Abstract
Background:
Ropivacaine, a newer local anesthetic (LA), has been increasingly used nowadays in different concentrations for peripheral nerve blocks. It has lesser cardiac toxicity and higher safety margin when compared to bupivacaine. Dexmedetomidine, a novel α2 agonist, is widely used as adjuvant to LA in peripheral nerve blocks to decrease the time of onset and increase the duration of the block. In this study, we evaluated the effect of dexmedetomidine as an adjuvant with 0.75% ropivacaine for interscalene brachial plexus block using nerve stimulator.
Aim:
This study aims to know the effect of using dexmedetomidine as an adjuvant to 0.75% ropivacaine in interscalene brachial plexuses block using nerve stimulator.
Settings and Designs:
Sixty patients scheduled for elective orthopedic surgery of the upper limb under interscalene block were considered in this prospective randomized controlled double-blind study. The study population was randomly divided into two groups with thirty patients in each group by using computerized randomization.
Materials and Methods:
Group R received 30 ml of 0.75% ropivacaine with 0.5 ml normal saline and Group RD received 30 ml of 0.75% ropivacaine with 50 μg of dexmedetomidine. The onset of sensory and motor blocks, duration of sensory and motor block, and patient satisfaction score were observed.
Results:
Both the groups were comparable in demographic characteristics. The onset of the sensory and motor block is earlier and statistically significant in Group RD (P < 0.05) when compared to Group R. The duration of sensory and motor blockade were significantly prolonged in Group RD (P < 0.0001).
Conclusion:
Addition of dexmedetomidine to 0.75% ropivacaine in interscalene brachial plexus block significantly shortened the time of onset of the block and prolongs the duration sensory and motor blockade.
Keywords: Dexmedetomidine, interscalene blocks, nerve stimulator, ropivacaine
INTRODUCTION
Regional blocks or peripheral nerve blocks are commonly used nowadays for upper limb orthopedic surgeries as an alternative to general anesthesia as it provides ideal operating conditions with complete muscle relaxation, stable intraoperative hemodynamics, and the associated sympathetic block, along with postoperative analgesia and early recovery avoiding systemic side effects.[1,2,3] Interscalene brachial plexuses block is one of the most widely used blocks for humerus and forearm surgeries.
Various local anesthetics (LAs) such as lignocaine and bupivacaine have been used for administering the blocks. Ropivacaine, a newer LA, has been increasingly used nowadays in different concentration for peripheral nerve blocks. It has lesser cardiac toxicity and higher safety margin when compared to bupivacaine.[4,5] It produces differential neural blockade with less motor block, hence well tolerated for postoperative analgesia and reduced cardiovascular and neurological toxicity.[6,7]
Many drugs such as clonidine[8] and fentanyl[9] have been used successfully as adjuvants with LAs for the early onset of the block and for prolonging the duration of the block. Recently, dexmedetomidine, a novel α2 agonist, having 8 times more affinity to α2 when compared to α1, is widely used as an adjuvant with various LAs in peripheral nerve blocks to decrease the time of onset and increase the duration of block. It has been shown to prolong the sensory and motor duration when added as an adjuvant to LA in nerve blocks.[10,11,12,13] Very few literatures are available on the usage of dexmedetomidine as an adjuvant to 0.75% ropivacaine in interscalene block using nerve stimulator. The ideal dose of dexmedetomidine for nerve blocks is still uncertain. We empirically chose 50 µg dexmedetomidine based on earlier studies.[10,11,12,13]
Hence, this study aims to know the effect of dexmedetomidine as an adjuvant to 0.75% ropivacaine in interscalene block using nerve stimulator.
MATERIALS AND METHODS
After obtaining the Institutional Ethics Committee approval, sixty patients belonging to the American society of Anesthesiologists (ASA) physical status I and II, aged about 18–60 years scheduled for upper limb surgery mainly humerus and forearm surgeries were selected for the study. Preoperatively patients were counseled and familiarized with the use of visual analog scale (VAS) pain score for the assessment of perioperative pain.
All the patients participating in the study were explained clearly about the purpose and nature of the study in their own understandable language and written informed consent was taken. Patients who were on beta-blockers, pregnant, with coagulopathy, skin infection at injection site, known allergy to LA, severe cardiopulmonary disease or neurological deficits in the operative arm or who refused regional anesthesia were excluded from the study.
A thorough preanesthetic evaluation was done. A routine preanesthetic examination was conducted assessing the following:
General condition of the patient
Airway assessment by Mallampati grading and rule of 1-2-3
A detailed examination of the cardiovascular system
A detailed examination of the respiratory system.
The following investigations were done in all patients:
Hemoglobin estimation
Urine examination for albumin, sugar, and microscopy
Standard 12-lead electrocardiogram (ECG)
X-ray chest
Blood sugar, fasting blood sugar/postprandial blood sugar
Blood urea, serum creatinine.
Patients were randomly allocated into two groups of thirty each using computer-generated randomization sequence. Patients in Group R received 30 ml of 0.75% ropivacaine with 0.5 ml of normal saline by electrical stimulation and patients in Group RD received 30 ml of 0.75% ropivacaine with 50 µg (0.5 ml) dexmedetomidine by electrical stimulation. The person who prepared the drug solution was different from the person who administered the block and the person who monitored the quality and duration of the block and the hemodynamics postoperatively.
All patients included in the study were premedicated with tablet alprazolam 0.5 mg and tablet ranitidine 150 mg orally at bedtime the previous night before surgery. They were kept nil orally 10 pm onwards on the previous night.
On arrival of the patient in the operating room, an 18-gauge intravenous cannula was inserted, and an infusion of 500 ml Ringer lactate was started. The patients were connected to, Mindray multi-parameter monitor which records heart rate (HR), noninvasive measurements of systolic blood pressure (SBP), diastolic BP (DBP), mean arterial pressure (MAP) and continuous ECG monitoring, respiratory rate (RR), and oxygen saturation (SpO2). The baseline SBP, DBP, MAP, and HR were recorded (basal parameters).
After positioning the patient on operating table with the head turned to opposite side, interscalene groove was identified by rolling the finger posterior to sternocleidomastoid muscle between the belly of the anterior and middle scalene muscle at the level of cricoid cartilage. Skin over the insertion site was infiltrated with 2% lignocaine.
Interscalene block was performed in all patients with the peripheral nerve stimulator (Stimuplex®, B Braun) connected to 3.5-cm, 22-gauge, short-bevel insulated stimulating needle by modified Winnies approach. The intensity of stimulating current was initially set to deliver 1 mA with an impulse duration of 0.1 ms. Thereafter, current was gradually decreased to 0.5 mA. The localization of the plexus was considered optimal when an output current of <0.5 mA caused the contraction of pectoralis muscle, deltoid, biceps or triceps. After eliciting motor response of any of these muscles, 30 ml of 0.75% ropivacaine with 0.5 ml of normal saline was given in Group R, in the increments of 5 ml after fixing the stimulating needle, aspirating in between to avoid inadvertent intrathecal or intravascular injection.
The same procedure mentioned above was repeated in patients of Group RD, and 30 ml of 0.75% ropivacaine with 50 mcg (0.5 ml) of dexmedetomidine was injected.
Hemodynamic parameters such as HR, SBP, DBP, SpO2, and RR were monitored at every 10 min interval till 1 h of LA injection and then every 20 min till 2nd h and thereafter every hour min till the end of surgery and postoperatively one hourly till first 24 h. Adverse events such as hypotension (20% decrease in relation to the baseline value), bradycardia (HR <60 bpm), hypoxemia (SpO2≤ 90%), and perioperative nausea and vomiting were recorded. Patient's perception of pain was assessed using VAS (0–10). Rescue analgesics in the form of injection fentanyl 1 µg/kg body weight was given in case patient complained of intraoperative discomfort at any time of surgery (VAS >3).
The following parameters were assessed.
Onset of sensory block
Sensory block was assessed by loss of sensation to pinprick over the C5-T1 dermatomes using a 3-points scale which is as follows:
0 - sharp pain, 1 - dull pain (analgesia), 2 - no pain (anesthesia).
Sensory onset time was defined as the time interval between the end of LA administration and establishment of score 2 on 3-point scale on all nerve territories.
Onset of motor block
Motor block was assessed using Bromage scale.
0 - Normal motor functions with full flexion and extension of the elbow, wrist, and fingers
1 - Decreased motor strength with the ability to move fingers only
2 - Complete motor blockade with the inability to move fingers.
Motor block onset time was defined as the time interval between the end of LA administration and complete motor block (score 2).
Duration of sensory block
Duration of sensory block was defined as the time interval between the end of LA administration and the complete resolution of anesthesia (score 0 on a 3-point scale) on all nerves.
Duration of motor block
Duration of motor block was defined as the time interval between the end of LA administration and the recovery of complete motor function (Score 0 on Bromage scale).
Patient satisfaction
Patient satisfaction score (PSS) was recorded after 24 h postoperatively as 5 - excellent, 4 - very good, 3 - good, 2 - fair, and 1 – poor.
In case of pain, supplementary analgesia with 1 µg/kg boluses of intravenous fentanyl was given. Block was considered inadequate when sensory anesthesia was not achieved within 30 min. General anesthesia was given subsequently to these patients who were then excluded from the study.
Statistical analysis
A sample size of 25 patients were needed to detect an intergroup difference of at least 10% in BP and HR with a power of 0.80 and α of 0.05. In order to make good for attrition rate, a total number of thirty patients in each group were included for the study. The independent samples t-test procedure compares means for two groups of cases. SPSS for windows (version 21.0, SPSS Inc., Chicago, IL, USA) was employed for data analysis. P < 0.05 was considered as significant.
RESULTS
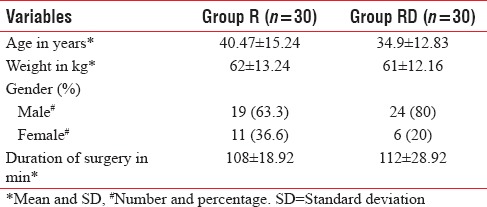
Sixty patients belonging to ASA physical status 1 and 11 undergoing humerus and forearm surgeries were included in the study. As shown in Table 1, patients in both groups were comparable with respect to baseline demographic characteristics.
Table 1.
The demographic profile and surgical characteristics of both groups
As shown in Table 2, the mean time for the onset of sensory block in Group R was 16 ± 1.93 min and in Group RD was 11.98 ± 2.01 min. The difference was statistically significant with earlier onset of sensory block in Group RD (P < 0.0001).
Table 2.
Onset of sensory and motor blockade, duration of sensory, motor block, patient satisfaction, and insufficient block
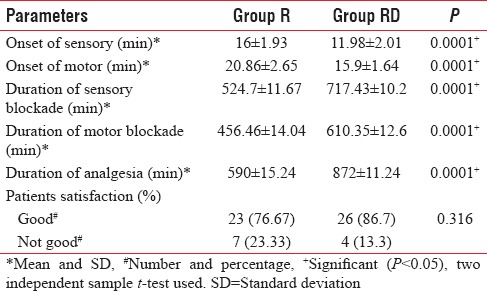
The mean time for the onset of motor block in Group R was 20.86 ± 2.65 min and in Group RD was 15.9 ± 1.64 min. The difference was statistically significant with earlier onset of sensory block in Group RD (P < 0.0001).
The mean duration of sensory block in Group R was 524.7 ± 11.67 min and in Group RD was 717.43 ± 10.2 min. The difference was statistically significant with earlier onset of sensory block in Group RD (P < 0.0001).
The mean duration of motor block in Group R was 456.46 ± 14.04 min and in Group RD was 610.35 ± 12.6 min. The difference was statistically significant with earlier onset of sensory block in Group RD (P < 0.0001). Total duration of analgesia in Group R was 590 ± 15.24 min and in Group RD was 872 ± 11.24 min. The difference was statistically significant.
Hemodynamic parameters such as HR, SBP, DBP, and MAP were measured in both the groups at 5, 10, 15, 20, 30, 45, 60, 90, 120 min and at 4, 6, 12, 18, and 24 h after giving block.
As shown in Graph 1, we observed a statistically significant difference (P < 0.05) in HR between two groups from 10 min after the block. In Group RD, HR was maximally reduced after 30 min of giving block, which returned to normal values at about 18 h. No patients required treatment for bradycardia.
Graph 1.
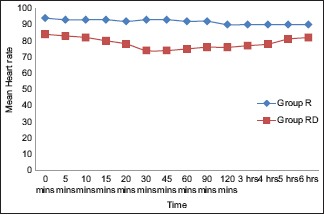
Comparison of heart rate between the groups at different intervals.
As shown in Graph 2, monitoring of MAP was done in both the groups at 5, 10, 15, 20, 30, 45, 60, 90, 120 min and at 4, 6, 12, 18, and 24 h after giving block. A statistically significant difference was observed in MAP from 10 min that returned to baseline after 6 h. Both the groups were comparable for RR and SpO2 at each interval, and the results were statistically insignificant. None of the patients in either group had technique or drug-related side effects or complications. Patients in both the groups had an equally good PSS.
Graph 2.
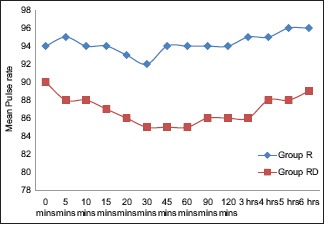
Comparison of the mean arterial pressure between the groups at different intervals.
DISCUSSION
Brachial plexus blocks are commonly used for upper limb orthopedic surgeries. Interscalene brachial plexus block is most often used for humerus and forearm surgeries. It was first described by Winnie in 1970, but the technique had a high incidence of major complications such as inadvertent injection in intrathecal space or vertebral artery injection for which the patients developed seizures. The technique was unpopular for a period of time when it underwent many modifications and has now proved its safety and advantages to general anesthesia in current studies.[14,15]
Various LAs such as lignocaine, bupivacaine, and ropivacaine have been used in peripheral nerve block. Though lignocaine causes profound muscle relaxation, it is associated with significant local nerve toxicity. Bupivacaine has serious cardiac toxicity if accidental intravascular injection occurs. Ropivacaine is a newer LA that is used in various concentrations of 0.25%, 0.5%, and 0.75% for nerve blocks. It has less cardiac toxicity when compared to bupivacaine. It causes a greater sensory and motor differential blockade than bupivacaine, which is dose dependent, with higher concentrations (1%) causing greater degree of motor blockade than lower concentration (0.5% and 0.75%).[16,17]
Many adjuvants such as clonidine,[8] fentanyl,[9] and magnesium[18] have been added to LA to hasten the onset time and prolong the duration of block. Recently, dexmedetomidine has been added to LA for regional block with good results. It has been found to significantly reduce the onset time and prolong the duration of sensory and motor block.[10,11,12,13,19,20] Dexmedetomidine is a newer congener of clonidine, which is an α2 agonist. Dexmedetomidine is a pharmacologically active d-isomer of medetomidine and is highly specific and selective α2 adrenoceptor agonist with α2:α1 binding selectivity ratio of 1620:1 as compared to 220:1 for clonidine, thus decreasing the unwanted side effects of α1 receptors. Presynaptic activation of α2 adrenoceptor in central nervous system inhibits the release of norepinephrine, terminating the propagation of pain signals and their postsynaptic activation inhibits sympathetic activity, thereby decreasing HR and BP.[21]
Various authors had used it in combination with LAs such as bupivacaine 0.25%,[10] levobupivacaine 0.5%,[13] and ropivacaine 0.5% and 0.75%.[19,20]
Esmaoglu et al. reported that addition of 100 µg of dexmedetomidine to levobupivacaine for axillary block causes significant prolongation sensory and motor blockade and also causes high incidence of bradycardia. However, when lower dose of dexmedetomidine was used, the incidence of bradycardia was not significant.[10,19,20]
In our study, we evaluated the effect of adding dexmedetomidine 50 µg as an adjuvant to 0.75% ropivacaine for interscalene brachial plexuses block using nerve stimulator. This dose of dexmedetomidine (50 µg) was chosen as earlier studies of higher doses of the drugs showed bradycardia.[13] We assessed onset time of sensory and motor block, total duration of sensory and motor block, and requirement of rescue analgesia. We found that addition of dexmedetomidine to 0.75% ropivacaine significantly shortened the onset time for sensory and motor block and also significantly extended the duration of sensory and motor block. The need for rescue analgesia in postoperative period was significantly reduced.
Marhofer et al.[19] has studied the effect of dexmedetomidine on 0.75% ropivacaine for ulnar nerve block. They found that the onset of motor block was faster, and the duration of motor block was significantly prolonged by the perineural administration of dexmedetomidine.
In another study done by Agarwal et al.,[22] dexmedetomidine was used as an adjuvant to bupivacaine in supraclavicular brachial plexuses block. Authors concluded that the addition of dexmedetomidine significantly shortens the onset time and prolongs the duration of sensory and motor block, which is similar to the results found by our study.
In 2014, Biswas et al.[23] studied the effect of adding dexmedetomidine as an adjuvant to levobupivacaine and found that it significantly shortens the sensory and motor onset time and prolongs the duration of sensory and motor block.
In our study, there was a significant prolongation of the duration of analgesia in the ropivacaine and dexmedetomidine group, and also the rescue analgesia was taken late than the plain ropivacaine group.
Limitations of our study
Fixed dose of dexmedetomidine (50 µg) was chosen empirically as higher doses of the drug showed significant bradycardia which was reported by Esmaoglu et al.[13]
We could not measure the relationship between dose and plasma concentration of ropivacaine and dexmedetomidine in our study as the facility is not available in our institution.
Combination of nerve stimulator and ultrasound-guided block would have been the better option than using nerve stimulator alone. As ultrasound is not available, we used nerve stimulator alone.
CONCLUSION
We conclude that addition of dexmedetomidine to 0.75% ropivacaine in interscalene brachial plexus block significantly shortens the time of onset of sensory and motor block and also extended the duration of sensory and motor blockade. However, further research work is needed to study the pharmacokinetics of dexmedetomidine when it is used as an adjuvant with LA and the effects of its systemic absorption.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McKenzie PJ, Loach AB. Local anaesthesia for orthopaedic surgery. Br J Anaesth. 1986;58:779–89. doi: 10.1093/bja/58.7.779. [DOI] [PubMed] [Google Scholar]
- 2.Jacob AK, Walsh MT, Dilger JA. Role of regional anesthesia in the ambulatory environment. Anesthesiol Clin. 2010;28:251–66. doi: 10.1016/j.anclin.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Kothari KC. Regional anesthesia techniques for orthopedic surgery. MJAFI. 2008;64:108–10. [Google Scholar]
- 4.Casati A, Fanelli G, Albertin A, Deni F, Anelati D, Antonino FA, et al. Interscalene brachial plexus anesthesia with either 0.5% ropivacaine or 0.5% bupivacaine. Minerva Anestesiol. 2000;66:39–44. [PubMed] [Google Scholar]
- 5.Klein SM, Greengrass RA, Steele SM, D’Ercole FJ, Speer KP, Gleason DH, et al. A comparison of 0.5% bupivacaine, 0.5% ropivacaine, and 0.75% ropivacaine for interscalene brachial plexus block. Anesth Analg. 1998;87:1316–9. doi: 10.1097/00000539-199812000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Kuthiala G, Chaudhary G. Ropivacaine: A review of its pharmacology and clinical use. Indian J Anaesth. 2011;55:104–10. doi: 10.4103/0019-5049.79875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClellan KJ, Faulds D. Ropivacaine: An update of its use in regional anaesthesia. Drugs. 2000;60:1065–93. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- 8.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 9.Sindjelic RP, Vlajkovic GP, Davidovic LB, Markovic DZ, Markovic MD. The addition of fentanyl to local anesthetics affects the quality and duration of cervical plexus block: A randomized, controlled trial. Anesth Analg. 2010;111:234–7. doi: 10.1213/ANE.0b013e3181e1e9ab. [DOI] [PubMed] [Google Scholar]
- 10.Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth. 2012;56:243–9. doi: 10.4103/0019-5049.98767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an α-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 12.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 13.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 14.Borgeat A, Dullenkopf A, Ekatodramis G, Nagy L. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology. 2003;99:436–42. doi: 10.1097/00000542-200308000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: A prospective study. Anesthesiology. 2001;95:875–80. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 16.McClure JH. Ropivacaine. Br J Anaesth. 1996;76:300–7. doi: 10.1093/bja/76.2.300. [DOI] [PubMed] [Google Scholar]
- 17.Finucane BT, Sandler AN, McKenna J, Reid D, Milner AL, Friedlander M, et al. A double-blind comparison of ropivacaine 0.5%, 0.75%, 1.0% and bupivacaine 0.5%, injected epidurally, in patients undergoing abdominal hysterectomy. Can J Anaesth. 1996;43(5 Pt 1):442–9. doi: 10.1007/BF03018104. [DOI] [PubMed] [Google Scholar]
- 18.Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59:21–7. doi: 10.1007/s12630-011-9604-5. [DOI] [PubMed] [Google Scholar]
- 19.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 20.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–62. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 21.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Bayl Univ Med Cent Proc. 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal S, Aggarwal R, Gupta P. Dexmedetomidine prolongs the effect of bupivacaine in supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2014;30:36–40. doi: 10.4103/0970-9185.125701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas S, Das RK, Mukherjee G, Ghose T. Dexmedetomidine an adjuvant to levobupivacaine in supraclavicular brachial plexus block: A randomized double blind prospective study. Ethiop J Health Sci. 2014;24:203–8. doi: 10.4314/ejhs.v24i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]


