Abstract
Ultrasound-guided regional anesthesia (UGRA), like other basic skills, should be learnt in a simulation laboratory before performing on the patient. Cadavers provide an ideal tool for learning sonoanatomy and skills required for performing UGRA. On the basis of preservation technique used, the cadavers can be formalin embalmed cadavers, Thiel cadavers (soft cadavers), and fresh frozen cadavers. We compared three types of cadavers for performing ultrasound-guided upper and lower limb blocks. We observed that fresh frozen and Thiel cadavers were less smelling and had more realistic appearance as compared to formalin embalmed cadavers. It was seen that Thiel cadavers were more flexible and hence, rotation of neck, shoulder and knee was easier. Although images seen in most cadavers were comparable with live subjects but, Thiel cadavers provided more realistic model.
Keywords: Cadaver, Thiel cadaver, ultrasound-guided regional anesthesia
INTRODUCTION
In the field of anesthesia, simulation-based training is used for airway management, regional anesthesia, perioperative medicine, and crisis resource management.[1] Cadavers provide an excellent opportunity for learning ultrasound-guided regional anesthesia (UGRA) without fear of harming the patient or wasting OT time. Major societies of regional anesthesia (American Society of Regional Anesthesia and Pain Medicine and European Society of Regional Anaesthesia and Pain Therapy) conduct cadaver-based seminars and workshops annually.[2] Traditionally, formalin embalmed and fresh cadavers were used for teaching UGRA. The development of Thiel cadavers using a preservation technique that retains full flexibility of limbs has improvised the training. There is literature available on the use of Thiel cadavers for learning UGRA but the technique is still not developed in India.[3,4,5,6,7,8] Recently, cadaver training and research facility was setup in our center and one of our co-authors (SL) gained experience in preparation of Thiel cadaver.
MATERIALS AND METHODS
We decided to compare different types of cadavers (four formalin, four fresh frozen, and one Thiel cadaver) for ultrasound-guided upper and lower limb blocks [Table 1]. All the cadavers had body mass index (BMI) <30 and were between 25 and 50 years of age. We compared the cadavers for the performance of UGRA (visibility of muscles, vessels, nerves), needling and tactile feedback. Neck, chest and axilla were scanned for interscalene, supraclavicular, infraclavicular, and axillary approach to brachial plexus. In the lower limb, we scanned inguinal region for femoral nerve and sciatic nerve was scanned in gluteal and popliteal region. Scanning of all nerves was performed using 5–10 MHz linear probe except sciatic nerve in gluteal region for which we used 2–5 MHz curvilinear probe. Transparent dressing (Tegaderm) was used to cover footprint of the probe. We used 100 mm, 22-gauge insulated nerve block needles (Stimuplex A, B. Braun, Melsungen AG, Germany) for the blocks.
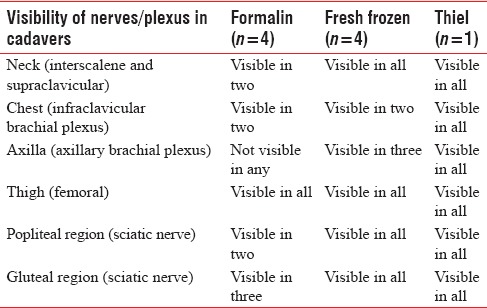
Table 1.
Summary of findings
RESULTS
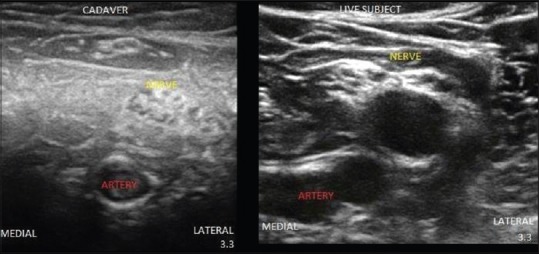
All the cadavers had comparable BMI and were in the same age groups. We found that formalin embalmed cadavers were rigid with stiff joints and had an unpleasant odor, fresh frozen cadavers were less rigid, and Thiel cadavers were the most flexible. Above the clavicle, the muscles (sternocleidomastoid and scalene muscles), vessels (internal jugular vein, common carotid artery) and plexus could be identified in most of the cadavers. The plexus could not be visualized in the neck region in two formalin cadavers [Figures 1 and 2]. Below the clavicle, the muscles (pectoralis major and minor) and subclavian vessels could be differentiated in all the cadavers, but plexus was not visible in two formalin and two fresh-frozen cadavers. Scanning was difficult in the axillary region in formalin cadavers and one fresh frozen cadavers. In the lower limb, femoral nerve, and vessels were visualized in all the cadavers [Figure 3]. Sciatic nerve was not visualized in popliteal and gluteal region in some formalin embalmed cadavers [Figures 4 and 5]. Needle visibility was comparable with live subjects in all the cadavers. Tissue planes and resistance could be appreciated in fresh frozen and Thiel cadavers.
Figure 1.
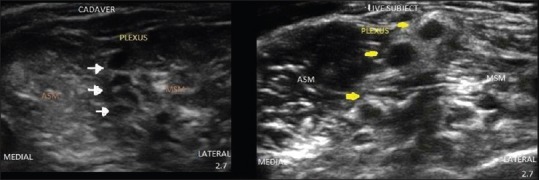
Ultrasound images of brachial plexus from scanning in interscalene region.
Figure 2.
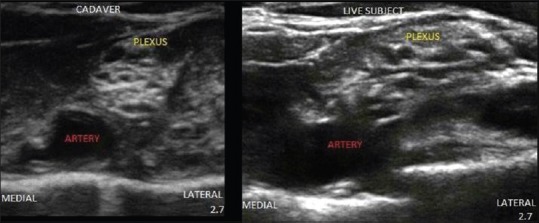
Ultrasound image of brachial plexus from scanning in supraclavicular region.
Figure 3.
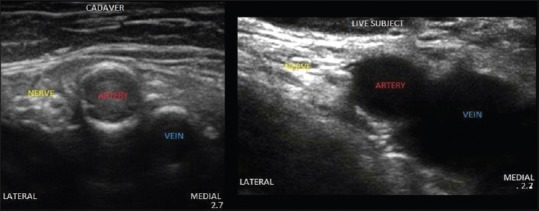
Ultrasound image from scanning of femoral nerve in inguinal region.
Figure 4.
Ultrasound image of sciatic nerve scanned in popliteal region.
Figure 5.
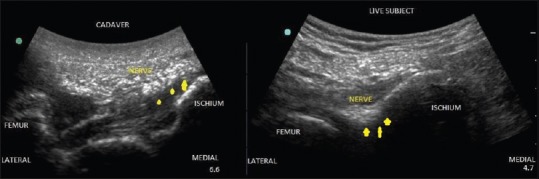
Ultrasound image of sciatic nerve captured in gluteal region.
DISCUSSION
The application of ultrasound has increased exponentionally over the last few decades. There is an ongoing effort to choose the best model available for learning UGRA. Simulator for learning UGRA include phantoms, cadavers, animal models, computer-based methods, and virtual reality models.[9] However, there is insufficient evidence regarding task fidelity, skill transfer, validity, and reliability for most of these tools.[10,11] Nonmeat phantoms have low background echogenicity which enhances the needle visibility. This can lead to false confidence with regard to clinical ability. On the other hand, meat phantoms have excellent feasibility, are cheap, can be constructed with minimal preparation and can be disposed off easily. Chuan et al. observed that meat models can be used to learn UGRA skills as adequately as a fresh frozen cadaver.[12] Animal models can be useful adjuncts, but they lack physical reality of actual human patient and have a limited lifespan (1–2 days).[13] Computer-based training imparts knowledge but is insufficient to improve the procedural skills.[14] Synthetic (bench) models are also available but these are expensive and dedicated faculty is required so they are within easy reach of many trainees.[10] The qualities of a simulator required for learning UGRA include visualization of anatomic structures, realistic feeling of needle progression, cost, and safety in terms of biohazards.[6,15] Cadavers can be used for learning sonoanatomy and practice skills such as hand-eye coordination, probe handling on irregular surface (like supraclavicular region) and probe needle alignment.[3] Due to the lack of blood flow and tissue movement, the cadavers provide optimal ultrasound image. The release of intracellular fluids from the dead cells increases the extracellular fluid around the needle. This watery background decreases echogenicity-enhancing the needle visibility.[9]
Cadavers differ in several aspects such as color, flexibility, quality of preserved tissue, cost, and storage on the basis of embalming method.[16] It is important to understand the strong and weak points of a cadaver to determine which preservation method is best suitable for a procedure. Depending on the preservation technique used, there are three types of cadavers. Traditionally, formalin embalmed cadavers have been used for learning various surgical skills. These cadavers are prepared using a solution of 4% formaldehyde, 95% ethanol, glycerol, phenol, and water perfused through femoral artery under pressure.[17] Formaldehyde is a strong tissue fixative with excellent antiseptic properties preventing the decay of cadavers. These cadavers are inexpensive and can be used for a long period. However, formaldehyde fixation causes rigidity, loss of tissue texture, consistency, surgical planes and has an unpleasant odor.[18,19] This led to a search for embalming fluids that contained little or no formalin. Fresh frozen cadavers were developed by keeping the cadavers in deep freezing refrigerator at −20°C for 3–4 days after serologic testing for infectious diseases (HIV, hepatitis B and C). During thawing process, the cadavers are left in the room temperature (18–20°C during the day and refrigerated in the night. This smooth gradient of temperature gain leads to reduced rigidity and realistic properties. These cadavers retain tissue consistency and offer a wide range of motion of joints.[20] However, these can be used for a very short period due to putrefaction. There is also a risk of exposure to microbiological agents such as viruses and bacteria.[6] To overcome these problems, soft embalmed Thiel cadaver was developed by Professor Thiel of University of Graz in 1992 using organic and inorganic compounds. The embalming mixture contains salts for fixation, boric acid for disinfection, morpholine as color preservative and minimal formaldehyde and chlorocresol.[21] The solution is infused through an artery and a vein and the cadaver is submerged in Thiel solution for 4–6 months. The embalming fluids used provide long lasting tissue preservation. These cadavers can be kept in plastic bags without refrigeration. Color, suppleness of skin, joint flexibility, and fascial integrity of the cadavers is retained. These have been used to simulate laparoscopic surgery, neurosurgery, and oral surgery.[22,23,24] In Thiel cadavers, due to fluid in tissues echogenicity of muscles increases which enhances the sonographic contrast between muscles and nerves.[21] This technique offers high standard of preservation without releasing harmful chemicals in the environment.
In our study, Inability to visualize the plexus in neck and axillary region in formalin embalmed cadavers could be due to difficulty in rotating the neck and shoulder as a result of rigidity. Rigidity depends on timing of embalming the cadaver and position of the cadaver. Tsui et al. described upper and lower limb blocks on formalin embalmed cadavers.[17,25] They concluded that imaging might not be ideal in all the cadavers but most images can be interpreted to some extent and can be used for training purpose. They also suggested that some block locations like axillary and subgluteal sciatic do not possess ideal imaging characteristics which we also encountered.[25] In this study, interindividual difference of nerves and landmark visibility could explain the inability to visualize the plexus in some cadavers despite similar embalming procedure and BMI. Age and BMI of the cadaver also influence the quality of imaging due to poor reflection from tissues in the elderly.[26] Hocking and McIntyre used unembalmed cadavers for teaching UGRA and observed that these cadavers offer realistic imaging and provide good tactile feedback.[20] In 2010, McLeod et al. used Thiel cadavers for UGRA and they found that intraneural injection and fluid around the nerves could be easily visualized in these cadavers.[3] Benkhadra et al. compared fresh cadavers and Thiel cadavers for UGRA in cervical region and concluded that Thiel cadavers offer safer and more realistic conditions than fresh cadavers.[6] Munirama et al. used sonoelastography to study the interstitial, intra-arterial, and intraneural spread of local anesthetics in Thiel cadavers and found that these can be used for objective assessment of the learning.[8,27] These cadavers can also be used for testing new technology and developing new techniques in UGRA.[5]
The drawbacks associated with the use of cadavers for UGRA include an inability to use nerve stimulator and lack of vascular anatomy. To overcome this problem, using a filler in the vascular system (dyed gelatin suspension (arterial filler), colored latex) or pneumatic devices have been tried to restore the appearance of vascular anatomy with good results.[21] Another deterrent is the preparation, preservation, and storage of cadavers which might not be available in all medical schools. For Thiel cadavers, expertise, and cost are another issue, but this is offset by excellent and repeatable conditions provided for simulation of UGRA.[21]
CONCLUSION
To summarize, cadaver is a promising tool which can be used to learn sonoanatomy and perform UGRA. All types of cadavers can be used, but Thiel embalmed cadavers provide optimal conditions for learning these skills.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lorello GR, Cook DA, Johnson RL, Brydges R. Simulation-based training in anaesthesiology: A systematic review and meta-analysis. Br J Anaesth. 2014;112:231–45. doi: 10.1093/bja/aet414. [DOI] [PubMed] [Google Scholar]
- 2.Sites BD, Chan VW, Neal JM, Weller R, Grau T, Koscielniak-Nielsen ZJ, et al. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anaesthesia and Pain Therapy Joint Committee recommendations for education and training in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2009;34:40–6. doi: 10.1097/AAP.0b013e3181926779. [DOI] [PubMed] [Google Scholar]
- 3.McLeod G, Eisma R, Schwab A, Corner G, Soames R, Cochran S. An evaluation of Thiel-embalmed cadavers for ultrasound-based regional anaesthesia training and research. Ultrasound. 2010;18:125–9. [Google Scholar]
- 4.Munirama S, McLeod GA, Eisma R, Schwab A, Corner G, Soames R, et al. Application of sonoelastography to regional anaesthesia: A descriptive study with the Thiel embalmed cadaver model. Ultrasound. 2012;20:41–8. [Google Scholar]
- 5.Munirama S, Joy J, Columb M, Habershaw R, Eisma R, Corner G, et al. A randomised, single-blind technical study comparing the ultrasonic visibility of smooth-surfaced and textured needles in a soft embalmed cadaver model. Anaesthesia. 2015;70:537–42. doi: 10.1111/anae.12925. [DOI] [PubMed] [Google Scholar]
- 6.Benkhadra M, Faust A, Ladoire S, Trost O, Trouilloud P, Girard C, et al. Comparison of fresh and Thiel's embalmed cadavers according to the suitability for ultrasound-guided regional anesthesia of the cervical region. Surg Radiol Anat. 2009;31:531–5. doi: 10.1007/s00276-009-0477-z. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Schwab A, McLeod G, Corner G, Cochran S, Eisma R, et al. Echogenic regional anaesthesia needles: A comparison study in Thiel cadavers. Ultrasound Med Biol. 2012;38:702–7. doi: 10.1016/j.ultrasmedbio.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Munirama S, Eisma R, Columb M, Corner GA, McLeod GA. Physical properties and functional alignment of soft-embalmed Thiel human cadaver when used as a simulator for ultrasound-guided regional anaesthesia. Br J Anaesth. 2016;116:699–707. doi: 10.1093/bja/aev548. [DOI] [PubMed] [Google Scholar]
- 9.Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;36:162–70. doi: 10.1097/aap.0b013e31820d4207. [DOI] [PubMed] [Google Scholar]
- 10.Cosman P, Hemli JM, Ellis AM, Hugh TJ. Learning the surgical craft: A review of skills training options. ANZ J Surg. 2007;77:838–45. doi: 10.1111/j.1445-2197.2007.04254.x. [DOI] [PubMed] [Google Scholar]
- 11.Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaesthesia. Anaesth Intensive Care. 2009;37:903–10. doi: 10.1177/0310057X0903700605. [DOI] [PubMed] [Google Scholar]
- 12.Chuan A, Lim YC, Aneja H, Duce NA, Appleyard R, Forrest K, et al. A randomised controlled trial comparing meat-based with human cadaveric models for teaching ultrasound-guided regional anaesthesia. Anaesthesia. 2016 Mar 19; doi: 10.1111/anae.13446. Doi: 10.1111/anae.13446. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Tabas JA, Rosenson J, Price DD, Rohde D, Baird CH, Dhillon N. A comprehensive, unembalmed cadaver-based course in advanced emergency procedures for medical students. Acad Emerg Med. 2005;12:782–5. doi: 10.1197/j.aem.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Woodworth GE, Chen EM, Horn JL, Aziz MF. Efficacy of computer-based video and simulation in ultrasound-guided regional anesthesia training. J Clin Anesth. 2014;26:212–21. doi: 10.1016/j.jclinane.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Bröking K, Waurick R. How to teach regional anesthesia. Curr Opin Anaesthesiol. 2006;19:526–30. doi: 10.1097/01.aco.0000245279.22658.57. [DOI] [PubMed] [Google Scholar]
- 16.Eisma R, Mahendran S, Majumdar S, Smith D, Soames RW. A comparison of Thiel and formalin embalmed cadavers for thyroid surgery training. Surgeon. 2011;9:142–6. doi: 10.1016/j.surge.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Tsui BC, Dillane D, Walji AH. Cadaveric ultrasound imaging for training in ultrasound-guided peripheral nerve blocks: Upper extremity. Can J Anaesth. 2007;54:392–6. doi: 10.1007/BF03022663. [DOI] [PubMed] [Google Scholar]
- 18.Lewis CE, Peacock WJ, Tillou A, Hines OJ, Hiatt JR. A novel cadaver-based educational program in general surgery training. J Surg Educ. 2012;69:693–8. doi: 10.1016/j.jsurg.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Brenner E. Human body preservation-old and new techniques. J Anat. 2014;224:316–44. doi: 10.1111/joa.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hocking G, McIntyre O. Achieving change in practice by using unembalmed cadavers to teach ultrasound-guided regional anaesthesia. Ultrasound. 2011;19:31–5. [Google Scholar]
- 21.Eisma R, Lamb C, Soames RW. From formalin to Thiel embalming: What changes? One anatomy department's experiences. Clin Anat. 2013;26:564–71. doi: 10.1002/ca.22222. [DOI] [PubMed] [Google Scholar]
- 22.Bregy A, Alfieri A, Demertzis S, Mordasini P, Jetzer AK, Kuhlen D, et al. Automated end-to-side anastomosis to the middle cerebral artery: A feasibility study. J Neurosurg. 2008;108:567–74. doi: 10.3171/JNS/2008/108/3/0567. [DOI] [PubMed] [Google Scholar]
- 23.Hölzle F, Franz EP, Lehmbrock J, Weihe S, Teistra C, Deppe H, et al. Thiel embalming technique: A valuable method for teaching oral surgery and implantology. Clin Implant Dent Relat Res. 2012;14:121–6. doi: 10.1111/j.1708-8208.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- 24.Giger U, Frésard I, Häfliger A, Bergmann M, Krähenbühl L. Laparoscopic training on Thiel human cadavers: A model to teach advanced laparoscopic procedures. Surg Endosc. 2008;22:901–6. doi: 10.1007/s00464-007-9502-7. [DOI] [PubMed] [Google Scholar]
- 25.Tsui BC, Dillane D, Pillay J, Ramji AK, Walji AH. Cadaveric ultrasound imaging for training in ultrasound-guided peripheral nerve blocks: Lower extremity. Can J Anaesth. 2007;54:475–80. doi: 10.1007/BF03022035. [DOI] [PubMed] [Google Scholar]
- 26.Kessler J, Moriggl B, Grau T. Ultrasound-guided regional anesthesia: Learning with an optimized cadaver model. Surg Radiol Anat. 2014;36:383–92. doi: 10.1007/s00276-013-1188-z. [DOI] [PubMed] [Google Scholar]
- 27.Munirama S, Satapathy AR, Schwab A, Eisma R, Corner GA, Cochran S, et al. Translation of sonoelastography from Thiel cadaver to patients for peripheral nerve blocks. Anaesthesia. 2012;67:721–8. doi: 10.1111/j.1365-2044.2012.07086.x. [DOI] [PubMed] [Google Scholar]


