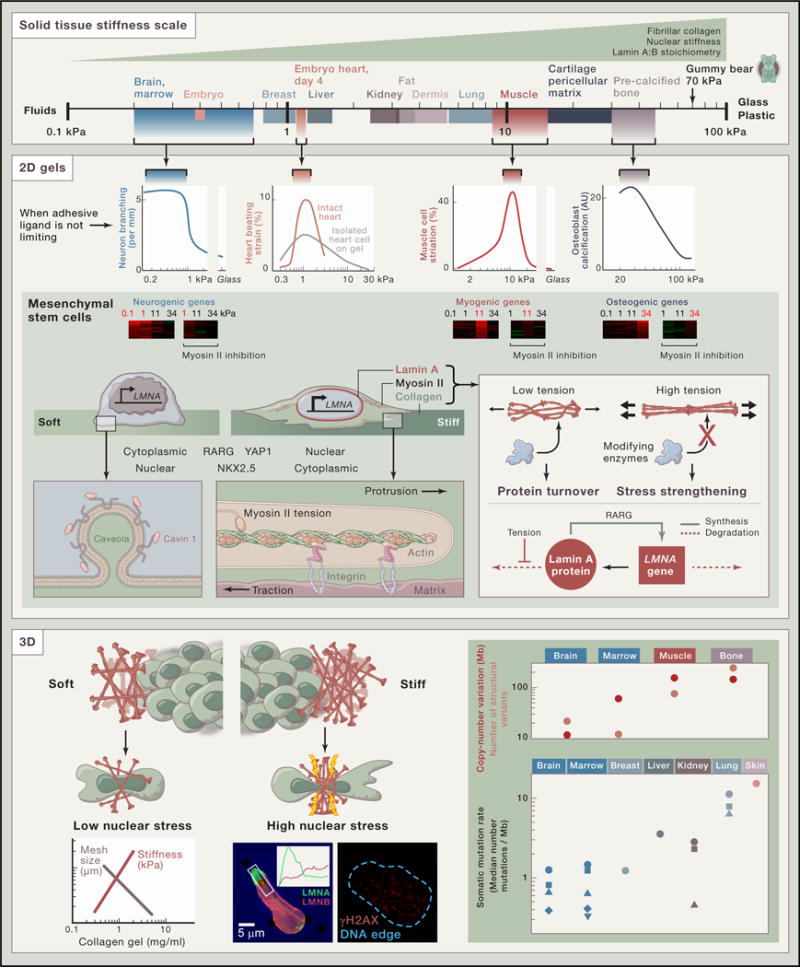
The human body harbors tissues that range in stiffness from the comparatively soft brain to the rigid bones of calcified collagen that define our frames. This spectrum supports and influences the underlying cell physiological processes. Cells apply forces and contractile tensions to probe the stiffness of the surrounding tissue, and they respond appropriately or, sometimes in pathogenesis, inappropriately.
Embryos are very soft cell aggregates, but the first organ to develop, the heart, stiffens within days (Majkut et al., 2013). Micro-stiffness, measured at the scale of a cell or a few cells and expressed in SI units of kiloPascal (kPa), quantifies the mechanical stress needed for a fractional change in length. For example, a gummy bear is squashed 10% by about 7 kPa of stress. Most tissues require far less stress to deform as much. If the stress is removed and the tissue springs back, as heart does, then it behaves as an elastic solid, but softer tissues such as brain do not fully recover and thus exhibit some similarities to a viscous fluid. Adult tissues–whether man, mouse, or other species–have a characteristic micro-stiffness that increases from soft brain and marrow to slightly stiffer breast matrix (Lopez et al., 2011), to much stiffer striated muscle and pre-calcified bone matrix (Swift et al., 2013).
The most abundant protein in adults is the structural protein collagen (specifically collagen I), and local tissue stiffness increases with the density of this and other fibrillar collagens, as does the stiffness of gels made with purified collagen. Within cells in normal tissues, the intermediate filament nuclear protein lamin A also increases with tissue stiffness, whereas the B-type lamins are nearly constant (Swift et al., 2013). The increase of lamin A level stiffens the nucleus, particularly the viscosity of the nucleus. In some diseases such as muscular dystrophy, fibrosis is evident as increased collagen and increased tissue stiffness (Engler et al., 2004), and lamin A is also increased.
Stiffness affects the functions of intact tissues as well as cells in culture. Very early in development, collagen I density increases in the heart to form a scaffold supporting cardiomyocyte’s rhythmic contractions (Majkut et al., 2013). Heart cells sense the difference: periodic beating is not only maximum for intact hearts with native stiffness, but also for single cardiomyocytes isolated from the same hearts and grown on 2D collagen-coated gels with heart-like stiffness. Striation of myosin II that drives contraction is also maximized by stiffness.
Early results showed that in 2D cultures neurons branch best on gels that are soft like brain (Flanagan et al., 2002), skeletal myotubes striate best on gels with similar stiffness to muscle (Engler et al., 2004), and osteoblasts are most osteogenic on stiffer gels like that of pre-calcified bone. Gene expression of mesenchymal stem cells (MSCs) was likewise shown to be induced toward neurogenesis in cultures on soft gels, toward myogenesis on intermediate stiffness gels, and toward osteogenesis on the stiffest gels, with all of these responses dependent on myosin II applying forces via adhesions to the gels (Engler et al., 2006).
Cell shape is downstream of matrix stiffness and requires the coordinated activity of many factors. Within F-actin rich protrusions, F-actin binds matrix via integrin-anchored complexes, and further back in the cell body the F-actin network also binds myosin II filaments (Giannone et al., 2007). Myosin II thereby applies tension to the matrix, and if the matrix is stiff and if the adhesive ligand is abundant, then (1) integrin adhesions are stabilized, (2) myosin II tractions on the matrix increase further, and (3) a flattened lamellipodia attaches and spreads on stiff matrix. If the matrix is soft, then processes (1)–(3) fail and cells do not spread. Plasma membrane remodeling in response to local environment is also implicated by multiple observations, including an increased abundance of the caveloae factor cavin 1 as a function of tissue stiffness (Swift et al., 2013).
Regulatory pathways respond to and reflect the local matrix environment as well. For example, key MSC transcription factors localize differentially depending on the microstiffness of the substrate. In cells grown on soft gels, RARG and YAP1 are cytoplasmic with NKX2.5 being nuclear, but these factors translocate when the cells are cultured on stiff gels to the nucleus or cytoplasm, respectively. For normal regulation of lamin A, high tension in cells on stiff matrix stresses the nucleus and stabilizes lamin A protein, which recruits a large amount of RARG from cytoplasm into the nucleus (via a shared binding factor), so that RARG can drive lamin A expression to be high on stiff gels as well as within stiff tissues (Swift et al., 2013). Mechanobiological gene circuits can thus be controlled by tension-suppressed solubilization and degradation of filamentous systems, in a “use it or lose it” mechanism. Epigenetic mechanisms involved in matrix mechanosensing remain an active topic of study.
Invasive migration of cancer cells into nearby normal tissue occurs in many processes, and soft tissues with low collagen matrix levels and larger pores are expected to be more permissive to this colonization. Indeed, invasion through small pores in stiff matrices can cause high nuclear stress, sufficient to disrupt the nuclear lamina and increase DNA damage as well as cell death (Harada et al., 2014). Genomic changes in cancer are surprisingly well organized by the scale of normal or host tissue micro-stiffness. Chromosome copy-number changes and translocations in childhood cancers (Chen et al., 2014) increase with tissue micro-stiffness as strongly as somatic mutations across diverse cancers (Alexandrov et al., 2013).
For the childhood cancers, the data are for medulloblastoma (brain), acute lymphoblastic leukemia (marrow), embryonic rhabdomyosarcoma (muscle), and osteosarcoma (bone). Symbols in the lower plot are for the brain cancers glioma low grade (
 ), glioblastoma (
), glioblastoma (
 ), neuroblastoma (
), neuroblastoma (
 ), medulloblastoma (
), medulloblastoma (
 ); the marrow cancers multiple myeloma (
); the marrow cancers multiple myeloma (
 ), lymphoma B cell (
), lymphoma B cell (
 ), chronic lymphocytic leukemia (
), chronic lymphocytic leukemia (
 ), acute myeloid leukemia (
), acute myeloid leukemia (
 ), acute lymphoblastic leukemia (
), acute lymphoblastic leukemia (
 ); the kidney cancers clear cell (
); the kidney cancers clear cell (
 ) papillary cell (
) papillary cell (
 ), chromophobe (
), chromophobe (
 ); and the lung cancers squamous (
); and the lung cancers squamous (
 ), adenocarcinoma (
), adenocarcinoma (
 ), and small cell (
), and small cell (
 ).
).
Acknowledgments
Seminal contributions from many groups to the topic of matrix mechanosensing were not able to be included due to length restrictions of this SnapShot, but some can be found within the references. This work was supported by the National Institutes of Health, National Cancer Institute (U54-CA193417); National Heart, Lung, and Blood Institute (R21-HL128187); National Institute of Diabetes and Digestive and Kidney Diseases (P01-DK032094); the American Heart Association (14GRNT20490285); and the National Science Foundation (Materials Research Science and Engineering Center).
References
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, et al. St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome Project Cell Rep. 2014;7:104–112. doi: 10.1016/j.celrep.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neuroreport. 2002;13:2411–2415. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Döbereiner HG, Freund Y, Borisy G, Sheetz MP. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE. J Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JI, Kang I, You WK, McDonald DM, Weaver VM. Integr Biol (Camb) 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Curr Biol. 2013;23:2434–2439. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]


