagic diet: a large apex predator specializes on small prey. Funct Ecol 27, 710–717. [Google Scholar]
- Oritsland T (1977) Food consumption of seals in the Antarctic pack ice. In: Adaptations within Antarctic Ecosystems, Third Symposium on Antarctic Biology (ed. Llano GA), Scientific Committee for Antarctic Research.
- Pauly D, Trites AW, Capuli E, et al. (1998) Diet composition and trophic levels of marine mammals. ICES J Mar Sci 55, 467–481. [Google Scholar]
- Penney RL, Lowry G (1967) Leopard seal predation of Adelie penguins. Ecology 48, 878–882. [DOI] [PubMed] [Google Scholar]
- Reichmuth C, Holt MM, Mulsow J, et al. (2013) Comparative assessment of amphibious hearing in pinnipeds. J Comp Physiol A 199, 491–507. [DOI] [PubMed] [Google Scholar]
- Revell LJ, Harmon LJ, Collar DC (2008) Phylogenetic signal, evolutionary process, and rate. Syst Biol 57, 591–601. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008) R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Scheel D‐M, Slater GJ, Kolokotronis S‐O, et al. (2014) Biogeography and taxonomy of extinct and endangered monk seals illuminated by ancient DNA and skull morphology. ZooKeys 409, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniff DB, Bengtson JL (1977) Observations and hypotheses concerning the interactions among crabeater seals, leopard seals, and killer whales. J Mammal 58, 414–416. [Google Scholar]
- Sivertsen E (1954) A Survey of the Eared Seals (family Otariidae) with Remarks on the Antarctic Seals Collected by M/K “Norvegia” in 1928–1929. Oslo: Det Norske Videnskaps Akademi. [Google Scholar]
- Taylor MA (1987) How tetrapods feed in water: a functional analysis by paradigm. Zool J Linn Soc 91, 171–195. [Google Scholar]
- Troughton E (1947) Furred Animals of Australia. New York, NY: Charles Scribner's Sons. [Google Scholar]
- Tucker S, Bowen WD, Iverson SJ, et al. (2009) Sources of variation in diets of harp and hooded seals estimated from quantitative fatty acid signature analysis (QFASA). Mar Ecol Prog Ser 384, 287–302. [Google Scholar]
- Uhen MD (2007) Evolution of marine mammals: back to the sea after 300 million years. Anat Rec 290, 514–522. [DOI] [PubMed] [Google Scholar]
- Walker TR, Boyd IL, McCafferty DJ, et al. (1998) Seasonal occurrence and diet of leopard seals (Hydrurga leptonyx) at Bird Island, South Georgia. Antarct Sci 10, 75–81. [Google Scholar]
- Villegas‐Amtmann S, Costa DP, Tremblay Y, et al. (2008) Multiple foraging strategies in a marine apex predator, the Galapagos sea lion Zalophus wollebaeki . Mar Ecol Prog Ser 363, 299–309. [Google Scholar]
- Weinberg SM, Naidoo SD, Bardi KM, et al. (2009) Face shape of unaffected parents with cleft affected offspring: combining three‐dimensional surface imaging and geometric morphometrics. Orthod Craniofac Res 12, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth A (2000) A kinematic study of suction feeding and associated behavior in the long‐finned pilot whale, Globicephala melas . Mar Mamm Sci 16, 299–314. [Google Scholar]
- Werth AJ (2007) Adaptations of the cetacean hyolingual apparatus for aquatic feeding and thermoregulation. Anat Rec 290, 546–568. [DOI] [PubMed] [Google Scholar]
- Wiley EO (1981) Phylogenetics: The Theory and Practice of Phylogenetic Systematics. New York City: John Wiley. [Google Scholar]
- Wilke F (1954) Seals of Northern Hokkaido. Am Soc Mammal 35, 218–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Time‐calibrated phylogeny of phocids modified from Scheel et al. (2014) and Fulton & Strobeck (2010).
Fig. S2. Plots showing PAM groupings (four and eight groups, respectively) from the unsupervised Random Forest analyses.
Fig. S3.Results of the mean decreasing accuracy measure of variable importance from the supervised Random Forest analysis.
Table S1. Sex, age, location and museum accession numbers for each specimen included in the study.
Table S2. Results of the PAM groupings for each specimen from the unsupervised Random Forest analyses.
Table S3. Positive and negative eigenvalues for the first six cranial principal components.
Table S4. Positive and negative eigenvalues for the first four mandible principal components.
Table S5. Mahalanobis distances among the feeding strategies in the cranial CVA.
Table S6. Positive and negative coefficients for the three cranial canonical variates.
Table S7. Mahalanobis distances among the feeding strategies in the mandible CVA.
Table S8. Positive and negative coefficients for the three mandible canonical variates.


