Abstract
Key point
Erythropoietin (Epo) treatment may induce myogenic differentiation factor (MyoD) expression and prevent apoptosis in satellite cells (SCs) in murine and in vitro models.
Endurance training stimulates SC proliferation in vivo in murine and human skeletal muscle.
In the present study, we show, in human skeletal muscle, that treatment with an Epo‐stimulating agent (darbepoetin‐α) in vivo increases the content of MyoD+ SCs in healthy young men. Moreover, we report that Epo receptor mRNA is expressed in adult human SCs, suggesting that Epo may directly target SCs through ligand‐receptor interaction.
Moreover, endurance training, but not Epo treatment, increases the SC content in type II myofibres, as well as the content of MyoD+ SCs.
Collectively, our results suggest that Epo treatment can regulate human SCs in vivo, supported by Epo receptor mRNA expression in human SCs. In effect, long‐term Epo treatment during disease conditions involving anaemia may impact SCs and warrants further investigation.
Abstract
Satellite cell (SC) proliferation is observed following erythropoitin treatment in vitro in murine myoblasts and endurance training in vivo in human skeletal muscle. The present study aimed to investigate the effects of prolonged erythropoiesis‐stimulating agent (ESA; darbepoetin‐α) treatment and endurance training, separately and combined, on SC quantity and commitment in human skeletal muscle. Thirty‐five healthy, untrained men were randomized into four groups: sedentary‐placebo (SP, n = 9), sedentary‐ESA (SE, n = 9), training‐placebo (TP, n = 9) or training‐ESA (TE, n = 8). ESA/placebo was injected once weekly and training consisted of ergometer cycling three times a week for 10 weeks. Prior to and following the intervention period, blood samples and muscle biopsies were obtained and maximal oxygen uptake () was measured. Immunohistochemical analyses were used to quantify fibre type specific SCs (Pax7+), myonuclei and active SCs (Pax7+/MyoD+). ESA treatment led to elevated haematocrit, whereas endurance training increased . Endurance training led to an increase in SCs associated with type II fibres (P < 0.05), whereas type I fibres showed no changes. Both ESA treatment and endurance training increased Pax7+/MyoD+ cells, whereas only ESA treatment increased the total content of MyoD+ cells. Epo‐R mRNA presence in adult SC was tested with real‐time RT‐PCR using fluorescence‐activated cell sorting (CD56+/CD45−/CD31−) to isolate cells from a human rectus abdominis muscle and was found to be considerably higher than in whole muscle. In conclusion, endurance training and ESA treatment may separately stimulate SC commitment to the myogenic program. Furthermore, ESA‐treatment may alter SC activity by direct interaction with the Epo‐R expressed on SCs.
Key points
Erythropoietin (Epo) treatment may induce myogenic differentiation factor (MyoD) expression and prevent apoptosis in satellite cells (SCs) in murine and in vitro models.
Endurance training stimulates SC proliferation in vivo in murine and human skeletal muscle.
In the present study, we show, in human skeletal muscle, that treatment with an Epo‐stimulating agent (darbepoetin‐α) in vivo increases the content of MyoD+ SCs in healthy young men. Moreover, we report that Epo receptor mRNA is expressed in adult human SCs, suggesting that Epo may directly target SCs through ligand‐receptor interaction.
Moreover, endurance training, but not Epo treatment, increases the SC content in type II myofibres, as well as the content of MyoD+ SCs.
Collectively, our results suggest that Epo treatment can regulate human SCs in vivo, supported by Epo receptor mRNA expression in human SCs. In effect, long‐term Epo treatment during disease conditions involving anaemia may impact SCs and warrants further investigation.
Abbreviations
- DAPI
4′,6‐diamidino‐2‐phenylindole
- eMHC
embryonic myosin heavy chain
- Epo
erythropoietin
- Epo‐R
erythropoietin receptor
- ESA
erythropoietin‐stimulating agent
- FACS
fluorescence‐activated cell sorting
- FBS
fetal bovine serum
- MyoD
myogenic differentiation factor
- nMHC
neonatal myosin heavy chain
- Pax7
paired box transcription factors 7
- SC
satellite cell
maximal oxygen uptake
Introduction
Satellite cells (SCs), which are located between the basal lamina and the plasma membrane in skeletal myofibres (Mauro, 1961), have been extensively verified to comprise a bona fide population of muscle stem cells and to play an essential role during muscle regeneration (Collins et al. 2005; Lepper et al. 2011; von Maltzahn et al. 2013). In addition to regeneration, SCs are the source of additional myonuclei during myofibre hypertrophy, and provide additional transcriptional capacity (Bruusgaard et al. 2010; McCarthy et al. 2011; Fry et al. 2014 a), although recent evidence suggests this is not completely essential for muscle hypertrophy (McCarthy et al. 2011) or the prevention of age‐related muscle atrophy (Fry et al. 2015). The function of SCs is profoundly influenced by physical cues from the extracellular matrix (Urciuolo et al. 2013) and soluble factors released from the immediate SC niche or the systemic environment, including several growth factors and cytokines (Tatsumi et al. 2002; McKay et al. 2008; Kandalla et al. 2011; Toth et al. 2011; Price et al. 2014; Tierney et al. 2014; Farup et al. 2015). Notably, factors delivered from the systemic environment have been shown to rejuvenate impaired stem cell function in vivo in old mice (Conboy et al. 2005; Elabd et al. 2014) and in vitro in human myoblasts (Carlson et al. 2009).
Erythropoietin (Epo) is a 30.4 kDa glycoprotein found in the circulation (Lombardero et al. 2011) and may directly or indirectly influence skeletal muscle and SCs (Jia et al. 2009). Epo is secreted form the kidneys and is the predominant factor for regulating the red blood cell pool by stimulating the proliferation and differentiation of erythroid progenitor cells, mainly in a hypoxia‐dependent manner (Lombardero et al. 2011). Epo is a ligand and acts by interaction with the Epo receptor (Epo‐R), which is present at the surface of the erythroid progenitor cells (Tilbrook & Klinken, 1999). Furthermore, the Epo‐R has been identified in a variety of other cell types and tissues, including skeletal muscle (Lombardero et al. 2011). Ogilvie et al. (2000) were the first to report Epo‐R expression in cultured primary murine myoblasts and the C2C12 cell line, which indicates that Epo may have a potential role in skeletal muscle function and myogenesis. Subsequently, the Epo‐R has been identified in fractions of the sarcolemma (Lundby et al. 2008), cultured primary muscle derived cells (Rundqvist et al. 2009) and in fetal myoblasts (Launay et al. 2010; Lamon et al. 2014) from humans. These findings led to investigations of a potential role of Epo–Epo‐R interactions in skeletal muscle and especially in SCs and myoblasts. However, recent studies have questioned the validity of previously utilized antibodies, thereby questioning whether the Epo‐R is expressed in human skeletal muscle or SCs (Christensen et al. 2015 b). Interestingly, to our knowledge, no studies have investigated whether the Epo‐R is expressed in freshly sorted (i.e. non‐cultured) primary SCs from adult human muscle.
In C2C12 cells, Epo appears to enhance proliferation and delay differentiation by the induction of MyoD expression and repression of myogenin expression (Ogilvie et al. 2000). Furthermore, treatment with Epo has been shown to induce faster muscle regeneration after muscle trauma in rodents (Rotter et al. 2008; Jia et al. 2009). However, recent studies have questioned the effect of Epo on myogenizes because in vitro Epo treatment of rat or human myoblasts failed to impact cell proliferation or differentiation (Launay et al. 2010; Lamon et al. 2014). However, to what extent these in vitro conditions and results mimic the in vivo effects of Epo in human skeletal muscle and SCs remains to be investigated.
In addition to the potential effects on SCs, Epo can increase the maximal oxygen uptake () and endurance capacity by increasing the red blood cell pool (Thomsen et al. 2007). Endurance training is another well‐described strategy for increasing and endurance capacity, although partly through mechanisms other than Epo treatment. In relation to SCs, endurance training is only sparsely investigated. The results obtained from rodents indicate that the quantity of SCs increases after endurance training (Kurosaka et al. 2009; Shefer et al. 2010). However, human studies have shown more ambiguous results, with one group finding no response in the SC pool following endurance training (Snijders et al. 2011), whereas other groups report an increase in the SC pool (Charifi et al. 2003; Verney et al. 2008). Furthermore, specific increases in SC content in type I fibres (Fry et al. 2014 b), hybrid fibres (Joanisse et al. 2013) and type II fibres (Verney et al. 2008) have been reported following endurance training. This indicates a potential fibre type specific SC response following endurance training; however, from the present literature, it is unclear which fibre type specific SCs are the most responsive to endurance training. In addition to SC content, Joanisse et al. (2013) reported an increased myogenic commitment of SCs (identified as Pax7−/MyoD+) after a short period (6 weeks) of high intensity endurance training in humans, indicating that endurance training may induce SC myogenic commitment. However, the effect of a longer, traditional endurance training protocol, as well as the combination with Epo treatment, is yet to be investigated.
The present study aimed to investigate (1) the separate and additive role of an erythropoiesis‐stimulating agent (ESA) and endurance training on the fibre type specific SC quantity; (2) the ability of ESA treatment and endurance training to induce myogenic commitment of SCs; and (3) whether a potential effect of Epo can be ascribed to a direct effect of Epo on the SCs (through SC expression of the Epo‐R). We hypothesized that (1) both ESA treatment and endurance training increase the SC pool; (2) both interventions increase the quantity of SCs committed to the myogenic programme; and (3) Epo‐R is expressed in freshly isolated SCs/myoblasts from adult human skeletal muscle.
Methods
Ethical approval
All participants were given oral and written information, and provided their written consent to participate in accordance with the Declaration of Helsinki. The study was approved by the Local Ethical Committee of Central Denmark Region (M‐20110035), and was registered at www.clinicaltrails.gov with the clinical trial number NCT01320449.
Subjects
Thirty‐eight healthy and untrained men participated in the study. Prior to enrolment, each participant underwent a physical examination and electrocardiogram measurements to ensure that potential ESA treatment was safe. Inclusion criteria were: age 18–35 years, body mass index between 18 and 29 kg/m2, normal blood pressure (<135/85 mmHg), moderate to low maximal oxygen uptake ( < 50 ml min−1 kg−1), haematocrit <45% and non‐smoker. Subjects were informed not to change their physical activity level and dietary habits throughout the study period. The results regarding metabolic changes and adaptations in skeletal muscle morphology have been published previously (Christensen et al. 2013; Larsen et al. 2014).
Experimental design
In a randomized and parallel design, the subjects were assigned to one of the following four groups: sedentary‐placebo (SP, n = 9), sedentary‐ESA (SE, n = 9), training‐placebo (TP, n = 10) or training‐ESA (TE, n = 10). Two participants were excluded in the TE group: one as a result of a groin injury and one because of poor compliance (i.e. final TE, n = 8). Additionally, after training, one subject (TP group) revealed having performed resistance training throughout the study and was therefore excluded from the analyses (i.e. final TP, n = 9). Hence, 35 subjects were included in the final analyses.
The subjects were in a single‐blinded manner treated subcutaneously once weekly for 10 weeks with either ESA (darbepoetin‐α, Aranesp; Amgen, Thousand Oaks, CA, USA) or placebo (isovolumetric saline). The estimated half‐life of darbepoetin‐α in chronic kidney disease patients not undergoing dialysis is 70 h (information provided by Amgen). For the first 3 weeks, the dose was 40 μg, and this was then reduced to 20 μg for the remaining 7 weeks. The initial three and four subjects in the SE and TE groups, respectively, were injected two times weekly for the first 3 weeks, which led to a higher haematocrit than expected (up to 50–54%). As a consequence, the injections were reduced to once weekly. Additionally, a few subjects were occasionally given placebo injections instead of ESA (SE, n = 4 and TE, n = 4) to keep the haematocrit value below 55% (one to four injections per subject; 19 in total). Haematocrit was measured once weekly until the end of the study period. One week prior to treatment and throughout the study, all participants were supplemented with 100 mg iron/day orally (Ferrosulfat; Ferro Duretter; GlaxoSmithKline, Brentford, UK) to avoid iron deficiency (Fisher, 2003).
The training groups performed three weekly supervised exercise sessions on an indoor ergometer throughout the 10‐week study period (Monark Ergomedic 928 E; Monark, Varberg, Sweden). The training protocol was based on a previously described progressive overload protocol, which has led to improvements in different endurance performance parameters (Vissing et al. 2012). Each training session consisted of a 5 min warm up, a main set and a 5 min cool down. Each of the following three main sets was performed once weekly: 40 min (day 1, continuous), 2 × 20 min with 5 min active recovery between intervals (day 2, long intervals) and 8 × 5 min with 1 min active recovery between intervals (day 3, short intervals). Target workloads for the first week were calculated based on oxygen uptake after a 5 min warm up at 140 W and (Larsen et al. 2014). At the following training sessions, the training instructors, who were blinded to treatment regimen (ESA/placebo), determined the target workload for each training session. A calculated progression scheme was not used in the present study because the improvements in relation to ESA treatment were unknown. If the ESA group had been able to train at a higher intensity, such a scheme would have induced an error as a result of unequal training stimuli, with the ESA group training at a lower relative intensity. Instead, the participants were encouraged to complete the main sets with the highest possible intensity. The aim was not only to increase the absolute intensity and magnitude of work performed every week, but also to ensure equal conditions in the two training groups despite different supplementation. A 2 W‐max test replaced a training session in weeks 4 and 8 to optimally target the workloads. A detailed description of the training protocol has been reported previously (Larsen et al. 2014).
Examination days
The subjects participated in an examination day before and after the 10‐week training/treatment period. They arrived at the research unit by taxi after an overnight fast (water intake was allowed). The subjects were informed not to participate in physical activity, change their dietary habits or drink alcohol 3 days prior to the examination days. The last training session was performed 60–72 h prior to the last examination day to minimize potential acute exercise effects. A series of tests were performed on the examination days; however, only analysis of muscle biopsies and blood samples are presented in the present study. A detailed description of the examination days has been published previously (Christensen et al. 2013).
Muscle biopsies
The same skilled physician obtained all skeletal muscle biopsies from vastus lateralis under local anaesthesia with a Bergström needle. The biopsies were collected early in the morning in a resting and non‐stimulated situation. Muscle biopsies were mounted in Tissue‐Tek (OCT compound; Sakura Finetek Europe BV, Alphen aan den Rijn, The Nederlands), immediately frozen in liquid nitrogen‐cooled isopentane and subsequently stored at −80°C until further analyses. Cryosections (10 μm) were cut at −18°C with pre‐ and post‐training sections placed on the same slide. Biopsies with freeze damages were excluded (12 samples in total).
Blood samples
Blood samples were once weekly (7 days after injection) during the training/treatment period to monitor the haematocrit value. Blood samples were centrifuged for 10 min at 4°C and 3200g, and haematocrit was measured on a Sysmex (Sysmex, Ballerup, Denmark) at the Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark.
To quantify the effect of darbepoetin‐α treatment on Epo/darbepoetin‐α levels, we collected serum samples from the SE group before the intervention and 7 days after the final injection. We were unable to identify an assay that could discriminate between endogenous Epo and darbepoetin‐α; however, because the combined concentration of both would determine Epo‐R signalling, we accepted this limitation. A commercially available kit (Quantikine IVD, Human Epo Immunoassay, #DEPOO; R&D Systems, Minneapolis, MN, USA) was utilized to measure serum endogenous Epo/darbepoetin‐α levels, as reported previously (Christensen et al. 2015 b). This assay has been validated and shown to react effectively with both endogenous Epo and darbepoetin‐α (Allon et al. 2002). A standard curve, ranging from 0.078 to 5 ng ml−1 darbepoetin‐α (Aranesp), was used (Allon et al. 2002; Christensen et al. 2015 b). In addition, a standard curve for endogenous Epo included in the assay, ranging from 2.5 to 200 mIU ml−1 (Human Erythropoietin Quantikine IVD; R&D Systems), was utilized to validate the ELISA kit performance. Both standard curves were linear and resulted in similar relative changes pre‐to‐post in the SE group, suggesting that the assay detected both endogenous Epo and darbepoetin‐α.
test
All subjects performed a test before and after the training period, independent of the examination days. The test was conducted on an ergometer bicycle (Monark Ergomedic 828E; Monark, Varberg, Sweden) and subjects were instructed to refrain from food and liquid intake (water was allowed) 2 h prior to the test. The test consisted of a 5 min warm‐up at 140 W; subsequently, the workload was increased by 35 W every 1 min until exhaustion. Subjects maintained a constant pedalling rate at 70 rpm throughout the test. Oxygen uptake was measured every 10 s (AMIS 2001; Innovision, Odense, Denmark) and was calculated as the highest mean of three consecutive measurements.
SC, myonuclei and central nuclei analysis
The number of SCs associated with type I, type II and hybrid (type I/II) fibres was determined using a staining protocol inspired from Joanisse et al. (2013). Sections were fixed in Histofix (Histolab, Gothenborg, Sweden) followed by 1.5 h in blocking buffer [2% BSA, 5% fetal bovine serum (FBS), 2% goat serum, 0.2% Triton X, 0.1% sodium azide]. Sections were then incubated overnight at 4°C with primary antibodies against Pax7 (1:500; catalogue no. MO15020; Neuromics, Edina, MN, USA) and Laminin (1:500; catalogue no. Z0097; Dako, Glostrup, Denmark). The next day, the sections were washed in PBS followed by incubation in appropriate secondary antibodies; Alexa‐fluor 568 goat‐anti‐mouse (1:200; catalogue no. A11004; Invitrogen A/S, Naerum, Denmark) and Alexa‐fluor 488 goat‐anti‐rabbit (1:500; catalogue no. A11008; Invitrogen A/S) at room temperature for 1.5 h. The sections were re‐fixed in Histofix for 4 min to preserve antigen/antibody interactions and then re‐blocked (5% goat serum in PBS) for 2 h at room temperature. Sections were then incubated overnight at 4°C with primary antibodies against myosin heavy chain I (1:500; Clone A4.951; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and myosin heavy chain II (1:1000; catalogue no. ab91506; Abcam, Cambridge, UK). The next day, the sections were washed in PBS and then incubated in appropriate secondary antibodies; Alexa‐fluor 488 goat‐anti‐mouse and Alexa‐fluor 350 goat‐anti‐rabbit (1:500; catalogue no. A11001 and catalogue no. A11046; Invitrogen A/S) for 2 h at room temperature. Finally, the sections were washed in PBS and mounted in medium containing 4′,6‐diamidino‐2‐phenylindole (DAPI) to visualize nuclei (Prolog Gold anti‐fade reagent, catalogue no. P36935; Invitrogen A/S). The sections were allowed to dry overnight in dark and then stored at −20°C until further analysis. Appropriate negative control stainings were utilized to confirm specific binding of the secondary antibodies.
All sections were visualized and analysed using a DM2000 fluorescence microscope and a Hi‐resolution Colour DFC camera (Leica, Stockholm, Sweden) combined with an image analysis software (Leica Qwin, version 3.2; Leica). All images were obtained at 20× magnification, and the number of Pax7+ cells (SCs) associated with type I, type II or hybrid fibres was quantified and centrally located nuclei were counted. The mean number of fibres counted per sample were 88 (range 16–272), 118 (range 24–281) and 5 (range 0–52) type I, type II and hybrid fibres, respectively. During the analysis, three samples were excluded because of poor staining quality and therefore insufficient ability to detect Pax7+ cells. The DAPI positive cells lying with the centre inside the basal lamina were counted as myonuclei. The investigator was blinded to pre/post sections and subject randomization.
Embryonic and neonatal myosin heavy chain analysis
Sections used for immunohistochemical analysis of embryonic myosin heavy chain (eMHC) and neonatal myosin heavy chain (nMHC) positive fibres were first incubated for 1 h in blocking buffer (2% BSA, 5% FBS, 2% goat serum, 0.2% Triton X, 0.1% sodium azide). Sections were then stained for eMHC (1:100; Clone F1.652; Developmental Studies Hybridoma Bank) or nMHC (1:100; NCL‐MHCn; Novocastra, Newcastle upon Tyne, UK) combined with laminin (1:500; catalogue no. Z0097, Dako) and incubated at 4°C overnight (eMHC staining) or 2 h at room temperature (nMHC staining). This was followed by incubation with the secondary antibody Alexa‐fluor 488 goat‐anti‐mouse and Alexa‐fluor 568 goat‐anti‐rabbit (1:500; catalogue no. A11011 and catalogue no. A11001; Invitrogen A/S) for 2 and 1.5 h, respectively. Finally, sections were mounted in a mounting medium containing DAPI (Prolog Gold anti‐fade reagent, catalogue no. P36935, Invitrogen A/S) and stored at −20°C. The visualization and counting of the eMHC and nMHC positive fibres were made with the same microscope and camera as described previously.
Analysis of myogenic activation
Following fixation and 1.5 h in blocking buffer (2% BSA, 5% FBS, 2% goat serum, 0.2% Triton X, 0.1% sodium azide), sections were incubated at 4°C overnight with the primary antibodies against Pax7 (1:500, catalogue no. MO15020, Neuromics) and MyoD1 (1:750; catalogue no. ab133627, Abcam, UK). The next day, sections were washed in PBS and incubated with secondary Alexa‐fluor 568 goat‐anti‐mouse and Alexa‐fluor 488 goat‐anti‐rabbit (1:200; catalogue no. A11004 and catalogue no. A11008; Invitrogen A/S) for 2 h at room temperature. Finally, sections were washed in PBS, mounted in medium containing DAPI (Prolog Gold anti‐fade reagent, catalogue no. P36935; Invitrogen A/S), and stored at −20 °C.
Cell preparation and fluorescence‐activated cell sorting (FACS) isolation
To examine whether adult human SCs or other mononuclear cell populations residing in the skeletal muscle microenvironment express the Epo‐R, we isolated primary cells from adult human muscle. Muscle tissue (15 g) was obtained from the musculus rectus abdominis during aortic aneurism surgery with patient (n = 1) consent and in accordance with the acceptance from the Local Ethical Committee of Central Denmark Region (reference no. 1‐10‐72‐20‐13) as described previously (Skov et al. 2015). Muscle was transported from the hospital to the laboratory in less than 30 min in ice‐cold Hepes‐based buffer (15 mm Na‐Hepes, 122 mm NaCl, 9 mm HCl, 2.8 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 1.27 mm CaCl2 and 5 mm d‐glucose).
Cell preparations were performed in agreement with previous works (Mozzetta et al. 2013; Tierney et al. 2014). For FACS, the muscle was mechanically minced with sterile scissors and enzymatically digested in DPBS (Gibco, Life Technologies, Naerum, Denmark) containing 2 mg ml−1 Collagenase A (Roche Diagnostics, Basel, Switzerland), 3 mg ml−1 Dispase II (Roche) and 10 μg ml−1 DNase I (Roche) for 90 min at 37°C. Following digestion, an HBSS solution (1:100 Penstrep, Gibco; 0.2% BSA) was added and the suspension was filtered twice through 70 and 40 μm cell strainers, respectively. The suspension (containing primary mononuclear cells and debris) was suspended in freezing buffer (70% Dulbecco's modified Eagle's medium, Gibco; 20% FBS, 10% DMSO), immediately frozen and stored for 1 week at −80°C until FACS analysis. The frozen cell suspension was thawed until only a small amount of ice was left and suspended in PBS + 10% FBS. The cells were then incubated in blocking buffer (HBSS, 1:100 Penstrep, 1:10 goat serum, 1% DNase, 0.2% BSA) on ice for 10 min followed by staining with primary antibodies against CD45‐FITC (1:30, Clone 5B1; MACS Miltenyi Biotec, Lund, Sweden), CD31‐FITC (1:30, Clone MEM‐05; Immunotools, Friesoythe, Germany), CD90‐PE (1:200, Clone 5E10; eBioscience, San Diego, CA, USA) and CD56‐PC7 (1:30, catalogue no. A51078; Beckman Coulter, Indianapolis, IN, USA) in blocking buffer at 4°C in darkness for 30 min. Cells were finally washed and resuspended in HBSS solution (1:100 Penstrep, 0.2% BSA). Non‐stained cells and single colour controls were prepared in combination with the primary (full colour) samples. Cell sorting was performed at the local FACS‐core facility with technical assistance using a FACS‐AriaIII cell sorter (BD Bioscience, San Jose, CA, USA). Gating strategies were optimized through multiple earlier experiments, which included various unstained, single colour and full colour samples.
Muscle SCs were isolated as Lin− (CD45−, CD31−)/CD56+ cells; mesenchymal progenitors (CD90+ cells) were isolated as Lin−/CD90+ cells; Lin+ cells were isolated as CD45+/CD31+ cells. The purity of the population was checked following the sorting and yielded >96%, 95% and 99% for the CD56+, CD90+ and Lin+ cell populations, respectively. Immediately upon sorting of the cells, pellets were frozen at −80°C awaiting gene expression analysis.
Gene expression analysis
RNA extraction
The frozen cell pellets (50,000–160,000), as well as the unsorted cells (preFACS), were resuspended in 1 ml of TriReagent. Furthermore, 5 and 25 mg muscle tissue pieces were homogenized in 1 ml of TriReagent (Molecular Research Centre, Cincinnati, OH, USA) containing five stainless steel balls of 2.3 mm in diameter (BioSpec Products, Bartlesville, OK, USA) and one silicon‐carbide sharp particle of 1 mm (BioSpec Products) by shaking in a FastPrep®‐24 instrument (MP Biomedicals, Illkirch, France) at speed level 4 for 15 s. To obtain complete homogenization of tissue, the shaking process was repeated with cooling on ice between the shaking step (to avoid heating of the sample). Following resuspension or homogenization, bromo‐chloropropane was added to separate the samples into an aqueous and an organic phase. After isolation of the aqueous phase, RNA was precipitated using isopropanol after addition of 80 μg of glycogen (10814‐010; Life Technologies). The RNA pellet was then washed in ethanol and subsequently dissolved in 10 μl of RNAse‐free water. Total RNA concentrations were determined with the RiboGreen assay (R11490; Life Technologies).
Real‐time RT‐PCR
In total, 8–17 ng of total RNA (max 4 ul) was converted into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, Valencia, CA, USA) and 1 μm poly‐dT (Invitrogen A/S) in accordance with the manufacturer's instructions (Qiagen). For each target mRNA, 0.25 μl of cDNA was amplified in a 25 μl SYBR Green PCR containing 1 × Quantitect SYBR Green Master Mix (Qiagen) and 100 nm of each primer (Table 1). The amplification was monitored in real time using the MX3005P Real‐Time PCR machine (Stratagene, La Jolla, CA, USA). The C t values were related to a standard curve made with known concentrations of cloned PCR products or DNA oligonucleotides (UltramerTM oligos; Integrated DNA Technologies, Inc., Leuven, Belgium) with a DNA sequence corresponding to the sequence of the expected PCR product. The specificity of the PCR products was confirmed by melting curve analysis after amplification. RPLP0 mRNA was chosen as the internal control.
Table 1.
Primers for real‐time RT‐PCR
| mRNA | Genbank | Sense | Anti‐sense |
|---|---|---|---|
| RPLP0 | NM_053275.3 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
| PAX7 | NM_002584.2 | AGCATCGACGGCATCCTGGG | CGTGAATGTGGTCCGACTGCG |
| THY1 | NM_006288.4 | ACTCCACCACTCTGGCCATTCC | GCTGATGCCCTCACACTTGACC |
| COL1A1 | NM_000088.3 | GGCAACAGCCGCTTCACCTAC | GCGGGAGGACTTGGTGGTTTT |
| EPOR | NM_000121.3 | CTGGTGCTGCTGACCGTGCT | GGCCTTCAAACTCGCTCTCTGG |
Statistical analysis
Data were checked for normality by QQ‐plots and plots of residuals vs. fitted values. One‐way ANOVA was used to assess differences in anthropometric data between the four experimental groups. A paired t test was utilized to compare pre‐to‐post changes in endogenous Epo and darbepoetin‐α levels in the SE group. A three‐way ANOVA with training, ESA and time as factors was used to examine interactions between groups for haematological and changes, as well as changes in SC and myonuclei content changes. When interactions were observed in the ANOVA analysis, a linear comparison post hoc analysis was made to evaluate time and group effects. Data for eMHC, nMHC, Pax7/MyoD and all data related to hybrid fibres were non‐normally distributed. For these data, a Kruskal–Wallis test was used to examine the additive effect of ESA treatment and training. This was followed by a Wilcoxon–Mann–Whitney test to examine the potential effect of ESA treatment and training individually. P < 0.05 was considered statistically significant. Statistical analyses were performed in STATA, version 13 (StataCorp, College Station, TX, USA).
Results
Anthropometric data
There were no significant differences in anthropometric data between the four groups, apart from a small difference in mean age (P < 0.05) (Table 2).
Table 2.
Anthropometric data at baseline
| Sedentary‐placebo | Sedentary‐ESA | Training‐placebo | Training‐ESA | Interaction | |
|---|---|---|---|---|---|
| (n = 9) | (n = 9) | (n = 9) | (n = 8) | P value | |
| Age (years) | 25 ± 2 | 23 ± 1 | 21 ± 1# | 25 ± 2 | 0.046* |
| Weight (kg) | 79.2 ± 3.3 | 82.1 ± 3.4 | 74.2 ± 2.7 | 78.8 ± 5.9 | 0.519 |
| Height (m) | 1.82 ± 0.02 | 1.87 ± 0.02 | 1.83 ± 0.02 | 1.81 ± 0.04 | 0.359 |
| Body mass index (kg/m2) | 24.0 ± 1.0 | 23.5 ± 0.6 | 22.2 ± 0.8 | 23.9 ± 1.2 | 0.419 |
Anthropometric data for all groups. Data are the mean ± SE. #Training‐placebo was significantly different from sedentary‐placebo and training‐ESA (P < 0.05).
Maximal oxygen uptake and treatment data
Training and ESA treatment increased significantly after the 10‐week study period (P < 0.001), although no additive effect was observed for combined training and ESA treatment (Table 3). ESA treatment, but not placebo, increased haemoglobin and haematocrit (P < 0.001) (Table 3). Training compliance was on average 97% in TP and 98% in TE. Throughout the study period, iron status did not change significantly (Table 3) (Christensen et al. 2013).
Table 3.
Maximal oxygen uptake and treatment data
| Sedentary‐placebo | Sedentary‐ESA | Training‐placebo | Training‐ESA | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| (l·min−1) | 3.37 ± 0.20 | 3.41 ± 0.23 | 3.56 ± 0.13 | 4.10 ± 0.23***##$$ | 3.28 ± 0.18 | 3.86 ± 0.13***$ | 3.05 ± 0.20 | 3.82 ± 0.22*** |
| (ml·min−1·kg−1) | 42.7 ± 2.5 | 43.2 ± 2.7 | 43.6 ± 1.6 | 50.1 ± 2.8***#$ | 43.9 ± 1.7 | 52.2 ± 0.9***##$$$ | 40.3 ± 2.9 | 49.2 ± 2.0***# |
| Haemoglobin (mmol·l−1) | 9.2 ± 0.2 | 9.0 ± 0.1 | 9.0 ± 0.1 | 10.0 ± 0.1***###$$$§§§ | 9.1 ± 0.2 | 8.9 ± 0.2 | 9.0 ± 0.2 | 10.2 ± 0.1***###$$$§§§ |
| Haematocrit (%) | 42.8 ± 0.7 | 41.8 0.5 | 42.1 ± 0.5 | 47.2 ± 0.5***###$$$§§§ | 41.8 ± 0.6 | 41.4 ± 0.5 | 42.1 ± 0.9 | 47.7 ± 0.6***###$$$§§§ |
, haemoglobin and haematocrit pre and post 10 weeks of sedentary‐placebo, sedentary‐ESA, training‐placebo or training‐ESA treatment. Data are the mean ± SE. Difference between pre and post within group; ***P < 0.001. Different from sedentary‐placebo post; #P < 0.05; ## P < 0.01; ### P < 0.001. Different from sedentary‐placebo pre, $ P < 0.05; $$ P < 0.01; $$$ P < 0.001. Different from training‐placebo post, §§§(P < 0.001).
Serum levels of combined endogenoues Epo and darbepoetin‐α in the SE group were 0.15 ng ml−1 (range 0.075–0.22 ng ml−1) pre‐intervention and 0.18 ng ml−1 (range 0.03–0.34 ng ml−1) 7 days after the final injection of darbepoetin‐α (P = 0.19) (Christensen et al. 2015 b).
Fibre type specific satellite cells
Figure 1 illustrates the assessment of fibre type specific SC content from immunohistochemical staining. The fibre type distribution at pre‐training was 41.6 ± 2.6%, 56.7 ± 2.9% and 1.7 ± 0.7% for type I, type II and hybrid fibres, with no group differences detected. The fibre type distribution did not change in any of the groups post‐intervention (groups combined: 41.9 ± 2.3%, 55.3 ± 2.4% and 2.8 ± 1.0% for type I, type II and hybrid fibres). Hybrid fibres were detected in 11 of 23 samples pre‐intervention and 21 of 29 samples post‐intervention, although they were equally dispersed between all groups.
Figure 1. Fibre type specific Pax7+ cell identification .
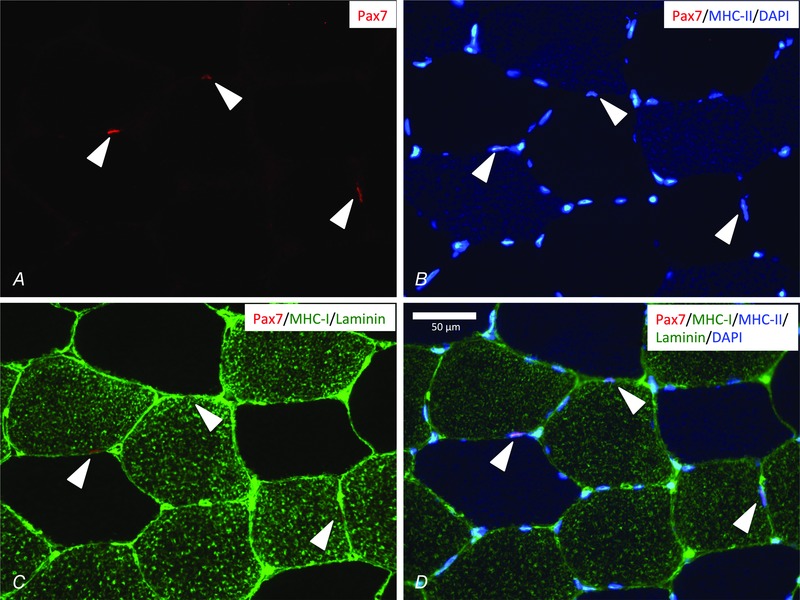
Representative muscle cross‐section stained for Pax7 (red, A), nuclei (DAPI) and MHC‐II (blue, B), MHC‐I and laminin (green, C) and merged in (D). Three Pax7+ cells are identified (cones) and all of them associated with type I fibres (MHC‐I+). Scale bar = 50 μm.
The number of SCs per fibre related to type I, type II or hybrid fibres is shown in Fig. 2. For SCs per fibre associated with type II fibres, we observed a training × time interaction (P < 0.05) (Fig. 2 B), which was supported by a significant training × time interaction for SC per myonuclei in type II fibres (P < 0.05) (Table 4). The post hoc analysis revealed an increase (P < 0.05) (Fig. 2 B) in SCs/fibre in type II fibres in the training groups (TP + TE), whereas no changes were detected in the sedentary groups (SP + SE). Furthermore, a pre‐training difference in SCs/fibre in type II fibres was observed between the training and sedentary groups (P < 0.05) (Fig. 2 B). Endurance training did not impact SC quantity in type I or hybrid fibres (Fig. 2 A and C). In general, very few hybrid fibres (example shown in Fig. 2 Da–c) were detected in the samples (the individual number of fibres per sample is shown in Fig. 2 C), thereby limiting the number of possible SCs to be detected. In samples where hybrid fibres were observed, we included 8.5 (range 1–52) hybrid fibres on average for the analysis shown in Fig. 2 C. This limits the mean detection threshold to 0.12 (1/8.5).
Figure 2. Satellite cell content pre‐ and post‐intervention period .
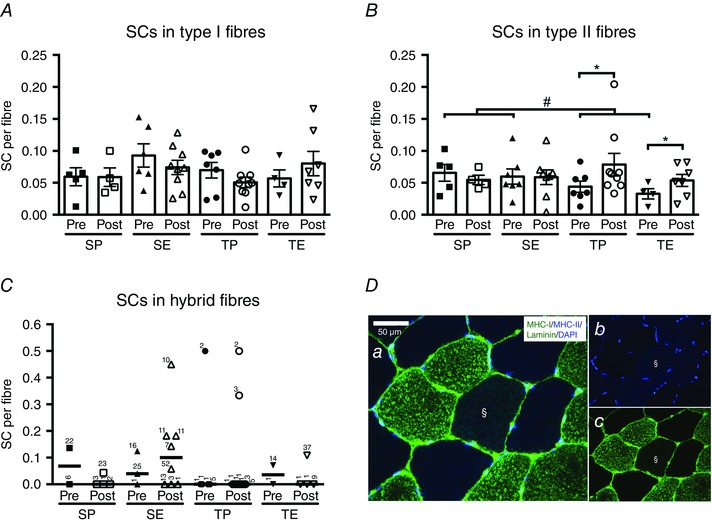
Satellite cells (SCs) associated with type I (A), type II (B) or hybrid (C) fibres pre and post 10 weeks of sedentary placebo (SP), sedentary ESA treatment (SE), training placebo (TP) or training ESA treatment (TE). One double positive (MHC‐I+/MHC‐II+) fibre is shown in (D) (§). SC content was expressed per fibre and data presented as individual values and the group mean ± SE (A and B) or individual values and medians (C). C, the number of fibres counted for each data point is shown next to the individual data point. Differences between pre and post are denoted by an asterisk (*P < 0.05). Group difference is denoted by a hash symbol (#P < 0.05).
Table 4.
Myonuclei and SCs per 100 myonuclei pre and post intervention period
| Sedentary‐placebo | Sedentary‐ESA | Training‐placebo | Training‐ESA | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Myonuclei/fibre | ||||||||
| Fibre type I | 2.03 ± 0.19 | 1.91 ± 0.16 | 1.685 ± 0.201 | 1.42 ± 0.18 | 1.72 ± 0.12 | 1.55 ± 0.12 | 2.01 ± 0.27 | 1.44 ± 0.12 |
| Fibre type II | 2.21 ± 0.24 | 2.06 ± 0.17 | 2.03 ± 0.182 | 1.91 ± 0.29 | 1.84 ± 0.25 | 1.965 ± 0.25 | 2.18 ± 0.29 | 2.02 ± 0.27 |
| Hybrid fibres | 2a | 2.50 (0.0–3.0) | 2.75 (0.0–3.0) | 1.05 (0.0––4.0) | NA | 0 (0.0–2.0) | 0.67 (0.0–1.3) | 2.38 (0.0–2.7) |
| SC/100 myonuclei | ||||||||
| Fibre type I | 2.5 ± 1.0 | 3.0 ± 0.7 | 5.0 ± 0.8 | 5.5 ± 1.7 | 3.7 ± 0.8 | 2.9 ± 0.4 | 2.6 ± 0.7 | 5.3 ± 1.1 |
| Fibre type II | 2.7 ± 1.1 | 2.3 ± 0.7 | 2.9 ± 0.5 | 3.2 ± 0.5 | 2.0 ± 0.3 | 3.1 ± 0.6* | 1.2 ± 0.5 | 3.2 ± 0.6* |
| Hybrid fibres | 6.8a | 0 (0.0–2.2) | 1.3 (0.0–4.6) | 8.3 (0.0–18.2) | NA | NA | 2.7 (0.0–5.4) | 0 (0.0–4.6) |
Myonuclei per fibre and satellite cells per myonclei in type I, type II and hybrid fibres pre and post 10 weeks of sedentary‐placebo, sedentary‐ESA, training‐placebo or training‐ESA treatment. Data are the mean ± SE for type I and type II fibres and median (range) for hybrid fibres. Significant change from pre is indicated by * (P < 0.05). a n = 1 included in analysis. NA, not available.
In addition, there was no main effect of ESA treatment on SC quantity.
Myogenic cell activation
In the present study, we differed between the following cell pools; cells expressing MyoD regardless of Pax7 (MyoD+), proliferating SCs expressing Pax7 and MyoD (Pax7+/MyoD+) and differentiating SCs expressing MyoD and not Pax7 (Pax7−/MyoD+) (McKay et al. 2012; Joanisse et al. 2013; Yin et al. 2013). Figure 3 A–C exemplifies the assessment of a Pax7+/MyoD+ cell (white cone) and a Pax7+MyoD− cell (yellow cone). No MyoD+ cells were found in any of the groups at baseline before the intervention (Fig. 3 D). Furthermore, no MyoD+ cells were identified in the SP group after the 10 weeks. We found no interaction between ESA treatment and training on MyoD expression. However, we did observe a main effect of ESA treatment with an increase in MyoD+ cells (P < 0.05) (Fig. 3 D), as well as Pax7+/MyoD+ cells (P < 0.05) (Fig. 3 E). Furthermore, we noted a tendency towards an ESA treatment effect on Pax7−/MyoD+ cells (P = 0.085) (Fig. 3 F). As for the effect of training, we found an overall increase in Pax7+/MyoD+ cells in the training groups (P < 0.05) (Fig. 3 E). This was supported by a difference between the training and the sedentary groups after training (P < 0.05) (Fig. 3 E). In addition, a tendency towards an increase in MyoD+ cells induced by training was observed (P = 0.068) (Fig. 3 D).
Figure 3. MyoD+ satellite cell identification pre‐ and post‐intervention period .
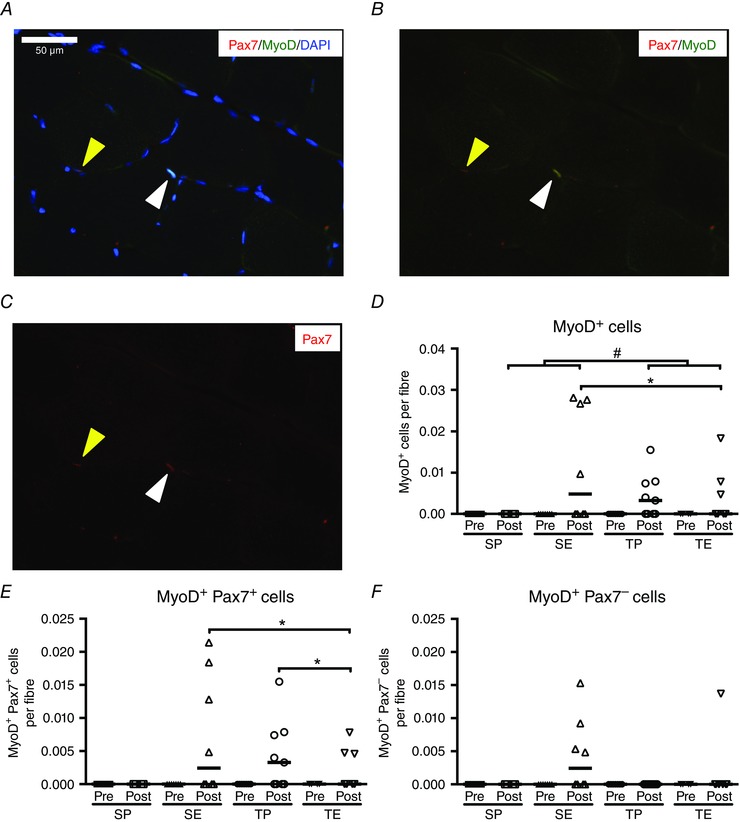
Representative muscle cross‐section stained for Pax7 (red, A), MyoD (green, B) and nuclei (DAPI, blue, C). One Pax7+/Myod+ cell (white cone) and one Pax7+/MyoD− (yellow cone) are identified. Scale bar = 50 μm. Quantification of MyoD+ cells (D), MyoD+/Pax7+ cells (E) and MyoD+/Pax7− cells (F) pre and post 10 weeks of sedentary placebo (SP), sedentary ESA treatment (SE), training placebo (TP) or training ESA treatment (TE). Cell content is expressed per fibre and data are presented as individual values and medians. Differences between pre and post are denoted by an asterisk (*P < 0.05). Group difference is denoted by a hash symbol (#P < 0.05).
Fibre type specific myonuclei
Myonuclei per fibre in type I, fibre type II and hybrid fibres were not significantly influenced by training or ESA treatment and no significant interactions were observed (Table 4).
Indices of remodelling
Quantification of fibres expressing the early isoforms of MHC, embryonic and neonatal MHC, in combination with detection of centrally located nuclei, was carried out to evaluate muscle fibre remodelling. Only three subjects expressed eMHC, and therefore the data are not presented graphically. Data on central nuclei in type I and II fibres (Fig. 4 A and B), as well as fibres expressing neonatal MHC (Fig. 4 C), were regularly distributed across all time‐points and no significant interactions or differences related to ESA treatment or endurance training were observed.
Figure 4. Indices of fibre remodelling or regeneration pre‐ and post‐intervention period .
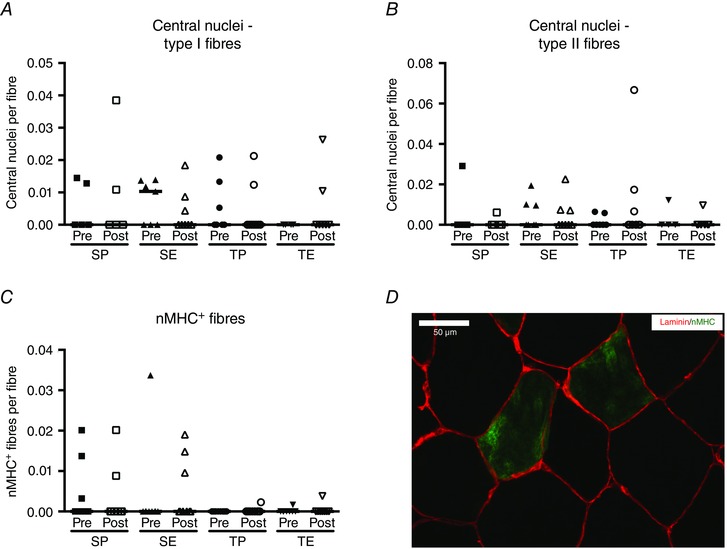
Central nuclei identified in type I (A) or type II (B) fibres, as well as fibres expressing neonatal (n)MHC (C) pre and post 10 weeks of sedentary placebo (SP), sedentary ESA treatment (SE), training placebo (TP) or training ESA treatment (TE). Two nMHC+ fibres (green) are shown in (D) with basal lamina delineated by laminin (red). Central nuclei content or nMHC+ fibres are expressed per fibre and data are presented as individual values and medians.
EpoR expression in isolated adult human primary cells
To test whether SCs express the EpoR receptor, cells were isolated from a human rectus abdominis muscle and FACS sorted. Contour plots from the FACS procedure are shown in Fig. 5 A–C. SCs were identified as CD56+Lin− (17.9% of parent population) (Fig. 5 C) and the subsequent gene expression analysis showed these cells to express high levels of Pax7 (Table 5), indicating that these were SCs. Mesenchymal progenitor cells identified as CD90+Lin− (4.7% of parent population) (Fig. 5 B) were also collected and shown to express high levels of CD90/Thy‐1 and COL1A1 (Table 5) in accordance with previous studies (Uezumi et al. 2014; Arrighi et al. 2015). Lin+ cells (CD45+CD31+, 13.3% of parent population) (Fig. 5 A) and the pre‐FACS isolation sample showed a generally low expression for all of the above transcripts (Table 5).
Figure 5. Epo‐R mRNA expression in adult human satellite cells .
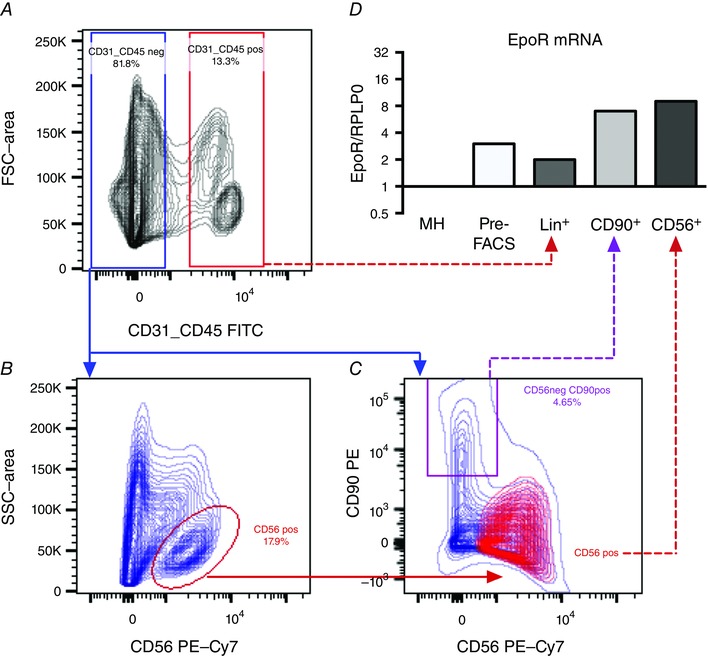
A–C, contour plots indicating the FACS strategy are shown. Three cell populations were identified; CD56+ cells (satellite cells) [CD56+/Lin (CD45/CD31)−], CD90+ cells (CD90+/Lin−) and Lin+ (CD45+/CD31+). As shown in (C), the CD56+ and CD90+ cell populations were largely negative for CD90 and CD56, respectively. In addition to FACS sorted cells, Epo‐R expression was determined in whole muscle homogenate (MH) and a pre‐FACS sample. mRNA expression levels of Epo‐R (D) were quantified and normalized to mRNA expression of ribosomal protein large P0 (RPLP0). Each target was expressed relative to whole muscle homogenate (MH). Data (n = 1) are shown on a log2 scale.
Table 5.
Quantitative RT‐PCR results from primary human FACS isolated cells obtained from skeletal muscle
| MH | Pre‐FACS | CD56+ | CD90+ | Lin+ | |
|---|---|---|---|---|---|
| Pax7 | 1 | <4.4 | 80 | <0.9 | <1.3 |
| Thy‐1/CD90 | 1 | <1.9 | 0.3 | 55 | <0.6 |
| COL1A1 | 1 | 11 | 2 | 53 | 0.2 |
Pax7, Thy‐1 (CD90) and COL1A1 mRNA expression in whole muscle homogenate (MH), pre‐FACS isolation sample (Pre‐FACS), isolated CD56+/CD90−/Lin‐ [CD45/CD31−] cells (CD56+), CD90+/CD56−/Lin− cells (CD90) and CD45+/CD31+/CD56−/CD90− cells (Lin+). Data are presented as cDNA molecules normalized to ribosomal protein large P0 expression and further normalized to expression level in MH (n = 1 included in analysis). When below detection level (one molecule) this was indicated by ‘<’, followed by the calculated value for one cDNA molecule.
Epo‐R transcript was detected in all the sorted cell populations (Fig. 5 D), although with the greatest expression in the SCs (9‐fold compared to whole muscle homogenate) and the mesenchymal progenitor cells (7‐fold compared to whole muscle homogenate).
Discussion
Skeletal muscle SCs have distinct and important roles during muscle regeneration and also influence the muscle microenvironment during remodelling and hypertrophy. In the present study, we investigated the potential separate and additive effect of ESA treatment and endurance training on SCs in humans. The main findings were: (1) endurance training increased the SC pool in type II fibres; (2) ESA treatment and endurance training both increased Pax7+/MyoD+ and ESA treatment increased the overall content of MyoD+ cells; and (3) the effect of ESA treatment could be mediated by Epo‐Epo‐R interaction since the Epo‐R is expressed in primary SCs isolated from adult human skeletal muscle.
The effects of ESA treatment
Ever since the Epo‐R was first identified in C2C12 myoblasts and primary muscle derived mononuclear cells, the effect of Epo and its possible role in muscle regeneration and remodelling has been investigated in several models. In murine cell cultures, myoblasts proliferate (and differentiation is delayed) in response to Epo treatment, possibly through increased and persistent MyoD expression (Ogilvie et al. 2000). In the present study, we found no effect of ESA treatment on the size of the SC pool in type I, type II or hybrid (MHC‐I+/MHC‐II+) fibres. This would suggest that ESA treatment has no influence on size of the SC pool in healthy young men following 10 weeks treatment. Conversely, we did observe an effect of ESA treatment on the overall MyoD+ cell content, as well as Pax7+/MyoD+ cell content, and also a tendency towards an increase in cells expressing Pax7−/MyoD+. These findings indicate that ESA treatment can induce MyoD expression in SCs and potentially increase the content of SC committed to the myogenic programme. Because we did not detect any changes in SC content, these findings suggest that Epo directs the SCs to myogenic commitment without self‐renewal, potentially decreasing the SCs pool over time. In rodents, induction of MyoD expression through the IL6‐STAT3 pathway has been shown to promote myogenic commitment while at the same time inhibiting self‐renewal, potentially exhausting the SC pool (Tierney et al. 2014). Most of the MyoD+ cells detected were also Pax7+, indicating that these cells were still in a proliferative stage of their cell cycle programme (Yin et al. 2013). Epo is a mitogenic factor that traditionally is known to activate mitogenic signalling pathways in erythroid progenitors to induce proliferation and, at later stages, differentiation into mature erythrocytes (Lombardero et al. 2011). Earlier studies have indicated that Epo may also have a role in cell cycle regulation in myoblasts because Epo‐R overexpression induced greater MyoD expression compared to control myoblasts (Jia et al. 2009). However, although Epo induces myoblast proliferation through MyoD expression, it may also prevent or delay differentiation by reducing myogenin expression (Ogilvie et al. 2000). This may explain why we were unable to detect a difference in central nuclei or myonuclei.
Our findings on Epo‐R mRNA expression in isolated adult human SCs suggest that the effect of Epo on MyoD expression could be mediated by direct interaction with the Epo‐R. Unfortunately, we were unable to determine the potential content of Epo‐R protein in our cells as a result of lack of valid antibodies for immunohisto‐ or cytochemistry. Interestingly, Lamon et al. (2014) did detect Epo‐R protein expression in human myoblasts by combining western blotting and antibody verification using liquid chromatography tandem mass spectrometry. However, in contrast to our in vivo results on MyoD+ SCs, in vitro Epo treatment of myoblasts failed to alter cell proliferation or protein synthesis (Lamon et al. 2014). These differences may relate to the to usage of human myoblasts compared to adult freshly isolated SCs or the inherent difference between in vivo and in vitro conditions. With regard to the latter, we did observe Epo‐R mRNA in CD90+ (Lin−) cells isolated from adult skeletal muscle, indicating that these cells may also be responsive to ESA treatment. The CD90+Lin− cells may represent a mesenchymal progenitor cell population, present in multiple tissues, including skeletal muscle, and this has recently been reported to have a significant role in regulating SCs (e.g. by induction of MyoD expression in SCs in co‐culture experiments) (Joe et al. 2010; Uezumi et al. 2010; Roberts et al. 2013; Uezumi et al. 2014; Farup et al. 2015). In addition to directly targeting the SCs, Epo may target the CD90+ cells and thereby impact the SCs by release of trophic factors such as follistatin (Roberts et al. 2013), which could provide one explanation for the differences between in vivo and in vitro results. Interestingly, this cell population may also reside in the bone marrow and stimulate erythropoiesis through secretion of paracrine factors such as KIT‐ligand (Roberts et al. 2013). Thus, it is possible that ESA treatment may stimulate production and secretion of paracrine factors from the CD90+ cells residing in both muscle and bone marrow to impact the function and activity of SCs, as well as erythroid progenitors in muscle bone marrow, respectively (Roberts et al. 2013); however, this speculation requires more detailed investigation.
In relation to the ESA treatment and the resulting increased haematocrit and erythrocyte count (Christensen et al. 2015 a), endogenous Epo production would expectedly be lowered. However, the half‐life of the ESA agent (darbepoetin‐α) is ∼70 h and thus the once‐per‐week injection in most subjects should result in an elevated Epo‐R signalling for a substantial part of the treatment period. We observed a 24% (non‐significant, P = 0.19) increase in combined darbepoetin‐α and endogenous Epo levels 7 days after the final injection (Christensen et al. 2015 b). One subject displayed a low post treatment value (0.03 ng ml−1) preventing the detection of a significant increase. However, these data suggests that, despite lowered endogenous Epo levels, the ESA treatment was able to counter this 7 days after injection.
Besides SC content and activity, we quantified myonuclei and central nuclei content, as well as the expression of embryonic or neonatal MHC. However, neither of these measures displayed any significant change in relation to ESA treatment. In relation to myonuclei and central nuclei, our data indicate that ESA treatment delay or prevent SC differentiation (Ogilvie et al. 2000) or, alternatively, that we were unable to detect minor changes in myonuclei content from relatively few SCs undergoing terminal differentiation. In agreement with the lack of change in myonuclei content, ESA treatment did not change myofibre size or myofibre pheonotype as described previously (Larsen et al. 2014).
The effect of endurance training
An increase in SCs associated with fibres expressing MHC‐II was observed in the present study, whereas no changes were observed in SCs associated with fibres expressing MHC‐I or hybrid fibres (MHC‐I+/MHC‐II+). The hybrid fibre analysis was performed based on recent results by Joanisse et al. (2013) who reported a specific upregulation of hybrid fibres, as well as SCs associated with hybrid fibres, following high intensity endurance training. The very few hybrid fibres observed after training in the present study may relate to the length of the training period, which, in the present study, was 10 weeks compared to 6 weeks in the study by Joanisse et al. (2013). The changes related to alterations in MHC isoform expression, myofibre remodelling and SC proliferation, as observed by Joanisse et al. (2013), could have been present earlier in the present study. In addition, the few hybrid fibres in the present study, as well as reported by Joanisse et al. (2013), limit the ability to reliably detect SCs in hybrid fibres because SC quantification is know to be influenced by the number of fibres in the sample (Mackey et al. 2009). In agreement with our findings, Verney et al. (2008) reported an increase in SCs specifically associated with type II fibres. Conversely, Fry et al. (2014 b) recently reported an increase in SCs associated with type I fibres following endurance training in previously sedentary adult subjects, in contrast to our results and those of Verney et al. (2008). This apparent discrepancy may relate to the type of endurance exercise performed during the training sessions. Although the subjects in the study by Fry et al. (2014 b) performed continuous endurance exercise at 70% of heart rate maximim, the exercise in the present study, as well as in the study by Verney et al. (2008), consisted of both moderate intensity (at 70% heart rate max), as well as high intensity intervals (at 85–95% heart rate maximum). Thus, whether the high intensity intervals are needed to sufficiently activate the larger motor units and thereby fibres expressing MHC‐II remains speculative. Results previously reported from the present study showed an increased content of type IIa fibres (by ATPase histochemistry) and a decrease in type IIx fibres following endurance training, indictaing that the type II fibres were indeed stimulated by the exercise (Larsen et al. 2014). Collectively, endurance training appears to induce changes in fibre type specific SC pool in an exercise intensity‐dependent manner (Verney et al. 2008; Joanisse et al. 2013; Fry et al. 2014 b). The effect of endurance exercise is supported by cross‐sectional data where endurance master athletes were able to maintain the SC content in type II compared to type I fibres, when normalized to fibre area (Mackey et al. 2014).
In addition to SC content, we found an increased number of Pax7+/MyoD+ cells and a trend towards an increased number of MyoD+ cells following endurance training, indicating that endurance training stimulates myogenic commitment of the SCs. Joanisse et al. (2013) compared the fibre type specific MyoD expression in SCs following 6 weeks of high intensity endurance training. They reported increased levels of Pax7−/MyoD+ cells and a trend towards increased content Pax7+/MyoD+ cells in hybrid fibres compared to type I and II fibres after training (Joanisse et al. 2013). This was interpreted as an increased content of differentiating SCs following training, in particular in the hybrid fibres (Joanisse et al. 2013). In the present study, we were technically unable to determine the fibre type specificity of the MyoD expressing cells; however, fibre type specific determination may also be hampered by the generally low content of MyoD+ cells in our study. In agreement with Joanisse et al. (2013), our results suggest that endurance training increases the content of proliferating and/or differentiating myogenic precursors from the SC pool (i.e. increase in MyoD+/Pax7+/− cells) (Yin et al. 2013). It should be noted that we observed no MyoD+ cells at pre‐training or in our sedentary control group, emphasizing that, in the non‐perturbed muscle, the SCs are generally quiescent (Yin et al. 2013). Taken together, endurance training increases the SC pool in a fibre type specific manner and enhances the activity of myogenic precursors from the SC pool.
The interaction between ESA treatment and endurance training
ESA treatment and endurance training constitute two different interventions, with some of the same documented effects, including improved aerobic capacity, skeletal muscle remodelling from fast to slow oxidative phenotypes and SC activation (Jones & Carter, 2000; Ogilvie et al. 2000; Thomsen et al. 2007; Cayla et al. 2008; Joanisse et al. 2013). Therefore, we hypothesized that the effects of ESA treatment and endurance training on SCs would be additive. In the present study we did not find any combined effect of ESA treatment and endurance training on SC quantity or myogenic cell activation, suggesting that the two interventions have no additive effects as hypothesized. Interestingly, the increase in type II fibre SC content with endurance training, both with and without ESA treatment, suggests that training could prevent a potential decrease in the SC pool from the Epo treatment. We should emphasize that our study design may have been underpowered to detect a potential additive effect and any conclusions solely based on our data will suffer from a risk of a statistical type II error.
In summary, in vivo ESA treatment of sedentary adult young men was associated with increased MyoD expression in SCs, although no effects on overall SC content were observed. We did detect Epo‐R mRNA in freshly isolated adult human SCs and the effect of Epo could therefore be mediated directly through Epo‐Epo‐R interaction on the SCs. By contrast to ESA treatment, endurance training increased the SC content in type II fibres and also increased SC expression of MyoD. However, we found no additive effect of ESA treatment and endurance training on SC quantity, activity or other measures of remodelling.
Additional information
Competing interest
The authors declare that they have no competing interests.
Author contributions
BC and JF conceived and designed the research. BC and BN conducted the clinical trial. AH and JF performed the experiments. AH, BC, URM, PS and JF analysed the data. AH, BC, URM, PS and JF interpreted the results of the experiments. AH and JF drafted the manuscript. AH, BC, BN, URM, MH, PS and JF edited and revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Funding is gratefully acknowledged from the Danish Council for Independent Research in Medical Sciences (grant numbers 271‐08‐0647 and 10‐09‐4021).
Acknowledgement
We thank Kirsten Nyborg Rasmussen, Lone Kvist, Susanne Sørensen, Lisa Buus, Elsebeth Hornemann, Eva Schriver, Hanne F. Petersen, Janni M. Jensen, Anja Jokipii and Gitte K. Hartvigsen for technical assistance. Frank de Paoli is thanked for providing clinical access to muscle tissue for cell isolation. The FACS core facility at Aarhus University is thanked for technical assistance for cell sorting. Pier Lorenzo Puri and his laboratory is thanked for assistance with the development of cell isolation protocol. The F1.652 and A4.591 monoclonal antibodies were developed by Helen M. Blau and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA, USA.
References
- Allon M, Kleinman K, Walczyk M, Kaupke C, Messer‐Mann L, Olson K, Heatherington AC & Maroni BJ (2002). Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clin Pharmacol Ther 72, 546–555. [DOI] [PubMed] [Google Scholar]
- Arrighi N, Moratal C, Clement N, Giorgetti‐Peraldi S, Peraldi P, Loubat A, Kurzenne JY, Dani C, Chopard A & Dechesne CA (2015). Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 6, e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA & Gundersen K (2010). Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci USA 107, 15111–15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M & Conboy I (2009). Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 1, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayla JL, Maire P, Duvallet A & Wahrmann JP (2008). Erythropoietin induces a shift of muscle phenotype from fast glycolytic to slow oxidative. Int J Sports Med 29, 460–465. [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L & Denis C (2003). Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28, 87–92. [DOI] [PubMed] [Google Scholar]
- Christensen B, Ludvigsen M, Nellemann B, Kopchick JJ, Honore B & Jorgensen JO (2015. a). Serum proteomic changes after randomized prolonged erythropoietin treatment and/or endurance training: detection of novel biomarkers. PLoS ONE 10, e0117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B, Nellemann B, Larsen MS, Thams L, Sieljacks P, Vestergaard PF, Bibby BM, Vissing K, Stodkilde‐Jorgensen H, Pedersen SB, Moller N, Nielsen S, Jessen N & Jorgensen JO (2013). Whole body metabolic effects of prolonged endurance training in combination with erythropoietin treatment in humans: a randomized placebo controlled trial. Am J Physiol Endocrinol Metab 305, E879–E889. [DOI] [PubMed] [Google Scholar]
- Christensen B, Nellemann B, Thorsen K, Nielsen MM, Pedersen SB, Ornstrup MJ, JO JO & Jessen N (2015. b). Prolonged erythropoietin treatment does not impact gene expression in human skeletal muscle. Muscle Nerve 51, 554–561. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA & Morgan JE (2005). Stem cell function, self‐renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL & Rando TA (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. [DOI] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, Kung S, Jiang KP & Conboy IM (2014). Oxytocin is an age‐specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun 5, 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farup J, Madaro L, Puri PL & Mikkelsen UR (2015). Interactions between muscle stem cells, mesenchymal‐derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis 6, e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW (2003). Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 228, 1–14. [DOI] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont‐Versteegden EE, McCarthy JJ & Peterson CA (2014. a). Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28, 1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont‐Versteegden EE, McCarthy JJ & Peterson CA (2015). Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA & Peterson CA (2014. b). Fibre type‐specific satellite cell response to aerobic training in sedentary adults. J Physiol 592, 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Warin R, Yu X, Epstein R & Noguchi CT (2009). Erythropoietin signaling promotes transplanted progenitor cell survival. FASEB J 23, 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse S, Gillen JB, Bellamy LM, McKay BR, Tarnopolsky MA, Gibala MJ & Parise G (2013). Evidence for the contribution of muscle stem cells to nonhypertrophic skeletal muscle remodeling in humans. FASEB J 27, 4596–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA & Rossi FM (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM & Carter H (2000). The effect of endurance training on parameters of aerobic fitness. Sports Med 29, 373–386. [DOI] [PubMed] [Google Scholar]
- Kandalla PK, Goldspink G, Butler‐Browne G & Mouly V (2011). Mechano Growth Factor E peptide (MGF‐E), derived from an isoform of IGF‐1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev 132, 154–162. [DOI] [PubMed] [Google Scholar]
- Kurosaka M, Naito H, Ogura Y, Kojima A, Goto K & Katamoto S (2009). Effects of voluntary wheel running on satellite cells in the rat plantaris muscle. J Sports Sci Med 8, 51–57. [PMC free article] [PubMed] [Google Scholar]
- Lamon S, Zacharewicz E, Stephens AN & Russell AP (2014). EPO‐receptor is present in mouse C2C12 and human primary skeletal muscle cells but EPO does not influence myogenesis. Physiol Rep 2, e00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen MS, Vissing K, Thams L, Sieljacks P, Dalgas U, Nellemann B & Christensen B (2014). Erythropoietin administration alone or in combination with endurance training affects neither skeletal muscle morphology nor angiogenesis in healthy young men. Exp Physiol 99, 1409–1420. [DOI] [PubMed] [Google Scholar]
- Launay T, Hagstrom L, Lottin‐Divoux S, Marchant D, Quidu P, Favret F, Duvallet A, Darribere T, Richalet JP & Beaudry M (2010). Blunting effect of hypoxia on the proliferation and differentiation of human primary and rat L6 myoblasts is not counteracted by Epo. Cell Prolif 43, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan CM (2011). An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardero M, Kovacs K & Scheithauer BW (2011). Erythropoietin: a hormone with multiple functions. Pathobiology 78, 41–53. [DOI] [PubMed] [Google Scholar]
- Lundby C, Hellsten Y, Jensen MB, Munch AS & Pilegaard H (2008). Erythropoietin receptor in human skeletal muscle and the effects of acute and long‐term injections with recombinant human erythropoietin on the skeletal muscle. J Appl Physiol (1985) 104, 1154–1160. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Karlsen A, Couppe C, Mikkelsen UR, Nielsen RH, Magnusson SP & Kjaer M (2014). Differential satellite cell density of type I and II fibres with lifelong endurance running in old men. Acta Physiol (Oxf) 210, 612–627. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen‐Moller J, Holm L & Kadi F (2009). Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40, 455–465. [DOI] [PubMed] [Google Scholar]
- Mauro A (1961). Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont‐Versteegden EE & Peterson CA (2011). Effective fiber hypertrophy in satellite cell‐depleted skeletal muscle. Development 138, 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA & Parise G (2008). Co‐expression of IGF‐1 family members with myogenic regulatory factors following acute damaging muscle‐lengthening contractions in humans. J Physiol 586, 5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA & Parise G (2012). Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J 26, 2509–2521. [DOI] [PubMed] [Google Scholar]
- Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, Sacco A & Puri PL (2013). Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med 5, 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie M, Yu X, Nicolas‐Metral V, Pulido SM, Liu C, Ruegg UT & Noguchi CT (2000). Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem 275, 39754–39761. [DOI] [PubMed] [Google Scholar]
- Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J & Rudnicki MA (2014). Inhibition of JAK‐STAT signaling stimulates adult satellite cell function. Nat Med 20, 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJ, Zhao Q, Caballero OL, Larder R, Coll AP, O'Rahilly S, Brindle KM, Teichmann SA, Tuveson DA & Fearon DT (2013). Depletion of stromal cells expressing fibroblast activation protein‐alpha from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 210, 1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter R, Menshykova M, Winkler T, Matziolis G, Stratos I, Schoen M, Bittorf T, Mittlmeier T & Vollmar B (2008). Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J Orthop Res 26, 1618–1626. [DOI] [PubMed] [Google Scholar]
- Rundqvist H, Rullman E, Sundberg CJ, Fischer H, Eisleitner K, Stahlberg M, Sundblad P, Jansson E & Gustafsson T (2009). Activation of the erythropoietin receptor in human skeletal muscle. Eur J Endocrinol 161, 427–434. [DOI] [PubMed] [Google Scholar]
- Shefer G, Rauner G, Yablonka‐Reuveni Z & Benayahu D (2010). Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS ONE 5, e13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov M, de Paoli FV, Lausten J, Nielsen OB & Pedersen TH (2015). Extracellular magnesium and calcium reduce myotonia in isolated ClC‐1 inhibited human muscle. Muscle Nerve 51, 65–71. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, Hansen D, Dendale P & van Loon LJ (2011). Continuous endurance‐type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve 43, 393–401. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE & Allen RE (2002). Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13, 2909–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen JJ, Rentsch RL, Robach P, Calbet JA, Boushel R, Rasmussen P, Juel C & Lundby C (2007). Prolonged administration of recombinant human erythropoietin increases submaximal performance more than maximal aerobic capacity. Eur J Appl Physiol 101, 481–486. [DOI] [PubMed] [Google Scholar]
- Tierney MT, Aydogdu T, Sala D, Malecova B, Gatto S, Puri PL, Latella L & Sacco A (2014). STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med 20, 1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbrook PA & Klinken SP (1999). The erythropoietin receptor. Int J Biochem Cell Biol 31, 1001–1005. [DOI] [PubMed] [Google Scholar]
- Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA & Parise G (2011). IL‐6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS ONE 6, e17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Ikemoto‐Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y & Tsuchida K (2014). Identification and characterization of PDGFRalpha+ mesenchymal progenitors in human skeletal muscle. Cell Death D 5, e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S & Tsuchida K (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA & Bonaldo P (2013). Collagen VI regulates satellite cell self‐renewal and muscle regeneration. Nat Commun 4, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl‐Aulin K & Denis C (2008). Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve 38, 1147–1154. [DOI] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH & Jessen N (2012). AMPK vs mTORC1 signaling: genuine exercise effects of differentiated exercise in humans. Response to letter to editor by Dr A. K. Yamada. Scand J Med Sci Sports 22, 580–581. [DOI] [PubMed] [Google Scholar]
- von Maltzahn J, Jones AE, Parks RJ & Rudnicki MA (2013). Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA 110, 16474–16479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Price F & Rudnicki MA (2013). Satellite cells and the muscle stem cell niche. Physiol Rev 93, 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


