Abstract
Background:
Midazolam has been commonly used orally for premedication in children. A search for a better alternative continues to overcome its side effects. Recently alpha-2 agonists, clonidine, and dexmedetomidine have been used for premedication in children.
Aim:
To study and compare the efficacy of oral clonidine, oral dexmedetomidine, and oral midazolam for premedication in pediatric surgical patients.
Settings and Design:
This prospective, randomized, double blind study was conducted in a tertiary care hospital.
Materials and Methods:
The study was conducted in ninety children of either sex, in the age group of 4–12 years and the American Society of Anesthesiologists Physical status I, posted for ophthalmic surgery. Patients were randomly allocated to one of the three groups of thirty patients each: Group M: Oral midazolam 0.5 mg/kg body weight, Group D: Oral dexmedetomidine 4 μg/kg body weight, and Group C: Oral clonidine 4 μg/kg body weight. Patients were assessed for sedation, anxiolysis, and change in heart rate and blood pressure in the preoperative area. Behavior of children at separation from parents, mask acceptance, and side effects if any were noted.
Statistical Analysis:
Data analysis was performed by unpaired Student's t-test and Chi-square test.
Results:
Children in oral midazolam group achieved faster onset of sedation, higher sedation score, and lower anxiety score as compared to other two groups. The Group D and Group M were comparable as regards behavior at separation from parents (P = 0.236), but Group D was significantly better than Group C (P = 0.031). The three groups were comparable as regards providing satisfactory mask acceptance (P = 0.163). A number of children with easy separation from parents and excellent mask acceptance were significantly more in Group M as compared to Groups C and D (P = 0.028 and P = 0.012, respectively). Group C and Group D showed a lower mean arterial pressure at 45 min (P < 0.001) and 60 min after premedication (P < 0.001) as compared to Group M.
Conclusion:
Oral midazolam is superior to the oral clonidine, and oral dexmedetomidine with faster onset of sedation, higher sedation score, lower anxiety score, and greater number of children with easy separation and excellent mask acceptance.
Keywords: Clonidine, dexmedetomidine, midazolam, pediatric, premedication
INTRODUCTION
Surgery and anesthesia may cause considerable stress for both parents and children. The anticipation of pain, separation from family, and fear of surgery are few of the factors that trigger perioperative anxiety in children.[1] Premedication causes sedation and reduction of anxiety during separation from parents. It also provides a calm and cooperative child for smooth induction of anesthesia.
Midazolam, a benzodiazepine, has been routinely used orally for premedication in children scheduled for surgery.[2] It has a rapid onset and short duration of action. It is reliable in achieving sedation and anxiolysis.[3,4] However, search for a better alternative continues due to concerns such as bitter taste, cognitive impairment, long-term behavioral disturbances, paradoxical reactions, hiccups, and respiratory depression.[5,6]
Clonidine, a centrally acting alpha-2 agonist, has been shown to reliably produce preoperative sedation and anxiolysis in children.[7] It has excellent bioavailability after oral administration although the onset of action is slow.[8,9]
Dexmedetomidine is a newer, centrally acting alpha-2 agonist with a more selective action on alpha-2 adrenoceptors and a shorter half-life as compared to clonidine. It has been shown to produce effective sedation, anxiolysis, and analgesia in children without respiratory depression.[10]
This study was undertaken to compare the efficacy of oral dexmedetomidine, clonidine, and midazolam for premedication in children undergoing ophthalmic surgery. We compared their effects on preoperative sedation, anxiolysis, separation from parents, mask acceptance, and preoperative hemodynamic parameters.
MATERIALS AND METHODS
After due clearance from the Institutional Ethics Committee and after obtaining written informed consent from a parent or a legal guardian, this prospective, randomized, double-blind study was conducted in ninety children in the age group of 4–12 years, of either sex and the American Society of Anesthesiologists (ASA) Physical status 1, posted for ophthalmic surgery.
Patients with known allergy to dexmedetomidine, clonidine or midazolam, developmental delay or mental retardation, history of any sedative or analgesic intake, ASA physical status ≥2, those with upper respiratory tract infection and intraocular pressure >20 mmHg were excluded from the study.
Patients were randomly allocated by computer-generated random numbers to one of the three groups of thirty each: Group M: Oral midazolam 0.5 mg/kg body weight, Group D: Oral dexmedetomidine 4 µg/kg body weight, and Group C: Oral clonidine 4 µg/kg body weight. An intravenous preparation of a drug (midazolam 5 mg/ml solution, dexmedetomidine 100 µg/ml solution and clonidine 150 µg/ml solution) was mixed with apple juice, diluted to total volume of 0.2 ml/kg body weight, and given orally.
Before administration of the premedication, the children were brought to the preoperative room along with their parents. Hemodynamic parameters such as heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), oxygen saturation (SpO2), and degree of sedation and anxiety were monitored and recorded at baseline, and every 15 min after premedication until the patient was shifted to the operation theater.
Response of the child to acceptance of premedication was assessed on a three-point scale:
1 = Accepts it/likes the taste
2 = Accepts it/but dislikes the taste
3 = Spits/vomits the premedication.
Score of 1 or 2 was considered as satisfactory acceptance of oral premedication.
Degree of sedation was assessed on a three-point scale:
1 = Awake
2 = Drowsy
3 = Asleep.
Score of 2 or 3 was considered as acceptable sedation.
Anxiety was assessed on a five-point scale:
1 = Quiet and comfortable
2 = Uneasy
3 = Worried or anxious
4 = Very worried or very upset
5 = Frightened or terrified.
Score of 1 or 2 was considered as acceptable anxiolysis.
The child was separated from the parents and taken to the operating room 60 min after the administration of premedication. Behavior of the child on separation from parents was assessed and graded according to a four-point scale, i.e., the parental separation anxiety scale (PSAS):[11]
1 = Easy separation
2 = Whimpers but is easily reassured
3 = Cries and cannot be easily reassured, but not clinging to parents
4 = Cries and clinging to parents.
A PSAS score of 1 and 2 were considered as acceptable separation from parents.
In the operating room, electrocardiograph, noninvasive blood pressure (BP), and SpO2 monitors were attached. Inhalation induction was performed using halothane (0.5–2.5%) in oxygen and nitrous oxide mixture in 1:2 ratio.
Mask acceptance was observed and scored using a four-point Likert scale, i.e., mask acceptance scale:[10]
1 = Excellent (unafraid, cooperative, and accepts mask easily)
2 = Good (slight fear of mask, easily reassured)
3 = Fair (moderate fear of mask, not calmed with reassurance)
4 = Poor (terrified, crying, or combative).
The score of 1 and 2 were considered as satisfactory mask acceptance.
An intravenous line was secured and fentanyl 2 µg/kg was given intravenously for analgesia, and muscle relaxation was provided with intravenous vecuronium bromide 0.1 mg/kg. Proseal laryngeal mask airway (LMA) of an appropriate size was inserted 3 min after the administration of the muscle relaxant. Anesthesia was maintained with isoflurane (1–1.5%), oxygen and nitrous oxide in a ratio of 1:2, and supplemental doses of vecuronium bromide. Rectal paracetamol suppository in the dose of 40 mg/kg was inserted after LMA placement for analgesia.
Hemodynamic parameters such as HR, SBP, DBP, MAP, and SPO2 were monitored intraoperatively. Inhalational agent was switched off 5 min before the end of surgery. At the end of surgery, effect of muscle relaxant was reversed with neostigmine (0.05 mg/kg) and glycopyrrolate (0.01 mg/kg) given intravenously. After ensuring adequate reversal, LMA was removed. Patients were shifted to the postoperative recovery room for observation and were given oxygen inhalation by venturi mask.
In the postoperative room, patients were monitored for hemodynamic parameters and side effects such as hypotension and bradycardia were noted. Other side effects such as respiratory depression, fall in saturation, shivering, vomiting, and hiccups if any were noted for 2 h postoperatively.
The anesthesiologist who monitored the patient, scored the patient's behavior, and collected the data was blind to the study drug administered.
Our primary outcomes were number of patients showing acceptable separation from parents and satisfactory mask acceptance. The secondary outcomes were time of onset of sedation, mean sedation score, and mean anxiety score.
Statistical analysis
Based on a pilot study, minimum required sample size is 25 patients in each group to detect 35% (from 85% to 50%) difference in satisfactory mask acceptance between midazolam and the other two drugs (clonidine and dexmedetomidine) at the 0.05 level of significance and to provide 80% power to the study. To account for dropouts, it was decided to take thirty patients in each group. All values are reported as mean ± standard deviation or percentage of patients. Data analysis for numerical data was performed by unpaired Student's t-test. Data analysis for categorical data was performed by Fisher's exact test or Chi-square test to detect differences for the scores. Analysis of variance was performed for comparing the differences between the three groups and unpaired t-test across two groups. The statistical significance of quantitative variables was calculated by Student's t-test/nonparametric Wilcoxon Mann–Whitney in case data did not follow a normal distribution. The level of statistical significance was taken as P < 0.05. The data were analyzed using SPSS statistical software (SPSS version 17 (SPSS IL, Chicago, IL, USA)).
RESULTS
The three groups were comparable with respect to age, gender, and weight [Table 1]. All the children in the midazolam, clonidine, and dexmedetomidine groups accepted the oral drug mixed with apple juice. Six (20%) children in midazolam group accepted the premedication but disliked the taste as compared to none in clonidine and dexmedetomidine groups (P = 0.0314). Satisfactory acceptance of premedication was present in all the children in the three groups (P = 1.000).
Table 1.
Demographic data

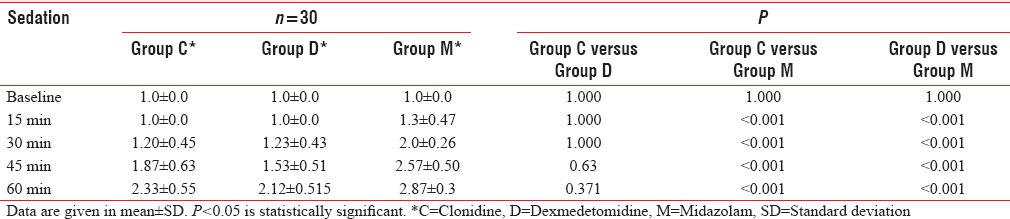
Baseline sedation score was comparable in all the three groups. Onset of sedation was at 15 min with midazolam and at 30 min with both clonidine and dexmedetomidine. Mean sedation score was significantly higher with midazolam at 30, 45, and 60 min as compared to the other two drugs (P < 0.001) [Table 2]. At 60 min after premedication, 29 (96.67%) children in clonidine group, 27 (90%) children in dexmedetomidine group, and 30 (100%) children in midazolam group had achieved satisfactory sedation scores of 2 or 3, and there was no significant difference between the groups (P = 0.637). However, only 11 (36.6%) children in clonidine group and 7 (23.3%) children in dexmedetomidine group as compared to 26 (86.6%) children in midazolam group were asleep with sedation score of 3. Significantly, more children were asleep in midazolam group as compared to Groups C and D (P < 0.001). There was no significant difference between Groups C and D (P = 0.394).
Table 2.
Sedation score
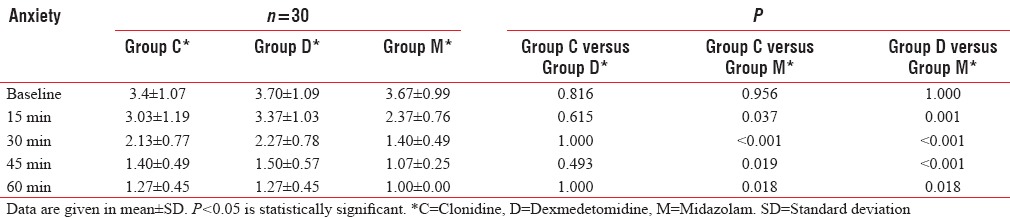
The baseline anxiety scores were comparable between the three groups (P = 0.483). The mean anxiety score at 60 min was significantly less with midazolam as compared to clonidine and dexmedetomidine groups (P = 0.018) [Table 3]. All 30 (100%) children in each group had achieved satisfactory anxiolysis by 60 min after premedication. In Group M, all the 30 (100%) children were quiet and comfortable as compared to only 22 (73.3%) children each in Groups C and D (P = 0.0045).
Table 3.
Anxiety score
Acceptable parent separation scores of 1 and 2 were achieved in 100% of the children in Group D, 90% of the children in Group M, and 80% of the children in Group C. The Group D and Group M were comparable as regards behavior at separation from parents (P = 0.236). Group M was comparable to Group C (P = 0.46), but Group D was significantly better than Group C (P = 0.031) for achieving acceptable separation from parents. However, in Group M, significantly, greater number of children had easy separation from parents (score of 1) as compared to Groups C and D (P = 0.028 and P = 0.012, respectively) [Figure 1].
Figure 1.
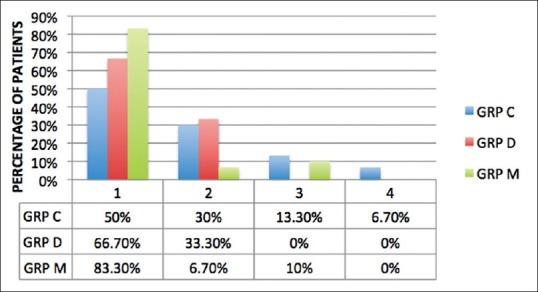
Parent separation anxiety score. Data are expressed as percentage of patients. PSAS: Parental separation anxiety score, C: Clonidine, D: Dexmedetomidine, M: Midazolam.
All the three groups were comparable as regards satisfactory mask acceptance (P = 0.163). A number of children with excellent mask acceptance were more in Group M as compared to Groups C and D, and the difference was statistically significant (P = 0.040 and P = 0.005, respectively). There was no statistically significant difference between Group C and D (P = 0.52) [Figure 2].
Figure 2.
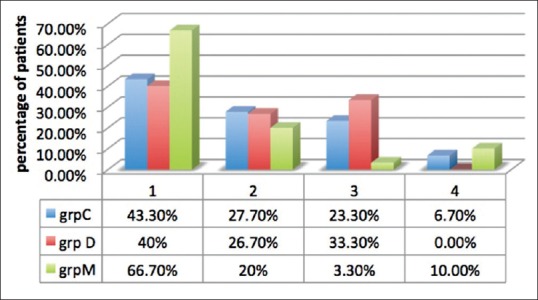
Mask acceptance score. Data are expressed as percentage of patients. C: Clonidine, D: Dexmedetomidine, M: Midazolam, MAS: Mask acceptance score.
There was a statistically significant fall in HR and mean arterial BP from baseline at 45 and 60 min after premedication in all the groups (P < 0.001) [Figures 3 and 4]. The fall in HR was comparable across all the three groups (P > 0.05). On comparing the MAP between the groups, it was found that Group C and Group D had a lower value at 45 min (P < 0.001) and 60 min after premedication (P < 0.001) as compared to Group M [Figure 4].
Figure 3.
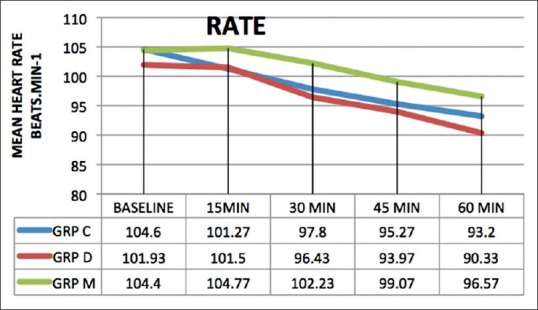
Preoperative heart rate at different time intervals. Data are expressed as mean values in beats/min. HR: Heart rate, C: Clonidine, D: Dexmedetomidine, M: Midazolam.
Figure 4.
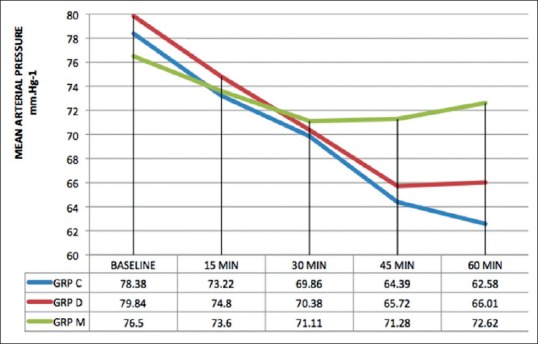
Preoperative mean arterial pressure at different time intervals. Data are expressed as mean values in mmHg. MAP: Mean arterial pressure, C: Clonidine, D: Dexmedetomidine, M: Midazolam.
There was no significant difference in the incidence of side effects between the three groups. The incidence of vomiting was 3.3%, and that of hiccups was 3% in Group M as compared to none in Groups C and D.
DISCUSSION
This prospective, double-blind, randomized controlled study demonstrated that oral clonidine 4 µg/kg, dexmedetomidine 4 µg/kg, and midazolam 0.5 mg/kg provided effective sedation, anxiolysis, acceptable separation from parents, and satisfactory mask acceptance in children between 4 and 12 years of age undergoing ophthalmic surgery under general anesthesia. Oral midazolam, however, was significantly better than the other two drugs as greater proportion of children showed easy separation from parents and excellent mask acceptance.
The oral route of administration of the drugs was used as it is the most acceptable and a nontraumatic route, especially in children.[12] The total volume of the oral premedication was kept at 0.2 ml/kg which is far below the gastric fluid volume of 0.4 ml/kg above which the risk of aspiration increases.[13]
Bioavailability of oral dexmedetomidine is only 16% due to extensive first-pass metabolism but that by buccal mucosa is 82% and that of intramuscular route is 104%.[14] However, it is difficult to make children hold liquid in their mouth for few minutes for absorption by buccal mucosa and hence, we used oral route of administration of the drug. In a study in adults, after oral administration of dexmedetomidine 2 μg/kg, the maximum serum concentration of 0.11 ± 0.04 μg/L was achieved in 2.2 ± 0.5 h after a lag time of 0.6 ± 0.3 h.[14] The plasma concentration of dexmedetomidine that confers sedation in children is 0.4–0.8 μg/L.[15] This would mean that a dose of 6–8 μg/kg of oral dexmedetomidine would be required to produce effective concentration in children for sedation if this data are extrapolated on children. Zub et al. were the first to recommend the dose of 3–4 μg/kg of oral dexmedetomidine for premedication to reduce anxiety in children undergoing surgical procedures.[16] They suggested that intravenous preparation of dexmedetomidine could be used orally with acceptable palatability.[16] Mountain et al. used oral dexmedetomidine in dose of 4 µg/kg and found it comparable to 0.5 mg/kg midazolam in reducing anxiety in children during mask acceptance and separation from parents without any adverse effects such as hypotension and bradycardia.[10] Hence, we used oral dexmedetomidine 4 µg/kg in our study.
Bioavailability of oral clonidine mixed with apple fruit drink in children was shown to be 55.4% by Larsson et al.[9] Bioavailability of 2.5 µg/kg rectal clonidine is 95% and produces effective plasma concentration of 0.77 ng/ml.[17] Larsson et al. observed that oral clonidine would be required in a dose of 4–5 µg/kg for premedication if 2.5 µg/kg after rectal administration and 1.25 µg/kg after intravenous administration are effective doses in children.[9] Mikawa et al. found that oral clonidine 4 µg/kg provided a better behavior on separation from parents and mask acceptance than oral clonidine 2 µg/kg.[8] Almenrader et al. found premedication with oral clonidine 4 µg/kg and oral midazolam 0.5 mg/kg effective and comparable in providing satisfact’ory mask acceptance in children.[18] Based on these studies, we decided to use oral clonidine in dose of 4 µg/kg.
Bioavailability of oral midazolam varies from 15% to 27% in children.[19] Feld et al. as early as in 1990 in their study stated that oral midazolam 0.5–0.75 mg/kg was effective and safe for premedication in pediatric surgical patients.[2] Later, McMillan et al. found that oral midazolam in dose of 0.5 mg/kg was safe and effective for premedication, but a dose of 0.75 mg/kg caused more side effects such as loss of balance, blurred vision, and dysphoric reactions while offering no additional benefit.[20] We decided to use oral midazolam in dose of 0.5 mg/kg in our study.
It was noted that 20% of children in midazolam group did not like the taste of drug as compared to none in clonidine and dexmedetomidine groups, inspite of mixing the study drug with sweet apple juice to mask its taste before oral administration. Similar to our study, Almenrader et al. found that 24% of children in oral midazolam group did not like the taste of the drug as compared to none in oral clonidine group.[18] However, as none of the children spit out the combination of midazolam and apple juice, it can be inferred that it had a satisfactory acceptance.
In our study, onset of sedation was significantly delayed in clonidine and dexmedetomidine groups (30 min for both) as compared to midazolam group (15 min). Similar results were found by Kamal et al. in their study.[21] They compared oral dexmedetomidine 3 µg/kg with oral midazolam 0.5 mg/kg in 60 children of age group 3–10 years. They found the onset of sedation faster in midazolam group (28.4 ± 13.7 min vs. 39.5 ± 14.3 min) as compared to dexmedetomidine group. Almenrader et al. found that oral clonidine was required to be administered 45 min before induction to achieve optimum sedation, whereas satisfactory sedation with midazolam could be achieved in 30 min after ingestion.[18]
Children given midazolam were more sedated and less anxious as compared to those given clonidine and dexmedetomidine at 15, 30, 45, and 60 min after administering premedication. Although there was no significant difference between the groups in the number of children who were drowsy or asleep, most of the children (86%) given midazolam were asleep at 60 min after premedication.
A previous study has shown that oral clonidine requires 105–120 min to reach peak sedative effect in children.[8] Oral dexmedetomidine in adults has been shown to take 2.2 ± 0.5 h to reach peak plasma concentration with a lag time of 0.6 ± 0.3 h.[14] No such study on oral dexmedetomidine in children has been published. Payne et al. observed that the peak serum levels of midazolam were achieved at 60 min following oral administration of 0.5 mg/kg in children.[19] In our study, patients were shifted to operation theater at 60 min after administering premedication and by this time, peak effect of clonidine and dexmedetomidine had not been reached and that of midazolam had been achieved. This may be the reason why midazolam was noted to be a better sedative.
In contrast to our study, Almenrader et al. found higher sedation scores in children receiving oral clonidine 4 µg/kg as compared to those in oral midazolam 0.5 mg/kg group. The children in their study were in younger age group of 1–6 years as compared to those in our study who were 4–12 years old. They also provided favorable quiet environment conducive to sleep to the children in their study.[18] The lack of a quiet and favorable environment for sleep, difference in age, and culture of the children could be few of the reasons why children in clonidine group in our study achieved lower sedation scores as compared to their study.
Acceptable parent separation scores of 1 and 2 were achieved in 100% of children in Group D, 90% of children in Group M, and 80% of children in Group C. A number of children with easy separation (score 1) were significantly more (83.3%) in midazolam group as compared to dexmedetomidine group (66.7%) and clonidine group (50%).
There was no significant difference between the three groups as regards satisfactory mask acceptance (P > 0.05). A number of children with excellent mask acceptance were significantly higher in oral midazolam (66.7%) group than in clonidine (43.3%) and dexmedetomidine (40%) groups. Better behavior of children at separation from parents and better mask acceptance in midazolam group could be because of its more pronounced sedative and anxiolytic effects due to action on gamma-aminobutyric acid receptors. Oral clonidine and oral dexmedetomidine decrease sympathetic outflow and increase parasympathetic outflow from locus coeruleus in central nervous system where they induce electroencephalographic activity similar to natural sleep.[22] It is due to this that any external stimuli such as taking the patient inside the operation theater and mask application can lead to arousal from sleep in patients administered clonidine and dexmedetomidine.
Fazi et al. also found better anxiolysis on separation from parents and at mask induction in children (4–12 years of age) given oral midazolam 0.5 mg/kg than those given oral clonidine 4 µg/kg, even though they separated children from parents at 75 ± 25 min after premedication in clonidine group.[23]
Mountain et al. found no significant difference between children (age group 1–6 years) administered oral midazolam 0.5 mg/kg or oral dexmedetomidine 4 µg/kg as regards acceptable behavior at separation from parents and satisfactory mask acceptance.[10]
Akin et al. also found that a significantly greater proportion of children (82.2%) in nasal midazolam (0.2 mg/kg) group had satisfactory mask acceptance during induction as compared to those (60%) in nasal dexmedetomidine (1 µg/kg) group. Similar to our study, there was no difference between the groups in achieving acceptable separation from parents.[24]
Yuen et al. found nasal dexmedetomidine 1 µg/kg similar to oral midazolam 0.5 mg/kg for providing satisfactory separation from parents and satisfactory mask acceptance at induction. However, in their study, children in the dexmedetomidine group were more sedated.[25] This may be because they used intranasal route of administration of dexmedetomidine, which reaches peak effect faster and has higher bioavailability as compared to oral dexmedetomedine. In healthy adults, Iirola et al. showed that peak plasma concentrations of intranasal dexmedetomidine were reached in 38 (15–60) min and its median (range) bioavailability was 65% (35–93%).[26] Yuen et al. in another study found median (range) time for the onset of sedation to be 25 (25–45) min and duration of action to be 85 (55–100) min in children (2–9 years of age) given intranasal dexmedetomidine.[27]
A meta-analysis by Sun et al. demonstrated that dexmedetomidine premedication is superior to midazolam premedication for producing satisfactory sedation on parent separation and mask acceptance. However, they also suggested that their conclusions should be viewed in the light of heterogeneity caused by differences in various studies as regards the characteristics of the children, age, doses, and routes of the premedicant drugs used, time of separation from parents after premedication, types of surgeries, and scores for evaluation for each outcome.[28]
Clonidine and dexmedetomidine caused a significantly greater fall than midazolam in MAP at 45 and 60 min after the administration of premedication. The HR began to fall from baseline at 30 min after premedication in all three groups and reached a lowest value at 60 min. The three groups were comparable as regards fall in HR. Kamal et al. found oral dexmedetomidine comparable to oral midazolam for fall in HR and BP. during preoperative period, but intraoperatively and postoperatively the fall was significantly more in dexmedetomidine group than in midazolam group.[21] No clinically adverse hemodynamic effects and need for any pharmacological intervention were noticed in all the three groups. Clonidine and dexmedetomidine cause a centrally mediated decrease in the sympathetic nervous system outflow and also an increase in vagal activity, which manifests as peripheral vasodilatation and decrease in SBP and HR.[22]
No significant side effects such as hypotension, bradycardia, respiratory depression, oxygen desaturation, and apnea were noticed during the study in all the three groups.
Limitations of our study include (1) intravenous formulations of the drugs were used as oral preparations of the drugs were not available. Mixing of the drug with apple juice could change pH of the drug and its absorption. (2) Children were separated from parents at 60 min after administering premedication by which time peak effect of clonidine may not have reached. However, in a busy operation theater with rapid turnover of patients, it was difficult to wait longer than this.
Pharmacokinetics and pharmacodynamics of drugs may differ in younger and older children. More studies with larger sample sizes are needed to find the optimum doses, time for peak effect, and the effects of these drugs in different age groups.
Dexmedetomidine at present is not approved for use in children in any country. There are number of small studies and case reports in literature showing its use in children.[10,21,24,25,26,27,28] However, additional large prospective studies in pediatric patients are warranted to further evaluate its safety and efficacy.
CONCLUSION
We conclude that oral dexmedetomidine 4 µg/kg is comparable to oral midazolam 0.5 mg/kg and superior to oral clonidine 4 µg/kg for providing acceptable, separation from parents in children. All the three drugs were comparable for providing satisfactory mask acceptance. Oral midazolam was superior to the other two drugs for providing easy separation from parents and excellent mask acceptance in children. Oral midazolam had faster onset of sedation and provided higher sedation scores and lower anxiety scores as compared to the other two groups. All three drugs are safe and effective for premedication in children when given orally.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Egan KJ, Ready LB, Nessly M, Greer BE. Self-administration of midazolam for postoperative anxiety: A double blinded study. Pain. 1992;49:3–8. doi: 10.1016/0304-3959(92)90180-J. [DOI] [PubMed] [Google Scholar]
- 2.Feld LH, Negus JB, White PF. Oral midazolam preanesthetic medication in pediatric outpatients. Anesthesiology. 1990;73:831–4. doi: 10.1097/00000542-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gutstein HB, Johnson KL, Heard MB, Gregory GA. Oral ketamine preanesthetic medication in children. Anesthesiology. 1992;76:28–33. doi: 10.1097/00000542-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, Wang SM, et al. Midazolam: Effects on amnesia and anxiety in children. Anesthesiology. 2000;93:676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Bergendahl H, Lönnqvist PA, Eksborg S. Clonidine in paediatric anaesthesia: Review of the literature and comparison with benzodiazepines for premedication. Acta Anaesthesiol Scand. 2006;50:135–43. doi: 10.1111/j.1399-6576.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergendahl H, Lönnqvist PA, Eksborg S. Clonidine: An alternative to benzodiazepines for premedication in children. Curr Opin Anaesthesiol. 2005;18:608–13. doi: 10.1097/01.aco.0000191891.44314.36. [DOI] [PubMed] [Google Scholar]
- 7.Wright PM, Carabine UA, McClune S, Orr DA, Moore J. Preanaesthetic medication with clonidine. Br J Anaesth. 1990;65:628–32. doi: 10.1093/bja/65.5.628. [DOI] [PubMed] [Google Scholar]
- 8.Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Efficacy of oral clonidine premedication in children. Anesthesiology. 1993;79:926–31. doi: 10.1097/00000542-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Larsson P, Nordlinder A, Bergendahl HT, Lönnqvist PA, Eksborg S, Almenrader N, et al. Oral bioavailability of clonidine in children. Paediatr Anaesth. 2011;21:335–40. doi: 10.1111/j.1460-9592.2010.03397.x. [DOI] [PubMed] [Google Scholar]
- 10.Mountain BW, Smithson L, Cramolini M, Wyatt TH, Newman M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J. 2011;79:219–24. [PubMed] [Google Scholar]
- 11.Dashiff CJ, Weaver M. Development and testing of a scale to measure separation anxiety of parents of adolescents. J Nurs Meas. 2008;16:61–80. doi: 10.1891/1061-3749.16.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Kain ZN, Mayes LC, Bell C, Weisman S, Hofstadter MB, Rimar S. Premedication in the United States: A status report. Anesth Analg. 1997;84:427–32. doi: 10.1097/00000539-199702000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Coté CJ, Goudsouzian NG, Liu LM, Dedrick DF, Szyfelbein SK. Assessment of risk factors related to the acid aspiration syndrome in pediatric patients-gastric ph and residual volume. Anesthesiology. 1982;56:70–2. doi: 10.1097/00000542-198201000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56:691–3. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care – a pooled analysis. Paediatr Anaesth. 2009;19:1119–29. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 16.Zub D, Berkenbosch JW, Tobias JD. Preliminary experience with oral dexmedetomidine for procedural and anesthetic premedication. Paediatr Anaesth. 2005;15:932–8. doi: 10.1111/j.1460-9592.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 17.Lönnqvist PA, Bergendahl HT, Eksborg S. Pharmacokinetics of clonidine after rectal administration in children. Anesthesiology. 1994;81:1097–101. doi: 10.1097/00000542-199411000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Almenrader N, Passariello M, Coccetti B, Haiberger R, Pietropaoli P. Premedication in children: A comparison of oral midazolam and oral clonidine. Paediatr Anaesth. 2007;17:1143–9. doi: 10.1111/j.1460-9592.2007.02332.x. [DOI] [PubMed] [Google Scholar]
- 19.Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989;37:267–72. doi: 10.1007/BF00679782. [DOI] [PubMed] [Google Scholar]
- 20.McMillan CO, Spahr-Schopfer IA, Sikich N, Hartley E, Lerman J. Premedication of children with oral midazolam. Can J Anaesth. 1992;39:545–50. doi: 10.1007/BF03008315. [DOI] [PubMed] [Google Scholar]
- 21.Kamal K, Soliman D, Zakaria D. Oral dexmedetomidine versus oral midazolam as premedication in children. Ain Shams J Anesthesiol. 2008;1:1–18. [Google Scholar]
- 22.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 23.Fazi L, Jantzen EC, Rose JB, Kurth CD, Watcha MF. A comparison of oral clonidine and oral midazolam as preanesthetic medications in the pediatric tonsillectomy patient. Anesth Analg. 2001;92:56–61. doi: 10.1097/00000539-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. 2012;22:871–6. doi: 10.1111/j.1460-9592.2012.03802.x. [DOI] [PubMed] [Google Scholar]
- 25.Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: A double-blinded randomized controlled trial. Anesth Analg. 2008;106:1715–21. doi: 10.1213/ane.0b013e31816c8929. [DOI] [PubMed] [Google Scholar]
- 26.Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67:825–31. doi: 10.1007/s00228-011-1002-y. [DOI] [PubMed] [Google Scholar]
- 27.Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia. 2010;65:922–9. doi: 10.1111/j.1365-2044.2010.06453.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Lu Y, Huang Y, Jiang H. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Paediatr Anaesth. 2014;24:863–74. doi: 10.1111/pan.12391. [DOI] [PubMed] [Google Scholar]


