Abstract
Context:
Two-thirds of patients undergoing coronary artery bypass grafting (CABG) surgery report moderate to severe pain, particularly with ambulatory or respiratory effort.
Aims:
The aim of this study is to compare the analgesic effect of perioperative thoracic epidural fentanyl with bupivacaine and intravenous fentanyl in patients undergoing CABG surgery.
Settings and Design:
The study was a prospective, randomized, nonblinded comparative study.
Materials and Methods:
A total of 60 patients coming under the American Society of Anesthesiologists Class III who were posted for CABG surgery were recruited in this study. The patients were randomized into one of two groups, higher thoracic epidural analgesia (HTEA) group receiving general anesthesia with thoracic epidural analgesia (TEA) in the postoperative period, and intravenous fentanyl analgesia group receiving general anesthesia with fentanyl infusion in the postoperative period. The pain was assessed at 4 h after extubation when the patient was fully awake, then at 8, 12, 18, and 24 h. Both groups received intravenous tramadol 100 mg as rescue analgesia whenever visual analog scale score was 5 and above. Heart rate, mean arterial pressure (MAP), sedation scores, and physiotherapy cooperation were also assessed.
Statistical Analysis Used:
The numerical data were analyzed using an independent t-test, repeated-measures ANOVA, and Mann–Whitney U-test.
Results:
Pain at rest and on cough was significantly lower in HTEA patients as compared to control group. Patients HTEA group got less frequent rescue analgesia than the control group. Physiotherapy cooperation was significantly better in HTEA patients at 4, 12, and 24 h postextubation. They also had significantly lower heart rate, MAP, and sedation scores.
Conclusion:
Perioperative TEA using fentanyl with bupivacaine provided optimal postoperative analgesia at rest and during coughing in patients following CABG surgery as compared to postoperative analgesia with intravenous fentanyl. It also resulted in optimal postoperative hemodynamic status, good cooperation to chest physiotherapy with less sedation.
Keywords: Analgesia, coronary artery bypass graft, fentanyl, intravenous, thoracic epidural
INTRODUCTION
Two-thirds of patients undergoing coronary artery bypass grafting (CABG) surgery report moderate to severe pain, particularly with ambulatory or respiratory effort.[1] The prospect of moderate or severe pain is a common concern of patients when contemplating cardiac operations. The principal intent of regional analgesic technique is to substantially reduce or possibly eliminate postoperative pain. Inadequate pain relief is a paramount cause of respiratory impairment and complications. The desired objective of limiting the extent of decreased pulmonary function would be a worthy goal if effective pain relief could be achieved without respiratory depression. We aimed to compare the analgesic effect of perioperative thoracic epidural fentanyl with bupivacaine and conventional intravenous fentanyl in patients undergoing CABG surgery. The secondary objective was to find out whether higher thoracic epidural offered any beneficial effect on the hemodynamic status postoperatively in these patients.
MATERIALS AND METHODS
The study was designed was a prospective, randomized, nonblinded comparative study. After obtaining approval from the Ethical Committee of the hospital and informed consent, sixty patients posted for CABG surgery were recruited and were randomly allocated into two equal groups by computer-generated random sequence of numbers.
A sample size of thirty in each group was selected based on Cohen's d derived from the data published by Royse et al.[2] where patients with the difference in pain score at rest, on day 1, between epidural versus morphine group, was 0.02 versus 0.8 (P = 0.008) with a Cohen's d of (0.78/0.2) = 3.9. Given the high value of Cohen's d from the previously published report, our sample size (thirty in each group) would give us more than 95% power to detect the same effect at the significance threshold of 0.05.
Sixty male patients aged 45–70 years with activated partial thromboplastin time (APTT) ≤45 s, prothrombin time (PT) (international normalized ratio [INR]) ≤1.5, platelets ≥80,000/dl, good left ventricular systolic function ejection fraction (EF) >50% who had discontinued aspirin and clopidogrel 7 days preoperatively were included in the study. Exclusion criteria were a patient refusal, infection at puncture site, APTT ≥45 s, PT (INR) 1.5, platelets ≤80,000/dl, clopidogrel within last 7 days of the procedure, and coexisting liver disease. They were all coming under American Society of Anesthesiologists (ASA) Class III and presented with triple vessel disease.
The patients were randomized into one of two groups, higher thoracic epidural analgesia (HTEA) group (Group A) receiving general anesthesia with thoracic epidural analgesia (TEA) in the postoperative period, and intravenous fentanyl analgesia group (Group B) receiving general anesthesia with fentanyl infusion in the postoperative period. The anesthetic technique described in the fentanyl infusion group was the standard technique at the time of the investigation. Hence, the fentanyl infusion group was considered a control group. Pain was measured by visual analog scale (VAS) 0–10. Patients were informed about the details and interpretations of VAS in the preoperative period.
All patients underwent a standardized anesthetic management. All patients received lorazepam 2 mg, ranitidine 150 mg, allopurinol 400 mg, Vitamin C, Vitamin A, and Vitamin E orally at night before surgery and on the morning of the surgery with sips of water. The day before the surgery, patients allocated to the epidural group had an epidural catheter inserted at T2–3/T3–4 levels under all aseptic precautions in the Intensive Care Unit (ICU), under local anesthesia and intravenous sedation with midazolam. During the procedure, electrocardiogram (ECG), SpO2 and noninvasive blood pressure were monitored. Epidural catheter was threaded with bevel facing up and 4–6 cm catheter length was placed inside the space. Placement was confirmed with a test dose consisting of 3 ml of 2% lignocaine with 1:200,000 adrenaline. Patients were shifted back to ward 1 h after the procedure.
On arrival in the operating theater, a pulse oximeter probe and ECG electrodes were applied. After local anesthesia, a large bore peripheral line and radial artery catheter were inserted. Induction was done with intravenous fentanyl 10 µg/kg and midazolam 1–2 mg. The trachea was intubated following pancuronium 12 mg after spraying of cords with 10% lignocaine, with 8–8.5 size endotracheal tube.
Maintenance of anesthesia was done with isoflurane, oxygen, and air mixture. Muscle relaxation was maintained with appropriate pancuronium 1–2 mg. Maintenance analgesia was done by intravenous fentanyl infusion at 2 mcg/kg/h. Just before sternotomy and before going on cardiopulmonary bypass an additional dose of fentanyl 2 mcg/kg was administered. Adequate bolus doses of pancuronium and midazolam were repeated while going in and coming out of cardiopulmonary pump.
Extracorporeal circulation (ECC) was induced using a primed membrane oxygenator (Medtronic Affinity). A cold potassium–magnesium cardioplegic solution (Plegiogard) was given for myocardial preservation accompanied by topical cooling. According to our routine, nonpulsatile flow was used during bypass and while during aortic cross clamp a pulsatile flow was used. This implies a mean blood pressure in the range of 40–60 mmHg. All patients were cooled to 30°C. Prior, patients were given heparin 3–4 mg/kg until full heparinization (activated clotting time [ACT] >480 s), which was reversed after termination of ECC with protamine sulfate 2–4 mg/kg (ACT normalized). Rewarming was started after the removal of aortic cross clamp and inotropic support with dopamine 5 mcg/kg/min was started when the patient was rewarmed to 34°C. One packed red blood cell unit was transfused to all patients after weaning from bypass to stabilize the circulation and to increase the hemoglobin value to about 10 g%.
All patients were electively ventilated in the ICU in synchronized intermittent mandatory ventilation with pressure support mode using Datex-Ohmeda Carestation Ventilator. Analgesia was started only when patient was awake and consciousness was assessed. HTEA group received 0.1% bupivacaine with 2 mcg/ml fentanyl at rate of 0.1 ml/kg/h through the epidural catheter, whereas control group received intravenous fentanyl infusion at 0.5–2 mcg/kg/h adjusted according to pain score.
All patients received, as needed, small doses of intermittent midazolam for sedation. The patients were warmed with a warming blanket until central temperature was 37°C. The patients were extubated when awake, with adequate spontaneous ventilation and when in a stable hemodynamic state. The postcardiopulmonary bypass inotropic supports were standardized as dopamine 5 mcg/kg/min in all patients. Hypotension requiring addition of any support was noted.
The pain was assessed using VAS starting at 4 h after extubation of the patient when the patient is fully awake. It was assessed every 4th hour in the first 12 h and 6th hour in the next 12 h after the extubation. Both groups received intravenous tramadol 100 mg as rescue analgesia whenever VAS score was 5 and above. Timing, frequency, and total amount of rescue analgesia was noted.
The hemodynamic variables such as systolic, diastolic, mean arterial blood pressure, and heart rate were recorded from 4th h postoperatively after receiving the patient in the ICU. It was measured every 4th hourly for first 12 h and 6th hourly for subsequent 12 h. All the patients had invasive arterial monitoring lines for blood pressure assessment whereas for heart rate monitoring continuous ECG was used.
Physiotherapy cooperation was assessed and scored [Table 1] along with pain at 4 h after extubation of the patient and every 4th hourly in the first 12 bupivacaine and 6th hourly in the next 12 h after the extubation. Sedation was assessed from 4th h postoperatively. It was measured every 4th hourly for first 12 h and 6th hourly for next 12 h. Sedation scales were recorded using the 6-point scale [Table 1].
Table 1.
Physiotherapy cooperation scores

Data were analyzed using SPSS statistics 20 software (IBM, Bengaluru, India). The numerical data were analyzed using independent t-test, repeated-measures ANOVA and Mann–Whitney U-test. Corresponding “P” values were also calculated. The level of significance was set at P < 0.05.
RESULTS
Distributions of patients in both groups were similar with respect to age, sex, height, weight, and ASA physical status. The mean EF and preoperative hemodynamic parameters were also comparable.
Pain at rest and on cough was significantly lower (P < 0.05) at 4th, 12th, and 24th h assessment time postextubation in HTEA patients as compared to control group [Table 2]. Mean VAS score for pain on cough was lower in HTEA group most of the time in postextubation period. Patients HTEA group got less frequent rescue analgesia than the control group (8 vs. 30 times, P = 0.001), [Figure 1]. Physiotherapy cooperation was significantly better in HTEA patients at 4th, 12th, and 24th h postextubation as compared to control group [Figure 2].
Table 2.
Pain at rest
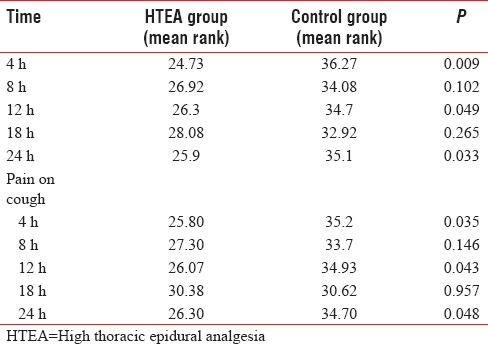
Figure 1.
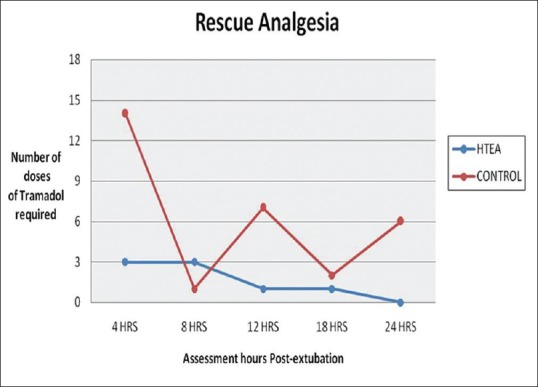
Comparison of rescue analgesia.
Figure 2.
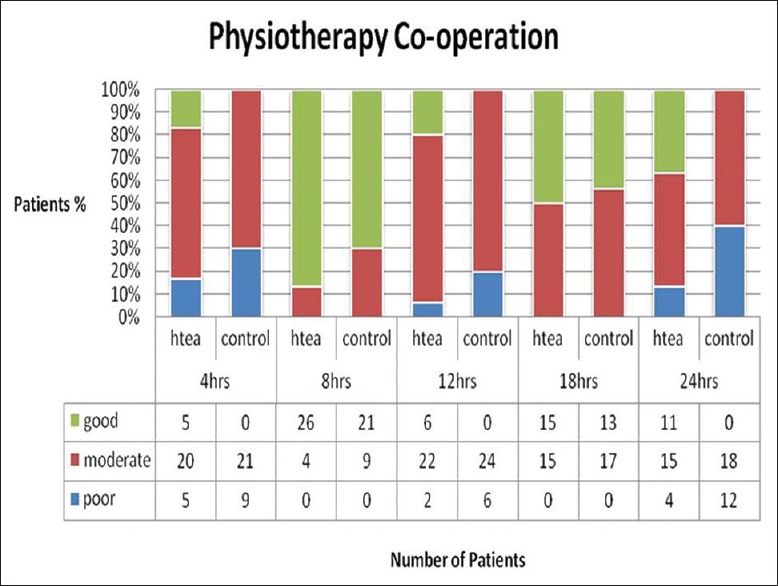
Comparison of physiotherapy cooperation.
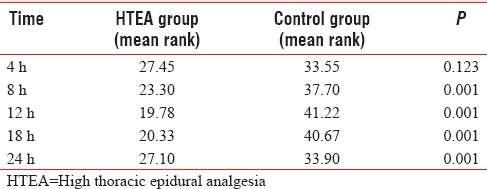
HTEA group had significantly lower heart rate and mean arterial pressure (MAP) 8th and 12th h [Table 3]. Sedation score was significantly lower (P < 0.001) at 8, 12.18, and 24 h assessment time postextubation in HTEA patients [Table 4].
Table 3.
Postoperative hemodynamics
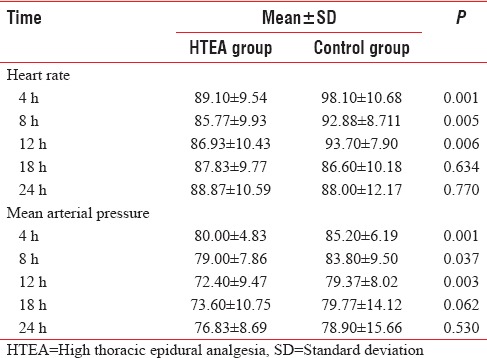
Table 4.
Sedation score
DISCUSSION
CABG is the most common type of cardiac surgery, accounting for 800,000 operations annually worldwide.[3] There is a worldwide trend for “fast-track” cardiac anesthesia, incorporating smaller doses of opioids, and aiming for rapid awakening after surgery.[4] Cardiac surgery has evolved substantially over the past decade, with improvements in surgical techniques now making it possible for some patients to avoid cardiopulmonary bypass. Anesthesia has also evolved with the advent of newer agents and techniques allowing for “fast-tracking” of patients, most notably a reduction in large doses of long-acting intravenous opioids.
There are a large variety of analgesic techniques, the most common still being intravenous opioids. TEA has revolutionized postoperative pain management and offers the prospect of almost painless cardiac surgery. Adequate postoperative pain relief increases patient's comfort, modulates the stress response, minimizes the effects of surgery on pulmonary function, and allows early patient ambulation.
Bupivacaine was taken as 0.1% solution with fentanyl 2 mcg/ml as an epidural infusion as there had been no reports of significant breakthrough pain and complications such as significant hypotension and motor block with this dose has been documented in patients undergoing CABG surgery. In Group B, we chose continuous intravenous fentanyl infusion at 1–2 mcg/kg/h as per the guidelines of the international association for the study of pain for acute postoperative pain. Fentanyl shows good analgesic efficacy with cardiovascular stability, even in critically ill patients. Continuous intravenous infusions of fentanyl have been used to provide postoperative analgesia after major surgeries. Infusion of fentanyl, especially at rates of 1.5–0.5 mcg/kg/h, provides good to excellent postoperative analgesia. At rest, the quality of analgesia remains stable, with movement (ambulation, coughing), it decreases significantly, even with higher infusion rates.
In this study, we found a significant reduction in pain at rest and with cough at 4th, 12th, and 24th h assessment time. Whereas VAS scores were insignificantly lower in Group A than Group B at 8th and 18th h assessment time. Use of additional rescue analgesia in Group B may be the reason for this.
As a variant from our study, Hansdottir et al.[5] showed contrasting data with no differences in VAS scores between patient-controlled (PC) TEA and PC analgesia with morphine. However, in their study, VAS scores were reported to be >5 in both groups on the 2nd and 3rd postoperative days, which is an unacceptable value during postoperative pain therapy. They observed that a continuous epidural infusion of a local anesthetic and an opioid seems to provide more efficient analgesia than PC-TEA after cardiac surgery as used in their study. In another article, Salvi et al.[6] documented VAS scores for pain during coughing in the first 24-h period to be <2 in all patients.
In this study, the difference in analgesia was evident both while the patients were at rest and on coughing maneuvers. Better dynamic pain relief (on coughing) in our study is probably explained by the synergistic action of combination of local anesthetic and opioid. Epidurally administered opioids produce segmental analgesia[7] and improve the quality and duration of sensory block produced by local anesthetics,[8] which may explain the better pain relief compared to intravenous analgesia.
Tachycardia and in particular hypertension are major problems after CABG surgery. The inability of even high fentanyl doses to prevent hypertension associated with intense surgical stimulation has been reported. In this study, the mean of heart rate, MAP, systolic, and diastolic blood pressure were lower in the HTEA group with statistically significant difference at 8th, 12th, and 18th h assessment time (P < 0.05).
It is a common understanding and observation that reduction in pain following postoperative anesthesia should correlate with corresponding changes in the hemodynamic parameters. Lower, but normotensive arterial blood pressure and heart rate values in epidural group reflect sympathetic blockade and suppressed stress response. However, in a study like this, where we have averaged the pain scores and hemodynamic parameters across a sample of patients these correlations can be difficult to interpret. The multifactorial nature of both pain sensitivity and hemodynamic parameters is well known, and it depends on a variety of covariates, such as age, gender, hemoglobin levels, and the presence of other comorbid conditions such as diabetes and hypertension. A study like this which uses univariate models of averaged pain scores and hemodynamic changes cannot adjust for the relevant covariates and hence the direct association of the pain scores with changes in blood pressure and heart rate, cannot be predicted and tested with certainty. To observe the exact effect would require a study with significantly larger sample size which can reduce the noise due to the covariates or which can have sufficient sample size to adjust for the covariates.
TEA offers several benefits: it reduces perioperative stress, the incidence of myocardial ischemia and postoperative pain, and it is also likely that it reduces complications. Despite these advantages, epidural anesthesia has not gained widespread acceptance in cardiac surgery because of concerns regarding the potential development of such squeal as spinal cord injury (associated with neuraxial hematoma), epidural infection, and hypotension (secondary to sympatholysis induced by anesthetic blockade).
The risk of neuraxial hematoma, generally regarded as the most serious complication of epidural anesthesia, has been thoroughly investigated.[9,10,11,12] In particular, a statistical calculation of the risk of paraplegia secondary to hematoma formation in the cardiac surgical population who underwent HTEA, carried out using data from all relevant small studies published before 2000, showed that the risk varied between a minimum 1:150,000 and maximum of 1:1500, with a 95% confidence interval.[13]
Following certain guidelines minimizes the risk for the development of epidural hematoma in patients undergoing epidural anesthesia during cardiac surgery. Significant breaching of such protocols will likely increase the risk of hematoma formation. Studies by Chakravarthy et al.[14] further support the worldwide experience of use of HTEA with anticoagulation in cardiac surgery without any serious complications. In this study, none of the patients developed any neurological squeal and clinical signs of epidural hematoma or neurological injury. There was no incidence of epidural infection, delayed recovery, respiratory depression, and residual muscle weakness in any of the patient.
CONCLUSION
Perioperative TEA using fentanyl with bupivacaine provided optimal postoperative analgesia at rest and during coughing in patients following CABG surgery as compared to postoperative analgesia with conventional intravenous fentanyl. The high thoracic epidural also resulted in optimal postoperative hemodynamic status, good cooperation to chest physiotherapy with less sedation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Sethulaxmi Rajan for preparing the tables and graph.
REFERENCES
- 1.Macguire B, Royse C, Royse A, Duane M, Pang J. Lung function following cardiac surgery is not affected by postoperative ventilation time. Ann Thorac Cardiovasc Surg. 2000;6:13–8. [PubMed] [Google Scholar]
- 2.Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomized trial of high thoracic epidural analgesia for coronary artery bypass surgery. Ann Thorac Surg. 2003;75:93–100. doi: 10.1016/s0003-4975(02)04074-2. [DOI] [PubMed] [Google Scholar]
- 3.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: A systematic review and analysis. Heart. 2003;89:767–72. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantatos A, Silvers AJ, Myles PS. Analgesia best practice after cardiac surgery. Anesthesiol Clin. 2008;26:591–602. doi: 10.1016/j.anclin.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten SE. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: A randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology. 2006;104:142–51. doi: 10.1097/00000542-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Salvi L, Sisillo E, Brambillasca C, Juliano G, Salis S, Marino MR. High thoracic epidural anesthesia for off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2004;18:256–62. doi: 10.1053/j.jvca.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Ginosar Y, Riley ET, Angst MS. The site of action of epidural fentanyl in humans: The difference between infusion and bolus administration. Anesth Analg. 2003;97:1428–38. doi: 10.1213/01.ANE.0000081793.60059.10. [DOI] [PubMed] [Google Scholar]
- 8.Kanai A, Osawa S, Suzuki A, Ozawa A, Okamoto H, Hoka S. Regression of sensory and motor blockade, and analgesia during continuous epidural infusion of ropivacaine and fentanyl in comparison with other local anesthetics. Pain Med. 2007;8:546–53. doi: 10.1111/j.1526-4637.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 9.Williams J. Thoracic epidural anaesthesia for cardiac surgery. Can J Anesth. 2002;49:7R. [Google Scholar]
- 10.Wheatley RG, Schug SA, Watson D. Safety and efficacy of postoperative epidural analgesia. Br J Anaesth. 2001;87:47–61. doi: 10.1093/bja/87.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Castellano JM, Durbin CG., Jr Epidural analgesia and cardiac surgery: Worth the risk? Chest. 2000;117:305–7. doi: 10.1378/chest.117.2.305. [DOI] [PubMed] [Google Scholar]
- 12.Ho AM, Chung DC, Joynt GM. Neuraxial blockade and hematoma in cardiac surgery: Estimating the risk of a rare adverse event that has not (yet) occurred. Chest. 2000;117:551–5. doi: 10.1378/chest.117.2.551. [DOI] [PubMed] [Google Scholar]
- 13.Casalino S, Mangia F, Stelian E, Novelli E, Diena M, Tesler UF. High thoracic epidural anesthesia in cardiac surgery: Risk factors for arterial hypotension. Tex Heart Inst J. 2006;33:148–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarthy M, Thimmangowda P, Krishnamurthy J, Nadiminti S, Jawali V. Thoracic epidural anesthesia in cardiac surgical patients: A prospective audit of 2,113 cases. J Cardiothorac Vasc Anesth. 2005;19:44–8. doi: 10.1053/j.jvca.2004.11.008. [DOI] [PubMed] [Google Scholar]


