Abstract
Background
Although abnormal left ventricular (LV) geometric patterns have prognostic value for morbidity and mortality, their possible association with silent cerebrovascular disease has not been extensively evaluated.
Methods
We examined 665 participants in the Cardiovascular Abnormalities and Brain Lesions (CABL) study who underwent transthoracic echocardiography and brain magnetic resonance imaging. Participants were divided into 4 geometric patterns: normal geometry (n=397), concentric remodeling (n=89), eccentric hypertrophy (n=126), and concentric hypertrophy (n=53). Subclinical cerebrovascular disease was defined as silent brain infarcts (SBI) and white matter hyperintensity volume (WMHV; expressed as log-transformed percentage of the total cranial volume).
Results
SBIs were observed in 94 participants (14%). Mean log-WMHV was −0.97±0.93. Concentric hypertrophy carried the greatest risk for both SBI (adjusted odds ratio [OR], 3.39; p<0.001) and upper quartile of log-WMHV (adjusted OR, 3.35; p<0.001), followed by eccentric hypertrophy (adjusted OR, 2.52; p=0.001 for SBI, and 1.96; p=0.004 for log-WMHV). Concentric remodeling was not associated with subclinical brain disease. In subgroup analyses, concentric and eccentric hypertrophy were significantly associated with SBI and WMHV in both genders and non-obese participants, but differed for SBI by age (all ages for eccentric hypertrophy, only patients ≥70 years for concentric hypertrophy) and by race-ethnicity (Hispanics for eccentric hypertrophy, Blacks for concentric hypertrophy; no association in Whites).
Conclusions
LV hypertrophy, both with eccentric or concentric pattern, was significantly associated with subclinical cerebrovascular disease in a multiethnic stroke-free general population. LV geometric patterns may carry different risks for silent cerebrovascular disease in different sex, age, race-ethnic, and body size subgroups.
Keywords: brain magnetic resonance imaging, echocardiography, left ventricular mass, relative wall thickness, subclinical cerebrovascular disease
Introduction
Stroke is the fifth leading cause of death and the leading cause of disability among Americans.1 In population-based studies, the prevalence of asymptomatic vascular brain lesions is substantially higher than that of clinically overt disease. Silent brain infarcts (SBIs) and white matter hyperintensities (WMHs), both manifestations of subclinical cerebrovascular disease, are commonly seen on brain magnetic resonance imaging (MRI) scans of elderly adults.2 SBIs are focal areas of infarcts, presumed to result from the occlusion of a single small perforating artery supplying the subcortical areas of the brain,3 whereas WMHs are considered areas of leukoaraiosis (loss of white matter) because of chronic hypoperfusion of the white matter and disruption of the blood–brain barrier, leading to chronic leakage of plasma into the white matter.4 Both SBIs and WMHs carry an increased risk of subsequent stroke, cognitive impairment, dementia, and death.5–8
An increased left ventricular (LV) mass, also known as LV hypertrophy (LVH), is a manifestation of cardiac remodeling strongly associated with unfavorable cardiovascular outcomes, independent of traditional risk factors including arterial hypertension.9–12 Recently, there have been reports of incremental risk associated with abnormal LV geometry beyond the simple LV mass increase. From LV mass and relative wall thickness (RWT), 3 abnormal geometric patterns (i.e. concentric hypertrophy, eccentric hypertrophy, and concentric remodeling) can be identified 13 that appear to carry different risks for cardiovascular events, including stroke.14–18
To date, the association between LV geometric patterns and silent cerebrovascular disease has not been extensively evaluated. The purpose of this study was to investigate the relationship between LV geometric patterns and silent cerebrovascular disease in an unselected sample of a multiethnic stroke-free, predominantly elderly general population.
Methods
Study population
The study population was derived from the Cardiovascular Abnormalities and Brain Lesions (CABL) study, designed to assess the cardiovascular predictors of silent cerebrovascular disease in a community-based cohort. CABL based its recruitment on the Northern Manhattan Study (NOMAS), an epidemiological study carried out in New York City. Details about the population and enrollment of NOMAS have been published previously.19 Participants were invited to participate in an MRI substudy beginning in 2003 and were eligible for the MRI cohort if they: 1) were at least 50 years of age; 2) had no contraindications to MRI; and 3) did not have a prior diagnosis of stroke. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo a more extensive cardiovascular evaluation were included in CABL. Participants for whom echocardiography and brain MRI information were available constitute the final sample of the present study. Written informed consent was obtained from all study participants. The study was approved by the Institutional Review Boards of Columbia University Medical Center and the University of Miami.
Risk factor assessment
Cardiovascular risk factors were ascertained through direct examination and interviews conducted by trained research assistants. Among the variables used in the analysis, hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg (mean of 2 readings obtained in sitting position) or a participant self-reported history of hypertension or antihypertensive medication use. Diabetes mellitus was defined by the participants’ self-report of history, current use of insulin or hypoglycemic agents, or a fasting glucose of ≥126 mg/dL, tested on ≥2 occasions in each participant. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, a participant’s self-report of hypercholesterolemia, or the use of lipid-lowering medications. Body mass index (BMI) was calculated using height and weight (kg/m2), and obesity was defined as BMI ≥30 kg/m2.
Echocardiographic examination
Echocardiographic examination was performed using a commercially available system (iE 33, Philips, Andover, Massachusetts, USA) by a trained, registered cardiac sonographer according to a standardized protocol. LV end-diastolic diameter (LVDD), LV end-systolic diameter (LVSD), interventricular septum (IVS), and posterior wall (PW) thickness were measured in the standard manner.20 LV ejection fraction was obtained by using the Simpson’s methods from apical 4- and 2-chamber views.20 LV mass was calculated with a validated formula: LV mass =0.8(1.04[(IVS+LVDD+PW)3-LVDD3]+0.6),21 and indexed for body surface area. LVH was defined as LV mass index greater than the 90th percentile of the participants without conditions associated with LV hypertrophy such as arterial hypertension, diabetes mellitus, obesity, and cardiovascular diseases (LV mass index: 124.0 g/m2 for men, and 111.75 g/m2 for women). RWT was calculated according to this formula: 2×(PW/LVDD).13 The 90th percentile of the sample without risk factors described above (RWT: 0.52 for men, and 0.60 for women) was used as the upper limit of normal. Four LV geometric patterns were identified on the basis of LV mass index and RWT: normal (normal LV mass, normal RWT), concentric remodeling (normal LV mass, abnormal RWT), eccentric hypertrophy (abnormal LV mass, normal RWT), and concentric hypertrophy (abnormal LV mass, abnormal RWT).13 In additional analyses, we also used cut-point for LVH (LV mass index; ≤115 g/m2 vs. >115 g/m2 for men and ≤95 g/m2 vs. >95 g/m2 for women) as published by the American Society of Echocardiography (ASE).20
Image acquisition and interpretation of brain MRI
A detailed description of the assessment of subclinical cerebrovascular lesions has been published previously.22,23 In brief, brain imaging was performed on a 1.5-T MRI system (Philips Medical Systems). SBIs were defined as either a cavitation on the fluid-attenuated inversion recovery sequence of at least 3 mm in size, distinct from a vessel (owing to the lack of signal void on T2 sequence), and of equal intensity to cerebrospinal fluid in the case of lacunar infarction, or as a wedge shaped cortical or cerebellar area of encephalomalacia with surrounding gliosis consistent with infarction attributable to distal arterial branch occlusion. Interobserver agreement for SBI detection was 93.3%.23 WMH volume (WMHV) analysis was based on a fluid attenuated inversion recovery image and performed by using the Quantum 6.2 package on a Sun Microsystems Ultra 5 workstation. WMHV was expressed as proportion of total cranial volume to correct for differences in head size, and log-transformed (log-WMHV) to achieve a normal distribution for analysis as a continuous variable. The upper quartile of log-WMHV (log-WMHV4) was used as the dependent variable in the categorical analyses. All measurements were performed blinded to participant clinical information.
Statistical analysis
Categorical variables are presented as numbers and percentages, and were compared using the Chi-square test. Continuous variables are expressed as mean ± standard deviation, and were compared using t-test or analysis of variance. Univariate and multivariate logistic regression analyses were used to study the association of LV geometric patterns with SBI and log-WMHV4, adjusting for significant potential cofactors (variables associated with subclinical brain disease with p<0.1 in the univariate analysis) in the multivariate logistic regression models. Unadjusted and adjusted odds ratios (ORs) were calculated for the entire study group and in sex, age, race-ethnic, and BMI subgroups. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (grant nos. R01NS36286 to M.D.T. and R37NS29993 to R.L.S./M.S.E.). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript and its final contents.
Results
Population characteristics
The baseline characteristics of the study population are listed in Table 1. Mean age of the study participants was 71±9 years, and 275 (41%) were male. Among the 665 participants, 397 (60%) were classified as having normal geometry, 89 (13%) concentric remodeling, 126 (19%) eccentric hypertrophy, and 53 (8%) concentric hypertrophy. Significant differences were observed among the LV geometry groups regarding age, gender, prevalence of hypertension, diabetes mellitus, and atrial fibrillation.
Table 1.
Comparison of clinical characteristics and echocardiographic parameters according to LV geometric patterns.
| Normal (n=397) |
Concentric remodeling (n=89) |
Eccentric hypertrophy (n=126) |
Concentric hypertrophy (n=53) |
p value | |
|---|---|---|---|---|---|
| Age, years | 69.5±9.5 | 73.1±7.4 | 73.8±9.1 | 75.3±8.2 | <0.001 |
| Male gender, n (%) | 144 (36.3) | 63 (70.8) | 40 (31.8) | 28 (52.8) | <0.001 |
| Hypertension, n (%) | 287 (72.3) | 73 (82.0) | 112 (88.9) | 49 (92.5) | <0.001 |
| Diabetes, n (%) | 91 (22.9) | 25 (28.1) | 51 (40.5) | 19 (35.9) | <0.001 |
| Hypercholesterolemia, n (%) | 247 (62.2) | 62 (69.7) | 88 (69.8) | 36 (67.9) | 0.296 |
| History of CAD, n (%) | 24 (6.1) | 5 (5.6) | 10 (7.9) | 4 (7.6) | 0.856 |
| Atrial fibrillation, n (%) | 14 (3.5) | 7 (7.9) | 13 (10.3) | 3 (5.7) | 0.024 |
| Body mass index, kg/m2 | 27.6±4.7 | 28.3±4.3 | 28.4±4.8 | 27.1±4.5 | 0.145 |
| Race/ethnicity | 0.091 | ||||
| Black, n (%) | 57 (14.4%) | 20 (22.5%) | 15 (11.9%) | 13 (24.5%) | |
| White, n (%) | 54 (13.6%) | 17 (19.1%) | 14 (11.1%) | 7 (13.2%) | |
| Hispanic, n (%) | 278 (70.0%) | 49 (55.1%) | 95 (75.4%) | 33 (62.3%) | |
| Other, n (%) | 8 (2.0%) | 3 (3.4%) | 2 (1.6%) | 0 (0%) | |
| Echocardiographic parameters | |||||
| LV end-diastolic diameter, mm | 44.7±4.0 | 41.4±3.2 | 49.7±4.5 | 43.5±4.1 | <0.001 |
| LV end-systolic diameter, mm | 28.2±4.1 | 26.6±3.2 | 32.1±6.9 | 26.9±6.0 | <0.001 |
| LV septal wall thickness, mm | 10.4±1.2 | 12.4±1.1 | 12.4±1.4 | 14.5±1.6 | <0.001 |
| LV posterior wall thickness, mm | 10.2±1.0 | 12.1±0.8 | 11.9±0.9 | 14.2±1.9 | <0.001 |
| LV ejection fraction, % | 63.4±6.7 | 64.3±4.9 | 59.4±11.0 | 62.9±9.0 | <0.001 |
| LV mass index, g/m2 | 90.8±14.7 | 97.5±13.7 | 136.4±20.1 | 139.9±23.9 | <0.001 |
| Relative wall thickness | 0.46±0.06 | 0.59±0.05 | 0.48±0.06 | 0.66±0.11 | <0.001 |
| Left atrial diameter, mm | 38.7±4.8 | 39.7±4.1 | 42.3±5.1 | 40.9±4.2 | <0.001 |
Values are mean ± standard deviation or n (percentage). CAD = coronary artery disease, and LV = left ventricle.
Association of LV geometric patterns and silent cerebrovascular disease
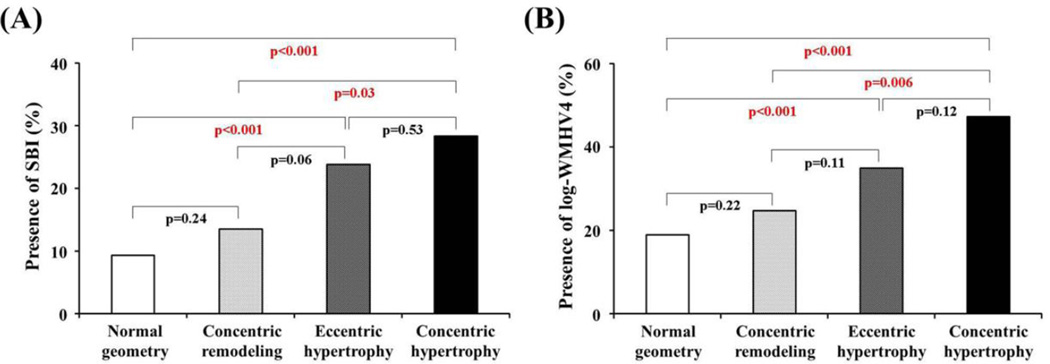
Presence of SBI was detected in 94 participants (14%); mean log-WMHV was −0.97±0.93 (median −1.11, min −3.79, max 1.74). The prevalence of SBI was greatest in concentric hypertrophy (28.3%), followed by eccentric hypertrophy (23.8%), concentric remodeling (13.5%) and normal geometry (9.3%) (p<0.001; Figure 1A). A similar trend was observed for the prevalence of log-WMHV4 (concentric hypertrophy: 47.2%, eccentric hypertrophy: 34.9%, concentric remodeling: 24.7%, and normal geometry: 18.9%; p<0.001; Figure 1B). After adjustment for pertinent covariates, concentric hypertrophy carried the greatest risk for both SBI (adjusted OR 3.39, p<0.001) and log-WMHV4 (adjusted OR 3.35, p<0.001), followed by eccentric hypertrophy (adjusted OR 2.52, p=0.001 for SBI; adjusted OR 1.96, p=0.004 for log-WMHV4) (Tables 2 and 3). Concentric remodeling was not associated with either SBI or log-WMHV4 in the adjusted analyses. When we used the ASE criteria for LVH (>115 g/m2 for men and >95 g/m2 for women), adjusted ORs for concentric hypertrophy were 3.18 and 2.85 (both p<0.001) for SBI and log-WMHV4, respectively, and those for eccentric hypertrophy were 1.86 and 1.60 (p=0.026 and 0.031) for SBI and log-WMHV4, respectively. Multivariate analysis was repeated including left atrial diameter as a covariate. Similar results were obtained, although ORs and p values were slightly different. Concentric hypertrophy carried the greatest risk for both SBI (adjusted OR 3.35, p=0.001) and log-WMHV4 (adjusted OR 3.45, p<0.001), followed by eccentric hypertrophy (adjusted OR 2.43, p=0.003 for SBI; adjusted OR 1.91, p=0.009 for log-WMHV4).
Figure 1.
Comparison of the prevalence of SBI (A) and that of log-WMHV4 (B) according to the LV geometric patterns.
LV = left ventricle, SBI = silent brain infarcts, and WMHV = white matter hyperintensity volume.
Table 2.
Association between LV geometric pattern and SBI.
| Concentric remodeling | Eccentric hypertrophy | Concentric hypertrophy | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
| All participants |
1.52 (0.756–3.041) p=0.241 |
1.33 (0.655–2.712) p=0.428 |
3.04 (1.787–5.174) p<0.001 |
2.52 (1.449–4.364) p=0.001 |
3.84 (1.933–7.633) p<0.001 |
3.39 (1.682–6.846) p<0.001 |
| Men | 1.55 (0.632–3.789) p=0.339 |
1.15 (0.419–3.126) p=0.792 |
5.00 (2.133–11.7) p<0.001 |
4.08 (1.591–10.5) p=0.003 |
3.71 (1.383–9.976) p=0.009 |
3.57 (1.208–10.6) p=0.021 |
| Women | 1.30 (0.364–4.678) p=0.684 |
1.38 (0.383–4.996) p=0.621 |
2.29 (1.144–4.566) p=0.019 |
2.13 (1.044–4.322) p=0.038 |
3.89 (1.471–10.3) p=0.006 |
3.88 (1.444–10.4) p=0.007 |
| Age <70 years |
0.94 (0.203–4.364) p=0.939 |
0.78 (0.163–3.731) p=0.756 |
3.36 (1.302–8.669) p=0.012 |
2.78 (1.038–7.427) p=0.042 |
3.11 (0.616–15.7) p=0.170 |
1.89 (0.332–10.8) p=0.473 |
| Age ≥70 years |
1.50 (0.666–3.363) p=0.330 |
1.41 (0.621–3.214) p=0.410 |
2.46 (1.280–4.708) p=0.007 |
2.19 (1.121–4.289) p=0.022 |
3.06 (1.393–6.705) p=0.005 |
3.15 (1.418–6.990) p=0.005 |
| Black | 2.38 (0.659–8.602) p=0.186 |
2.09 (0.566–7.700) p=0.269 |
2.60 (0.646–10.4) p=0.179 |
2.19 (0.533–8.976) p=0.278 |
8.33 (2.167–32.0) p=0.002 |
6.44 (1.621–25.6) p=0.008 |
| White | 0.67 (0.129–3.436) p=0.628 |
0.57 (0.104–3.155) p=0.523 |
1.36 (0.316–5.892) p=0.678 |
1.58 (0.350–7.138) p=0.552 |
2.00 (0.334–12.0) p=0.448 |
2.13 (0.337–13.5) p=0.422 |
| Hispanic | 1.09 (0.357–3.318) p=0.882 |
0.95 (0.306–2.949) p=0.929 |
3.91 (2.048–7.465) p<0.001 |
2.96 (1.492–5.878) p=0.002 |
2.72 (1.010–7.320) p=0.048 |
2.49 (0.913–6.816) p=0.075 |
| BMI ≥30 kg/m2 |
1.18 (0.300–4.605) p=0.817 |
1.14 (0.289–4.497) p=0.853 |
2.35 (0.883–6.256) p=0.087 |
2.30 (0.814–6.513) p=0.116 |
3.76 (0.994–14.2) p=0.051 |
3.59 (0.934–13.8) p=0.063 |
| BMI <30 kg/m2 |
1.67 (0.744–3.760) p=0.213 |
1.39 (0.601–3.219) p=0.442 |
3.45 (1.827–6.509) p<0.001 |
2.77 (1.425–5.381) p=0.003 |
3.87 (1.736–8.631) p=0.001 |
3.48 (1.519–7.967) p=0.003 |
All comparisons were with normal LV geometry.
Adjusted for hypertension, diabetes mellitus, and atrial fibrillation.
BMI = body mass index, CI = confidential interval, LV = left ventricle, OR = odds ratio, and SBI = silent brain infarcts.
Table 3.
Association between LV geometric pattern and upper quartile of log-WMHV.
| Concentric remodeling | Eccentric hypertrophy | Concentric hypertrophy | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
| All participants |
1.41 (0.819–2.427) p=0.215 |
1.24 (0.712–2.174) p=0.443 |
2.30 (1.477–3.592) p<0.001 |
1.96 (1.237–3.117) p=0.004 |
3.83 (2.114–6.950) p<0.001 |
3.35 (1.821–6.166) p<0.001 |
| Men | 1.99 (0.959–4.146) p=0.065 |
1.72 (0.792–3.735) p=0.171 |
3.15 (1.420–7.003) p=0.005 |
2.57 (1.099–5.989) p=0.029 |
3.25 (1.322–8.011) p=0.010 |
3.08 (1.181–8.038) p=0.021 |
| Women | 1.11 (0.423–2.889) p=0.838 |
1.04 (0.389–2.788) p=0.936 |
1.97 (1.155–3.374) p=0.013 |
1.76 (1.008–3.059) p=0.047 |
5.53 (2.351–13.0) p<0.001 |
4.72 (1.973–11.3) p<0.001 |
| Age <70 years |
1.41 (0.448–4.450) p=0.556 |
1.11 (0.344–3.572) p=0.863 |
0.78 (0.220–2.775) p=0.703 |
0.63 (0.171–2.341) p=0.494 |
5.47 (1.502–20.0) p=0.010 |
3.25 (0.811–13.0) p=0.096 |
| Age ≥70 years |
1.10 (0.574–2.093) p=0.780 |
1.07 (0.557–2.065) p=0.835 |
2.25 (1.314–3.844) p=0.003 |
2.14 (1.233–3.715) p=0.007 |
2.30 (1.157–4.581) p=0.018 |
2.37 (1.182–4.762) p=0.015 |
| Black | 1.44 (0.503–4.147) p=0.494 |
1.27 (0.425–3.805) p=0.668 |
1.44 (0.446–4.674) p=0.539 |
1.35 (0.402–4.507) p=0.630 |
7.22 (1.771–29.5) p=0.006 |
6.93 (1.577–30.4) p=0.010 |
| White | 1.54 (0.407–5.815) p=0.525 |
1.76 (0.425–7.276) p=0.436 |
2.00 (0.512–7.813) p=0.319 |
2.58 (0.628–10.6) p=0.189 |
3.75 (0.714–19.7) p=0.118 |
4.93 (0.892–27.2) p=0.067 |
| Hispanic | 1.17 (0.529–2.568) p=0.705 |
1.00 (0.449–2.244) p=0.992 |
2.89 (1.704–4.889) p<0.001 |
2.23 (1.279–3.893) p=0.005 |
2.96 (1.359–6.440) p=0.006 |
2.48 (1.123–5.468) p=0.025 |
| BMI ≥30 kg/m2 |
2.08 (0.749–5.758) p=0.160 |
1.96 (0.689–5.595) p=0.207 |
2.68 (1.162–6.177) p=0.021 |
3.23 (1.276–8.181) p=0.013 |
2.37 (0.658–8.554) p=0.186 |
2.26 (0.606–8.462) p=0.225 |
| BMI <30 kg/m2 |
1.24 (0.649–2.366) p=0.516 |
1.05 (0.537–2.055) p=0.886 |
2.28 (1.341–3.892) p=0.002 |
1.80 (1.035–3.136) p=0.037 |
4.53 (2.271–9.038) p<0.001 |
3.92 (1.928–7.965) p<0.001 |
All comparisons were with normal LV geometry.
Adjusted for hypertension, diabetes mellitus, and atrial fibrillation.
BMI = body mass index, CI = confidential interval, LV = left ventricle, OR = odds ratio, and WMHV = white matter hyperintensity volume.
Effect of gender, age, race-ethnicity, and obesity
The association of LV geometric patterns with silent cerebrovascular disease in gender, age, race-ethnic, and BMI subgroups is also shown in Table 2 and 3. Concentric and eccentric hypertrophy were associated with SBI and log-WMHV4 in both men and women. In women, concentric hypertrophy was more strongly associated with silent cerebrovascular disease than eccentric hypertrophy, although similar risk was observed in men between the two LVH patterns. In older participants (age ≥70 years), concentric and eccentric hypertrophy were associated with both SBI and log-WMHV4, whereas only eccentric hypertrophy was associated with SBI and no geometric pattern was associated with log-WMHV4 in participants <70 years. The association of concentric hypertrophy with silent cerebrovascular disease was greatest in Blacks (adjusted OR 6.44, p=0.008 for SBI and adjusted OR 6.93, p=0.01 for log-WMHV4), whereas there was no significant association between LV geometric abnormalities and silent cerebrovascular disease in Whites. In Hispanics, eccentric hypertrophy was associated with both SBI (adjusted OR 2.96, p=0.002) and log-WMHV4 (adjusted OR 2.23, p=0.005), but concentric hypertrophy was only associated with log-WMHV4 (adjusted OR 2.48, p=0.025). In non-obese participants (BMI <30 kg/m2), concentric and eccentric hypertrophy were associated with both SBI and log-WMHV4, whereas only eccentric hypertrophy was associated with log-WMHV4 and no geometric pattern was associated with SBI in obese participants (BMI ≥30 kg/m2).
Discussion
To the best of our knowledge, this is the largest study to investigate the association of LV mass and geometric patterns with silent cerebrovascular disease in a stroke-free sample of a multiethnic general population. We showed that concentric hypertrophy carried the greatest risk for silent cerebrovascular disease, independent of traditional risk factors, followed by eccentric hypertrophy. While LVH was associated with subclinical brain disease regardless of geometric pattern, differences by sex, age, race-ethnic and BMI were observed in the relationship between LV geometric pattern and silent cerebrovascular disease.
LV geometric pattern and silent cerebrovascular disease
The prevalence of stroke is estimated at approximately 3.0% 1 and that of SBI is higher (from 8% to 28%).5, 24–26 SBI and WMHV are important subclinical cerebral abnormalities because of their associations with increased risk of subsequent stroke, cognitive impairment, dementia, and death.5–8 Although LV geometric abnormalities have been reported to be associated with unfavorable cardiovascular outcomes,14, 15, 17, 18 limited data is available regarding relationship with silent cerebrovascular disease. Kohara et al. showed that LV hypertrophy was associated with lacunar lesions in 150 Japanese hypertensive patients.27 Selvetella et al. found that concentric hypertrophy was associated with more pronounced asymptomatic cerebrovascular damage compared with eccentric hypertrophy in 195 Italian hypertensive patients.28 However, these studies were relatively small and no studies in the U.S. focused on the relationship between LV geometric pattern and silent cerebrovascular disease. In our larger sample, concentric hypertrophy carried the greatest independent risk for both SBI and WMHV, followed by eccentric hypertrophy, whereas concentric remodeling was not associated with silent cerebrovascular disease. Our results are consistent with previous studies that showed the greatest risk for adverse cardiovascular outcomes as being associated with concentric hypertrophy.14, 15, 17, 18 Furthermore, we have previously reported in a case-control study that concentric hypertrophy carried the greatest risk for first ischemic stroke among the LV geometric abnormalities.16 In our study, abnormal LV geometry in the presence of normal LV mass (i.e., concentric remodeling) was not associated with subclinical brain disease, indicating a modulating effect of LV geometry on stroke risk when LVH is present, but no independent effect. Our results persisted even after we replacing ASE criteria for LVH for internally derived criteria. In addition, although left atrial enlargement may play an important role in the association of LV hypertrophy with subclinical cerebrovascular disease,29 both LVH patterns remained significantly associated with subclinical cerebrovascular disease in our study after adjustment for left atrial diameter.
The underlying mechanisms by which LVH may play a role in silent cerebrovascular disease is unclear, but we hypothesize several potential explanations. First, because concentric hypertrophy was associated with the greatest LV mass index, it may be a marker of more advanced end-organ damage in the cerebral vasculature. Second, LVH, especially concentric, is known to be associated with increased carotid artery intima-media thickening.30, 31 A progressive increase in intima-media thickness was previously described, from the lowest values in patients with normal geometry (0.68mm) to intermediate in those with concentric remodeling (0.76mm) and eccentric hypertrophy (0.81mm) and to the highest level in patients with concentric hypertrophy (0.87mm) in 1074 hypertensive patients.30 Because carotid artery disease is an established risk for silent cerebrovascular disease,32 its association with LVH might be involved in the explanation of our results. Finally, elevated serum insulin levels have been reported in hypertensive patients with LVH33 that could be associated with silent cerebrovascular disease. Indeed, Kario et al. found that hyperinsulinemia was associated with silent lacunar infarction in 123 asymptomatic hypertensive patients.34 Regardless of the possible mechanisms, our data suggest that the assessment of LV geometric pattern can provide useful information about the risk of subclinical brain disease. Whether a more aggressive pharmacological treatment intervention and risk factor control, and consequent reverse LV remodeling, may affect the future incidence of silent or clinical stroke and resulting cognitive decline is a hypothesis that deserves further investigation.
Gender, age, racial and body size difference
Interestingly, gender, age, race-ethnic, and body size differences were observed in the relation between LV geometric patterns and silent cerebrovascular disease. In women, concentric hypertrophy had an approximately 2-fold higher risk for SBI and WMHV compared to eccentric hypertrophy, whereas concentric and eccentric hypertrophy carried a similar risk in men. Furthermore, concentric hypertrophy showed strong association with silent cerebrovascular disease in older (age ≥70 years) participants, in Blacks, and in non-obese participants, although no significant association was observed in participants <70 years, in Whites, and in obese participants.
The reasons for these differences are not clear, but previous studies demonstrated a different response to the same stimuli between men and women.35, 36 Krumholz et al. showed that although isolated systolic hypertension was associated with increased LV mass in men and women, the geometric pattern differed by sex, as women demonstrated a pattern of concentric hypertrophy, whereas an eccentric pattern was observed in men.35 Kuch et al. also reported gender-specific differences in the adaptation of the LV to the combination of hypertension and obesity, with post-menopausal women being more susceptible to developing concentric hypertrophy.36 Zabalgoitia et al. also reported that older women (age ≥65 years) showed a greater prevalence of concentric hypertrophy than younger women and men.37 As far as race-ethnic differences are concerned, high systolic blood pressure levels were shown to be more strongly associated with concentric hypertrophy in Blacks (OR 3.74, p<0.001) than in Whites (OR 1.50, p=0.037), suggesting a greater susceptibility to develop LVH in Blacks.38 Rodriguez et al. showed the prevalence of concentric hypertrophy in Hispanics was greater than in Whites and lower than in Blacks.39 These findings may be involved in explaining part of the race-ethnic differences in the relationship of LV geometric pattern and subclinical cerebrovascular disease in our study. No consistent association has yet been identified between obesity and SBI,32 although obesity is an established risk factor for LVH. On the other hand, Yamashiro et al. showed that abdominal visceral fat measured by computed tomography, but not BMI, was related to subclinical cerebrovascular disease in 506 Japanese subjects.40 Because BMI does not reflect body fat volume accurately, future studies are needed to investigate the impact of LV geometry on SBI in relation to abdominal adiposity.
Study limitations
Our study has several limitations. The study sample included participants over 50 years of age and with a high prevalence of cardiovascular risk factors, which might not allow generalization to populations with different demographic composition and risk profiles. The sample size of the study was relatively small (n=665), although adequate to evaluate the associations under examination because of the older age and high frequency of cardiovascular risk factors in the study group. In particular, the smaller sample size of the White and Black subgroups may have affected the race-ethnic comparisons. Furthermore, because of the cross-sectional design of the study, we could show an association between LV geometric pattern and subclinical cerebrovascular disease, but could not establish a cause-effect relationship.
Conclusions
LVH was significantly associated with the presence of subclinical cerebrovascular disease in a multiethnic stroke-free sample of the general population, and the risk was higher in the presence of a concentric pattern. Gender, age, race-ethnic, and body size differences existed in the relationship between LV geometric patterns and silent cerebrovascular disease that deserve further attention.
Acknowledgments
The authors wish to thank Janet De Rosa, MPH (project manager), Rui Liu, MD, Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (data).
Disclosures
This work was supported by the National Institute of Neurological Disorders and Stroke [grant number R01 NS36286 to MDT and R01 NS29993 to RLS/MSE].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 3.Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988;19:1074–1082. doi: 10.1161/01.str.19.9.1074. [DOI] [PubMed] [Google Scholar]
- 4.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 6.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Longstreth WT, Jr, Arnold AM, et al. White matter hyperintensity on cranial magnetic resonance imaging: A predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 8.Liebetrau M, Steen B, Hamann GF, et al. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: A population-based study in 85-year-old subjects. Stroke. 2004;35:1816–1820. doi: 10.1161/01.STR.0000131928.47478.44. [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 10.Koren MJ, Devereux RB, Casale PN, et al. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 11.Ghali JK, Liao Y, Simmons B, et al. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831–836. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- 12.Liao Y, Cooper RS, Durazo-Arvizu R, et al. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997;29:641–647. doi: 10.1016/s0735-1097(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 13.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 14.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the framingham heart study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 15.Ghali JK, Liao Y, Cooper RS. Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol. 1998;31:1635–1640. doi: 10.1016/s0735-1097(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 16.Di Tullio MR, Zwas DR, Sacco RL, et al. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke. 2003;34:2380–2384. doi: 10.1161/01.STR.0000089680.77236.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavie CJ, Patel DA, Milani RV, et al. Impact of echocardiographic left ventricular geometry on clinical prognosis. Prog Cardiovasc Dis. 2014;57:3–9. doi: 10.1016/j.pcad.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Oktay AA, Lavie CJ, Milani RV, et al. Current perspectives on left ventricular geometry in systemic hypertension. Prog Cardiovasc Dis. 2016 doi: 10.1016/j.pcad.2016.09.001. in press. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Khatri M, Rundek T, et al. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 22.Wright CB, Paik MC, Brown TR, et al. Total homocysteine is associated with white matter hyperintensity volume: The Northern Manhattan Study. Stroke. 2005;36:1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willey JZ, Moon YP, Paik MC, et al. Lower prevalence of silent brain infarcts in the physically active: The Northern Manhattan Study. Neurology. 2011;76:2112–2118. doi: 10.1212/WNL.0b013e31821f4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price TR, Manolio TA, Kronmal RA, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 25.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 26.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 27.Kohara K, Zhao B, Jiang Y, et al. Relation of left ventricular hypertrophy and geometry to asymptomatic cerebrovascular damage in essential hypertension. Am J Cardiol. 1999;83:367–370. doi: 10.1016/s0002-9149(98)00870-4. [DOI] [PubMed] [Google Scholar]
- 28.Selvetella G, Notte A, Maffei A, et al. Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke. 2003;34:1766–1770. doi: 10.1161/01.STR.0000078310.98444.1D. [DOI] [PubMed] [Google Scholar]
- 29.Patel DA, Lavie CJ, Milani RV, et al. Left atrial volume index predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin Proc. 2011;86:730–737. doi: 10.4065/mcp.2010.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuspidi C, Mancia G, Ambrosioni E, et al. Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: Results from the Assessment Prognostic Risk Observational Survey (APROS) J Hum Hypertens. 2004;18:891–896. doi: 10.1038/sj.jhh.1001759. [DOI] [PubMed] [Google Scholar]
- 31.Di Bello V, Carerj S, Perticone F, et al. Carotid intima-media thickness in asymptomatic patients with arterial hypertension without clinical cardiovascular disease: Relation with left ventricular geometry and mass and coexisting risk factors. Angiology. 2009;60:705–713. doi: 10.1177/0003319708329337. [DOI] [PubMed] [Google Scholar]
- 32.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: A systematic review of population-based cohorts. BMC Med. 2014;12:119. doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdecchia P, Reboldi G, Schillaci G, et al. Circulating insulin and insulin growth factor-1 are independent determinants of left ventricular mass and geometry in essential hypertension. Circulation. 1999;100:1802–1807. doi: 10.1161/01.cir.100.17.1802. [DOI] [PubMed] [Google Scholar]
- 34.Kario K, Matsuo T, Kobayashi H, et al. Hyperinsulinemia and hemostatic abnormalities are associated with silent lacunar cerebral infarcts in elderly hypertensive subjects. J Am Coll Cardiol. 2001;37:871–877. doi: 10.1016/s0735-1097(00)01172-4. [DOI] [PubMed] [Google Scholar]
- 35.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 36.Kuch B, Muscholl M, Luchner A, et al. Gender specific differences in left ventricular adaptation to obesity and hypertension. J Hum Hypertens. 1998;12:685–691. doi: 10.1038/sj.jhh.1000689. [DOI] [PubMed] [Google Scholar]
- 37.Zabalgoitia M, Rahman SN, Haley WE, et al. Comparison in systemic hypertension of left ventricular mass and geometry with systolic and diastolic function in patients <65 to > or = 65 years of age. Am J Cardiol. 1998;82:604–608. doi: 10.1016/s0002-9149(98)00404-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Chen W, Ruan L, et al. Differential effect of elevated blood pressure on left ventricular geometry types in black and white young adults in a community (from the Bogalusa Heart Study) Am J Cardiol. 2011;107:717–722. doi: 10.1016/j.amjcard.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez CJ, Diez-Roux AV, Moran A, et al. Left ventricular mass and ventricular remodeling among hispanic subgroups compared with non-hispanic blacks and whites: MESA (Multi-ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;55:234–242. doi: 10.1016/j.jacc.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashiro K, Tanaka R, Tanaka Y, et al. Visceral fat accumulation is associated with cerebral small vessel disease. EurJ Neurol. 2014;21:667–673. doi: 10.1111/ene.12374. [DOI] [PubMed] [Google Scholar]