Abstract
Although CD4 T-cells are a major target for HIV, recent work has demonstrated the ability of macrophages despite expressing relatively low levels of CD4, to be a target of the virus. Our recent study has found that the presence of growth factors not only play a role in the phenotype of these monocyte-derived-macrophages, but also are an important aspect of the permissiveness of these cells to infection. The work utilized cellular and biophysical methods to examine Siglec-1 on macrophages as a primary receptor in HIV-1 infection. These findings support the notion that Siglec-1 and macrophages and their interactions with the HIV-1 envelope should be considered in HIV-1 vaccine development.
Keywords: Siglec-1, HIV-1, macrophages, GM-CSF, M-CSF
Macrophages are terminally differentiated, non-dividing cells, and play an important role in the innate and adaptive immune response. In addition to their phagocytic capabilities, macrophages also act as professional antigen presenting cells (APC) by presenting pathogen derived peptides via the MHC-II pathway to CD4+ T cells, activating CD8+ cytotoxic T-cells (CTL) and dendritic cells (DC) by cross-presentation of HIV-1 antigens [1] and exosomes [2]. In humans, macrophages arise from circulating or resident monocytes and their differentiation and effector functions are largely dependent on the surrounding microenvironment [3] [4]. Thus, the prevailing pattern of stimulation within the macrophage microenvironment leads to considerable plasticity in the tissue macrophage phenotype [5]. Once differentiated, macrophages exhibit marked phenotypic heterogeneity, and can become long-lived cells with specialized functions.
Macrophages are present in various tissues (e.g. microglia in the brain, alveolar macrophages in the lung, or Kupffer cells in the liver), and their life spans are largely determined by their immunological roles and tissue localizations. Thus inflammatory macrophages derived from circulating monocytes die after a few days [6], whereas microglia or alveolar macrophages can live from several weeks up to years [7].
Macrophages are important targets of HIV-1, and represent specialized viral reservoirs, with the ability to store HIV-1 particles in intracellular compartments [8, 9], which can be refractory to the effects of broadly neutralizing antibodies [10]. Thus, infectious HIV-1 within macrophages generally seems to be protected from neutralizing antibodies, further complicating its eradication. Due to their dissemination over different tissues and their capacity to infiltrate virtually all organs including the brain, macrophages could critically contribute to the spread of HIV-1 and HIV-related pathologies [11]. Indeed, macrophages have been implicated as key cells that influence mother-to-child transmission of HIV-1 [12].
CD4 molecules and chemokine receptors have been identified as major receptor/coreceptors utilized by HIV-1 for infection of target cells. However, unlike CD4+ T cells, macrophages and monocyte-derived macrophages express relatively low levels of CD4 on the cell surface further confounding the role of this molecule in HIV-1 entry into macrophages. Recently, the role of sialic acid-binding immunoglobulin-like lectin-1 (Siglec-1) in HIV-1 infection of myeloid cells has been investigated, and shown to play an important role in HIV-1 pathogenesis in both dendritic cells [13, 14] and macrophages [15, 16].
This highlight will focus on (i) the effect of environmental milieu on Siglec-1 expression on macrophages, (ii) the role of Siglec-1 in HIV-1 infection of macrophages, and (iii) the interaction of Siglec-1 with the virus envelope.
Fifteen human Siglec receptors have been identified [17]. These are sialic acid specific type I lectins that belong to the Ig superfamily. Human Siglec-1, a 175-185 kDa glycoprotein, also known as sialoadhesin or CD169, selectively binds to 2,3-linked sialic acid residues. Siglec-1 is unique among all Siglecs in having an extremely long 17 Ig-like C2 extracellular domains which extends the V-set domains from the glycocalix of the cell thus facilitating interaction with external ligands (trans interaction) [18] [19], while other members of the Siglec family contain a lower number of C2-type domains and interact with sialyated molecules exposed on the same cell (cis interaction) [20]. Siglec-1 also lacks the tyrosine binding signaling motif within its cytoplasmic domain. While the primary role of Siglecs is to serve as mediators of cell adhesion [20], it is being increasingly recognized that they can also serve as receptors for pathogens [21], and as molecules that can deliver inhibitory signals similar to killer cells immunoglobulin-like receptors (KIRs). In DCs, Siglec-1 serves as a receptor for capturing HIV-1 through its Ig-like V set domain by binding to host-derived glycosphingolipid (ganglioside GM3) that is incorporated in the membrane of HIV-1 particles. This interaction between Siglec-1 and GM3 is independent of the HIV envelope protein [22], while in macrophages the capture of HIV-1 is through the interaction of Siglec-1 and HIV-1 envelope protein [15, 16].
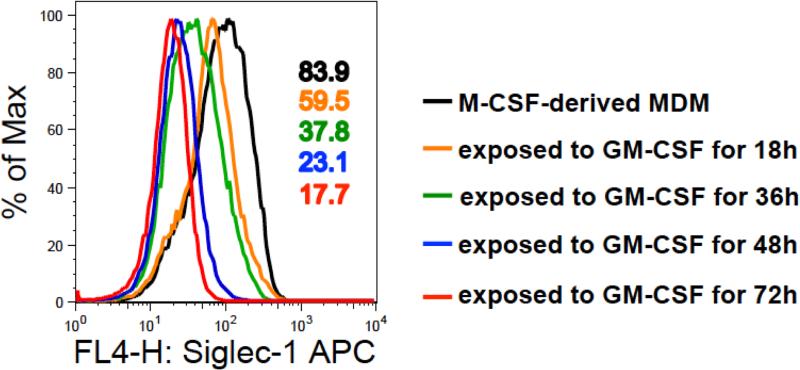
Studies have shown that Siglec-1 expression is influenced by the cytokines present in the environmental milieu induced as a result of chronic inflammation [23, 24]. Upregulation of Siglec-1 on human and nonhuman primate monocytes after HIV-1 or SIV infection respectively, has been demonstrated early after infection and then an increase as the disease progresses to the chronic phase [25, 26]. In our recent study, we have also shown that Siglec-1 expression on in vitro monocyte-differentiated macrophages is impacted by the environmental milieu [16]. Using multiple donors, the analyses of the number of Siglec-1 receptors per macrophage revealed that macrophages derived in culture media containing macrophage colony stimulating factor (M-CSF) had 5.5-fold higher number of Siglec-1 receptors than their counterparts derived in culture media containing granulocyte macrophage colony stimulating factor (GM-CSF). However, the relatively high expression of Siglec-1 on M-CSF-derived macrophages was not static and was affected when the M-CSF-derived monocyte derived macrophages (MDM) were transferred to an environment containing GM-CSF. Indeed, exposure of M-CSF-derived MDM to GM-CSF resulted in a gradual decrease in Siglec-1 expression (Figure 1).
Figure 1. Effect of M-CSF and GM-CSF on Siglec-1 expression on monocyte-derived macrophages.
Monocytes were differentiated into macrophages (MDM) following in vitro culture in media containing 50 ng/ml macrophage colony stimulating factor (M-CSF) for 5 days. The M-CSF-derived MDM were subsequently exposed to 50 ng/ml granulocyte macrophage colony stimulating factor (GM-CSF) for varying time periods. Cells were harvested, stained with anti-Siglec-1 (CD169)-APC, and then analyzed for Siglec-1 expression by flow cytometry on a FACSCalibur. Data analysis was performed on gated CD14+ cells using FlowJo 8.8.6 software. The histograms show the effect of GM-CSF on Siglec-1 expression. M-CSF-derived MDM in the absence of GM-CSF (black); M-CSF-derived MDM exposed to GM-CSF for 18 h (brown), for 36 h (green), for 48 h (blue), and for 72 h (red). Values in the histogram plot represent the MFI of Siglec-1 expression under the respective conditions.
Our work showed that anti-Siglec-1 antibody was able to completely inhibit HIV-1 infection of both M-CSF and GM-CSF-derived macrophages, while the other anti-Siglec mAbs were only able to partially inhibit (25-45%) the infectivity. Furthermore, HIV-1 infectivity in the presence of anti-CD4 mAb or anti-CCR5 mAb was inhibited by 50% or 20%, respectively. These data indicate that Siglec-1 is a primary receptor for HIV-1 infection in macrophages.
Our study demonstrated that M-CSF-derived MDM were more permissive to HIV-1 infection compared to the GM-CSF-derived MDM. These results were attributed to the increased presence of Siglec-1 receptors in the M-CSF-derived MDM. Taken together, these findings suggest a relationship between M-CSF, Siglec-1 expression, and HIV infection in MDM. Using qRT-PCR to examine the association of viral RNA with MDM revealed that masking the Siglec-1 receptor on the cells resulted in near complete inhibition of HIV-1 infection. Interestingly, such extensive degree of inhibition was not observed with either CD4 or CCR5, further highlighting the importance of Siglec-1 in permissiveness of MDM to HIV-1.
The heavily glycosylated HIV-1 envelope is presumed to bind to Siglec-1 through glycoprotein interactions with the receptor. Sialic acid residues are present in N-linked glycosylation sites of the variable regions of the envelope glycoprotein. We demonstrated that sialic acid, 2’,3’ sialyllactose (GM3), blocked Siglec-1 receptor and subsequently modulated the infection of M-CSF-derived MDM.
Our study demonstrated that the V1V2 region of HIV-1 envelope bound to the Siglec-1 receptor on MDM. Biophysical measurements using SPR determined that both monomers and trimers were able to bind to Siglec-1 at nano Molar affinity. We also observed that there was a direct protein-protein interaction between the V1V2 region of HIV-1 envelope and Siglec-1. These interactions were disrupted in the presence of competing sialic acid, indicating the importance of glycosylation on the interaction between the virus envelope and the macrophage receptor. Indeed, pre-incubation of MDM with V1V2 envelope protein, caused significant masking of the Siglec-1 receptor, with a resultant significant inhibition of HIV-1 infection. Treatment of V1V2 envelope proteins with PNGase F to remove sialyations, or neuraminidase A to remove glycans, abrogated the ability of the envelope proteins to inhibit HIV-1 infection of MDM. Each treatment modulated the ability of the protein to block inhibition of viral replication by 50%, highlighting the importance of sialyation as well as glycosylation in the interaction between the HIV-1 envelope and Siglec-1 on MDM.
In addition to CD4 T cells, macrophages, and more specifically M-CSF derived MDM serve as additional targets for HIV-1 infection and replication through the Siglec-1 receptor. It is possible that monocytes and tissue macrophages could also serve as reservoirs for HIV-1, since Siglec-1 has been shown to be induced to high levels on blood monocytes early after HIV-1 infection. The persistence of upregulated levels of Siglec-1 induced by elevated levels of cytokines during HIV-1 infection could have a negative inhibitory effect on the immune system and contribute to immune dysregulation seen in AIDS patients. In summary, our work shows a comprehensive examination of the interactions between HIV-1 and Siglec-1 responsible for HIV-1 entry and replication.
Acknowledgements
The authors were supported by a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD) and a grant from the NIAID, NIH (AI102725). The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army or the Department of Defense.
Abbreviations
- APC
Antigen presenting cells
- DC
dendritic cells
- GM-CSF
granulocyte macrophage colony stimulating factor
- HIV-1
Human immunodeficiency virus-1
- mAb
monoclonal antibody
- M-CSF
macrophage colony stimulating factor
- Siglec-1
Sialic acid-binding immunoglobulin-like lectin-1
- qRT-PCR
quantitative real time polymerase chain reaction
Footnotes
Author contributions
O.J. and M.R. conceived the paper, O.J., J.K., M.R. wrote the paper.
To cite this article: Ousman Jobe, et al. The role of Siglec-1 in HIV-1/macrophage interaction. Macrophage 2016; 3: e1435. doi: 10.14800/ Macrophage.1435.
Conflicting interests
The authors have declared that no conflict of interests exist.
References
- 1.Buseyne F, Le Gall S, Boccaccio C, Abastado JP, Lifson JD, Arthur LO, et al. MHC-I restricted presentation of HIV-1 virion antigens without viral replication. Nat Med. 2001;7:344349. doi: 10.1038/85493. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Liu Y, Yang C, Kang L, Wang M, Hu J, et al. Macrophages transfer antigens to DCs by releasing exosomes containing dead cell-associated antigens partially through ceramide-dependent pathway to enhance CD4 T cell response. Immunology. 2016;149:157–171. doi: 10.1111/imm.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 5.Norton SE, Dunn ET, McCall JL, Munro F, Kemp RA. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin Transl Immunology. 2016;5:e76. doi: 10.1038/cti.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 7.Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life-span of alveolar macrophages. Am J Respir Cell Mol Biol. 2008;38:380–385. doi: 10.1165/rcmb.2007-0224RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudin R, Berre S, Cunha de Alencar B, Decalf J, Schindler M, Gobert FX, et al. Dynamics of HIV-containing compartments in macrophages reveal sequestration of virions and transient surface connections. PLoS One. 2013;8:e69450. doi: 10.1371/journal.pone.0069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan J, Sattentau QJ. The HIV-1-containing macrophage compartment: a perfect cellular niche? Trends Microbiol. 2013;21:405–412. doi: 10.1016/j.tim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Koppensteiner H, Banning C, Schneider C, Hohenberg H, Schindler M. Macrophage internal HIV-1 is protected from neutralizing antibodies. J Virol. 2012;86:2826–2836. doi: 10.1128/JVI.05915-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 12.Ometto L, Zanotto C, Maccabruni A, Caselli D, Truscia D, Giaquinto C, et al. Viral phenotype and host-cell susceptibility to HIV-1 infection as risk factors for mother-to-child HIV-1 transmission. AIDS. 1995;9:427–434. [PubMed] [Google Scholar]
- 13.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, et al. Interferon inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013;9:e1003291. doi: 10.1371/journal.ppat.1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou Z, Chastain A, Moir S, Ford J, Trandem K, Martinelli E, et al. Siglecs facilitate HIV-1 infection of macrophages through adhesion with viral sialic acids. PLoS One. 2011;6:e24559. doi: 10.1371/journal.pone.0024559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe O, Trinh HV, Kim J, Alsalmi W, Tovanabutra S, Ehrenberg PK, et al. Effect of cytokines on Siglec-1 and HIV-1 entry in monocyte-derived macrophages: the importance of HIV-1 envelope V1V2 region. J Leukoc Biol. 2016;99:1089–1106. doi: 10.1189/jlb.2A0815-361R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crocker PR, Mucklow S, Bouckson V, McWilliam A, Willis AC, Gordon S, et al. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 1994;13:4490–4503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97:288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 20.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 21.Chang YC, Nizet V. The interplay between Siglecs and sialylated pathogens. Glycobiology. 2014;24:818–825. doi: 10.1093/glycob/cwu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izquierdo-Useros N, Lorizate M, Contreras FX, Rodriguez-Plata MT, Glass B, Erkizia I, et al. Sialyllactose in viral membrane gangliosides is a novel molecular recognition pattern for mature dendritic cell capture of HIV-1. PLoS Biol. 2012;10:e1001315. doi: 10.1371/journal.pbio.1001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.York MR, Nagai T, Mangini AJ, Lemaire R, van Seventer JM, Lafyatis R. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonists. Arthritis Rheum. 2007;56:1010–1020. doi: 10.1002/art.22382. [DOI] [PubMed] [Google Scholar]
- 24.Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, et al. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology. 2015;12:37. doi: 10.1186/s12977-015-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Kuyl AC, van den Burg R, Zorgdrager F, Groot F, Berkhout B, Cornelissen M. Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PLoS One. 2007;2:e257. doi: 10.1371/journal.pone.0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaroenpool J, Rogers KA, Pattanapanyasat K, Villinger F, Onlamoon N, Crocker PR, et al. Differences in the constitutive and SIV infection induced expression of Siglecs by hematopoietic cells from non-human primates. Cell Immunol. 2007;250:91–104. doi: 10.1016/j.cellimm.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]