SUMMARY
The Hypertension Community has three conflicting dilemmas, a goal systolic pressure of 120 mmHg or less (The SPRINT Trials), 40% of our 60,000,000 hypertensives still sustain blood pressures above 140/90 mmHg and our most potent antihypertensive drug minoxidil sits on the side-lines, imprisoned in the FDA’s Black Box Designation. My solutions to these dilemmas are: #1. Review of the facts of our most potent antihypertensive drug minoxidil which is essentially free of toxicity, #2. Treatment focus on the fundamental cause of High Blood Pressure, excess dietary sodium and, #3. Prevention of, and/or reversal of, the fundamental mechanism of worsening hypertension, arteriolar hypertrophy.
My focus at U.T. Southwestern in Dallas was on extremely severely hypertensive patients with a quantifiable, measurable complication of high blood pressure, progression of nephrosclerotic damage to kidneys. This model had the greatest likelihood of exposing fundamental dis-regulatory mechanisms in hypertensive patients (which it did) and the potential for study of the most relevant antihypertensive drug interactions to achieve optimal blood pressure control (which it did). By maintaining diastolic pressures at 80 mmHg or less in the first NIH supported, long term randomized clinical trial to save the kidneys, the bases for a fundamental blood pressure support mechanism (arteriolar hypertrophy) was illuminated but not fully described until now. This fundamental hypertensinogenic mechanism results from HBP but with time and severity, becomes its own raison d’être.
I am now 84 years of age. As a result of a stroke 20 years ago which caused permanent double vision and because of poor blood pressure control with triple therapy I started using minoxidil 5 mg/day along with atenolol and occasional furosemide. Now, along with some dietary salt restriction, my resting blood pressure is 110/65–125/75 and, in spite of > 30 year history of HBP, I have no retinal arteriolar hypertrophy nor arcus senilis (Dr. Schwartz-U. of Miami) which is almost universally present at this age. YES, prevention of, or reversal of, arteriolar hypertrophy should be a central focus of HBP treatment. I simply wish to share a bit of accumulated wisdom that might be of use to others.
INTRODUCTION
Results of the recent SPRINT Trials (1,2) demand that we and the Food & Drug Administration (FDA) reassess the clinical use of minoxidil (Loniten), our most potent antihypertensive drug. The goal systolic blood pressure in the SPRINT Trials in the Strict Control Group was 120 mmHg. Complications of High Blood Pressure (HBP) in this group were reduced except that renal function appeared to decline in the Strict Control Group (discussed below with edema and the PRAS Syndrome). An embarrassing fact to our hypertension community is that over 40% of hypertensives’ blood pressure is sustained above 140/90 mmHg in the USA (3). Even though Minoxidil is virtually free of toxicity the FDA has essentially shut down its use by a Black Box Designation. This designation resulted from two side effects (cardiac symptoms and edema) that could have been, and should have been controlled by companion drugs which, in fact, potentiate minoxidil’s efficacy.
Chidsey’s prophetic analysis (4) in 1973 of minoxidil’s role was ignored by the FDA and by the medical community. He noted that “minoxidil’s extraordinary efficacy when combined with a diuretic and beta-receptor blocker, lack of tolerance for two years, and its paucity of side effects suggests that this agent may be of great value in the chronic therapy of hypertension”.
Our interest in minoxidil was precipitated by its historic role in arresting the tragic procedure of removing both kidneys of patients with refractory hypertension and advanced nephrosclerosis (5). It soon became apparent that far better blood pressure control was needed to “save-the-kidneys” and that more understanding of drug interactions with minoxidil and blood pressure support mechanisms might enable us to achieve better blood pressure control. These interactions extend to fascinating intra-renal mechanisms involving arteriolar hypertrophy, adrenergic receptors, renin-angiotensin and the increasingly recognized cause of reversible renal failure from drugs, the Pseudo Renal Artery Stenosis Syndrome (PRASS) (6). Interestingly, mechanisms of PRASS (to be discussed) were the cause of poor kidney outcomes in the SPRINT trial in the Elderly (2) in the Strict Blood Pressure Control Group (37 events) vs Conventional (13 events) blood pressure control group. All other indices of outcomes were improved in the “Strict” (<120 mmHg systolic pressure) control group.
Our early goal was to reduce diastolic blood pressure to <80 mmHg (minoxidil was required in all patients) in the most severely hypertensive nephrosclerosis patients prior to randomization into two groups. In one randomized group we attempted to maintain diastolic pressures below 80 mmHg and in the other to permit diastolic pressure to be maintained above 90 mmHg by reducing drug dosages. Analogies to this strategy was subsequently applied in the two SPRINT Trial Designs (1,2).
The interactions reviewed herein relate to impressive activation of the sympathetic nervous system and renal retention of sodium leading to edema. Because of the power of the four mechanisms causing edema from minoxidil, emphasis is placed on restriction of dietary sodium by documenting the real Minimum Daily Requirement (MDR) for sodium (<0.2 G/Day).
When compared to most other antihypertensive drugs minoxidil is unique in several ways:
-
#1
Minoxidil dilates pre-capillary arterioles. Arteriolar hypertrophy (discussed later) results from from High Blood Pressure (HBP). Because of encroachment on the arteriolar lumen it becomes a fundamental hypertensinogenic mechanism independent of the initiating cause.
-
#2
Minoxidil is as an agonist drug. Receptor blockers or enzyme inhibitors suppress only the ambient level of activity of their specific blood pressure support mechanism whereas an agonist has a full range of pharmacologic activity.
-
#3
Minoxidil has a 3–4 day duration of action. It binds strongly to its arteriolar site of action via a covalent (sulfydryl) linkage. Therefore its pharmacologic effects are cumulative with constant daily dosing for up to a week at which time its efficacy reaches a plateau; once daily dosage should suffice. This pharmacologic property of minoxidil may be the determining factor of its greater efficacy in clinical use than the vasodilator hydralazine (Apresoline).
-
#4
Minoxidil stimulates/maintains hair growth. This effect is highly variable from patient to patient and a mixed bag indeed. Because its site and mechanism of action is on vascular smooth muscle leading to increased blood flow, future minoxidil research should include diseases potentially related to ischemia. These include age-related hearing loss and penile erectile dysfunction for which use-patents (not mine) are on file. If age related dementias are due to ischemia minoxidil might have efficacy therein as well.
Fortunately, we had a large coterie of severely hypertensive patients at Parkland Hospital in Dallas in whom we could do full dose-response curves of each of the types of antihypertensive drugs superimposed on two or on three other drugs at fixed doses. We monitored indices of each blood pressure support mechanism including supine and standing Serum Renin Activity (SRA) and plasma norepinephrine (NE) concentrations and vasoconstrictor effects using the selective antagonists P-113 (Saralasin) for angiotensin (Ang II) and phentolamine or prazosin for NE. In some studies we measured cardiac output, venous compliance, peripheral and pulmonary vascular resistance and, of course, blood pressures with various drug combinations. Mechanistic studies of these interactions and blood pressure support mechanisms were also done in genetic hypertensive and normotensive animals and in isolated perfused rat kidneys.
PHARMACOLOGY
My focus is on the mechanisms of side effects of minoxidil and control thereof by companion drugs with the goal of “opening the door” for physicians to take advantage of its unique efficacy. For traditional aspects of minoxidil pharmacology see reviews (7–9).
#1. CARDIAC REFLEX EFFECTS
Minoxidil’s antihypertensive site of action is arteriolar smooth muscle; active-agonist dilation at this site decreases peripheral resistance thereby lowering blood pressure. Lowering of blood pressure by any mechanism other than suppressing of, or blocking sympathetic neuronal effects, causes reflex activation of the sympathetic neural release of norepinephrine (Figure 1) onto peripheral alpha (alphaAR) and beta-adrenergic receptors (betaAR).
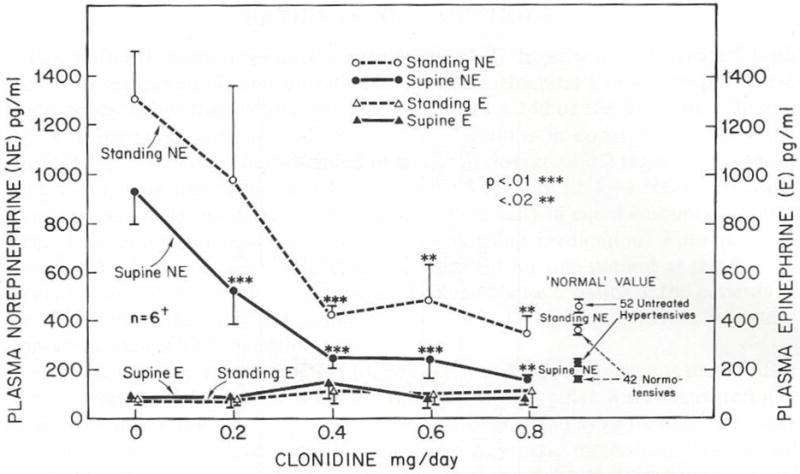
FIG 1.
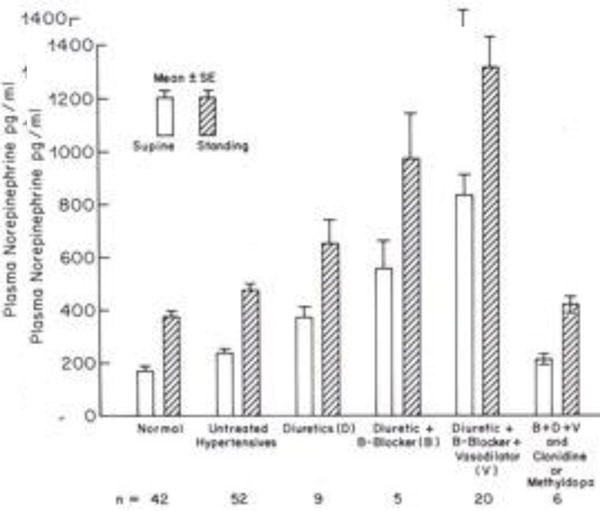
Plasma NE concentrations after 10 minutes supine and after two min. standing, in normotensives, untreated hypertensives, with diuretics, + beta-blockers, + hydralazine or minoxidil, + clonidine or methyl-dopa.
Failure to control this reflex activation (Figure 2) or its effects, especially with higher doses of minoxidil, causes increased heart rate, myocardial contractility, arrhythmias and even myocardial infarction. Failure of physicians to control this minoxidil-induced reflex activation contributed to the FDA’s Black-Box Designation, thereby restricting and limiting minoxidil usage to drug-resistant hypertension and scaring physicians away from its use because of potential litigation.
FIG 2.
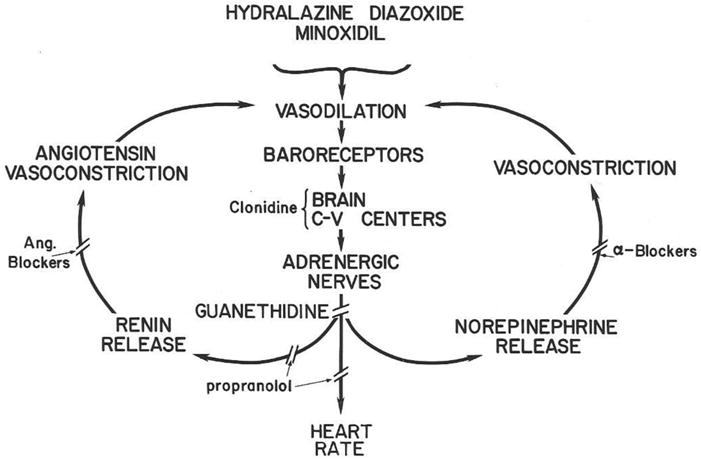
Vasodilator-beta-blocker interactions. Vasodilation lowers blood pressure, signals to the C-V center in the brain to activate release of NE onto peripheral alpha and beta-adrenoceptors. Angiotensin and norepinephrine constriction, increased cardiac output and sodium retention limit blood pressure lowering of vasodilator drugs unless drugs are used to interfere with the three limbs of the reflexes. (Reprinted with permission from New England Journal of Medicine, WA Pettinger, Minoxidil and the Treatment of Severe Hypertension, Vol 303, page 992. Copyright 1980 Massachusetts Medical Society).
Plasma concentrations of norepinephrine (NE) with various antihypertensive drug combinations are shown in Figure 1. Hypertensives tended to have slightly (not statistically significant) higher plasma norepinephrine levels and there was a gradation throughout more severely hypertensive patients as a function of the requirement for more powerful drug combinations. High plasma NE concentrations were reversed by clonidine or methyldopa which lower blood pressure by their site of action on alpha2-adrenoceptors (10) (alpha2A) in the cardiovascular control center in the brain (11).
Veins are like a sewer for disposal of NE released from sympathetic nerve endings so its concentration in venous blood is an index of sympathetic neuronal activity. Circulating levels of NE are normally below the level for vasoconstriction. However, the circulating levels (Figure 1) with higher doses of minoxidil are in the range of circulating NE concentrations in pheochromocytoma. Interestingly, the effects of excess NE released from sympathetic nerve terminals directly onto receptors (ie. due to reflex activation) contrasts remarkably from effects of high circulating NE levels from pheochromocytoma, a concept deserving of further comment in a discussion of minoxidil edema and effects of clonidine (see below) on the edema.
Hydralazine (Apresoline) and reflex cardiac stimulation
Hydralazine, like minoxidil is an agonist vasodilator but is also a specific angiotensin receptor blocker (ARB) (12). In the model (ganglionic blockaded, anesthetized rat) in which this property was discovered vasodilators shifted angiotensin and norepinephrine dose response curves to the right equally except for hydralazine. Hydralazine shifted the DRC for angiotensin much further to the right than NE and therefore is also a selective ARB. The shift of DRCs by 40+ vasodilators correlated quantitatively with antihypertensive activity in genetic hypertensive and DOCA-sodium hypertensive rats (12). Interestingly, the enzyme inhibiting capacity of phosphodiesterase inhibitors (caffeine, theophylline, and 20 other un-named drugs) were directly proportional to their antihypertensive and vasodilating potencies (12), thus linking causality of these three properties.
Because of this combined pharmacologic property (ARB plus vasodilator), hydralazine caused cardiac arrhythmias and infarctions in the emergency room of Parkland Hospital when hydralazine was administered intravenously for extreme blood pressure elevation. Consequently, we abandoned its use in this manner. Alternatively, orally administered minoxidil has been used for rapid control of extreme elevations of blood pressure (13,14) and I have used it this way as well. Alpert (13) started with a 20 mg dose of minoxidil superimposed on a beta-blocker and furosemide and added 5–20 mg four hours later if necessary. One could start with 5–10 mg initially (after a beta-blocker) and add to the dose at 3–4 hour intervals with careful monitoring. Importantly, it is simpler and easier to use than nitroprusside or even diazoxide. However, if heart failure-pulmonary edema are present nitroprusside infusion is preferable because of veno-dilation and reduction of venous return by this drug, thereby reducing cardiac work-load.
Even with the combination of pharmacologic effects, hydralazine is less potent as a blood pressure lowering agent than minoxidil as first noted by Chidsey (4) and in our own experience. Keep in mind however, that hydralazine was a major component of the drug combination used in the transformative VA Cooperative studies coordinated by Fries (15,16), so this drug clearly has efficacy in controlling blood pressure and preventing complications. Note the expression of Fries’ wisdom of the use of reserpine (site of action is in the CNS like clonidine and methyl-dopa) in Figure 2. Hydralazine causes headaches and occasional lupus erythematosis at doses of 200 mg/day and higher.
#2. MINOXIDIL EDEMA
Four physiologic mechanisms contribute to edema from minoxidil, each of which are dependent on excess dietary sodium (see below). These mechanisms are:
#1. Capillary Leakage
A function of precapillary arterioles is to prevent excess blood flow into capillaries when blood pressure is temporarily increased. Long-standing high blood pressure from any cause leads to hypertrophy of this circular smooth muscle (Figure 3), encroachment on the lumen and increased passive resistance to blood flow. Therefore arteriolar hypertrophy becomes a fundamental mechanism of sustaining high blood pressure per se. Arteriolar dilation, especially with minoxidil, increases blood flow into capillaries thereby increasing their transmural pressure gradient which shifts water into extracellular spaces thereby contributing to edema formation, which is highly variable among patients.
FIG 3.

Progressive Arteriolar Hypertrophy with duration and severity of blood pressure elevation. The outer fibrous layer is fixed in circumference so hypertrophy encroaches on the lumen and becomes a major passive mechanism for maintenance of hypertension independent of the original cause. My goal in treatment is to reverse this hypertrophy by sustaining diastolic blood pressure <80 mmHg for months, thereby lessening this fundamental hypertensinogenic mechanism.
#2. Reduced Glomerular Filtration Pressure
Because salt excretion is partially pressure dependent as described by Guyton (17), the extraordinary lowering of blood pressure capacity of minoxidil (combined with other drugs) increases passive (independent of aldosterone) renal retention of sodium and water thereby contributing to edema.
#3. Renal Nerve Released NE
This mechanism for minoxidil-induced edema is due to increased sympathetic nerve release of NE directly onto alpha1As in the kidney which causes sodium retention (18–20). This mechanism of sodium-water retention predominates over the high circulating NE levels (as in pheochromocytoma-see below) which suppress release of NE through the inhibitory action of presynaptic alpha2-adrenoceptors (10).
Interestingly, vasopressin also has a direct renal effect of retaining sodium in addition to its water retaining action (21). Since we did not study any vasopressin antagonists nor blood levels of this peptide we cannot exclude an important salt and water retaining role of vasopressin in minoxidil edema. Interestingly, excess antidiuretic hormone (vasopressin) secretion syndrome in myxedema is associated with low serum sodium (even less than 105 meq/liter) (22) which appears inconsistent with a direct renal effect of sodium retention by this hormone. However, mineralocorticoids potentiate vasopressin’s effects on salt and water retention. Thus, the volume expansion mechanism suppressing aldosterone secretion appears to be absolute in continuing urinary sodium excretion (in spite of serum sodium concentrations as low as 100 meq/liter) in the syndrome of Inappropriate Secretion of Antidiuretic Hormone (22).
#4. Beta adrenoceptor mediated renin release
Finally, sympathetic nerve activation stimulates renin release via a beta-adrenoceptor (23,24) on the juxtaglomerular apparatus, angiotensin formation and synthesis and release of aldosterone, the sodium-retaining hormone. This minoxidil-augmented renin release is largely blocked by beta-adrenoceptor blocking agents, a major blood pressure lowering mechanism in this antihypertensive drug combination in man and in animals (23,24).
Contrasting effects on edema of high sympathetic nerve-released NE versus high circulating NE as in pheochromocytoma. Minoxidil-induced sympathetic nerve released NE onto renal tubules induces reabsorption of sodium causing edema (#3 above). Alternatively, high circulating levels of NE from pheochromocytoma have two opposite effects on salt and water excretion. One is to constrict veins which forces stored blood to the heart, and by the Starling Mechanism, propels the excess blood to the kidney and forces salt and water excretion (17). The second mechanism of high circulating NE in pheochromocytoma to increase salt and water excretion is to activate pre-synaptic alpha2As which inhibit sympathetic neuronal release of NE (10) on renal tubules and thereby to reduce sodium retention. Intravascular volume depletion by these two mechanisms in pheochromocytoma can be so severe that patients may even fall from the sitting position because of orthostatic hypotension. Alternatively, orthostatic hypotension is absent with minoxidil because of this drug’s four cumulative mechanisms (see above) causing renal retention of salt and water resulting in volume expansion.
Why not just use the alpha1AB prazosin to prevent minoxidil’s reflex mediated salt-retention-edema (#3 above)?” What a fine idea! However, after three days of prazosin administration, alpha2As multiply, move into the post-synaptic site on renal tubules and mediate nerve stimulated salt and water retention (25), the otherwise exclusive domain of alpha1As. A similar multiplication of alpha2As with prazosin and substitution for alpha2As on veins may explain temporary (2–3 days) orthostatic hypotension with alpha1ABs and very short term benefits in congestive heart failure (26). I don’t know whether some degree of tolerance (to relief of urethral obstruction) occurs when alpha1ABs are used to relieve urethral obstruction from prostatic hypertrophy.
Failure of physicians to optimally combine counteracting drugs with minoxidil to control effects of the reflex activation caused cardiac stimulation and edema. On the flip side of this issue is the occasional patient with poor cardiac conduction as in the “sick-sinus-syndrome”. In some patients with this syndrome I have used minoxidil in lower doses (ie. 10 mg) without a beta-blocker to enhance conduction through activation of beta-adrenergic receptors in the heart. Such use of minoxidil is possible because of its constant, long duration of increased sympathetic neuronal release of NE for which tolerance does not develop. This mechanism substituted for having pace-makers installed in some of my patients.
Cause and effect relationships of antihypertensive drug interactions (Figure 2) were confirmed using selective blockers (prazosin, saralasin, propranolol) of each receptor type. BetaAB such as propranolol or atenolol, when added to minoxidil, lower blood pressure impressively by inhibiting the exaggerated renin release (23,24). The renin-angiotensin limb in Figure 2 can become a critical support mechanism when minoxidil is used alone (28) as shown in Figure 3. Without a betaAB such as propranolol SRA is high and infusion of the A2B saralasin can cause extreme lowering (27) of blood pressure (Figure 4).
FIG 4.
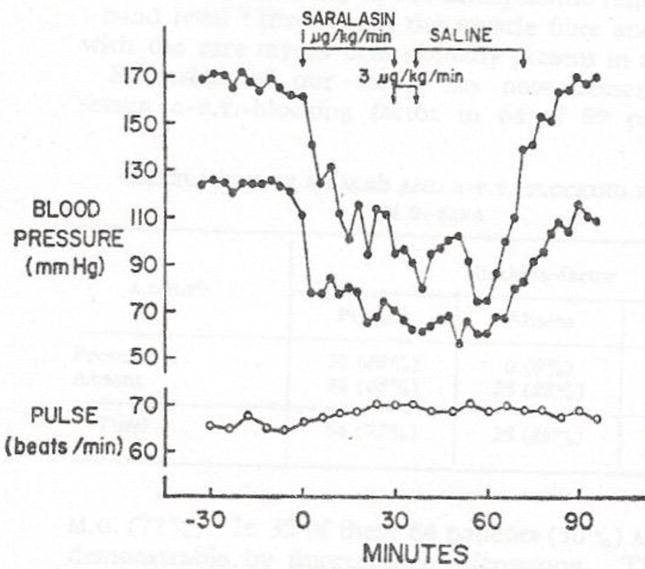
Severe angiotensin-dependence of blood pressure with vasodilator (minoxidil) plus diuretic without beta-blocker. Saralasin, is an angiotensin blocker (P-113-Norwich). Blood pressure was reduced from 170/124 to70/50 by Saralasin infusion. Later propranolol reduced control blood pressure to 130/88 and saralasin infusion hardly reduced blood pressure further, indicating a shift away from angiotensin dependence. Clearly, inhibition of renin release by a beta-blocker is a powerful antihypertensive mechanism in the vasodilator-beta blocker drug interaction. (Reprinted with permission from the Lancet, WA Pettinger and K Keeton, Hypotension During Angiotensin Blockade with Saralasin, Vol 1, page 1387. Copyright 1975 Elsevier)
Since converting enzyme inhibitors such as Captopril, Ang II RB or inhibitors of the renin enzyme have similar anti-angiotensin profiles (28), these drugs should be used very carefully with a vasodilator.
EDEMA AND THE MINIMUM DAILY REQUIREMENT (MDR) FOR SODIUM
Excess dietary sodium is a prerequisite for edema from minoxidil and is the cause of high blood pressure in the vast majority of hypertensive patients. So how much sodium do we really need, or “What is the Real MDR for sodium?”
Here are two scientific fact bases for the MDR for sodium:
-
#1
Millions of people with dietary sodium intake of < 9 milliequivalents (meq)/day (0.2 grams) have no high blood pressure nor its complications and don’t even have obesity nor diabetes (29,30). Their blood pressure does not even increase with age and their exercise tolerance is excellent. I constructed a pseudo-dose response curve by combining blood pressure as a function of dietary sodium intake from the Intersalt studies (29,30), and the salt-blood pressure study of Mente et. al. (31) in Figure 5. The Mente Study also indicated a quantitative relationship between salt intake and blood pressure but over a narrow and very high range of salt intake. Clearly, the MDR for sodium is < 0.2 grams/day.
-
#2
I personally conducted studies in hypertensive patients ingesting diets containing 9 meq/day of sodium (0.207 grams) in NIH supported Clinical Research Centers at the U. of Texas Southwestern Medical School in Dallas, at Vanderbilt in Nashville, TN and at NIH’s Clinical Center in Bethesda, MD. Note the placement on the dose-response curve of 0.207 grams sodium/day.
FIG 5.
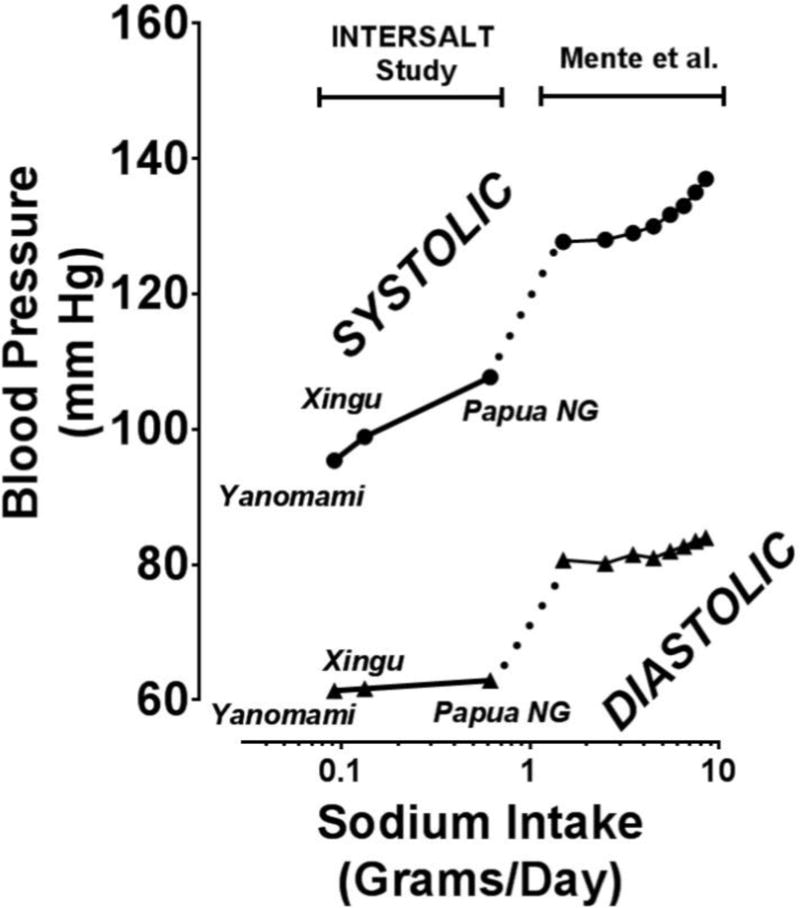
Dose-Response Relationship between dietary sodium in the Yanomami, the Xingua and Papua New Guineans on the left and from Mente on the right.
Blood pressure was reduced in every patient by reducing sodium intake for two weeks but none to the level associated with the same salt intake for people shown in Figure 5; why not? I suspect that failure to lower blood pressure over a short time interval (two weeks) of lowered salt intake was due to residual arteriolar hypertrophy (Figure 3) in my patients that had resulted from long-standing high blood pressure (see below). Clearly the MDR for sodium is < 0.2 grams/day and political pressure should be put upon the USDA to reduce this toxic addictive poison (kills more people than opiates, car accidents and terrorists’ guns combined) on food labels in our grocery stores. I suspect that there would be no minoxidil-edema if salt intake were reduced to 0.2 grams/day of sodium. Reduction even to 1.0 gram/day might eliminate most of the diuretic requirement in the vasodilator-beta-blocker drug combination.
Dr. Frieden’s recent Viewpoint Title in JAMA (32) “Sodium Reduction-Saving Lives by Putting Choice Into consumers’ Hands” is incisive except for two issues: #1. He fails to demand of the USDA to use a scientific basis for labelling of foods with the real MDR for sodium of <0.2 grams/day. And #2, a recommendation for ready availability at low cost of urine sodium and creatinine measurements for people who really care about preventing a stroke and other HBP complications. Now few are aware of their sodium intake relative to the 0.2 gram/day value in Figure 5. Measurements of urine sodium-creatinine are analogous to monitoring of blood pressure with a cuff for hypertensives or blood sugar for diabetics.
High Pulmonary Vascular Resistance and Minoxidil
Pulmonary vascular resistance and pressure increases in proportion to peripheral vascular resistance (33,34) and hypertension. Thus, the isolated reports of high pulmonary vascular resistance associated with minoxidil are due to the pre-minoxidil status. In fact, minoxidil blunts pulmonary hypertension from hypoxia (35).
MINOXIDIL & SAVING THE KIDNEYS IN REFRACTORY HYPERTENSION
To the reader it may seem ironic that a stroke and its sequelae were a cause of my writing of this update on minoxidil. JP’s average blood pressure for three years in the outpatient clinic of Parkland Hospital had been 239/139 and it had destroyed 90% of his kidney function. He fit the policy at that time (5) of removing both kidneys to save his life. However, in the admitting office he had a stroke with coma and no longer a candidate for hemodialysis so was admitted for stabilization and transfer to a nursing home to die. I happened to be his attending physician and had just received the research drug minoxidil from Upjohn. After three days of minoxidil his blood pressure was normal and in two weeks he walked out of the hospital with a cane and two months later returned to work.
One year later we had accumulated similar experiences (minus the stroke) in 11 patients and published “Minoxidil, an Alternative to Nephrectomy For Refractory Hypertension” (5) because of which this horrendous procedure was soon abandoned. Instead of cardiac problems JP.’s huge cardiac hypertrophy had been reduced to normal voltage on his ECG and he had had no arhythmias. His kidney function had improved by >50 % from his remaining 10% and it appeared that this huge problem of severe hypertension-neprhrosclerosis was solved.
But not quite! One to two years later several of the patients kidney function was deteriorating. Fortunately, Mitch and Walser (36) had published a straight line of decay of kidney function (Figure 6) in patients like JP which predicted the date for beginning hemodialysis in individual patients to within a few months; what a tragic, depressing perspective! JPs decay of renal function shown in Figure 7 prior to minoxidil fit perfectly with Mitch and Walser’s observations. Unfortunately, another straight line of decay (Figure 7), albeit at a slower rate, predicted that he would require hemodialysis in 6–7 years which actually occurred. Because his blood pressures were in the 145/95 range, we suspected that further lowering of blood pressure might reduce the downward slope of his decay curve. Nevertheless, the capacity of good blood pressure control to change the slope of the Mitch-Walser Curve was very encouraging.
FIG 6.
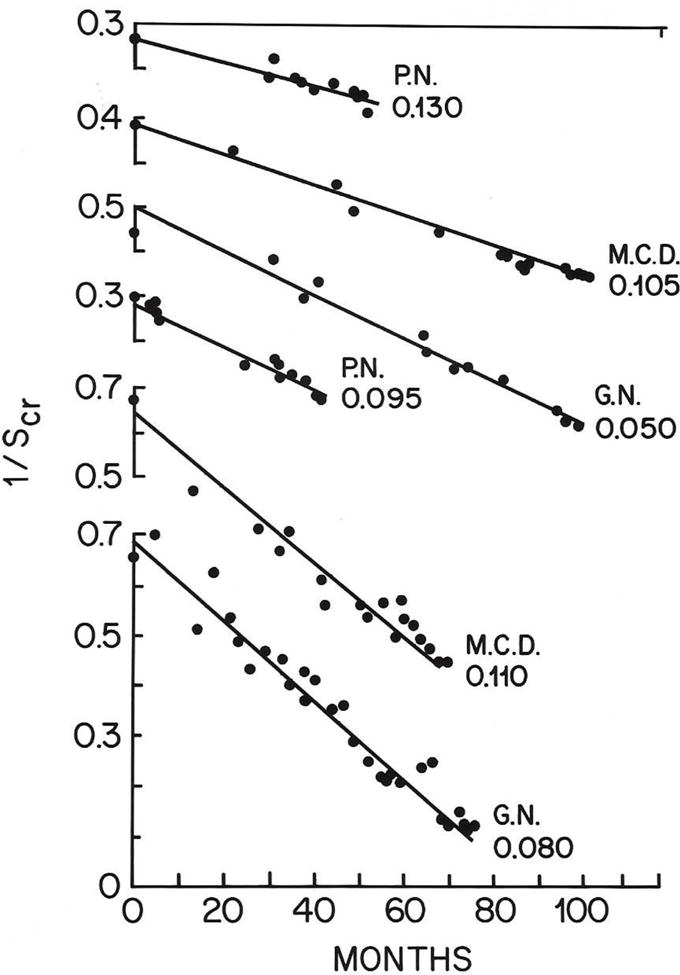
Slopes of Decay in Kidney Function using the reciprocal of the serum creatinine concentration versus time in six patients. Time to hemodialysis was predictable in each patient. Can this slope be improved by optimal blood pressure control? = the core issue in our NIH supported randomized clinical trial. trial? (Reprinted with permission from the Lancet, WE Mitch et al, A Simple Method of Estimating Progression of Chronic Renal Failure, Vol 2, page 1326. Copyright 1976 Elsevier)
FIG 7.
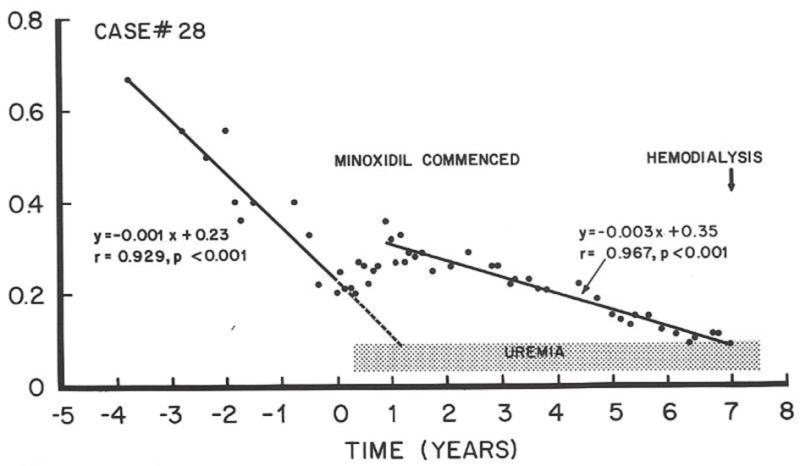
Rate of decay of renal function in JP before starting minoxidil on the left; then improvement of function with minoxidil for one year, followed by a new decay curve predicting need for hemodialysis after seven years of minoxidil. ? Can the rate (slope) of decay of renal function be altered by antihypertensive drugs?
In fact, another patient had three slopes of decay of renal function. The first was rapid decay of renal function, the second with the triple combination of minoxidil, propranolol and diuretic and the third with an increase dose of minoxidil to 60 mg/day plus clonidine (Figure 8). The latter combination controlled blood pressure in the range of 120/70 mmHg and renal function was sustained for 14 years at which time I lost contact with CW.
FIG 8.
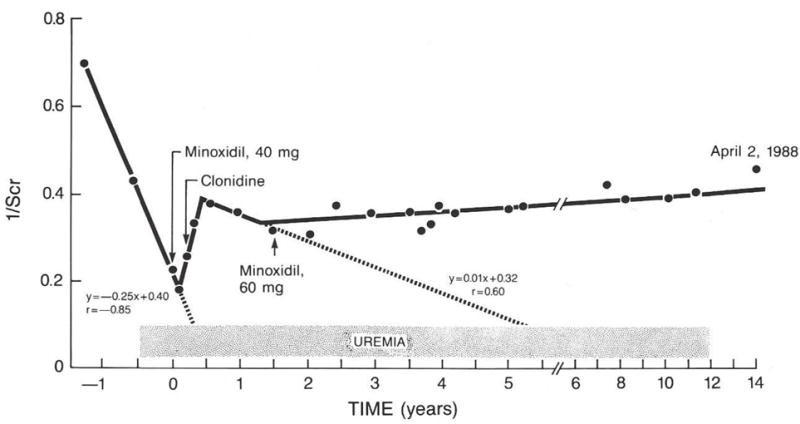
Three slopes of decay of renal function in CW. One before minoxidil; at time “0” to six months temporary improvement and then a new rate of decay predicting hemodialysis in five years. Increased dose of minoxidil and optimized clonidine dosage (Figure 9) resulted in blood pressures in the range of 120/70 mmHg. No further decay occurred for 13 years at which time I lost contact with his physician in Boston.
The stage was set to pursue every aspect of interactions of antihypertensive drugs with each blood pressure support mechanism, one of which is shown in Figure 9. The informative Dose Response Curve (DRC) for clonidine suppression of plasma NE (37), blood pressure and plasma renin activity (Figure 10) emphasizes the role of the DRC as the Pharmacologist’s Sword. Wouldn’t the world be better if each physician could draw the DRC for every drug she/he prescribes? Or, if definitive DRCs were required before sale of all dietary supplements?
FIG 9.
Dose Response of Clonidine Suppression of High Plasma NE in minoxidil-BB treated severely hypertensive patients. The DRC for both supine and standing NE reached a plateau with 0.4 mg/day. Epinephrine concentrations were normal and unaffected by clonidine at any dose. (Reprinted from the Journal of Cardiovascular Pharmacology, HC Mitchell and WA Pettinger, Dose Response of Clonidin on Plasma Catecholamines in the Hypernoradrenergic State Associated with Vasodilator beta-blocker Therapy, vol 3, page 650. Copyright 1981 Wolters Kluwer)
FIG 10.
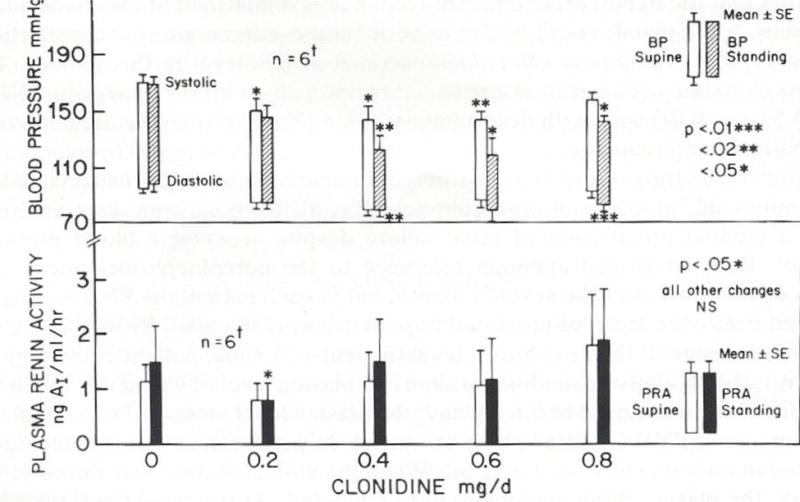
Dose Response to clonidine of supine and standing systolic and diastolic blood pressures and plasma renin activity in refractory hypertensive patients treated with minoxidil-BB. Maximum lowering of blood pressures occurred at 0.4 mg/day as with suppression of plasma NE. However, with 0.8 mg/day higher plasma renin activities and blood pressures were associated with diuresis-weight loss; these fascinating mechanisms are discussed in the text. (Reprinted from the Journal of Cardiovascular Pharmacology, HC Mitchell and WA Pettinger, Dose Response of Clonidin on Plasma Catecholamines in the Hypernoradrenergic State Associated with Vasodilator beta-blocker Therapy, vol 3, page 651. Copyright 1981 Wolters Kluwer)
Maximum lowering of blood pressure and of the high plasma NE concentrations occurred at or below 0.4 mg/day of clonidine. Higher doses of clonidine produced no additional lowering of plasma NE nor blood pressure; in fact, blood pressure tended to increase. Also, higher doses are associated with increased withdrawal symptoms and very HBP with discontinuation of this drug. Consequently, I recommend 0.2 mg of clonidine at night (enhances sleep) and 0.1 mg in the AM; I add another 0.1 mg at noon but only if it is really necessary.
Not only did clonidine lower blood pressure further but appeared to cause diuresis (38). This effect is expected due to suppression of high reflex-induced NE release directly onto renal alpha1-adrenoceptors which, in turn, cause renal retention of sodium and water (19–20). Theoretically, there are two sites of clonidine suppression of NE release; one is in the cardiovascular control center in the brain which suppresses peripheral sympathetic nerve activity and the other is on the sympathetic nerve terminal through activation of alpha2As suppressive to NE release (10). The resulting diuresis is sufficient to increase SRA (38) even though alpha2As are inhibitory to renin release.
Now if all of this seems a bit complicated just place yourself in CW’s position or that of his lovely wife Julia, a Cardiologist. She grasped the significance of the decay curves shown in Figure 8 for CW and Figure 7 for JP so was motivated to take any measures to save his kidneys. Most importantly, keep in mind the potential for improvement (reduced need for drugs) if diastolic blood pressure is maintained below 80 mmHg for two to six months (38) which improvement appears due to reversal of arteriolar hypertrophy (see below).
The First NIH-Supported Randomized Trial Attempting to Save Kidneys
The design of the study (38) is shown in Figure 11. Eighty-seven non-diabetic, severely hypertensive patients with advanced nephrosclerosis qualified for the study (39). To qualify they had severe refractory hypertension requiring minoxidil to control (hence the focus on minoxidil), have lost more than half of their kidney function and to be sufficiently responsible and compliant to sustain diastolic blood pressure control at <80 mm Hg for 2–6 months prior to randomization. 10% of the original group had failed to sustain diastolic pressures below 80 mmHg so were not randomized but continued to be followed in the study. In one randomized group (1/2) diastolic pressures were permitted to rise above 90 mmHg by reducing medication dosage. In the group randomized to lower blood pressures, we attempted to maintain diastolic pressures below 80 mmHg. Diastolic pressure was selected because of less variability than in systolic blood pressure.
FIG 11.
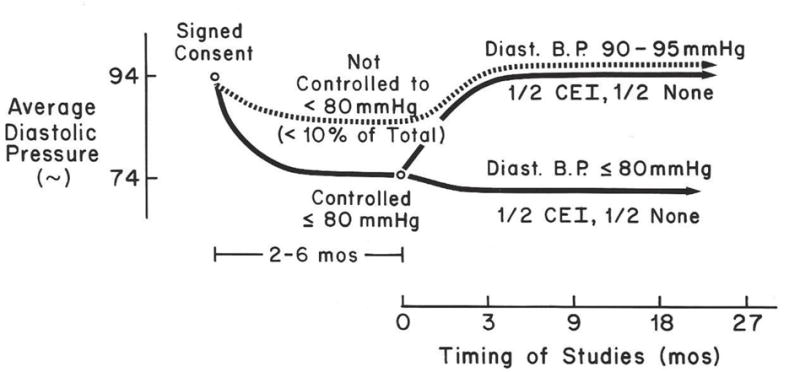
Design of the first NIH supported, randomized, long term clinical trial attempting to establish a goal blood pressure to optimally conserve kidney function in previously refractory hypertensive patients with advanced nephrosclerosis. (Reprinted with permission from Hypertension, William A. Pettinger et al, Long-term Improvement in Renal Function after Short-term Strict Blood Pressure Control in Hypertensive Nephrosclerosis, vol 13, page 767. Copyright 1989 Wolters Kluwer)
Our NIH-supported, randomized, long term clinical trial achieved the following:
-
#1
As a result of 2–6 months of blood pressure control at <80 mmHg in both patient groups, kidney function improved for three years. Antihypertensive drug requirement was remarkably reduced in both groups (38). My opinion is that this quality of blood pressure control for this time interval caused reversal of arteriolar hypertrophy. This result contrasted with Mitch & Walser’s conclusion that, “renal disease progression once established progresses irrevocably” (36) in such patients.
-
#2
Simultaneous studies (40) reversed the concept that hyper-filtration is the mechanism for progressive loss of kidney function studied in rats (41,42). Minoxidil induced hyperfiltration in hypertensive nephrosclerosis patients while simultaneously improving kidney function over a three year period. Thus, hyper-filtration cannot be a major mechanism of destroying kidney function in our patients as suggested by studies in rats (41,42). Minoxidil-induced hyper-filtration may, in fact, explain why hemodialysis may be discontinued in some minoxidil treated patients (44) and should be systematically studied for delay of, or reducing hemodialysis.
-
#3
Discovery of, and understanding of the mechanism of the PRAS Syndrome originally described for minoxidil (6) but subsequently found to be related to severe reversible loss of renal function with other antihypertensive drugs (43,44). (See below)
-
#4
Final results of the hypertension nephrosclerosis trial were reported by Toto et.al. (39). Mean diastolic blood pressure in the Strict Group was 81+/− 1 and in the conventional group 87 +/− 1 mmHg. The mean slopes of decay of renal function in both groups were not significantly different from -0-. However, by combining results in all patients there was a slight but significant decline of renal function. While it is not possible to identify a specific goal diastolic pressure I recommend 2–6 months of blood pressure control at or < 80 mmHg by whatever drug combination is required. For long term maintenance try to keep resting diastolic pressure < 83 mmHg and the lower the better without hypotensive symptoms. Reduction of dietary salt intake to <1.0 grams/day will help greatly.
THE Pseudo-Renal Artery Stenosis Syndrome (PRASS) and Arteriolar Hypertrophy
PRASS was first described in minoxidil treated patients (6) because it was the only antihypertensive drug sufficiently effective to control HBP in refractory patients (5). Reduction of systemic arterial pressure by minoxidil (plus companion drugs) along with extreme narrowing of arteriolar lumen due to hypertrophy reduced filtration pressure in the glomerulus to such an extent that little filtration occurred. Also, this reduced pressure triggered huge rates of renin release but the perfusion pressure was so low that even angiotensin constriction of the efferent arteriole was unable to maintain filtration, as in unilateral renal artery stenosis as previously discussed. Thus, the syndrome consists of reversible very high concentrations of serum creatinine and urea (nearly to uremic levels), high SRA and edema with weight gain.
Two of these abnormalities, increased renin release and increased tubular reabsorption of sodium and water, occur in unilateral renal artery stenosis but functioning of the opposite kidney compensates for the increased reabsorption so split function studies are required to demonstrate this altered function. However, in bilateral renal artery stenosis, excess tubular reabsorption is so severe that patients present with extreme HBP and acute heart failure with pulmonary edema. The PRASS syndrome is therefore like bilateral renal artery stenosis and, in fact, stenosis is bilateral because it involves all of the pre-capillary arterioles in both kidneys.
Some of these functional abnormalities also occur with ACE Inhibitors because of the following paradigm. Renin is released from the juxta-glomerular apparatus located on the afferent arteriole into the glomerular capillaries heavily laden with converting enzyme which forms Ang II. Ang II constricts the efferent arteriole which increases feedback pressure to the glomerulus and maintains filtration in spite of decreased filtration pressure from artery stenosis. In normal circumstances renin-angiotensin concentration is low, so ACEI do not decrease glomerular filtration rate normally. However, decreases of glomerular filtration pressure increase renin release to such an extent that glomerular filtration depends, at least in part, on Ang II constriction of the efferent arteriole. This phenomenon explains the worst outcome, apparent renal failure, in the Strict control group of SPRINT Trial in the elderly (2). Diuretics and ACEI potentiate the PRASS dysfunctions (43).
There are several other important aspects of arteriolar hypertrophy. A major function of pre-capillary arterioles is to prevent excess flow of blood into capillaries during temporary increases in blood pressure. Excess flow into capillaries increases transmural pressure in thin capillary walls forcing water and salt into extracellular spaces and edema. An anatomic feature of arterioles (Figure 3) includes a fixed dimension fibrous outer layer, a circular muscle and a thin inner lining which extends into and, in fact, comprises the capillary wall. This thin lining is the site of exchange of oxygen, carbon dioxide, sugar, creatine-creatinine and urea in all tissues. Capillaries in the glomerulus of the kidney constitute the filtering mechanism, the first step in urine formation. Malignant hypertension occurs when the onset of extreme elevation of blood pressure occurs rapidly, before sufficient smooth muscle hypertrophy develops to prevent the excess blood flow into capillaries.
Arteriolar hypertrophy was first demonstrated in the hindlimb arterioles of dogs (circa 1948) made hypertensive by renal artery constriction. The hypertrophy was prevented by partial constriction of one femoral artery thereby reducing perfusion pressure of that hindlimb. This paradigm may explain why blood pressure in my patients on short term (2–3 weeks) restricted salt intake (0.207 grams sodium) failed to decrease to the levels existing in people whose salt intake had been limited throughout their life span (30,31). It would be anticipated that reversal of the hypertrophic smooth muscle encroachment on the lumen of arterioles caused by chronic hypertension would require significant time to reverse.
One might argue that emphasis on arteriolar hypertrophy and its potential reversal is inappropriate in an update of minoxidil. However, this concept is useful in explaining improvement of renal function after only 2–6 months of antihypertensive therapy noted above (38).
MINOXIDIL-HAIR GROWTH-ROGAINE
Increased hair growth is highly variable with minoxidil administration. Two women age 20 and 24 were so offended by body hair growth that they said that they would rather die, so stopped taking minoxidil; within six months both were dead. Twin brothers (CB & HB) age 59, had black hair growth over their bodies which was reminiscent of the appearance of bears; both had hair growth in their ear canals which required trimming. Two women noted lengthening and thickening of eye lashes requiring trimming to prevent scratching on their eye-glasses. CB and one elderly obese woman had roughening of facial features but preferred having blood pressure controlled with minoxidil, the only drug effective enough to do so. In most patients increased hair growth was not a problem and was, in fact, a beneficial side effect, especially in men.
Topically applied minoxidil has to be absorbed through the skin, circulate to the hair follicles and only then does it produce hair growth. It is much easier to take a 5 mg tablet of minoxidil but the oral form is included in the Black Box Designation by the FDA; how unfortunate since the off-patent pill is so inexpensive.
The dose-response curve (DRC) for minoxidil-induced hair growth is unknown. However, from my own experience and observations I suspect that the maximum induction of hair growth occurs with 5–10 mg/day. Interestingly, the dose-response curve for increased renal blood flow is biphasic with higher doses (30–40 mg/day) actually decreasing renal blood flow (46). Possibly the lowered systemic blood pressure plus renal vasoconstrictor effects of angiotensin plus NE could reduce renal blood flow at higher doses is one possibility. Whether a similar peak skin blood flow at 5–10 mg/day occurs is unknown.
CONCLUSIONS
-
#1
Reversal of arteriolar hypertrophy by controlling diastolic pressure <80mmHg for 2–6 months in our minoxidil trial appears to be a fundamental determinant of conserving kidney function in high risk patients with nephrosclerosis. Whether minoxidil may play a unique role in remodeling of arterioles should be thoroughly investigated (47).
-
#2
Arteriolar hypertrophy is closely linked to the PRAS Syndrome, the cause of reversible major loss of kidney function due to blood pressure reduction by minoxidil and by anti-renin-angiotensin agents.
-
#3
Edema from minoxidil is caused by at least four mechanisms, each of which is dependent on dietary sodium in excess of the real MDR for sodium. Thus, the USDA must resort to fact-based science as reviewed herein, instead of profit-driven industries for MDR for sodium labeling on food items in our grocery stores.
-
#4
Since excess dietary sodium is the basic cause of the vast majority of HBP and minoxidil edema is dependent on it, hypertensive patients should be “let-out-of-the-dark” concerning dietary intake of sodium. As with blood sugar for diabetics and blood pressure cuffs for hypertensives they should have ready access to inexpensive measurements of urinary excretion of sodium and of creatinine for monitoring of dietary sodium.
-
#5
Minoxidil is underutilized (48) due to the FDA’s Black Box Designation which should be eliminated and appropriate recommendations for its optimal use inserted into the package insert.
-
#6
Excellent control of moderate hypertension can be achieved or augmented using doses of 5–10 mg/day of minoxidil with a betaAB, and with long term use, the need for the beta-blocker may not even be required. Because of erratic HBP control with triple therapy and permanent diplopia from a small stroke 20 years ago, I have personally used minoxidil 5 mg/day plus 25 mg of atenolol each morning. If I exercise or do manual work in the afternoon I take another 25 mg of atenolol. On the occasion that I dine at a restaurant I use furosemide 20 mg. the subsequent morning. My personal experience is a motive for writing this essay. My resting blood pressures run 115/65 to 130/75.
-
#7
Minoxidil is valuable in hypertensive patients on hemodialysis because of cardiovascular stability and, in fact, urine output and renal function can improve sufficiently with minoxidil to discontinue hemodialysis in some patients.
-
#8
Use patents for minoxidil are on file (not mine) for prevention of age-related hearing loss (ie restoration of tiny hair cells on the cochlea which are lost with age-related hearing loss) and for restoration or maintenance of penile erection in patients with long standing hypertension. Interestingly, patients in our long term minoxidil project noted restoration of penile erection; whether it was due to improved general health or a specific benefit of minoxidil is unknown. Those were pre-Viagra days so we were not specifically monitoring this issue.
Acknowledgments
Dr. Helen Mitchell (deceased) monitored patients, maintained records and contributed to each manuscript. The $100,000,000.00/year tax base from Dallas County for care of indigent patients at Parkland Hospital provided the unique opportunity for Dr. Mitchell and I to:
-
#1
Accumulate the relatively homogeneous group of severely hypertensive nephrosclerosis patients for the controlled randomized study.
-
#2
Pursue the multiple projects of drug interactions which permitted far better control of blood pressure than ever achieved in our patient population.
-
#3
The first NIH supported, randomized, long term clinical trial attempting to establish a goal blood pressure for preventing HBP complications. A subsequent multi-center clinical trial organized and funded by NIH ($65,000,000) had no chance of success because of results of our trial; we reduced the rate of loss of renal function to “not-different-from-0”.
ABREVIATIONS
- HBP
High Blood Pressure
- NE
Norepinephrine
- SRA
Serum Renin Activity
- Ang II
Angiotensin-2
- alpha1A
Alpha1-Adrenoceptor
- alpha2-A
Alpha2-adrenoceptor
- ARB
Angiotensin Receptor Blocker
- PRASS
Pseuro-Renal-Artery-Stenosis Syndrome
- FDA
Food and Drug Administ
- DRC
Dose Response Curve (The Pharmacologist’s Sword).
References
- 1.SPRINT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control; The SPRINT Research Group. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson JD, et al. Intensive vs Standard Blood Pressure control and Cardiovascular Disease Outcomes in Adults Aged>75 years. JAMA. 2016;315(24):2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chobanian AV. SPRINT Results in Older Patients. JAMA. 2016;315(24):2669–2670. doi: 10.1001/jama.2016.7070. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb TB, Katz FH, Chidsery CA. Combined therapy with vasodilator drugs and beta-adrenergic blockade in hypertension: a comparative study of minoxidil and hydralazine. Circulation. 1972;45:571–582. doi: 10.1161/01.cir.45.3.571. [DOI] [PubMed] [Google Scholar]
- 5.Pettinger WA, Mitchell HC. Minoxidil – An alternative to nephrectomy for refractory hypertension. N Engl J Med. 1973;289:167–171. doi: 10.1056/NEJM197307262890401. [DOI] [PubMed] [Google Scholar]
- 6.Pettinger WA, Mitchell H-C, Lee HC, Redman H. The pseudo-renal artery stenosis syndrome. Am J Hypertension. 1989;2(5) doi: 10.1093/ajh/2.5.349. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme DW, Freyburger WA, Graham BE, et al. Pharmacologic properties of minoxidil: a new hypotensive agent. J Phharmacol Exp There. 1973;184:662–670. [PubMed] [Google Scholar]
- 8.Campese VM. Minoxidil: A Review of its Pharmacological Properties and Therapeutic Use. Drug Evaluations. 2012;222(4):257–278. doi: 10.2165/00003495-198122040-00001. [DOI] [PubMed] [Google Scholar]
- 9.Pettinger WA. Drug Therapy: Minoxidil and the treatment of severe hypertension. N Engl J Med. 1980;303:922–926. doi: 10.1056/NEJM198010163031607. [DOI] [PubMed] [Google Scholar]
- 10.Berthelsen S, Pettinger WA. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977;21:595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- 11.Morris M, Campbell WB, Pettinger WA. Renin and hemodynamic changes via central adrenergic, cholinergic, and sodium receptor mechanisms in conscious rats. Proc Soc Exp Biol Med. 1976;151:101–104. doi: 10.3181/00379727-151-39152. [DOI] [PubMed] [Google Scholar]
- 12.Pettinger WA, Sheppard H, Palkoski Z, Renyi E. Angiotensin antagonism and antihypertensive activity of phosphodiesterase inhibiting agents. Life Sci. 1973;12:49–62. doi: 10.1016/0024-3205(73)90061-1. [DOI] [PubMed] [Google Scholar]
- 13.Alpert MA, Bauer JH. Rapid Control of Severe Hypertension With Minoxidil. Arch Intern Med. 1982;142(12):2099–2104. [PubMed] [Google Scholar]
- 14.Wood BC, Sharma JN, Crouch TT. Rapid control of BP with minoxidil. JAMA. 1979 Jan 12;241(2):163. [PubMed] [Google Scholar]
- 15.Fries Veterans Administration Cooperative Study Group on Antihyper-tensive Agents. JAMA. 1967;202:1028–1034. [Google Scholar]
- 16.Fries Veterans Administration Cooperative Study Group on Antihyper-tensive Agents. JAMA. 1970;213:143–152. [Google Scholar]
- 17.Guyton AC. The surprising kidney-fluid mechanism for pressure control: its infinite gain! Hypertension. 1990;16:725–730. doi: 10.1161/01.hyp.16.6.725. [DOI] [PubMed] [Google Scholar]
- 18.Smyth DD, Umemura S, Pettinger WA. alpha1-adrenoceptor selectivity of phenoxybenzamine in the rat kidney. J Pharmacol Exp Ther. 1984;230:387–392. [PubMed] [Google Scholar]
- 19.Osborn Jl, Holdaas H, Thames MD, DiBona GF. Renal adrenergic mediation of antinatriuretic and renin secretion responses to low frequency renal nerve stimulation in the dog. Cir Res. 1983;53:298–305. doi: 10.1161/01.res.53.3.298. [DOI] [PubMed] [Google Scholar]
- 20.Smyth DD, Umemura S, PETTINGER WA. Renal nerve stimulation causes alpha1-adrenoceptor mediated sodium retention but not alpha2-adrenoceptor antagonism of vasopressin. Circ Res. 1985;57:304–311. doi: 10.1161/01.res.57.2.304. [DOI] [PubMed] [Google Scholar]
- 21.Smyth DD, Umemura S, Pettinger WA. Alpha2-adrenoceptor antagonism of vasopressin-induced changes in sodium excretion. Am J Physiol. 1985;248:F767–772. doi: 10.1152/ajprenal.1985.248.6.F767. [DOI] [PubMed] [Google Scholar]
- 22.Pettinger WA, Talner L, Ferris TF. Inappropriate secretion of antidiuretic hormone due to myxedema. N Engl J Med. 1965;272:362–364. doi: 10.1056/NEJM196502182720707. [DOI] [PubMed] [Google Scholar]
- 23.Pettinger WA, Keeton K. Altered renin release and propranolol potentiation of vasodilatory drug hypotension. J Clin Invest. 1975;55:236–243. doi: 10.1172/JCI107927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettinger WA, Mitchell HC. Renin release, saralasin and the vasodilator-beta-blocker drug interaction in man. N Engl J Med. 1975;292:1214–1217. doi: 10.1056/NEJM197506052922304. [DOI] [PubMed] [Google Scholar]
- 25.Smyth DD, Umemura S, Pettinger WA. Renal alpha2-adrenergic receptors multiply and mediate sodium retention after prazosin treatment. Hypertension. 1986;8:323–331. doi: 10.1161/01.hyp.8.4.323. [DOI] [PubMed] [Google Scholar]
- 26.Graham RM, Thornell IR, Gain JM, Bagnoli C, Oates HF, Stokes GS. Prazosin: the first-dose phenomenon. Br Med J. 1976 Nov 27;2(6047):1293–1294. doi: 10.1136/bmj.2.6047.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettinger WA, Keeton K. Hypotensin during angiotensin blockade with saralasin. Lancet. 1975;1:1387–1388. doi: 10.1016/s0140-6736(75)92305-3. [DOI] [PubMed] [Google Scholar]
- 28.Gavras H, Brunner H, Gavras I, Laragh J. Hypotension with ACEI SQ 20881. Lancet. 1974:353. doi: 10.1016/s0140-6736(74)91734-6. [DOI] [PubMed] [Google Scholar]
- 29.Intersalt The INTERSALT Cooperative Research Group. INTERSALT: An international study of electrolyte excretion and blood pressure: results for 24-hour urinary sodium and potassium excretion. Br Med J. 1988;297:319–28. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Intersalt Mancilha-Carvalho JJ, Baruzzi RG, Howard PF, et al. Blood pressure in four remote populations in the INTERSALT study. Hypertension. 1989;14:238–46. doi: 10.1161/01.hyp.14.3.238. [DOI] [PubMed] [Google Scholar]
- 31.MENTE A, et al. Association of Urinary Sodium and Potassium Excretion With Blood Pressure. New England Jour of Med. 2014;371(7) doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 32.Frieden TR. Sodium Reduction—Saving Lives by Putting Choice Into Consumers’ Hands. Online JAMA. 2016 Jun 1; doi: 10.1001/jama.2016.799. [DOI] [PubMed] [Google Scholar]
- 33.Atkins JM, Mitchell HC, PETTINGER WA. Increased pulmonary vascular resistance with systemic hypertension. Am J Cardiol. 1977;39:802–807. doi: 10.1016/s0002-9149(77)80030-1. [DOI] [PubMed] [Google Scholar]
- 34.Klotman PE, Grim CE, Weinberger MH, Judson W. The Effects of Minoxidil on Pulmonary and Systemic Hemodynamics in Hypertensive Man. Circulation…. doi: 10.1161/01.cir.55.2.394. [DOI] [PubMed] [Google Scholar]
- 35.Peth S, Karle, Dehnert C, Bartsch P, Mairbaurl H. K+ Channel Activation with Minoxidil Stimulates Nasal-Epithelial Ion Transport and Blunts Exaggerated Hypoxic Pulmonary Hypertension. High Altitude Medicine & Biology. 2006;7(1):54–63. doi: 10.1089/ham.2006.7.54. [DOI] [PubMed] [Google Scholar]
- 36.Mitch WE, Walser M, et al. A simple method of estimating progression of chronic renal failure. Lancet. 1976;2:1326–8. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell HC, Pettinger WA. Dose response of clonidine on plasma catecholamines in the hypernoradrenergic state associated with vasodilator beta-blocker therapy. J of Cardiovas Pharmacol. 1981;3:647–654. doi: 10.1097/00005344-198105000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Pettinger WA, Lee H-C, Reisch J, Mitchell HC. Long term improvement in renal function after short term strict blood pressure control in hypertensive nephrosclerosis. Hypertension. 1989;13(6):766–772. doi: 10.1161/01.hyp.13.6.766. [DOI] [PubMed] [Google Scholar]
- 39.Toto R, Mitchell H, Smith R, McIntyre D, Pettinger WA. “Strict” Blood Pressure Control and Progression of Renal Disease in Hypertensive Nephrosclerosis. Kidney Int. 1995 Sep;48(3):851–859. doi: 10.1038/ki.1995.361. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Mitchell H, VanDreal P, Pettinger WA. Hyperfiltration and conservation of renal function in hypertensive nephrosclerosis patients. Am Jour Kid Dis. 1993;121(4 Suppl):68–74. doi: 10.1016/0272-6386(93)70076-b. [DOI] [PubMed] [Google Scholar]
- 41.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse reaction to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 42.Anderson S, Meyer TW, Reneke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HC, Pettinger WA. Diuretics Potentiate the Angiotensin Converting-Enzyme Inhibitor-Associated Acute Renal Dysfunction. Clinical Nephrology. 1992;38(4):236–237. [PubMed] [Google Scholar]
- 44.Toto RD, Mitchell HC, Lee HC, Milam C, Pettinger WA. Reversible renal insufficiency due to angiotensin converting enzyme inhibitors in Hypertensive Nephrosclerosis. Ann Int Med. 1991;115(7):513–519. doi: 10.7326/0003-4819-115-7-513. [DOI] [PubMed] [Google Scholar]
- 45.Javier R, Dumler F, Park JK, Bok DV, Riley RW, Levin NW. Long Term Treatment With Minxidil in Patients with Severe Renal Failure. J Cardiovascular Pharmacology. 1980;2:1–10. doi: 10.1097/00005344-198000022-00008. [DOI] [PubMed] [Google Scholar]
- 46.Terry J, Granger S, Chen B, Rysavy J, Lefkowitz D, Oldemeyer B, Lee HC, Pettinger WA. Adjusted Resistive Index: A method to estimate rapidly renal blood flow: preliminary validation in hypertensives. J Ultrasound Med. 1993;12:751–756. doi: 10.7863/jum.1993.12.12.751. [DOI] [PubMed] [Google Scholar]
- 47.Schriffrin S. Small Artery Remodeling in Hypertension: Can it be Corrected? The American Journal of the Medical Sciences. 2001;322(1):7–11. doi: 10.1097/00000441-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Sica D. Minoxidil: An Underused Vasodilator for Treating Resistant Severe Hypertension. The Journal of Clinical Hypertension. 2004;6:283–287. doi: 10.1111/j.1524-6175.2004.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


