Abstract
Background:
Despite the advent of modern injection techniques, palatal injection continues to be a painful experience for children.
Aims:
To compare the pain experienced during extraction of maxillary primary molars with conventional lignocaine anesthesia versus lignocaine and articaine buccal infiltration in children aged 6–14 years.
Materials and Methods:
A prospective randomized triple blinded study was conducted with ninety children (n = 90), randomly allocated to receive lignocaine conventional anesthesia (Group I [control group]), and buccal infiltration using articaine (Group II [articaine group]) or lignocaine (Group III [lignocaine group]). A composite score of self-report (faces pain scale-revised), behavioral measure (face legs activity cry consolability scale), and a physiological response (pulse rate) was measured following maxillary primary molar extraction.
Statistical Analysis Used:
To test the mean difference between two groups, Students’ t-test was used and among the three groups, one-way ANOVA with post hoc test was used.
Results:
Articaine group had significantly lower pain scores for self-report (P < 000.1) and behavioral measures (P < 000.1) while there was no significant difference (P > 0.05) between articaine and control groups during primary maxillary molar extraction.
Conclusion:
Maxillary primary molar extraction procedure can be successfully accomplished by bypassing the palatal injection. Articaine buccal infiltration can be considered as an alternative to conventional local anesthesia for the extraction of maxillary primary molars.
Keywords: Articaine, buccal administration, children, lignocaine, local anesthesia, maxillary primary molars, palatal injection
INTRODUCTION
Local anesthetics (LA) are essential in dentistry for appropriate pain control as they inhibit nociception generated during surgical and dental procedures.[1] Palatal injections are ranked among the most painful intraoral injections[2] and direct experience of administering the palatal anesthesia is considered to be the frequent source of fear during pediatric dental invasive procedures. Palatal injections are painful due to tight adherence of palatal mucosa to its underlying periosteum and its rich neural innervations,[3] displacement of mucoperiosteum results in increased pain rather than the needle piercing the mucosa.[4]
A number of methods and techniques have been suggested to reduce the discomfort due to infiltration of LA agents, including transcutaneous electronic nerve stimulation,[5] topical anesthetic application,[6] precooling of the palate,[7] computerized injection systems,[8] pressure administration,[9] and eutectic mixture of LA,[10] though none of them gained universal acceptance.
Maxillary molars removal without palatal injection is possible due to relatively thin porous bone of posterior buccal maxilla that facilitates the diffusion of any local anesthetic.[11] LA should be able to diffuse through soft and hard tissues by a property of diffusability, and anesthetic requirement is not as high as that required for routine conservative dental treatment.[12]
Articaine can diffuse through soft and hard tissues more reliably than other LA so that maxillary buccal infiltration of articaine provides palatal soft tissue anesthesia.[1] The basis for the prevalent use of articaine is due to the belief that it has better diffusion through soft tissue and bone, rapid onset, excellent quality of anesthesia, and lower degree of toxicity than lidocaine. Articaine provides complete anesthesia even by infiltration technique due to its superior tissue penetration capability.[13] Lidocaine is the most widely used local anesthetic in medicine as well as dentistry. It is evident that extraction of the permanent maxillary tooth is possible with single buccal infiltration of 2% lidocaine without the need for palatal injection.[14]
This study was conducted to determine the efficacy of 4% of articaine hydrochloride and 2% of lignocaine hydrochloride for maxillary primary molar tooth extraction without the need of palatal injection in children aged 6–14 years. The study hypothesized that; there would be no significant difference among the three groups in all the outcome measures.
MATERIALS AND METHODS
This study was registered with Clinical Trial Registration of India (CTRI registration number: CTRI/2016/02/006605).
Ethics
This study was approved by the Institutional Ethical Committee and the University of Health Sciences under protocol number D138407005/2014. Informed statement of consent was obtained from parents/caregivers before their child's participation in the research.
Study design and participants
A prospective, randomized, equivalence, quantitative, closed label study with a balanced allocation ratio of 1:1:1 was carried out. The study population consisted of individuals and their parents/caregivers, who attended the department of pediatric dentistry and those referred from orthodontics of local dental college and hospital. Children aged 6–14 years were selected based on the following eligibility criteria:
Inclusion criteria
Cooperative children
Children with definite indications for extraction of primary first or second maxillary molar
No history of intra-oral injections
Maxillary molars with 2/3 of root should be present
Children who can fully understand the given instructions.
Exclusion criteria
Whose parents or caregivers did not give consent for the study
Children allergic to lidocaine/articaine
Children with underlying vascular or immunological disease.
Pilot study was carried out with ten participants with the same characteristics of the main population to confirm the applicability of the proposed methodology. After the pilot study, the required adjustments were made to the methodology before the development of the main study. The sample size was calculated based on the detected difference of 0.3 value obtained from the pilot study with a power of 80% with minimum possible error of 0.05. The sample size arrived at 28, and 5% was added. Hence, a total of thirty children have been allocated to each treatment.
Research team
An experienced pediatric dentist performed all the injections who was blinded to the anesthetic solutions while another experienced pediatric dentist performed the extraction procedure. An experienced investigator not related to the study has ascertained outcomes from the child. The statistical analyst was blinded regarding the three groups and decoded only after the analysis of the results.
Recruitment and randomization
The treatment allocation was predetermined by generating randomization list using GraphPad StatMate version 1.01i (GraphPad Software, Inc., Armonk, NY: IBM Corp). Children were allocated sequentially into one of the three groups.
Treatment procedure
In the control group, the mucobuccal fold near the concerned teeth was dried with gauge, followed by application of topical anesthetic gel (benzocaine 2%) with the help of cotton applicator for about 30 s. Later, 1.7 ml of anesthetic solution (2% lidocaine with adrenaline 1:80,000 buccally [1.5 ml] and palatally [0.2 ml]), was injected under aseptic conditions using a 27-gauge needle at a rate of approximately 1 ml/min. To achieve effective buccal and palatal anesthesia, the procedure was delayed for at least 5 min. Objective signs were checked with the help of number 9. Molt periosteal elevator, first on the contralateral side followed by the anesthetized side. The tooth was extracted using a standard protocol, with minimal elevation of the palatal gingiva. Similar procedure was followed in other two groups, except that 1.7 ml of 4% articaine was injected buccally in articaine group, and 1.7 ml of 2% lignocaine was injected buccally in lignocaine group.
Outcome measures
For all the groups, the pulse oximeter device was attached to the left index finger, and three readings of pulse rate were recorded before, during, and after the extraction, and the mean was calculated, respectively. Faces pain scale-revised (FPS-R) score was recorded after the extraction and face legs activity cry consolability (FLACC) score was recorded perioperatively.
Statistical analysis
The data were entered in the Microsoft excel spreadsheet 2013. The statistical analysis was performed using IBM SPSS Version 20.0 (Armonk, NY: IBM Corp.). To test the mean difference among two groups’ Student's t-test was used and to test the mean difference between three or more groups ANOVA one-way with post hoc (Tukey honest significant difference) test was used. All the efficacy parameters were presented as absolute change from baseline. All P values having < 0.05 were considered as statistical significant (P < 0.05).
RESULTS
A total number of children assessed for eligibility, recruitment, randomization allocation, and numbers analyzed were illustrated [Figure 1].
Figure 1.
CONSORT flow diagram.
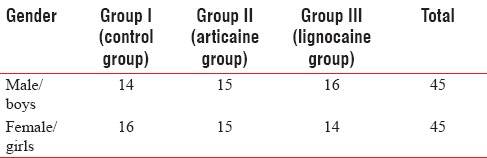
A total of ninety children (45 boys and 45 girls), who required extraction of one or more maxillary primary molars were included in the study. The mean age of the children was 9.74 ± 1.9 years. There was no significant difference among the groups regarding age or gender (independent t-test, P < 0.01) [Table 1].
Table 1.
Gender-wise distribution of sample among the groups
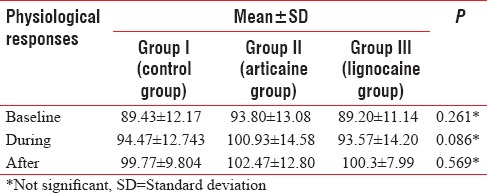
Intergroup comparison of the mean values of physiological parameters (heart rate) at baseline (P = 0.26), during extraction (P = 0.08), and after extraction (P = 0.56) revealed that they were not statistically significant among the three groups [Table 2].
Table 2.
Comparison of mean value of the physiological responses before, during, and after extraction in the control and test groups
Intergroup comparison of the mean values of self-report scale (FPS-R) after extraction and FLACC during extraction revealed that there was a highly statistical significant difference among the three groups (P < 0.0001) [Table 3].
Table 3.
Comparison of mean value of the self-report faces pain scale (revised) score and face legs activity cry consolability score in the control and test groups
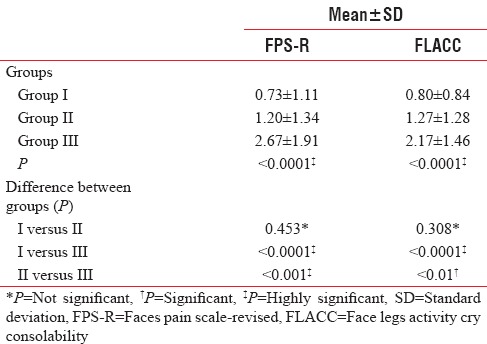
There was no statistical significant difference in the mean reduction of pain scores between the conventional and articaine groups with FPS-R and FLACC (P > 0.05) [Table 3].
The mean FLACC and FPS-R scores were found to be higher in the lignocaine group, compared to the conventional group, indicating that the pain experienced among the children in the lignocaine group was higher compared to the conventional group. This difference in the pain was found to be statistically significant (P < 0.0001) [Table 3].
The mean FLACC and FPS-R scores were found to be higher in the lignocaine group, compared to the articaine group, indicating a statistically significant difference in the pain, between the lignocaine and articaine groups [Table 3].
No adverse events were observed during the course of the study.
DISCUSSION
Anxiety and fear may develop due to poorly managed injection pain during childhood, and these reactions may not develop if appropriate measures are taken to reduce the pain associated with injections.[15] Customarily, adequate surface anesthesia could not be achieved for palatal muvosa through topical anesthetics, due to poor drug penetration through the highly keratinized tissue, firmly attached tissue inhibiting tissue distention created by the pressure of the injected solution (volume dependent), and the decreased tissue buffering capacity.[16]
Three approaches to measure pain in children include self-report, observational or behavioral, and physiological. A composite measure including self-report and one or more of these other approaches would be ideal for measurement of pain.[17] Hence, the FPS-R, the FLACC scale, and pulse rate were used for measuring self-report, behavioral, and physiological measures, respectively. FLACC and FPS-R were considered to be valid and reliable instruments for pain measurement, improving management of pain in children, and adolescents.[18]
In this study, there was no significant difference in the pain perception between control and articaine groups during extraction of maxillary primary molars. The results of this study are in concordance with the many studies[19,20,21,22,23,24] while another study had contradictory outcomes, in which they could not establish the presence of 4% articaine HCl at the palatal tissues after buccal injection.[25]
This study showed that pain perception was high in the lignocaine group compared to articaine group during the extraction of primary molars. The study results states that 4% articaine offers better clinical performance than 2% lignocaine particularly regarding providing adequate palatal anesthesia with only buccal infiltration as inferred by other others.[26,27,28]
Pain perception in lignocaine group was higher compared to that in control group during the extraction of maxillary primary molars, which are in concordance with one study[29] while contradictory to the results of few other studies.[14,30,31]
A study reported by Mittal M et al., in which articaine failed to provide adequate palatal anesthesia during the extraction of primary maxillary molars, contradictory to the results of this study.[32]
In this study, articaine buccal infiltration showed better success than lignocaine buccal infiltration, which can be explained by the fact that articaine is unique among amide LA due to the presence of thiophene ring, which makes it better lipid soluble. Due to its higher lipid solubility, articaine diffuses better through soft tissues than do other anesthetics, thereby achieving higher intraneural concentration, more extensive longitudinal spreading, and better conduction blockade. Articaine (2% and 4%) is better than lidocaine (2% and 4%) in depressing the compound action potential of the A fibers in the isolated rat sural nerve.[33] In addition, ionic channels are blocked even in lower concentrations with the thiophene derivative (articaine), compared to the benzene derivative (lidocaine).[34] A study noted that none of the younger subjects (below 20 years) in the articaine group had pain during extraction.[21] This can be ascribed to the fact that the maxillary bone becomes more sclerotic with age, thereby diminishing the bone penetrating effect of articaine. This study was conducted in children below 14 years, which might be the reason for enhanced vestibulo-palatal diffusion of articaine, thus providing effective palatal anesthesia with buccal infiltration only.
Delivering comfortable palatal anesthesia is a practice builder that will increase both patient's trust and treatment acceptance at the same time reduces personal stress levels.[2] Avoidance of the palatal injection completely when it is not necessary is desirable Using 1.7 ml of 4% articaine, we can avoid palatal injection during extraction of primary molars.
Limitation of the study was not utilizing similar concentration of drug in the injection solutions for buccal infiltration anesthesia. However, more randomized clinical trials with higher sample size should be conducted to synthesize higher levels of evidence to use this child-friendly approach in pediatric dentistry.
CONCLUSION
Maxillary primary molar extraction procedure can be successfully accomplished by bypassing the palatal injection. Children were more comfortable with articaine buccal infiltration (low-pain scores) than lignocaine buccal infiltration.
Financial support and sponsorship
Departmental resources.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Malamed S. Handbook of Local Anesthesia. 6th ed. St. Louis, MO: CV Mosby; 2013. [Google Scholar]
- 2.McArdle BF. Painless palatal anesthesia. J Am Dent Assoc. 1997;128:647. doi: 10.14219/jada.archive.1997.0265. [DOI] [PubMed] [Google Scholar]
- 3.Ram D, Peretz B. Administering local anaesthesia to paediatric dental patients-current status and prospects for the future. Int J Paediatr Dent. 2002;12:80–9. doi: 10.1046/j.1365-263x.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 4.Harker T. What injection? Br Dent J. 1997;182:50. doi: 10.1038/sj.bdj.4809297. [DOI] [PubMed] [Google Scholar]
- 5.Meechan JG, Winter RA. A comparison of topical anaesthesia and electronic nerve stimulation for reducing the pain of intra-oral injections. Br Dent J. 1996;181:333–5. doi: 10.1038/sj.bdj.4809252. [DOI] [PubMed] [Google Scholar]
- 6.Meechan JG. Effective topical anesthetic agents and techniques. Dent Clin North Am. 2002;46:759–66. doi: 10.1016/s0011-8532(02)00035-6. [DOI] [PubMed] [Google Scholar]
- 7.Harbert H. Topical ice: A precursor to palatal injections. J Endod. 1989;15:27–8. doi: 10.1016/S0099-2399(89)80094-9. [DOI] [PubMed] [Google Scholar]
- 8.Friedman MJ, Hochman MN. A 21st century computerized injection system for local pain control. Compend Contin Educ Dent. 1997;18:995–1000. [PubMed] [Google Scholar]
- 9.Kravitz J. The palatal press and roll anesthesia technique. Pract Proced Aesthet Dent. 2006;18:242–244. 5. [PubMed] [Google Scholar]
- 10.Munshi AK, Hegde AM, Latha R. Use of EMLA: Is it an injection free alternative? J Clin Pediatr Dent. 2001;25:215–9. doi: 10.17796/jcpd.25.3.hn62713500418728. [DOI] [PubMed] [Google Scholar]
- 11.Shields PW. Local anaesthesia and applied anatomy. Aust Dent J. 1986;31:319–25. doi: 10.1111/j.1834-7819.1986.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DH, Sowray JH. Local Analgesia in Dentistry. 3rd ed. Guildford: Wright; 1987. [Google Scholar]
- 13.Oertel R, Richter K, Weile K, Gramatté T, Berndt A, Feller K. A simple method for the determination of articaine and its metabolite articainic acid in dentistry: Application to a comparison of articaine and lidocaine concentrations in alveolus blood. Methods Find Exp Clin Pharmacol. 1993;15:541–7. [PubMed] [Google Scholar]
- 14.Sekhar GR, Nagaraju T, KolliGiri, Nandagopal V, Sudheer R, Sravan Is palatal injection mandatory prior to extraction of permanent maxillary tooth: A preliminary study. Indian J Dent Res. 2011;22:100–2. doi: 10.4103/0970-9290.80006. [DOI] [PubMed] [Google Scholar]
- 15.Milgrom P, Coldwell SE, Getz T, Weinstein P, Ramsay DS. Four dimensions of fear of dental injections. J Am Dent Assoc. 1997;128:756–66. doi: 10.14219/jada.archive.1997.0301. [DOI] [PubMed] [Google Scholar]
- 16.Primosch RE, Robinson L. Pain elicited during intraoral infiltration with buffered lidocaine. Am J Dent. 1996;9:5–10. [PubMed] [Google Scholar]
- 17.Champion G, Goodenough B, Von Baeyer C, Thomas W. Measurement of pain by self-report. Progress in pain research and management. Vol. 10. WA: IASP Press Seattle; 1998. pp. 123–60. [Google Scholar]
- 18.Da Silva FC, Santos Thuler LC, de Leon-Casasola OA. Validity and reliability of two pain assessment tools in Brazilian children and adolescents. J Clin Nurs. 2011;20:1842–8. doi: 10.1111/j.1365-2702.2010.03662.x. [DOI] [PubMed] [Google Scholar]
- 19.Uckan S, Dayangac E, Araz K. Is permanent maxillary tooth removal without palatal injection possible? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:733–5. doi: 10.1016/j.tripleo.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Fan S, Chen WL, Yang ZH, Huang ZQ. Comparison of the efficiencies of permanent maxillary tooth removal performed with single buccal infiltration versus routine buccal and palatal injection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:359–63. doi: 10.1016/j.tripleo.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 21.Somuri AV, Rai AB, Pillai M. Extraction of permanent maxillary teeth by only buccal infiltration of articaine. J Maxillofac Oral Surg. 2013;12:130–2. doi: 10.1007/s12663-012-0396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luqman U, Majeed Janjua OS, Ashfaq M, Irfan H, Mushtaq S, Bilal A. Comparison of articaine and lignocaine for uncomplicated maxillary exodontia. J Coll Physicians Surg Pak. 2015;25:181–4. [PubMed] [Google Scholar]
- 23.Oertel R, Rahn R, Kirch W. Clinical pharmacokinetics of articaine. Clin Pharmacokinet. 1997;33:417–25. doi: 10.2165/00003088-199733060-00002. [DOI] [PubMed] [Google Scholar]
- 24.Evans G, Nusstein J, Drum M, Reader A, Beck M. A prospective, randomized, double-blind comparison of articaine and lidocaine for maxillary infiltrations. J Endod. 2008;34:389–93. doi: 10.1016/j.joen.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Ozeç I, Tasdemir U, Gümüs C, Solak O. Is it possible to anesthetize palatal tissues with buccal 4% articaine injection? J Oral Maxillofac Surg. 2010;68:1032–7. doi: 10.1016/j.joms.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Hassan S, Rao BH, Sequeria J, Rai G. Efficacy of 4% articaine hydrochloride and 2% lignocaine hydrochloride in the extraction of maxillary premolars for orthodontic reasons. Ann Maxillofac Surg. 2011;1:14–8. doi: 10.4103/2231-0746.83145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma K, Sharma A, Aseri M, Batta A, Singh V, Pilania D, et al. Maxillary posterior teeth removal without palatal injection -truth or myth: A dilemma for oral surgeons. J Clin Diagn Res. 2014;8:ZC01–4. doi: 10.7860/JCDR/2014/10378.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darawade DA, Kumar S, Budhiraja S, Mittal M, Mehta TN. A clinical study of efficacy of 4% articaine hydrochloride versus 2% lignocaine hydrochloride in dentistry. J Int Oral Health. 2014;6:81–3. [PMC free article] [PubMed] [Google Scholar]
- 29.Kumaresan R, Srinivasan B, Pendayala S. Comparison of the effectiveness of lidocaine in permanent maxillary teeth removal performed with single buccal infiltration versus routine buccal and palatal injection. J Maxillofac Oral Surg. 2015;14:252–7. doi: 10.1007/s12663-014-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badcock ME, McCullough MJ. Palatal anaesthesia for the removal of maxillary third molars as practised by oral and maxillofacial surgeons in Australia and New Zealand. Aust Dent J. 2007;52:329–32. doi: 10.1111/j.1834-7819.2007.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 31.Yadav S, Verma A, Sachdeva A. Buccal injection of 2% lidocaine with epinephrine for the removal of maxillary third molars. Anesth Prog. 2013;60:95–8. doi: 10.2344/0003-3006-60.3.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal M, Sharma S, Kumar A, Chopra R, Srivastava D. Comparison of anesthetic efficacy of articaine and lidocaine during primary maxillary molar extractions in children. Pediatr Dent. 2015;37:520–4. [PubMed] [Google Scholar]
- 33.Potocnik I, Tomsic M, Sketelj J, Bajrovic FF. Articaine is more effective than lidocaine or mepivacaine in rat sensory nerve conduction block in vitro. J Dent Res. 2006;85:162–6. doi: 10.1177/154405910608500209. [DOI] [PubMed] [Google Scholar]
- 34.Borchard U, Drouin H. Carticaine: Action of the local anesthetic on myelinated nerve fibres. Eur J Pharmacol. 1980;62:73–9. doi: 10.1016/0014-2999(80)90482-3. [DOI] [PubMed] [Google Scholar]


