Abstract
Formation of the costimulatory axis between the B7-2 and CD28 coreceptors is critical for T-cell activation. Superantigens, Gram-positive bacterial virulence factors, cause toxic shock and sepsis by hyperinducing inflammatory cytokines. We report a novel role for costimulatory receptors CD28 and B7-2 as obligatory receptors for superantigens, rendering them therapeutic targets. We show that by engaging not only CD28 but also its coligand B7-2 directly, superantigens potently enhance the interaction between B7-2 and CD28, inducing thereby T-cell hyperactivation. Using a conserved twelve amino-acid domain, superantigens engage both B7-2 and CD28 at their homodimer interfaces, sites far removed from where these receptors interact, implying that inflammatory signaling can be controlled through the receptor homodimer interfaces. Short B7-2 and CD28 dimer interface mimetic peptides bind diverse superantigens, prevent superantigen binding to cell-surface B7-2 or CD28, attenuate inflammatory cytokine overexpression, and protect mice from lethal superantigen challenge. Thus, superantigens induce a cytokine storm by mediating not only the interaction between MHC-II molecule and T-cell receptor but critically, by promoting B7-2/CD28 coreceptor engagement, forcing the principal costimulatory axis to signal excessively. Our findings highlight the B7/CD28 interaction as a bottleneck in signaling for expression of inflammatory cytokines. B7-2 and CD28 homodimer interface mimetic peptides prevent superantigen lethality by blocking the superantigen-host costimulatory receptor interaction.
Keywords: bacterial superantigens, inflammatory cytokine storm, toxic shock, costimulatory receptors, B7-2, B7-2 dimer interface peptides, CD28
Introduction
Superantigens from Staphylococcus aureus and Streptococcus pyogenes induce toxic shock and sepsis by activating an immune response, orders of magnitude beyond that elicited by ordinary antigens. Superantigens exploit the main axis of T-cell activation by binding directly as intact proteins to most major histocompatibility class II (MHC-II) and T-cell receptor (TCR) molecules outside their antigen-binding domains, linking them and bypassing restricted presentation of conventional antigens which typically activate <<1% of T cells, thereby activating up to 20-30% of T cells [1-3]. The exaggerated inflammatory response (‘cytokine storm’) that results is harmful to the host.
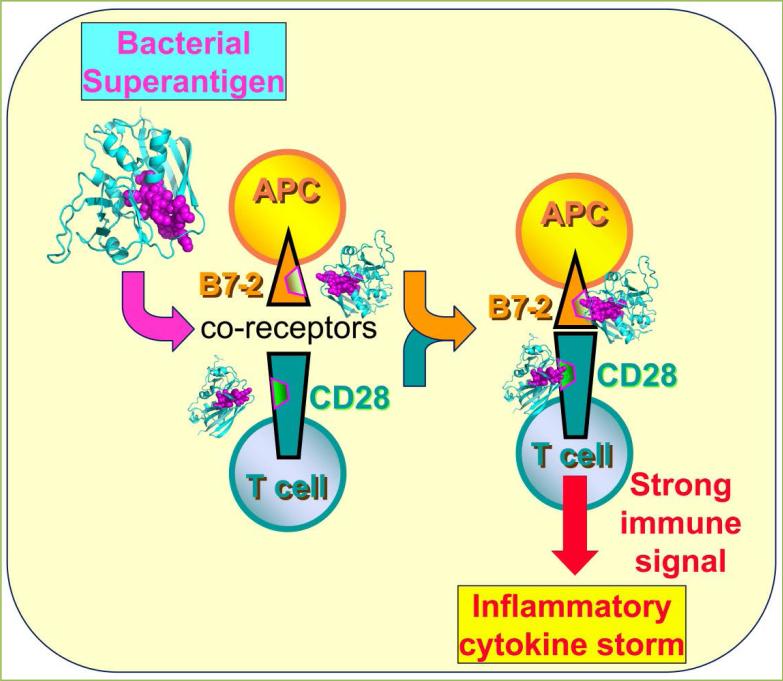
Until recently, it was thought that this is the mechanism through which superantigens hyperinduce an inflammatory response. However, in our search for superantigen antagonists, we discovered that superantigens directly facilitate not one but two synaptic events between antigen-presenting cell and T cell: (1) interaction of MHC-II with TCR, and (2) interaction between the primary costimulatory receptors B7-2 and CD28, potently enhancing formation of the B7-2/CD28 costimulatory axis that is critical for T-cell activation. Indeed, preventing the direct binding of a superantigen molecule to the B7-2 or CD28 costimulatory receptor, with short peptide mimetics of the contact domains in any one of these three proteins, suffices to protect from lethal toxic shock. Our finding is that engagement of B7-2 and CD28 receptors can be regulated via their homodimer interfaces, a property exploited by the superantigens to their advantage (Fig. 1).
Figure 1. Superantigens induce a cytokine storm by binding to B7-2 and CD28, enhancing costimulatory axis formation.
Two SEB molecules bind, through their accessible β-strand/hinge/α-helix domain (magenta spheres), B7-2 and CD28 in their extracellular domains at the homodimer interfaces (outlined in magenta), thereby potently enhancing B7-2/CD28 engagement and inflammatory signaling. For clarity, simultaneous engagement of TCR and MHC-II molecule by SEB and the second monomer in the CD28 dimer were omitted.
A conserved domain in superantigens is essential for their function
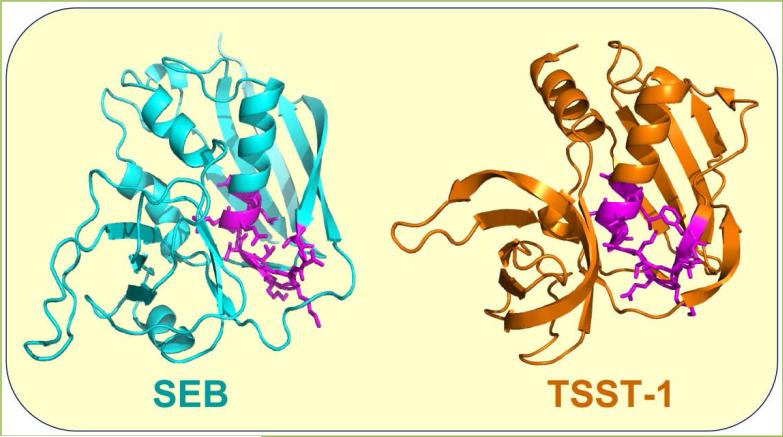
Induction of human inflammatory cytokine gene expression by divergent superantigens is inhibited by a short peptide that protects mice from their lethal effect [4]. The peptide shows homology to a β-strand-hinge-α-helix superantigen domain of 12 amino acids, remote from the MHC-II and TCR binding sites. This finding was surprising because it was believed initially that the region of the superantigen molecule, in which this domain is located, is dispensable for function. The broad family of superantigens exhibits high conservation within this domain and despite sequence differences among diverse superantigens, shows overall spatial conservation of the amino acid backbone [4]. Thus, although toxic shock syndrome toxin-1 (TSST-1) shares only 6% overall sequence identity with staphylococcal enterotoxin B (SEB), its β-strand/hinge/α-helix domain has a similar backbone fold (Fig. 2).
Figure 2. The β-strand/hinge/α-helix superantigen domain: spatial conservation of the amino acid backbone.
In structures of SEB (3SEB.pdb) and TSST-1 (4OHJ.pdb), backbone and side chains of the β-strand/hinge/α-helix domain are depicted in magenta.
The conservation of the β-strand/hinge/α-helix domain and broad-spectrum protective efficacy of peptide mimetics of this domain demonstrated that it exerts an essential function in eliciting a cytokine storm and lethality, but the nature of this function remained unknown [4]. We hypothesized that this domain, which is accessible (Fig. 2), might serve to engage an as yet unknown receptor.
CD28 is an obligatory receptor for superantigens
CD28 is the principal costimulatory receptor. CD28 has a critical function in regulating the immune response by providing the second signal mandatory for T-cell activation [5-7]. Constitutively expressed on T cells as a homodimer, CD28 must interact with either of its two B7 coreceptors in order to transduce the signal essential for an immediate T-cell response [6-9]. The coreceptor B7-2 (CD86) is expressed constitutively on the antigen-presenting cell (APC) whereas B7-1 (CD80) is induced later in the immune response [9, 10]. The B7-2/CD28 interaction thus regulates early signaling by antigens [11, 12]. Apart from its B7 coreceptors needed for costimulation, CD28 was not thought to engage any other ligand.
A first hint in our search for a putative novel superantigen receptor was provided by the finding that a peptide mimetic of the superantigen β-strand/hinge/α-helix domain (Fig. 2) inhibited inflammatory cytokine gene expression in human peripheral blood mononuclear cells (PBMC) in the absence of any superantigen, induced by anti-CD28/anti-CD3 monoclonal antibodies, which models the normal T cell-mediated immune response, or by anti-CD28 alone, yet not by anti-CD3 alone [13]. We considered the intriguing possibility that the peptide might compete with the anti-CD28 antibody for its binding site in CD28 and by extension, that the intact superantigen molecule might bind directly to CD28. To test this concept, we next screened a random phage display library for peptides that bind to CD28 and are displaced from CD28 by SEB. Indeed, such peptides could be recovered; they strongly inhibited SEB-mediated induction of inflammatory cytokines in human PBMC and protected mice from lethal SEB challenge [13]. Confocal microscopy then showed that labeled SEB colocalizes fully with CD28 expressed on the surface of CD28-transfected cells, providing a strong indication that even in the absence of MHC-II and TCR, the superantigen will bind directly to the extracellular domain of the CD28 receptor [13]. Direct binding experiments then documented that through its β-strand/hinge/α-helix domain, SEB binds directly to CD28 [13].
We next sought to map the binding site for superantigen toxins within CD28. This proved a difficult challenge [14]. Since we knew that a peptide mimetic of the β-strand/hinge/α-helix domain in SEB blocks cytokine gene expression in human PBMC induced by anti-CD28, we mapped the epitope of this monoclonal antibody, speculating that it might be the site where the SEB mimetic peptide binds, and by extension, holo-SEB. A peptide having the 9 amino-acid CD28 epitope sequence indeed showed potent antagonist activity against SEB in human PBMC [13]. CD28 exhibits extensive homology with the related costimulatory receptor, CTLA-4 [5]. The CD28 epitope aligned with a completely distinct sequence in CTLA-4 that constitutes part of the CTLA-4 homodimer interface. Another portion of the composite homodimer interface in CTLA-4 is located well over 100 amino acids upstream. Should the CD28 dimer interface, which at the time had not yet been resolved, be involved in binding SEB, we argued, then a peptide in CD28 having an 8 amino-acid sequence, located at the corresponding site upstream yet totally distinct from the equivalent sequence in CTLA-4, might also be able to antagonize SEB. This proved indeed to be the case [13]. Both of these CD28 mimetic peptides bound superantigens, blocked cytokine gene induction in human PBMC by superantigens, including by TSST-1, and protected mice from lethal superantigen challenge [13, 15]. Crystallographic analysis [16] confirmed our hunch that we had identified the CD28 dimer interface correctly; five peptides, spanning all dimer interface residues, each proved a superantigen antagonist [13].
Thus, through the β-strand-hinge-α-helix domain essential for superantigen action [4], superantigens engage CD28 directly at its homodimer interface [13]. Blocking access of a superantigen to CD28, with peptide mimetics of the CD28 dimer interface or of the β-strand-hinge-α-helix superantigen domain, suffices to block signaling for overexpression of inflammatory cytokines in human PBMC and to protect mice from lethal toxic shock [4, 13, 15]. This work established that CD28 is not only a costimulatory receptor but in a novel role, serves as an obligatory receptor for superantigens. Nonetheless, the mechanism underlying the indispensable role of binding CD28 in superantigen signaling remained elusive.
B7-2 is an obligatory receptor for superantigens
The foregoing studies led us to ask whether the primary coligand of CD28, B7-2, might also have a function in superantigen action. Once again, our peptide mimetic of the β-strand-hinge-α-helix domain in superantigens proved useful. Induction of inflammatory cytokine gene expression by anti-CD3 together with the soluble extracellular domain of B7-2, a model for joint signaling through the TCR and B7-2/CD28 costimulatory pathway, was inhibited by this peptide [17]. The observation that a superantigen mimetic peptide inhibits signaling dependent on B7-2 could be explained, on one hand, by the direct interaction of this peptide with CD28 that we had already demonstrated [13], resulting in attenuation of CD28 signaling. Alternatively, however, the explanation might be that the peptide binds B7-2 and thereby inhibits signaling through the B7-2/CD28 axis. We thus hypothesized that through its β-strand-hinge-α-helix domain, the superantigen might engage B7-2 directly.
Our hunch was borne out by direct binding studies [17]. SEB bound selectively to the surface of cells transfected to express B7-2. This indicated that even in the absence of its CD28, MHC-II and TCR receptors, SEB binds directly to cell-surface B7-2. Indeed, SEB bound soluble B7-2 extracellular domain protein when either was immobilized, as judged by direct immunoassay or by surface plasmon resonance, and as the free proteins in solution, shown by microscale thermophoresis. As for CD28, the superantigen binds to B7-2 with micromolar affinity [17]. Mutation of two highly conserved residues in the β-strand/hinge/α-helix domain of SEB that are critical for binding of CD28 [13], abrogated binding of SEB to cell-surface B7-2, demonstrating specificity. Direct binding analysis showed that soluble B7-2 binds to the peptide mimetic of the β-strand-hinge-α-helix domain. Thus, using the same domain, the superantigen engages B7-2 and CD28.
To map the binding site for superantigen in B7-2, we created a set of short peptides from the extracellular domain of this coreceptor. Only peptides that overlapped with residues in the B7-2 crystallographic homodimer interface [5] were capable of inhibiting SEB-mediated induction of inflammatory cytokines in PBMC, and of protecting mice from SEB lethality, whereas peptides that fall outside the dimer interface failed to do so. The B7-2 dimer interface mimetics bound diverse superantigens directly, with a KD in the micromolar range, demonstrating that we had mapped the dimer interface as the binding site [17]. The hydrophilic B7-2 dimer interface [18] has no known role in costimulatory signaling and is well separated from the CD28 binding site. Whereas CD28 is a strong, covalent homodimer [16], B7-2 forms only an extremely weak, noncovalent homodimer and exists mostly as a monomer on the cell surface [12, 19]. This renders the binding of superantigens into the B7-2 dimer interface even more remarkable. Considering that B7-2 and CD28 function each as direct superantigen receptor and that the superantigen engages both through its β-strand/hinge/α-helix domain, at any given time a single superantigen molecule can bind to only one of these two costimulatory ligands. Accordingly, two superantigen molecules are needed to engage both CD28 and B7-2 (Fig. 1).
Once we had evidence that superantigens use the same domain - the β-strand/hinge/α-helix domain - to engage both CD28 and B7-2, we predicted that dimer interface mimetic peptides derived from either receptor would compete with both intact cell-surface receptors for superantigen, to inhibit binding of superantigen to either CD28 or B7-2. This turned out to be indeed the case [17]. Thus, the superantigen not only uses its β-strand/hinge/α-helix domain to engage either B7-2 or CD28 at their dimer interface but peptide mimetics of either dimer interface possess dual antagonist activity, blocking binding of superantigen to either receptor in a reciprocal manner.
In conclusion, to elicit an inflammatory cytokine response, SEB must engage not only CD28 but also its coligand B7-2, raising the question how these direct interactions promote strong T-cell activation.
Mechanism: the superantigen strongly enhances the B7-2/CD28 interaction
A single superantigen molecule facilitates the MHC-II/TCR interaction by binding simultaneously to both receptors, linking them together [3]. By contrast, our finding is that a single superantigen molecule cannot bind both CD28 and B7-2 at the same time [17]. Nonetheless, we hypothesized that the binding of superantigen molecules to each B7-2 and CD28 might enhance B7-2/CD28 receptor engagement. To examine this concept, we expressed CD28 or B7-2 on the surface of transfected cells and studied the effect of SEB on binding of soluble B7-2 and CD28, respectively. This strategy allowed us to measure the B7-2/CD28 interaction in the absence of multiple ligand-receptor interactions that underlie synapse formation between the antigen-presenting cell and the T cell, involving not only MHC-II/TCR but also other costimulatory ligand pairs whose expression could change upon SEB exposure, resulting in changes in synapse strength.
SEB strongly enhanced binding of B7-2 to cell-surface CD28, even at low concentrations. Conversely, binding of CD28 to cell-surface B7-2 was stimulated by an order of magnitude by SEB. Here, too, enhanced binding was detectable already at low SEB concentrations [17]. We next used flow cytometry to validate that SEB promotes B7-2/CD28 synapse formation between cells that express B7-2 and CD28, respectively, in their native state on the cell membrane. A splice variant of B7-2 unable to bind CD28 failed to support significant cell-cell adhesion, demonstrating specificity. Even though flow cytometry will not distinguish a synapse formed by a single intercellular B7-2/CD28 pair from one supported by multiple pairs, rendering it less sensitive than binding of the soluble coreceptors, SEB had a pronounced stimulatory effect on B7-2/CD28-mediated intercellular synapse formation, observed already at low SEB concentrations [17]. These results provide a mechanism for why the superantigen must bind B7-2 and CD28 directly: this strongly upregulates the B7-2/CD28 interaction, the primary costimulatory axis mandatory for T-cell activation (Fig. 1).
Conclusion
We have shown that through its β-strand/hinge/α-helix domain, the superantigen binds not only to the homodimer interface of CD28 but also to the homodimer interface of its coligand, B7-2, and provide a molecular mechanism for how this dual binding achieves excessive signaling for T-cell activation. Although the two receptor dimer interfaces are remote from the domains where CD28 and B7-2 engage one another, by binding both dimer interfaces, the superantigen potently enhances the interaction between B7-2 and CD28 (Fig. 1). Thus, superantigens directly facilitate not one but two events in the formation of the immune synapse between antigen-presenting cell and T cell: interaction of MHC-II with TCR, and interaction of B7-2 with CD28. These results reveal a hitherto unknown, novel function for the superantigen: to promote formation of the B7-2/CD28 costimulatory axis, critical for full T-cell activation. Binding of superantigen to each B7-2 and CD28 may be accommodated as shown in Fig. 1, although conceivably, binding of the superantigen to only one receptor within a given pair, rather than to both, may suffice for T cell hyperactivation, as long as both are engaged within the immunological synapse, which contains multiple copies of each of these receptors. We have shown that the superantigen enhances the interaction between B7-2 and CD28 even without the need for engaging MHC-II and TCR. To our knowledge, this is the first example whereby a pathogen-associated molecule, in this case a bacterial superantigen toxin, hyperinduces cytokines by strongly enhancing formation of the B7-2/CD28 costimulatory receptor axis.
We provide a novel, host-oriented therapeutic approach to block the indispensible interaction of the superantigen with B7-2 and CD28 dimer interfaces, through short peptide mimetics of the B7-2 dimer interface. Such peptides bind diverse superantigens, prevent binding of superantigen to cell-surface B7-2 or CD28, inhibit superantigen-mediated induction of inflammatory cytokines in human PBMC, and are effective antagonists in vivo, protecting mice from lethal superantigen challenge [17].
Acknowledgments
This work was supported by grants UC1AI067231 and 2U54AI057168 from the United States National Institute of Allergy and Infectious Diseases.
Abbreviations
- APC
antigen-presenting cell
- MHC-II
major histocompatibility class II molecule
- PBMC
peripheral blood mononuclear cells
- SEB
staphylococcal enterotoxin B
- TCR
T-cell receptor
- TSST-1
toxic shock syndrome toxin-1
Footnotes
To cite this article: Raymond Kaempfer, et al. Bacterial superantigen toxins induce a lethal cytokine storm by enhancing B7-2/CD28 costimulatory receptor engagement, a critical immune checkpoint. Receptor Clin Invest 2017; 4: e1500. doi: 10.14800/rci.1500.
Author Contributions
R.K., Z.R., R.L. and G.A. designed research, R.L., A.P., D.H., G.A. and Z.R. performed experiments, R.K., Z.R. and R.L. analyzed the results.
Conflicting interests
The authors have declared that no conflict of interests exists.
References
- 1.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH, Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leder L, Llera A, Lavoie PM, Lebedeva MI, Li H, Sékaly RP, et al. A mutational analysis of the binding of staphylococcal enterotoxins B and C3 to the T cell receptor beta chain and major histocompatibility complex class II. J Exp Med. 1998;187:823–833. doi: 10.1084/jem.187.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med. 2000;6:414–421. doi: 10.1038/74672. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 7.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 8.Lindsten T, Lee KP, Harris ES, Petryniak B, Craighead N, Reynolds PJ, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 9.Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 10.Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, et al. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol Lett. 2006;104:70–75. doi: 10.1016/j.imlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Arad G, Levy R, Nasie I, Hillman D, Rotfogel Z, Barash U, et al. Binding of superantigen toxins into the CD28 homodimer interface is essential for induction of cytokine genes that mediate lethal shock. PLoS Biol. 2011;9:e1001149. doi: 10.1371/journal.pbio.1001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaempfer R, Arad G, Levy R, Hillman D, Nasie I, Rotfogel Z. CD28: direct and critical receptor for superantigen toxins. Toxins (Basel) 2013;5:1531–1542. doi: 10.3390/toxins5091531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran G, Tulapurkar ME, Harris KM, Arad G, Shirvan A, Shemesh R, et al. A peptide antagonist of CD28 signaling attenuates toxic shock and necrotizing soft-tissue infection induced by Streptococcus pyogenes. J Infect Dis. 2013;207:1869–1877. doi: 10.1093/infdis/jit104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 17.Levy R, Rotfogel Z, Hillman D, Popugailo A, Arad G, Supper E, et al. Superantigens hyperinduce inflammatory cytokines by enhancing the B7-2/CD28 costimulatory receptor interaction. Proc Natl Acad Sci USA. 2016;113:E6437–E6446. doi: 10.1073/pnas.1603321113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Schwartz JC, Almo SC, Nathenson SG. Crystal structure of the receptor-binding domain of human B7-2: insights into organization and signaling. Proc Natl Acad Sci USA. 2003;100:2586–2591. doi: 10.1073/pnas.252771499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatia S, Edidin M, Almo SC, Nathenson SG. Different cell surface oligomeric states of B7-1 and B7-2: implications for signaling. Proc Natl Acad Sci USA. 2005;102:15569–15574. doi: 10.1073/pnas.0507257102. [DOI] [PMC free article] [PubMed] [Google Scholar]