Abstract
Acetaminophen (APAP) overdose is the major cause of acute liver failure in the US. Prompt liver regeneration is critical for recovery after APAP hepatotoxicity, but mechanisms remain elusive. Extracellular matrix (ECM)-mediated signaling via integrin-linked kinase (ILK) regulates liver regeneration after surgical resection. However, the role of ECM signaling via ILK in APAP toxicity and compensatory regeneration is unknown, which was investigated in this study using liver-specific ILK knockout (KO) mice. ILK KO and wild-type (WT) mice were treated with 300 mg/kg APAP, and injury and regeneration were studied at 6 and 24 h after APAP treatment. ILK KO mice developed lower liver injury after APAP overdose, which was associated with decreased JNK activation (a key mediator of APAP toxicity). Further, higher glutathione levels after APAP treatment and lower APAP protein adducts levels, along with lower levels of CYP2E1, suggest decreased metabolic activation of APAP in ILK KO mice. Interestingly, despite lower injury, ILK KO mice had rapid and higher liver regeneration after APAP overdose accompanied with increased β-catenin signaling. In conclusion, liver-specific deletion of ILK improved regeneration, attenuated toxicity after APAP overdose, and decreased metabolic activation of APAP. Our study also indicates that ILK-mediated ECM signaling plays a role in the regulation of CYP2E1 and may affect toxicity of several centrilobular hepatotoxicants including APAP.
Key words: Extracellular matrix (ECM), Drug-induced liver injury, Hepatocyte proliferation, β-Catenin, Cytochrome P450 2E1
INTRODUCTION
Acetaminophen (APAP) is a commonly used over-the-counter analgesic and antipyretic drug. APAP is considered very safe at therapeutic doses, but intentional or unintentional APAP overdose could result in liver toxicity progressing to acute liver failure (ALF). APAP overdose is one of the major causes of ALF in the Western world, contributing to almost 50% of the ALF cases. N-Acetylcysteine (NAC) is the current standard of care for APAP overdose patients, which is effective only at an early stage1. APAP-induced liver injury involves bioactivation of APAP to reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which then binds to cellular proteins, specifically mitochondrial proteins, and triggers an intracellular signaling cascade ultimately resulting in liver necrosis2. Liver injury after APAP overdose is also followed by compensatory liver regeneration, which is important for inhibition of progression of injury and final recovery3. Various targets directed to attenuate liver injury or stimulate liver regeneration have been investigated in the past to develop therapeutic strategy for APAP-induced ALF3–5.
Extracellular matrix (ECM) communication with cells is considered vital for normal cellular functions. ECM transmits intracellular signals by interacting with transmembrane adhesion proteins known as integrins. Integrin-linked kinase (ILK) is a Ser/Thr kinase, which interacts with the cytoplasmic domain of β1 integrin and relays integrin-mediated ECM signaling. ILK binds to proteins PINCH and Parvin to form a ternary complex referred as IPP complex and act as a hub to modulate a variety of cellular signaling pathways involved in cell proliferation, differentiation, and survival. ILK mediates its effects by acting as adaptor protein as well as by its protein kinase activity6,7.
ECM remodeling is an important adaptive response to altered liver homeostasis. Alteration in ECM plays a crucial role in regulating regenerative response of liver after partial hepatectomy (PHX). Specifically, ILK-mediated ECM signaling exhibits mitoinhibitory effects in hepatocytes8. Liver-specific deletion of ILK results in increased hepatocyte proliferation along with defect in termination of liver regeneration after PHX resulting in mice with increased liver size8. Further, enhanced and prolonged liver regeneration were observed in liver-specific ILK knockout (KO) mice after administration of nuclear receptor agonists, such as TCBOPOP and phenobarbital9,10.
ILK-mediated ECM signaling is known to regulate hepatocyte differentiation and survival11–13. ILK signaling has been reported to play an important role in fibrogenesis and wound-healing response in various chronic liver injury models14–16. Alteration in ECM is also known to occur after toxin-induced acute liver injury, with its role largely unexplored. ILK signaling was found to be important for survival in the CCl4 model of acute liver injury17. On the contrary, liver-specific deletion of ILK was reported to protect from FAS-induced apoptosis and liver failure, which was associated with upregulation of survival signaling13. However, the role of ILK-dependent or -independent ECM signaling in APAP-induced liver injury (a clinically relevant model of fulminant liver failure) and subsequent compensatory liver regenerative response to APAP toxicity is completely unexplored. The objective of the current study was to investigate the role of ILK in APAP-induced liver toxicity and compensatory liver regeneration. Mice with liver-specific ILK deletion were used as a model to study the role of ILK in acetaminophen overdose. Here we report that liver-specific deletion of ILK improved regeneration, attenuated toxicity after APAP overdose, and decreased metabolic activation of APAP.
MATERIALS AND METHODS
Animals, Treatment, and Tissue Harvesting
Liver-specific ILK KO mice were generated by mating ILK-floxed mice with mice expressing Cre-recombinase under α-fetoprotein enhancer albumin promoter12. Cre+ animals were considered as ILK KO and confirmed by genotyping. Cre− littermates were used as controls and represented as wild-type (WT) mice. All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities at the University of Kansas Medical Center under a standard 12-h light/dark cycle with access to chow and water ad libitum. The Institutional Animal Care and Use Committee at the University of Kansas Medical Center approved all studies. APAP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in warm 0.9% saline. ILK KO or WT mice (n = 3 to 5) were treated with 300 mg/kg APAP, intraperitoneally (IP). Mice were fasted 12 h before administration of APAP, and food was returned to the mice immediately after APAP treatment. Mice were sacrificed at 0, 6, and 24 h following APAP treatment by cervical dislocation under isofluorane anesthesia, and blood and livers were collected. Serum samples were obtained from the blood and used for further analysis. Liver sections were prepared for histological analysis as described previously18.
Histological Analysis and Serum ALT Measurement
Paraffin-embedded liver sections (4 μm thick) were used for hematoxylin and eosin (H&E) staining. H&E-stained slides were used to score for percentage necrotic area. Serum alanine aminotransferase (ALT) was measured by kinetic assay method using the Infinity ALT kit (Thermo Fisher Scientific, Pittsburgh, PA, USA), according to the manufacturer’s protocol.
Antibodies
All primary and secondary antibodies used for Western blot analysis were obtained from Cell Signaling Technologies (Danvers, MA, USA) unless stated otherwise. Active β-catenin antibody was purchased from EMD Millipore (Billerica, MA, USA). CYP2E1 and Ki-67 antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-APAP protein adduct antibody, as previously described19,20, was a gift from Dr. Lance Pohl, National Heart, Lung, and Blood Institute (NHIBL), National Institutes of Health (NIH).
Immunofluorescence
Ki-67 immunofluorescence staining was done on freshly frozen sections (4 μm thick) as described previously21. Briefly, after attaining room temperature, sections were fixed in formaldehyde [10% in phosphate-buffered saline (PBS)] and permeabilized with Triton X-100 (0.1% in PBS). After washing with PBS, sections were blocked in donkey serum (10% in PBS) for 1 h and then incubated overnight with the Ki-67 primary antibody (1:500 diluted in 1% donkey serum in PBS). The next day, after washing with PBS, sections were incubated in the donkey anti-rabbit secondary antibody (1:500 dilution) for 1 h. 4′,6-Diaminido-2-phenylindole (DAPI)-containing medium (ProLong Gold Antifade Reagent with DAPI; Invitrogen, Eugene, OR, USA) was used for final mounting of sections after washing in PBS.
Protein Isolation and Western Blotting
Protein estimation and Western blot analysis were performed using individual samples of protein extracts prepared from frozen liver tissues as previously published without any modifications18. Densitometric analysis of Western blot images was done using ImageJ software.
Glutathione Analysis
Total hepatic glutathione (GSH) levels were measured in liver homogenates by kinetic assay method using Glutathione Assay Kit (Sigma-Aldrich), according to the manufacturer’s protocol and as described previously22.
Statistical Analysis
Data presented in the form of bar graphs show mean ± standard error of the mean (SEM). Significant difference between WT and ILK KO groups was determined using Student’s t-test. Statistically significant difference between groups was represented in graphs indicating p < 0.05, p < 0.01 and p < 0.001.
RESULTS
Attenuated Liver Injury After APAP Overdose in ILK KO Mice
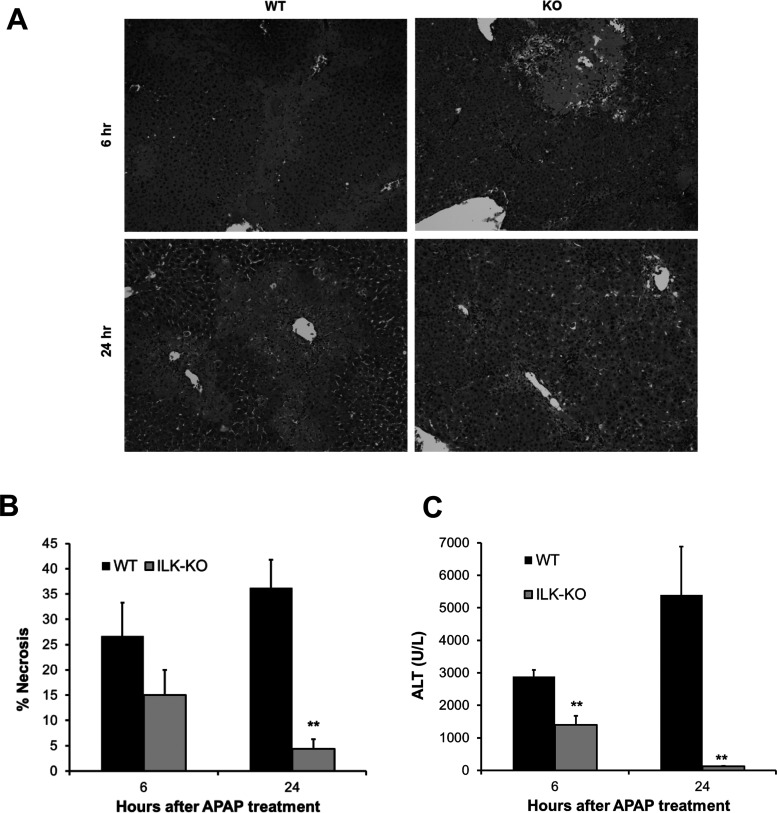
The role of ILK in APAP-induced liver injury was investigated, in mice, using liver-specific ILK deletion strategy. Liver sections were obtained from WT or ILK KO mice at various time points after APAP treatment and stained with H&E. Histopathological analysis of liver sections revealed centrilobular necrosis, a hallmark of APAP toxicity, at 6 and 24 h after APAP administration in WT mice. Interestingly, there was lower liver necrosis at both time points in ILK KO mice, especially at 24 h after APAP (Fig. 1A). These data were further confirmed by scoring the H&E-stained liver section for percentage necrotic area (Fig. 1B). Further, analysis of serum ALT, a marker to liver injury, corroborated that there was extensive injury in WT mice after APAP administration, which was attenuated in ILK KO mice, and the effect was especially remarkable at 24 h (Fig. 1C). It should be noted that there were significant zones of liver necrosis observed in ILK KO at 6 h after APAP treatment (as evidenced by necrosis scoring analysis of H&E-stained liver sections), although lower compared to WT mice, but this was followed by rapid resolution of injury at 24 h after APAP, where we observed very infrequent necrotic patches with minimal remaining zone of necrosis and decreased ALT levels. In contrast, WT mice displayed higher initial injury at 6 h after APAP, but injury progressed further at 24 h after APAP as evidenced by spread of necrotic zones and elevation of ALT levels.
Figure 1.
Attenuated liver injury after acetaminophen (APAP) overdose in integrin-linked kinase (ILK) knockout (KO) mice. (A) Representative photomicrographs of hematoxylin and eosin (H&E)-stained liver sections. (B) Bar graph showing percent necrosis area based on H&E-stained liver sections. (C) Bar graph showing serum aminotransferase (ALT) levels. All samples were collected from wild-type (WT) or ILK KO mice treated with 300 mg/kg APAP. Significant difference between groups at **p < 0.01.
Improved Liver Regeneration After APAP Overdose in ILK KO Mice
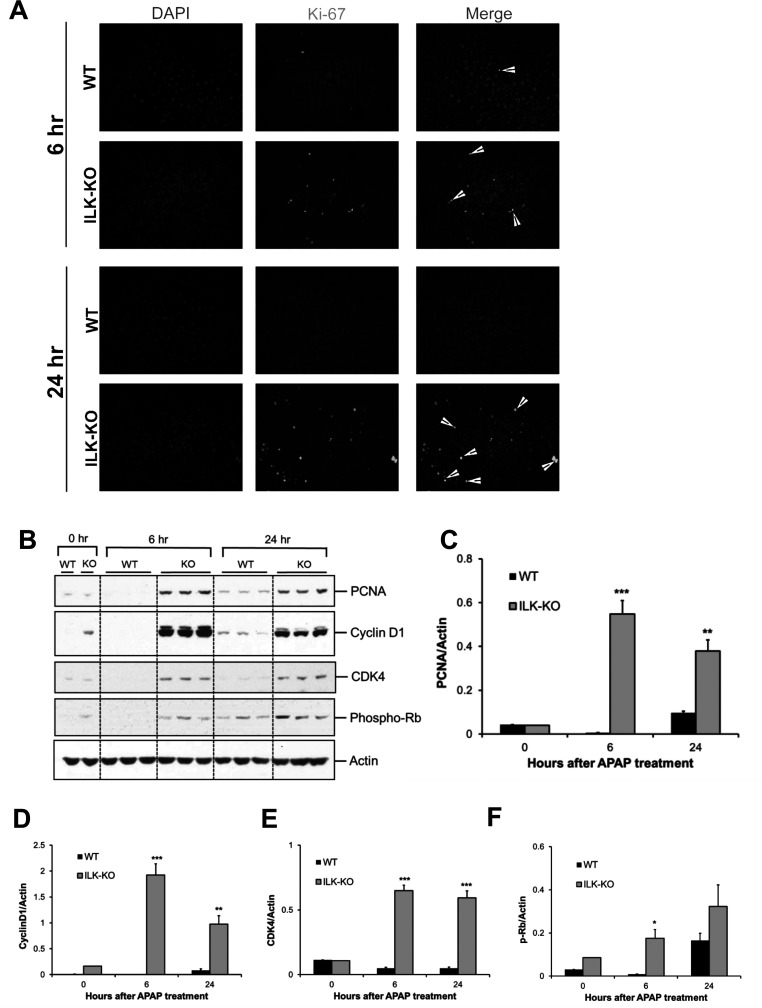
We next investigated the effect of liver-specific deletion of ILK on liver regeneration after APAP-induced liver injury. Liver regeneration was studied using Ki-67 immunofluorescence staining and Western blot analysis of proliferating cell nuclear antigen (PCNA), cyclin D1, CDK4, and phosphorylated Rb after administering a toxic dose of APAP (300 mg/kg, IP) in WT or liver-specific ILK KO mice. ILK KO mice displayed a notably higher number of Ki-67-positive cells compared to WT mice at both 6 and 24 h after APAP treatment (Fig. 2A). Similarly, there was remarkable increase in PCNA expression at 6 and 24 h after APAP treatment in ILK KO mice. WT mice showed a relatively lower increase in PCNA expression, only after 24 h of APAP administration (Fig. 2B and C). Expression of cyclin D1, a key regulator of cell cycle entry and cell proliferation, followed a pattern similar to PCNA with striking increase specifically in ILK KO mice at 6 and 24 h after APAP administration (Fig. 2B and D). During cell cycle progression, cyclin D1 induction and binding to CDK4 cause phosphorylation (i.e., inactivation) of Rb, which results in activation of many proregenerative genes23. Similar to PCNA and cyclin D1 expression, CDK4 expression was remarkably increased in ILK KO mice at both time points after APAP treatment (Fig. 2B and E). Further, phosphorylation of Rb was significantly higher in ILK KO mice compared to WT mice at 6 h after APAP administration, and a similar trend was also observed at 24 h after APAP administration (Fig. 2B and F). These data demonstrate that liver-specific deletion of ILK in mice results in early and enhanced liver regeneration after APAP-induced liver injury and correlates with decreased progression of injury and early recovery in ILK KO mice.
Figure 2.
Improved liver regeneration after APAP overdose in ILK KO mice. (A) Representative photomicrographs showing Ki-67 immunofluorescence performed on frozen liver sections of WT or ILK KO mice treated with 300 mg/kg APAP. (B) Western blot analysis of proliferating cell nuclear antigen (PCNA), cyclin D1, CDK4, and phospho-Rb using total cell extract of liver of WT or ILK KO mice treated with 300 mg/kg APAP. All samples were collected at 0, 6, and 24 h after APAP treatment. Densitometric analysis showing quantification of protein expression of (C) PCNA, (D) cyclin D1, (E) CDK4, and (F) phospho-Rb relative to actin as loading control. Significant difference between groups at *p < 0.05, **p < 0.01, and ***p < 0.001.
Differential Activation of Proregenerative Signaling Pathways After APAP Overdose in ILK KO Mice
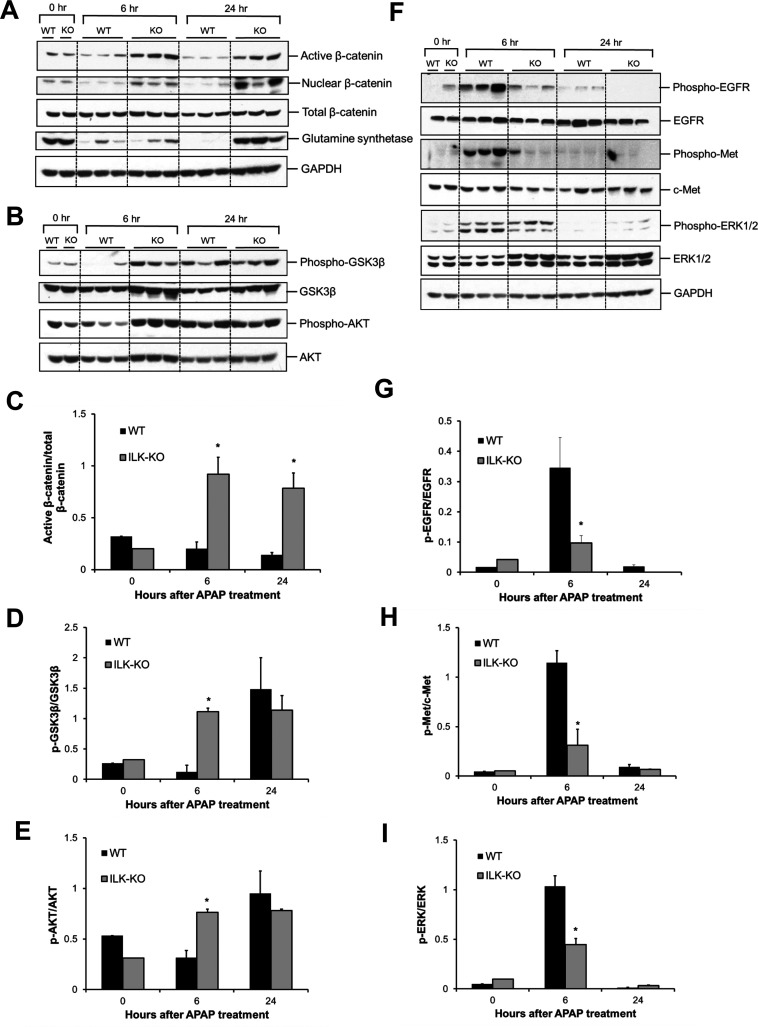
We next investigated mechanisms of improved liver regeneration in ILK KO mice. Signaling pathways, which are known to regulate liver regeneration after APAP-induced liver injury, were studied. β-Catenin signaling plays an important role in stimulating liver regeneration after PHX or APAP toxicity3,24–26. We observed a striking increase in the expression of active β-catenin (unphosphorylated and stable form) at both time points (6 and 24 h) after APAP administration, specifically in ILK KO mice (Fig. 3A and C). Increase in expression of active β-catenin was further corroborated by increased levels of nuclear β-catenin at 6 and 24 h after APAP administration, specifically in ILK KO mice compared to WT mice (Fig. 3A). This was further correlated with increased cyclin D1 induction (Fig. 2B and D), glutamine synthetase protein expression (Fig. 3A) (both of which are known targets of β-catenin27), and improvement of other regeneration parameters in ILK KO mice.
Figure 3.
Differential activation of proregenerative signaling after APAP overdose in ILK KO mice. (A) Western blot analysis of active β-catenin, nuclear β-catenin, total β-catenin, glutamine synthetase, (B) phospho-GSK3β, GSK3β, phospho-AKT, and AKT using total cell extract (unless specified) of liver of WT or ILK KO mice treated with 300 mg/kg APAP. Densitometric analysis showing (C) activation of β-catenin, (D) inactivation of GSK3β, and (E) activation of AKT, respectively, based on Western blot images shown in (A) and (B). (F) Western blot analysis of phospho-EGFR, EGFR, phospho-Met, c-Met, phospho-ERK1/2, and ERK1/2 using total cell extract of liver of WT or ILK KO mice treated with 300 mg/kg APAP. (G–I) Densitometric analysis showing quantification of expression proteins shown in (F). All samples were collected at 0, 6, and 24 h after APAP treatment. Significant difference between groups at *p < 0.05.
Glycogen synthase kinase 3β (GSK3β) is a negative regulator of many proteins involved in cell proliferation including β-catenin28. Our previous study has demonstrated positive correlation between inactivation of GSK3β and liver regeneration after APAP-induced liver injury3. We found an early inactivation (phosphorylation) of GSK3β (at 6 h after APAP), specifically in ILK KO mice, but not in WT mice. In contrast, GSK3β phosphorylation was increased in the WT group only at the later time point (24 h after APAP) and was comparable between the two groups at this time point (Fig. 3B and D). A similar pattern was observed for phosphorylated AKT (which is a known upstream regulator of GSK3β and causes phosphorylation of GSK3β28) at 6 and 24 h after APAP (Fig. 3B and E).
Growth factors such as epidermal growth factor (EGF) and hepatocyte growth factor (HGF) are primary mitogens for hepatocytes and play an important role in liver regeneration after PHX29. We investigated the status of these growth factor signaling pathways in our model. Phosphorylation (i.e., activation) of both EGFR and Met receptors (which are cell surface receptors for EGF and HGF, respectively) was increased at 6 h after APAP treatment in both groups, but activation was remarkably higher in the WT group compared to the ILK KO group. Phosphorylation of these receptors returned to basal levels at 24 h after APAP in both groups (Fig. 3F–H). A similar pattern was observed for ERK1/2 phosphorylation (i.e., activation), which is a downstream mediator of growth factor signaling (Fig. 3F and I). These data indicate that there was differential activation of β-catenin signaling in ILK KO mice correlating with increased liver regeneration, but signaling via growth factor receptors was activated more in WT mice, which showed lower regenerative response.
Attenuated Injury Signaling in Liver of ILK KO Mice After APAP Overdose
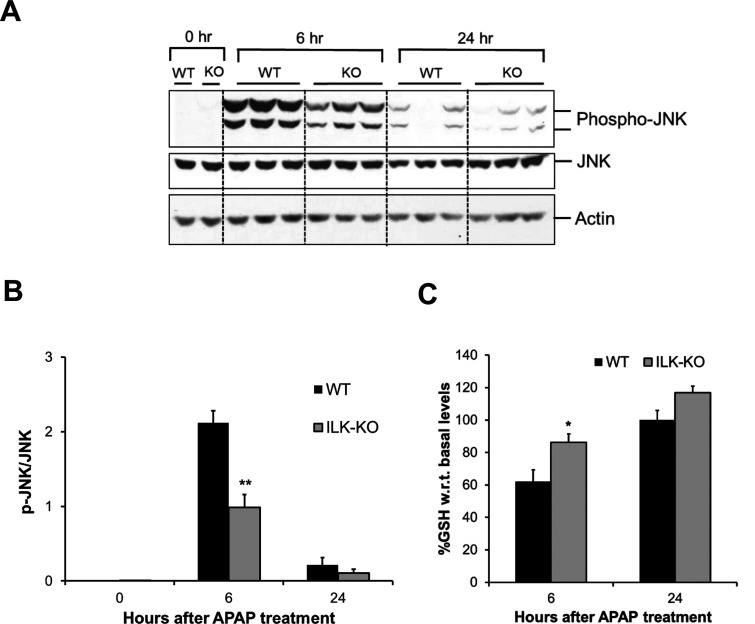
We further studied cellular signaling known to be involved in initiation of APAP toxicity. Phosphorylation-mediated activation of JNK is considered as one of the important early signaling events in APAP-induced liver injury. Phosphorylation of JNK and its translocation to mitochondria lead to exacerbation of mitochondrial oxidant stress, which ultimately results in mitochondrial permeability transition (MPT) and liver necrosis2. As expected, there was a remarkable increase in phosphorylation of JNK at 6 h after APAP treatment in WT mice, which was significantly reduced at 24 h after APAP treatment. Although, there was significant activation of JNK at 6 h after APAP treatment in ILK KO mice, the activation was lower compared to that in WT mice, corroborating with lower initial injury observed in ILK KO mice. Similar to WT mice, JNK activation was greatly reduced at 24 h after APAP treatment in ILK KO mice (Fig. 4A and B).
Figure 4.
Attenuated injury signaling in liver of ILK KO mice after APAP overdose. (A) Western blot analysis of phospho-JNK and JNK using total cell extract of liver of WT or ILK KO mice treated with 300 mg/kg APAP. All samples were collected at 0, 6, and 24 h after APAP treatment. (B) Densitometric analysis showing quantification of protein expression of phospho-JNK relative to JNK. (C) Liver glutathione levels represented as percentage of basal levels in WT or ILK KO mice. Significant difference between groups at *p < 0.05 and **p < 0.01.
APAP bioactivation results in the formation of NAPQI, a reactive metabolite, which undergoes conjugation with liver GSH followed by excretion. This results in depletion of GSH (around 90% of GSH is depleted within the first 30 min after toxic doses of APAP) and binding of excess NAPQI to cellular proteins leading to APAP protein adduct formation and toxicity2. Recovery of GSH levels after initial depletion can regulate progression of injury by decreasing APAP-induced oxidative stress. We measured total GSH levels in livers of WT and ILK KO mice after APAP treatment. Both groups had comparable basal GSH levels. The WT mice showed around 60% GSH compared to basal level at 6 h after APAP treatment, and GSH levels returned back to basal values at 24 h after APAP treatment. ILK KO mice had significantly higher GSH levels at 6 h after APAP treatment compared to WT mice (Fig. 4C). This suggests that there is lesser GSH depletion in ILK KO mice in the first place, and/or there is faster recovery of GSH levels in ILK KO mice, which may have a role to play in decreased toxicity in ILK KO mice.
Altered APAP Metabolic Activation in ILK KO Mice
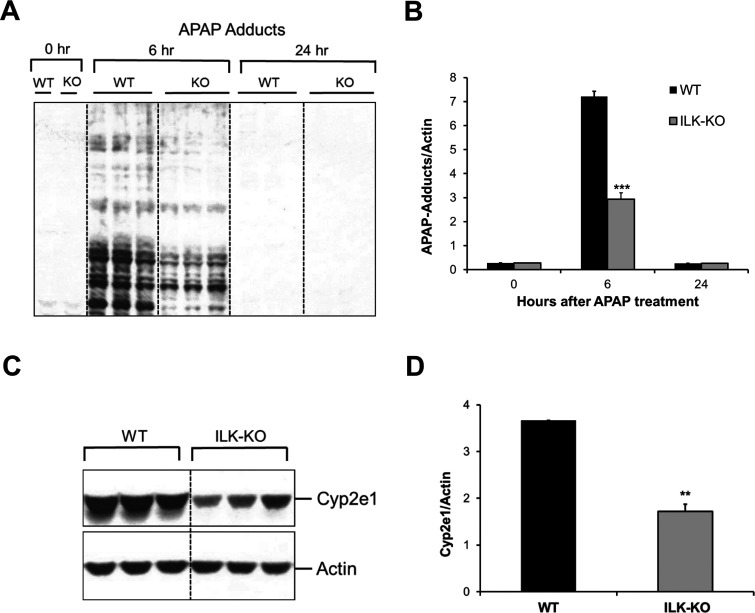
Higher GSH levels after APAP treatment in ILK KO mice suggested the possible role of altered APAP metabolism behind observed effects on APAP toxicity. We investigated APAP metabolic activation parameters in ILK KO mice. We measured APAP protein adduct levels using immunoblotting, which is an indicator of APAP metabolism to toxic metabolite NAPQI. As expected, APAP adduct levels increased strikingly at 6 h after APAP treatment in WT mice and decreased remarkably by 24 h after APAP treatment. Interestingly, APAP protein adduct levels were significantly lower in ILK KO mice at 6 h after APAP treatment compared to WT mice (Fig. 5A and B), suggesting that APAP metabolism and bioactivation to toxic metabolite are compromised in ILK KO mice, which resulted in decreased APAP protein adduct formation. CYP2E1 is the major enzyme that is involved in the metabolism of APAP to NAPQI30. Therefore, we measured liver CYP2E1 protein expression in ILK KO mice (Fig. 5C and D). There was a significantly lower basal CYP2E1 expression in ILK KO mice compared to WT mice (Fig. 5C and D). These data suggest that ECM-mediated signaling through ILK can alter APAP metabolic enzyme CYP2E1, which leads to altered APAP metabolism and thus affects APAP toxicity.
Figure 5.
Altered APAP metabolic activation in ILK KO mice. (A) Western blot analysis showing APAP protein adduct levels in liver of WT or ILK KO mice treated with 300 mg/kg APAP. All samples were collected at 0, 6, and 24 h after APAP treatment. (B) Densitometric analysis showing quantification of APAP protein adduct levels shown in (A). (C) Western blot analysis showing CYP2E1 protein expression in the liver of WT or ILK KO mice at 0 h. (D) Densitometric analysis showing quantification of CYP2E1 levels shown in (C). Significant difference between groups at **p < 0.01 and ***p < 0.001.
DISCUSSION
Interaction of ECM with cells is important for maintaining cellular/tissue homeostasis. Previous studies show that ILK-mediated ECM signaling inhibits hepatocyte proliferation, and ILK deletion results in enhanced proliferation after PHX or treatment with nuclear receptor agonists8–10. Changes in ECM occur after toxin-induced liver injury, and ILK signaling has been reported to play a role in the process of injury development, and subsequent recovery and survival response13,14,17. APAP overdose-induced liver injury is one of the major causes of ALF in the Western world, contributing to almost 50% of the ALF cases1. Studies show that increased liver regeneration is a key factor in inhibiting progression of injury and recovery from APAP overdose3–5. The role of ECM or ILK-mediated ECM signaling in liver injury and compensatory liver regeneration after APAP overdose is not known. Targeting ECM signaling or ILK-mediated ECM signaling can be a potential approach for developing a novel therapeutic strategy for APAP toxicity. We investigated the role of ILK in APAP toxicity and compensatory liver regeneration using liver-specific ILK deletion model in mice.
APAP-induced liver necrosis was attenuated in ILK KO mice at both the investigated time points, with the effect more remarkable at the later time point. Furthermore, JNK activation, which is the major initial signal involved in APAP toxicity, was significantly reduced. Decreased CYP2E1 levels leading to decreased APAP metabolism (as suggested by lower APAP protein adduct levels and higher GSH levels) was found to be the most possible explanation for the decrease in initial injury signaling and toxicity in ILK KO mice. Liver-specific ILK deletion resulted in rapid and enhanced liver regeneration after APAP-induced liver injury. Early and robust regenerative response along with accompanying rapid recovery in ILK KO mice after APAP overdose may have a part to play in attenuated progression of liver injury in addition to altered metabolism, especially at the later time point. This is supported by our data that significant liver necrosis and activation of JNK were observed at the early time point (6 h) even in the ILK KO group (although lower than the WT group), but injury was mostly resolved by 24 h, correlating with robust regenerative response. In contrast, injury progressed with time in the case of WT mice, where regenerative response was lower. Further, decreased initial APAP-induced liver toxicity and related stress might have provided proliferative advantage in the case of ILK KO mice, contributing to higher regeneration. ILK-mediated ECM signaling is known to exert inhibitory effects on hepatocyte proliferation and is one of the major “termination signals” for liver regeneration. ILK KO mice, which lack this inhibitory signal and thus have proliferative advantage due to termination defect, exhibit increased liver regeneration after PHX as well as after administration of nuclear receptor agonists8–10. Both these models do not involve massive liver injury. These findings indicate that lack of ILK induces a termination defect in hepatocytes, which might be persistent even in the model of drug-induced liver injury, and this termination defect could be manifested in the disproportionately higher liver regeneration as compared to the extent of APAP-induced liver injury. However, further studies using alternative strategies to inhibit ILK signaling are required to confirm the direct role of ILK in liver regeneration after APAP-induced liver injury.
Previously, we showed that β-catenin signaling was specifically activated during the regenerative response after moderate APAP overdose, but was inhibited after severe APAP overdose where regenerative response was also inhibited. However, growth factor signaling via EGFR and c-Met was dose-dependently stimulated and remained highly activated even after severe APAP overdose where regeneration was inhibited3. Consistent with these findings, in this study we found that β-catenin signaling was activated in ILK KO mice, which had robust regenerative response after APAP overdose, but was not activated in WT mice where regenerative response was lower. These data along with our previous findings3,26 highlight the importance of β-catenin signaling in stimulating liver regeneration after APAP toxicity and thus could be a potential therapeutic target for regenerative therapy after APAP overdose. Further, similar to our previous work, we found that growth factor signaling pathways remained highly activated in WT mice, but still, regenerative response was lower, questioning the importance of these pathways as target for potential regenerative therapies. Additionally, our results indicate that mechanisms of enhanced liver regeneration after ILK deletion might be model dependent, as both β-catenin signaling and growth factor signaling via HGF were found to be involved in the PHX model, and we found specific association of β-catenin signaling but not HGF signaling in the APAP overdose model8. The preponderance of β-catenin signaling in the ILK KO mice following APAP administration and the decreased activation of EGFR and Met suggest that the accelerated regeneration in the ILK KO mice after chemical toxicity may be Wnt driven.
Our results showed that ILK-mediated ECM signaling can regulate CYP2E1 protein expression, which is supported by a previous analysis of global gene expression profile in ILK KO mice12. CYP2E1 is a major liver metabolic enzyme, found predominantly in the centrilobular region. Apart from APAP, CYP2E1 is responsible for metabolic activation of many other hepatotoxins and carcinogens. Thus, it is possible that ILK-mediated ECM signaling may affect acute and chronic toxicity of other hepatotoxicants. At transcriptional level, Cyp2e1 gene expression is controlled by transcription factor hepatocyte nuclear factor 1α (HNF1α)30, deletion of which resulted in decreased gene expression of Cyp2e1 in mice31. In addition to HNF1α, β-catenin is also known to regulate Cyp2e1 transcription along with zone-specific expression of other centrilobular genes in the liver32. However, our results (that β-catenin signaling was not altered at basal levels in ILK KO mice and increased after APAP treatment, while CYP2E1 levels were decreased in ILK KO mice) suggest the potential role of β-catenin-independent mechanisms (such as regulation by HNF1α) in the alteration of CYP2E1 protein in ILK KO mice, which need further investigation. The exact signaling mechanisms involved in ILK-mediated CYP2E1 regulations were not pursued in this study.
In conclusion, liver-specific deletion of ILK in mice improved liver regeneration, attenuated liver toxicity after APAP overdose, and decreased metabolic activation of APAP. Our study also indicated that ILK-mediated ECM signaling plays a crucial role in the regulation of CYP2E1 and may affect toxicity of several carcinogens and hepatotoxicants including APAP.
ACKNOWLEDGMENTS
This work was supported by P20 RR021940, 5T32ES007079-34, 1R01DK098414, and AASLD/ALF Liver Scholar Award to U.A.
REFERENCES
- 1. Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: A curable disease by 2024? J Hepatol. 2015;62(1S):S112–20. [DOI] [PubMed] [Google Scholar]
- 2. Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 2014;184(11):3013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donahower BC, McCullough SS, Hennings L, Simpson PM, Stowe CD, Saad AG, Kurten RC, Hinson JA, James LP. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther. 2010;334(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu B, Colletti LM. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7(1):20–31. [DOI] [PubMed] [Google Scholar]
- 7. Donthamsetty S, Bhave VS, Mars WM, Bowen WC, Orr A, Haynes MM, Wu C, Michalopoulos GK. Role of PINCH and its partner tumor suppressor Rsu-1 in regulating liver size and tumorigenesis. PLoS One 2013;8(9):e74625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Apte U, Gkretsi V, Bowen WC, Mars WM, Luo JH, Donthamsetty S, Orr A, Monga SP, Wu C, Michalopoulos GK. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology 2009;50(3):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donthamsetty S, Bowen W, Mars W, Bhave V, Luo JH, Wu C, Hurd J, Orr A, Bell A, Michalopoulos G. Liver-specific ablation of integrin-linked kinase in mice results in enhanced and prolonged cell proliferation and hepatomegaly after phenobarbital administration. Toxicol Sci. 2010;113(2):358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donthamsetty S, Bhave VS, Kliment CS, Bowen WC, Mars WM, Bell AW, Stewart RE, Orr A, Wu C, Michalopoulos GK. Excessive hepatomegaly of mice with hepatocyte-targeted elimination of integrin linked kinase following treatment with 1,4-bis [2-(3,5-dichaloropyridyloxy)] benzene. Hepatology 2011;53(2):587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun. 2007;353(3):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gkretsi V, Apte U, Mars WM, Bowen WC, Luo JH, Yang Y, Yu YP, Orr A, St-Arnaud R, Dedhar S, Kaestner KH, Wu C, Michalopoulos GK. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology 2008;48(6):1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donthamsetty S, Mars WM, Orr A, Wu C, Michalopoulos GK. Protection against Fas-induced fulminant hepatic failure in liver specific integrin linked kinase knockout mice. Comp Hepatol. 2011;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem. 2006;281(34):24863–72. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology 2006;44(3):612–22. [DOI] [PubMed] [Google Scholar]
- 16. Shafiei MS, Rockey DC. The function of integrin-linked kinase in normal and activated stellate cells: Implications for fibrogenesis in wound healing. Lab Invest. 2012;92(2):305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duncan MB, Yang C, Tanjore H, Boyle PM, Keskin D, Sugimoto H, Zeisberg M, Olsen BR, Kalluri R. Type XVIII collagen is essential for survival during acute liver injury in mice. Dis Model Mech. 2013;6(4):942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolfe A, Thomas A, Edwards G, Jaseja R, Guo GL, Apte U. Increased activation of the Wnt/beta-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J Pharmacol Exp Ther. 2011;338(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: Role of inducible nitric oxide synthase. Hepatology 2002;35(2):289–98. [DOI] [PubMed] [Google Scholar]
- 20. Matthews AM, Roberts DW, Hinson JA, Pumford NR. Acetaminophen-induced hepatotoxicity. Analysis of total covalent binding vs. specific binding to cysteine. Drug Metab Dispos. 1996;24(11):1192–6. [PubMed] [Google Scholar]
- 21. Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-specific deletion of hepatocyte nuclear factor-4alpha in adult mice results in increased hepatocyte proliferation. Am J Physiol Gastrointest Liver Physiol. 2013;304(1): G26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhushan B, Borude P, Edwards G, Walesky C, Cleveland J, Li F, Ma X, Apte U. Role of bile acids in liver injury and regeneration following acetaminophen overdose. Am J Pathol. 2013;183(5):1518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fausto N. Liver regeneration. J Hepatol. 2000;32(1 Suppl):19–31. [DOI] [PubMed] [Google Scholar]
- 24. Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC Jr, Dar MJ, Khillan J, Dai C, Monga SP. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology 2010;51(5):1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin Cancer Biol. 2011;21(1):44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175(3):1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torre C, Benhamouche S, Mitchell C, Godard C, Veber P, Letourneur F, Cagnard N, Jacques S, Finzi L, Perret C and others. The transforming growth factor-alpha and cyclin D1 genes are direct targets of beta-catenin signaling in hepatocyte proliferation. J Hepatol. 2011;55(1):86–95. [DOI] [PubMed] [Google Scholar]
- 28. Kaidanovich-Beilin O, Woodgett JR. GSK-3: Functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab Dispos. 2007;35(1):1–8. [DOI] [PubMed] [Google Scholar]
- 31. Cheung C, Akiyama TE, Kudo G, Gonzalez FJ. Hepatic expression of cytochrome P450s in hepatocyte nuclear factor 1-alpha (HNF1alpha)-deficient mice. Biochem Pharmacol. 2003;66(10):2011–20. [DOI] [PubMed] [Google Scholar]
- 32. Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology 2006;43(4):817–25. [DOI] [PubMed] [Google Scholar]