Abstract
Context:
Propofol is a commonly used induction agent during general anesthesia. As a sole agent, it does not provide any strong analgesic effect. The nitrous oxide (N2O) used along with propofol for induction of anesthesia augments the induction characteristics and reduces the dose of propofol.
Aims:
To study the effects of inhaled N2O on the induction dose and time of propofol during general anesthesia and also its hemodynamic response and adverse effects.
Settings and Design:
The present research is a prospective, randomized, double-blind comparative study.
Subjects and Methods:
The study population consisted of eighty patients aged 18–60 years from either sex, American Society of Anesthesiologists physical status 1 and 2 which were scheduled for various elective surgical procedures under general anesthesia. The patients were randomly allocated into two groups comprising forty patients in each group. All patients were premedicated with glycopyrrolate 0.2 mg, ondansetron 4 mg, and fentanyl 1 μg/kg intravenously. Group FN received breathing mixture of gases (67% N2O @ 4 L/min and 33% O2 @ 2 L/min), and propofol and Group FO received 100% O2 @ 6 L/min and propofol. The different hemodynamic parameters (heart rate, mean arterial pressure, systolic blood pressure, diastolic blood pressure, and SpO2) were measured.
Statistical Analysis:
All observations were analyzed using Chi-square test, Student's t-test, and analysis of variance.
Results:
The mean induction time and dose were significantly less in Group FN as compared to Group FO (P < 0.05). The mean induction time was 172 ± 32 s in Group FN as compared to 242 ± 43 s in Group FO (P < 001), whereas the mean induction dose was 56.10 ± 13.92 mg in Group FN as compared to 81.67 ± 17.64 mg in Group FO (P < 0.05). The hemodynamic parameters remained stable with no complications.
Conclusion:
The coadministration of N2O during induction of anesthesia with propofol not only reduced the induction dose of propofol but also reduced induction time significantly. Furthermore, it provided stable hemodynamics without any complications.
Keywords: Dose, hemodynamics, induction, nitrous oxide, propofol
INTRODUCTION
Propofol has been accepted in recent years as most commonly used agent for intravenous induction of anesthesia. Induction, with propofol is smooth, rapid, has rapid awakening and orientation times, better intubating conditions, and upper airway integrity.[1] The major disadvantage associated with propofol is a significant decrease in arterial blood pressure and heart rate (HR) when used at higher doses which is particularly detrimental for the elderly and high-risk patients.[2,3]
During general anesthesia, various methods are available to reduce the induction dose requirements of propofol particularly concomitant use of nitrous oxide (N2O) as inhalational agent; opioids, barbiturates, and benzodiazepines as intravenous agents; local anesthetics and priming dose of propofol.[1,4] The inhalation of 66% N2O concentration before induction of anesthesia using propofol leads to a 44% reduction in the required dose of propofol for loss of response to verbal commands and also reduction in the induction time.[4,5,6]
N2O has been used for more than 150 years because of its analgesic, anxiolytic, and anesthetic properties and thus clearly safe for most patients.[7,8] However, certain properties of N2O restricts its use during general anesthesia such as second gas effect, diffusion hypoxia, expansion of gas-filled cavities, and postoperative nausea and vomiting.[7] N2O is a frequently used adjunct to propofol anesthesia. Although it reduces the requirement of propofol for induction and maintenance, the effect of both drugs on overall hemodynamics remain controversial. In healthy humans, the addition of 70% N2O to bolus propofol at a dose of 2.5 mg/kg did not alter hemodynamic variables.[9,10]
Hence, the present study was undertaken primarily to study the effects of inhaled N2O in reducing the induction dose requirements of propofol with induction time along with hemodynamic variations and adverse effects.
SUBJECTS AND METHODS
After obtaining Institutional Ethical Committee clearance and written informed consent, this prospective, randomized and double-blinded study was undertaken at the Department of Anaesthesiology, JLN Medical College, Ajmer, Rajasthan. A total of 80 American Society of Anesthesiologists (ASA) physical status 1 and 2 patients, aged 18–60 years, of either sex, scheduled for various elective surgical procedures under general anesthesia were included in our study.
The exclusion criteria included pregnant female, morbidly obese patients, patients with ASA physical status 3 and above, allergy to anesthetic agents used in our study, and relative contraindications to the use of N2O during induction such as raised intracranial pressure, bowel obstruction, pneumothorax, air embolism, or middle ear surgeries.
The patients were randomly allocated into two groups of forty patients each using closed envelope technique in Group FN and Group FO.
All the patients underwent preanesthetic evaluation before surgery. The procedure of general anesthesia was explained while taking written and informed consent. All patients were kept nil per oral overnight and tablet alprazolam 0.25 mg was given night before surgery to reduce anxiety.
On arrival to operation theater, all the standard monitors were attached to the patient and baseline HR, mean arterial pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and SpO2 were recorded. All patients were premedicated with glycopyrrolate 0.2 mg, ondansetron 4 mg, and fentanyl 1 µg/kg. After 3 min of preoxygenation with 100% oxygen, the closed circuit was primed with 4 L/min of N2O with 2 L/min of O2 and patients were asked to breath into it via face mask using closed circuit breathing system for 1 min (Group FN) and 6 L/min of O2 (Group FO) and then propofol infusion started at a rate of 20 mg/min. The infusion was stopped at loss of response to verbal commands and induction was confirmed by disappearance of eyelash reflex. The induction time was calculated from start of propofol infusion to loss of response to verbal commands and the induction dose as the amount of drug administered in that duration. Patients were given vecuronium 0.1 mg/kg and after 3 min of IPPV with 100% O2, patients were intubated using cuffed endotracheal tube of appropriate size and then maintained with O2, N2O, and halothane. All the patients were monitored for HR, MAP, SBP, DBP, and SpO2 at specific intervals, preoperatively (T1), before start of propofol infusion (T2), 1 min after start of infusion (T3), 2 min after start of infusion (T4), 3 min after start of infusion (T5), 4 min after start of infusion (T6), end of infusion (T7), before intubation (T8), after intubation (T9), perioperatively (T10), before extubation (T11), and after extubation (T12). Any complications associated with their use, for example, apnea, vomiting, laryngospasm, and involuntary movements were also noted.
Statistical analysis
The sample size for our study was calculated based on assuming a power of 80%. All the data were reported as mean values ± standard deviation (SD). Statistical analysis of demographic data (age, weight, and gender) was done using Chi-square test. The comparison between the two groups for the induction dose, induction time, and hemodynamic parameters (HR, MAP, SBP, DBP, and SpO2) was done using Student's t-test/Fisher exact test to find the significance of study parameters on categorical scale between the two groups and P values were calculated. Levene's test has been done to assess the homogeneity of variance. Nonparametric data were compared with Kruskal–Wallis test. The SSP SAS 9.2 (SAS Institute Inc., NC, USA), SPSS 15.0 (SPSS Inc., Chicago, IL, USA), Stata 10.1 (StataCorp LP, Texas, USA), MedCalc 9.0.1 (MedCalc Software bvba, Ostend, Belgium), Systat 12.0 (Systat Software. Inc., San Jose, USA), and R environment version 2.11.1 (Free Online Software) were used for the analysis of data.
RESULTS
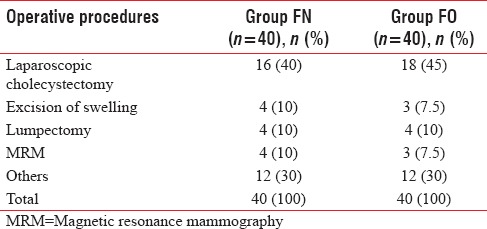
The demographic data were comparable for age, gender, and weight (P > 0.05), [Table 1]. The distribution of ASA physical status classification and operative procedures was also comparable in two groups (P > 0.05), [Tables 1 and 2].
Table 1.
Demographic data
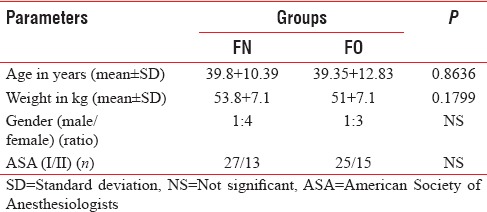
Table 2.
Distribution of operative procedures in both groups
In Group FN, 72.50% of patients were induced in <200 s and only 27.50% of patients required more than 200 s for induction of anesthesia. Whereas in Group FO, only 30% of patients were induced in <200 s and 70% required more than 200 s, so the mean induction time (mean ± SD) was significantly less in Group FN (172.18 ± 32.16) as compared to Group FO (242.86 ± 43.47) (P < 0.001), [Table 3 and Figure 1].
Table 3.
Distribution of induction time in both groups
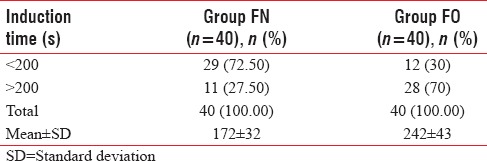
Figure 1.
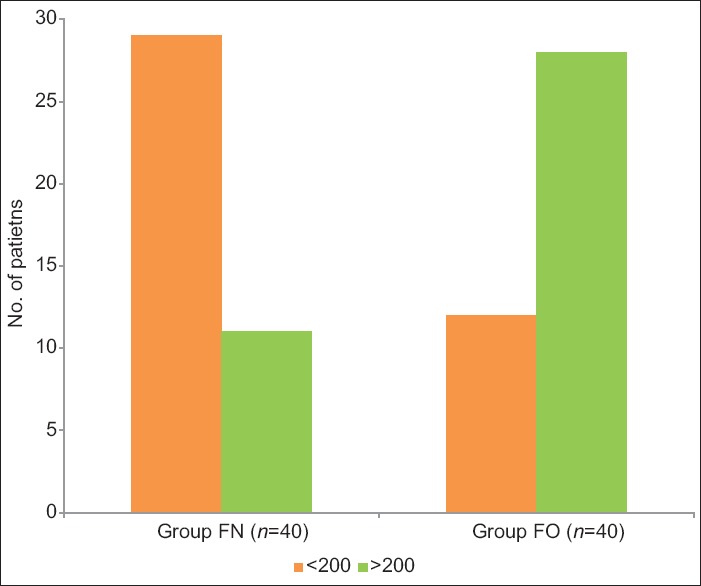
Distribution of induction time (s) in both groups.
In Group FN, 30% of patients required a dose of 0.5–1.0 mg/kg for induction, 70% of patients required 1.0–1.5 mg/kg, and none of the patients required more than 1.5 mg/kg. However, in Group FO, 57% of patients required more than 1.5 mg/kg and 42% required 1.0–1.5 mg/kg but none of the patients were induced in <1.0 mg/kg. Hence, the induction dose (mean ± SD) was significantly less in Group FN (56.10 ± 13.92) as compared to Group FO (81.67 ± 17.64) P < 0.05, [Table 4 and Figure 2].
Table 4.
Distribution of induction dose in both groups
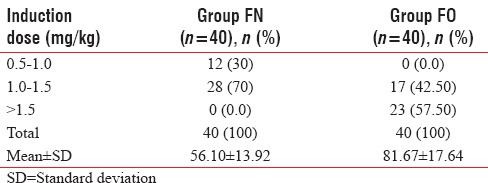
Figure 2.
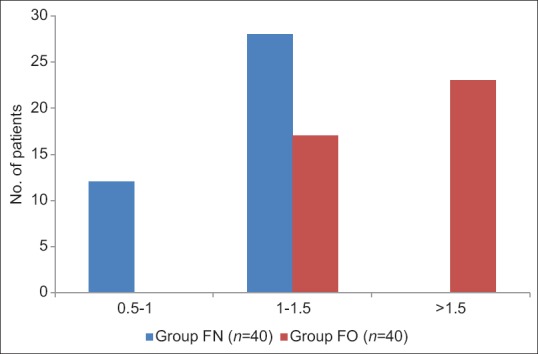
Distribution of induction dose (mg/kg) in both groups.
In Groups FN and FO, the mean baseline HR was 82.8 ± 14.02 and 82.5 ± 15.41, respectively, which were comparable (P > 0.05). There was no significant rise in HR till the end of infusion in both groups. However, there was a significant rise in HR after intubation (T9) in both groups, (P < 0.05), but clinically not significant as increase in HR were only 13% and 12% in Groups FN and FO, respectively. On extubation (T12), there was no significant increase in HR in the Group FN (87 ± 8.52), whereas a significant rise in HR was observed in Group FO (90.62 ± 9.52) (P < 0.05) [Table 5 and Figure 3]. The baseline MAP was comparable in both groups, but there was a significant drop in MAP at the end of propofol infusion 91.2 ± 9.44 and 90.45 ± 6.85 in Groups FN and FO, respectively, but clinically not significant as drop in MAP was only 9% and 12% only in both groups and no significant change were observed after intubation (T9), intraoperative period (T10), and after extubation (T12) [Table 6 and Figure 4].
Table 5.
Comparison of heart rate (bpm) in both groups
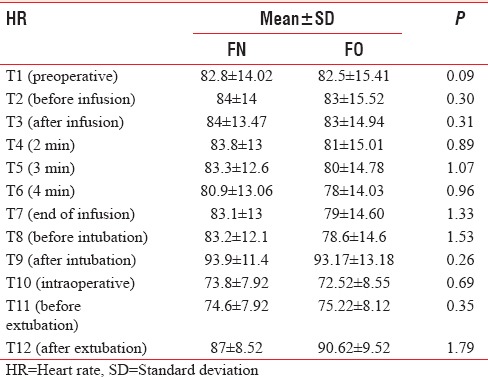
Figure 3.
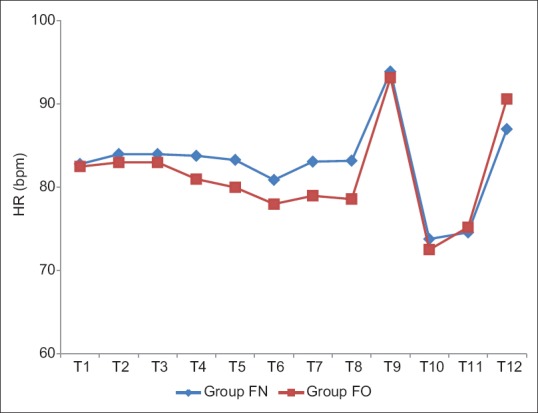
Comparison of heart rate (bpm) in both groups.
Table 6.
Comparison of mean arterial pressure (mmHg) in both groups
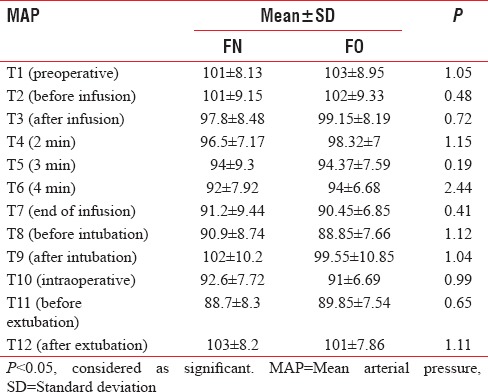
Figure 4.
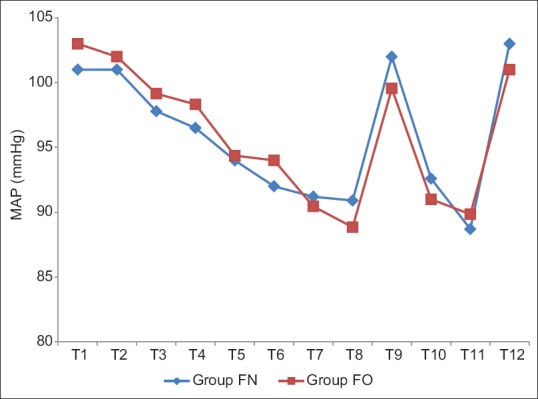
Comparison of mean arterial blood pressure in both groups.
The baseline mean SBP and DBP were comparable in between two groups. There was a significant drop in SBP and DBP at the end of infusion in both Groups FN and FO (P < 0.05) but clinically not significant as reduction in SBP and DBP were 5% and 8%, and 9% and 13%, respectively. There was no significant change in SBP and DBP observed after intubation (T9), intraoperative period (T10), and after extubation (T12) in both groups [Tables 7 and 8, Figures 5 and 6].
Table 7.
Comparison of systolic blood pressure (mmHg) in both groups
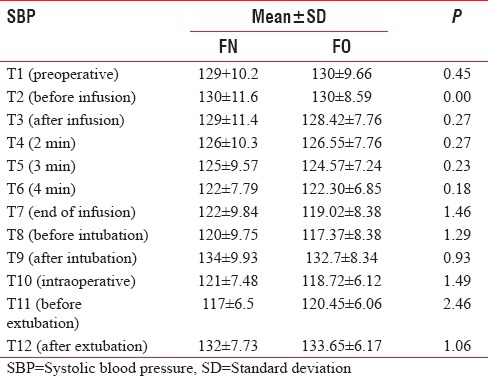
Table 8.
Comparison of diastolic blood pressure (mmHg) in both groups
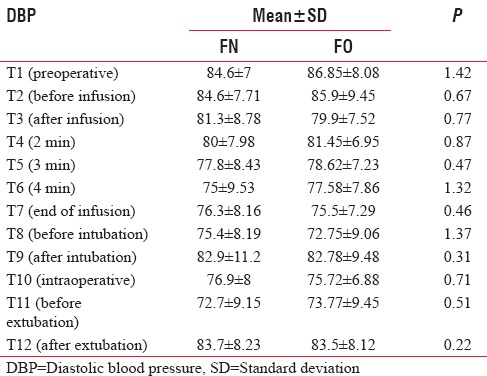
Figure 5.
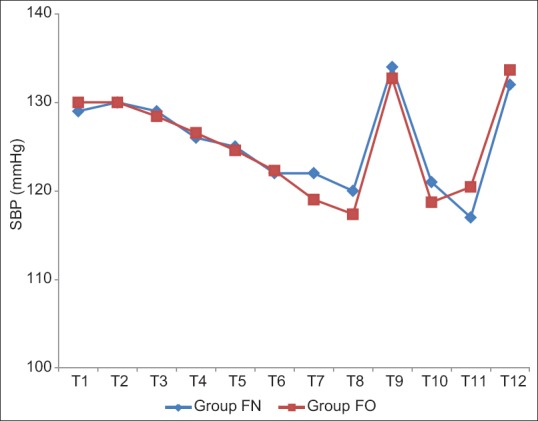
Comparison of mean systolic blood pressure in both groups.
Figure 6.
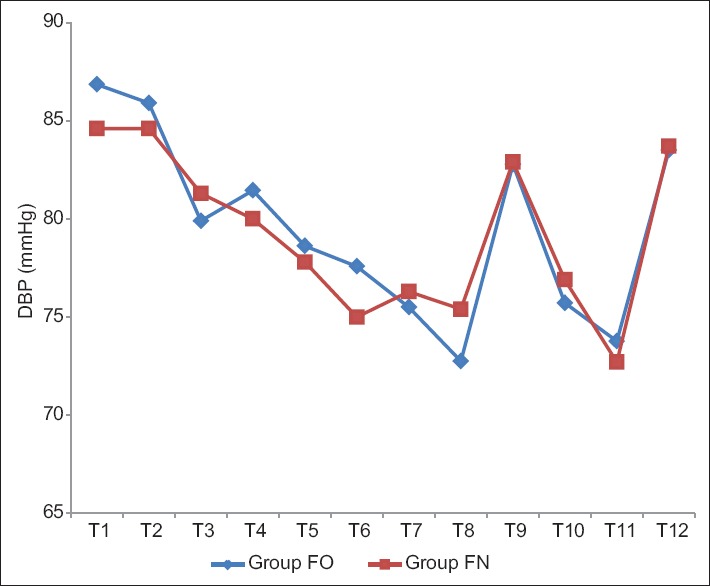
Comparison of mean diastolic blood pressure in both groups.
The intergroup analysis showed stable MAP, SBP, and DBP after induction of anesthesia in both groups (P > 0.05). The baseline SpO2 was 98.8 ± 1.08 and 99.17 ± 1.03 in Group FN and Group FO, respectively, which were statistically comparable. The mean SpO2 remained 100% after 2 min of infusion till the patient extubated in both groups (P > 0.05) [Table 9 and Figure 7]. The patients in both groups did not experience any adverse effects and any complication.
Table 9.
Comparison of oxygen saturation in both groups
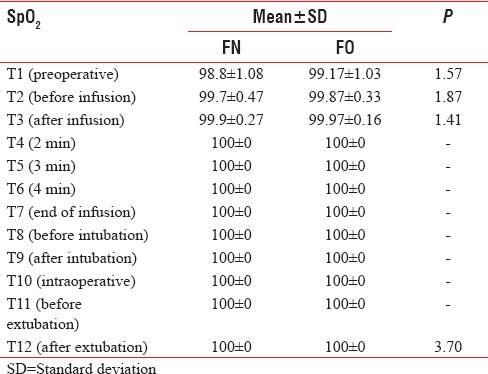
Figure 7.
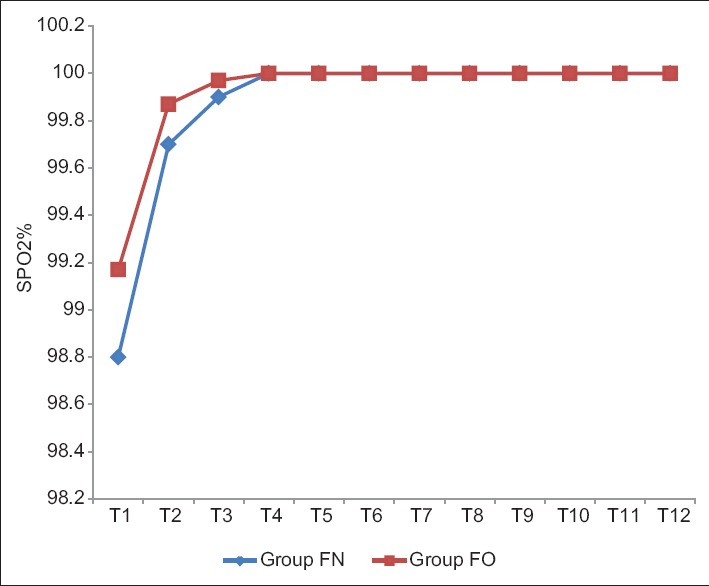
Comparison of arterial oxygen saturation in both groups.
DISCUSSION
Propofol is a commonly used intravenous induction agent during general anesthesia. This is used alone or in combination with benzodiazepines, opioids, or inhalational agents.[11] Induction with propofol is smooth and rapid associated with better intubating conditions and rapid recovery from anesthesia. The combination of other volatile or inhalational anesthetic agents and analgesics increased the induction characteristics and reduces the dose of propofol.[4,12] However, rapid induction and higher dose of propofol are associated with profound hypotension and other hemodynamic changes which require vasopressors or inotropic support.[13,14]
N2O has been used for the last several years as an essential part of general anesthesia. However, its use in general anesthesia has steadily declined in Western European countries during the past few decades,[12,15] but still used as a most common inhalational agent in developing countries because of its cost effectiveness.[16] Xenon may be an available alternative to N2O for supplementation of general anesthesia as it is more potent but due to its high cost, it does not seems to replace N2O in near future particularly in developing countries. It is commonly used along with intravenous induction agents and volatile anesthetic agents reducing the dose of intravenous induction agents such as propofol or minimal alveolar concentration of volatile anesthetic agents.[4,7,14] The administration of 65% N2O has been shown to reduce the propofol requirements by 30–50% which in turn leads to less cardiovascular and respiratory depression and cost saving.[4,17]
The use of N2O along with propofol for induction of anesthesia has not been studied extensively, so our study was designed to determine the effects of inhaled N2O on the induction dose and time of propofol and its hemodynamic response. The faster and smoother induction with improved oxygenation has been reported with concomitant use of N2O, so currently there is no clinically relevant evidence for withdrawal of N2O use in anesthetic practice.[12]
In our study, propofol was administered by infusion at a rate of 20 mg/min. The induction dose and induction time both were significantly less in Group FN as compared to Group FO. We found that induction time is prolonged in Group FO, but coadministration of 67% N2O along with 33% O2 (Group FN) significantly reduced the induction time concurs with the study of Ng and Hwang and Singh,[4,14,18] In Group FN, 72.50% of the patients were induced in <200 s but only 30% of patients required <200 s for induction in Group FO. However, none of the patients required more than 200 s for induction of anesthesia in a study by Singh showed better results as compared to our study regarding induction time. In an another study by Ng and Hwang, the number of patients induced under 200 s were 90% in group receiving 66% N2O in O2, thereby shortened the induction time which is almost similar to our study.
The mean induction time (seconds) was significantly less in Group FN (172 ± 32) as compared to Group FO (242 ± 43) P < 0.0001, which is almost 30% less than the control group. However, the induction times were reduced more in the studies done by Ng and Hwang and Singh, 111.38 ± 35.93 and 132.7 ± 57.0, respectively, and Dominguez et al. as compared to our study.[4,14,18]
The mean induction dose (mg) was significantly less in Group FN (56.10 ± 13.92) as compared to Group FO (81.67 ± 17.64) P < 0.05. Our findings showed 35% reduction in the induction dose of propofol with concomitant use of N2O; however, there was almost 40% reduction in induction dose of propofol in the studies done by Ng and Hwang and Singh, which are almost similar to our study.[4,14] The reduction in induction dose of propofol may be due to analgesic, anxiolytic, and hypnotic properties of N2O as well as slower infusion rate (20 mg/min) of propofol that we have studied and also supported by other authors, Peacock et al., Ng and Hwang and others.[19]
Several studies have undertaken to compare the infusion rates of propofol (20–500 mg/min) on induction time and induction dose. In a study by Peacock et al.,[19] the reduction in infusion rate of propofol for induction increased the induction time along with no reduction in induction dose of propofol used. However, concomitant administration of N2O reduced the induction dose of propofol.[4] Slower infusion rates (20 mg/min) of propofol with N2O not only reduced the total dose required for induction but also achieved more acceptable induction time as shown in our study.[1,4,20,21]
In our study, none of the patients in Group FN, who received 67% N2O with 33% O2, desaturated at any point of induction with an infusion rate of 20 mg/min of propofol. The SpO2 remained at median baseline value of 100%. This was similar to study by Singh and Ng and Hwang both. This concurs with an another study also done by Khoo et al.,[22] John et al., which showed that concomitant use of N2O with oxygen did not result in desaturation of patients during a slow propofol infusion for induction and not compromise oxygenation. They concluded that preinduction inhalation of N2O (50%) in O2 appears to be an effective and acceptable method of preoxygenating the patient and augmenting the propofol induction of anesthesia as shown in our study. This was the major concern of our study to adopt this technique.[12]
The hemodynamic variations in both groups were also compared in our study. There was no significant change in HR at the end of infusion during induction in Group FN and FO, in contrast to our study by Singh where there was a continuous increase in HR till 10 min after induction. However, in our study, there was a significant rise in HR after intubation in both groups (P < 0.05) but clinically not significant. This may be due to fall in HR by propofol which may be compensated by increased sympathetic stimulation by N2O.[9,23] The hemodynamic effects of N2O are due to its property to stimulate the sympathetic nervous system; however, it is a direct myocardial depressant, but arterial blood pressure, cardiac output, and HR are unchanged or slightly elevated because of sympathetic stimulation and catecholamine release.[24] The study by Mckinney and Fee showed decrease in HR as the study population has average age of 70 years who have limited responsiveness of cardiovascular system toward the sympathetic stimulation.[25] There was no significant change in SBP, DBP, and MAP observed after intubation, intraoperative period, and after extubation in both groups. The various studies have demonstrated reduction up to 26–28% of SBP, 19% of DBP, and 11% of MAP after induction with propofol at a dose of 2 mg/kg.[13] However, in our study, reduction in 2–4% of HR, 9–12% of MAP, 5–8% of SBP, and 9–13% of DBP were observed which were significantly less as compared to other studies. This may be due to significant reduction in induction dose of propofol by use of N2O and slower infusion (20 mg/min) of propofol. Kumar et al.[1] observed complications such as hypotension in control (8%) and study (1%) groups but none of the patients in our study have any complications, for example, hemodynamic instability, laryngospasm, desaturation, involuntary movements, apnea, or delayed extubation.
We have studied the effects of inhaled N2O on the induction dose and time of propofol along with hemodynamic variations and any adverse effects or complications, during induction of anesthesia. However, we did not studied the acceptance or feasibility of concomitant administration of N2O with oxygen during induction of anesthesia, but none of the patients experienced any unpleasant sensation or desaturation during induction.
CONCLUSION
In conclusion, the coadministration of N2O during induction of anesthesia with propofol not only reduced the induction dose of propofol but also reduced induction time significantly thereby reducing adverse effects of propofol. Hence, N2O should only be used in the induction of anesthesia in patients scheduled for elective surgeries and not to be used in patients with anticipated difficult intubation and low cardiorespiratory reserve simultaneously evaluating the risk-benefit profile for the use of N2O. The hemodynamic parameters (HR, SBP, DBP, MAP, and SpO2) remained stable without any adverse effects and complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kumar AA, Sanikop CS, Kotur PF. Clinical investigation effect of priming principle on the induction dose requirements of propofol – A randomized clinical trial. Indian J Anaesth. 2006;50:283–7. [Google Scholar]
- 2.Rasooli S, Parish M, Mahmoodpoor A, Moslemi F, Sanaie S, Faghfuri S. The effect of intramuscular ephedrine in prevention of hypotension due to propofol. Pak J Med Sci. 2007;23:893–7. [Google Scholar]
- 3.Hashiba E, Hirota K, Suzuki K, Matsuki A. Effects of propofol on bronchoconstriction and bradycardia induced by vagal nerve stimulation. Acta Anaesthesiol Scand. 2003;47:1059–63. doi: 10.1034/j.1399-6576.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- 4.Ng JM, Hwang NC. Inhaling nitrous oxide reduces the induction dose requirements of propofol. Anesth Analg. 2000;90:1213–6. doi: 10.1097/00000539-200005000-00040. [DOI] [PubMed] [Google Scholar]
- 5.Duarte LT, Duval Neto GF, Mendes FF. Nitrous oxide use in children. Rev Bras Anestesiol. 2012;62:451–67. doi: 10.1016/S0034-7094(12)70145-9. [DOI] [PubMed] [Google Scholar]
- 6.Davidson JA, Macleod AD, Howie JC, White M, Kenny GN. Effective concentration 50 for propofol with and without 67% nitrous oxide. Acta Anaesthesiol Scand. 1993;37:458–64. doi: 10.1111/j.1399-6576.1993.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 7.de Vasconcellos K, Sneyd JR. Nitrous oxide: Are we still in equipoise? A qualitative review of current controversies. Br J Anaesth. 2013;111:877–85. doi: 10.1093/bja/aet215. [DOI] [PubMed] [Google Scholar]
- 8.Turan A, Mascha EJ, You J, Kurz A, Shiba A, Saager L, et al. The association between nitrous oxide and postoperative mortality and morbidity after noncardiac surgery. Anesth Analg. 2013;116:1026–33. doi: 10.1213/ANE.0b013e31824590a5. [DOI] [PubMed] [Google Scholar]
- 9.Shiga T, Wajima Z, Inoue T, Ogawa R. Nitrous oxide produces minimal hemodynamic changes in patients receiving a propofol-based anesthetic: An esophageal Doppler ultrasound study. Can J Anaesth. 2003;50:649–52. doi: 10.1007/BF03018705. [DOI] [PubMed] [Google Scholar]
- 10.Carlier S, Van Aken H, Vandermeersch E, Thorniley A, Byttebier G. Does nitrous oxide affect the hemodynamic effects of anesthesia induction with propofol? Anesth Analg. 1989;68:728–33. [PubMed] [Google Scholar]
- 11.Ben-Shlomo I, Finger J, Bar-Av E, Perl AZ, Etchin A, Tverskoy M. Propofol and fentanyl act additively for induction of anaesthesia. Anaesthesia. 1993;48:111–3. doi: 10.1111/j.1365-2044.1993.tb06846.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolfgang B, Vladimir C, Stefan DH, Nicola D, Walid H, Jan H, et al. The current place of nitrous oxide in clinical practice: An expert opinion-based task force consensus statement of the European society of anaesthesiology. Eur J Anaesthesiol. 2015;32:517–20. doi: 10.1097/EJA.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 13.Claeys MA, Gepts E, Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988;60:3–9. doi: 10.1093/bja/60.1.3. [DOI] [PubMed] [Google Scholar]
- 14.Singh S. Does inhalational nitrous oxide affect induction dose of propofol and haemodynamic? J Anesthesiol. 2014;2:32–9. [Google Scholar]
- 15.Enlund M, Edmark L, Revenäs B. Ceasing routine use of nitrous oxide-a follow up. Br J Anaesth. 2003;90:686–8. doi: 10.1093/bja/aeg108. [DOI] [PubMed] [Google Scholar]
- 16.Lyratzopoulos G, Blain KM. Inhalation sedation with nitrous oxide as an alternative to dental general anaesthesia for children. J Public Health Med. 2003;25:303–12. doi: 10.1093/pubmed/fdg068. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Aramaki Y, Watanabe K, Higuchi H, Satoh T. Co-administration of nitrous oxide reduces the pressor response against oro-tracheal intubation during induction of anesthesia with propofol infusion at a low rate. Masui. 2001;50:405–9. [PubMed] [Google Scholar]
- 18.Domínguez VC, Bellolio PC. Influence of inhaled nitrous oxide on the induction doses of propofol and thiopental assessed by auditory evoked potentials. Rev Esp Anestesiol Reanim. 2007;54:475–9. [PubMed] [Google Scholar]
- 19.Peacock JE, Lewis RP, Reilly CS, Nimmo WS. Effect of different rates of infusion of propofol for induction of anaesthesia in elderly patients. Br J Anaesth. 1990;65:346–52. doi: 10.1093/bja/65.3.346. [DOI] [PubMed] [Google Scholar]
- 20.Stokes DN, Hutton P. Rate-dependent induction phenomena with propofol: Implications for the relative potency of intravenous anesthetics. Anesth Analg. 1991;72:578–83. doi: 10.1213/00000539-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Uzun S, Ozkaya BA, Yilbaş SO, Ayhan B, Sahin A, Aypar U. Effects of different propofol injection speeds on blood pressure, dose, and time of induction. Turk J Med Sci. 2011;41:397–401. [Google Scholar]
- 22.Khoo ST, Woo M, Kumar A. Preoxygenation techniques: The value of nitrous oxide. Acta Anaesthesiol Scand. 1993;37:23–5. doi: 10.1111/j.1399-6576.1993.tb03591.x. [DOI] [PubMed] [Google Scholar]
- 23.Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725–33. doi: 10.1097/00000542-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Edmond I. Eger II. Inhaled anesthetics: Uptake and distribution. In: Miller RD, editor. Miller's Anaesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. pp. 539–60. [Google Scholar]
- 25.McKinney MS, Fee JP. Cardiovascular effects of 50% nitrous oxide in older adult patients anaesthetized with isoflurane or halothane. Br J Anaesth. 1998;80:169–73. doi: 10.1093/bja/80.2.169. [DOI] [PubMed] [Google Scholar]


