Abstract
Guidelines conflict regarding recommendations for prostate-specific antigen (PSA) screening for early detection of prostate cancer. The United States Preventive Services Task Force (USPSTF) assigned a grade of D (recommending against screening) for men 75 and older in 2008 and for men of all ages in 2012. We reviewed temporal trends in rates of screening before and after the 2012 recommendation based on a literature search for studies published between 2011/01/01–2016/10/03 on PSA utilization patterns, changes in prostate cancer incidence and biopsy patterns, and how the recommendation has shaped physician and patient attitudes about PSA screening and subsequent ordering of other screening tests. Rates of PSA screening decreased by 3–10 percentage points among all age groups and within most U.S. geographic regions. Rates of prostate biopsy and prostate cancer incidence have declined in unison, with a notable shift towards higher grade, stage and risk upon detection. Despite the recommendation, some physicians reported ongoing willingness to screen appropriately selected men, and men largely reported intending to continue to ask for the PSA test. In the coming years, we expect to have a better picture of whether these decreased rates of screening will impact prostate cancer metastasis and mortality.
Keywords: prostate cancer, screening, USPSTF, incidence, prostate-specific antigen
INTRODUCTION
Prostate-specific antigen (PSA) screening for early detection and timely treatment of prostate cancer (PC) is controversial. Two large-scale randomized clinical trials focused on the efficacy of PSA screening: the European Randomized Study of Screening for Prostate Cancer (ERSPC) and the Prostate, Lung, Colorectal and Ovarian (PLCO) trial. The ERSPC found a PC mortality rate ratio of 0.79 (95% CI 0.68–0.98) in favor of screening after 11 years of follow-up1; however, the PLCO trial found no significant reduction in PC-specific mortality within the screening arm compared to the control arm2. These two trials also elucidated the harms of screening, such as false-positive results leading to unnecessary biopsies and treatment side effects including erectile dysfunction and incontinence. In 2012, after again considering the benefits and harms, the United States Preventive Services Task Force (USPSTF) concluded “there is moderate certainty that the benefits of PSA-based screening for prostate cancer do not outweigh the harms”, particularly because “the harms of PSA-based screening for prostate cancer include a high rate of false-positive results and accompanying negative psychological effects, high rate of complications associated with diagnostic biopsy, and—most important—a risk for overdiagnosis coupled with overtreatment.”3 Prior to 2008, the USPSTF had assigned a grade of I to PSA screening, indicating there was insufficient evidence of either the merits or harms of screening to make an informed recommendation4. In 2008, the USPSTF changed their recommendation to a D grade for PSA screening in men 75 and older, stating that the benefits do not outweigh the harms in this population5. In May 2012 (following a draft recommendation in October, 2011), the USPSTF updated their recommendation to a D grade for PSA screening in all men, discouraging against the practice altogether3.
It is understandable why a guideline group recommends against PSA-screening as a response to the non-optimal screening and treatment practices seen in the U.S. including excessive rates of screening among older men with limited life expectancy or multiple comorbidities, unlikely to benefit6–8. These men have been screened at rates equal to, or even in excess of, those of younger, healthier men, who are more likely to benefit from PSA testing, early detection, and treatment. Similarly, there has been excessive overtreatment of men with low risk tumors in the U.S. over the past two decades9, contributing to treatment-related harm and deterioration in quality of life. It is only in recent years that active surveillance for low risk tumors has been increasing (40% in 2010–2013 as compared to ~10% during 1990–2009).9
The USPSTF recommendation is one of several guidelines for PSA screening. Others include those issued by the American Cancer Society, American Urological Association, European Association of Urology, National Comprehensive Cancer Network and American College of Physicians. Most guidelines emphasize shared decision-making10; however, they diverge in areas such as age to start and stop screening, screening intensity and the PSA level at which to refer a patient for prostate biopsy10–13. Many of the guidelines agree that screening does not benefit men with a life expectancy of less than 10 years12, 13.
In this review, we examine temporal trends in PSA screening and PC incidence that have emerged in the years following the 2012 USPSTF recommendation against PSA screening in all men. We also evaluate how this recommendation has shaped physician and patient attitudes about PSA screening. Finally, we discuss the implications of these trends with respect to PSA screening practice and PC outcomes.
METHODS
We utilized PubMed.gov to search MEDLINE, the U.S. Library of Medicine database of over 22 million indexed citations to articles published in approximately 5,600 biomedical journals from over 80 countries. We searched for publications about PSA screening and/or PC that specifically referenced the 2012 USPSTF recommendation. Appendix 1 contains the full search strategy employed. Articles were limited to those published in English between 2011/01/01– 2016/10/03. Two of the authors (SVC and MJR) screened the results, independently categorizing them for inclusion/exclusion based on title and/or abstract. In cases of discrepancy, inclusion was based on consensus. The full text of each article selected for inclusion was retrieved. We included original articles and commentaries/editorials.
In total, the search yielded 119 articles, of which 63 were selected for inclusion, the majority from the database search and a handful of which were identified through hand search.
In addition, and to avoid publication bias, we searched Embase (which indexes conference proceedings as a source) on all of the journal titles that publish the proceedings from each of the following associations’ annual conferences: European Association of Urology (EAU), American Urological Association (AUA), American Society of Clinical Oncology (ASCO), American Academy of Family Physicians (AAFP), and the Society of General Internal Medicine (SGIM) of which abstracts that did not overlap with concurrent publications were included. The search yielded 135 abstracts of which 26 were included.
RESULTS
PSA screening rates
PSA testing patterns overall
Since its inception in the late 1980s, PSA screening has been widespread in the United States as a method for secondary prevention of PC and for reducing risk of PC-specific death14. However, the recent literature suggests that PSA screening rates have decreased in response to the 2012 USPSTF recommendation6, 7, 15–33 (Table 1). A study conducted by Li et al., using the National Health Interview Survey (NHIS), reported that screening rates dropped from 31.8% in 2008 to 24.2% in 201320. A cross-sectional study by Abdollah et al., employing the 2012 and 2014 Behavioral Risk Factor Surveillance System (BRFSS), investigated how many men ≥50 underwent PSA testing in the previous year. They found a nationwide decrease, from 34.9% (95% CI 34.4–35.4) in the 2012 survey (reflecting rates of 2011) to 31.9% (95% CI 31.4–32.4) in the 2014 survey (reflective of 2013)15, 16. Jemal et al. analysed Surveillance, Epidemiology and End Results (SEER) data on men ≥50 who responded to an NHIS and found an 18% (95% CI 11–25) relative decrease in PSA testing, from 37.8% in 2010 to 30.8% in 201319. Also utilizing data from the NHIS, Sammon et al. found that 46.5% of men aged ≥50 received screening in 2010 compared to 41.3% in 2013 (P<0.001).23
Table 1.
Papers selected for qualitative synthesis after the USPSTF 2012 recommendation against PSA screening
| Outcome | Main Finding | References |
|---|---|---|
|
PSA
Testing Patterns |
Decrease in PSA screening rates | 6, 7, 15–33,38 |
| No change /specialty-dependent PSA screening rates (PCPs vs. urologists) | 17, 28, 37, 39, 41 | |
| PSA screening rates changed (decreased) in men aged: | ||
| 40–49 | 32 | |
| 50+ | 15, 16, 19, 22, 23, 74 | |
| 50–54 | 24 | |
| 50–59 (decrease) | 6, 17 | |
| 50–59 (increase) | 21 | |
| 50–70 | 26, 31 | |
| 55–64 | 74 | |
| 55–69 | 18 | |
| 60+ | 21 | |
| 60–64 | 24 | |
| 60–74 | 6 | |
| 65–74 | 74 | |
| 70+ | 18, 21, 32 | |
| 75+ | 6, 32,37 | |
| Inconsistent changes in PSA screening practices among high-risk men | 18, 27, 38 | |
|
Other
Tests and Clinical Work-up |
Fewer DREs | 25, 26, 40 |
| Fewer clinical work-ups for elevated PSA | 22, 42, 43 | |
| Urology referrals at higher PSA values | 39, 43, 44 | |
| Increased utilization of risk-stratified screening approaches | 32, 45 | |
|
Biopsy Patterns |
Fewer biopsies performed | 30, 42, 43, 46–50 |
| No change in biopsy rates | 22, 45, 51 | |
| More positive biopsies | 43, 63, 64 | |
| Fewer low-risk/grade cancers detected | 46, 47, 57, 62 | |
| Fewer intermediate/high-risk/grade cancers | 47, 57, 62, 49 | |
| More intermediate/high-risk/grade cancers | 43, 44, 46, 50, 64, 65, 66 | |
| Decrease in PC incidence | Decrease in PC incidence | 19, 22, 47, 57, 59, 60 |
| Decrease in loco-regional PC | 19, 60, 62 | |
| Increase in clinical stage T3a+ | 44 | |
| No change in distant-stage PC | 44, 60 | |
| Increase in distant-stage PC in men 75+ | 44, 19 | |
| Increase in metastatic disease at diagnosis | 43, 61 | |
|
Effects
on longer-term outcomes |
Fewer radical prostatectomies performed | 72 |
| Higher Gleason grade and extraprostatic extensions at radical prostatectomy | 72 | |
| Fewer patients with low/intermediate risk presenting for radiation oncology | 73 | |
Model-projected outcomes in the U.S. during
years 2013–2025 without PSA:
|
75, 76 | |
|
Physician Attitudes |
Aware of the recommendation, most still
willing to consider screening appropriate candidates |
63, 70, 71 |
|
Patient Attitudes |
Most agreed with USPSTF, but still intended to continue screening | 67, 68 |
| Majority pro-screening; anti-screening opinions increased | 69 | |
Several studies investigated whether there was regional variation in PSA screening rates following the 2012 USPSTF recommendation. Abdollah et al. examined interstate differences in PSA screening rates before and after the recommendation. Many states experienced a decrease, the most pronounced of which was a drop of 7.5% in both Alabama and Alaska15; some states reported no change in screening rates while a few reported increases. Rezaee et al., utilizing a database covering southeastern Michigan, generated a linear model to ascertain changes in PSA testing and transrectal ultrasound usage22. Their study, consisting of 3,647 men aged ≥50, found that average PSA utilization rates were high both before and after the recommendation (72.1% in 2010–2011; 79.3% in 2013–2014; P=0.48). Despite the 7% increase, however, the authors noted a downward trend in the post-USPSTF period.
PSA testing by age group
Screening is often indicated for men ages 55–6911, 13 because the ERSPC trial results showed a significant reduction in risk of PC mortality in this population1. Other guidelines recommend starting at age 4512, 34, 35, due to growing evidence that an elevated baseline PSA can be predictive of future lethal disease36. Despite these discrepancies, when PSA screening rates following the 2012 recommendation were stratified by age, decreases were noted across all groups, including those for which screening may be most beneficial6, 18, 20, 21, 24, 25, 29, 31–33,26 . Drazer et al., using the NHIS, reported that for men aged 50–59, rates of screening decreased from 33.2% to 24.8% from 2010–2013; for men 60–74, screening decreased from 51.2% to 43.6%; and for men ≥75, screening decreased from 43.9% to 37.1%6. Aslani et al. reviewed 2008–2012 data from a large healthcare system in northeastern Ohio, where the greatest decrease in screening was in men 50–59 years of age, significantly after the first publications of ERSPC and PLCO in 2009, albeit not statistically significant in the post-2012 period17. Similarly analysing the influence of the 2009 ERSPC and PLCO publications, Zeliadt et al. found that PSA screening during 2005 to 2010 decreased by 3% and 2.7% in men aged 40–54 and 55–74, respectively, with a continuing decline in men ≥75 that likely began following the USPSTF’s 2008 recommendation against screening in this population29.
Sammon et al. reviewed data from the 2000, 2005, 2010 and 2013 versions of the NHIS and found a significant decrease in PSA testing between 2010–2013: from 36% (95% CI 34–37) to 31% (95% CI 30–33). Stratification by age revealed the most significant decreases in men aged 50–54, from 23% (95% CI 20–26) to 18% (95% CI 15–21), and 60–64, from 45% (95% CI 41–79) to 35% (95% CI 32–39)23. Furthermore, these two age groups were less likely to be screened in 2013 than 2010, with an odds ratio (OR) of 0.71 (95% CI 0.66–1.10) for 50–54 and 0.69 (95% CI 0.54–0.89) for 60–64. Likewise, examination of population-based institutions in Michigan and Massachusetts found PSA testing rates in these states increased to 27% and 32%, respectively, between 2000–2008, with subsequent decreases to a mean of 25% in 2009–2012 and 23% in 2013–201418. Stratification by age produced similar decreases in both the 55–69 and ≥70 populations, at 33% and 31%, respectively. In a population-based cohort of privately insured patients, PSA screening rates did not change between 2008 and 2013 for patients aged 50–74, but decreased significantly for men 75+.37
Frendl et al. also reported a 25% decrease in PSA screening among men at higher risk for developing PC because of African-American race and/or family history of PC.18 Nevertheless, Turini et al., using 2012 BRFSS data, found that despite the USPSTF recommendation, African-American men still had higher odds of being screened compared with white men (OR 1.51; 95% CI 1.37–1.67)27. Moreover, one study conducted in a group of primary care clinics concluded that the USPSTF guideline was not followed in such a way that the no-screening message was brought equally to all men; men with a family history were more likely and African American men less likely to be screened, and older men received a more balanced counselling on PSA testing.38
PSA testing patterns by specialty
PSA screening is often part of a routine physical examination, meaning that PCPs are largely responsible for initiating discussions about screening. Measuring the proportion of screening by specialty, Aslani et al. found that within their regional hospital network (2008–2012), 64.9% of PSA tests were ordered by internists and 23.7% by family physicians, while only 6.1% were ordered by urologists and 1.3% by hematologist/oncologists17. Several studies looked at differences in PSA test ordering among PCPs21, 25, 26, 28, 31, 32, 39. Using the National Ambulatory Medical Care Survey (NAMCS), Shoag et al. found that rates of PSA testing and digital rectal examination (DRE) by PCPs fell 39% and 64%, respectively, after the 2012 recommendation25. A similar trend was suggested by Yates et al. in a survey of 73 primary-care providers, among whom 75% changed their PSA screening practice, mainly offering fewer PSA tests; however 50% still offered PSA to men >70 years. When asked, “has the USPSTF statement in 2012 changed your practice of recommending DRE?,” 36% reported performing fewer DREs40.
From 2011 to 2012, Cohn et al. noted, PSA testing by PCPs decreased from 8.6% to 7.6% (P=0.0001); stratification by age revealed the largest decreases in men 40–49 (5.6% to 4.6%, P=0.004) and 70–79 (7.9% to 6.2%, P=0.0074)32. Miller et al. evaluated whether being a new (vs “established”) patient impacted the likelihood of screening in the primary care setting. Among their established patients, screening decreased 9.9% (P<0.01) through mid-2014, most notably for patients in their 60s (10.8% decrease, P<0.01) and >70 (18.2% decrease, P<0.01). New patients saw a 4.1% overall reduction, with a 27.6% decrease for patients >70 but a 12.3% increase for patients 50–59 (P<0.01).21
The USPSTF recommendation may have differentially impacted PSA testing practice among physicians with different specialties. Aslani et al. found that within their network of 7 hospitals, of which 6 are considered suburban/rural, urologists had the most significant decrease in PSA test ordering17. There was no significant change among family physicians. Conversely, Meyer, Zavaski et al. found that, using the 2010 and 2012 NAMCS, PSA testing substantially decreased from 36.5% to 16.4% within the primary care setting but displayed a non-significant decrease in the urology setting, from 38.7% to 34.5%28, 41. This discrepancy may be reflective of differences in practitioner attitudes and/or geographic variation between institutions. Moreover, a study by Kim et al. evaluated changes in PSA testing practice between urologists and radiation oncologists in response to the USPSTF recommendation. Urologists were more likely than radiation oncologists to recommend screening for men 40–49 and 50–59, (OR 3.09 and 3.81, respectively), but less likely to recommend screening for men 75–79 (OR 0.66) and >80 (OR 0.45).33 Similarly, Cohn et al. analyzed data from the North Shore University health system enterprise data warehouse and noted a decrease in PSA testing in primary care, predominantly in older men (aged 70–79; 9.1% pre vs. 5.6% post, P <0.001) and in men without a history of an elevated PSA.32
Effects on other screening tests
Several studies have demonstrated the effects of the USPSTF recommendation on further assessments for PC, including urologic referrals, rescreening, PCA3 testing and repeat PSA testing. McGinley et al. found a 16.4% decrease in clinical work-ups for men with elevated PSA42, Gaylis et al. found a 19% decrease in urologic referrals for elevated PSA43, and Hutchinson et al. found that although PSA testing rates did not change at their institution, PCPs were referring patients at increasingly higher PSA values, at an average of 2.56 ng/ml in 2012, 2.72 ng/mL in 2013, 3.06 ng/ml in 2014 and 3.84 ng/ml from 2015 onwards39. Dalela et al. tracked risk group stratification in the National Cancer Database (NCDB), noting that 13.9% of patients had a PSA level >20 ng/ml in 2013 versus 11.5% in 201144. Gaylis et al. found that, within their large urology practice, median pre-biopsy PSA levels steadily increased from 7.0 ng/ml to 8.1 ng/ml (P=0.0006) and the proportion of men with PSA levels >10 ng/ml increased from 28% to 38% from 2011–201443.
Although some studies suggest a trend toward indiscriminate decreases in screening, others suggest the adoption of more risk-stratified approaches. Cohn et al. found that rescreening rates at their institution decreased significantly in patients with a highest previous PSA value <2.5 ng/ml32. After the recommendation, Perez et al. found, more patients received PCA3 testing (27% vs. 11%; P<0.01) and more received repeat PSA testing (82% vs. 72%; P=0.02)45.
PC incidence
Biopsy rates
Considering the widespread reduction in rates of screening after the USPSTF recommendation, it is unsurprising that lower rates of prostate biopsy were reported as well, with decreases ranging from 13–34%30, 42, 43, 46–50. Bhindi et al. analysed prostate biopsy rates at a large hospital network in Toronto, where the median number of biopsies per month decreased from 58 (IQR 54.5–63.0) to 35.5 (IQR 27.0–41.0)47. Since prostate biopsies may be diagnostic or to investigate possible recurrence after treatment, first-time biopsies were analysed separately; these also decreased, from a median of 42.5 (IQR 37.5–57.5) to 24.0 (IQR 19.0–32.5).
Other studies, however, found no such declines. Although Rezaee and colleagues reported a drop in biopsy rates between the years 2010–11 and 2013–14 (12.6% vs. 8.1%; P<0.001), they found no significant decline in biopsy utilisation22. Similarly, Perez et al. noted that while urologists at their institution were recommending biopsy to fewer new patients (16% pre vs. 24% post; P=0.03), the overall proportion of biopsies performed before and after the recommendation was unchanged, at 44.3% and 45.5%, respectively (P=0.8)45. One study from a large community urology practice, reported no change–or less than a 5% change per year–in the number of biopsies in the first three years post USPSTF 201251.
Overall PC incidence
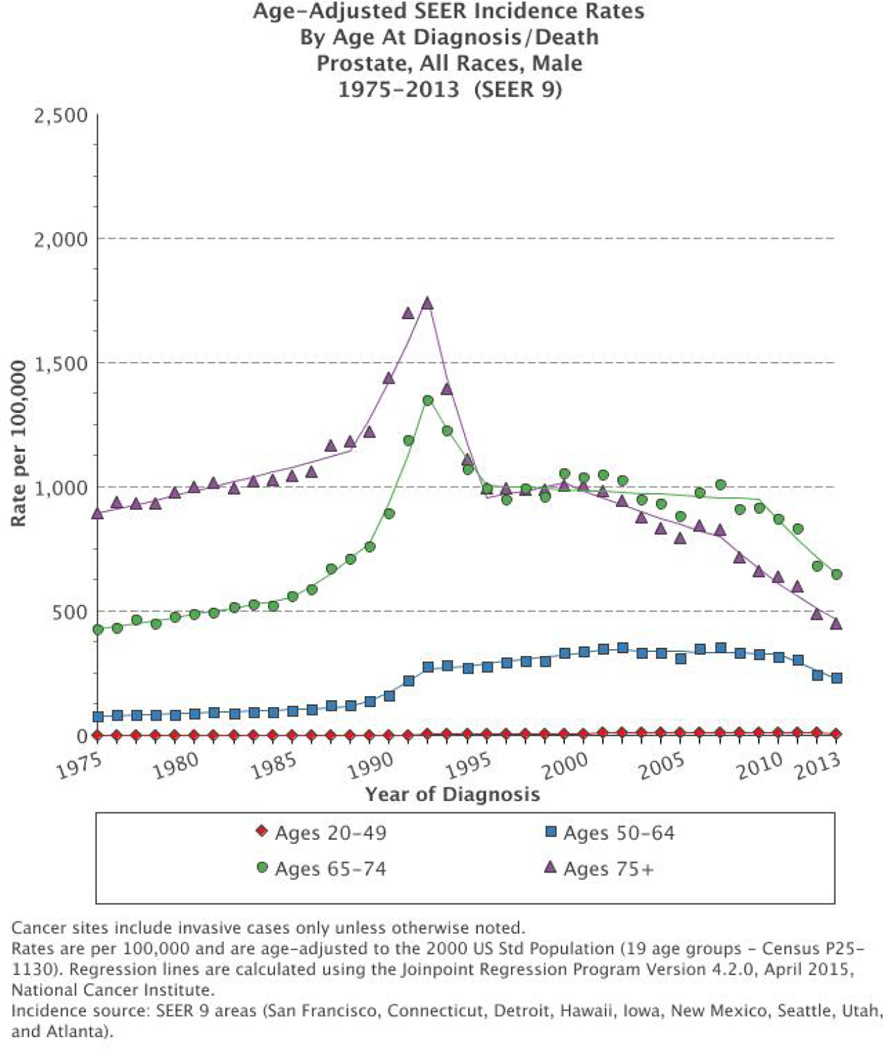
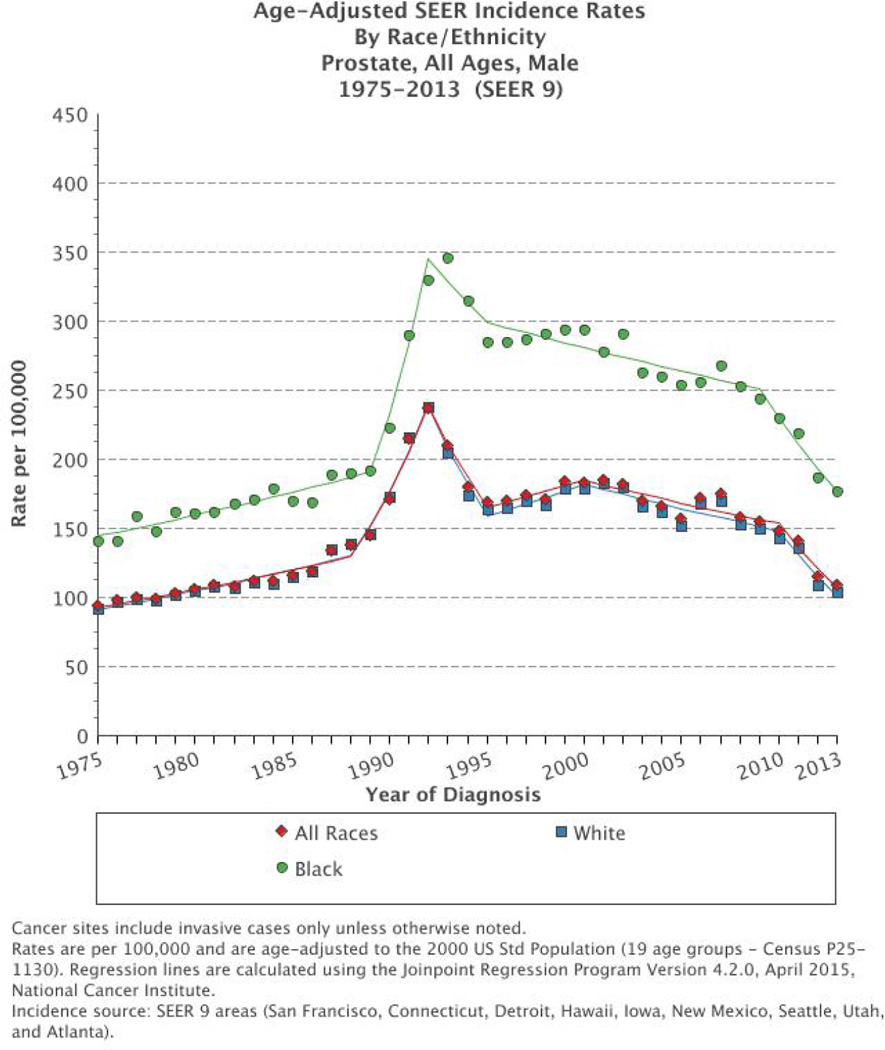
The PSA era in the U.S., beginning in the mid-late 1980s with its commercial introduction together with landmark studies in the early 1990’s showing that PSA outperforms DRE as a screening test52, caused an initial, rapid surge in PC incidence in the U.S.; according to SEER data, age-adjusted PC incidence increased 82% from 1986 to 1991 among men ≥6553. However, PC incidence has been declining since the early-mid 1990s i.e. even before the USPSTF 2008 and 2012 recommendations, and today, levels are below pre-PSA era rates54. This is likely multifactorial (rate of dissemination of PSA screening, lead time, secular trends in the absence of screening and overdiagnosis)55 and a combination of early detection of tumors at a curable stage and overdetection of indolent disease. The initial trend peak in PC incidence has been interpreted as a “harvest effect”, i.e. a depletion of previously undiagnosed and accumulated cases from the pool of prevalent preclinical cases from previous’ years. In addition, in the 1980’s there was an increase in the use of transurethral resection of the prostate (TURP) for benign prostatic hyperplasia with incidental PC detection upon pathologic review of the TURP-specimens56. The decline in PC incidence in the late 1990s represents a clearing out of prevalent cases54. In more recent years, including the USPSTF 2008 recommendation against PSA in men over the age of 75 and the conflicting publications from ERSPC and PLCO, decreased rates of PSA screening have likely contributed to the observed decreased incidence of PC19, 47, 57, 58. SEER data indicate that, between 2011, when the USPSTF released their draft recommendation, and 2013, PC incidence declined each year in comparison to the preceding year in all ages and races (Figures 1, 2, and 3); the age-adjusted rate declined from 147.9 per 100,000 men in 2010 to 141.5 in 2011, 115.2 in 2012 and 108.6 in 2013.59 . Among men ≥50, there were 33,519 fewer cases of PC diagnosed between 2011–201219.
Figure 1.
Age-adjusted prostate cancer incidence rate in the Surveillance, Epidemiology and End Results (SEER 9) database by age at diagnosis from 1975 to 2013 in the USA. Rates are per 100,000 and age-adjusted to the 2000 US standard population. The figure is extracted with permission from the SEER Database. The figure is extracted with permission from the SEER Database. https://seer.cancer.gov/
Figure 2.
Age-adjusted prostate cancer incidence rates in the Surveillance, Epidemiology and End Results (SEER) database by age at diagnosis from 1975 to 2013 in the USA. Rates are per 100,000 and age-adjusted to the 2000 US standard population. The figure is extracted with permission from the SEER Database. The figure is extracted with permission from the SEER Database. https://seer.cancer.gov
Figure 3.
Age-adjusted prostate cancer incidence rates in the Surveillance, Epidemiology and End Results (SEER 9) database by race/ethnicity, from 1975 to 2013 in the USA. Rates are per 100,000 and age-adjusted to the 2000 US standard population. The figure is extracted with permission from the SEER Database. The figure is extracted with permission from the SEER Database. https://seer.cancer.gov
Using data from the NCDB, Barocas et al. found that in the year after the release of the 2011 USPSTF recommendation draft, PC diagnoses fell 12.2% in the first month (P<0.01) and continued to decrease by 1.8% per month (P<0.01), leading to an overall decline of 28%57. This translated to 1363 fewer cases the first month and a further decline of 164 cases per month. In contrast, colon cancer diagnosis rates remained stable over the same period. Rezaee et al. found that men were less likely to be diagnosed with PC (OR 0.81, 95% CI 0.74–0.89) after the USPSTF recommendation22. Assuming no change in the natural history of PC itself, this decline in PC incidence can likely be attributed to lower rates of screening.
PC incidence by stage
Analysing stage-specific rates of PC incidence, Jemal et al. found that, in men 50 years and older, from 2008–2012, declines were largely among local/regional cancers19. The decrease in early-stage PC incidence persisted through 2013, but at a slower pace.60 Distant-stage cancer rates remained stagnant except in men ≥75, where the incidence per 100,000 men increased significantly, from 57.7 (99% CI 53.2–62.3) in 2011 to 65.0 (99% CI 60.3–69.9) in 2012.19 Between 2012 and 2013, however, incidence rates for distant-stage disease remained unchanged.60
From the NCDB, a small increase in diagnoses of clinical stage T3a or higher was noted, from 4.0% in 2011 to 4.8% in 201344. Gaylis et al. also reported a small but noticeable rise in the incidence of of metastatic disease upon diagnosis: 5.0% in 2011, 5.8% in 2012 and 7.7% in 201343. Utilizing joinpoint regression to model annual percentage changes (APC) in the incidence of PC based on stage in the NCDB during 2007 to 2013, Weiner et al. noted a decrease in the incidence of low-risk PC, and an increase in the incidence of metastatic PC (APC: 7.1%, P<0.05 from 2007 to 2013; or 72% more than that of 2004); most pronounced in men aged 55–69.61 These findings cannot be entirely explained by reactions to the USPSTF recommendations, because the trends started earlier. Plausible explanations are multifactorial and the authors speculate in other influences in screening guidelines/practices, alterations in the biological aggressiveness of PC or, less likely, changes in case ascertainment.61 Using the same methodology, Herget et al. analyzed data from SEER between 2007 and 2012 and found a −19.6% APC in PC incidence beginning in May 2011 (before the USPSTF draft release in October 2011), a decline that was observed in all age groups, and with the greatest decline in men 75+, following the USPSTF 2008 recommendation. There was a decline in low- and high-grade tumors as well as stage I/II and III tumors. The authors consider changes in risk factors of population demographics less likely to explain changes in PC incidence, than changes in screening practices. Trends also coincide with the simultaneous publications of the two conflicting results of RCTs of PSA-screening, ERSPC and PLCO, which published their first results in 2009.29, 62
Biopsy outcomes and risk groups at diagnosis
Several studies evaluated whether the 2012 recommendation catalysed a shift towards more adverse biopsy findings such as advanced clinical TNM stage and higher Gleason grade disease. Three studies found that the rate of positive biopsies increased following the recommendation to cease screening: Gaylis et al. found an increase from 46% to 50%43, Rosenberg and colleagues reported a 29% increase63, and Olsson et al. noted annual increases, from 39% in 2010–11 to 41.4% in 2013, 42.6% in 2014 (P<0.001) and 46% in 2015 (P<0.001)64. Similarly, Banerji et al. reported that post-USPSTF, the number of biopsies at Virginia Mason containing no PC and Gleason score 6 PC decreased by 36% and 15%, respectively46.
Cessation of PSA screening might reduce diagnosis of low-risk cancers that are unlikely to be fatal; however, there is concern that the USPSTF’s blanket rejection could similarly decrease detection of more aggressive cancers for which intervention would be indicated. Newly diagnosed cases of PC stratified by risk group within the University Health Network in Toronto showed that the median number of low-risk cancers decreased from 8.5 (IQR 6.5–10.5) to 5.5 per month (IQR 4.0–7.0; P=0.012), and the median number of intermediate- to high-risk cancers (Gleason 7–10) decreased from 17.5 (IQR 14.5–21.5) to 10.0 (IQR 9.0–12.0; P<0.001)47. Similarly, Barocas et al., using the NCDB, reported significant decreases in PC incidence across all D’Amico risk groups one year after the USPSTF recommendation: low-risk, intermediate-risk and high-risk disease decreased by 37.9%, 28.1%, and 23.1%, respectively, with an additional slight but non-significant decrease in non-localized disease57.
Conversely, other studies demonstrate migration towards higher-risk PCs. Dalela et al. also utilized NCDB data and found a decrease in low-risk cases, from 31.9% in 2011 to 25.9% in 2013, and a corresponding increase in intermediate-risk (43.5% to 45.1%) and high-risk (24.5% to 29%) cases (all P<0.001)44. Additionally, men in their cohort were less likely to be diagnosed with low-risk disease (OR 0.74) and slightly more likely to be diagnosed with intermediate- or high-risk disease (OR 1.05 and 1.22, respectively). Among 580 men in the Inland Empire Health Plan, Algotar et al. noted an increase in high-grade disease at diagnosis within 3 years of the 2012 recommendation (75.5% post vs 56.5% pre; P=0.01), together with an increase in direct medical costs of care.65 Reporting on data from 87,562 men extracted from the National Oncology Data Alliance, Hall et al. noted a rise in the percentage of men with intermediate or higher risk PC by 2.9% per year after 2011 (P<0.003).66
Banerji et al. similarly noted that, post-USPSTF, patients had an adjusted relative risk of 1.25 (95% CI 1.02–1.52; P=0.036) of a D’Amico high-risk cancer diagnosis46.
Several studies also reported shifts in Gleason grades. Gaylis et al. reported an increase in the proportion of Gleason 8 cancers, from 21% in 2011 to 30% in 2014 (P=0.0001)43, and Dalela et al. reported an increase from 17% in 2011 to 21.2% in 201344. Olsson et al. noted an increase in Gleason 8–10 cancers, from 9% in 2011 to 19% in 2014; analysis of all biopsy cores containing Gleason 8–10 patterns found an increase from 14.8% in 2010–11 to 19.7% in 2013, 26.1% in 2014 and 25.4% in 201564. However, Sterling et al. at the Veterans’ Administration Medical Center in Brooklyn found that detection of Gleason score 8–10 cancers in African-American men decreased from 10.2% in 2010–12 to 3.3% in 2013–1449.
Attitudes towards PSA screening
Patient attitudes
Our search yielded three studies examining men’s reactions to the 2012 USPSTF recommendation and whether it impacted their perceptions about PSA screening. Squiers et al. surveyed 1089 men aged 40–74 to determine if they agreed with the recommendation and would adhere to it67. Although 62% of respondents agreed with the recommendation, only 13% intended to actually cease screening; 54% intended to continue and 33% were undecided. Factors associated with continued screening were African-American race, PSA testing within the past two years, high income and a moderate to high level of concern over developing PC. Similarly, Maurice et al. examined 54 patient questionnaires from primary care and urology clinics taken before and after reading opposing PSA screening guidelines (AUA and USPSTF). Initially, 52% of patients reported good or very good comprehension of PSA screening recommendations. After they read the guidelines, claims of good or very good understanding increased to 65%, and support for PSA screening decreased non-significantly yet remained high (87% to 80%; P=0.6)68.
Prabhu et al. at New York University undertook a novel approach, amassing all PC-related Twitter posts in each 24-hour period after the release of the draft in October 2011 and final recommendation in May 201269. The draft version generated 2042 tweets and the finalised version generated 5357; however, only a small minority (9% and 4%, respectively) articulated an opinion about screening. While the majority of opinionated tweets were pro-screening, the proportion of anti-screening tweets increased from 22% in 2011 to 32% in 2012 (P=0.03).
Primary care physician attitudes
Three studies examined the extent to which the 2012 USPSTF recommendation influenced PCP’s attitudes about PSA screening. All three found that PCPs were largely informed about the guideline. Tasian et al. and Rosenberg et al. noted that, of all available guidelines, PCPs were most influenced by those of the USPSTF63, 70. Within the Johns Hopkins Community Physicians practice, 49.2% of PCPs agreed or strongly agreed with the recommendation, 36.0% disagreed or strongly disagreed and the rest neither agreed nor disagreed71. However, despite these sentiments, 37.7% said they would not change their screening practices, while only 1.8% expected to cease routine PSA testing; 21.9% stated they were much less likely to screen and 38.6% were somewhat less likely. Interestingly, Pollack et al. found that clinicians who were active advocates of PSA screening were less likely to abandon the practice than those who, after a discussion of benefits and risks, allowed their patients to decide (11.9% vs. 32.6%; P=0.01). Furthermore, a national cross-sectional survey by Tasian and colleagues found that only 51% of 3,010 PCPs discussed PSA screening with patients, while 64% reported ordering the test70. Finally, Rosenberg et al., conducted a survey at the 2015 American Academy of Family Practice annual meeting and found that 22.9% of respondents do not recommend PSA screening63. Of the 78% that do, 50% begin at age 45 and 41% begin at 55.
Effects on long-term outcomes
At present, only four years have passed since the USPSTF issued a D grade to PSA screening. It is too soon to ascertain whether this recommendation will have an effect on PC-related mortality. At one institution, the total number of radical prostatectomies decreased by 35% and the proportion of Gleason score 6 from 24% to 12% (P<0.01) in the first three years post USPSTF 2012 compared to three years prior. At the same time, the post-2012 group had a significantly higher proportion of Gleason scores 4+3=7 and 8, and extraprostatic extension; however, whether this is due to less PSA-screening or improved patient selection is difficult to tell.72 Similarly, within a network of radiation oncology clinics, the number of men presenting with PC for radiation oncology care decreased during 2007–2013; low and intermediate risk patients decreased significantly, however there was no short-term change in high risk or metastatic disease and longer-term data is unknown.73 The USPSTF 2012 recommendation may also have influenced practice patterns in other continents, with a steady nationwide decline in the rates of PSA-testing, prostate biopsy and prostatectomy seen in Australian Medicare data.74
Nevertheless, mathematical simulation models have been developed to estimate the number of future PC deaths that might have been avoided with early detection and treatment with curative intent. Under current screening rates, it is estimated that 710,000–1.12 million men would be overdiagnosed between 2013 and 2025, yet 36,000–57,000 deaths from PC would be prevented75, 76. Abandonment of PSA screening would prevent all cases of overdiagnosis but fail to prevent 100% of avoidable deaths, leading to a 13%–20% increase in deaths from PC. It would also cause incidence of metastatic disease to increase more than twofold. Even if screening were restricted to men under 70, a predicted 13,000–22,000 additional deaths would occur, and the incidence of metastatic disease at diagnosis would increase 46%–57%.
Critiques/Commentaries
Proponents
Since the USPSTF issued their draft recommendation in late 2011, there has been debate over whether their blanket rejection of PSA screening was justified. Those who support the USPSTF cite the ERSPC and PLCO trials: the former found a moderate decrease in PC-specific mortality and the latter found no decrease at all77, 78, with the study’s power to detect a difference in PC mortality between trial arms being limited by excessive opportunistic PSA testing in the control arm79, 80. Michael LeFevre, MD, a member of the USPSTF, points out that while the benefits may not be fully clear due to mixed results, the harms of screening are well-defined— overdiagnosis, biopsy complications and overtreatment77. Barry and Nelson note the lack of population-based data linking the recommendation to increased advanced-stage PC81. Richard Ablin, PhD, notes that PSA is inadequate as a diagnostic marker for PC because it lacks specificity78; therefore, the USPSTF recommendation may lead to development of specific biomarkers for PC that are superior to PSA.
In the New England Journal of Medicine, Brett and Ablin point out that other guidelines emphasize shared decision-making between patients and practitioners, but the less-than-definitive results from the randomized clinical trials have made it difficult to derive any conclusive benefit from screening82.
Opponents
The USPSTF’s guideline has been cited for creating confusion among patients and clinicians alike25, 63, 81, 83–86 and more physician education is warranted40. The USPSTF recommends against PSA screening in all men but simultaneously states that “clinicians should understand the evidence but individualize decision making to the specific patient or situation.”3 These ideas are essentially in conflict. Editorialising in The Journal of Urology, Samir Taneja, MD, wrote: “The mass confusion regarding interpretation of guidelines and application in practice is the result of a recommendation that is not particularly intuitive. How does one prevent prostate cancer death if one is not looking for prostate cancer?”87 In response to the October 2011 draft, the AUA responded by saying “the USPSTF—by disparaging the [PSA] test—is doing a great disservice to the men worldwide who may benefit from the PSA test”88…
Moreover, clinicians and researchers have challenged the recommendation because the USPSTF excluded relevant data. Catalona and Walsh note the failure to consider epidemiologic data from the PSA era, which show a 40% reduction in PC mortality and a 75% decline (from 21% to 4%) in advanced-stage disease upon diagnosis85, 89–91. The USPSTF also stressed the morbidity associated with radical prostatectomy and radiotherapy, yet overlooked the morbidity associated with advanced-stage disease, such as spinal cord compression81, 92. In addition, although their decision was largely based on the PLCO trial, the USPSTF did not account for methodological limitations within that trial.
Given these limitations, researchers have questioned why the USPSTF did not consider modelling data as evidence of the benefits of screening. Etzioni and Thompson claimed modelling data was necessary for obtaining a complete picture about PSA screening, as the limitations and variability within the two large trials may have dampened its true benefits93. Through statistical models, they demonstrated that extrapolation of the ERSPC results yielded a fivefold greater benefit in favour of screening94, while modelling of the PLCO trial revealed insufficient statistical power to detect differences between the screening and control arms93, 94.
Several researchers have suggested the USPSTF upgrade their recommendation for PSA screening to a grade C95, 96, meaning: “clinicians may provide this service to selected patients depending on individual circumstances. However, for most persons without signs or symptoms there is likely to be only a small benefit from this service.”3 McNaughton-Collins and Barry explain that a grade C is more fitting, considering the benefit of screening noted in the ERSPC trial, and—unlike a blanket rejection that might influence physicians to abandon the practice altogether—it would involve the patient in the decision of whether or not to screen95. It might also decrease haphazard screening of men who are unlikely to benefit. Volk and Wolf concur: while overtreatment is a disadvantage of PSA screening, some men are willing and eager to accept the side effects of treatment to eradicate their cancer, regardless of risk96. However, a blanket rejection of PSA screening with a D grade wholly eliminates the patient from the discussion. A grade of C would restore patient participation and facilitate individualized shared decision-making (SDM). Today, all professional guideline groups agree that PSA screening ought to take place in the context of SDM between an individual man and his provider10. However, there is evidence that SDM, as currently practiced, is sub-par97–99.There is evidence that that one in four PCPs order the PSA without discussing it100, or do not engage in SDM as “by the book”; only 10%-33% of patient-provider communications cover essential domains of SDM.101, 102 Using data from the 2013 Health Information National Trends Survey, Meyer et al suggested differential counseling by sociodemographic factors, with greater odds of being counseled on the potential adverse side-effects of treatment in older men and men with a prior cancer history, yet these were also more likely to undergo PSA testing41. More research is certainly needed on the practical aspects of implementing SDM at the point of care.
DISCUSSION
In this review, we have summarized the growing body of literature on temporal trends post-USPSTF in PSA testing and PC incidence patterns, as well as attitudes and opposing views.
Overall, we found widespread decreases in PSA testing rates, with the magnitude varying between cohorts, age groups and physician specialty. While screening rates in men >75 continued to decline, in part due to the USPSTF’s 2008 recommendation, and concurrent decreases were also noted in younger men, current screening rates suggest it is still a common practice in the U.S. There is now an aggregated body of evidence that, in the years following the 2012 USPSTF recommendation, fewer younger men, who may benefit from being screened, are being screened (under-screening), while there is still a substantial proportion of older men being screened, most likely without benefit (over-screening). Men at high risk of developing PC also received less screening18, 27, possibly a consequence of a lack of special recommendation for this population. While some physicians are inclined to adopt risk-stratified screening practices through further clinical workup to ascertain disease aggressiveness and longer screening intervals for patients with low PSA levels32, 45 others have opted to indiscriminately abandon the practice altogether.
Furthermore, differences in PSA testing rates and attitudes among physicians of various specialties elucidate the ways in which different physicians may be interpreting the recommendations. PCPs, who are largely influenced by the USPSTF’s recommendations63, 70, 71, were more accepting of the guideline than urologists28. The guidelines also had differential impact on clinical administration of PSA testing among urologists and radiation oncologists33. These differences suggest that physicians may not have uniformly translated the guidelines into a change in clinical practice.
We also noted an overall decrease in PC incidence in the years following the 2012 USPSTF recommendation19, 22, 47, 57. PSA screening favours detection of PCs that are low risk and often confined within the prostate, so with systematic reduction of screening, rates of low-risk cancer incidence have also decreased19, 47, 57. This decline may be indicative of the intended effect of the USPSTF recommendation—i.e., reducing rates of overdiagnosis and overtreatment of low-risk cancers. However, a simultaneous reduction in the detection of intermediate- and high-risk cancers significantly hinders the likelihood of cure by delaying timely diagnosis and treatment.
In accordance with lower rates of screening and PC incidence, biopsy rates at some institutions have declined simultaneously42, 43, 46–50. It is possible that apprehension towards PSA screening generated by this guideline has led to fewer asymptomatic, disease-free men receiving prostate biopsies. However, this might concurrently lead to more men with PC being diagnosed at later stages, once their cancers have precipitated symptoms that would justify clinical investigation. Indeed, increased rates of advanced disease upon detection were noted43, characterized by increased pre-biopsy PSA levels39, 43, 44 higher Gleason scores43, 50, 64 and more advanced clinical TNM stage44, 46. This risk-group/stage migration towards higher risk, more advanced PCs may be indicative of more appropriate selection of men for prostate biopsy. Of concern were notable decreases in PC incidence across all risk groups47, which—assuming no change in the natural history of PC—could indicate that high-risk cancers are evading detection.
Many physicians were aware of the USPSTF recommendation; however, while only a small percentage stated intent to follow the guideline subsequent to its release and eliminate PSA screening from their practice63, 70, 71, the nationwide decreases might suggest otherwise. Similarly, patients who were informed about the guideline agreed with it but intended to continue screening67, 68. Despite the USPSTF, patients are still choosing the PSA test.
Dr. Penson raises concerns “that we will soon return to the pre-PSA era, when men presented with locally advanced and/or metastatic disease and our only treatment option was androgen deprivation therapy and palliative care” and calls for abandoning the “one size fits all” approach to screening in favor or more personalized screening strategies, to keep the benefits and decrease the harms of screening.103
CONCLUSIONS
In the four years since the 2012 USPSTF recommendation against PSA screening, PSA testing rates and PC incidence have decreased. The long-term consequences of this rejection of PSA screening have yet to be elucidated. The USPSTF is currently (as of October 2016) in the process of reviewing the evidence and preparing to updating their recommendation.
KEY POINTS.
This review examines temporal trends in rates of screening and prostate cancer incidence before and after the USPSTF 2012 recommendation against PSA.
Rates of PSA screening, prostate biopsy and overall prostate cancer incidence declined in the first few years after the recommendation.
There was a notable shift towards higher grade, stage and risk upon detection.
Some physicians reported ongoing willingness to screen appropriately selected men.
Men largely reported intending to continue to ask for the PSA test.
Longer follow-up is needed to tell whether the decreased rates of PSA screening will impact prostate cancer metastasis and mortality.
Acknowledgments
Funding: Dr. Sigrid Carlsson’s work on this paper was supported in part by a Cancer Center Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30-CA008748). Dr. Carlsson is also supported by a post-doctoral research grant from AFA Insurance.
Biographies
Katherine Fleshner has studied pharmacology at McGill University, Montreal, Canada and is now a first year medical school student at Schulich School of Medicine and Dentistry, University of Western Ontario, Canada. This work was done as part of Fleshner’s Summer student internship at the Departments of Surgery/Urology and Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, USA.
Sigrid V. Carlsson is an assistant attending epidemiologist at the Departments of Surgery and Epidemiology and Biostatistics at Memorial Sloan Kettering Cancer Center, New York, USA. She is an associate professor of experimental urology affiliated with the Institute of Clinical Sciences at Sahlgrenska Academy at Gothenburg University, Gothenburg, Sweden. She is an MD, with a PhD in urology, both from Gothenburg University and has a MPH in quantitative methods from Harvard School of Public Health, Boston, USA. She is an investigator of the Swedish section of the European Randomized Study of Screening for Prostate Cancer (ERSPC).
Monique J. Roobol is an epidemiologist and associate professor at the department of Urology at Erasmus University Medical Center, Rotterdam, The Netherlands. She is the principal investigator of the Rotterdam section of the ERSPC, the web-based study on active surveillance PRIAS and the Movember GAP on Active Surveillance. She has co-authored more than 250 publications in peer-reviewed journals.
Appendix 1
MEDLINE search
((“diagnosis”[Subheading] OR “diagnosis”[All Fields] OR “screening”[All Fields] OR “mass screening”[MeSH Terms] OR (“mass”[All Fields] AND “screening”[All Fields]) OR “mass screening”[All Fields] OR “screening”[All Fields] OR “early detection of cancer”[MeSH Terms] OR (“early”[All Fields] AND “detection”[All Fields] AND “cancer”[All Fields]) OR “early detection of cancer”[All Fields]) OR “prostate-specific antigen”[MeSH Terms] OR PSA[All Fields]) AND (USPSTF[All Fields] OR “Preventive Services Task Force”[All Fields]) AND (“prostate cancer”[All Fields] OR “prostatic neoplasms”[MeSH Terms]) AND (“2011/01/01”[PDAT] : “2017/12/31”[PDAT])
Embase search
‘european urology’:jt OR ‘journal of urology’:jt OR ‘journal of clinical oncology’:jt OR ‘american family physician’:jt OR ‘journal of general internal medicine’:jt AND USPSTF AND ([conference abstract]/lim OR [conference paper]/lim)
Footnotes
COI: MJR is on the advisory board of OPKO. KF and SVC has no conflict of interest to declare.
References
- 1.Schroder FH, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriole GL, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 4.USPSTF. U.S. Preventive Services Task Force. Screening for prostate cancer: recommendation and rationale. Ann Intern Med. 2002;137:915–916. doi: 10.7326/0003-4819-137-11-200212030-00013. [DOI] [PubMed] [Google Scholar]
- 5.USPSTF. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 6.Drazer MW, Huo D, Eggener SE. National Prostate Cancer Screening Rates After the 2012 US Preventive Services Task Force Recommendation Discouraging Prostate-Specific Antigen-Based Screening. J Clin Oncol. 2015;33:2416–2423. doi: 10.1200/JCO.2015.61.6532. [DOI] [PubMed] [Google Scholar]
- 7.Drazer MW, et al. National trends in prostate cancer screening among older American men with limited 9-year life expectancies: evidence of an increased need for shared decision making. Cancer. 2014;120:1491–1498. doi: 10.1002/cncr.28600. [DOI] [PubMed] [Google Scholar]
- 8.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304:1584–1591. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson SV, Roobol MJ. What’s new in screening in 2015? Curr Opin Urol. 2016;26:447–458. doi: 10.1097/MOU.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter HB, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidenreich A, et al. Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol. 2013;64:347–354. doi: 10.1016/j.eururo.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Qaseem A, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158:761–769. doi: 10.7326/0003-4819-158-10-201305210-00633. [DOI] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 15.Abdollah F, et al. The impact of 2011 united states preventive services task force panel update on PSA screening practice: A nationwide, and state-by-state level analyses. Journal of Urology. 2016;195:e388–e389. [Google Scholar]
- 16.Abdollah FFH, et al. The impact of 2012 United States Preventive Services Task Force (USPSTF) panel update on PSA screening practice: A nationwide, and state-by-state level analyses. European Urology, Supplements. 2016;15:e91–e91a. [Google Scholar]
- 17.Aslani A, et al. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191:1737–1742. doi: 10.1016/j.juro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Frendl D, et al. Impact of guidelines on prostate cancer screening in a population-based setting, 2000–2014: Preliminary results from the first AUA data grant. Journal of Urology. 2016;195:e543. [Google Scholar]
- 19.Jemal A, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314:2054–2061. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Berkowitz Z, Hall IJ. Decrease in prostate cancer testing following the US preventive services task force (USPSTF) recommendations. J Am Board Fam Med. 2015;28:491–493. doi: 10.3122/jabfm.2015.04.150062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C, et al. United States Preventive Services Task Force prostate cancer screening guidelines were associated with age and race dependent changes in primary care prostate specific antigen based screening at a tertiary care center (Abstr MP37-05) J Urol. 2016;195:e498. [Google Scholar]
- 22.Rezaee ME, Ward CE, Odom BD, Pollock M. Prostate cancer screening practices and diagnoses in patients age 50 and older, Southeastern Michigan, pre/post 2012. Prev Med. 2016;82:73–76. doi: 10.1016/j.ypmed.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Sammon J, et al. Age dependent variation in the effect of physician recommendations to undergo prostate specific antigen (PSA) screening following the United States preventive services task force 2012 statement against PSA screening. Journal of Urology. 2016;195:e248–e249. [Google Scholar]
- 24.Sammon JD, et al. Prostate-specific antigen screening after 2012 US Preventive Services Task Force recommendations. JAMA. 2015;314:2077–2079. doi: 10.1001/jama.2015.7273. [DOI] [PubMed] [Google Scholar]
- 25.Shoag J, et al. Decline in Prostate Cancer Screening by Primary Care Physicians: An Analysis of Trends in the Use of Digital Rectal Examination and Prostate Specific Antigen Testing. J Urol. 2016;196:1047–1052. doi: 10.1016/j.juro.2016.03.171. [DOI] [PubMed] [Google Scholar]
- 26.Shoag J, et al. Decline in prostate specific antigen screening among primary care physicians in response to United States preventative services task force recommendations. Journal of Urology. 2016;195:e282. [Google Scholar]
- 27.Turini GI, Gjelsvik A, Golijanin D, Pareek G, Renzulli JI. The role of patient race and ethnicity in predicting physician recommendation of prostate-specific antigen (PSA) testing (Abstr PD09-07) J Urol. 2016;195:e236. [Google Scholar]
- 28.Zavaski M, et al. Differences in prostate specific antigen testing among urologists and primary care providers in the United States following the 2011 USPSTF recommendations. Journal of Urology. 2016;195:e389–e390. doi: 10.1001/jamainternmed.2015.7901. [DOI] [PubMed] [Google Scholar]
- 29.Zeliadt SB, et al. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103:520–523. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 30.Greene R, Tausch T, Perez D, Shellmyer M, Sutherland D. An examination of PSA utilization and referral patterns in a large, integrated health care system following the us preventative services task force PSA recommendations. Journal of Urology. 2013;189:e513. [Google Scholar]
- 31.Werntz R, Acevedo AM, Conlin M, Amling C. Trends in PSA utilization by primary care physicians: Impact of the USPSTF recommendation. Journal of Urology. 2015;193:e897. [Google Scholar]
- 32.Cohn JA, et al. Primary care physician PSA screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol. 2014;32:41, e23–e30. doi: 10.1016/j.urolonc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Kim SP, et al. A national survey of radiation oncologists and urologists on recommendations of prostate-specific antigen screening for prostate cancer. BJU Int. 2014;113:E106–E111. doi: 10.1111/bju.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll PR, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Canc Netw. 2016;14:509–519. doi: 10.6004/jnccn.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vickers AJ, Eastham JA, Scardino PT, Lilja H. The Memorial Sloan Kettering Cancer Center Recommendations for Prostate Cancer Screening. Urology. 2016 doi: 10.1016/j.urology.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilja H, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117:1210–1219. doi: 10.1002/cncr.25568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SP, et al. Contemporary National Trends of Prostate Cancer Screening Among Privately Insured Men in the United States. Urology. 2016 doi: 10.1016/j.urology.2016.06.067. [DOI] [PubMed] [Google Scholar]
- 38.Misra-Hebert AD, et al. Prostate cancer screening trends in primary care practices in a large integrated healthcare system: 2007–2015. Journal of General Internal Medicine. 2016;31:S372–S373. [Google Scholar]
- 39.Hutchinson R, et al. Testing and referral patterns in the years surrounding the US Preventive Services Task Force recommendation against prostate-specific antigen screening. Cancer. 2016 doi: 10.1002/cncr.30330. [DOI] [PubMed] [Google Scholar]
- 40.Yates J, et al. Changes in primary care provider practice patterns since 2012: Impact of the USPSTF guideline statement. Journal of Urology. 2015;193:e175. doi: 10.1016/j.urpr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Meyer C, et al. Differences in prostate specific antigen testing among urologists and primary care providers in the United States following the 2011 USPSTF recommendations. European Urology, Supplements. 2016;15:e90. doi: 10.1001/jamainternmed.2015.7901. [DOI] [PubMed] [Google Scholar]
- 42.McGinley KF, McMahon GC, Brown GA. Impact of the US Preventive Services Task Force Grade D Recommendation: Assessment of Evaluations for Elevated Prostate-specific Antigen and Prostate Biopsies in a Large Urology Group Practice Following Statement Revision. Rev Urol. 2015;17:171–177. [PMC free article] [PubMed] [Google Scholar]
- 43.Gaylis F, et al. The change in prostate cancer presentation and diagnosis coinciding with screening recommendations. Journal of Clinical Oncology. 2016;34 [Google Scholar]
- 44.Dalela D, et al. Impact of the 2012 United States preventive services task force recommendation against prostate specific antigen screening on prostate cancer risk group stratification. Journal of Urology. 2016;195:e546–e547. [Google Scholar]
- 45.Perez TY, et al. Impact of the 2012 United States Preventive Services Task Force statement on prostate-specific antigen screening: analysis of urologic and primary care practices. Urology. 2015;85:85–89. doi: 10.1016/j.urology.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 46.Banerji JS, et al. Prostate Needle Biopsy Outcomes in the Era of the U.S. Preventive Services Task Force Recommendation against Prostate Specific Antigen Based Screening. J Urol. 2016;195:66–73. doi: 10.1016/j.juro.2015.07.099. [DOI] [PubMed] [Google Scholar]
- 47.Bhindi B, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519–1524. doi: 10.1016/j.juro.2014.11.096. [DOI] [PubMed] [Google Scholar]
- 48.Gershman B, et al. Impact of prostate-specific antigen (PSA) screening trials and revised PSA screening guidelines on rates of prostate biopsy and postbiopsy complications. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Sterling W, Mehta K, Schreiber D, Karanikolas N, Weiss J. Prostate biopsy in the era of contemporary USPSTF guidelines (Abstr PD26-03) J Urol. 2016;195:e645. [Google Scholar]
- 50.Gejerman G, et al. USPSTF PSA screening guidelines result in higher Gleason score diagnoses (Abstr PD09-03) J Urol. 2016;195:e234–e235. [Google Scholar]
- 51.Lipsitz DU, Eshet G, White JS. A comparison of prostate biopsy rates among urologists in a large group practice and implications of PSA screening. Journal of Clinical Oncology. 2015;33 [Google Scholar]
- 52.Catalona WJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 53.Potosky AL, Miller BA, Albertsen PC, Kramer BS. The role of increasing detection in the rising incidence of prostate cancer. JAMA. 1995;273:548–552. [PubMed] [Google Scholar]
- 54.Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152–156. doi: 10.1093/jncimonographs/lgs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Etzioni R, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 56.Merrill RM, Feuer EJ, Warren JL, Schussler N, Stephenson RA. Role of transurethral resection of the prostate in population-based prostate cancer incidence rates. Am J Epidemiol. 1999;150:848–860. doi: 10.1093/oxfordjournals.aje.a010090. [DOI] [PubMed] [Google Scholar]
- 57.Barocas DA, et al. Effect of the USPSTF Grade D Recommendation against Screening for Prostate Cancer on Incident Prostate Cancer Diagnoses in the United States. J Urol. 2015;194:1587–1593. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 58.Ryerson AB, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NCI. National Cancer Institute. http://www.cancer.gov.
- 60.Jemal A, et al. Prostate Cancer Incidence Rates 2 Years After the US Preventive Services Task Force Recommendations Against Screening. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.2667. [DOI] [PubMed] [Google Scholar]
- 61.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004–2013) Prostate Cancer Prostatic Dis. 2016 doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 62.Herget KA, Patel DP, Hanson HA, Sweeney C, Lowrance WT. Recent decline in prostate cancer incidence in the United States, by age, stage, and Gleason score. Cancer Med. 2016;5:136–141. doi: 10.1002/cam4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg M, Crawford D, Newmark J, Steiner M. Use of PSA screening guidelines among primary care physicians (Abstr MP39-01) J Urol. 2016;195:e541. [Google Scholar]
- 64.Olsson C, Anderson A, Kapoor D. Initial prostate cancer detection before and after United States Preventive Services Task Force recommendation on prostate cancer screening (Abstr MP39-04) J Urol. 2016;195:e542. [Google Scholar]
- 65.Algotar AM, Singh P, Billins J, Thomazin G. Change in prostate biopsy outcomes and costs of care for prostate cancer in underserved population after changes in USPSTF guidelines. Journal of Clinical Oncology. 2016;34 [Google Scholar]
- 66.Hall MD, Schultheiss TE, Farino G, Wong JYC. Increase in higher risk prostate cancer cases following new screening recommendation by the US Preventive Services Task Force (USPSTF) Journal of Clinical Oncology. 2015;33 [Google Scholar]
- 67.Squiers LB, et al. Prostate-specific antigen testing: men’s responses to 2012 recommendation against screening. Am J Prev Med. 2013;45:182–189. doi: 10.1016/j.amepre.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Maurice MJ, Abouassaly R. Patient opinions on prostate cancer screening are swayed by the United States Preventative Services Task Force recommendations. Urology. 2014;84:295–299. doi: 10.1016/j.urology.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 69.Prabhu V, et al. Twitter response to the United States Preventive Services Task Force recommendations against screening with prostate-specific antigen. BJU Int. 2015;116:65–71. doi: 10.1111/bju.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tasian GE, et al. PSA screening: determinants of primary-care physician practice patterns. Prostate Cancer Prostatic Dis. 2012;15:189–194. doi: 10.1038/pcan.2011.59. [DOI] [PubMed] [Google Scholar]
- 71.Pollack CE, Noronha G, Green GE, Bhavsar NA, Carter HB. Primary care providers’ response to the US Preventive Services Task Force draft recommendations on screening for prostate cancer. Arch Intern Med. 2012;172:668–670. doi: 10.1001/archinternmed.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rotker K, Brito J, Edmund L, Matoso A, Renzulli J. Upstaging in radical prostatectomy in the post-PSA ERA: An effect of delayed diagnosis or better patient selection? Journal of Urology. 2016;195:e57–e58. doi: 10.1016/j.humpath.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Su KW, et al. Changes in prostate cancer presentation for radiation oncology care after USPSTF recommendations, 2007–2013. Journal of Clinical Oncology. 2015;33 [Google Scholar]
- 74.Zargar H, van den Bergh R, Moon D. The impact of the United States Preventive Services Task Force (USPTSTF) recommendations against prostate-specific antigen (PSA) testing on PSA testing in Australia. 2016 doi: 10.1111/bju.13602. [DOI] [PubMed] [Google Scholar]
- 75.Gulati R, et al. Expected population impacts of discontinued prostate-specific antigen screening. Cancer. 2014;120:3519–3526. doi: 10.1002/cncr.28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fenner A. Prostate cancer: USPSTF screening recommendation could lead to greater numbers of avoidable deaths. Nat Rev Urol. 2014;11:481. doi: 10.1038/nrurol.2014.209. [DOI] [PubMed] [Google Scholar]
- 77.Lefevre M. PSA screening: the USPSTF got it right. J Fam Pract. 2013;62:617, 619. [PubMed] [Google Scholar]
- 78.Ablin RJ. The United States Preventive Services Task Force recommendation against prostate-specific antigen screening--point. Cancer Epidemiol Biomarkers Prev. 2012;21:391–394. doi: 10.1158/1055-9965.EPI-12-0058. [DOI] [PubMed] [Google Scholar]
- 79.Shoag JE, Mittal S, Hu JC. Reevaluating PSA testing rates in the PLCO trial. N Engl J Med. 2016;374:1795–1796. doi: 10.1056/NEJMc1515131. [DOI] [PubMed] [Google Scholar]
- 80.Pinsky PF, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clin Trials. 2010;7:303–311. doi: 10.1177/1740774510374091. [DOI] [PubMed] [Google Scholar]
- 81.Barry MJ, Nelson JB. Patients present with more advanced prostate cancer since the USPSTF screening recommendations. J Urol. 2015;194:1534–1536. doi: 10.1016/j.juro.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 82.Brett AS, Ablin RJ. Prostate-cancer screening--what the U.S. Preventive Services Task Force left out. N Engl J Med. 2011;365:1949–1951. doi: 10.1056/NEJMp1112191. [DOI] [PubMed] [Google Scholar]
- 83.Scherger JE. PSA screening: the USPSTF got it wrong. J Fam Pract. 2013;62:616, 618. [PubMed] [Google Scholar]
- 84.Slomski A. USPSTF finds little evidence to support advising PSA screening in any man. JAMA. 2011;306:2549–2551. doi: 10.1001/jama.2011.1804. [DOI] [PubMed] [Google Scholar]
- 85.Catalona WJ, et al. What the U.S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Ann Intern Med. 2012;157:137–138. doi: 10.7326/0003-4819-157-2-201207170-00463. [DOI] [PubMed] [Google Scholar]
- 86.Carlsson S, et al. Prostate cancer screening: facts, statistics, and interpretation in response to the US Preventive Services Task Force Review. J Clin Oncol. 2012;30:2581–2584. doi: 10.1200/JCO.2011.40.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taneja SS. Re: National Prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Urol. 2016;195:350–351. doi: 10.1016/j.juro.2015.10.166. [DOI] [PubMed] [Google Scholar]
- 88.Lacy SS. Association urges men to speak with their physicians about the value of prostate cancer testing. Linthicum, MD: American Urological Association; 2011. [Google Scholar]
- 89.Catalona WJ. The United States Preventive Services Task Force recommendation against prostate-specific antigen screening--counterpoint. Cancer Epidemiol Biomarkers Prev. 2012;21:395–397. doi: 10.1158/1055-9965.EPI-12-0059. [DOI] [PubMed] [Google Scholar]
- 90.Walsh PC. Re: Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. J Urol. 2012;188:1181. doi: 10.1016/j.juro.2012.06.129. [DOI] [PubMed] [Google Scholar]
- 91.Walsh PC. Re: Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. J Urol. 2012;187:1267–1268. doi: 10.1016/j.juro.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Schroder FH, Words of wisdom. Re: Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Eur Urol. 2012;61:423–424. doi: 10.1016/j.eururo.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 93.Etzioni RD, Thompson IM. What do the screening trials really tell us and where do we go from here? Urol Clin North Am. 2014;41:223–228. doi: 10.1016/j.ucl.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Etzioni R, et al. Limitations of basing screening policies on screening trials: The US Preventive Services Task Force and Prostate Cancer Screening. Med Care. 2013;51:295–300. doi: 10.1097/MLR.0b013e31827da979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNaughton-Collins MF, Barry MJ. One Man at a Time - Resolving the PSA Controversy. N Engl J Med. 2011 doi: 10.1056/NEJMp1111894. [DOI] [PubMed] [Google Scholar]
- 96.Volk RJ, Wolf AM. Grading the new US Preventive Services Task Force prostate cancer screening recommendation. JAMA. 2011;306:2715–2716. doi: 10.1001/jama.2011.1893. [DOI] [PubMed] [Google Scholar]
- 97.Han PK, et al. National evidence on the use of shared decision making in prostate-specific antigen screening. Ann Fam Med. 2013;11:306–314. doi: 10.1370/afm.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wyatt KD, et al. Peering into the black box: a meta-analysis of how clinicians use decision aids during clinical encounters. Implement Sci. 2014;9:26. doi: 10.1186/1748-5908-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vickers AJ, Edwards K, Cooperberg MR, Mushlin AI. A simple schema for informed decision making about prostate cancer screening. Ann Intern Med. 2014;161:441–442. doi: 10.7326/M14-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Volk RJ, et al. Primary care physicians’ use of an informed decision-making process for prostate cancer screening. Ann Fam Med. 2013;11:67–74. doi: 10.1370/afm.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 102.Bhuyan SS, et al. Patient-Provider Communication About Prostate Cancer Screening and Treatment: New Evidence From the Health Information National Trends Survey. Am J Mens Health. 2015 doi: 10.1177/1557988315614082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Penson DF. Re: Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. J Urol. 2016;196:105–106. doi: 10.1016/j.juro.2016.04.031. [DOI] [PubMed] [Google Scholar]