Summary
The proteasome inhibitors Carfilzomib (Cfz) and Bortezomib (Btz) are used successfully to treat MM, but have not shown clinical efficacy in solid tumors. Here we show that clinically achievable inhibition of the β5 site of the proteasome by Cfz and Btz does not result in loss of viability of triple-negative breast cancer cell lines. We use site-specific inhibitors and CRISPR-mediated genetic inactivation of β1 and β2 to demonstrate that inhibiting a second site of the proteasome, particularly the β2 site, sensitizes cell lines to Btz and Cfz in vitro and in vivo. Inhibiting both β5 and β2 suppresses production of the soluble, active form of the transcription factor Nrf1 and prevents the recovery of proteasome activity through induction of new proteasomes. These findings provide a strong rationale for the development of dual β5 and β2 inhibitors for the treatment of solid tumors.
eTOC
Weyburne et al. demonstrate that clinically achievable inhibition of the proteasome is not cytotoxic to triple-negative breast cancer cells, and that co-inhibition of the β2 site dramatically sensitizes cells to FDA-approved β5 inhibitors by blocking recovery of proteasome activity.

Introduction
The proteasome is a multi-subunit 2.5 MDa proteolytic complex that plays an essential role in protein quality control in mammalian cells. In cancer cells, mutations, hypoxia (Fels et al., 2008), glucose deprivation (Ma and Hendershot, 2004), and oxidative stress (Deshaies, 2014; Reuter et al., 2010) increase the production of misfolded proteins, resulting in a higher load on the proteasome. In addition, aneuploidy leads to excessive production of the individual subunits of multi-subunit complexes (Deshaies, 2014; Oromendia et al., 2012), further increasing the requirement for protein quality control by the ubiquitin-proteasome pathway. The load on the proteasome is especially high in multiple myeloma (MM), a cancer of the plasma cells, because these cells are highly secretory. Proteasome inhibition in MM causes a toxic buildup of proteins and leads to apoptosis (Bianchi et al., 2009; Deshaies, 2014; Obeng et al., 2006). Three proteasome inhibitors, bortezomib (Btz, Velcade), ixazomib (Ixz, Ninlaro), and carfilzomib (Cfz, Kyprolis), are approved by the FDA for the treatment of MM (Kisselev et al., 2012).
In vitro, many cell lines derived from solid tumor cells are as sensitive to proteasome inhibitors as MM cells (Adams et al., 1999; Garnett et al., 2012). However, Btz has not demonstrated efficacy in clinical trials in solid tumors, either alone or in combination with other therapies (Dou and Zonder, 2014). Pharmacodynamics data from clinical trials demonstrate that Btz equally inhibits proteasomes in solid tumors and blood (Papandreou et al., 2004; Trinh et al., 2012), ruling out poor tumoral penetration as an explanation for the lack of activity. Furthermore, ixazomib, a second-generation Btz analogue with better tissue penetration, did not generate significant responses in a phase I trial in solid tumors (Smith et al., 2015). The reasons for lack of clinical activity of the currently approved proteasome inhibitors are unknown.
In an effort to determine why proteasome inhibitors are not active in solid tumors we chose to focus on triple-negative breast cancers (TNBC), i.e., cancers that do not express the estrogen receptor, progesterone receptor, or human epidermal growth factor receptor 2. TNBCs are characterized by early age of onset, short time to relapse, and high aggressiveness (Foulkes et al., 2010). Fifty to seventy percent of TNBCs are of the basal-like molecular subtype according to gene expression profiles (Bertucci et al., 2008; Lehmann et al., 2011). In a genome-wide siRNA screen that compared in vitro-transformed basal-like and myoepithelial breast cell lines, knock-down of proteasome genes selectively decreased the viability of the basal-like line (Petrocca et al., 2013). Basal-like TNBC cell lines were more sensitive to Btz than estrogen receptor-positive lines. Proteasome inhibitors also decreased tumor growth of basal-like TNBC xenografts in mice.
A possible explanation for the lack of response to proteasome inhibitors in solid tumors is insufficient potency. The 26S proteasome is composed of a cylindrical 20S core that is capped by one or two regulatory 19S particles (Kisselev and Goldberg, 2001). The 20S contains three types of active site subunits on each of its β-rings. The active site of the β1 subunit cleaves after acidic residues (caspase-like), β2 cleaves after basic residues (trypsin-like), and β5 cleaves after hydrophobic residues (chymotrypsin-like) (Groll et al., 1999). Btz, Cfz, and Ixz primarily inhibit β5, which was shown by yeast mutational analysis to be the most important active site in protein degradation (Arendt and Hochstrasser, 1997; Chen and Hochstrasser, 1996; Heinemeyer et al., 1997). Pharmaceutical companies have focused on targeting the β5 site due to its primary role in protein degradation and the cell permeability of hydrophobic peptides (Goldberg, 2012). The β1 and β2 subunits, which have not been specifically targeted by existing pharmaceuticals, also contribute to protein degradation (Kisselev et al., 2006). Although Btz and Ixz can, at higher concentrations, inhibit the β1 site, neither drug inhibits the β2 site (de Bruin et al., 2016). We have developed specific inhibitors of the β1 and β2 sites and demonstrated that co-inhibiting either the β1 or β2 site increases the cytotoxicity of β5 inhibitors in MM cell lines (Britton et al., 2009; Geurink et al., 2013; Kraus et al., 2014; Mirabella et al., 2011). The β2 inhibitor LU-102 overcomes Btz and Cfz resistance in primary MM cells ex vivo, and also reduces xenograft tumor growth in Cfz-treated mice in vivo (Kraus et al., 2014). Thus, inhibiting a second active site of the proteasome could also increase the potency of proteasome inhibitors in TNBCs and other solid tumors.
In this study, we demonstrate that clinically achievable concentrations of Btz and Cfz do not cause death in TNBC cell lines. We then use specific inhibitors of the β1 or β2 sites, as well as CRISPR-generated mutations of β1 or β2, to demonstrate that inhibition of a second active site increases the cytotoxicity of the β5 inhibitors. Furthermore, we show that inhibition of β2 activity sensitizes cells to Cfz and Btz more effectively than does inactivation β1 activity.
We determine that β2 inhibition in combination with β5 inhibition blocks the accumulation of soluble, active Nrf1 (TCF11, encoded by NFE2L1 gene), a transcriptional activator of proteasomal genes that is responsible for the “bounce-back” response that restores proteasome activity (Radhakrishnan et al., 2014; Radhakrishnan et al., 2010; Sha and Goldberg, 2014). Knockdown of Nrf1 sensitizes cells to proteasome inhibitors (Radhakrishnan et al., 2010; Sha and Goldberg, 2014; Steffen et al., 2010). Nrf1 is associated with the ER membrane, and normally (i.e. if the proteasome is not inhibited) is rapidly degraded. Upon partial proteasome inhibition, Nrf1 is proteolytically processed to a soluble, active C-terminal fragment that enters the nucleus and activates transcription. If proteasome activity is more fully inhibited, Nrf1 is incorporated into protein aggregates and becomes insoluble (Sha and Goldberg, 2016). We show that β2 inhibition sensitizes cells to β5 inhibitors by suppressing production of soluble, active Nrf1 and preventing the recovery of proteasome activity. These results demonstrate the importance of further development of inhibitors of the β2 site of the proteasome.
Results
TNBC cells are sensitive to Btz and Cfz only when two active sites of the proteasome are inhibited
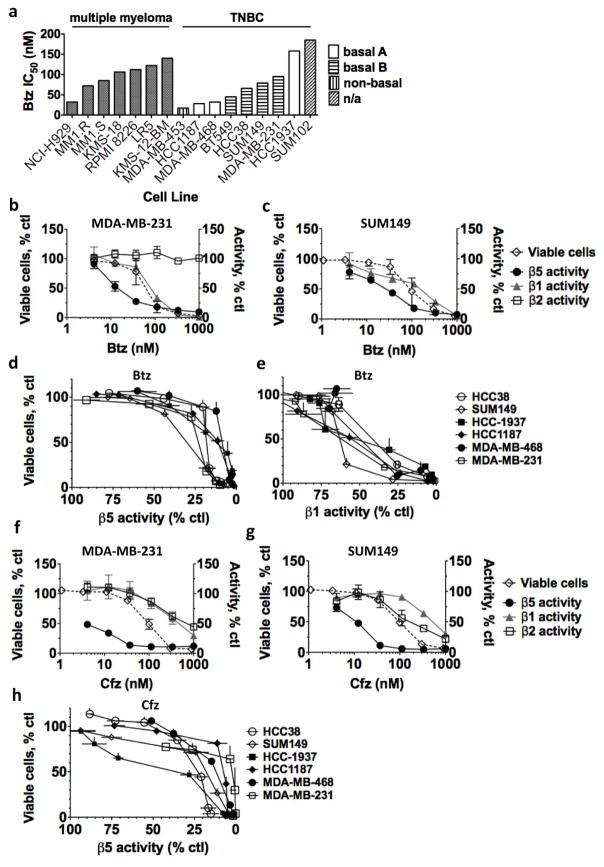
Patients receive Btz as an intravenous or subcutaneous bolus, and the concentration peaks in the blood within 1 h and then decreases rapidly (Papandreou et al., 2004). Therefore, to achieve clinical relevance, cells derived from all recently defined subtypes of TNBC (Lehmann et al., 2016) were tested for sensitivity to a 1-h pulse treatment with Btz. After Btz treatment, we cultured the TNBC cells in drug-free media and assayed cells at 48 h. All cell lines, including the stem-like cell line HCC-38 (Charafe-Jauffret et al., 2009), were highly sensitive to Btz (Fig. 1a). In fact, on average, they were more sensitive than MM cells treated under identical conditions (Fig. 1a) [although decreased production of immunoglobulins may desensitize cultured MM to proteasome inhibitors (Edwards et al., 2009)]. By contrast, primary human mammary epithelial cells (pHMEs) were not sensitive to a 1-h pulse treatment with 1 μM Btz (not shown). Petrocca et al. (2013) found that proteasome inhibitors were more cytotoxic to basal-like (Basal A) TNBC cell lines than to myoepithelial-like (Basal B) lines, but we did not observe this distinction in the cell lines we tested when a 1-h pulse treatment was used (Fig. 1a).
Figure 1. The sensitivity of TNBC cells to Btz and Cfz does not correlate with inhibition of the β5 site.
a) TNBC and MM cell lines were treated with Btz for 1h, recovered in drug-free media for 48h, then assayed for viable cells with Alamar Blue. Dose-response curves were generated by plotting the averages of 2–5 biological replicates and used to determine the average IC50. See Table 1 for confidence intervals. MM data are from (Shabaneh et al., 2013). TNBC subtype assignments are from (Charafe-Jauffret et al., 2009). b,c) Cells were treated with Btz for 1h, then immediately assayed for proteasome inhibition using site-specific substrates from Proteasome-Glo. In a parallel experiment, viable cells were quantified 48h after treatment as in (a). d,e) Viable cells were plotted against inhibition of the β5 and β1 sites after Btz treatment. f,g) Cells were treated with Cfz for 1h, and analyzed as in (b). h) Viable TNBC cells were plotted against inhibition of the β5 site after Cfz treatment. Values in (b–h) are mean ± S.E.M of 2–3 biological replicates.
To determine whether the loss of viability of TNBC cell lines occurs with a clinically achievable degree of proteasome inhibition, the activities of the individual active sites were measured immediately after a 1h treatment of cells with Btz. In clinical trials in endocrine-resistant breast cancer, the amount of β5 inhibition after Btz treatment in biopsied tumor tissue was similar to the proteasome inhibition in blood (50–80%) (Trinh et al., 2012). Therefore, we inferred that treatment with Btz can achieve up to 80% β5 inhibition inside breast tumors in patients. In MDA-MB-231 cells, ~30 nM Btz caused 80% inhibition of the β5 site but induced only ~20% loss of viability (Fig. 1b). Surprisingly, we noticed that viability correlated not with inhibition of the β5 site, but with the onset of inhibition of β1 in MDA-MB-231 (Fig. 1b), SUM149 (Fig. 1c), and four other TNBC cell lines (Fig. 1d, e). We next treated cells with Cfz, which is a slightly more potent and specific β5 inhibitor. The greater inhibition of the β5 site in TNBC cells did not translate into greater cytotoxicity (Table 1), and as with Btz there was a poor correlation between β5 inhibition and cytotoxicity (Fig. 1h). Only a slight decrease (~15%) in the viability of Cfz-treated cell lines was observed upon 90% β5 inhibition, which is clinically achievable by Cfz in human blood (O’Connor et al., 2009; Papadopoulos et al., 2015) (Fig. 1h). Greater cytotoxicity was usually observed only at concentrations where the β1 and β2 sites were at least partially inhibited. These data demonstrate that TNBC cells that are treated with proteasome inhibitors can survive clinically achievable inhibition of the β5 site, but lose viability when an additional site is inhibited.
Table 1.
LU-102 is a stronger sensitizer to Cfz and Btz than NC-021 in a panel of TNBC cell lines.
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Cfz | Cfz+ NC-021 |
Cfz+ LU-102 |
Btz | Btz+ NC-021 |
Btz+ LU-102 |
|
| Cell line | IC50 (C.I., nM) | |||||
| MDAMB-231 | 100 (85–118) | 18 (12–26) | 1 (n.d.) | 95 (74–125) | 45 (34–58) | 11 (9.5–13.5) |
| MDAMB-468 | 19 (13–28) | 0.8 (0.04–18) | 0.4 (0.2–1.3) | 32 (28–37) | 19 (15–24) | 7 (6–9) |
| SUM149 | 100 (67–133) | 19 (8–32) | 2 (n.d.) | 79 (64–98) | 42 (34–52) | 15 (13–17) |
| HCC38 | 133 (116– 224) | 56 (45–70) | 10 (8–14) | 66 (59–75) | 50 (44–56) | 18 (15–22) |
| HCC1187 | 60 (36–100) | 24 (16–36) | 1 (0.02–20) | 62 (46–82) | 56 40–75 |
16 (12–21) |
| HCC1937 | 162 (113– 200) | 50 (32–75) | 15 (9–25) | 158 (106– 216) | 125 (97–161) | 19 (15–25) |
Cells were treated with a range of concentrations of Btz or Cfz in combination with either 3 μM LU-102 or 5 μM NC-021 for 1h, and the Alamar Blue assay was performed 48h later. Results of 2–4 biological replicates were averaged and plotted in dose-response curves that were used to determine IC50. LU-102 and NC-021 inhibited β2 and β1 sites respectively by >95% in all cell lines. C.I., 95% confidence interval.
Site-specific inhibition of the β2 site sensitizes TNBC cells to β5 inhibitors better than site-specific inhibition of the β1 site
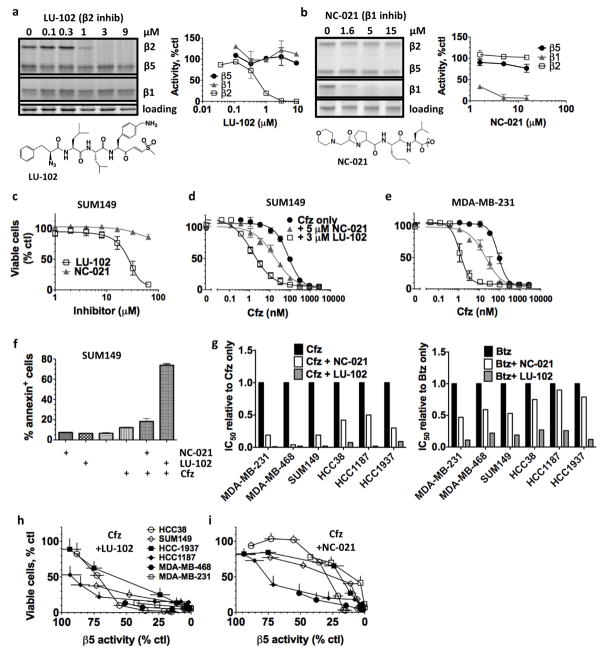
We next sought to determine which of the two additional active sites (β1 or β2), when co-inhibited, more effectively increases cytotoxicity of β5 inhibitors. LU-102 is a β2-specific peptide vinyl sulfone developed by our group (Kraus et al., 2014), and NC-021 is a novel peptide epoxyketone that is a more potent β1 inhibitor than the previously published inhibitor NC-001 (Britton et al., 2009) (Fig. S1a). Both LU-102 and NC-021 inactivate their respective sites by irreversibly binding to the catalytic threonine (Kisselev et al., 2012). To monitor the ability of LU-102 and NC-021 to inhibit their presumed targets, we used activity-based probes (ABPs, (Verdoes et al., 2010)), which can only label the subunit if the active site is not inhibited. A one-hour treatment with 3 μM LU-102 and with 5 μM NC-021 inhibited >95% of their respective activities with high specificity, as evidenced by the absence of ABP labeling of the β2 or β1 subunits in LU-102 and NC-021-treated cells respectively (Fig. 2a, b; Fig. S1b). At these concentrations, neither NC-021 nor LU-102 alone caused a decrease in the number of viable cells or in cell growth over five days (Fig. 2c; S1c), and LU-102 did not inhibit cathepsins B, H, L or S even after 6h treatment (Fig. S1d).
Figure 2. Inhibition of the β2 subunit sensitizes cells to β5 inhibitors better than inhibition of the β1 subunit.
a,b) SUM149 cells were treated for 1h with LU-102 or NC-021. The active site probes MV-151 and BODIPY-NC-001 (Verdoes et al., 2010) were used to determine site inhibition in cell extracts. Band intensities were quantified by densitometry. The graphs are averages ± S.E.M of two independent experiments. c) SUM149 cells were treated with NC-021 or LU-102 for 1h and viable cells were determined with Alamar Blue after 48h. d,e) Cells were treated with Cfz, Cfz+5μM NC-021, or Cfz+3μM LU-102 for 1h and viable cells were determined after 48h. Combination indexes are listed in Fig S1e. f) SUM149 cells were treated with 100nM Cfz, 3μM LU-102, and 5μM NC-021 for 1h. Cells were stained with Annexin V after 36h. Values are mean ± S.E.M of 2–3 biological replicates. g) Average IC50s were plotted relative to the average IC50 for Btz or Cfz only. h,i) Viable cells after 1h co-treatment with Cfz + 3μM LU-102 (h) or Cfz + 5μM NC-021 (i) were plotted against inhibition of β5 sites by Cfz (as in Fig. 1h). NC-021 and LU-102 do not alter inhibition of the β5 sites by Cfz (not shown).
We next tested co-treatment of NC-021 or LU-102 with Cfz, which we chose as a β5 inhibitor because it is more β5-specific than Btz. Treatment with either 5 μM NC-021 or 3 μM LU-102 dramatically sensitized SUM-149 and MDA-MB-231 cells to Cfz (Fig. 2d, e). The combination indexes (CIs) of Cfz and LU-102 co-treatment indicated strong synergism (Fig. S1e) (CIs for Cfz+NC-021 could not be calculated because NC-021 as a single agent does not reduce viable cells). Strikingly, LU-102 had a stronger effect than NC-021 in increasing sensitivity to Cfz. Specifically, in SUM149 cells, co-treatment with NC-021 decreased the IC50 ~5-fold over the IC50 of Cfz alone, while co-treatment with LU-102 decreased the IC50 ~50-fold. Treatment of SUM149 cells with 100 nM Cfz induced apoptosis in 6% of cells, co-treatment with NC-021 increased this number to 12%, but co-treatment with LU-102 produced 68% apoptotic cells (Fig. 2f). A similar trend was also seen in MDA-MB-231 cells (Fig. S1f). The combination of Btz or Cfz with β2 inhibition decreased viable cells more than did the combination with β1 inhibition across a panel of TNBC cell lines (Fig. 2g, Table 1).
In all TNBC cell lines tested, when Cfz was used to treat cells together with LU-102, Cfz was cytotoxic (>75%) when it inhibited the β5 site by ~50% (achievable at most clinical doses)(Fig. 2h). In contrast, together with NC-021, Cfz was only cytotoxic by >75% in most TNBC cell lines if it inhibited the β5 site by 80% or more (achievable only by the maximal tolerated clinical doses)(Fig. 2i). Overall, co-inhibition of the β2 and β5 sites in TNBC cell lines causes a stronger pro-apoptotic effect than co-inhibition of the β1 and β5 sites, and allows Cfz to exert cytotoxicity over a wider range of clinically achievable concentrations.
Genetic inactivation of the β2 site sensitizes cells to β5 inhibition more than inactivation of the β1 site
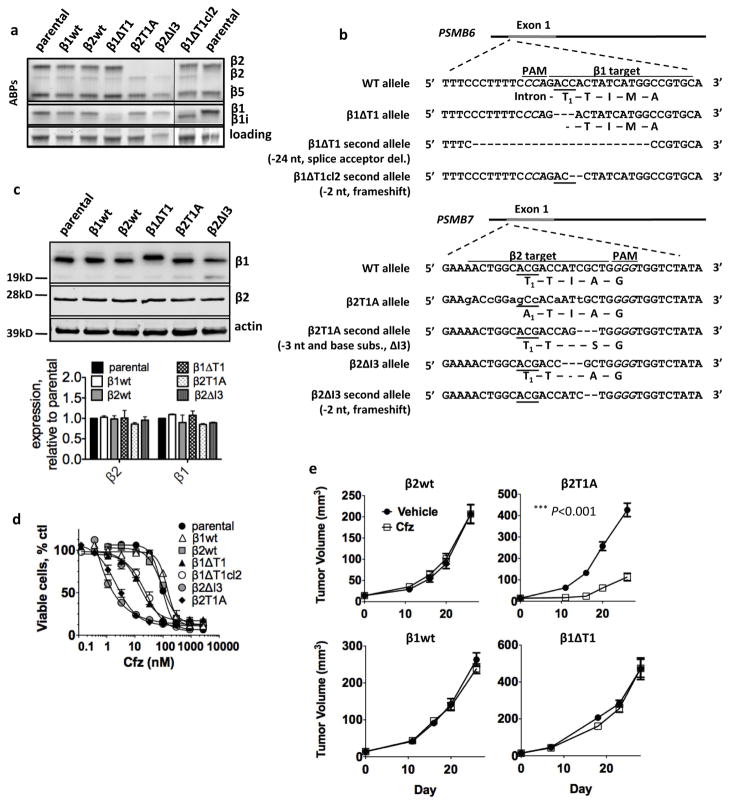
To confirm the results we obtained with site-specific inhibitors, we inactivated the β1 and β2 subunits via a genetic approach. We employed CRISPR genome editing to mutate the catalytic threonines of the β1 (gene: PSMB6) or β2 (gene: PSMB7) sites into alanines in MDA-MB-231 cells. Clones were initially selected based on the lack of β2 or β1 activity (Fig. 3a), and the presence of mutations was then confirmed by Sanger sequencing. We isolated a clone with the intended substitution of the catalytic Thr with Ala in the β2 site (β2T1A), and a clone with deletion of the Ile3 residue two residues downstream of the catalytic Thr1 (β2ΔI3) (Fig. 3b). The second allele for β2T1A was rendered dysfunctional by deletion of Ile3, and the second allele for β2ΔI3 was inactivated by frameshift. We also isolated a clone lacking the catalytic Thr of the β1 site (β1ΔT1). A second ΔT1 clone, β1ΔT1cl2, had the same ΔT mutation but a different mutation in the second allele and hence was independent. The activity assay for β1ΔT1 and β1ΔT1cl2 exhibits a faint band slightly below the β1 position (Fig. 3a), but this band is attributed to cross-reaction of the probe with the β5 site (see Fig. S2a). We believe that we isolated deletion mutants more readily than substitutions because the odds of non-homologous end joining DNA repair (NHEJ), which can generate 3-nucleotide in-frame deletions, are higher than the odds of homologous recombination (HR), which was used to introduce the T1A mutation (Maruyama et al., 2015). None of the mutations perturbed subunit expression, even if the second allele was frameshifted (Fig. 3c). The non-targeted active sites remained fully functional (Fig. 3a, quantified in S2b). These results are in agreement with previous data that ΔT1 or T1A mutations lead to fully assembled but inactive archaebacterial proteasomes (Seemuller et al., 1995). CRISPR-generated clones that did not exhibit loss of activity and that express the same levels of proteasome subunits were used as appropriate controls in these experiments (β1wt, β2wt).
Figure 3. Mutant CRISPR cell lines with inactive β2 subunits are more effectively sensitized to β5 inhibition than cells with inactive β1 subunits.
a) Activity of each active site was determined with activity-based probes. “i” designates subunits of lymphoid-tissue specific immunoproteasomes. The faint band between β1 and β1i in the β1ΔT1 mutant is due to BODIPY-NC-001 cross-reacting with the β5/5i subunit (see Fig. S2a). b) Sequences of mutant alleles. T1 is the active site threonine. Lowercase letters indicate CRISPR-introduced T1A and synonymous mutations. subs.= substitution, del.=deletion. c) Western blot confirming expression of mutant subunits. d) Cells were treated with Cfz for 1h and viable cells were determined at 48h. Data are averages ± S.E.M. of 3–7 biological replicates. e) Cells were injected into the mammary fat pads of female NSG mice. Day 0 indicates the day that tumors were deemed palpable, defined as 14mm3. Mice were treated intravenously with 1.5 mg/kg Cfz twice-weekly on consecutive days. ***P<0.001, two-way ANOVA, compared to vehicle control, n=8–14/group (Table S1).
We first used the mutant cell lines to rule out the possibility that off-target effects were contributing to the biological action of LU-102 and NC-021. As predicted, LU-102 no longer decreases viability of both β2 mutant cell lines, and NC-021 no longer decreases viability of the β1ΔT1 mutant cell line (Fig. S2c). Therefore, LU-102 and NC-021 sensitize cells to Cfz by inhibiting β2 and β1, respectively, rather than by any off-target effect. These data also eliminate the possibility that low-level expression of the β2i and β1i subunits of immunoproteasomes, which are inhibited by LU-102 (Geurink et al., 2013) and NC-021 respectively (not shown), compensate for the loss of β2 and β1 activity in the mutants.
Most importantly, both of the β2 mutant clones were more sensitive to Cfz than the wild type clones, and they were also more sensitive than the β1 mutants (Fig. 3d). The differential viabilities between mutant cell lines were proteasome inhibitor-dependent: the cell lines did not show differential viabilities when treated with the DNA-damaging agent doxorubicin (Fig. S2d). These data confirm the inhibitor-based approach (Figure 2) and demonstrate that the β2 site is a better co-target of β5-directed inhibitors than the β1 site.
We next tested whether inhibition of β2 enhances Cfz activity in a xenograft tumor model of TNBC in mice. Orthotopic tumors of β2T1A, β1ΔT1, β1wt and β2wt CRISPR-engineered lines were grown in the mammary fat pad of female NSG mice. When tumors became palpable, mice were injected twice weekly with 1.5 mg/kg Cfz (half of the maximal tolerated dose (Ichikawa et al., 2012)). β1wt, β2wt, and β1ΔT1 tumors did not respond to Cfz treatment (Fig. 3e). In contrast, the growth of β2T1A tumors was markedly reduced. At the end of the study we observed induction of the β1i and β2i subunit in both vehicle- and Cfz-treated tumors (Fig. S2e). β2T1A tumors were Cfz-sensitive despite the increased expression of the β2i subunit, suggesting that a compound that inhibits both β2 and β2i (e.g., LU-102) may be even more effective. β1- and β2-mutant tumors grew faster than wild-type tumors, matching the growth rates seen with the cell lines in vitro (Fig. S2f); the different growth rates could be due to random puromycin selection of faster or slower growing cells. Taken together, these data show that inhibition of the β2 site, but not the β1 site, sensitizes TNBC to Cfz in vivo.
Co-inhibition of β5 and β2 increases ubiquitylated protein accumulation and inhibits recovery of proteasome activity
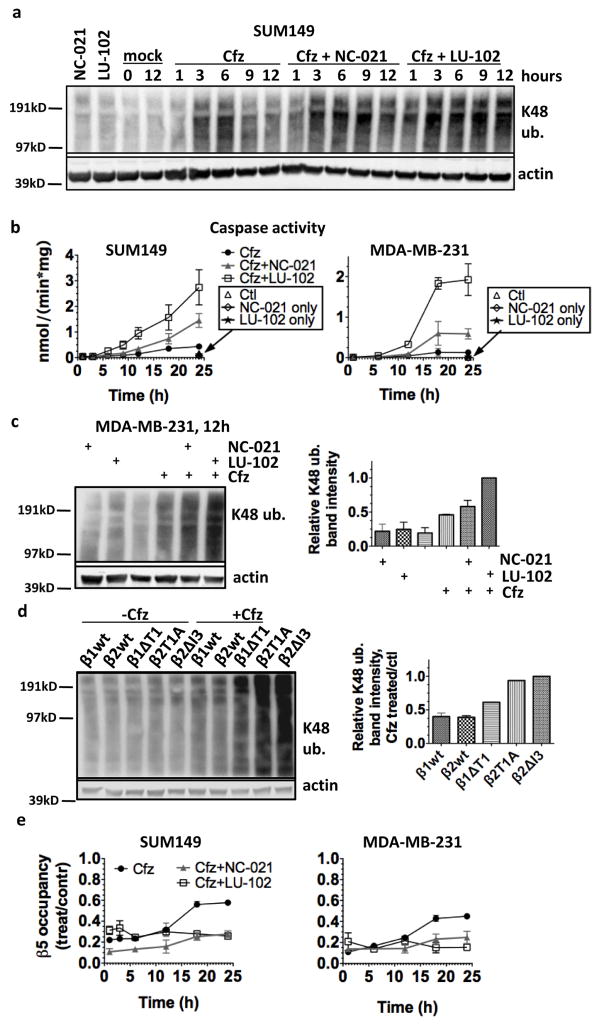
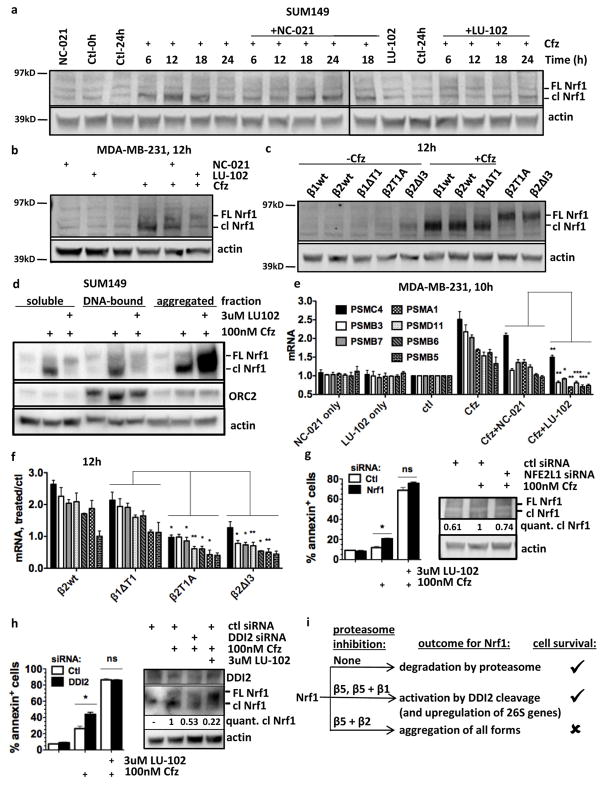
We next asked why β2 inhibitors sensitize cells to β5 inhibitors better than β1 inhibitors. We initially hypothesized that co-inhibition of β5 and β2 results in a greater reduction in protein breakdown than either inhibition of β5 alone or co-inhibition of β5 and β1. Blocking proteasome function leads to an accumulation of ubiquitylated proteins, and assessing their accumulation is a widely used approximation assay of proteasome inhibition. We measured the levels of ubiquitin conjugates in cells treated with 100nM Cfz, the concentration at which LU-102, but not NC-021, sensitized cells to Cfz-induced apoptosis (Fig. 2f). This is a clinically relevant concentration because the inhibition of the β5 site is similar to the inhibition observed in human blood at the maximal tolerated dose of Cfz (~90%, Fig. 1fg). Treatment of SUM149 cells with 100nM Cfz alone caused a moderate accumulation of K48-linked ubiquitin conjugates, which peaked at 6 h and then decreased (Fig. 4a). Co-treatment with NC-021 prolonged the accumulation for an additional 3h, and cells co-treated with LU-102 did not clear ubiquitinylated proteins by 12h, when caspases begin to become activated in this cell line (Fig. 4b). A stronger blockage of ubiquitin conjugate clearance by co-inhibiting β5 and β2 than by co-inhibiting β5 and β1 was also observed when the MDA-MB-231 cell line was treated with these inhibitors, as well as in the CRISPR-generated active site mutant lines (Fig. 4c, d). We wondered if the clearance of ubiquitin conjugates in Cfz-treated cells indicated a restoration of proteasome activity. Indeed, the clearance of conjugates coincided with the recovery of β5 activity in the Cfz-treated cells (Fig. 4e, S3), which occurred before the onset of apoptosis (Fig. 4b). Cells treated with both NC-021 and Cfz also recovered β5 activity, although at a slower rate. However, co-inhibition of β2 with LU-102 completely blocked the recovery of proteasome activity in the Cfz-treated cells.
Figure 4. β2 inhibition increases accumulation of ubiquitinated protein and inhibits proteasome activity recovery in Cfz-treated cells.
a,c) Cells were treated for 1h with 100 nM Cfz, 3 μM LU-102, and 5 μM NC-021, harvested at the times indicated, and analyzed by western blot. b) Cells were treated as in (a) and harvested. Caspase activity was determined in cell extracts using the caspase substrate Ac-DEVD-amc. d) CRISPR cell lines were treated for 1h with 100nM Cfz and harvested at 9h, then analyzed by western blot. e) Cells were treated for 1h with the same concentrations of inhibitors as in (a) and (b), and β5 occupancy was determined with activity-based probes as in Fig. 2ab. See Fig. S3 for β1 and β2 occupancies. Data are presented as mean ± S.E.M of 2–3 biological replicates.
Co-inhibition of β5 and β2 suppresses production of soluble, active Nrf1 and prevents induction of proteasome genes
Because Cfz is an irreversible inhibitor, synthesis of new proteasomes is the most likely mechanism for proteasome activity recovery after Cfz treatment. As discussed in the Introduction, Nrf1 activates transcription of all 26S genes after proteasome inhibition, allowing cells to escape the cytotoxic effects of proteasome inhibitors (Radhakrishnan et al., 2010; Sha and Goldberg, 2014; Steffen et al., 2010). It has been observed that partial inhibition of the proteasome causes cleavage of the transmembrane domain of Nrf1 to release soluble, transcriptionally active Nrf1, which remains in the supernatant of cell lysate after centrifugation at 10,000×g for 10 min in 1% TX-100 (Sha and Goldberg, 2014). Strong inhibition of the proteasome, however, results in inclusion of both processed and unprocessed forms of Nrf1 into insoluble protein aggregates (Sha and Goldberg, 2016). We detected the active, cleaved form of Nrf1 (soluble in 0.5% CHAPS) in Cfz-treated SUM149 cells at 6h (Fig. 5a), preceding the recovery of proteasome activity (Fig. 4e). The accumulation of active Nrf1 reached a maximum at 12h and 18h, then returned nearly to pre-treatment levels at 24h. In cells co-treated with NC-021, peak accumulation was delayed until 18h (Fig. 5a). However, co-treatment with LU-102 and Cfz suppressed the generation of active Nrf1. LU-102 and inactivation of β2 by mutation in Cfz-treated MDA-MB-231 cells also suppressed production of active Nrf1. Instead, accumulation of the higher-molecular weight, full-length form of Nrf1 was observed (Fig. 5b, c). The identity of the higher molecular weight band was verified to be the full-length form of Nrf1, not the glycosylated form, by comparison to cell treatment with the p97/VCP inhibitor NMS-873 (which causes accumulation of glycosylated Nrf1 (Sha and Goldberg, 2014)) (Fig. S4). Because of the composition of the extraction buffer, these experiments (Fig. 5a–c) followed soluble and membrane-bound proteins. Next, we used DNase and 0.5 M KCl extraction to determine whether the active Nrf1 in Cfz-treated SUM149 cells was associated with chromatin (Fig. 5d). The remaining pellet was sequentially lysed in 2% SDS to liberate proteins from insoluble protein aggregates. This step revealed a large amount of aggregated (but not chromatin-bound) Nrf1, both cleaved and full-length, in cell co-treated with Cfz and LU-102 (Fig. 5d). Thus, a combination of Cfz and LU-102 suppresses Nrf1 by causing its aggregation.
Figure 5. Co-inhibition of β5 and β2 suppresses soluble, active Nrf1 and prevents upregulation of proteasome genes.
a–c) Cells were treated for 1h with 100nM Cfz, 3 μM LU-102, and/or 5 μM NC-021 (a,b), or 100 nM Cfz (c), harvested at times indicated, lysed with CHAPS buffer, and analyzed by western blot. Cfz+NC-021 18h is loaded on both gels to allow comparison. Representative results of 2 experiments are shown. FL=full-length, cl=cleaved. d) SUM149 cells were treated for 1h and harvested at 16h. The soluble fraction was isolated with CHAPS buffer, the pellet was treated with DNase and high-salt to isolate the chromatin-bound fraction, and aggregates were subsequently solubilized by sonication in 2% SDS. Fractions were analyzed by western blot. Origin Recognition Complex subunit 2 (ORC2) is a marker for chromatin-bound proteins. e) MDA-MB-231 cells were treated as in (a), harvested at 10h, and mRNA was measured by RT-PCR. mRNA levels are relative to the control gene PGK1. f) Cells were treated as in (c), harvested at 12h, and mRNA was measured by RT-PCR. (g,h) Cells were treated with 60nM control, Nrf1, or DDI2 siRNA, then treated with indicated compounds for 1h. Samples for westerns were harvested at 16h. Remaining cells were stained with Annexin V after 36h. i) Model for the effect of proteasome inhibition on Nrf1 regulation. ***P<0.001, **P<0.01, *P<0.05, unpaired t-test. Data in (e–h) are presented as mean ± S.E.M of 2–5 biological replicates.
To determine whether aggregation blocks the transcriptional activity of Nrf1, we measured the levels of mRNA for proteasome subunit genes by RT-PCR at 10h, when the rapid recovery of proteasome activity begins (Fig. 4e) but no caspase cleavage is evident (Fig. 4b). As expected, treatment with Cfz alone caused a strong induction of genes encoding 26S subunits (Fig. 5e). Co-treatment with either LU-102 or NC-021 decreased 26S subunit mRNA levels, but LU-102 caused a larger decrease. This result is consistent with the finding that LU-102 co-treatment causes Nrf1 aggregation but NC-021 co-treatment does not. Treatment of β2wt and β1ΔT1 cells with Cfz also caused an increase of genes encoding 26S subunits, but treatment of β2T1A and β2ΔI3 cells did not (Fig. 5f). Thus, LU-102 co-treatment blocks Cfz-induced Nrf1 activation and production of new proteasomes.
If Nrf1 is involved in survival following proteasome inhibitor treatment, then knockdown of Nrf1 with siRNA would decrease cell viability in Cfz-treated cells. However, knockdown of Nrf1 in Cfz+LU-102 treated cells would not affect viability, as the Nrf1 in these cells was already inactivated by aggregation. Although we only achieved ~25% knockdown of active Nrf1, we indeed saw a significant increase in apoptotic Cfz-treated SUM149 cells, but no change in cells treated with Cfz+LU-102 (Fig. 5g). To further test the importance of Nrf1-mediated proteasome production as a pro-survival mechanism that is inhibited by LU-102 co-treatment, we knocked down DDI2, an aspartyl protease involved in cleavage of Nrf1 to its active form (Koizumi et al., 2016; Lehrbach and Ruvkun, 2016). We found that knocking down DDI2 in SUM149 cells with siRNA also caused a significant increase in apoptosis upon Cfz-treatment, while there was no change in cells treated with Cfz+LU-102 (Fig. 5h). Therefore, TNBC cells survive Cfz treatment due to Nrf1 cleavage leading to upregulation of proteasome genes. On the other hand, co-treatment with Cfz and LU-102 causes cell death by causing Nrf1 aggregation and suppressing the compensatory production of proteasomes (see model in Fig. 5i).
LU-102 sensitizes cell lines derived from other solid tumor types to Cfz and Btz
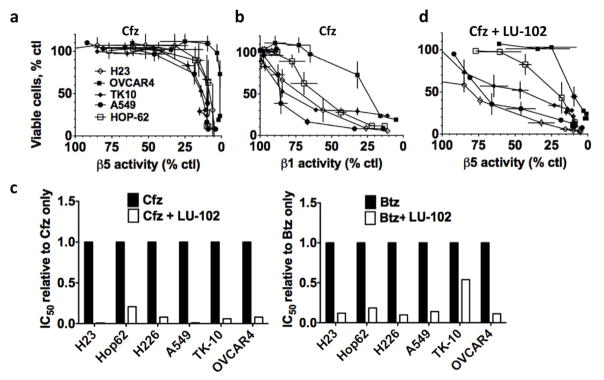
We next queried the applicability of our results to other solid tumor types. As in TNBC, we found that there was also a poor correlation between β5 inhibition and cytotoxicity in cells lines derived from lung, renal, and ovarian cancer treated with Cfz (Fig. 6a). Again, greater cytotoxicity was observed with the onset of inhibition of a second site at higher concentrations of Cfz (Fig. 6b). The combination of Btz or Cfz with a non-toxic LU-102 concentration decreased viable cells in all of the solid tumor cell lines tested (Fig. 6c and Table S2). In some lung cancer and renal cancer lines treated with Cfz and LU-102, Cfz was >75% cytotoxic when it inhibited the β5 site by ~50%, which is achievable with most clinical doses (Fig. 6d). Therefore, inhibition of β2 sensitizes different solid tumors to FDA-approved proteasome inhibitors.
Figure 6. LU-102 sensitizes cell lines derived from other solid tumors to Cfz and Btz.
a,b) Percentage of viable cells were plotted against inhibition of the β5 or β1 sites after 1h treatment with Cfz. Cells lines are derived from lung (A549, H23, Hop-62, H226), renal (TK-10), or ovarian (OVCAR4) cancer. c) Average IC50s were plotted relative to the average IC50 for Btz or Cfz only. d) Percentage of viable cells were plotted against inhibition of the β5 site after 1h treatment with Cfz + 3 μM LU-102. LU-102 did not reduce viability when used as a single agent and did not alter inhibition of the β5 site by Cfz (not shown).
Discussion
The proteasome inhibitors Btz and Cfz have greatly advanced the treatment of MM, but have not yet shown clinical efficacy against solid tumors, despite demonstrating marked cytotoxicity against some such cancer subtypes (e.g. TNBC) in vitro. While poor drug penetration into tumors may account for the lack of efficacy in solid tumors, pharmacodynamic data from two different clinical trials with Btz have demonstrated similar inhibition of the proteasome inside tumors as in blood (Papandreou et al., 2004; Trinh et al., 2012). This study presents evidence that these inhibitors lack clinical activity against proteasome-dependent TNBC cells (Petrocca et al., 2013) and other solid tumor cells because clinically achievable inhibition of the proteasome is insufficient to induce cytotoxicity. We also demonstrate that inhibition of two proteasomal active sites increases the cytotoxicity of proteasome inhibitors in TNBC, and that co-inhibition of the β5 and β2 sites provides stronger anti-neoplastic effect than co-inhibition of the β5 and β1 sites.
To the best of our knowledge, this is the first report in the literature describing CRISPR-mediated site-directed mutagenesis of proteasomal active sites. We took advantage of the CRISPR-engineered cell lines to demonstrate unequivocally that LU-102 and NC-021 exert their biological effects by binding to proteasomal catalytic sites (Fig. S2c). Treatment of the CRISPR cell lines with Cfz confirmed that β2 is a better co-target. The distinct effects of the β1 and β2 mutations were even more striking in a xenograft model, in which only the β2 mutation sensitized tumor cells to sub-toxic doses of Cfz.
We found that co-inhibiting β2 in Cfz-treated cells (in which β5 is inhibited) suppresses generation of the active form of the transcription factor Nrf1, which activates the expression of genes encoding proteasome subunits. The activation of Nrf1 upon proteasome inhibition requires the protease DDI2 (Koizumi et al., 2016; Lehrbach and Ruvkun, 2016; Sha and Goldberg, 2016), which cleaves the ER-bound precursor of Nrf1 to a smaller form. However, in order for the cleaved Nrf1 to be active, it must also remain soluble. Findings of Sha and Goldberg indicate that treatment with high concentrations of proteasome inhibitors, which block all three sites, causes Nrf1 aggregation (Sha and Goldberg, 2016). Aggregation likely causes Nrf1 to lose activity, contributing to the unusual observation that Nrf1-mediated proteasome gene expression can only be induced in cells treated with low, but not high, concentrations of proteasome inhibitors (Sha and Goldberg, 2014). Similar to these observations, we find that β5 proteasome inhibition stimulates generation of soluble processed Nrf1 and recovery of proteasome activity, but β2 and β5 inhibition causes Nrf1 aggregation and blocks recovery. Our work suggests that inhibition of the β2 and β5 sites is sufficient to induce the aggregation of Nrf1, even with the compensatory expression of the immunoproteasome subunit β2i in β2T1A and β2ΔI3 cells (Fig S2b).
The FDA-approved proteasome inhibitors (e.g., Btz, Ixz, Cfz) do not inhibit the β2 site (de Bruin et al., 2016). Only one inhibitor in clinical trials, marizomib, inhibits both the β5 and β2 sites in whole blood of human patients (Levin et al., 2016). However, marizomib also inhibits β1 and crosses the blood-brain barrier (BBB). It has a unique pattern of CNS toxicity in phase I clinical trials (Harrison et al., 2016; Richardson et al., 2016). Thus, there is a need to develop a β2 inhibitor that cannot cross the BBB. Cfz does not cross the BBB, and a combination with a BBB-impermeable β2–specific inhibitor would result in more targeted, less toxic proteasome inhibition.
In summary, we show that an inhibitor of the β2 site dramatically increases the efficacy of FDA-approved proteasome inhibitors in TNBC, indicating the importance of developing additional β2 inhibitors and testing them in TNBC patients, and patients with other solid tumors.
Significance
Triple Negative Breast Cancers (TNBCs) are aggressive solid tumors that affect younger women and have the worst prognosis of any breast tumor type. TNBCs do not have targeted therapies and new treatments are needed. Although the proteasome inhibitors Btz and Cfz have been clinically tested in solid tumors, they have not been effective. Our work demonstrates that clinically relevant concentrations of Btz and Cfz are not active in cell lines derived from TNBC or solid tumors of different organs. This finding suggests that stronger inhibition of the proteasome is necessary for efficacy in human patients. We show stronger anti-neoplastic effects can be achieved by co-inhibiting the β5 and β2 active sites, a combination that is not targeted by FDA-approved agents. We generate cell lines with CRISPR-Cas9 inactivation of β1 and β2, and use them to confirm the findings obtained with inhibitors. Inactivation of β2 additionally sensitizes TNBC tumors in vivo to Cfz. The CRISPR-Cas9 cell lines are also used to demonstrate the high specificity of our active site-specific inhibitors, setting a standard for small molecule inhibitor validation. We find that co-inhibition of β5 and β2 causes aggregation of Nrf1, which blocks upregulation of proteasome genes and prevents recovery of proteasome activity. Overall, this work proposes that solid tumors have an intrinsic level of resistance to FDA-approved proteasome inhibitors. The ability of these cells to robustly recover proteasome activity contributes to resistance. Recovery involves Nrf1-mediated expression of proteasome genes, but can be blocked by co-inhibiting β2 to cause Nrf1 aggregation and inactivation. In summary, this study provides a rationale for developing β2 proteasome inhibitors for the treatment of solid tumors.
Experimental Procedures
Cell culture
MDA-MB-231, MDA-MB-468, HCC38, HCC1937 and HCC1187 cells were obtained from ATCC. SUM149 and SUM102 cells were obtained from Asterand. SUM149 cells were validated at the Vermont Cancer Center DNA Analysis Facility by STR DNA fingerprinting using the Promega GenePrint® 10 System according to manufacturer’s instructions (Promega #B9510). SUM102 does not have a profile in the Cell Line Integrated Molecular Authentication database (CLIMA) or in the German Collection of Microorganisms and Cell Cultures database (DSMZ), but the profile did not match any other known DNA fingerprints in the database. Medium composition for each cell line is provided in the supplement.
Inhibitors
LU-102 was synthesized as described (Geurink et al., 2013). Btz and Cfz were obtained from LC Laboratories. NMS-873 was obtained from Xcess Biosciences. NC-021 was synthesized as described for NC-001 (Britton et al., 2009), and purified by HPLC to 98%+ purity. The compound was observed as a mixture of cis/trans Pro isomers interconverting on the NMR time scale. NMR spectrum is provide in the Supplement.
Activity assays
Proteasomal activity in intact cells was determined with luminescent site-specific substrates using the ProteasomeGlo assay (Promega) as described in our previous study (Britton et al., 2009). To determine activity with activity-based probes (ABPs), cells were lysed in 0.25M sucrose, 2mM EDTA, 1mM ATP, 1mM DTT, 0.05% digitonin in 50mM tris-HCl, pH 7.5. Extracts were treated with 5 μM BODIPY-NC-001 then 20 μM MV-151 for 20 minutes each at 37°C. Extracts were then fractionated on 10% Bis-Tris gels (Genscript) using MOPS running buffer to separate subunits. Gels were analyzed by fluorescent densitometry using the Typhoon imager. Proteasome bands were identified based on their well-characterized mobility and ABP modification pattern (Britton et al., 2009; Mirabella et al., 2011). Subsequent Coomassie staining was used as loading control.
Cell viability and apoptosis assays
Viable cells were measured with Alamar Blue mitochondrial dye conversion assay 48h after drug treatment. Caspase activity was measured with the caspase substrate Ac-DEVD-amc as described (Britton et al., 2009). Annexin staining was performed using the Alexa Fluor 488 Annexin V/Dead Cell apoptosis kit from Molecular Probes, and quantified with flow cytometry.
Preparation of cell extracts for immunoblots
Cells were lysed in CHAPS-buffer (0.5% CHAPS, 10% glycerol, 5mM MgCl2, and 1mM EDTA in 50mM tris-HCl, pH 7.5) for 15 min on ice, and centrifuged at 20,000g for 15 min. To isolate chromatin-bound proteins (Fig. 5d), the remaining pellet was then incubated with Turbo DNase for 20 min RT. After centrifuging at 20,000g for 3 min, the remaining pellet was incubated in high-salt lysate buffer (20 mM Tris pH 7.5, 500 mM KCl, 300 mM sucrose, 5 mM MgCl2, 1 mM EDTA, 0.5% NP40, 2 mM ATP) for 15 min on ice, pelleted at 20,000 g, and the supernatant was combined with the supernatant from DNase treatment. To isolate the insoluble aggregated fraction of proteins, the remaining pellet was suspended in 2% SDS in 50 mM Tris-Hcl pH 7.4 and sonicated 15s at 30% amplitude. List of antibodies used are provided in the Supplemental section.
siRNA transfection
siRNA complexes were made with Hiperfect (Qiagen) following the manufacturer’s protocol. Complexes were added dropwise to cells at the time of plating, and cells were allowed to attach for 24 h before starting treatment. siRNAs were obtained from GE Dharmacon: NFE2L1 (L-019733-00-0010); non-targeting siRNA #1 (D-001810-01-05); and a custom DDI2 siRNA (Sense: 5′ GCCAAGUAGUGAUGCUUUA 3′ (Koizumi et al., 2016)).
RT-PCR
mRNA was extracted with the RNeasy Mini kit (Qiagen), and cDNA was synthesized using iScript cDNA synthesis kit (Biorad). Real-time RT-PCR was performed using ABsolute Blue qPCR ROX Mix (Thermo Scientific) on a Bio-Rad C1000 thermal cycler.
CRISPR-Cas9 genome editing
Guide RNAs were designed to disrupt PSMB6 (β1) and PSMB7 (β2) genes in the vicinity of the Thr1 codons. Guide RNAs targeted the following genomic sites: 5′-GCACGGCCATGATAGTGGTC-3′ (β1, PSMB6) and 5′-AACTGGCACGACCATCGCTG-3′ (β2, PSMB7). The sequence CACC was added to the 5′ end of the gRNAs to facilitate ligation into the lentiCRISPRv2 vector (Addgene) containing Cas9. The substitution mutant β2T1A was produced with lipid transfection. The lentiCRISPRv2 plasmid, as well as a pBluescript II KS(+) plasmid with homologous recombination vector containing the mutation, were co-transfected into MDA-MB-231 cells with Lipofectamine3000. Because we were not able to isolate a β1 substitution mutant, we next attempted deletion mutants. The deletion mutants β1ΔT1 and β2ΔI3 were produced with lentivirus. The gRNAs were transfected with Lipofectamine 3000 (Invitrogen) into 293FTs to produce lentiviruses, which were then collected and used to transduce MDA-MB-231 cells. Because proteasome genes are essential, only cells with mutations that did not affect expression were viable. For both the deletion mutants and the substitution mutant, positive clones were selected with puromycin, and colonies were separated using ring cloning. Colonies were screened for lack of subunit activity with the active site probe activity assay. The control cell lines β1wt and β2wt were derived from colonies that underwent either the lentiviral transduction (β2wt) or Lipofectamine transfection (β1wt) and puromycin selection, which did not lead to inactivation of targeted sites. Mutations were confirmed by sequencing. For sequencing, PCR fragments from the positive clones were ligated into pGEM-T Easy (Promega) and amplified in E. coli.
Statistics
Unpaired student t-test was used for statistical analysis unless otherwise indicated, with p-value less than 0.05 as the criteria for significant difference. Statistical data was calculated with GraphPad Prism.
Mice
Six-week old female NSG mice were obtained in-house from the Transgenics and Genetic Constructs Shared Resource at Dartmouth. Three million cells were injected subcutaneously into the mammary fat pad. Beginning when tumors were palpable, Cfz was injected intravenously in 10% captisol (sulfobutylether-β-cyclodextrin) in 10mM buffer citrate pH 5.5. Treatments were given twice weekly on consecutive days (days 1, 2, 7, 8, 14, 15, 21, and 22). Tumor volume was measured with calipers. All procedures were carried out according to an IACUC-approved protocol.
Supplementary Material
Highlights.
Clinically relevant concentrations of FDA-approved β5 inhibitors are not cytotoxic
Specific inhibitors of the β1 or β2 sites sensitize cells to β5 inhibitors
CRISPR-engineered mutations of β1 or β2 also sensitize cells to β5 inhibitors
Inactivation of β2 suppresses Nrf1-dependent recovery of proteasome activity
Acknowledgments
These studies were supported by grant from Susan G. Komen for the Cure (to AFK), NCI (MDC), and NIGMS (A Goldberg). We are grateful to Scott Gerber for high-resolution mass-spectrometry, Steve Fiering and Jennifer Fields at the Norris Cotton Cancer Center Mouse Modeling Shared Resource for providing mice, to the Molecular Biology and Genomics Shared Resource for DNA sequencing, and to the University of Vermont DNA analysis facility for cell line identification. The Shared Resources of the Norris Cotton Cancer Center were supported by the NCI Cancer Center Support Grant 5P30 CA023108-37.
Footnotes
Author Contributions
E.W. and A.K. conceived the study and designed experiments, with involvement of M.C for CRISPR experiments and of Z.S. and A.G. for Nrf1 experiments. E.W., O.W., D.W., and Z.S. conducted experiments. E.W., O.W., D.W., Z.S., M.C., A.G., and A.K. analyzed data. A.P. synthesized NC-021. G.d.B. and H.O. synthesized LU-102. E.W. and A.K wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- Arendt CS, Hochstrasser M. Identification of the yeast 20S proteasome catalytic centers and subunit interactions required for active-site formation. Proc Natl Acad Sci U S A. 1997;94:7156–7161. doi: 10.1073/pnas.94.14.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Cervera N, Esterni B, Hermitte F, Viens P, Birnbaum D. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–240. doi: 10.1002/ijc.23518. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F, Orsi A, Pasqualetto E, Mezghrani A, Calbi V, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113:3040–3049. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- Britton M, Lucas MM, Downey SL, Screen M, Pletnev AA, Verdoes M, Tokhunts RA, Amir O, Goddard AL, Pelphrey PM, et al. Selective inhibitor of proteasome’s caspase-like sites sensitizes cells to specific inhibition of chymotrypsin-like sites. Chem Biol. 2009;16:1278–1289. doi: 10.1016/j.chembiol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- de Bruin G, Xin BT, Kraus M, van der Stelt M, van der Marel GA, Kisselev AF, Driessen C, Florea BI, Overkleeft HS. A set of activity-based probes to visualize human (immuno)proteasome activities. Angew Chem Int Ed Engl. 2016;55:4199–4203. doi: 10.1002/anie.201509092. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ. Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol. 2014;12:94. doi: 10.1186/s12915-014-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou QP, Zonder JA. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr Cancer Drug Targets. 2014;14:517–536. doi: 10.2174/1568009614666140804154511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Lwin ST, Fowler JA, Oyajobi BO, Zhuang J, Bates AL, Mundy GR. Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. Am J Hematol. 2009;84:268–272. doi: 10.1002/ajh.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, Koong AC, Koumenis C. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer Res. 2008;68:9323–9330. doi: 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, Greninger P, Thompson IR, Luo X, Soares J, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurink PP, van der Linden WA, Mirabella AC, Gallastegui N, de Bruin G, Blom AE, Voges MJ, Mock ED, Florea BI, van der Marel GA, et al. Incorporation of non3 natural amino acids improves cell permeability and potency of specific inhibitors of proteasome trypsin-like sites. J Med Chem. 2013;56:1262–1275. doi: 10.1021/jm3016987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL. Development of proteasome inhibitors as research tools and cancer drugs. J Cell Biol. 2012;199:583–588. doi: 10.1083/jcb.201210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll M, Heinemeyer W, Jager S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci U S A. 1999;96:10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Mainwaring P, Price T, Millward MJ, Padrik P, Underhill CR, Cannell PK, Reich SD, Trikha M, Spencer A. Phase 1 clinical trial of marizomib (NPI-0052) in patients with advanced malignancies including multiple myeloma: study NPI-0052-102 final results. Clin Cancer Res. 2016;22:4559–4566. doi: 10.1158/1078-0432.CCR-15-2616. [DOI] [PubMed] [Google Scholar]
- Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH. The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem. 1997;272:25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, Barnard J, Nevarez S, Goldman BI, Kirk CJ, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. 2012;64:493–503. doi: 10.1002/art.33333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–758. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, van der Linden WA, Overkleeft HS. Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol. 2012;19:99–115. doi: 10.1016/j.chembiol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Irie T, Hirayama S, Sakurai Y, Yashiroda H, Naguro I, Ichijo H, Hamazaki J, Murata S. The aspartyl protease DDI2 activates Nrf1 to compensate for proteasome dysfunction. eLIFE. 2016:5. doi: 10.7554/eLife.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M, Bader J, Geurink PP, Weyburne ES, Mirabella AC, Silzle T, Shabaneh TB, van der Linden WA, de Bruin G, Haile SR, et al. The novel beta2-selective proteasome inhibitor LU-102 synergizes with bortezomib and carfilzomib to overcome proteasome inhibitor resistance of myeloma cells. Haematologica. 2014;100:1350–1360. doi: 10.3324/haematol.2014.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLIFE. 2016:5. doi: 10.7554/eLife.17721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin N, Spencer A, Harrison SJ, Chauhan D, Burrows FJ, Anderson KC, Reich SD, Richardson PG, Trikha M. Marizomib irreversibly inhibits proteasome to overcome compensatory hyperactivation in multiple myeloma and solid tumour patients. Br J Haematol. 2016 doi: 10.1111/bjh.14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat Biotechnol. 2015;33:538–542. doi: 10.1038/nbt.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabella AC, Pletnev AA, Downey SL, Florea BI, Shabaneh TB, Britton M, Verdoes M, Filippov DV, Overkleeft HS, Kisselev AF. Specific cell-permeable inhibitor of proteasome trypsin-like sites selectively sensitizes myeloma cells to bortezomib and carfilzomib. Chem Biol. 2011;18:608–618. doi: 10.1016/j.chembiol.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26:2696–2708. doi: 10.1101/gad.207407.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos KP, Siegel DS, Vesole DH, Lee P, Rosen ST, Zojwalla N, Holahan JR, Lee S, Wang Z, Badros A. Phase I study of 30-minute infusion of carfilzomib as single agent or in combination with low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2015;33:732–739. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, Pien CS, Millikan RE, Tu SM, Pagliaro L, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- Petrocca F, Altschuler G, Tan SM, Mendillo ML, Yan H, Jerry DJ, Kung AL, Hide W, Ince TA, Lieberman J. A genome-wide siRNA screen identifies proteasome addiction as a vulnerability of basal-like triple-negative breast cancer cells. Cancer Cell. 2013;24:182–196. doi: 10.1016/j.ccr.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, den Besten W, Deshaies RJ. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. Elife. 2014;3:e01856. doi: 10.7554/eLife.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Molecular cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Zimmerman TM, Hofmeister CC, Talpaz M, Chanan-Khan AA, Kaufman JL, Laubach JP, Chauhan D, Jakubowiak AJ, Reich S, et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood. 2016;127:2693–2700. doi: 10.1182/blood-2015-12-686378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Proteasome from Thermoplasma acidophilum: a threonine protease. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol. 2014;24:1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Goldberg AL. Reply to Vangala et al.: Complete inhibition of the proteasome reduces new proteasome production by causing Nrf1 aggregation. Curr Biol. 2016;26:R836–837. doi: 10.1016/j.cub.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabaneh TB, Downey SL, Goddard AL, Screen M, Lucas MM, Eastman A, Kisselev AF. Molecular basis of differential sensitivity of myeloma cells to clinically relevant bolus treatment with bortezomib. PLoS One. 2013;8:e56132. doi: 10.1371/journal.pone.0056132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Kalebic T, Infante JR, Siu LL, Sullivan D, Vlahovic G, Kauh JS, Gao F, Berger AJ, Tirrell S, et al. Phase 1 study of ixazomib, an investigational proteasome inhibitor, in advanced non-hematologic malignancies. Invest New Drugs. 2015;33:652–663. doi: 10.1007/s10637-015-0230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen J, Seeger M, Koch A, Kruger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Trinh XB, Sas L, Van Laere SJ, Prove A, Deleu I, Rasschaert M, Van de Velde H, Vinken P, Vermeulen PB, Van Dam PA, et al. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancer. Oncol Rep. 2012;27:657–663. doi: 10.3892/or.2011.1562. [DOI] [PubMed] [Google Scholar]
- Verdoes M, Willems LI, van der Linden WA, Duivenvoorden BA, van der Marel GA, Florea BI, Kisselev AF, Overkleeft HS. A panel of subunit-selective activity-based proteasome probes. Org Biomol Chem. 2010;8:2719–2727. doi: 10.1039/c001036g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.