Abstract
Background:
Organophosphorus compound poisoning (OPCP) is a major public health problem in developing countries like India. Atropine and oximes remain the main-stay of management. Magnesium sulfate (MgSO4) has shown benefit in the management of OPCP.
Aims:
This study was designed to assess the effect of MgSO4 on outcome in OPCP patients admitted to Intensive Care Unit (ICU).
Settings and Design:
Double-blind prospective randomized clinical trial in an ICU of tertiary care institution.
Methods:
One hundred patients (50 in each group) of OPCP, confirmed by history and syndrome of OPCP with low plasma pseudocholinesterase, aged between 18 and 60 years were studied. Magnesium group (Group M) received 4 g of 20% MgSO4 infusion over 30 min at admission to ICU, control group (Group C) received normal saline placebo in the same manner. Patients were assessed for the need for intubation, requirement of atropine, duration of mechanical ventilation, duration of ICU stay, and its effect on mortality.
Statistical Analysis:
Chi-square test and Fisher's exact test for categorical data, independent sample t-test, and paired t-test for nominal data.
Results:
Demographics and basal serum magnesium levels were comparable. Atropine requirement was higher in Group C (74.82 ± 22.39 mg) compared to Group M (53.11 ± 45.83 mg) (P < 0.001). A total of 33 patients in Group C and 23 patients in Group M required intubation, respectively (P = 0.043). The mean duration of mechanical ventilation was 4.51 ± 2 days in Group C compared to 4.13 ± 1.6 days in Group M (P = 0.45). ICU stay was 5.36 ± 2.018 days in Group C compared to 4.54 ± 1.581 days in Group M (P = 0.026). There was no significant difference in mortality between the groups.
Conclusion:
Four grams of MgSO4 given to OPCP patients within 24 h of admission to ICU, decreases atropine requirement, need for intubation, and ICU stay.
Keywords: Atropine, Intensive Care Unit, magnesium sulfate, mechanical ventilation, organophosphorus compound poisoning
INTRODUCTION
Organophosphorus compounds (OPCs) are widely used as pesticides across Asian countries. In South Asian countries like India, it is one of most common cause for suicidal deaths and Intensive Care Unit (ICU) admissions.[1] It is a major public health problem killing 300,000 patients per year worldwide,[2,3] and in the developing countries mortality due to it is 10–20%.[4] OPC binds irreversibly to acetylcholinesterase in the plasma, red cells, cholinergic synapses in the central nervous system and peripheral nervous system, and autonomic ganglia resulting in classical cholinergic symptoms.[5] Standard management involves reducing the OPCs (OPCP) absorption by decontamination of skin and gastric lavage, and administration of atropine and oximes.[1] Atropine competitively antagonizes acetylcholine accumulation, acting at muscarinic, and nicotinic receptors. Oximes reactivate acetylcholinesterase by removing phosphoryl group. Early intervention with oximes reduces the incidence of the intermediate syndrome, which is characterized by early or late neuromuscular weakness and altered consciousness with an incidence of 15–30%.[6] However, some recent studies and a Cochrane analysis have questioned the role of oximes in the management of OPCP.[2,7] Hence, various drugs such as clonidine, sodium bicarbonate, fresh frozen plasma and magnesium sulfate (MgSO4) have been tried in combination with oximes and atropine.[8] MgSO4 is an inhibitor of acetylcholine release in the central nervous system central nervous system, peripheral sympathetic and parasympathetic synapses. It inhibits calcium channels at the presynaptic nerve terminals that trigger the release of acetylcholine and increases hydrolysis of some pesticides. It decreases the arrhythmias associated with OPCP and atropine, in central nervous system decreases overstimulation by OPCP, acting on N-methyl-D-aspartate receptor, and reverses neuromuscular weakness in the peripheral nervous system.[2,9,10] Literature review revealed very few studies assessing the effect of MgSO4 on ICU stay and outcome in patients with OPCP. Hence, a double-blind, randomized controlled trial was undertaken to study the to study the effects of MgSO4 in OPCP.
METHODS
This prospective, randomized, double-blind, placebo-controlled study was conducted between May 2014 and May 2015. Institutional Ethical Committee approval was taken, informed written consent was obtained from the patient relatives. Patients aged between 18 and 60 years presenting with history, clinical syndrome of OPCP, admitted to ICU within 24 h of ingestion, and with low pseudocholinesterase levels (<5320 IU/L) were included in the study. Postcardiac arrest patients, patients with a history of multiple poisoning, cardiac failure, renal failure, liver failure, epilepsy or bleeding tendencies, and those who refuse to give consent, were excluded from the study. Decontamination of skin and gastrointestinal tract was done as per standard institutional protocol. Injection Atropine intravenous (IV) was given as 2 mg boluses, till signs of atropinization was achieved (heart rate more than 80 beats/min, systolic blood pressure more than 90 mm of Hg, drying of oral, and tracheobronchial secretions) followed by a titrated infusion to maintain heart rate of 80–100 beats/min. Injection pralidoxime IV 30 mg/kg as loading dose, followed by 8–10 mg/kg/h infusion over 48 h was also given. Patients who developed acute respiratory failure and neuromuscular weakness were intubated and mechanically ventilated. Using a computer generated randomisation sequence, 110 patients were allocated into two groups and group allocation not revealed to observers. The nurse who administered the drug, the patient who received it and the doctor who observed the findings were all blinded to the contents of the syringe. On admission to ICU, Group M received injection MgSO4 20% 4 g (diluted to 50 ml using normal saline), by slow infusion over 30 min. Group C received a placebo 50 ml normal saline in the same manner. Intensive monitoring of heart rate, blood pressure (noninvasive blood pressure), oxygen saturation (SpO2), and electrocardiogram was done throughout the ICU stay. Patients who developed desaturation (SpO2 < 90%), proximal neck muscle weakness, Glasgow Coma Scale <8 during the ICU stay were intubated and put on mechanical ventilation. Fluid and nutritional management, criteria for extubation were followed uniformly in both groups. Patients were monitored for hypotension, arrhythmias, and treated as per the standard institutional protocol during the ICU stay. Patients who remained stable for 24 h postextubation were shifted out of ICU. Serum magnesium levels were done at admission and at 24 h after admission. Serum pseudocholinesterase levels were monitored on alternate days, and other investigations were done as warranted by the patient's condition. Primary outcomes measures observed were atropine requirement per day, number of patients requiring intubation and mechanical ventilation, day of intubation, duration of mechanical ventilation, ICU stay, and mortality. Sample size calculation was based on the outcome measures (duration of mechanical ventilation). The average duration of mechanical ventilation in our ICU was 7.4 ± 4.2 days (based on the last 6 months statistics). To reduce the mechanical ventilation duration by 33%, keeping power at 80% and confidence interval at 95%, the minimal sample size required was 45 in each group. For better validation of results, the sample size was taken as 50 in each group. Statistical analysis was done using statistical package for social sciences (SPSS Inc., Chicago, IL, USA) version 17. Statistical tools used were: Chi-square tests and Fisher's exact test were used for categorical data, Yates correction was applied as appropriate. Multiple comparisons were performed using an analysis of variance test for repeated measurements. Paired Student's t-test was used to compare parametric data before and after intervention. P < 0.05 was considered statistically significant.
RESULTS
A total of 120 patients presenting to the emergency department with history and clinical features suggestive of OPCP were screened, of which 110 were included in the study (Group C, n = 53 and Group M, n = 57). Three patients in Group C, seven patients in Group M were excluded, and fifty patients in each group were considered for final analysis [Figure 1]. Demographic parameters and basal pseudocholinesterase levels were comparable between the groups [Table 1]. Average atropine requirement per patient per day was higher in Group C (74.82 ± 22.39 mg/patient/day) compared to Group M (53.11 ± 45.83 mg/patient/day) which was statistically significant (P < 0.001). For the first 4 days, atropine consumption was significantly lower in Group M compared to Group C [Figure 2]. Number of patients requiring intubation and mechanical ventilation was significantly higher in Group C (33 patients) compared to Group M (23 patients) (P = 0.043). Seventeen patients in Group C and 16 patients in Group M required intubation within 24 h of admission to ICU (P = 0.83). After 24 h, 16 patients of the remaining 33 patients in Group C required intubation compared to seven patients of remaining 34 patients in Group M, this was clinically and statistically significant (P = 0.012). The median number of days of ventilation was 4 days (interquartile range [IQR] 0–14) in Group C, whereas in Group M it was 3 (IQR 0–8) days. The mean duration of mechanical ventilation was 4.51 ± 2 days in Group C compared to 4.13 ± 1.6 days in Group M (P = 0.45). The average duration of ICU stay was longer in Group C (5.36 ± 2.018 days) compared to Group M (4.54 ± 1.581 days) (P = 0.026) which was statistically significant [Table 2]. Serum pseudocholinesterase levels were comparable between the two groups during ICU stay [Figure 3]. The mean serum magnesium levels were significantly lower in Group C (2.116 ± 0.0.2385 mg/dl) compared to Group M (2.246 ± 0.3189 mg/dl) (P = 0.023). Four patients in Group C and three patients in Group M died (P = 0.695) (odds ratio 0.734, 95% confidence interval, 0.15 to 3.46). One patient in Group C required tracheostomy.
Figure 1.
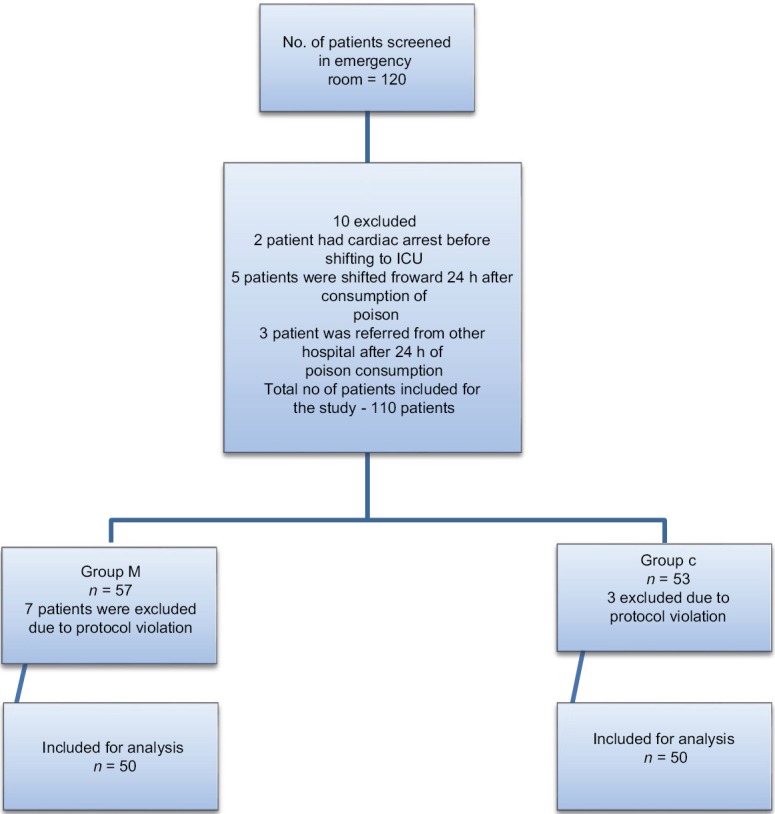
Consort diagram showing the number of patients included and analyzed.
Table 1.
Demographic variables, basal pseudocholinesterase, and magnesium levels
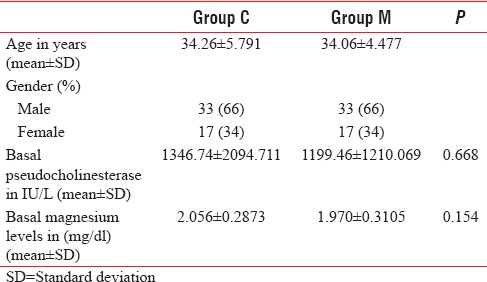
Figure 2.
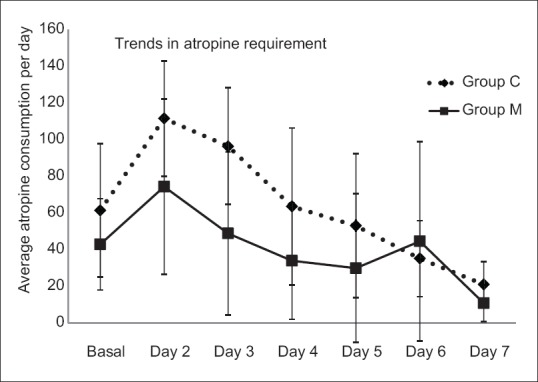
Daily average atropine requirement.
Table 2.
Outcome parameters
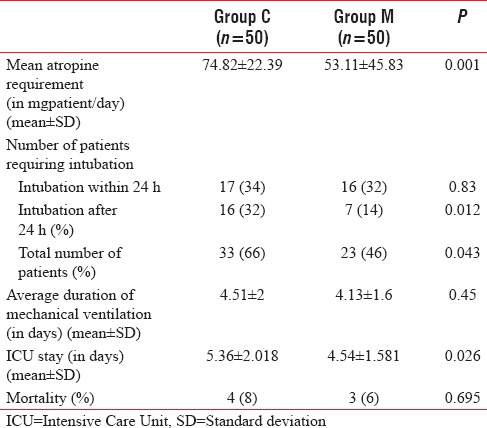
Figure 3.
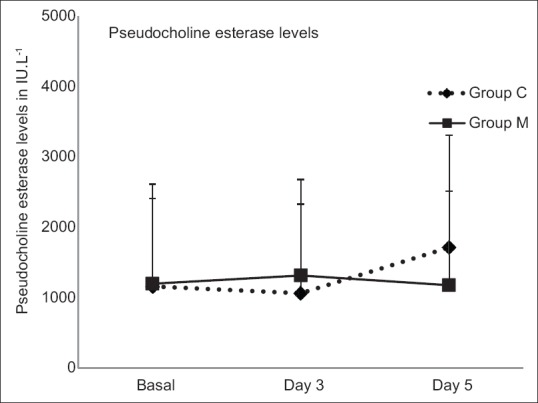
Average pseudo cholinesterase levels.
DISCUSSION
This study shows that patients with OPCP receiving 4 g of MgSO4 within 24 h of ICU admission reduces the requirement of atropine, need for intubation, and decreases the length of stay in ICU; however, there was no statistically significant difference in the duration of mechanical ventilation and in mortality between the groups. Clinical features of OPCP were classified based on (a) receptor-specific manifestations-muscarinic and nicotinic,[11] (b) based on the time of occurrence <24 h, 24 h to 2 weeks, and beyond 2 weeks.[5] Most of the signs and symptoms of OPCP are the result of excessive muscarinic receptor stimulation. Severe acute toxicity results from reduced central drive, neuromuscular dysfunction, and bronchorrhea leading to respiratory arrest and death within 30 min of consumption.[2] Patients who do survive acute toxicity, develop neuromuscular weakness classified into (i) Type 1 paralysis, weakness within 24 h, (ii) Type 2 paralysis weakness after 24 h to 2 weeks (intermediate syndrome),[12] (iii) Type 3 paralysis after 2 weeks (organophosphorus-induced polyneuropathy).[13] Management of OPCP has not changed much since 1955, with atropine and oximes being main-stay.[2] The therapeutic value of oximes is in neuromuscular recovery,[14] but its effects in reactivating enzyme acetylcholinesterase is questionable. Many recent studies and analysis have questioned the effectiveness, dosing, timing and in fact, in some, it was found to be harmful.[7] There are ongoing studies assessing the efficacy of fresh frozen plasma, nicotinic receptor antagonist, beta-adrenergic agonist, lipid emulsions, organophosphorus hydrolases, and magnesium in the management of OPCP.[2,15] In an animal study, MgSO4 showed benefit by reducing cholinergic stimulation after OPCP.[16] Magnesium has been successfully used in the management of cardiac arrhythmias due to OPCP.[17] A clinical study showed reversion of the neuroelectrophysiological defects due to OPCP.[18] In an earlier study, 4 g of MgSO4 when administered with in the first 24 h of OPCP consumption, reduced mortality, and duration of hospitalization.[19] A more recent study compared 4 g, 8 g, 12 g, and 16 g of MgSO4 in the management of OPCP patients and found better outcomes with increasing doses. MgSO4 at all doses was well-tolerated.[20] However, a dose of 4 g was selected for this study, keeping in mind the ease of administration and need for less intense monitoring of magnesium levels. Although an increase in serum magnesium levels was noted following the administration of MgSO4, it was within the therapeutic range, and none of the patients had clinical side effects attributable to magnesium. The observations concur with that of other studies.[19,20] The requirement of atropine in OPCP may vary with the severity of poisoning.[21] There is conflicting evidence regarding the effect of magnesium on atropine requirement.[19,20] However, we noted a significant reduction in atropine requirement in patients receiving magnesium. The need for intubation and mechanical ventilation varies between 50% and 88% depending on the severity of poisoning.[22] In our study, there was an overall 30% reduction in the need for intubation and mechanical ventilation in magnesium group compared to control group highlighting the beneficial effect of magnesium at the neuromuscular junction. By decreasing acetylcholine release and facilitating, the metabolism of OPCP, magnesium is found to reduce the incidence of intermediate syndrome.[20] This may be the reason for the significant reduction in the need for intubation after initial 24 h in magnesium group in this study. This finding is similar to that of the previous studies.[20] The average duration of mechanical ventilation observed in this study is in agreement with observations of the previous studies,[14,23,24] there was no difference in the duration of mechanical ventilation in patients receiving magnesium compared to control. A single dose of MgSO4 may not be enough to maintain a sustained therapeutic level which may influence the duration of mechanical ventilation.[20] Reduction in the number of patients requiring intubation and mechanical ventilation has reduced the duration of ICU stay in patients receiving magnesium in this study, which further supports the beneficial role of magnesium in the management of OPCP. The overall mortality rate in this study is comparable with the previous studies,[23,24,25,26,27] but there was no significant difference in mortality between groups. Basher et al. noted reduced mortality with increasing doses of MgSO4.[20] There are some limitations in this study. First, the patients were followed up only during ICU stay; hence, we are not able to comment about the effect of MgSO4 on the duration of hospitalization. Second, daily assessment of magnesium levels was not carried out due to financial constraints, which might have thrown some light on the possible correlation between magnesium levels and outcome measures. As the primary focus of this study was to observe the effect of MgSO4 on the need for mechanical ventilation and ICU stay, it is not sufficiently powered to comment on the effect of MgSO4 on mortality. Future multicentric studies with larger sample size, daily and different dosing of magnesium with frequent measurement of magnesium levels to maintain the therapeutic levels, may be needed to fully assess the effect of magnesium on outcome in OPCP.
CONCLUSION
MgSO4 4 g when given to OPCP patients within 24 h of admission as single dose reduces requirement of atropine, need for intubation, and duration of ICU stay. It does not influence the duration of mechanical ventilation and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.South Asian Cochrane Network and Centre. Interventions for Acute Organophosphate Poisoning. c2012. [Last cited on 2013 Jun 10]. Available from: http://www.cochrane-sacn.org/toxicology/files/Evidence%20regarding%20OP%20poisoning%20treatments.pdf .
- 2.Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: The old and the (possible) new. Br J Clin Pharmacol. 2016;81:462–70. doi: 10.1111/bcp.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Wig N, Chaudhary D, Sood NK, Sharma BK. Changing pattern of acute poisoning in adults: Experience of a large North-West Indian Hospital. J Assoc Phys India. 1997;45:194–7. [Google Scholar]
- 4.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter JV, Sudarsan TI, Moran JL. Clinical features of organophosphate poisoning: A review of different classification systems and approaches. Indian J Crit Care Med. 2014;18:735–45. doi: 10.4103/0972-5229.144017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamil H. Organophosphorus insecticide poisoning. J Pak Med Assoc. 1989;39:27–31. [PubMed] [Google Scholar]
- 7.Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011;(2):CD005085. doi: 10.1002/14651858.CD005085.pub2. doi: 10.1002/14651858.CD005085.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Palaniappen V. Muruganathan A, Geetha T, editors. Current Concepts in the Management of Organophosphorus Compound Poisoning. India: Association of physician. 2013. [Last accessed on 2016 May 16]. 33 pp. Available from: <http://www.apiindia.org/medicine_update_2013/chap95.pdf> .
- 9.Blain PG. Organophosphorus poisoning (acute) Clin Evid. 2011. [Last accessed on 2016 May 16, Last cited on 2012 Feb 27]. Available from: http://www.ncbi.nlm.nih.gov/pu bmed/21575287 .
- 10.Naguib M, Lien CA, Meistelman C. Pharmacology of neuromuscular blocking drugs. In: Miller RD, Eriksson LI, Cohen NH, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Millers? Anesthesia. 8th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2015. p. 982. [Google Scholar]
- 11.Namba T. Cholinesterase inhibition by organophosphorus compounds and its clinical effects. Bull World Health Organ. 1971;44:289–307. [PMC free article] [PubMed] [Google Scholar]
- 12.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–3. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 13.Aygun D, Onar MK, Altintop BL. The clinical and electrophysiological features of a delayed polyneuropathy developing subsequently after acute organophosphate poisoning and it's correlation with the serum acetylcholinesterase. Electromyogr Clin Neurophysiol. 2003;43:421–7. [PubMed] [Google Scholar]
- 14.Ahmed SM, Das B, Nadeem A, Samal RK. Survival pattern in patients with acute organophosphate poisoning on mechanical ventilation: A retrospective intensive care unit-based study in a tertiary care teaching hospital. Indian J Anaesth. 2014;58:11–7. doi: 10.4103/0019-5049.126780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayananda VP, Bhaskara B, Pateel GN. A study of effectiveness of fresh frozen plasma in organophosphorous compound poisoning in reducing length of intensive care unit stay and in reducing need for tracheostomy. Anesth Essays Res. 2016;10:268–72. doi: 10.4103/0259-1162.171455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petroianu G, Toomes LM, Petroianu A, Bergler W, Rüfer R. Control of blood pressure, heart rate and haematocrit during high-dose intravenous paraoxon exposure in mini pigs. J Appl Toxicol. 1998;18:293–8. doi: 10.1002/(sici)1099-1263(199807/08)18:4<293::aid-jat509>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Kiss Z, Fazekas T. Organophosphates and torsade de pointes ventricular tachycardia. J R Soc Med. 1983;76:984–5. doi: 10.1177/014107688307601124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh G, Avasthi G, Khurana D, Whig J, Mahajan R. Neurophysiological monitoring of pharmacological manipulation in acute organophosphate (OP) poisoning. The effects of pralidoxime, magnesium sulphate and pancuronium. Electroencephalogr Clin Neurophysiol. 1998;107:140–8. doi: 10.1016/s0013-4694(98)00053-4. [DOI] [PubMed] [Google Scholar]
- 19.Pajoumand A, Shadnia S, Rezaie A, Abdi M, Abdollahi M. Benefits of magnesium sulfate in the management of acute human poisoning by organophosphorus insecticides. Hum Exp Toxicol. 2004;23:565–9. doi: 10.1191/0960327104ht489oa. [DOI] [PubMed] [Google Scholar]
- 20.Basher A, Rahman SH, Ghose A, Arif SM, Faiz MA, Dawson AH. Phase II study of magnesium sulfate in acute organophosphate pesticide poisoning. Clin Toxicol (Phila) 2013;51:35–40. doi: 10.3109/15563650.2012.757318. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya K, Phaujdar S, Sarkar R, Mullick OS. Serum creatine phosphokinase: A probable marker of severity in organophosphorus poisoning. Toxicol Int. 2011;18:117–23. doi: 10.4103/0971-6580.84263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajeev H, Arvind MN. Study of clinical and biochemical parameters in predicting the need for ventilator support in organophosphorus compound poisoning. J Evol Med Dent Sci. 2013;49:9555–70. [Google Scholar]
- 23.Banday TH, Tathineni B, Desai MS, Naik V. Predictors of morbidity and mortality in organophosphorus poisoning: A case study in rural hospital in Karnataka, India. N Am J Med Sci. 2015;7:259–65. doi: 10.4103/1947-2714.159331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiran BR, Vishwas GK, Dheeraj RP, Chiragbabu PS. Organo phosphorus poisoning cases requiring mechanical ventilation: A retrospective study at tertiary hospital. Indian J Appl Res. 2014;4:435–7. [Google Scholar]
- 25.Hussain AM, Sultan ST. Organophosphorus insecticide poisoning: Management in surgical intensive care unit. J Coll Physicians Surg Pak. 2005;15:100–2. [PubMed] [Google Scholar]
- 26.Yamashita M, Yamashita M, Tanaka J, Ando Y. Human mortality in organophosphate poisonings. Vet Hum Toxicol. 1997;39:84–5. [PubMed] [Google Scholar]
- 27.Safdar A, Saeed A, Muhammad NR. Organophosphorus poisoning: Emergency management in intensive care unit. Professional. 2003;10:308–14. [Google Scholar]


