Abstract
Background:
Intravenous regional anesthesia (IVRA) is safe, technically simple, and cost-effective technique compared to general anesthesia with success rates of 94–98% for upper and lower limb surgeries. The main disadvantage of this procedure is its limited duration for surgery, lack of postoperative analgesia, and tourniquet pain. To overcome this disadvantage, various adjuvants to lignocaine have been studied from time to time.
Aim:
To compare the analgesic efficacy of dexmedetomidine and midazolam as adjuncts to lignocaine for IVRA for forearm and hand surgeries.
Setting and Design:
The study was conducted by the Department of Anaesthesia of Medical College and patients posted for elective as well as the emergency forearm and hand surgeries were included in the study. It was a prospective comparative study.
Materials and Methods:
Sixty patients of either sex belonging to the American Society of Anesthesiologists Class I and II, in the age range of 18–65 years, scheduled for upper limb orthopedic surgery, either elective or emergency, were included in the study. All patients were administered IVRA in this prospective, double-blind, randomized study. Patients enrolled in the study were randomly divided into two groups of thirty each. Group M-received 40 ml of 0.5% lignocaine with midazolam 50 μg/kg and Group D-received 40 ml of 0.5% lignocaine with dexmedetomidine 1 μg/kg. Time of onset of sensory block, duration of analgesia, total dose of fentanyl given, intraoperative blood pressure, oxygen saturation, heart rate, postoperative analgesia, and adverse effects were recorded and compared between the groups.
Statistical Analysis Used:
The statistical evaluation was performed using SPSS version 17.0 software. All values were calculated with a 95% confidence interval. The parameters were expressed as mean ± standard deviation and t-test was used for comparing demographic and clinical data. For comparisons, P < 0.05 was considered statistically significant.
Results:
Mean duration of analgesia was 93 ± 28 min in dexmedetomidine group and 84 ± 28 min in midazolam group, and onset of sensory block was comparable in both groups.
Conclusion:
Dexmedetomidine and midazolam, when used as adjuvants to lignocaine for IVRA, significantly improve the intraoperative conditions by providing superior quality of block. The superiority of one over the other could not be established as midazolam produced the early onset of block and less requirement of fentanyl, whereas dexmedetomidine when added to IVRA provided longer duration of analgesia (93 ± 28 min) in comparison to midazolam (84 ± 28 min).
Keywords: Adjuvants, dexmedetomidine, intravenous regional anesthesia, lignocaine, midazolam
INTRODUCTION
Intravenous regional anesthesia (IVRA), first described by August Bier in 1902, is technically simple and reliable, with success rates between 94% and 98%.[1] IVRA is a method of producing analgesia in the distal part of a limb by IV injection of dilute solution of a local anesthetic solution into the vein of the same limb while circulation to the limb is occluded by the application of tourniquet. Different agents have been used as additive to local anesthetic for IVRA including phencyclidines, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, and muscle relaxants.[2]
Dexmedetomidine is approximately eight times more selective toward the α2 adrenoceptors than clonidine. It decreases anesthetic requirements by up to 90% and induces analgesia in patients.[3] It has been used successfully in combination with local anesthetics for procedures such as spinal, epidural, brachial blocks, and intravenous regional anesthesia. A large number of studies have shown that dexmedetomedine when added to lignocaine improves the quality of anesthesia and perioperative analgesia in intravenous regional anesthesia without causing side effects.
Midazolam, a short-acting benzodiazepine, is roughly 1.5–2 times as potent as diazepam and has a greater hypnotic effect than diazepam because it interferes with gamma-aminobutyric acid (GABA) reuptake.[4] Midazolam is primarily used preoperatively as an anxiolytic and sedative-hypnotic agent. It is available in IV, intramuscular, oral, sublingual, rectal, and intranasal preparations. The role of midazolam in regional anesthesia has been successfully studied. Midazolam has been shown to improve the quality of anesthesia and perioperative analgesia when added to lignocaine in IVRA without causing side effects. GABA receptors have also been found in peripheral nerves and midazolam reduces A-delta and C-fiber-evoked activity when given IV.
No study till date has compared the analgesic efficacy of dexmedetomidine and midazolam as additives in IVRA. We compared the onset and duration of analgesia during IVRA using lignocaine with midazolam, and lignocaine with dexmedetomidine and assessed postoperative analgesic requirement of the patients.
MATERIALS AND METHODS
This prospective randomized study was conducted after obtaining clearance from the Institutional Ethics Committee, and written informed consent from all patients. Patients of the American Society of Anesthesiologists (ASA) physical Status I and II, aged between 18 and 65 years scheduled for forearm and hand surgeries in the distal region of upper limb under IVRA, were included in this study after fulfilling inclusion and exclusion criteria. Those with a history of allergic reaction to lignocaine, significant cardiovascular disease, sickle cell disease, highly nervous and uncooperative patients, patient with crush injury or open wound, history of opioid dependence, drug or alcohol abuse, psychiatric disorder, peripheral vascular disease, and neurological diseases were excluded from the study. At the preoperative visit, in the evening before surgery, the visual analog scale (VAS) scoring system was explained to all the patients.
Sample size calculation was done on the basis of the previous study. The primary outcome was taken as pain-free period and assuming 85% of study power and 5% α error; the minimum sample size was calculated to be 27 patients per group were required. Therefore, thirty patients in each group were planned. Patients were assigned randomly into two groups by computer-generated random number chart, and patients were assigned respective group by sealed opaque envelope technique. The anesthesiologist who prepared the study solution for Bier's block did not participate in the study. Attending anesthetist was not aware of the composition of the drug composition used for block. Patients in Group M received lignocaine along with midazolam (50 µg/kg), and Group D received lignocaine and dexmedetomidine (1 µg/kg). The lignocaine used in the study was 2% preservative-free and constant (200 mg) in all groups. To make the total volume of test solution 40 ml, normal saline (NS) (0.9%) was added.
No premedication was given to any of the patients. Standard monitoring was used in all patients, which included noninvasive blood pressure, heart rate, electrocardiogram, and pulse oximetry. Before administration of IVRA, an infusion of 0.9% NS was started in the normal limb. A 20-gauge IV cannula was inserted into the distal vein of the extremity that was to be operated. Two tourniquets were placed, and arm was exsanguinated using an Esmarch bandage. The proximal tourniquet was inflated to 100 mmHg above the systolic pressure of the patient. The absence of radial artery pulsations and failure of pulse oximetry tracing in the ipsilateral index finger was confirmed. Forty milliliters of test solution was injected over 90 s by an anesthesiologist who was blinded to the drug being administered.
After the injection of different study solutions, the onset of sensory block (defined as loss of pain sensation) was determined by the pin prick method, with a 22-gauge hypodermic needle, distal to the proximal tourniquet. The distal tourniquet was inflated followed by the deflation of proximal tourniquet. Assessment of tourniquet pain score was made on the basis of VAS (0 = no pain, 10 = worst pain). If the patient had complained of tourniquet pain, 1 μg/kg of fentanyl with 0.1 mg/kg ondansetron was given IV and in case of persistent pain, same dose of fentanyl was repeated after 5 min. Time to first analgesic demand, and sedation score (Ramsay sedation score) were compared. Postoperatively, patients received diclofenac 75 mg IV if VAS was >5.
Statistical analysis
The sample size was chosen after reviewing many randomized control studies on the same subject. The statistical evaluation was performed using SPSS version 17.0 software (IBM). All values were calculated with a 95% confidence interval. The parameters were expressed as mean ± standard deviation and t-test was used for comparing demographic and clinical data. Analysis of variance technique was used for comparison between the two groups for parametric data. Chi-square test was used for nonparametric data. For comparisons, P < 0.05 was considered statistically significant.
RESULTS
Patients in both groups were comparable with regard to age, sex, weight, and ASA status [Table 1]. The mean age was 33.03 ± 13.57 years in Group D and 37.67 ± 14.39 years in Group M. The difference in mean age was statistically insignificant (P = 0.205). Thus, two groups were comparable with regard to age distribution.
Table 1.
Demographic profile
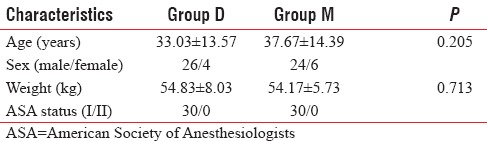
In the study population, male patients constituted 86.7% and 80% in Group D and Group M, respectively, and female patients constituted 13.3% and 20% in Group D and Group M, respectively. The difference between the two groups regarding weight distribution was statistically insignificant (P = 0.713) [Table 1].
The mean time of onset of sensory block was 8.01 ± 4.02 min in Group D and 6.49 ± 4.26 min in Group M [Figure 1]. The difference in mean time of onset of the sensory block between Group D and Group M was statistically insignificant (P > 0.05) [Table 2 and Figure 1].
Figure 1.
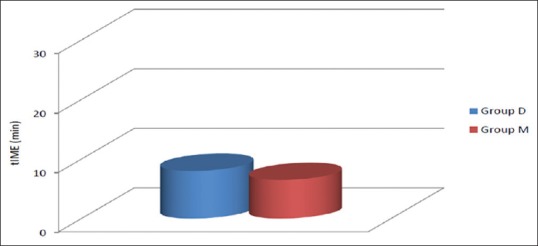
Onset of sensory block (mean ± standard deviation).
Table 2.
Block characteristics
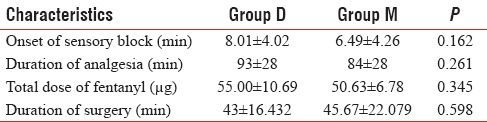
The mean of total dose of fentanyl given in Group D was 55 ± 10.69 µg and 50.00 ± 6.78 µg in Group M [Figure 2]. Difference was statistically insignificant [Figure 2]. The mean duration of analgesia was 93 ± 28 min in Group D and 84 ± 28 min in Group M. Difference in duration was insignificant [Table 2 and Figure 2].
Figure 2.
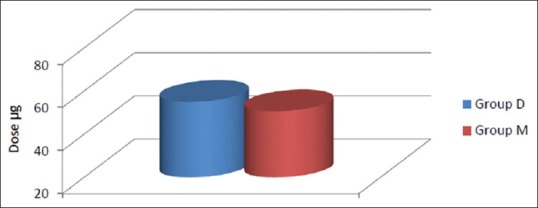
Mean dose of fentanyl required.
Comparison of hemodynamics and sedation
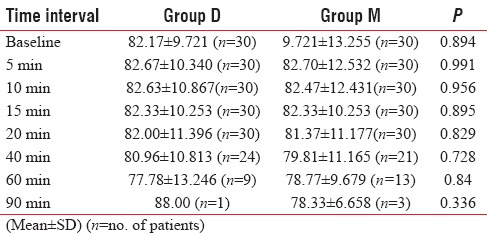
Patients in both groups were similar with regard to heart rate at various time intervals [Table 3].
Table 3.
Comparison of heart rate
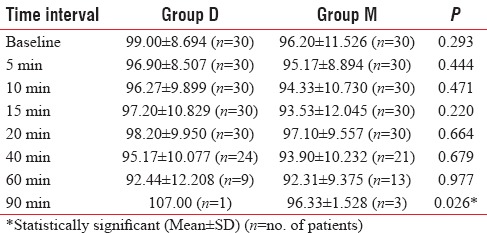
With regard to mean arterial pressure, there were no statistically significant differences between the two groups except at 90 min time point, where it was higher in Group D (P = 0.023) [Table 4] which can be explained by weaning off of dexmedetomidine effect.
Table 4.
Comparison of mean arterial pressure
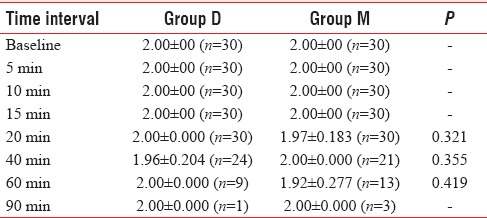
With regard to sedation score, there were no statistically significant differences between the two groups [Table 5].
Table 5.
Comparison of sedation score
The difference in oxygen saturation between two groups at all time points was statistically insignificant.
DISCUSSION
IVRA technique is widely used for upper limb surgeries. The advantages of IVRA are high indices of reliability, rapid onset of analgesia, and good muscle relaxation. The main limitations of IVRA are tourniquet pain, limited duration of surgery, and absence of postoperative analgesia.
In several studies, it was tried to find a local anesthetic mixture that allows relief from tourniquet pain and prolonged duration of analgesia after tourniquet release. NSAIDs (ketorolac, ibuprofen, dexketoprofen, paracetamol, lornoxicam), opioids (morphine, fentanyl, tramadol, and pentazocine), and the combination of opioid and muscle relaxant (atracurium, mivacurium, pancuronium) have been studied without demonstrating clear advantage.[2]
Holmes, Janardhan, and Venkata Rao had advocated the use of double tourniquet method with the second tourniquet on the anesthetized portion on the extremity distal to the proximal one to prevent tourniquet pain and discomfort.[1,5] Hence, in the present study, double tourniquet was used.
In this study, mean time of onset of sensory block was 8.01 ± 4.02 min in Group D and 6.49 ± 4.26 min in Group M, which was statistically insignificant.
According to Tahawy et al., the mean time of onset of sensory block was 2.93 ± 0.86 min in dexmedetomidine group, who received 20 ml of 1% lignocaine with 0.5 µg/kg dexmedetomidine diluted with NS to make total volume of 40 ml and was 3.07 ± 0.86 min in magnesium sulfate group, who received 20 ml of 1% lignocaine with 5 ml of 20% magnesium sulfate diluted with 15 ml NS to make total volume of 40 ml. The difference between two groups regarding mean time of onset of sensory block was statistically insignificant (P = 0.554). They observed that mean time of onset of sensory block was less in dexmedetomidine group in comparison to magnesium sulfate group. In contrast with present study, mean time of onset of sensory block was delayed in dexmedetomidine group (8.01 ± 4.02 min) as compared to midazolam group (6.49 ± 4.26 min). This may be because of premedication with midazolam 0.5 mg/kg orally in contrast to our study where no premedication was given. Local anesthetic property of midazolam and its synergistic action with lignocaine and dexmedetomidine can explain the early onset of sensory block in their study. They concluded that dexmedetomidine was superior to magnesium sulfate as an adjuvant regarding tourniquet pain and hemodynamic stability. Hence, the findings of our study are in accordance with observations made by Tahawy et al. regarding hemodynamic stability in Group D.[6]
According to Kumar et al., the mean time of onset of sensory block was 6.5 ± 0.88 min in Group L (control group), who received 10 ml of 2% lignocaine diluted with NS to make a total volume of 20 ml and was 1.15 ± 0.50 min in Group LK, who received 0.5 mg/kg ketamine along with 2% 10 ml of lignocaine diluted with NS to make total volume 20 ml and was 4.30 ± 0.60 min in Group LD, who received 1 µg/kg dexmedetomidine along with 2% 10 ml of lignocaine diluted with NS to make total volume 20 ml. Differences between LK and LD were statistically significant. That means onset was delayed in Group LD (4.30 ± 0.60 min). In contrast to the present study, it was 8.01 ± 4.02 min in dexmedetomidine group, which can be explained by the concentration of lignocaine. Kumar et al. used 1% in contrast to our study, it was 0.5% lignocaine. Similarly, in this study, results were in favor of observations made by Kumar et al. that addition of dexmedetomidine as an adjunct in IVRA improves the quality of anesthesia and perioperative analgesia without causing side effects.[7]
Abosedira compared clonidine and dexmedetomidine in IVRA, and he concluded that significantly lesser number of patients required reduced amount of analgesics (fentanyl) in dexmedetomidine group.[8]
Kashefi et al. and Honarmand et al. concluded that midazolam (50 µg/kg) when added to lignocaine for IVRA shortens the onset of sensory and motor block and improves the quality of anesthesia and postoperative analgesia.[9,10]
One study by Memis et al. was conducted in 2004 on dexmedetomidine as an adjuvant to lignocaine in IVRA, which was having level of evidence II for improving the quality of anesthesia in IVRA.[11] Various adjuvants had been added from time to time to local anesthetics to prolong the period of analgesia and to improve the quality of anesthesia, but no study had been done for comparison of dexmedetomidine and midazolam when added to lignocaine in IVRA. Hence, it is worthwhile to compare the dexmedetomidine and midazolam when added lignocaine in IVRA.
CONCLUSION
This study showed that effects of dexmedetomidine (1 µg/kg) and midazolam (50 µg/kg) as adjuncts in lignocaine (0.5%) IVRA were comparable statistically for the onset of sensory block and duration of analgesia. However, the onset of sensory block was earlier with midazolam (6.49 ± 4.26 min vs. 8.01 ± 4.02 min), and duration of analgesia was longer with dexmedetomidine (93 ± 28 min vs. 84 ± 28 min). Thus, we concluded that both drugs had a similar duration of analgesia and are equally good for IVRA without any respiratory and cardiovascular side effects.
Strength
IVRA is a simple, reliable, noninvasive method for the upper limb ambulatory procedures. Using IVRA with dexmedetomidine or midazolam to lignocaine, we can improve the quality of block, and this can be the ideal technique for emergency upper limb procedures where risk of general anesthesia is high or facilities for ultrasound-guided brachial plexus block or experienced person for giving blind upper limb block may not be available.
Limitations
The quality of anesthesia assessment by patient and surgeons could have been added for the quality of block. The main limitation was lack of postoperative analgesia and limited duration of anesthesia. We compared two drugs as adjuvants to lignocaine for IVRA without taking control patient population of plain lignocaine for IVRA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Holmes CM. Intravenous regional analgesia. A useful method of producing analgesia of the limbs. Lancet. 1963;1:245–7. doi: 10.1016/s0140-6736(63)90954-1. [DOI] [PubMed] [Google Scholar]
- 2.Choyce A, Peng P. A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth. 2002;49:32–45. doi: 10.1007/BF03020416. [DOI] [PubMed] [Google Scholar]
- 3.Mizrak A, Gul R, Erkutlu I, Alptekin M, Oner U. Premedication with dexmedetomidine alone or together with 0.5% lidocaine for IVRA. J Surg Res. 2010;164:242–7. doi: 10.1016/j.jss.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Pieri L. Preclinical pharmacology of midazolam. Br J Clin Pharmacol. 1983;16(Suppl 1):17S–27S. doi: 10.1111/j.1365-2125.1983.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janardhan TN, Venkata Rao M. Intravenous regional analgesia – A clinical study. Indian J Anaesth. 1969;17:230–8. [Google Scholar]
- 6.Tahawy ME, Shaaban AR, Ahmad A. Comparison between dexmedetomidine and magnesium sulfate as adjuvants for intravenous regional anesthesia. Ain Shams J Anaesthesiol. 2015;8:129–33. [Google Scholar]
- 7.Kumar A, Sharma D, Datta B. Addition of ketamine or dexmedetomidine to lignocaine in intravenous regional anesthesia: A randomized controlled study. J Anaesthesiol Clin Pharmacol. 2012;28:501–4. doi: 10.4103/0970-9185.101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abosedira MA. Adding clonidine or dexmedetomidine to lidocaine during Bier's block. J Med Sci. 2008;7:660–4. [Google Scholar]
- 9.Kashefi P, Montazeri K, Honarmand A, Safavi M, Hosseini HM. The analgesic effect of midazolam when added to lidocaine for intravenous regional anaesthesia. J Res Med Sci. 2011;16:1139–48. [PMC free article] [PubMed] [Google Scholar]
- 10.Honarmand A, Safavi M, Nemati K, Oghab P. The efficacy of different doses of Midazolam added to Lidocaine for upper extremity Bier block on the sensory and motor block characteristics and postoperative pain. J Res Pharm Pract. 2015;4:160–6. doi: 10.4103/2279-042X.162359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Memis D, Turan A, Karamanlioglu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98:835–40. doi: 10.1213/01.ane.0000100680.77978.66. [DOI] [PubMed] [Google Scholar]


