Abstract
The present review aims to provide a systematic overview on tensile transmission along myofascial chains based on anatomical dissection studies and in vivo experiments. Evidence for the existence of myofascial chains is growing, and the capability of force transmission via myofascial chains has been hypothesized. However, there is still a lack of evidence concerning the functional significance and capability for force transfer. A systematic literature research was conducted using MEDLINE (Pubmed), ScienceDirect and Google Scholar. Studied myofascial chains encompassed the superficial backline (SBL), the back functional line (BFL) and the front functional line (FFL). Peer‐reviewed human dissection studies as well as in vivo experiments reporting intermuscular tension transfer between the constituents of a myofascial chain were included. To assess methodic quality, two independent investigators rated studies by means of validated assessment tools (QUACS and PEDro Scale). The literature research identified 1022 articles. Nine studies (moderate to excellent methodological quality) were included. Concerning the SBL and the BFL, there is moderate evidence for force transfer at all three transitions (based on six studies), and one of two transitions (three studies). One study yields moderate evidence for a slight, but not significant force transfer at one transition in the FFL. The findings of the present study indicate that tension can be transferred between some of the examined adjacent structures. Force transfer might have an impact in overuse conditions as well as on sports performance. However, different methods of force application and measurement hinder the comparability of results. Considering anatomical variations in the degree of continuity and histological differences of the linking structures is crucial for interpretation. Future studies should focus on the in vivo function of myofascial continuity during isolated active or passive tissue tensioning.
Keywords: anatomy trains, fascia, myofascial continuity, tension transfer
Introduction
Fascia is a mechanically active tissue with proprioceptive and nociceptive functions (Yahia et al. 1992; Schleip et al. 2005; Stecco et al. 2006, 2007, 2008, 2013b; Bhattacharya et al. 2010; Tesarz et al. 2011). In contrast to prior assumptions, it builds an extensive tensegrity network linking the skeletal muscles of the human body (Myers, 1997a,b, 2014; Wilke et al. 2016). This bodywide myofascial continuity holds particular significance because fascial tissues are able to change their tensional state. The presence of contractile cells has been demonstrated for the crural fascia (Staubesand & Li, 1996), the thoracolumbar fascia (TLF; Schleip et al. 2005) and the pectoral fascia (Stecco et al. 2008). Based on calculations, several authors suggest that the amount of force created by these cells is sufficient to influence musculoskeletal dynamics (Schleip et al. 2005; Willard et al. 2012). Although the existence of myofibroblasts represents a plausible explanation for stiffness changes, it most likely is not the only cause. In a recent experiment, Schleip and colleagues found that the lumbar fascia modifies its tensional state also in the absence of cellular contraction (Schleip et al. 2012a). In parallel to the stiffness change, an analogue alteration of the water content was observed. The authors therefore conclude that hydration might be the driving factor influencing fascial tension. Another theory proposes that muscle contraction directly stretches the overlying fascia, thereby altering connective tissue stiffness (Findley et al. 2015).
Regardless of the underlying mechanisms, the capability of fascia to modify its mechanical properties has potential implications for therapy and training. If tension can be increased and decreased in response to individual movement characteristics, it might be transmitted to neighbouring structures. Recent studies have demonstrated this for antagonistic and synergistic muscles (Maas et al. 2001; Huijing et al. 2003, 2007, 2011; Bojsen‐Moller et al. 2010; Yucesoy, 2010). However, the direction of load transfer in these trials was lateral. In a systematic review, Wilke et al. (2016) showed that there is good evidence for the existence of three myofascial chains proposed by Myers (1997a,b, 2014): the superficial backline (SBL: plantar fascia, gastrocnemius, hamstrings, erector spinae); the back functional line (BFL: latissimus dorsi, contralateral glutueus maximus, vastus lateralis); and the front functional line (FFL: adductor longus, contralateral rectus abdominis, pectoralis major). Therefore, the aim of the present study was to provide a systematic overview on longitudinal in‐series muscular force transmission along myofascial chains based on cadaveric studies and in vivo experiments.
Materials and methods
The systematic review was conducted adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (Liberati et al. 2009; Moher et al. 2009) and the recommendations of Wager & Wiffen (2011) for ethical publishing of systematic reviews.
A systematic literature research was conducted between May 2013 and May 2014 by two independent investigators (FK, JW). Relevant articles were identified using Medline (Pubmed), ScienceDirect and Google Scholar (each 1900–2014). Studies focusing on force transmission through direct myofascial connections between the components of the following myofascial chains were targeted: SBL, BFL and FFL. Inclusion criteria consisted of: (i) study type anatomical dissection study or in vivo experimental study; (ii) load or force transfer via myofascial continuity as one/main outcome parameter; (iii) studied myofascial connection as part of the investigated myofascial chain; and (iv) publication in a peer‐review‐journal. Studies of other types (e.g. case reports, model‐based calculations or animal studies) were excluded. The same applied to articles in languages other than German and English.
Search algorithms for the respective databases are listed in Table 1. The approach for Google Scholar was the same as in a previous systematic review (Wilke et al. 2016). Additionally, reference lists of all detected studies were checked. Data extraction was carried out by two independent investigators (FK, JW).
Table 1.
Literature search algorithms for the respective databases
| Database | Search term |
|---|---|
| MEDLINE (PubMed) | (“cadaver”[Mesh]) AND (“Fascia”[Mesh]) OR ((myofascial OR aponeurotic OR fascial) AND ((load OR tension OR strain) AND transfer)) |
| ScienceDirect | (cadaver) AND ((“load transfer”) OR (“tension transfer”) OR (“strain transfer”)) |
To evaluate study quality, two researchers independently rated the included dissection studies by means of the QUACS scale. It has been shown to be a reliable and valid tool to appraise the quality of observational cadaveric studies, and has been described elsewhere in detail (Wilke et al. 2015). Methodological quality of in vivo experimental studies was evaluated with the PEDro scale (Sherrington et al. 2000). Levels of evidence were classified as strong (consistent findings among multiple high‐quality studies), moderate (consistent findings among multiple low‐quality studies and/or one high‐quality study), limited (one low‐quality study), conflicting (inconsistent findings among multiple studies), or no evidence (no studies available) according to the recommendations of the Cochrane Collaboration Back Review Group (van Tulder et al. 2003).
Results
The initial literature research yielded 1022 publications. After removing duplicates and irrelevant papers as well as applying exclusion criteria, nine studies (Vleeming et al. 1989, 1995; van Wingerden et al. 1993; Carlson et al. 2000; Barker et al. 2004; Erdemir et al. 2004; Carvalhais et al. 2013; Norton‐Old et al. 2013; Cruz‐Montecinos et al. 2015) were included (Fig. 1). Study quality was moderate to excellent (Tables 2 and 3). Detailed information about the methodological approach for each enclosed study is available from Table 4, the main findings for each examined station of the included myofascial chains are summarized in Table 5.
Figure 1.
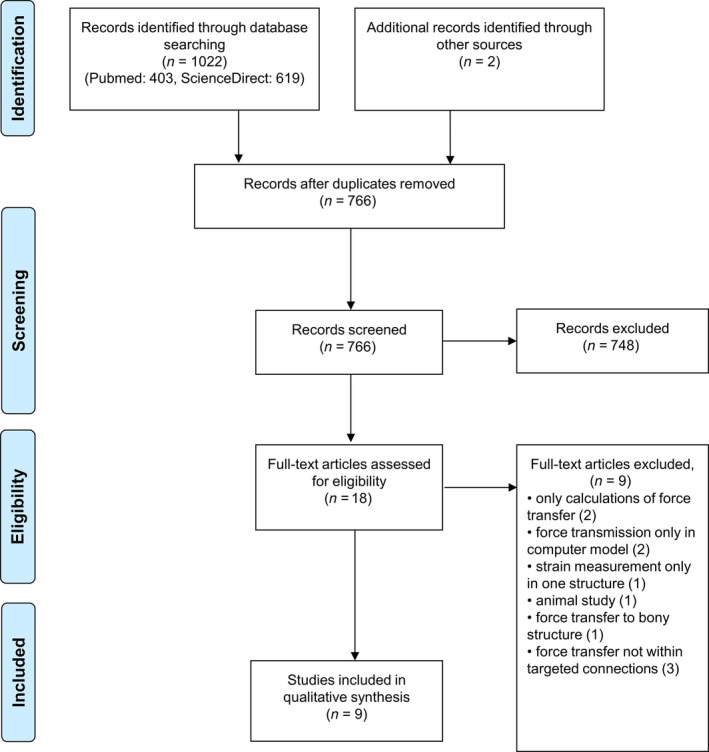
PRISMA flowchart displaying the literature research.
Table 2.
Methodological quality rated by the QUACS scale of the included dissection studies
| Study purpose | Sample data | Method of dissection | Condition of specimens | Education of investigator | More observers | Results precise | Statistics appropriate | Consistency of findings | Photographs included | Findings in context | Clinical relevance | Limitations addressed | Total score (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barker et al. 2004; | ✓ | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 77 |
| Carlson et al. 2000; | ✓ | – | – | – | – | – | ✓ | ✓ | n/a | ✓ | ✓ | ✓ | – | 58 |
| Erdemir et al. 2004; | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | n/a | ✓ | ✓ | ✓ | ✓ | 75 |
| Norton‐Old et al. 2013; | ✓ | ✓ | ✓ | ✓ | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 85 |
| van Wingerden et al. 1993; | ✓ | ✓ | ✓ | – | – | – | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | – | 69 |
| Vleeming et al. 1989; | – | – | ✓ | – | – | – | ✓ | n/a | ✓ | – | ✓ | ✓ | – | 42 |
| Vleeming et al. 1995 | ✓ | ✓ | – | – | – | – | ✓ | n/a | – | – | ✓ | ✓ | – | 42 |
n/a, not applicable.
Table 3.
Methodological quality rated by the PEDro scale of the included in vivo studies
| Eligibility criteria | Random allocation | Concealed allocation | Groups similar at baseline | Blinding of subjects | Blinding of observers | Blinding of assessors | Measures from at least 85% | All subjects included | Statistic comparison | Point measures and variance | Total score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carvalhais et al. 2013; | ✓ | ✓ | – | ✓ | – | – | – | ✓ | ✓ | ✓ | ✓ | 6/11 |
| Cruz‐Montecinos et al. 2015 | ✓ | n/a | n/a | n/a | n/a | – | – | ✓ | ✓ | ✓ | ✓ | 5/8 |
n/a, not applicable.
Table 4.
Methodological characteristics of included studies
| Study | Study type | Sample size | ♂ | ♀ | Age (± SD/range) | Methods of force application | Methods of transmitted force measurement | Methodological quality |
|---|---|---|---|---|---|---|---|---|
| Barker et al. 2004 | Cadaveric dissection | 8 | 2 | 6 | 83 (73–101) | Calibrated force manually applied in 1 N intervals parallel to muscle fascicle orientation | Strain gauge, fascial displacement (photographs), fascial area affected (photographs) | ‘substantial’ (77%) |
| Carlson et al. 2000 | Cadaveric dissection | 8 | ns | ns | 60–76 | Tension mechanically applied to Achilles tendon up to 500 N at varying MP joint angles (0 °, 15 °, 30 °, 45 °) | Strain‐gauge extensometer | ‘moderate’ (58%) |
| Carvalhais et al. 2013 | In vivo experimental study | 37 | 15 | 22 | 24.92 (3.21) | Passive and active LD tensioning | Passive stiffness recorded by isokinetic dynamometer, resting joint position | ‘good’ (6/10) |
| Cruz‐Montecinos et al. 2015 | In vivo experimental study | 17 | 17 | 0 | 22.76 (1.8) | Translation of STL and hamstrings induced by pelvic motion | Ultrasonographic measurement of deep fascial displacement | ‘good’ (5/8) |
| Erdemir et al. 2004 | Cadaveric dissection | 7 | 4 | 3 | 68.4 (22.5) | Special device for gait simulation, linear actuators pulling on AT | Fibre‐optic strain measurement | ‘substantial’ (75%) |
| Norton‐Old et al. 2013 | Cadaveric dissection | 10 | 5 | 5 | 84 (75–98) | Forces of 20 N and 50 N applied by a mechanical winch to the muscle according to its line of action | Foil‐type microstrain gauge | ‘excellent’ (85%) |
| van Wingerden et al. 1993 | Cadaveric dissection | 6 | 2 | 4 | 70–90 | Forces from 10 to 100 N applied with weights during simulated erect and flexed stance | Custom‐made buckle‐transducer | ‘moderate’ (69%) |
| Vleeming et al. 1995 | Cadaveric dissection | 10 | 6 | 4 | 65–90 | Traction of 50 N mechanically applied in the direction of muscle fibres | Estimation of traction effect by means of fascial displacement (photographs) | ‘moderate’ (42%) |
| Vleeming et al. 1989 | Cadaveric dissection | 12 | ns | ns | ns | Forces not exceeding 50 N applied to the muscle in the direction of the insertion | Visual inspection | ‘moderate’ (42%) |
AT, Achilles tendon; LD, latissimus dorsi; MP, metatarsophalangeal; ns, not stated; STL, sacrotuberous ligament.
Table 5.
Main findings for each examined station of the included myofascial chains
| Transition | No. of studies | Main findings | |
|---|---|---|---|
| SBL | Plantar fascia–Achilles tendon | 2 |
Carlson et al. 2000: tension transferred from AT to PF in all tested cases, greater force transmission at greater MP joint angles; 100 N applied to AT resulted in mean forces from 116 to 256 N, 500 N resulted in mean PF forces from 314 to 511 N (MP angle between 0 and 45 °) Erdemir et al. 2004: force applied to AT and force measured at PF well correlated (r = 0.76; P < 0.001); linear regression: almost 50% of AT force could be measured in the PF during simulated walking |
| Gastrocnemius–hamstrings | 1 | Cruz‐Montecinos et al. 2015: pelvic motion induced displacement of the MG deep fascia, indicating strain transfer between STL/hamstrings and gastrocnemius | |
| Biceps femoris–sacrotuberous ligament | 3 |
van Wingerden et al. 1993: Force transfer from BF to STL between 7 and 69%; high inter‐individual variance and significant differences between specimen with partially or fully fixed STL to ischial tuberosity; if only partially fixed, also the lateral deep part of the ligament continuous with the BF tendon, and significantly more force was transferred; no differences in erected vs. flexed stance Vleeming et al. 1995: traction on BF led to displacement of deep lamina of the TLF; no direct measurement of force transfer Vleeming et al. 1989: tension on STL greatest for traction on GM and BF (if fused); STL and BF fused in only 50%; no direct measurement of force transfer |
|
| BFL | Latissimus dorsi–contralateral gluteus maximus | 3 |
Barker et al. 2004: traction to LD and GM led to displacements at the TLF; traction to LD resulted in bilateral displacement and in greatest area of fascial displacement (Th12 to S1); traction to GM led to fascial displacement in posterior layer of TLF between L3 and S3, bilateral displacement varied between subjects; force of 10 N applied to LD or GM in direction of attaching muscles fascicles resulted in tensile force at L3 of 4.9/0.8 N in the posterior layer of TLF Carvalhais et al. 2013: passive LD tensioning led to lateral shift of hip joint resting position; active LD tensioning led to lateral sift of hip joint resting position and increased passive hip stiffness Vleeming et al. 1995: slight displacement of the superficial lamina of TLF when tensioning cranial fibres of LD; displacement homolaterally and to some extend contralaterally at L4‐S2 when tractioning caudal fibres of LD; tractioning GM led to smaller homolateral, but greater contralateral displacement to LD traction conditions |
| Gluteus maximus–vastus lateralis | – | No data available | |
| FFL | Adductor longus–contralateral rectus abdominis | 1 | Norton‐Old et al. 2013: force of 50 N applied to the AL resulted in a deformation in the contralateral rectus sheath between −0.64 and 1.11% compared to baseline length (mean: 0.23 ± 0.43%; P = 0.176); high inter‐individual variance of transferred strain |
| Rectus abdominis–pectoralis major | – | No data available |
AL, adductor longus; AT, Achilles tendon; BF, biceps femoris; BFL, back functional line; FFL, front functional line; GM, gluteus maximus; LD, latissimus dorsi; MG, medial gastrocnemius; MP, metatarsophalangeal; PF, plantar fascia; SBL, superficial backline; STL, sacrotuberous ligament; TLF, thoracolumbar fascia.
SBL
Two studies reporting force transfer between the plantar fascia and the Achilles tendon were found (moderate evidence). One trial identified force transfer between pelvic motion/hamstring movement and gastrocnemius muscle (moderate evidence), and three studies demonstrated force transfer between the hamstrings and the sacrotuberous ligament or the TLF, respectively (moderate evidence).
BFL
No study stating force transfer between the gluteus maximus and the vastus lateralis was found (no evidence). In contrast, three studies reported force transfer between the latissimus dorsi and the contralateral gluteus maximus, respectively, the TLF (moderate evidence).
FFL
One study reported transmission of force between the adductor longus and the contralateral distal rectus sheath, which turned out to be non‐significant when compared with baseline values (moderate evidence). No study examining tension transfer between the rectus abdominis and the pectoralis major muscle was detected.
Discussion
While evidence for the existence of morphological continuity between the skeletal muscles is growing (Stecco et al. 2009, 2013a,c), there is still a lack of research concerning the practical relevance of these connections. For the three myofascial chains that had been evidenced previously (Wilke et al. 2016) and were subject of this study, only nine studies reporting tension transfer between adjacent structures could be identified. This, however, is not unexpected, as recent histological and anatomical findings have changed the view of the biomechanical function of fascial tissues (van der Wal, 2009). To the authors’ knowledge, the present review is the first systematic approach to evaluate tension transfer along myofascial intermuscular connections.
The current research shows that tension can be transferred between at least some of the examined adjacent muscles, which challenges the traditional view that muscles function as independent actuators during physiological movements (Herbert et al. 2008; Maas & Sandercock, 2008; van der Wal, 2009). The possibility of load transfer between muscles encourages targeting entire myofascial chains in the evaluation process, therapy and exercise. Instead of focusing on single structures, muscles or joints, more holistic diagnostic and treatment approaches seem appropriate for overuse conditions or radiation pain symptoms that involve several structures of myofascial chain. However, three factors hamper the applicability of the current results to in vivo conditions.
First, there was considerable variation in the amount of force transfer. This might be explained by discrepancies concerning the tissue type and the degree of morphological continuity. For the transition from plantar fascia to the Achilles tendon, both included studies that conclude that considerable force is transferred.(Carlson et al. 2000; Erdemir et al. 2004) In contrast to this, force transfer between adductor longus and rectus abdominis was only marginal despite structural continuity (Norton‐Old et al. 2013). Though broad definitions of fascia (encompassing tendinous, aponeurotic and ligamentous tissue as well as the muscle fascia itself) emphasizing histological similarity have been proposed (Schleip et al. 2012b), different tissue types might explain the variance in transmitted force. Tissue hydration (Schleip et al. 2012a) as well as temperature (Sapin‐de Brosses et al. 2010) have been shown to alter tissue stiffness, and could contribute to the observed variance in force transfer. In vivo, mechanical stimulation as well as TGF‐β1 expression have been shown to alter myofibroblast activity (Hinz et al. 2001; Tomasek et al. 2002), which could in turn influence tissue stiffness and load transfer.
Second, the assessed outcomes and methods vary considerably between studies and examined body regions. Concerning the cadaveric studies, the type of force application differs widely between trials. The used methods range between mechanically applied forces (van Wingerden et al. 1993; Carlson et al. 2000; Barker et al. 2004; Norton‐Old et al. 2013), simulated gait with a special device (Erdemir et al. 2004), to manual traction with either little (Vleeming et al. 1995) or no description of applied force (Vleeming et al. 1989). Even more important, also methods of transmitted force or strain measurement vary extensively between the included studies. While some authors use sophisticated methods like electronic strain gauges (Carlson et al. 2000; Barker et al. 2004; Norton‐Old et al. 2013) or fibre‐optic force measurements (Erdemir et al. 2004), others report tension transfer based on visual inspection (Vleeming et al. 1989). Others use photographs and report fascial displacement of the affected fascial area as an outcome measure (Vleeming et al. 1995; Barker et al. 2004). The different measurement devices and outcome parameters limit comparability of the results. Interestingly, measurements of tensile force and fascial areas of displacement showed similar trends, but did not correlate significantly in one study (Barker et al. 2004). It seems as if the various outcome parameters do not necessarily represent the same physical or biomechanical tissue properties.
The third factor relates to the use of cadaver specimen for biomechanical testing. Fixation in formalin has been shown to increase cross‐linking in collagenous tissue (Chapman et al. 1990; Abe et al. 2003) and alter hyaluronic acid content (Lin et al. 1997), freezing and thawing of tendon specimen significantly changes their modulus (Clavert et al. 2001), all leading to altered biomechanical properties. Further, the architecture of muscular tissue concerning fibre bundle length and fibre pennation angle differ between cadaveric and in vivo measurements (Martin et al. 2001). In addition, the application of traction, even if applied in the fascicle direction, does most likely not adequately mimic muscular contraction. This raises the question to which extend the biomechanical behaviour of externally induced forces on formalin‐fixed or thawed cadaver specimens can be transferred to the in vivo behaviour of human connective and muscle tissue.
Despite these factors, the current results have several implications for clinical practice. Contracture of the gastrocnemius muscle or tightness of the Achilles tendon are associated with plantar fasciitis and heel pain (Goff & Crawford, 2011; Pascual Huerta, 2014; Solan et al. 2014). Tension transfer from a stiff gastrocnemius–Achilles tendon complex to the plantar fascia is a feasible explanation for these overuse conditions, which is supported by the current findings. The results consequently endorse stretching of the Achilles tendon in the treatment of plantar fasciitis and heel pain (Roxas, 2005; Healey & Chen, 2010; Patel & DiGiovanni, 2011; Garrett & Neibert, 2013). Also, with reference to the FFL, strength imbalances between adductor longus and lower abdominal muscles are associated with groin pain in athletes (Fricker et al. 1991; Anderson et al. 2001; Morales‐Conde et al. 2010; Choi et al. 2011). As in the one included study focusing on tension transfer between both muscles (Norton‐Old et al. 2013), tension was transferred in most of the examined specimens. Improving the strength of the abdominal muscles while releasing tension of the adductors seems to represent a promising treatment for patients with groin pain. Finally, regarding the impact on sport performance, force transfer via myofascial chains might influence strength development and range of motion (ROM) in multisegmental movements as well as during locomotion. Despite some methodological flaws, recent studies have yielded encouraging evidence that previous in vitro findings can be transferred to in vivo conditions. For example, ankle ROM seems to be affected by forward head posture (Hyong & Kim, 2012), passive hamstring stretching tended to increase cervical spine ROM (Hyong & Kang, 2013), self‐myofascial release on the plantar fascia increased sit‐and‐reach performance (Grieve et al. 2015), and ankle ROM seems to be affected not only by knee, but also hip position (Mitchell et al. 2008). These findings endorse incorporating entire myofascial chains in the strength and conditioning process and during flexibility training. Nonetheless, future studies should further investigate the in vivo tension transfer between adjacent myofascial structures or muscle groups.
Conclusion
The present systematic review points towards the fact that tension can be transferred between at least some of the investigated adjacent myofascial structures. However, the heterogeneity in methods of force application as well as the variety of outcome parameters used in the included studies hamper the comparability of the results. Considering anatomical variations in continuity as well as histological differences in the linking structures is crucial when interpreting results. Dissection studies and experiments should preferably be carried out in fresh cadavers, as fixation as well as freezing and thawing have been shown to alter biomechanical properties. In particular, future studies on the in vivo behaviour of adjacent structures should further investigate the practical relevance of the proposed intermuscular myofascial connections for exercise, prevention and rehabilitation.
Author contributions
Frieder Krause: concept and design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and approval of the article. Jan Wilke: concept and design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and approval of the article. Lutz Vogt: concept and design, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and approval of the article. Winfried Banzer: concept and design, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript and approval of the article.
Conflict of interest
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article, so they have no conflict of interest to declare.
References
- Abe M, Takahashi M, Horiuchi K, et al. (2003) The changes in crosslink contents in tissues after formalin fixation. Anal Biochem 318, 118–123. [DOI] [PubMed] [Google Scholar]
- Anderson K, Strickland SM, Warren R (2001) Hip and groin injuries in athletes. Am J Sports Med 29, 521–533. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G (2004) Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine 29, 129–138. [DOI] [PubMed] [Google Scholar]
- Bhattacharya V, Barooah PS, Nag TC, et al. (2010) Detail microscopic analysis of deep fascia of lower limb and its surgical implication. Indian J Plast Surg 43, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojsen‐Moller J, Schwartz S, Kalliokoski KK, et al. (2010) Intermuscular force transmission between human plantarflexor muscles in vivo . J Appl Physiol 109, 1608–1618. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Fleming LL, Hutton WC (2000) The biomechanical relationship between the tendoachilles, plantar fascia and metatarsophalangeal joint dorsiflexion angle. Foot Ankle Int 21, 18–25. [DOI] [PubMed] [Google Scholar]
- Carvalhais VOdC, Ocarino J, Araújo VL, et al. (2013) Myofascial force transmission between the latissimus dorsi and gluteus maximus muscles: an in vivo experiment. J Biomech 46, 1003–1007. [DOI] [PubMed] [Google Scholar]
- Chapman JA, Tzaphlidou M, Meek KM, et al. (1990) The collagen fibril – a model system for studying the staining and fixation of a protein. Electron Microsc Rev 3, 143–182. [DOI] [PubMed] [Google Scholar]
- Choi H, McCartney M, Best TM (2011) Treatment of osteitis pubis and osteomyelitis of the pubic symphysis in athletes: a systematic review. Br J Sports Med 45, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavert P, Kempf JF, Bonnomet F, et al. (2001) Effects of freezing/thawing on the biomechanical properties of human tendons. Surg Radiol Anat 23, 259–262. [DOI] [PubMed] [Google Scholar]
- Cruz‐Montecinos C, Gonzalez Blanche A, Lopez Sanchez D, et al. (2015) In vivo relationship between pelvis motion and deep fascia displacement of the medial gastrocnemius: anatomical and functional implications. J Anat 227, 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdemir A, Hamel AJ, Fauth AR, et al. (2004) Dynamic loading of the plantar aponeurosis in walking. J Bone Joint Surg Am, 86‐A, 546–552. [DOI] [PubMed] [Google Scholar]
- Findley T, Chaudhry H, Dhar S (2015) Transmission of muscle force to fascia during exercise. J Bodyw Mov Ther 19, 119–123. [DOI] [PubMed] [Google Scholar]
- Fricker PA, Taunton JE, Ammann W (1991) Osteitis pubis in athletes. Infection, inflammation or injury? Sports Med 12, 266–279. [DOI] [PubMed] [Google Scholar]
- Garrett TR, Neibert PJ (2013) The effectiveness of a gastrocnemius‐soleus stretching program as a therapeutic treatment of plantar fasciitis. J Sport Rehabil 22, 308–312. [DOI] [PubMed] [Google Scholar]
- Goff JD, Crawford R (2011) Diagnosis and treatment of plantar fasciitis. Am Fam Physician 84, 676–682. [PubMed] [Google Scholar]
- Grieve R, Goodwin F, Alfaki M, et al. (2015) The immediate effect of bilateral self myofascial release on the plantar surface of the feet on hamstring and lumbar spine flexibility: a pilot randomised controlled trial. J Bodyw Mov Ther 9, 544–552. [DOI] [PubMed] [Google Scholar]
- Healey K, Chen K (2010) Plantar fasciitis: current diagnostic modalities and treatments. Clin Podiatr Med Surg 27, 369–380. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Hoang PD, Gandevia SC (2008) Are muscles mechanically independent? J Appl Physiol (1985), 104, 1549–1550. [DOI] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, et al. (2001) Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijing PA, Maas H, Baan GC (2003) Compartmental fasciotomy and isolating a muscle from neighboring muscles interfere with myofascial force transmission within the rat anterior crural compartment. J Morphol 256, 306–321. [DOI] [PubMed] [Google Scholar]
- Huijing PA, van de Langenberg RW, Meesters JJ, et al. (2007) Extramuscular myofascial force transmission also occurs between synergistic muscles and antagonistic muscles. J Electromyogr Kinesiol 17, 680–689. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Yaman A, Ozturk C, et al. (2011) Effects of knee joint angle on global and local strains within human triceps surae muscle: MRI analysis indicating in vivo myofascial force transmission between synergistic muscles. Surg Radiol Anat 33, 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyong IH, Kang JH (2013) The immediate effects of passive hamstring stretching exercises on the cervical spine range of motion and balance. J Phys Ther Sci 25, 113–116. [Google Scholar]
- Hyong IH, Kim JH (2012) The effect of forward head on ankle joint range of motion and static balance. J Phys Ther Sci 24, 925–927. [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62, e1–e34. [DOI] [PubMed] [Google Scholar]
- Lin W, Shuster S, Maibach HI, et al. (1997) Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem 45, 1157–1163. [DOI] [PubMed] [Google Scholar]
- Maas H, Sandercock TG (2008) Are skeletal muscles independent actuators? Force transmission from soleus muscle in the cat J Appl Physiol (1985), 104, 1557–1567. [DOI] [PubMed] [Google Scholar]
- Maas H, Baan GC, Huijing PA (2001) Intermuscular interaction via myofascial force transmission: effects of tibialis anterior and extensor hallucis longus length on force transmission from rat extensor digitorum longus muscle. J Biomech 34, 927–940. [DOI] [PubMed] [Google Scholar]
- Martin DC, Medri MK, Chow RS, et al. (2001) Comparing human skeletal muscle architectural parameters of cadavers with in vivo ultrasonographic measurements. J Anat 199, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Bressel E, McNair PJ, et al. (2008) Effect of pelvic, hip, and knee position on ankle joint range of motion. Phys Ther Sport 9, 202–208. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 339, b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales‐Conde S, Socas M, Barranco A (2010) Sportsmen hernia: what do we know? Hernia 14, 5–15. [DOI] [PubMed] [Google Scholar]
- Myers TW (1997a) The ‘anatomy trains’. J Bodyw Mov Ther 1, 91–101. [Google Scholar]
- Myers TW (1997b) The ‘anatomy trains’: part 2. J Bodyw Mov Ther 1, 135–145. [Google Scholar]
- Myers TW (2014) Anatomy Trains: Myofascial Meridians for Manual and Movement Therapists. London: Churchill Livingstone. [Google Scholar]
- Norton‐Old KJ, Schache AG, Barker PJ, et al. (2013) Anatomical and mechanical relationship between the proximal attachment of adductor longus and the distal rectus sheath. Clin Anat 26, 522–530. [DOI] [PubMed] [Google Scholar]
- Pascual Huerta J (2014) The effect of the gastrocnemius on the plantar fascia. Foot Ankle Clin 19, 701–718. [DOI] [PubMed] [Google Scholar]
- Patel A, DiGiovanni B (2011) Association between plantar fasciitis and isolated contracture of the gastrocnemius. Foot Ankle Int 32, 5–8. [DOI] [PubMed] [Google Scholar]
- Roxas M (2005) Plantar fasciitis: diagnosis and therapeutic considerations. Altern Med Rev 10, 83–93. [PubMed] [Google Scholar]
- Sapin‐de Brosses E, Gennisson J, Pernot M, et al. (2010) Temperature dependence of the shear modulus of soft tissues assessed by ultrasound. Phys Med Biol 22, 1701–1718. [DOI] [PubMed] [Google Scholar]
- Schleip R, Klingler W, Lehmann‐Horn F (2005) Active fascial contractility: fascia may be able to contract in a smooth muscle‐like manner and thereby influence musculoskeletal dynamics. Med Hypotheses 65, 273–277. [DOI] [PubMed] [Google Scholar]
- Schleip R, Duerselen L, Vleeming A, et al. (2012a) Strain hardening of fascia: static stretching of dense fibrous connective tissues can induce a temporary stiffness increase accompanied by enhanced matrix hydration. J Bodyw Mov Ther 16, 94–100. [DOI] [PubMed] [Google Scholar]
- Schleip R, Jäger H, Klingler W (2012b) What is ‘fascia’? A review of different nomenclatures. J Bodyw Mov Ther 16, 496–502. [DOI] [PubMed] [Google Scholar]
- Sherrington C, Herbert RD, Maher CG, et al. (2000) PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther 5, 223–226. [DOI] [PubMed] [Google Scholar]
- Solan MC, Carne A, Davies MS (2014) Gastrocnemius shortening and heel pain. Foot Ankle Clin 19, 719–738. [DOI] [PubMed] [Google Scholar]
- Staubesand J, Li Y (1996) Zum Feinbau der Fascia cruris mit besonderer Berücksichtigung epi‐ und intrafaszialer Nerven. Manuelle Medizin 34, 196–200. [Google Scholar]
- Stecco C, Porzionato A, Macchi V, et al. (2006) Histological characteristics of the deep fascia of the upper limb. Ital J Anat Embryol 111, 105–110. [PubMed] [Google Scholar]
- Stecco C, Gagey O, Belloni A, et al. (2007) Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie 91, 38–43. [DOI] [PubMed] [Google Scholar]
- Stecco C, Porzionato A, Lancerotto L, et al. (2008) Histological study of the deep fasciae of the limbs. J Bodyw Mov Ther 12, 225–230. [DOI] [PubMed] [Google Scholar]
- Stecco A, Macchi V, Stecco C, et al. (2009) Anatomical study of myofascial continuity in the anterior region of the upper limb. J Bodyw Mov Ther 13, 53–62. [DOI] [PubMed] [Google Scholar]
- Stecco A, Antonio S, Gilliar W, et al. (2013a) The anatomical and functional relation between gluteus maximus and fascia lata. J Bodyw Mov Ther 17, 512–517. [DOI] [PubMed] [Google Scholar]
- Stecco A, Gesi M, Stecco C, et al. (2013b) Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep 17, 352. [DOI] [PubMed] [Google Scholar]
- Stecco C, Corradin M, Macchi V, et al. (2013c) Plantar fascia anatomy and its relationship with Achilles tendon and paratenon. J Anat 223, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesarz J, Hoheisel U, Wiedenhofer B, et al. (2011) Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 194, 302–308. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, et al. (2002) Myofibroblasts and mechano‐regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3, 349–363. [DOI] [PubMed] [Google Scholar]
- van Tulder TM, Furlan A, Bombardier C, et al. (2003) Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976), 28, 1290–1299. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Stoeckart R, Snijders CJ (1989) The sacrotuberous ligament: a conceptual approach to its dynamic role in stabilizing the sacroiliac joint. Clin Biomech (Bristol, Avon) 4, 201–203. [Google Scholar]
- Vleeming A, Pool‐Goudzwaard AL, Stoeckart R, et al. (1995) The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine (Phila Pa 1976), 20, 753–758. [PubMed] [Google Scholar]
- Wager E, Wiffen PJ (2011) Ethical issues in preparing and publishing systematic reviews. J Evid Based Med 4, 130–134. [DOI] [PubMed] [Google Scholar]
- van der Wal J (2009) The architecture of the connective tissue in the musculoskeletal system – an often overlooked functional parameter as to proprioception in the locomotor apparatus. Int J Ther Massage Bodywork 2, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke J, Krause F, Niederer D, et al. (2015) Appraising the methodological quality of cadaveric studies: validation of the QUACS scale. J Anat 226, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke J, Krause F, Vogt L, et al. (2016) What is evidence‐based about myofascial chains? A systematic review Arch Phys Med Rehabil 97, 454–61. [DOI] [PubMed] [Google Scholar]
- Willard FH, Vleeming A, Schuenke MD, et al. (2012) The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat 221, 507–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingerden JP, Vleeming A, Snijders CJ, et al. (1993) A functional‐anatomical approach to the spine‐pelvis mechanism: interaction between the biceps femoris muscle and the sacrotuberous ligament. Eur Spine J 2, 140–144. [DOI] [PubMed] [Google Scholar]
- Yahia L, Rhalmi S, Newman N, et al. (1992) Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand 63, 195–197. [DOI] [PubMed] [Google Scholar]
- Yucesoy CA (2010) Epimuscular myofascial force transmission implies novel principles for muscular mechanics. Exerc Sport Sci Rev 38, 128–134. [DOI] [PubMed] [Google Scholar]


