Abstract
In quadrupeds the musculature of the hindlimbs is expected to be responsible for generating most of the propulsive locomotory forces, as well as contributing to body support by generating vertical forces. In supporting the body, postural changes from crouched to upright limbs are often associated with an increase of body mass in terrestrial tetrapods. However, felids do not change their crouched limb posture despite undergoing a 300‐fold size increase between the smallest and largest extant species. Here, we test how changes in the muscle architecture (masses and lengths of components of the muscle‐tendon units) of the hindlimbs and lumbosacral region are related to body mass, to assess whether there are muscular compensations for the maintenance of a crouched limb posture at larger body sizes. We use regression and principal component analyses to detect allometries in muscle architecture, with and without phylogenetic correction. Of the muscle lengths that scale allometrically, all scale with negative allometry (i.e. relative shortening with increasing body mass), whereas all tendon lengths scale isometrically. Only two muscles' belly masses and two tendons' masses scale with positive allometry (i.e. relatively more massive with increasing body mass). Of the muscles that scale allometrically for physiological cross‐sectional area, all scale positively (i.e. relatively greater area with increasing body mass). These muscles are mostly linked to control of hip and thigh movements. When the architecture data are phylogenetically corrected, there are few significant results, and only the strongest signals remain. None of the vertebral muscles scaled significantly differently from isometry. Principal component analysis and manovas showed that neither body size nor locomotor mode separate the felid species in morphospace. Our results support the inference that, despite some positively allometric trends in muscle areas related to thigh movement, larger cats have relatively weaker hindlimb and lumbosacral muscles in general. This decrease in power may be reflected in relative decreases in running speeds and is consistent with prevailing evidence that behavioural changes may be the primary mode of compensation for a consistently crouched limb posture in larger cats.
Keywords: anatomy, biomechanics, effective mechanical advantage, locomotion, mammal, morphometrics
Introduction
In terrestrial tetrapods, where there are evolutionary increases in body masses there tend to be changes in limb posture from crouched to upright to avoid potential increases in stresses within the supportive tissues, whose relative strengths tend not to vary (Biewener, 1989, 1990, 2005). Extant felids are unusual in that they maintain the same crouched posture from the smallest species to the largest (Day & Jayne, 2007) throughout their ~1–300 kg range of body masses (Cuff et al. 2015). In addition, felids mostly capture prey using ambushes and short, high‐speed pursuits. Larger felids (above cheetah, Acinonyx jubatus, size) seem to suffer from reduced locomotor performance relative to their smaller relatives (e.g. range of speeds: Garland, 1983; Day & Jayne, 2007), which may be emphasised more strongly in Felidae than in some other mammals due to their conserved limb postures. Previous work on the scaling of the limb bones in felids shows that long bone lengths in both the hind‐ and forelimbs scale isometrically with body mass (Anyonge, 1993; Christiansen & Harris, 2005; Doube et al. 2009). However, diameters and cross‐sectional areas of those bones scale with positive allometry, meaning long bones become relatively more robust (and stiffer and stronger as a consequence) in larger felid species (Doube et al. 2009; Meachen‐Samuels & Van Valkenburgh, 2009, 2010; Lewis & Lague, 2010). Similar patterns have been found for vertebral dimensions in felids, indicating that some degree of skeletal allometry may help to support loads on the spine that might otherwise incur greater stresses as body mass increases. However, the lumbar region tends to show relatively weaker allometry than is observed in the cervicothoracic regions (Jones, 2015; Randau et al. 2016).
Muscles generate greater moments around joints partly by increasing moment arms (i.e. by lengthening the distance of muscle action from the joint), increasing the mechanical advantage of the muscles; e.g. as potentially present for the M. gastrocnemius on felid calcanei (Gálvez‐Lopéz & Casinos, 2012). Although larger animals might not forestall increases in tissue stresses if they do not straighten their limbs to increase their limbs' effective mechanical advantage (EMA) (Biewener, 1989, 1990, 2005), maintaining a crouched posture at larger body sizes may otherwise increase the ability to generate horizontal (as opposed to vertical) forces, needed in accelerations and manoeuvring. As the hindlimbs generally are the main propulsive drivers in the locomotion of felids, their muscles must be able to provide forces and power that are capable of generating the required forward movement and acceleration. Across mammalian quadrupeds, this force requirement tends to be largely achieved through an increase of the volume of hip extensor musculature (Alexander et al. 1981; Usherwood & Wilson, 2005; Williams et al. 2008, 2009). The same or similar extensor (e.g. antigravity) muscles must also be able to support the animal's body weight. The impulse (force‐time integral) required for this support is equivalent to the product of the animal's body weight and stride time (Alexander & Jayes, 1978). At faster speeds the foot is in contact with the ground for a shorter period of time (shorter stance time) and a smaller proportion of the stride (decreasing duty factor). Therefore, peak limb force must increase (Witte et al. 2004) and the muscles must be able to generate larger amounts of forces and joint moments to sustain this limb force.
In addition, during the swing phase the hindlimbs must be protracted quickly enough to reposition them in time for the next stance phase. This capacity for limb protraction is limited by the limbs' inertia (Lee et al. 2004), the internal muscle architecture (including maximal contraction velocity of the muscle fibres), and the moment arms of the muscles (Hudson et al. 2011a,b). In fast‐running tetrapods there tends to be a reduction in muscle mass towards the distal ends of limbs, in which the distal muscles transmit their forces down long tendons (Alexander et al. 1981; Alexander & Jayes, 1983; Payne et al. 2005; Smith et al. 2006, 2007; Hudson et al. 2011a,b). This tapering of the limbs reduces their inertial properties and therefore reduces the amount of power that would otherwise be required from the muscles to swing the limb (Hudson et al. 2011b). Additional energy savings are achieved by using long tendons to store elastic strain energy, contributing to the bouncing dynamics of locomotion and enabling the muscles to remain closer to optimal isometric activity during steady‐state locomotion (Alexander, 1984; Alexander & Maloiy, 1989). In addition to the limbs, the vertebral musculature is important for locomotion in quadrupeds, whether being used in active dynamic flexion and extension of the spine, or for stabilisation of the spine in larger taxa (Boszczyk et al. 2001).
Here we measure the architecture of the musculature of the hindlimb and lumbosacral vertebrae in a range of felid species, spanning almost their full spectrum of body sizes, to quantify patterns of musculoskeletal scaling and interpret their biomechanical consequences. This work follows that of Cuff et al. (2016) on scaling of the forelimb, cervical and thoracic musculature across extant felids. We hypothesise that, as in the forelimbs (Cuff et al. 2016), many of the muscles involved in limb and body support scale with positive allometry such that the muscles are more adept at supporting the increasing body masses. We further hypothesise that muscle fascicles scale with negative allometry (i.e. shortening), whereas tendons scale with positive allometry (i.e. lengthening), as is common in other cursorial tetrapods (Alexander, 1977; Pollock & Shadwick, 1994a,b). We finally predict that, as with the cervico‐thoracic vertebral muscles (Cuff et al. 2016), the lumbosacral musculature scales indistinguishably from isometry.
Methods
Muscle data collection
The methodological protocol used here is identical to that described in detail in Cuff et al. (2016). In brief, the species studied in this study were the black‐footed cat (Felis nigripes: NMS.Z.2015.90; male), domestic cat (Felis catus: Royal Veterinary College, JRH uncatalogued personal collection; female), caracal (Caracal caracal: NMS.Z.2015.89.1; male), ocelot (Leopardus pardalis: NMS.Z.2015.88; male), cheetah (Acinonyx jubatus: data from Hudson et al. 2009a,b), snow leopard (Panthera uncia: NMS.Z.2015.89.2; female), jaguar (Panthera onca: NMS.Z.2014.67.2; female), Sumatran tiger (Panthera tigris sondaica: NMS.Z.2015.91; female), and Asian lion (Panthera leo persica: NMS.Z.2015.128; female) (Table 1). No specimens were euthanised for the purposes of this research. The institutional abbreviation NMS refers to the National Museums Scotland, Department of Natural Sciences. All body mass and dissection data are included in the Supporting Information (Table S1).
Table 1.
Specimens dissected in this study. Sex: F = female, M = Male or Mix = both (unspecified)
| Common name | Species | Sex | Body mass (kg) | General condition |
|---|---|---|---|---|
| Black‐footed cat | Felis nigripes | F | 1.1 | Underweight |
| Domestic cat | Felis catus | F | 2.66 | Underweight |
| Caracal | Caracal caracal | M | 6.6 | Underweight |
| Ocelot | Leopardus pardalis | M | 9.6 | Overweight |
| Cheetah | Acinonyx jubatus | Mix | 33.1 average | Unknown |
| Snow leopard | Panthera uncia | F | 36 | OK |
| Jaguar | Panthera onca | F | 44 | OK |
| Sumatran tiger | Panthera tigris sondaica | F | 86 | OK |
| Asian lion | Panthera leo persica | F | 133 | Overweight |
Dissection
All specimens were frozen shortly after death and then defrosted (variably 24–48 h) prior to dissection except the Asian lion, which was dissected 1 day postmortem without any freezing or thawing. Initially, each specimen had the limbs from one side removed and refrozen, allowing for future dissection if the initial material was incomplete or damaged. The muscles from the hindlimb and vertebral column were dissected individually and muscle architecture was measured following standard procedures (e.g. Alexander et al. 1981; Hudson et al. 2011a). For each muscle the following architectural parameters were measured: muscle belly length and mass, tendon length and mass, muscle fascicle length and pennation angle (at least three for each muscle, but up to 10 for some specimens, depending on muscle size and variation of fascicle dimensions). These data were used to calculate physiological cross‐sectional area (PCSA) for each muscle using Eq. (1):
| (1) |
where density is 1060 kg m−3 (typical vertebrate muscle, Mendez & Keys, 1960), and then with Eq. (2):
| (2) |
In total 38 hindlimb muscles were measured for all nine species, producing up to 228 metrics per species, and three vertebral muscles, producing up to 18 metrics per species. For most species, fewer than 12 metrics were missing in total. The exception is the cheetah, as the data taken from Hudson et al. (2011a) yielded only 50% completeness for hindlimb measures (only muscle mass, fascicle length and PCSA were usable; no tendon measurements were provided).
Scaling (regression) analysis
The data for muscle belly length and mass, tendon length and mass, fascicle length, and PCSA were subjected to a series of scaling analyses. Where tendon lengths and masses could not be measured (because there were no tendons), those data were removed before scaling analyses. Metrics for which there were data from fewer than three species were removed, but only metrics with at least six measures will be discussed (although the results from metrics with fewer measures, if significant, are displayed in Tables 1, 2, 3, 4, 5, 6). The data were log10‐transformed, and then each logged metric was regressed against log10 body mass, using standardised reduced major axis (SMA) regression in the ‘smatr' package (Warton et al. 2012) in r 3.1.0 (R Core Team, 2014) software. Significances of the regression line relative to isometry and the correlation (r 2) between each metric and body mass were determined using bootstrapped 95% confidence intervals (2000 replicates). Isometry is defined as scaling patterns that match the slope expected for a given increase in body size (i.e. maintaining geometric similarity), and allometry represents increases or decreases from that slope. For the logged metrics, isometry is defined as follows: muscle or tendon masses scale against body mass with slope equal to 1.00; muscle or tendon lengths scale against body mass with a slope of 0.333 (i.e. length is proportional to mass1/3); and muscle PCSA scales against body mass with a slope of 0.667 (i.e. area is proportional to mass2/3).
Table 2.
RMA results for log muscle belly lengths against log body mass, displaying only those that differ significantly from an isometric slope value of 0.333. Results with significant r 2 are indicated in bold. No results were significant after phylogenetic correction. Upper and lower limits represent 95% confidence intervals of the slope, ‘slope P' represents statistical probability of the slope differing from isometry, the ‘r 2 P' shows the statistical significance of the correlation. All results including non‐significant patterns are provided in Supporting Information
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Piriformis | 0.167 | 0.101 | 0.276 | 0.013 | −1.43 | 0.722 | 0.008 | 8 |
| Peroneus brevis | 0.192 | 0.112 | 0.330 | 0.047 | −1.14 | 0.677 | 0.012 | 8 |
| Soleus | 0.212 | 0.147 | 0.304 | 0.021 | −1.06 | 0.863 | 0.001 | 8 |
| Gastrocnemius medialis | 0.262 | 0.216 | 0.317 | 0.022 | −1.14 | 0.963 | 0.000 | 8 |
| Semitendinosus | 0.279 | 0.242 | 0.322 | 0.023 | −0.980 | 0.980 | 0.000 | 8 |
| After correction | ||||||||
| None | ||||||||
Table 3.
Significant RMA (before and after phylogenetic correction) scaling results for log tendon lengths plotted against log body mass, displaying only those that differ from an isometric slope value of 0.333. Results with significant r 2 shown in bold. Column headings as in Table 2
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Superficial dig. flex. | 0.887 | 0.369 | 2.134 | 0.031 | −2.48 | 0.007 | 0.846 | 8 |
| After correction | ||||||||
| None | ||||||||
Table 4.
Significant RMA (before and after phylogenetic correction) scaling results for log muscle fascicle lengths plotted against log body mass, displaying only those that differ from an isometric slope value of 0.333. Results with significant r 2 are shown in bold. Column headings as in Table 2
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Dig. ext. lateralis | −0.185 | −0.300 | −0.114 | 0.022 | −1.26 | 0.684 | 0.006 | 9 |
| Vastus intermedius | 0.617 | 0.374 | 1.018 | 0.021 | −2.15 | 0.659 | 0.008 | 9 |
| Peroneus brevis | 0.716 | 0.349 | 1.469 | 0.038 | −2.60 | 0.234 | 0.187 | 9 |
| Psoas major | 0.936 | 0.417 | 2.101 | 0.019 | −2.11 | 0.580 | 0.078 | 6 |
| Adductor magnus | 1.20 | 0.567 | 2.523 | 0.002 | −2.02 | 0.162 | 0.282 | 9 |
| After correction | ||||||||
| None | ||||||||
Table 5.
Significant RMA (before and after phylogenetic correction) scaling results for log muscle body mass plotted against log body mass, displaying only those that differ from an isometric slope value of 1.00. Results with significant r 2 are shown in bold. Column headings as in Table 2
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Vastus intermedius | 0.796 | 0.650 | 0.976 | 0.033 | −2.619 | 0.947 | 0.000 | 9 |
| Gluteus medius | 1.22 | 1.12 | 1.33 | 0.001 | −2.800 | 0.991 | 0.000 | 9 |
| After correction | ||||||||
| Gluteus medius | 1.25 | 1.08 | 1.45 | 0.010 | 0.010 | 0.978 | 0.000 | 9 |
Table 6.
Significant RMA (before and after phylogenetic correction) scaling results for log tendon mass plotted against log body mass, displaying only those that differ from an isometric slope value of 1.00. Results with significant r 2 are shown in bold. Column headings as in Table 2
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Long dig. ext. | 1.57 | 1.06 | 2.31 | 0.029 | −4.610 | 0.841 | 0.001 | 9 |
| Superficial dig. flex. | 1.71 | 1.15 | 2.54 | 0.014 | −4.47 | 0.836 | 0.001 | 8 |
| Psoas major | 1.72 | 1.08 | 2.76 | 0.042 | −5.129 | 0.999 | 0.024 | 7 |
| After correction | ||||||||
| None | ||||||||
As closely related species tend to have characteristics more similar to each other, and as in felids large body masses are only found in a few clades (Cuff et al. 2015), we tested variables for phylogenetic signal. Each variable was analysed using the phylosignal function in the ‘picante' package (Kembel et al. 2010) in r, which measures phylogenetic signal using the K statistic. The phylogeny used for this analysis was from Piras et al. (2013), which was pruned to include only the taxa in this study. Metrics which were found to have significant phylogenetic signal underwent correction using independent contrasts in r, before the contrast data were subjected to SMA, as implemented in the ‘smatr' package (Warton et al. 2012) in r. However, as phylogenetic SMA does not tolerate missing data, each metric was analysed independently, dropping any taxa with missing data for that metric.
Principal components analysis and manovas
Principal component (PC) analyses were also carried out on the unlogged muscle data. As PC analyses require complete datasets, any missing values were imputed based on observed instances for each variable, using r 3.1.2 software. The imputed data were calculated iteratively until convergence was achieved (German & Hill, 2006; Ilin & Raiko, 2010). The resulting ‘complete' dataset was entered into past 2.17c (Hammer et al. 2001) software. The ‘allometric vs. standard' option within the ‘remove size from distances' tool was used to remove the effects of body size upon the metrics. The felid species were assigned to groups first by body size (i.e. small cat vs. big cat species, following Cuff et al. 2015; although here defined as Panthera vs. non‐Panthera species), and in a second analysis by locomotor mode (following Meachen‐Samuels & Van Valkenburgh, 2009; terrestrial: F. nigripes, Acinonyx jubatus, P. tigris, Panthera leo; scansorial: F. silvestris, C. caracal, L. pardalis, P. uncia, P. onca). Significant PC scores were then tested for body size and locomotory signal using manovas with and without phylogenetic correction in the ‘geomorph' package (Adams & Otarola‐Castillo, 2013) in r.
Results
Limb muscles
Prior to phylogenetic correction the belly lengths for M. piriformis, M. peroneus brevis, M. soleus, M. gastrocnemius medialis and M. semitendinosus all displayed significant negative allometry (i.e. relative shortening as body mass increases) (Table 2, Fig. 1). After phylogenetic correction, only the M. soleus remained significantly negatively allometric (Table 2, Fig. 2). None of the tendon lengths exhibited significant allometry before or after phylogenetic correction (Table 3). Prior to phylogenetic correction, the fascicle lengths for M. extensor digitorum lateralis and M. vastus intermedius showed significant allometry: the M. lateral digital extensor fascicles scaled with negative allometry (again, relative shortening), and M. vastus intermedius scaled with positive allometry (Table 4). After phylogenetic correction, no fascicle lengths scaled significantly differently from isometry (slope of 0.333) (Table 4).
Figure 1.
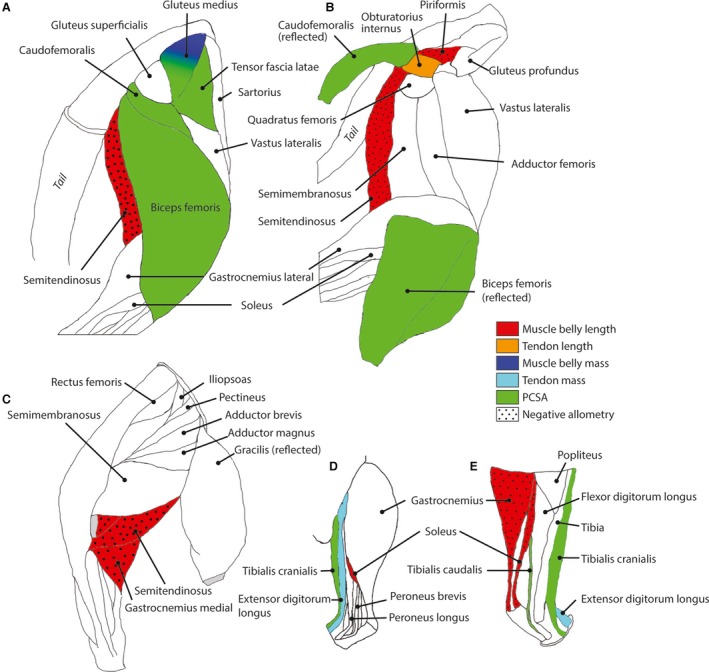
Muscles displaying potential allometry (prior to phylogenetic analysis) in the studied felid species are shown in colour; others as white; for a representative right hindlimb. (A) Lateral superficial muscles of hip and knee. (B) Lateral, deeper muscles of the hindlimb. (C) Medial muscles of the thigh and shank. (D) Lateral muscles of the lower leg. (E) Medial muscles of the lower leg. Red = muscle belly length; orange = tendon length; navy blue = muscle mass; light blue = tendon mass; green = PCSA. Stippling pattern is for negative allometry. Muscles not shown: M. psoas majorum (Table 6); M. vastus intermedius (Tables 4 and 5); M. lateral digital extensor (Table 4); M. superficial digital flexor (Table 6); M. peroneus brevis (Table 2).
Figure 2.
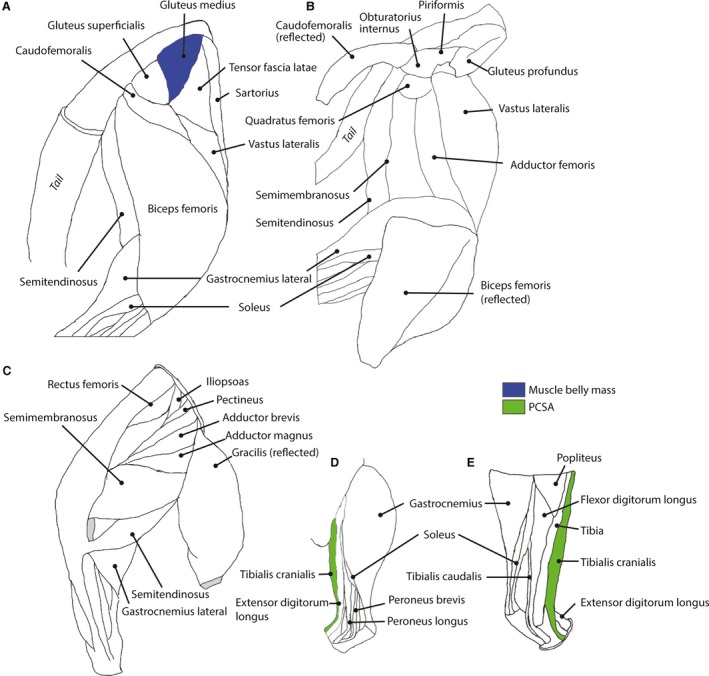
Muscles displaying potential allometry (after phylogenetic analysis) in the studied felid species are shown in colour; others as white; for a representative right hindlimb. (A) Lateral superficial muscles of hip and knee. (B) Lateral, deeper muscles of the hindlimb. (C) Medial muscles of the thigh and shank. (D) Lateral muscles of the lower leg. (E) Medial muscles of the lower leg. Navy blue = muscle mass; green = PCSA. Stippling pattern is for negative allometry.
For the muscle belly masses, two muscles initially showed significant allometry; the M. vastus intermedius scaled with negative allometry (i.e. relatively less massive with increasing body mass) and the M. gluteus medius scaled with positive allometry (Table 5, Fig. 1). After phylogenetic correction, only the M. gluteus medius retained significantly positive allometry (Table 5, Fig. 2). The tendon masses for the M. psoas major and M. extensor digitorum longus both showed significant positive allometry prior to phylogenetic correction, but no tendon masses scaled significantly differently from isometry after phylogenetic correction (Table 6). Before phylogenetic correction, seven muscles' PCSAs scaled with positive allometry (Table 7, Fig. 1) (i.e. relatively greater area with increasing body mass): the M. gluteus medius, M. gemelli, M. biceps femoris, M. tensor fascia latae, M. caudofemoralis, M. tibialis caudalis, and the M. tibialis cranialis. After phylogenetic correction, only the PCSA of the M. tibialis cranialis remained significantly positively allometric with body mass (Table 7, Fig. 2).
Table 7.
Significant RMA (before and after phylogenetic correction) scaling results for log physiological cross‐sectional area plotted against log body mass, displaying only those that differ from an isometric slope value of 0.667. Results with significant r 2 are shown in bold. Column headings as in Table 2
| Muscle | Slope | Lower limit | Upper limit | slope P | Intercept | r 2 | r 2 P | n |
|---|---|---|---|---|---|---|---|---|
| Before correction | ||||||||
| Biceps femoris | 0.862 | 0.680 | 1.09 | 0.037 | −4.18 | 0.929 | 0.000 | 9 |
| Caudal tibial | 0.977 | 0.790 | 1.21 | 0.003 | −4.90 | 0.943 | 0.000 | 9 |
| Gluteus medius | 1.00 | 0.769 | 1.31 | 0.008 | −4.39 | 0.910 | 0.000 | 9 |
| Tensor fascia latae | 1.05 | 0.725 | 1.52 | 0.022 | −4.75 | 0.821 | 0.001 | 9 |
| Gemelli | 1.10 | 0.739 | 1.64 | 0.021 | −5.05 | 0.832 | 0.002 | 8 |
| Tibialis cranialis | 1.12 | 0.847 | 1.49 | 0.003 | −5.08 | 0.897 | 0.000 | 9 |
| Caudofemoralis | 1.17 | 0.781 | 1.74 | 0.012 | −5.40 | 0.788 | 0.001 | 9 |
| After correction | ||||||||
| Tibialis cranialis | 1.14 | 0.698 | 1.85 | 0.036 | 0.017 | 0.743 | 0.006 | 9 |
| Caudofemoralis | 1.32 | 0.680 | 2.56 | 0.045 | −0.036 | 0.491 | 0.053 | 9 |
Vertebral muscles
None of the vertebral muscle metrics showed significant difference from isometry either before or after phylogenetic correction (Supporting Information Table S2).
Principal components analyses and phylogenetic manovas
PCA of all of the metrics for the hindlimb muscles alone produced eight significant PC axes according to the Joliffe cutoff, which is automatically generated in past. PC1 represented 28.5% of the total variance, PC2 was 15.4%, with PC3‐8 representing between 12.8 and 4.5% (Fig. 3). There was no significant separation between body size or locomotory groups using either a manova or phylogenetic manova of all PCs (P ≫ 0.05 in all analyses). Adding data from lumbosacral vertebral muscles did not improve the ability to distinguish among either body size or locomotor groupings (P ≫ 0.05).
Figure 3.
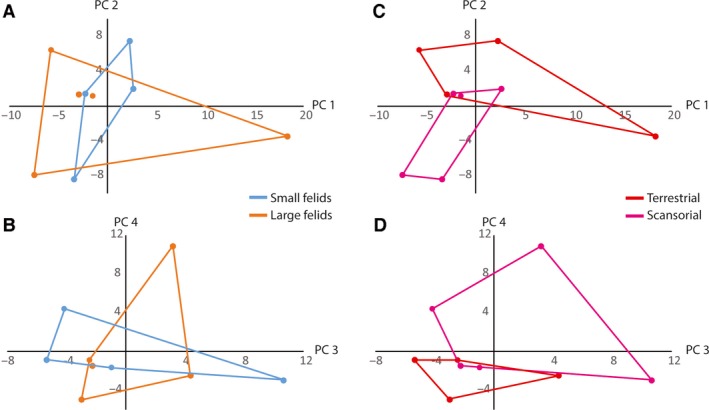
Principal component analysis of hind limb muscle architecture metrics. (A,B) Body size groups, with blue for small felids and orange for large felids (groupings follow Cuff et al. 2015). (C,D) Locomotory mode groups with red for terrestrial and pink for scansorial. (A,C) PC1 (28.48% of total variance) vs. PC 2 (15.39% of total variance). (C,D) PC3 (12.83% of total variance) vs. PC 4 (11.24% of total variance).
Discussion
In quadrupeds, the hindlimbs are usually the main propulsive drivers (Alexander, 1977; Alexander et al. 1981; Hudson et al. 2011a), and as such play more roles than just limb‐maintaining support against gravity. The muscles responsible for such roles are primarily the hip extensors (Alexander, 1977; Alexander et al. 1981; Usherwood & Wilson, 2005; Williams et al. 2008, 2009; Hudson et al. 2011a). Therefore it should be expected that these muscles will scale with at least isometry, or possibly positive allometry, for the muscle belly measurements and PCSA (a metric which is linked to force production). Our results showed that most thigh muscle metrics actually scaled isometrically, or at least with allometry that is indistinguishable from isometry, in our dataset. In the thigh only the M. gluteus medius, M. tensor fascia latae, M. caudofemoralis and M. biceps femoris have PCSAs that scale positively allometrically, with the M. biceps femoris (weakly positively allometric), and the M. gluteus medius being responsible for thigh extension (the rest are used in adduction or rotation). Because the muscles' cross‐sectional areas scaled isometrically proportional to mass2/3, most muscles of the thigh appear to be relatively weaker in larger species of felids.
In quadrupeds able to move rapidly, as taxa become larger, there tends to be a reduction in muscle mass towards the distal ends of limbs, in which the distal muscles transmit their forces down long tendons (Alexander & Jayes, 1983; Payne et al. 2005; Smith et al. 2006, 2007). Cheetahs have been noted to exhibit some similar degree of limb tapering (Hudson et al. 2011a,b). This reduction of distal limb muscle mass does not appear to be the case in felids in general, with all distal muscles' masses scaling isometrically, and only the tendon mass of M. extensor digitorum longus scaled with positive allometry. In felids, this would result in an increase in inertial properties and therefore require more work and power from the muscles to swing the hindlimbs (Hudson et al. 2011b), and with no apparent increase in elastic energy storage by the tendons (Alexander, 1984; Alexander & Maloiy, 1989), thereby reducing the overall efficiency of the hindlimbs in larger taxa. This may be because most felids have to retain limbs that are powerful enough for climbing and capturing prey as well as being ‘light' enough for fast locomotion. Perhaps owing to its fast pursuit of prey, the cheetah is the only felid that shows marked limb tapering and, as a consequence of its less powerful limbs, tends to feed on relatively smaller prey. Interestingly, a few muscle belly lengths actually scale with negative allometry (Table 4), but this length is not compensated for in any way with positively allometric tendons or muscle fascicles that display unambiguous negative allometry. Previous work indicates that the bone lengths of felid limbs scale isometrically (Anyonge, 1993; Christiansen & Harris, 2005; Doube et al. 2009), but if there is a shortening of some muscle bellies, and no corresponding increase in tendon lengths, there may potentially be some subtle positional changes of these muscles between the taxa or an increase in musculotendinous compliance (Roberts, 2002). Alternatively, with the small sample size, there may just be some outliers within our data, but this would require more specimens to test.
The lack of general allometric increase in muscle PCSAs suggests that felid limbs become relatively weaker at larger body sizes, especially with no reduction in distal limb muscle mass and no increase in tendon masses or lengths across most of the limb, and no change in limb posture (Day & Jayne, 2007; Zhang et al. 2012; Doube et al. 2009) as alternative compensatory mechanisms. As terrestrial mammals get larger, maintaining a crouched posture becomes increasingly energetically expensive due to the muscles of the limbs having to balance the moments incurred by the body weight, and the resulting vertical ground reaction forces. The advantage of remaining crouched is that it maximises the horizontal component of the ground reaction forces' moment arms, potentially allowing for increased locomotor performance in a horizontal direction (Biewener, 1989, 1990, 2005). However, as felid limb posture does not seem to change with body mass and the muscle force‐capacities (linked to PCSA) appear to decrease, it might be predicted that larger felids become relatively slower and incur greater metabolic costs during similar behaviours due to lower mechanical efficiency. Indeed, Day & Jayne (2007) found that the velocity of locomotion within felids (during walking) is broadly similar across all species, consistent with the theory of dynamic similarity (Alexander & Jayes, 1983). Furthermore, Garland (1983) found that larger cats (beyond an optimal body mass of ~ 40 kg) move more slowly than smaller ones. However, felids may partially compensate for the near‐isometric muscle scaling by the seemingly increased mechanical advantage of the felid calcaneus (Gálvez‐Lopéz & Casinos, 2012). Although evidence for allometry of that mechanical advantage is not strong, if present it may help counter the isometric scaling of the gastrocnemius, which is the largest (in terms of PCSA and thus force potential) antigravity muscle in the hindlimb, although further work is required on both the muscles and bones.
Muscle fascicle lengths are linked to contractile speed and range of motion, with longer fascicles able to contract faster and over a longer range of motion than smaller ones (Alexander, 1977; Alexander et al. 1981). Typically for most Carnivora, the fascicle lengths scale indistinguishably from isometry across the hindlimb (Alexander et al. 1981). Our results broadly fit this pattern of near‐isometric scaling, with one exception. In our dataset, inverse allometry of muscle fascicle length (where the slope is actually negative rather than only less than the isometric slope) was detected for the M. digitorum extensor lateralis. Thus, bigger cats have shorter fascicle lengths (in an absolute and relative sense) than smaller cats for the M. digitorum extensor lateralis, which becomes increasingly multipennate in form, resulting in a slower digital extension or more limited range of motion in larger cat species. What role this may play in their ecology and locomotion is, however, uncertain.
The limb muscles, nonetheless, do not work in isolation; the vertebral muscles also play important roles in support and locomotion. All vertebral muscles' metrics from the lumbosacral region scale isometrically in felids; therefore the vertebral muscles also seem to become relatively weaker with increasing body mass. However, this relative weakening of the musculature of the vertebral muscles may be compensated for by positive allometry of vertebrae and the resulting moment arms in other vertebral regions (Jones, 2015; Randau et al. 2016). The combined results for the vertebral muscles (here and Cuff et al. 2016) show that there is a relative reduction in force production capacity in the spinal musculature of larger felids. This lack of clear allometry of the intervertebral musculature may have consequences for the maximum extension of the spine (a vital component in maximising stride length and, therefore, maximum speed: Hildebrand, 1959), although positive allometry in the lever arms may compensate (Jones, 2015; Jones & Pierce, 2016). However, how the complex interactions of musculoskeletal anatomy, limb posture, range of spinal motion and gait relate to tissue stresses or safety factors across the body size range of Felidae remains unclear and deserves further study. We also accept there are limitations to the current study as all the individuals were captive, of varying degrees of health, and all of our measurements were from a single individual from each species (or, in the case of the lion and tiger, a single subspecies), and not all of the same sex (with the largest species all represented by females), but we have no reason to expect this would change our overall conclusions. For a more in‐depth discussion of these limitations see Cuff et al. (2016).
In the forelimbs of felids, only those metrics with the strongest allometric signals remained significantly different from isometry after phylogenetic correction (Cuff et al. 2016), and indeed broadly similar results were obtained for the hindlimbs of felids, with only two metrics of 228 displaying allometry after correction. With so many muscles scaling indistinguishably from isometry (or scaling only weakly allometrically), there is no separation of the taxa using PCAs or manovas when assessing body mass groupings (Cuff et al. 2015) or locomotor mode either before or after phylogenetic correction. This will remain an issue in muscle scaling studies at least until larger sample sizes are studied, particularly in felids, with many of the largest felids being closely related members of the genus Panthera (the exceptions being the cheetah and puma, which convergently evolved larger body sizes: Cuff et al. 2015). This close relationship of large‐bodied felids (i.e. Panthera) means that any potentially allometric patterns are more difficult to tease apart from the null hypothesis of similarity due to common ancestry, and it is thus more difficult to distinguish modest allometry from true isometry in the musculoskeletal system of Felidae. However, the dataset provided here is an important step forward in understanding how felid locomotor muscles scale with body mass, and future efforts can test our findings by building on this dataset.
Conclusions
Unlike the predominantly supportive, deceleratory and prehensile roles of the forelimb muscles, the musculature of the hindlimb is responsible for generating most of the acceleratory forces during typical (e.g. steady‐state) locomotion in felids. However, the majority of propulsive (and other) hindlimb muscles appear to scale isometrically across Felidae, with only the strongest allometries remaining significant after phylogenetic correction. As a consequence, larger felids have relatively weaker hindlimb muscles than those of their smaller relatives, consistent with the reduction in relative and even absolute locomotor speeds as observed in other studies (Garland, 1983; Day & Jayne, 2007). The vertebral muscles emphasise these results further, with all of the metrics scaling indistinguishably from isometry. Furthermore, multivariate analysis (PCA) of muscle metrics was unable to distinguish between locomotor modes and body mass difference, which may be due in part to the phylogenetic proximity of most large‐ and small‐bodied felids (Cuff et al. 2015).
Supporting information
Table S1. Raw muscle architecture measures for hindlimb and lumbosacral vertebrae.
Table S2. RMA results for all muscles before and after phylogenetic correction.
Acknowledgements
This work was funded by Leverhulme Trust grant RPG 2013‐124 to A.G. and J.R.H. A.C.K. thanks the Aspinall Foundation (Port Lympne Wild Animal Park), the Zoological Society of East Anglia (Banham Zoo), the Cat Survival Trust, Thrigby Hall Wildlife Gardens, Cromer Zoo and the Zoological Society of London (London Zoo) for donation of specimens used in this study. A.C.K. is grateful to the Negaunee Foundation for its support of the Curatorial Preparator at National Museums Scotland. We thank two anonymous reviewers for comments that improved the manuscript.
References
- Adams DC, Otarola‐Castillo E (2013) geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4, 393–399. [Google Scholar]
- Alexander R (1977) Allometry of the limbs of antelopes (Bovidae). J Zool Lond 183, 125–146. [Google Scholar]
- Alexander R (1984) Elastic energy stores in running vertebrates. Am Zool 24, 85–94. [Google Scholar]
- Alexander R, Jayes AS (1978) Vertical movements in walking and running. J Zool Lond 185, 27–40. [Google Scholar]
- Alexander R, Jayes AS (1983) A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J Zool 201, 135–152. [Google Scholar]
- Alexander R, Maloiy GMO (1989) Locomotion of African mammals. Sym Zool S 61, 163–180. [Google Scholar]
- Alexander R, Jayes AS, Maloiy GMO, et al. (1981) Allometry of the leg muscles of mammals. J Zool Lond 194, 539–552. [Google Scholar]
- Anyonge W (1993) Body mass in large extant and extinct carnivore. J Zoo 231, 339–384. [Google Scholar]
- Biewener AA (1989) Scaling body support in mammals: limb posture and muscle mechanics. Science 245, 45–48. [DOI] [PubMed] [Google Scholar]
- Biewener AA (1990) Biomechanics of mammalian terrestrial locomotion. Science 250, 1097–1103. [DOI] [PubMed] [Google Scholar]
- Biewener AA (2005) Biomechanical consequences of scaling. J Exp Biol 208, 1665–1676. [DOI] [PubMed] [Google Scholar]
- Boszczyk BM, Boszczyk AA, Putz R (2001) Comparative and functional anatomy of the mammalian lumbar spine. Anat Rec 264, 157–168. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Harris JM (2005) The body size of Smilodon (Mammalia:Felidae). J Morph 266, 369–384. [DOI] [PubMed] [Google Scholar]
- Cuff AR, Randau M, Head J, et al. (2015) Big cat, small cat: reconstructing body size evolution in living and extinct Felidae. J Evolution Biol 28, 1516–1525. [DOI] [PubMed] [Google Scholar]
- Cuff AR, Sparkes E, Randau M, et al. (2016) The scaling of postcranial muscles in cats (Felidae) I: forelimb, cervical, and thoracic muscles. J Anat 229, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day LM, Jayne BC (2007) Interspecific scaling of the morphology and posture of the limbs during the locomotion of cats (Felidae). J Exp Biol 210, 642–654. [DOI] [PubMed] [Google Scholar]
- Doube M, Wiktorowicz‐Conroy A, Christiansen P, et al. (2009) Three‐dimensional geometric analysis of felid limb bone allometry. PLoS ONE 4, e4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez‐Lopéz E, Casinos A (2012) Scaling and mechanics of the felid calcaneus: geometric similarity without differential allometric scaling. J Anat 220, 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr (1983) Scaling the ecological cost of transport to body mass in terrestrial mammals. Am Nat 121, 571–587. [Google Scholar]
- German A, Hill J (2006) Data Analysis Using Regression and Multilevel/Hierarchical Models (Analytical Methods for Social Research). New York: Cambridge University Press. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4, 9. [Google Scholar]
- Hildebrand M (1959) Motions of the running cheetah and horse. J Mamm 40, 281–495. [Google Scholar]
- Hudson PE, Corr SA, Payne‐Davis RC, et al. (2011a) Functional anatomy of the cheetah (Acinonynx jubatus) hindlimb. J Anat 218, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson PE, Corr SA, Payne‐Davis RC, et al. (2011b) Functional anatomy of the cheetah (Acinonynx jubatus) forelimb. J Anat 218, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilin A, Raiko T (2010) Practical approaches to principal component analysis in the presence of missing values. J Mach Learn Res 11, 1957–2000. [Google Scholar]
- Jones KE (2015) Evolutionary allometry of lumbar shape in Felidae and Bovidae. Biol J Linn Soc Lond 116, 721–740. [Google Scholar]
- Jones KE, Pierce SE (2016) Axial allometry in a neutrally buoyant environment: effects of the terrestrial aquatic transition on vertebral scaling. J Evol Biol 29, 594–601. doi:10.1111/jeb.12809. [DOI] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, et al. (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. [DOI] [PubMed] [Google Scholar]
- Lee DV, Stakebake EF, Walter RM, et al. (2004) Effects of mass distribution on the mechanics of level trotting in dogs. J Exp Biol 207, 1715–1728. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Lague MR (2010) Interpreting sabretooth cat (Carnivora; Felidae; Machariodontinae) postcranial morphology in light of scaling patterns in felids Carnivoran Evolution: New Views on Phylogeny, Form and Function. pp. 411–465, Cambridge: Cambridge University Press. [Google Scholar]
- Meachen‐Samuels J, Van Valkenburgh B (2009) Forelimb indicators of prey‐size preference in the Felidae. J Morphol 270, 729–744. [DOI] [PubMed] [Google Scholar]
- Meachen‐Samuels JA, Van Valkenburgh B (2010) Radiographs reveal exceptional forelimb strength in the sabretooth cat, Smilodon fatalis . PLoS ONE 5, e11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J, Keys A (1960) Density and composition of mammalian muscles. Metabolism 9, 184–188. [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, et al. (2005) Functional specialisation of pelvic limb anatomy in horses (Equus caballus) . J Anat 206, 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras P, Maiorino L, Teresi L, et al. (2013) Bite of the cats: relationships between functional integration and mechanical performance as revealed by mandible geometry. Syst Biol 62, 878–900. [DOI] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE (1994a) Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am J Physiol Regulat Integr Comp Physiol 266, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Pollock CM, Shadwick RE (1994b) Relationship between body mass and biomechanical properties of limb tendons in adult mammals. Am J Physiol Regulat Integr Comp Physiol 266, 1016–1021. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org/. [Google Scholar]
- Randau M, Goswami A, Hutchinson JR, et al. (2016) Cryptic complexity in felid vertebral evolution: shape differentiation and allometry of the axial skeleton. Zool J Linnean Soc. doi: 10.1111/zoj.12403. [Google Scholar]
- Roberts TJ (2002) The integrated function of muscles and tendons during locomotion. Comp Biochem Phys A 133, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. (2006) Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus). J Anat 209, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NC, Wilson AM, Jespers KJ, et al. (2007) Muscle moment arms of pelvic limb muscles of the ostrich (Struthio camelus). J Anat 211, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood JR, Wilson AM (2005) Biomechanics: no force limit on greyhound sprint speed. Nature 438, 753–754. [DOI] [PubMed] [Google Scholar]
- Warton DI, Duursma RA, Falster DS, et al. (2012) smatr 3 – an R package for estimation and inference about allometric lines. Methods Ecol Evol 3, 257–259. [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, et al. (2008) Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris). J Anat 213, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Usherwood JR, Jespers K, et al. (2009) Exploring the mechanical basis for acceleration: pelvic limb locomotor function during acceleration in the racing greyhound (Canis familiaris). J Exp Biol 212, 550–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte TH, Knill K, Wilson AM (2004) Determination of peak vertical ground reaction force from duty factor in the horse (Equus caballus). J Exp Biol 207, 3639–3648. [DOI] [PubMed] [Google Scholar]
- Zhang KY, Wiktorowicz‐Conroy A, Hutchinson JR, et al. (2012) 3D Morphometric and posture study of felid scapulae using statistical shape modelling. PLoS ONE 7, e34619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Raw muscle architecture measures for hindlimb and lumbosacral vertebrae.
Table S2. RMA results for all muscles before and after phylogenetic correction.


