Abstract
Key points
The increase in blood pressure observed during physical activities is exaggerated in patients with hypertension, exposing them to a higher cardiovascular risk.
Neural signals from the skeletal muscles appear to be overactive, resulting in this abnormal response in hypertensive patients.
In the present study, we tested whether the attenuation of these neural signals in hypertensive patients could normalize their abnormal increase in blood pressure during physical activity.
Attenuation of the neural signals from the leg muscles with intrathecal fentanyl injection reduced the blood pressure of hypertensive men during cycling exercise to a level comparable to that of normotensive men.
Skeletal muscle afferent overactivity causes the abnormal cardiovascular response to exercise and was reverted in this experimental model, appearing as potential target for treatment.
Abstract
Hypertensive patients present an exaggerated increase in blood pressure and an elevated cardiovascular risk during exercise. Although controversial, human studies suggest that group III and IV skeletal muscle afferents might contribute to this abnormal response. In the present study, we investigated whether attenuation of the group III and IV muscle afferent signal of hypertensive men eliminates the exaggerated increase in blood pressure occurring during exercise. Eight hypertensive men performed two sessions of 5 min of cycling exercise at 40 W. Between sessions, the subjects were provided with a lumbar intrathecal injection of fentanyl, a μ‐opioid receptor agonist, aiming to attenuate the central projection of opioid‐sensitive group III and IV muscle afferent nerves. The cardiovascular response to exercise of these subjects was compared with that of six normotensive men. During cycling, the hypertensive group demonstrated an exaggerated increase in blood pressure compared to the normotensive group (mean ± SEM: +17 ± 3 vs. +8 ± 1 mmHg, respectively; P < 0.05), whereas the increase in heart rate, stroke volume, cardiac output and vascular conductance was similar (P > 0.05). Fentanyl inhibited the blood pressure response to exercise in the hypertensive group (+11 ± 2 mmHg) to a level comparable to that of the normotensive group (P > 0.05). Moreover, fentanyl increased the responses of vascular conductance and stroke volume to exercise (P < 0.05), whereas the heart rate response was attenuated (P < 0.05) and the cardiac output response was maintained (P > 0.05). The results of the present study show that attenuation of the exercise pressor reflex normalizes the blood pressure response to cycling exercise in hypertensive individuals.
Key points
The increase in blood pressure observed during physical activities is exaggerated in patients with hypertension, exposing them to a higher cardiovascular risk.
Neural signals from the skeletal muscles appear to be overactive, resulting in this abnormal response in hypertensive patients.
In the present study, we tested whether the attenuation of these neural signals in hypertensive patients could normalize their abnormal increase in blood pressure during physical activity.
Attenuation of the neural signals from the leg muscles with intrathecal fentanyl injection reduced the blood pressure of hypertensive men during cycling exercise to a level comparable to that of normotensive men.
Skeletal muscle afferent overactivity causes the abnormal cardiovascular response to exercise and was reverted in this experimental model, appearing as potential target for treatment.
Abbreviations
- a.u.
arbitrary units
- APBM
ambulatory BP monitoring
- BL
baseline resting measurements
- BP
blood pressure
- CO
cardiac output
- CTRL
control exercise session before fentanyl injection
- FENT
exercise session after fentanyl injection
- HR
heart rate
- HT
hypertensive
- MBP
mean blood pressure
- MVC
maximal voluntary contraction
- NO
nitric oxide
- NT
normotensive
- SV
stroke volume
- TVC
total vascular conductance
Introduction
Regular exercise is recommended as a coping resource to lower blood pressure (BP) in hypertensive (HT) patients (Pescatello et al. 2015). However, in these patients, exercise is often accompanied by exaggerated increases in BP (Papademetriou et al. 1989; Kokkinos et al. 2002), leading to greater myocardial demand and risk of cardiovascular events, particularly when associated with asymptomatic cardiovascular diseases (Thompson et al. 2007). In addition, an exaggerated BP response to low‐intensity exercise is also a strong predictor of cardiovascular mortality (Mundal et al. 1996; Kurl et al. 2001). Hence, identification of the signals that drive the exaggerated pressor response to exercise in hypertension may help the development of interventions aiming to revert this abnormal response, reducing the potential risks of physical activity in this disease.
Cardiovascular responses to exercise are greatly influenced by adjustments of the autonomic nervous system, which are modulated by neural signals interacting within the brainstem. These signals originate from the brain (‘central command’) (Goodwin et al. 1972) and from group III and IV skeletal muscle afferent nerve endings (‘exercise pressor reflex’) in response to mechanical (mechanoreflex) and metabolic (metaboreflex) stimuli during muscular contractions (Coote et al. 1971; McCloskey & Mitchell, 1972; Fisher et al. 2015), as well as from the arterial and cardiopulmonary baroreflex, a negative feedback mechanism that adjusts cardiac and peripheral vascular autonomic neural outflow in a beat‐to‐beat basis, in response to abrupt changes in BP (Fadel & Raven, 2012). Evidence from animals suggests that both components of the exercise pressor reflex are overactive in hypertension (Leal et al. 2008). In HT humans, however, the BP response to handgrip exercise was either augmented (Delaney et al. 2010; Vongpatanasin et al. 2011; Greaney et al. 2015) or preserved (Rondon et al. 2006; Sausen et al. 2009), and responses to metaboreflex stimulation by muscle ischaemia after handgrip exercise were also controversial (Rondon et al. 2006; Sausen et al. 2009; Delaney et al. 2010). Furthermore, these findings are limited to upper limb exercise, whereas experiments involving leg exercise are lacking, and would have relevance for daily‐life activities. Thus, in HT humans, the involvement of the exercise pressor reflex in mediating the exaggerated pressor response to physical activity remains unclear.
Experiments with anaesthetized cats found that the administration of a μ‐opioid receptor agonist into the dorsal horn of the spinal cord attenuated the cardiovascular responses to electrically‐induced muscle contraction and passive stretch, denoting inhibition of the exercise pressor reflex (Meintjes et al. 1995). Recently, this model was adapted to humans, investigating cardiovascular responses to leg exercise after injection of the μ‐opioid receptor agonist fentanyl into the lumbar intrathecal (subarachnoid) space. In healthy young men, fentanyl reduced the BP response to cycling (Amann et al. 2010; Barbosa et al. 2015), without affecting muscle force production and central command activation (Amann et al. 2009). Using a similar approach, in the present study, we investigated the role of the exercise pressor reflex for the BP response of HT patients during cycling. We hypothesized that HT patients would present an exaggerated BP response, but this response would be normalized, i.e., reduced to the same level as that of normotensive (NT) subjects, following attenuation of the exercise pressor reflex with intrathecal fentanyl.
Methods
Ethical approval
All procedures were approved by the Ethical Committee for Research at Fluminense Federal University (CAAE: 09282812.3.0000.5243) and conformed to the Declaration of Helsinki. All individuals provided their written informed consent prior to participation.
Study population
Recreationally active and non‐smoking men were divided into two groups: non‐treated HT and NT (Table 1). They were screened for hypertension (HEM‐742INT; Omron Healthcare, Kyoto, Japan) on two separate days in a seated position, with three measures per day using cuffs of an adequate size with respect to their arm circumference. To confirm the diagnosis of hypertension, we used ambulatory BP monitoring (ABPM) (Dyna‐MAPA; Cardios, São Paulo, Brazil) because of its greater accuracy for describing the BP profile in the daily routine, avoiding ‘white‐coat’ hypertension (O'Brien et al. 2013). The average BP values obtained in 24 h ABPM are usually lower than those obtained by office BP measurements as a result of nocturnal dipping. The consensus values for the diagnosis of hypertension using 24 h ABPM are systolic BP >130 mmHg and/or diastolic BP >80 mmHg (Mancia et al. 2013; O'Brien et al. 2013). These values yielded 10 year cardiovascular risks similar to those associated with high BP on office measurement (i.e. 140/90 mmHg) (Kikuya et al. 2007). During BP measurements in the awake period, subjects should relax their arms beside the body or over a support, either in a standing or seated position. Measures were obtained every 20 min during the 24 h monitoring. Four subjects were unable to participate in the 24 h ambulatory BP monitoring (three HT and one NT) and were diagnosed by office BP measurements only, when presenting average systolic BP >140 mmHg and/or diastolic BP >90 mmHg (Mancia et al. 2013). None of the HT patients was under pharmacological treatment of hypertension. All subjects were free of diseases other than hypertension.
Table 1.
Subject characteristics
| HT | NT | |
|---|---|---|
| Number of subjects | 8 | 6 |
| Number of systolic hypertension | 7 | – |
| Number of diastolic hypertension | 6 | – |
| Number of systolic and diastolic hypertension | 5 | – |
| Age (years) | 49 ± 2 | 47 ± 5 |
| Weight (kg) | 86.7 ± 3.0 | 81.5 ± 6.8 |
| Height (cm) | 174 ± 2 | 176 ± 2 |
| Body mass index (kg m−2) | 28.7 ± 0.6 | 26.2 ± 2.3 |
| 24‐h systolic BP (mmHg) | 132 (130–177)* | 122 (113–127) |
| 24‐h diastolic BP (mmHg) | 84 (81–114)* | 76 (68–80) |
| Office systolic BP (mmHg) | 143 (142–145)* | 111 |
| Office diastolic BP (mmHg) | 84 (80–103) | 71 |
Twenty‐four hour BP measurements included 5 HT and 5 NT. Office BP measurements included 3 HT and 1 NT.
Data are the mean ± SEM or median (minimum – maximum). *P < 0.05 vs. NT.
Exercise protocol
Subjects were asked to avoid caffeine, alcohol and intense exercise for 24 h and to fast for 3 h prior to the experiment, which was conducted in a room with an ambient temperature of 22–24°C.
Figure 1 displays the timeline of experimental procedures. After a 30 min rest and instrumentation, subjects were positioned on a cycling ergometer (AB–RB 300 Magnetic Recumbent Bike; AIBI, Singapore) with support for the arms. The experiment comprised two exercise sessions. Initially, to assess a possible and undesired cephalad migration or vascular absorption of fentanyl that could attenuate cardiovascular responses to exercise by a direct effect on opioid receptors within the brainstem (Caringi et al. 1998), the HT group performed a static handgrip contraction for 2 min at 40% of the maximal voluntary contraction (MVC), with visual feedback of the force level. MVC of the hand was assessed in the first exercise session by squeezing a digital force transducer (Grip Force Transducer; ADInstruments, Sydney, Australia) with maximal effort for 2–3 s at least three times, with a 1 min interval. The greatest force achieved among trials ranging within 5% of each other was taken as the MVC.
Figure 1. Timeline of experimental procedures .
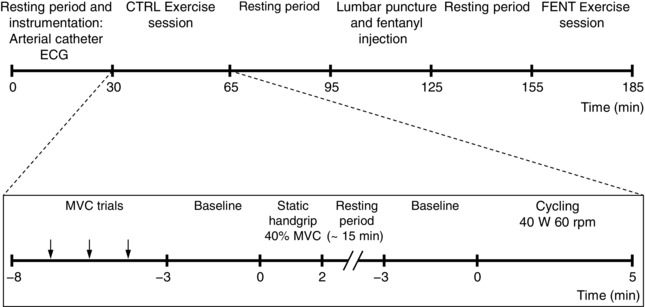
The FENT exercise session was identical to the CTRL exercise session, except for the MVC trials. Normotensive subjects performed the CTRL cycling only.
Fifteen minutes later, the subjects performed cycling exercise for 5 min at 40 W and 60 rpm, with visual feedback of the cadence. NT subjects also performed this cycling exercise protocol. Approximately 30 min after the end of the first session (CTRL; i.e. control exercise session before fentanyl injection), the HT group was provided with an intrathecal administration of fentanyl and then repeated the exercise protocol in a second exercise session. Therefore, HT subjects performed the CTRL session before the fentanyl session (FENT; i.e. exercise session after fentanyl injection). Static handgrip and leg cycling exercises were repeated within 29–37 min and 51–58 min, respectively, of fentanyl administration.
Subjects reported the rate of perceived exertion according to the modified Borg rating scale (Noble et al. 1983) at the end of each exercise trial, with scores ranging from 0 (minimal perception of effort) to 10 (maximal perception of effort).
Lumbar intrathecal fentanyl
After finishing CTRL cycling exercise, HT patients rested supine for 30 min and then underwent lumbar puncture. When seated with the trunk flexed, a 27 G, 9 cm Whitacre spinal needle was inserted into the L2–L3 or L3–L4 intervertebral intrathecal space. With presentation of spinal fluid, 1 ml of a solution containing 50 μg of fentanyl was injected through a filter. After fentanyl injection, the needle was removed. Then, subjects returned to the cycle ergometer and performed FENT exercise session. They remained upright until the end of the experiment and were instructed to report any sensations after fentanyl administration, including pruritus, nausea and dyspnoea (Chaney, 1995). To prevent hypotension and headache after lumbar puncture, subjects received 500 ml of saline solution at the end of the experiment and were followed for 1 week.
Cardiovascular measurements
Mean BP (MBP) was obtained from a catheter into the brachial or radial artery of the non‐exercising arm, linked to a transducer kept at the heart level (TruWave Disposable Pressure Transducer; Edwards Life Sciences, Irvine, CA, USA) and a monitor (Dialogue 2000; IBC‐Danica, Copenhagen, Denmark). Heart rate (HR) was recorded using an electrocardiogram (Dual Bio Amp and 5 Lead Shielded Cable; ADInstruments). These signals were recorded at a 1000 Hz sampling rate using an analogue‐to‐digital data acquisition system (PowerLab 16/35, software LabChart 7 for Mac OS; ADInstruments). Stroke volume (SV) was derived from the BP waveform using the Modelflow method (Wesseling et al. 1993) (Beatscope 1.1a; Finapres Medical Systems BV, Amsterdam, The Netherlands). Cardiac output (CO) was the product of HR and SV. Total vascular conductance (TVC) was the ratio of CO to MBP.
Statistical analysis
Resting baseline values (average from 2–3 min immediately pre‐exercise) and the responses to exercise [differences between values during exercise (average of the entire period of static handgrip or 1 min averages during cycling) and resting baseline (∆)] of the HT group in CTRL and FENT sessions were compared by Student's paired t test and two‐way repeated measures ANOVA (factors: Time of exercise × Fentanyl), whereas Student's t test for independent samples and two‐way repeated measures ANOVA (factors: Time of exercise × Group) were used for comparisons between groups (HT CTRL vs. NT, HT FENT vs. NT). When a significant interaction was found by ANOVA, Fisher's least significant difference post hoc tests were conducted for pairwise comparisons. Statistical analysis was performed using Statistica, version 10 (StatSoft Inc., Tulsa, OK, USA).
Results
Fentanyl‐related effects and responses to static handgrip
All experiments were completed within 80 min of fentanyl administration. None of the HT subjects reported nausea or felt dyspnoeic, although seven subjects reported pruritus over the legs, pelvis and abdomen. This symptom disappeared within less than 1 h after the end of the experiments.
HT subjects sustained the target force level during static handgrip in both trials (HT CTRL: 39.5 ± 0.3% MVC, HT FENT: 39.6 ± 0.3% MVC; P = 0.74). Also, the rate of perceived exertion was similar in both conditions (HT CTRL: 6 ± 0 a.u., HT FENT: 5 ± 1 a.u.; P = 0.25). Table 2 presents resting cardiovascular measurements and their responses to static handgrip. Following fentanyl administration, HT patients presented lower resting MBP (P = 0.02 vs. HT CTRL), accompanied by higher TVC (P = 0.05 vs. HT CTRL) and lower SV (P = 0.01 vs. HT CTRL), although HR (P = 0.36 vs. HT CTRL) and CO (P = 0.07 vs. HT CTRL) did not change. Cardiovascular responses to static handgrip were maintained after fentanyl administration (P > 0.05 for all comparisons). Further analysis considering only the first 30 s of static handgrip had equivalent results.
Table 2.
Cardiovascular measures at resting baseline and during 2 min static handgrip
| Baseline | Static handgrip | Difference | |
|---|---|---|---|
| Mean BP (mmHg) | |||
| HT CTRL | 117 ± 3* | 150 ± 4 | +37 ± 5 |
| HT FENT | 103 ± 6 | 134 ± 7 | +33 ± 3 |
| Heart rate (beats min−1) | |||
| HT CTRL | 67 ± 4 | 85 ± 5 | +19 ± 2 |
| HT FENT | 68 ± 3 | 84 ± 5 | +15 ± 2 |
| Stroke volume (mL) | |||
| HT CTRL | 77 ± 4* | 73 ± 4 | −8 ± 3 |
| HT FENT | 72 ± 4 | 69 ± 6 | −5 ± 2 |
| Cardiac output (L min−1) | |||
| HT CTRL | 5.2 ± 0.4 | 6.2 ± 0.6 | +0.8 ± 0.2 |
| HT FENT | 4.9 ± 0.4 | 5.8 ± 0.6 | +0.6 ± 0.2 |
| Total vascular conductance (mL min−1 mmHg−1) | |||
| HT CTRL | 45 ± 4* | 42 ± 4 | −6 ± 2 |
| HT FENT | 50 ± 6 | 45 ± 6 | −7 ± 1 |
Values are average from 2–3 min of rest before, and during the entire period of 2 min static handgrip at 40% MVC. Resting values and the differences between exercising and resting values were used for comparisons. HT CTRL, hypertensive subjects before fentanyl administration; HT FENT, hypertensive subjects after fentanyl administration. Data are the mean ± SEM. *P < 0.05 vs. HT FENT.
Responses to cycling
Subjects reported similar rates of perceived exertion (HT CTRL: 3 ± 0 a.u., HT FENT: 3 ± 1 a.u., NT: 4 ± 1 a.u.; P > 0.05 for all comparisons). Table 3 presents the resting cardiovascular measurements before each cycling trial. MBP was greater (P = 0.03) and TVC was smaller (P = 0.03) for HT CTRL compared to the NT group. HR, SV and CO were similar between groups (P > 0.05). Following fentanyl administration, the HT group presented lower MBP (P < 0.01 vs. HT CTRL) and higher TVC (P = 0.02 vs. HT CTRL), whereas resting HR, SV and CO remained similar (P > 0.05 vs. HT CTRL). An original BP recording of one HT patient during cycling is presented in Fig. 2. Changes in MBP during cycling are presented in Fig. 3, whereas HR, SV, CO and TVC are presented in Fig. 4. Cycling elicited an exaggerated increase of MBP in HT CTRL compared to NT (Interaction P = 0.02). HR, SV, CO and TVC also increased, although with the same magnitude in HT CTRL and NT (Interaction P > 0.05). Fentanyl decreased the MBP response of the HT subjects (Interaction P < 0.01 vs. HT CTRL), accompanied by a larger increase in TVC (Interaction P = 0.02 vs. HT CTRL). The MBP response of the HT subjects after fentanyl injection was similar to that of NT subjects (Interaction P = 0.75). Moreover, fentanyl not only attenuated the HR response of the HT subjects (Interaction P < 0.01 vs. HT CTRL), but also increased SV (Interaction P = 0.01 vs. HT CTRL), resulting in similar CO (Interaction P = 0.36 vs. HT CTRL). The post hoc tests indicated that the differences between‐groups or within‐group were apparent from the first to the fifth minute of cycling.
Table 3.
Cardiovascular variables before cycling exercise
| HT CTRL | HT FENT | NT | |
|---|---|---|---|
| Mean BP (mmHg) | 121 ± 4*, † | 109 ± 5 | 106 ± 5 |
| Heart rate (beats min−1) | 66 ± 3 | 67 ± 3 | 70 ± 4 |
| Stroke volume (mL) | 76 ± 4 | 74 ± 5 | 84 ± 5 |
| Cardiac output (L min−1) | 5.0 ± 0.4 | 5.0 ± 0.4 | 5.8 ± 0.3 |
| Total vascular conductance (mL min−1 mmHg−1) | 42 ± 4*, † | 47 ± 5 | 56 ± 3 |
Values are average from 2–3 min of rest before cycling. HT CTRL, hypertensive subjects before fentanyl administration; HT FENT, hypertensive subjects after fentanyl administration; NT, normotensive subjects. Data are the mean ± SEM. *P < 0.05 vs. NT. †P < 0.05 vs. HT FENT.
Figure 2. Representative original data .
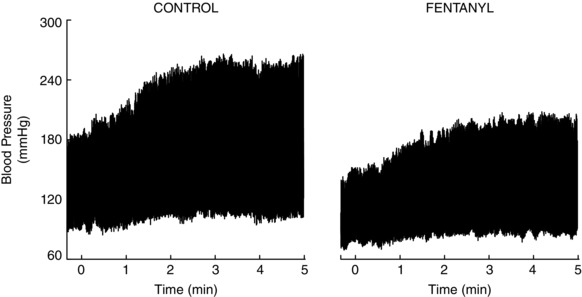
Blood pressure tracing from one hypertensive subject during the trials of cycling before and after intrathecal fentanyl administration.
Figure 3. Blood pressure response to cycling in hypertensive and normotensive subjects .
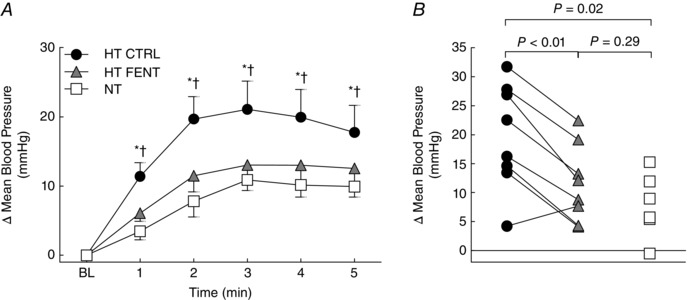
A, responses of mean BP during each minute of cycling. HT CTRL (black circles), hypertensive subjects before fentanyl administration; HT FENT (grey triangles), hypertensive subjects after fentanyl administration; NT (white squares), normotensive subjects; BL, resting baseline. Data are the mean ± SEM. *P < 0.05 HT CTRL vs. NT. †P < 0.05 HT CTRL vs. HT FENT. B, individual mean BP change from resting baseline at the second minute of cycling. Connecting lines indicate that, except for one individual, the BP response of every hypertensive subject to cycling exercise was lower after fentanyl. These statistical comparisons had equivalent results from the first to the fifth minutes. HT CTRL (black circles), hypertensive subjects before fentanyl administration; HT FENT (grey triangles), hypertensive subjects after fentanyl administration; NT (white squares), normotensive subjects.
Figure 4. Cardiovascular responses to cycling in hypertensive and normotensive subjects .
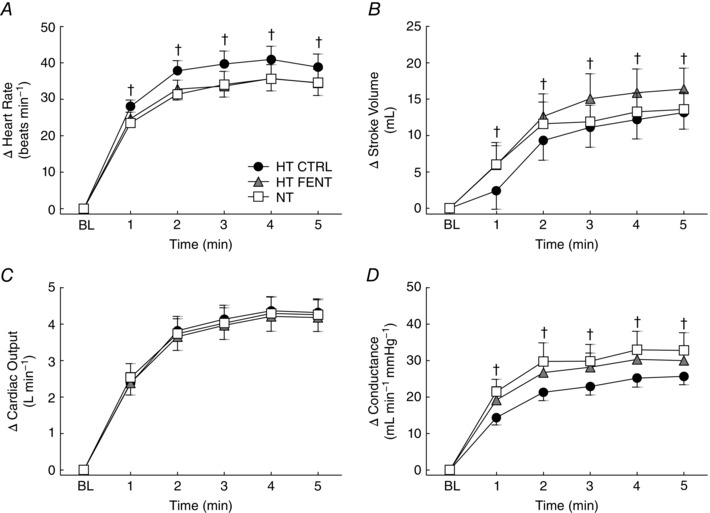
Responses of heart rate (A), stroke volume (B), cardiac output (C) and total vascular conductance (D) during each minute of cycling. HT CTRL (black circles), hypertensive subjects before fentanyl administration; HT FENT (grey triangles), hypertensive subjects after fentanyl administration; NT (white squares), normotensive subjects. BL, resting baseline. Data are the mean ± SEM. † P < 0.05 HT CTRL vs. HT FENT.
When subgroups of individuals with systolic HT, diastolic HT or concurrent systolic and diastolic HT were considered, statistical analyses to compare each of these HT subgroups with the NT group and to test whether fentanyl attenuated their cardiovascular responses led to the same conclusions.
Discussion
In clinical exercise tests using a treadmill, an abnormal increase of BP in HT patients was detected during the first 6 min of exercise, at intensities of ∼5–7 metabolic‐equivalents (Papademetriou et al. 1989; Kokkinos et al. 2002). The present study reproduced this condition in an experimental protocol. In detail, using beat‐to‐beat measurement, we show that the BP abnormally rises since the first minute of leg cycling exercise in HT patients. More importantly, we assessed the role of the group III and IV leg muscle afferent signal in this exaggerated BP response to leg exercise, using a lumbar intrathecal injection of fentanyl to attenuate the muscle afferent signal. Intrathecal fentanyl normalized the increase of BP during cycling in HT men, suggesting an overactivity of the exercise pressor reflex in this population. These findings demonstrate that a key mechanism driving an increased cardiovascular risk in HT patients can be corrected, with potential application for the development of treatments for this abnormal response to exercise.
Cardiovascular responses to cycling
During dynamic exercise, the integration of central command, the exercise pressor reflex and other neural signals within the brainstem modulates sympathetic and parasympathetic activity directed to the heart, evoking increases in CO, and also increasing sympathetic activity to peripheral blood vessels. In healthy conditions, the sympathetic vasoconstriction is attenuated in the exercising muscles because of a number of substances released from the active muscle, a physiological phenomenon termed functional sympatholysis (Remensnyder et al. 1962). The result is an increase in blood flow to the exercising muscles, with BP maintained in a narrow range. However, HT animals presented impaired functional sympatholysis, with significant decrease of vascular conductance in the exercising limb during superimposed sympathetic stimulation (Zhao et al. 2006; Mizuno et al. 2014; Thomas, 2015). Similarly, HT humans presented impaired functional sympatholysis in the exercising forearm, associated with an exaggerated increase of BP and sympathetic activity (Vongpatanasin et al. 2011). During one‐leg exercise, although the functional sympatholysis was not significantly impaired in HT humans, they consistently had lower leg vascular conductance compared to NT subjects (Mortensen et al. 2014). In the present study, although TVC responses to cycling only tended to be different between HT and NT subjects, a clear indication of blunted increase of TVC driving the exaggerated BP in the HT group is the effect of fentanyl with respect to reducing the BP response, accompanied by a greater increase of TVC and similar CO. Possibly, the attenuation of the exercise pressor reflex resulted in less sympathetic vasoconstriction, counterbalancing the effects of the impaired functional sympatholysis with regard to TVC and BP. In line with our findings, lumbar intrathecal fentanyl injection also resulted in a decrease of BP during leg exercise in heart‐failure patients, accompanied by an increase in the exercising leg blood flow and vascular conductance (Amann et al. 2014).
Regulation of HR during low‐intensity exercise is mostly attributed to the central command (Williamson et al. 1995), with the mechanoreflex having some influence (Williamson et al. 1995; Gladwell & Coote, 2002). We found that fentanyl decreased the HR response to cycling in the HT subjects, corroborating a possible overactivity of the mechanoreflex in HT patients (Greaney et al. 2015). However, the lower HR was compensated by a greater SV, and so the CO increase during cycling remained unchanged.
At the molecular level, two important factors associated with the overactive exercise pressor reflex in hypertension are the lower nitric oxide (NO) bioavailability and the greater oxidative stress mediated by angiotensin II (Smith et al. 2015; Thomas, 2015). NO modulates neural signalling in the nucleus tractus solitarius and rostral ventrolateral medulla, two areas related to autonomic and cardiovascular regulation. An increase of NO concentration in these areas significantly decreases BP and sympathetic activity, as well as their responses to exercise, in HT rats (Kishi et al. 2002; Leal et al. 2013 a; Smith et al. 2015), suggesting that NO bioavailability in the central nervous system may be decreased in hypertension. In this sense, oxidative enzymes activated by angiotensin II increase reactive oxygen species, such as superoxide anions. These substances rapidly react with NO, generating peroxynitrite, thus reducing NO bioavailability (Kishi & Hirooka, 2012). Peripherally, oxidative stress also decreases NO bioavailability in the skeletal muscle vasculature, resulting in impaired functional sympatholysis (Zhao et al. 2006) and, with the accumulation of metabolites, further sensitization of the muscle afferents (Smith et al. 2015; Thomas, 2015). Moreover, alterations in the expression of proteins related to NO formation (Murphy et al. 2013), oxidative stress (Felix & Michelini, 2007) and muscle afferent receptors (Mizuno et al. 2011) have been demonstrated in HT rats, indicating that multiple alterations are required, at the central and peripheral level, to significantly impair the homeostasis and cause an exaggerated pressor response to exercise.
Fentanyl‐related effects and cardiovascular responses to static handgrip
One major concern regarding the use of intrathecal fentanyl is the need to rule out the possibility of cephalad migration through the cerebrospinal fluid or its vascular absorption. A direct action of fentanyl on opioid receptors within the brainstem can decrease HR and BP responses to exercise (Caringi et al. 1998) and this would mislead interpretations concerning the role of muscle afferents during cycling. Therefore, we assessed cardiovascular responses to static handgrip, which are expected to be impaired only if fentanyl had migrated within the cerebrospinal fluid to a higher level of the spinal cord and the brainstem. Because these responses were not affected, along with the absence of nausea, dyspnoea and pruritus above the abdominal level, a central effect of fentanyl can be considered most improbable in our experiments. Moreover, studies with similar doses of fentanyl excluded its central effect by observing the hyperventilatory response to CO2 breathing and the preserved cardiovascular responses to arm exercise (Amann et al. 2010; Trinity et al. 2010).
Methodological aspects
The design of the present study presents some limitations worthy of consideration. We did not include sham injection or injection of saline and, considering the symptoms that could manifest after fentanyl injection, we did not blind the subjects about the pharmacological intervention. However, a previous study showed no difference between the absence of injection or saline injection (Amann et al. 2009), suggesting that the blinding of subjects is not crucial for the responses observed.
HT subjects presented a significant decrease of ∼12 mmHg in the resting MBP before the cycling session following fentanyl. Some level of decrease in resting MBP (∼3 mmHg) following fentanyl administration has been reported by our group and in other studies conducted in healthy young men (Amann et al. 2010; Trinity et al. 2010; Amann et al. 2011; Amann et al. 2014; Olson et al. 2014; Barbosa et al. 2015; Ives et al. 2015; Sidhu et al. 2015), raising the question of whether the stimulation of spinal opioid receptors could affect BP regulation at rest. Some possible mechanisms are a reduced activity of preganglionic sympathetic neurons as a result of activation of opioid receptors in the intermediolateral cell column of the spinal cord (Li & Han, 1984; D'Angelo et al. 1994), as well as a local anaesthetic effect (Gissen et al. 1987; Power et al. 1991) affecting sympathetic nerves. The psychological stress associated with the experimental procedures (Huang et al. 2013) and a hypotensive effect of cumulative exercise sessions (da Nobrega, 2005; Halliwill et al. 2013) might have affected resting BP. Nevertheless, a central effect of fentanyl is most improbable in our experiments because of the preserved MBP response to handgrip and an absence of sensory symptoms.
The blockade of the muscle afferent signal by fentanyl was probably incomplete, as a result of limited delivery to μ‐opioid receptor and afferent fibres expressing δ‐opioid receptors, including some mechanosensitive group III fibres (Leal et al. 2013 b).
Perspectives
The present study is the first to report that an overactive exercise pressor reflex can cause exaggerated increase of BP in HT individuals during low‐intensity leg exercise. These potentially dangerous elevations in BP possibly increase the cardiovascular risk during many daily activities (e.g. playing with children, cleaning duties, walking upstairs, etc.) (Ainsworth et al. 2011), limit regular exercise training, and are associated with an increased risk of developing chronic cardiovascular diseases. Correction of the exercise pressor reflex overactivity in HT patients would potentially protect them from the acute increased risk of cardiovascular events and improve safety during regular physical activities, which is beneficial for the cardiovascular system over the long term. From a clinical perspective, more research is needed to create a new intervention aiming to restore the normal exercise pressor reflex, probably via action on the free nerve endings of the group III and IV skeletal muscle afferents.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
Experiments were performed at Antonio Pedro University Hospital and Laboratory of Exercise Sciences, Fluminense Federal University, Brazil. TCB was involved in the conception and design of the experiments; the collection, assembly, analysis and interpretation of data; and the drafting of the first version of the manuscript. LVC, NHS and ACLN were involved in the conception and design of the experiments; the interpretation of data; the drafting of the manuscript; and the revision of the article for important intellectual content. IAF, EP, HNMR, VPG and NGR were involved in the collection, analysis and interpretation of data and the revision of the article for important intellectual content. All authors revised and approved the final version and submission of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by grants and scholarships from the Brazilian National Council of Scientific and Technological Development (CNPq – 87584/2013‐9), the Foundation for Research Support of Rio de Janeiro (FAPERJ – E‐26/110.097/2013), Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES – 99999.008878/2014‐05) and the Brazilian Funding Agency for Studies and Projects (FINEP – MCT/FINEP/CT‐INFRA‐PROINFRA‐01/2007). NHS was sponsored by the program Science without Borders (PVE 051/2012 – CNPq/CAPES).
Acknowledgements
The authors appreciate the time and effort expended by all volunteer subjects in this study. We would also like to thank Dr Lisbeth G. Jorgensen, Dr Alessandra C. Toledo‐Arruda, Dr Gustavo Mataruna and Igor R. C. Cardoso for their support during the experiments.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr , Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC & Leon AS (2011). 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43, 1575–1581. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2010). Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA (2009). Opioid‐mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR & Richardson RS (2011). On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589, 3855–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, Morgan DE, Gmelch B, Witman MA, Jonathan Groot H, Walter Wray D, Stehlik J & Richardson RS (2014). Group III/IV muscle afferents impair limb blood in patients with chronic heart failure. Int J Cardiol 174, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa TC, Fernandes IA, Magalhaes‐Jr N, Cavalcanti IL, Secher NH, Nobrega AC & Vianna LC (2015). Oscillatory blood pressure response to the onset of cycling exercise in men: role of group III/IV muscle afferents. Exp Physiol 100, 302–311. [DOI] [PubMed] [Google Scholar]
- Caringi D, Mokler DJ, Koester DM & Ally A (1998). Rostral ventrolateral medullary opioid receptor activation modulates pressor response to muscle contraction. Am J Physiol Heart Circ Physiol 274, H139–H146. [DOI] [PubMed] [Google Scholar]
- Chaney MA (1995). Side effects of intrathecal and epidural opioids. Can J Anaesth 42, 891–903. [DOI] [PubMed] [Google Scholar]
- Coote JH, Hilton SM & Perez‐Gonzalez JF (1971). The reflex nature of the pressor response to muscular exercise. J Physiol 215, 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo R, Anderson MT, Philip J & Eisenach JC (1994). Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 80, 1209–1215. [DOI] [PubMed] [Google Scholar]
- da Nobrega AC (2005). The subacute effects of exercise: concept, characteristics, and clinical implications. Exerc Sport Sci Rev 33, 84–87. [DOI] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299, H1318–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ & Raven PB (2012). Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix JV & Michelini LC (2007). Training‐induced pressure fall in spontaneously hypertensive rats is associated with reduced angiotensinogen mRNA expression within the nucleus tractus solitarii. Hypertension 50, 780–785. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN & Fadel PJ (2015). Autonomic adjustments to exercise in humans. Compr Physiol 5, 475–512. [DOI] [PubMed] [Google Scholar]
- Gissen AJ, Gugino LD, Datta S, Miller J & Covino BG (1987). Effects of fentanyl and sufentanil on peripheral mammalian nerves. Anesth Analg 66, 1272–1276. [PubMed] [Google Scholar]
- Gladwell VF & Coote JH (2002). Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol 540, 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI & Mitchell JH (1972). Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226, 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Edwards DG, Fadel PJ & Farquhar WB (2015). Rapid onset pressor and sympathetic responses to static handgrip in older hypertensive adults. J Hum Hypertens 29, 402–408. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Buck TM, Lacewell AN & Romero SA (2013). Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol 98, 7–18. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Webb HE, Zourdos MC & Acevedo EO (2013). Cardiovascular reactivity, stress, and physical activity. Front Physiol 4, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives SJ, Amann M, Venturelli M, Witman MA, Jonathan Groot H, Walter Wray D, Morgan DE, Stehlik J & Richardson RS (2015). The mechanoreflex and hemodynamic response to passive leg movement in heart failure. Med Sci Sports Exerc doi: 10.1249/MSS.0000000000000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuya M, Hansen TW, Thijs L, Bjorklund‐Bodegard K, Kuznetsova T, Ohkubo T, Richart T, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y & Staessen JA (2007). Diagnostic thresholds for ambulatory blood pressure monitoring based on 10‐year cardiovascular risk. Circulation 115, 2145–2152. [DOI] [PubMed] [Google Scholar]
- Kishi T & Hirooka Y (2012). Oxidative stress in the brain causes hypertension via sympathoexcitation. Front Physiol 3, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Ito K, Sakai K, Shimokawa H & Takeshita A (2002). Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke‐prone spontaneously hypertensive rats. Hypertension 39, 264–268. [DOI] [PubMed] [Google Scholar]
- Kokkinos PF, Andreas PE, Coutoulakis E, Colleran JA, Narayan P, Dotson CO, Choucair W, Farmer C & Fernhall B (2002). Determinants of exercise blood pressure response in normotensive and hypertensive women: role of cardiorespiratory fitness. J Cardiopulm Rehabil 22, 178–183. [DOI] [PubMed] [Google Scholar]
- Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J & Salonen JT (2001). Systolic blood pressure response to exercise stress test and risk of stroke. Stroke 32, 2036–2041. [DOI] [PubMed] [Google Scholar]
- Leal AK, Mitchell JH & Smith SA (2013. a). Treatment of muscle mechanoreflex dysfunction in hypertension: effects of L‐arginine dialysis in the nucleus tractus solitarii. Exp Physiol 98, 1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AK, Williams MA, Garry MG, Mitchell JH & Smith SA (2008). Evidence for functional alterations in the skeletal muscle mechanoreflex and metaboreflex in hypertensive rats. Am J Physiol Heart Circ Physiol 295, H1429–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AK, Yamauchi K, Kim J, Ruiz‐Velasco V & Kaufman MP (2013. b). Peripheral delta‐opioid receptors attenuate the exercise pressor reflex. Am J Physiol Heart Circ Physiol 305, H1246–H1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ & Han JS (1984). Depressor and bradycardic effect following intrathecal injection of [NMePhe3,D‐Pro4] morphiceptin in rats. Eur J Pharmacol 99, 91–95. [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B & Zannad F (2013). 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31, 1281–1357. [DOI] [PubMed] [Google Scholar]
- McCloskey DI & Mitchell JH (1972). Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes AF, Nobrega AC, Fuchs IE, Ally A & Wilson LB (1995). Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L‐7 dorsal horn of cats. Circ Res 77, 326–334. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Iwamoto GA, Vongpatanasin W, Mitchell JH & Smith SA (2014). Exercise training improves functional sympatholysis in spontaneously hypertensive rats through a nitric oxide‐dependent mechanism. Am J Physiol Heart Circ Physiol 307, H242–H251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Murphy MN, Mitchell JH & Smith SA (2011). Antagonism of the TRPv1 receptor partially corrects muscle metaboreflex overactivity in spontaneously hypertensive rats. J Physiol 589, 6191–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Gliemann L, Thaning P, Saltin B & Hellsten Y (2014). Exercise training modulates functional sympatholysis and alpha‐adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J Physiol 592, 3063–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E & Erikssen J (1996). Exercise blood pressure predicts mortality from myocardial infarction. Hypertension 27, 324–329. [DOI] [PubMed] [Google Scholar]
- Murphy MN, Mizuno M, Downey RM, Squiers JJ, Squiers KE & Smith SA (2013). Neuronal nitric oxide synthase expression is lower in areas of the nucleus tractus solitarius excited by skeletal muscle reflexes in hypertensive rats. Am J Physiol Heart Circ Physiol 304, H1547–H1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble BJ, Borg GA, Jacobs I, Ceci R & Kaiser P (1983). A category‐ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 15, 523–528. [PubMed] [Google Scholar]
- O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A & Zhang Y (2013). European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 31, 1731–1768. [DOI] [PubMed] [Google Scholar]
- Olson TP, Joyner MJ, Eisenach JH, Curry TB & Johnson BD (2014). Influence of locomotor muscle afferent inhibition on the ventilatory response to exercise in heart failure. Exp Physiol 99, 414–426. [DOI] [PubMed] [Google Scholar]
- Papademetriou V, Notargiacomo A, Sethi E, Costello R, Fletcher R & Freis ED (1989). Exercise blood pressure response and left ventricular hypertrophy. Am J Hypertens 2, 114–116. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, MacDonald HV, Ash GI, Lamberti LM, Farquhar WB, Arena R & Johnson BT (2015). Assessing the existing professional exercise recommendations for hypertension: a review and recommendations for future research priorities. Mayo Clin Proc 90, 801–812. [DOI] [PubMed] [Google Scholar]
- Power I, Brown DT & Wildsmith JA (1991). The effect of fentanyl, meperidine and diamorphine on nerve conduction in vitro. Reg Anesth 16, 204–208. [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH & Sarnoff SJ (1962). Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11, 370–380. [DOI] [PubMed] [Google Scholar]
- Rondon MU, Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM & Negrao CE (2006). Abnormal muscle metaboreflex control of sympathetic activity in never‐treated hypertensive subjects. Am J Hypertens 19, 951–957. [DOI] [PubMed] [Google Scholar]
- Sausen MT, Delaney EP, Stillabower ME & Farquhar WB (2009). Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105, 351–356. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Rossman MJ, Gmelch BS, Bledsoe AD, Richardson RS & Amann M (2015). Aging alters muscle reflex control of autonomic cardiovascular responses to rhythmic contractions in humans. Am J Physiol Heart Circ Physiol 309, H1479–H1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Leal AK, Murphy MN, Downey RM & Mizuno M (2015). Muscle mechanoreflex overactivity in hypertension: a role for centrally‐derived nitric oxide. Auton Neurosci 188, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD (2015). Functional sympatholysis in hypertension. Auton Neurosci 188, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, 3rd, Fulton JE , Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN & Costa F (2007). Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115, 2358–2368. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett‐O'Keefe Z, Runnels S, Morgan DE, Wray DW & Richardson RS (2010). Limb movement‐induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299, H1693–H1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams‐Huet B, Mitchell JH, Victor RG & Thomas GD (2011). Functional sympatholysis is impaired in hypertensive humans. J Physiol 589, 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling KH, Jansen JR, Settels JJ & Schreuder JJ (1993). Computation of aortic flow from pressure in humans using a nonlinear, three‐element model. J Appl Physiol (1985) 74, 2566–2573. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, Winchester PK, Zim S & Mitchell JH (1995). Instantaneous heart rate increase with dynamic exercise: central command and muscle‐heart reflex contributions. J Appl Physiol (1985) 78, 1273–1279. [DOI] [PubMed] [Google Scholar]
- Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W & Thomas GD (2006). Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II‐dependent hypertension. Hypertension 48, 637–643. [DOI] [PubMed] [Google Scholar]


