Abstract
Background:
The quest for an ideal sedative during regional anesthesia is on. Although propofol has been accepted as a sedative intraoperatively, it can be associated with troublesome hemodynamic changes. Dexmedetomidine is a new alpha 2 agonist used widely for sedation.
Aims:
In this study, we tried to compare equivalent doses of dexmedetomidine infusion with propofol with emphasis on their effect on the hemodynamics.
Settings and Design:
Prospective, single-blinded randomized controlled trial.
Materials and Methods:
In a single blinded study, 60 American Society of Anesthesiologists (ASA) I and II patients scheduled for forearm surgeries under brachial plexus block were randomized to receive either propofol (Group I) or dexmedetomidine (Group II) infusion. Ultrasound-guided supraclavicular brachial plexus block was given in all the patients. After confirming adequate motor and sensory blockade, they were administered an initial loading dose of the drug over 10 min followed by a maintenance dose till the end of the surgery. The rate of infusion was titrated to maintain Ramsay sedation score of 2–4. Intraoperative hemodynamic and respiratory effects were documented along with surgeon and patient satisfaction. Any adverse effect such as hypotension, bradycardia, nausea, and vomiting was also noted.
Statistical Analysis Used:
The data collected were evaluated using Stata version 10. P < 0.05 was considered statistically significant.
Results:
Heart rate decreased significantly in Group II (dexmedetomidine) while mean arterial pressure decreased significantly in Group I (propofol). There was no increase in the incidence of bradycardia or hypotension in either groups. Patient satisfaction score was significantly greater in Group II (dexmedetomidine) while surgeon satisfaction score was similar in both the groups.
Conclusion:
Dexmedetomidine at equivalent doses of propofol has a similar hemodynamic and respiratory effect, similar surgeon's satisfaction score, higher patient's satisfaction score, and no significant side effects in ASA I/II patients. Thus, dexmedetomidine may prove to be a valuable alternative to propofol for sedation in patients undergoing upper limb surgeries in brachial plexus block.
Keywords: Blood pressure, brachial plexus block, dexmedetomidine, heart rate, propofol, sedation
INTRODUCTION
Regional anesthesia offers several benefits over general anesthesia both from the patient's and anesthesiologist's point of view. These include an awake patient with patent airway reflexes and stable cardiovascular and respiratory parameters.[1,2] Managing an awake patient's stress and anxiety related to the surgery and unknown surrounding remains a challenge.[3] Various drugs and techniques have been used for this purpose. Propofol has been the gold standard of intraoperative sedation due to its rapid onset and offset of sedation and easy titratability.[4,5] The major disadvantage of propofol is its depressant action on the patient's hemodynamics and respiration.[6] Dexmedetomidine is a selective alpha 2 agonist with many desirable properties such as sedation, hypnosis, analgesia, anxiolysis with rapid onset–offset of sedation, and minimal effect on respiration.[7] Despite being used as a sedative widely, it is not free of adverse effects. Hypotension and bradycardia are the two commonly seen adverse effects.[8] Although there have been studies comparing both these drugs for Intensive Care Unit sedation, there are few studies comparing them in brachial plexus blocks.[9,10] In addition, there are no studies comparing them in the American Society of Anesthesiologists (ASA) I/II patients undergoing surgeries in brachial plexus block. Hence, in this study, we aimed to compare both these drugs with emphasis on their effect on the hemodynamic and respiratory profile.
MATERIALS AND METHODS
After approval from Institutional Ethics Committee, sixty ASA I and II patients between the age group 18–50 years were recruited for this randomized, single-blind, prospective study done over a period of 1 year. Inclusion criteria included ASA health status classes I and II patients undergoing forearm fixation surgeries under supraclavicular brachial plexus blocks. Patients with a history of chronic systemic disease, drug or alcohol abuse, allergy to any of the study medications, second- or third-degree heart block, and bleeding disorders were excluded from this study. The sample size was calculated as 30 in each group. Randomization was done using computer-generated random number table. Sequence allocation was done using sealed, opaque envelopes that were opened just before the procedure by the floor nurse. The patients were allocated into either of the 2 groups: Group I patients received propofol infusion (loading dose 75 µg/kg over 10 min followed by 12.5–75 µg/kg/min). Fifty milliliters 1% propofol was loaded undiluted in a 50 ml syringe. Patients in Group II received dexmedetomidine infusion (loading 1 µg/kg over 10 min followed by 0.2–0.6 µg/kg/h). Two milliliters dexmedetomidine (200 µg) was diluted to 50 ml with 0.9% normal saline in a 50 ml syringe.
On arrival in the operating theater (OT) holding area, 18 gauge intravenous cannula was secured in the dorsum of the contralateral hand. Preloading with 15 ml/kg ringers lactate was done over half an hour. On shifting to OT, standard monitors including pulse oximeter, noninvasive blood pressure, electrocardiography, and end-tidal carbon dioxide were connected. Nasal prong was inserted, and supplemental oxygen was administered at 2 L/min throughout the procedure. After recording baseline vitals, ultrasound-guided supraclavicular brachial plexus block was given using 20 cc of 0.5% bupivicaine. Sensory blockade was complete when there was complete loss to pinprick sensation along the distribution of radial, ulnar, and median nerve. Motor blockade was attained with the complete loss of finger movements. After confirming adequate sensory and motor blockade, drug infusion was started according to the group allocated A screen was applied, and the patient was unaware of the infusion being administered. Parameters including mean arterial pressure (MAP), heart rate (HR) and respiratory rate (RR), and peripheral oxygen saturation (SpO2) were recorded every 5 minutes until the termination of the surgery.
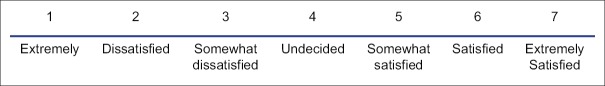
Intraoperatively, target sedation Ramsay sedation score (RSS) 2–4 was maintained by titrating the dose of the infusion (1 = awake, agitated, 2 = awake, cooperative, 3 = sleeping, responds to commands, 4 = sleeping, responds to loud verbal commands, 5 = sluggish response to auditory or light glabellar touch, and 6 = deeply sedated, no response) Adverse events including bradypnea (RR <10/min), desaturation (SpO2 <92%), bradycardia (HR <45 b.p.m), and hypotension (MAP <50 mm Hg) were recorded and treated with 4 L/min of oxygen via a nasal cannula, intravenous 0.5-mg atropine, and intravenous 10 ml/kg 0.9% saline, respectively. Pain on injection, dry mouth, nausea, and any event of hypertension (>20% increase in MAP) was also recorded. The surgeons were asked to rate their satisfaction immediately after the surgery using a 7-point likert-like verbal rating scale[6] [Figure 1]. Patients rated their satisfaction 10 h after the surgery using the same scale.
Figure 1.
Likert scale.
A pilot study had been done earlier to determine the sample size of this study. The 10 case pilot study showed that the mean MAP in Group I (propofol) was 86.37 ± 6.33 mm Hg and Group II (dexmedetomidine) was 80.42 ± 6.36 mm Hg. Taking 6 mm difference of MAP as clinically relevant, the sample size estimated at 95% power and 5% level of significance was 29 in each group. Hence, we took a sample size of 30 in each group.
Statistical analysis
Statistical analysis was carried out using Stata Version 10 (Stata Corp, Houston, Texas, USA). Baseline continuous variables of two groups were compared using t-test for assuming equal variance. Repeated measures analysis of variance was used to compare continuous variables. Post hoc testing was performed with Scheffé's F-test. P < 0.05 was considered statistically significant.
RESULTS
Three patients were excluded from the study due to inadequate block. General anesthesia had to be administered in these three patients. The two groups were comparable with respect to the following variables: age, sex, weight, ASA health status, and duration of surgery [Table 1].
Table 1.
Demography and other characteristics
Mean arterial pressure
There was no significant difference in the mean MAP of both the groups. The basal MAP in Group I (propofol) was 86.37 ± 6.33 mm Hg which reduced to 81.23 ± 5.98 mm Hg at the end of the surgery. This was statistically significant (P = 0.020). The mean MAP in Group II (dexmedetomidine) was 83.42 ± 6.25 mm Hg which remained somewhat constant till the end of surgery (83.58 ± 5.89 mm Hg) [Figure 2].
Figure 2.
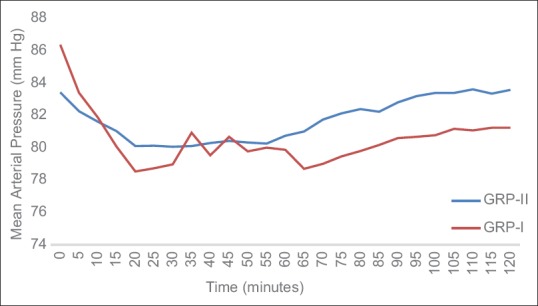
Mean arterial pressure in both groups.
Heart rate
The baseline HR in Group II (dexmedetomidine) was 81.9 ± 12.46 beats/min while that of Group I (propofol) was 83.8 ± 12.93 beats/min. The mean HR in Group II (dexmedetomidine) gradually decreased to 74.5 ± 12.27 beats/min while that in Group I (propofol) remained 83.6 ± 13.90 beats/min. The decrease in HR in Group II (dexmedetomidine) was statistically significant [Figure 3].
Figure 3.
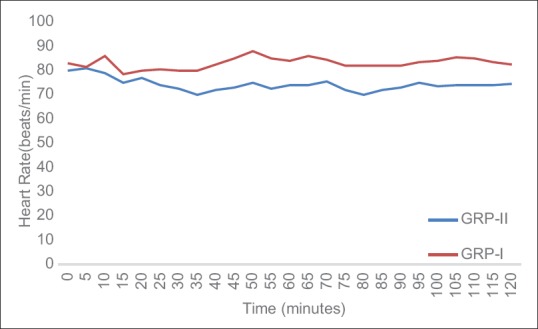
Trend of heart rate in both groups.
Peripheral oxygen saturation
There was no difference in peripheral SpO2 levels in both the groups in the intraoperative period. The SpaO2 values in all the patients in both the groups were above 92%.
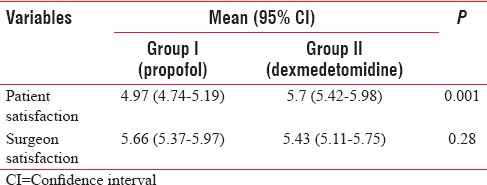
Patient and surgeon satisfaction
The mean level of patient's satisfaction score regarding the anesthetic technique was statistically higher in Group II (dexmedetomidine) when compared to Group I (P < 0.001). There was no difference in the mean values of satisfaction scores among surgeons in either of the groups [Table 2].
Table 2.
Satisfaction in both groups
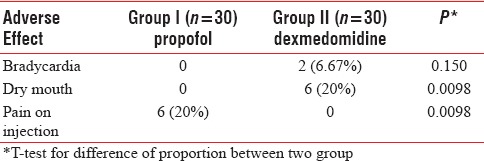
Adverse effects
There was no incidence of vomiting, hypotension, hypertension, or respiratory depression in both the groups. Bradycardia occurred in two patients in Group II while compared to none in Group I. This was not statistically significant. Six patients in Group II (dexmedetomidine) had dry mouth when compared to none patient in Group I and this difference was statistically significant. Pain on injection was complained by six patients in Group I (propofol) and none in Group II and this difference was statistically significant [Table 3].
Table 3.
Comparison of adverse effect in two groups
DISCUSSION
Our study shows that dexmedetomidine can be used as a useful alternative to propofol as an intraoperative sedative in forearm fixation surgeries under brachial plexus block. It provides good sedation with little hemodynamic compromise and more patient satisfaction.
Alpha 2 adrenoceptor agonists are being increasingly used in anesthesia and critical care due to their sedative, analgesic, and sympatholytic effects.[11] The unique feature of dexmedetomidine is deep sedation without respiratory compromise.[12,13] Satisfactory results have been found when dexmedetomidine has been used for sedation during regional and local anesthesia in all age groups.[12,13] Doses of both the drugs in this study were selected based on earlier studies on their sedative effects during regional anesthesia.[5,14,15]
The adverse effects of dexmedetomidine include hypotension and bradycardia. Alpha 2 agonists cause a temporary increase in blood pressure due to the activation of postjunctional alpha 2 receptors in the vessels. This is followed by decrease in the sympathetic outflow decreasing the blood pressure and HR both.[16] There are studies which have utilized dexmedetomidine for hypotensive anesthesia during maxillofacial, middle ear, and nasal surgeries.[17] The incidence of hypotension has been reported to be more after the loading dose. In fact, few authors have recommended the omission of the loading dose.[18] In our study, we used the loading dose followed by maintenance dose with no significant hypotension. This could be due to preloading and inclusion of only ASA I and II patients. These findings are similar studies done by Yektas et al. and Shah et al. where there was no hemodynamic compromise despite using a loading dose.[19,20] Dinardo observed in their study that multiple bolus doses and less age are strong predictors of occurrence of the initial hypertension.[21] We did not observe hypertension in our patients. This could be due to a single bolus dose used and the range of patients’ age (18–50 years). Patients who were administered propofol experienced a decrease in MAP. The fall in MAP could be due to the powerful inhibitory effect on the sympathetic outflow propofol has.[20]
HR was significantly lesser in Group II (dexmedetomidine) when compared to Group I. This is due to the sympatholytic and vagal mimetic effect of dexmedetomidine. The results are similar to the studies done by Dinardo, Mahmoud et al., and Al-Mustafa et al.[21,22,23]
There are very few studies in which dexmedetomidine has been used for intravenous sedation during brachial plexus block, with only two emphasizing its effects on the hemodynamic profile. In a study done by Bondok et al., the HR and MAP decreased in both the groups with no significant increase in bradycardia or hypotension in either of the groups. This is similar to the results in our study.[8] In a study done by Rutkowska, dexmedetomidine was used as a sedative in patients with end-stage renal disease undergoing surgeries in brachial plexus block.[10] Although there was decrease in HR and MAP, no significant increase in incidence of hypotention and bradycardia was noted. Both of these studies were done in patients with systemic renal or cardiac disease on various medications. Our study included only ASA I/II patients.
Dexmedetomidine has not been associated with respiratory depression despite profound levels of sedation.[23] In our study also, there was preservation of respiratory function with the use of dexmedetomidine. Few reports describe the incidents of respiratory depression during infusions of propofol for sedation.[24] However, in the present study, patients receiving propofol did not have significant respiratory depression. This preservation of respiratory function may be related to the frequent queries by the observers in the intraoperative period to assess the RSS. Each time the patient was evaluated for RSS score, the basal respiratory pattern would be affected.
Another finding in our study was that the surgeon's satisfaction score with patient's sedation was similar for both groups. Patient's satisfaction score with the anesthetic technique was higher in the Group II (dexmedetomidine) when compared to the Group I (propofol). This can be attributed to dexmedetomidine mimicking the natural sleep pathway.[25]
Dry mouth is a known side effect of α 2 agonist.[26] This was also seen in our study where there was significant increase in dry mouth in Group II (dexmedetomidine). There was a significant increase in pain on injection in Group I (propofol). This is attributed to the lipid solvent of propofol which activates the plasma kallekrein– kinin system producing bradykinin.[27]
The strength of the study was the inclusion criteria: ASA I/II patients. The studies done earlier included patients with ischemic heart disease or end-stage renal disease. Hence, the results in such studies might not be generalized to ASA I/II patients.
One of the drawbacks of the study was that RSS was used as a sedation scale. This was due to unavailability of the BIS (bispectral index) monitor in our institute. RSS is a subjective score and requires repeated external stimulus by the observant.[28] BIS provides real-time objective assessment of the patient's sedation.[29] The main aim of our study was to study both these drugs with emphasis on the hemodynamic parameters. We did not study the effect of both these drugs on analgesic requirements or sedation characteristics intraoperatively or postoperatively. This study was a single-blind study not double blind.
CONCLUSION
Dexmedetomidine can be used safely as a sedative in orthopedic forearm surgeries under brachial plexus block without significant compromise on the patients’ hemodynamic and respiratory profile. It has a similar surgeon satisfaction score, a higher patient satisfaction score with insignificant side effects compared to propofol. Thus, dexmedetomidine may prove to be a useful alternative to propofol for sedation in patients undergoing upper limb surgeries under ultrasound-guided supraclavicular brachial plexus block.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Asehnoune K, Albaladejo P, Smail N, Heriche C, Sitbon P, Gueneron JP, et al. Information and anesthesia: What does the patient desire? Ann Fr Anesth Reanim. 2000;19:577–81. doi: 10.1016/s0750-7658(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 2.De Andrés J, Valía JC, Gil A, Bolinches R. Predictors of patient satisfaction with regional anesthesia. Reg Anesth. 1995;20:498–505. [PubMed] [Google Scholar]
- 3.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subrahmanyam M, Sreelakshmi B. Comparison of total intravenous anaesthesia using propofol with or without sufentanil in laparoscopic cholecystectomies. Indian J Anaesth. 2009;53:467–74. [PMC free article] [PubMed] [Google Scholar]
- 5.Ghali A, Mahfouz AK, Ihanamäki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-Tenon's anesthesia. Saudi J Anaesth. 2011;5:36–41. doi: 10.4103/1658-354X.76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khurana P, Agarwal A, Verma R, Gupta P. Comparison of Midazolam and propofol for BIS-guided sedation during regional anaesthesia. Indian J Anaesth. 2009;53:662–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen DA, Daume JT. Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation. Anesth Analg. 2006;103:68–9. doi: 10.1213/01.ane.0000216289.52261.5e. [DOI] [PubMed] [Google Scholar]
- 8.Bondoka RS, El-Shalakany NA. The equisedative effect of dexmedetomidine versus propofol on supraclavicular brachial plexus block and recovery profile in patients with ischemic heart disease. Ain Shams J Anesthesiol. 2014;7:32–9. [Google Scholar]
- 9.Rutkowska K, Knapik P, Misiolek H. The effect of dexmedetomidine sedation on brachial plexus block in patients with end-stage renal disease. Eur J Anaesthesiol. 2009;26:851–5. doi: 10.1097/EJA.0b013e32832a2244. [DOI] [PubMed] [Google Scholar]
- 10.Ok HG, Baek SH, Baik SW, Kim HK, Shin SW, Kim KH. Optimal dose of dexmedetomidine for sedation during spinal anesthesia. Korean J Anesthesiol. 2013;64:426–31. doi: 10.4097/kjae.2013.64.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manne GR, Upadhyay MR, Swadia V. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth. 2014;58:726–31. doi: 10.4103/0019-5049.147164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason KP, Zgleszewski SE, Prescilla R, Fontaine PJ, Zurakowski D. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth. 2008;18:393–402. doi: 10.1111/j.1460-9592.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 14.Parikh DA, Kolli SN, Karnik HS, Lele SS, Tendolkar BA. A prospective randomized double-blind study comparing dexmedetomidine vs. combination of midazolam-fentanyl for tympanoplasty surgery under monitored anesthesia care. J Anaesthesiol Clin Pharmacol. 2013;29:173–8. doi: 10.4103/0970-9185.111671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith I, Monk TG, White PF, Ding Y. Propofol infusion during regional anesthesia: Sedative, amnestic, and anxiolytic properties. Anesth Analg. 1994;79:313–9. doi: 10.1213/00000539-199408000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 17.Durmus M, But AK, Dogan Z, Yucel A, Miman YC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. Eur J Anaesthesiol. 2007;24:447–53. doi: 10.1017/S0265021506002122. [DOI] [PubMed] [Google Scholar]
- 18.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 19.Yektas A, Gümüs F, Alagol A. Dexmedetomidine and propofol infusion on sedation characteristics in patients undergoing sciatic nerve block in combination with femoral nerve block via anterior approach. Braz J Anesthesiol. 2015;65:371–8. doi: 10.1016/j.bjane.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Shah PJ, Dubey KP, Sahare KK, Agrawal A. Intravenous dexmedetomidine versus propofol for intraoperative moderate sedation during spinal anesthesia: A comparative study. J Anaesthesiol Clin Pharmacol. 2016;32:245–9. doi: 10.4103/0970-9185.168172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinardo JA. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth. 2010;20:516–23. doi: 10.1111/j.1460-9592.2010.03299.x. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745–53. doi: 10.1213/ane.0b013e3181adc506. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mustafa MM, Badran IZ, Abu-Ali HM, Al-Barazangi BA, Massad IM, Al-Ghanem SM. Intravenous dexmedetomidine prolongs bupivacaine spinal analgesia. Middle East J Anaesthesiol. 2009;20:225–31. [PubMed] [Google Scholar]
- 24.Lee MH, Yang KH, Lee CS, Lee HS, Moon SY, Hwang SI, et al. The effect-site concentration of propofol producing respiratory depression during spinal anesthesia. Korean J Anesthesiol. 2011;61:122–6. doi: 10.4097/kjae.2011.61.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KH. Safe sedation and hypnosis using dexmedetomidine for minimally invasive spine surgery in a prone position. Korean J Pain. 2014;27:313–20. doi: 10.3344/kjp.2014.27.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth. 1999;83:397–404. doi: 10.1093/bja/83.3.397. [DOI] [PubMed] [Google Scholar]
- 28.Naithani U, Bajaj P, Chhabra S. Assessment of sedation in mechanically ventilated patients in intensive care unit. Indian J Anaesth. 2008;52:519. [Google Scholar]
- 29.Mahmood S, Parchani A, El-Menyar A, Zarour A, Al-Thani H, Latifi R. Utility of bispectral index in the management of multiple trauma patients. Surg Neurol Int. 2014;5:141. doi: 10.4103/2152-7806.141890. [DOI] [PMC free article] [PubMed] [Google Scholar]