Abstract
Key points
A post‐menopausal hot flush consists of profuse physiological elevations in cutaneous vasodilatation and sweating that are accompanied by reduced brain blood flow. These responses can be used to objectively quantify hot flush severity.
The impact of an exercise training intervention on the physiological responses occurring during a hot flush is currently unknown.
In a preference‐controlled trial involving 21 post‐menopausal women, 16 weeks of supervised moderate intensity exercise training was found to improve cardiorespiratory fitness and attenuate cutaneous vasodilatation, sweating and the reductions in cerebral blood flow during a hot flush.
It is concluded that the improvements in fitness that are mediated by 16 weeks of exercise training reduce the severity of physiological symptoms that occur during a post‐menopausal hot flush.
Abstract
A hot flush is characterised by feelings of intense heat, profuse elevations in cutaneous vasodilatation and sweating, and reduced brain blood flow. Exercise training reduces self‐reported hot flush severity, but underpinning physiological data are lacking. We hypothesised that exercise training attenuates the changes in cutaneous vasodilatation, sweat rate and cerebral blood flow during a hot flush. In a preference trial, 18 symptomatic post‐menopausal women underwent a passive heat stress to induce hot flushes at baseline and follow‐up. Fourteen participants opted for a 16 week moderate intensity supervised exercise intervention, while seven participants opted for control. Sweat rate, cutaneous vasodilatation, blood pressure, heart rate and middle cerebral artery velocity (MCAv) were measured during the hot flushes. Data were binned into eight equal segments, each representing 12.5% of hot flush duration. Weekly self‐reported frequency and severity of hot flushes were also recorded at baseline and follow‐up. Following training, mean hot flush sweat rate decreased by 0.04 mg cm2 min−1 at the chest (95% confidence interval 0.02–0.06, P = 0.01) and by 0.03 mg cm2 min−1 (0.02–0.05, P = 0.03) at the forearm, compared with negligible changes in control. Training also mediated reductions in cutaneous vasodilatation by 9% (6–12%) at the chest and by 7% (4–9%) at forearm (P ≤ 0.05). Training attenuated hot flush MCAv by 3.4 cm s−1 (0.7–5.1 cm s−1, P = 0.04) compared with negligible changes in control. Exercise training reduced the self‐reported severity of hot flushes by 109 arbitrary units (80–121, P < 0.001). These data indicate that exercise training leads to parallel reductions in hot flush severity and within‐flush changes in cutaneous vasodilatation, sweating and cerebral blood flow.
Key points
A post‐menopausal hot flush consists of profuse physiological elevations in cutaneous vasodilatation and sweating that are accompanied by reduced brain blood flow. These responses can be used to objectively quantify hot flush severity.
The impact of an exercise training intervention on the physiological responses occurring during a hot flush is currently unknown.
In a preference‐controlled trial involving 21 post‐menopausal women, 16 weeks of supervised moderate intensity exercise training was found to improve cardiorespiratory fitness and attenuate cutaneous vasodilatation, sweating and the reductions in cerebral blood flow during a hot flush.
It is concluded that the improvements in fitness that are mediated by 16 weeks of exercise training reduce the severity of physiological symptoms that occur during a post‐menopausal hot flush.
Abbreviations
- BP
blood pressure
- CBVC
cerebro‐vascular conductance
- CO
cardiac output
- CVC
cutaneous vascular conductance
- HR
heart rate
- HRR
heart rate reserve
- LSD
least significant difference
- MAP
mean arterial pressure
- MCAv
middle cerebral artery velocity
- SV
stroke volume
maximal oxygen consumption
Introduction
The primary symptom of the menopause is the experience of multiple hot flushes, which are associated with elevated lipids and, potentially, insulin resistance – all of which are risk factors for cardiovascular disease (Thurston et al. 2012 a,b). Self‐reported hot flush severity is linked to vascular inflammation and endothelial dysfunction in post‐menopausal women (Bechlioulis et al. 2010). A hot flush is described as a sudden and intense sensation of heat causing skin reddening and profuse sweating (Freedman, 2002). The observed elevations in cutaneous vasodilatation (∼80% increase from baseline) and sweating (5‐fold increases) during a hot flush that lasts 2–3 min are comparable to those mediated by 30 min of moderate intensity cycling or 60 min of passive heating, which tend to raise core body temperature by ∼0.4–0.6°C (or greater) (Wingo et al. 2010). Hot flushes can therefore be considered to be moderate‐to‐severe thermoregulatory events.
Hot flushes are physiologically defined as a transient and pronounced increase in sweat rate that is preceded by a rapid surge in cutaneous vasodilatation (Low et al. 2010). Nevertheless, the severity of a hot flush is typically only subjectively defined by the individual, using symptom‐based interpretations of the magnitude of sweating, cutaneous vasodilatation, dizziness and increases in heart rate (HR) (Sloan et al. 2001). The physical symptoms that describe hot flush severity using a self‐reported rating scale have recently been confirmed, with a series of studies describing physiological elevations in cutaneous vasodilatation, sweating and reductions in cerebral blood flow during a hot flush (Low et al. 2008, 2010; Lucas et al. 2013). Interventions aimed at relieving menopausal symptoms typically use self‐reported rating scales as outcome variables (Stearns et al. 2003; Daley et al. 2015). Nevertheless, no researcher to date has investigated if any of the physiological changes that occur during a hot flush, and define severity, are responsive to intervention.
Exercise training can improve the self‐reported frequency and severity of hot flushes (Karacan, 2010; Luoto et al. 2012; Moilanen et al. 2012). Our research group recently demonstrated that the reduction in weekly hot flush frequency with exercise training is associated with improvements in thermoregulatory control in symptomatic post‐menopausal women. These improvements were characterised by a lower resting core body temperature, improving the temperature threshold for the onset of cutaneous vasodilatation and sweating, alongside improved sweating sensitivity to passive heating (Bailey et al. 2015). Similarly, exercise training also improves cerebrovascular function in middle‐aged and elderly individuals (Ainslie et al. 2008; Bailey et al. 2013; Murrell et al. 2013). The mechanisms of hot flushes have often been attributed to a malfunctioning thermoregulatory control system (either centrally or peripherally) (Charkoudian & Stachenfeld, 2014; Freedman, 2014; Jayasena et al. 2015). If exercise training can improve thermoregulatory and cerebrovascular control in symptomatic post‐menopausal women, it is possible to suggest that this may be reflected in the physiological responses observed during a hot flush (e.g. attenuated cutaneous vasodilatation, sweating or cerebrovascular responses), which would positively impact upon hot flush severity.
Therefore, the aim of this study was to quantify the acute physiological thermoregulatory and (cerebro)vascular changes that occur during hot flushes prior to and following a 16 week exercise intervention in symptomatic post‐menopausal women. We hypothesised that exercise training attenuates the changes in cutaneous vasodilatation, sweat rate and cerebral blood flow during a hot flush.
Methods
Participants
As part of a pilot preference trial, initially powered for the primary outcome of reported hot flush severity, 21 healthy symptomatic post‐menopausal women were recruited from the gynaecology and reproductive medicine clinic at Liverpool Women's Hospital, local GP practices and via local advertisement. One to 4 years had elapsed since the participants experienced their last menstrual period, and they suffered more than four hot flushes across a 24 h period (self‐reported using a 7 day diary). All participants had no history of type II diabetes, or cardiovascular or respiratory disease, were non‐smokers, drank < 14 units of alcohol per week, and had no contraindications to exercise. Participants were excluded if they engaged in regular exercise (>2 h per week based on a self‐reported questionnaire), had used hormone replacement therapy within the last 6 months, or were currently prescribed metformin, vasoactive or blood pressure (BP)‐lowering medications. Participants were informed of the methods verbally and in writing before providing written informed consent. The study conformed to the Declaration of Helsinki and was approved by the National Research ethics committee.
Research design
Participants reported to the laboratory on two occasions before and after a 16 week exercise training or no‐exercise control intervention (four visits in total). For all visits, participants were asked to fast overnight, refrain from alcohol and exercise for 24 h, and caffeine for 12 h. Visit one consisted of a cardiorespiratory fitness assessment to determine maximal oxygen consumption () and a 7 day hot flush severity questionnaire. The second visit consisted of anthropometric measurement (height, weight and body mass index) and a physiological hot flush assessment. Thermoregulatory, haemodynamic and cerebrovascular responses were assessed during hot flushes at rest under normothermic conditions and during passive heating. All participants then undertook either 16 weeks of exercise or a no‐exercise control intervention – this choice being based on participant preference (Kowalski & Mrdjenovich, 2013). Visits 1 and 2 were repeated at follow‐up. Fourteen (n = 14, 52 ± 4 years) symptomatic women opted for the 16 week programme of supervised moderate intensity aerobic exercise training while seven (n = 7, 52 ± 6 years) symptomatic women opted for the no‐exercise control group. Participants in the no‐exercise control group were asked to continue with their normal daily routine in terms of physical activity, and received no contact with the research team throughout the 16 week intervention period.
Measurements
Cardiorespiratory assessment for peak oxygen consumption
A test of was completed on a treadmill using a modified Bruce protocol. Following 2 min of warm‐up at 2.2 km h−1 on a flat gradient, the initial workload was set at 2.7 km h−1 at a 5° gradient. Thereafter, stepwise increments in speed and gradient were performed each minute until volitional exhaustion. HR (12‐lead ECG) and rate of perceived exertion were monitored throughout (Borg, 1970). Peak oxygen uptake was calculated from expired gas fraction (Oxycon Pro, Jaegar, Hochberg, Germany) as the highest consecutive 15 s period of data in the final minute before volitional exhaustion.
Hot flush frequency and severity questionnaire
At baseline and follow‐up, participants completed a 7 day hot flush frequency and severity diary (Sloan et al. 2001). Participants recorded how many hot flushes they experienced on a daily basis as well as information regarding the severity of each hot flush on a scale of 1–4 (1 being mild, 2 moderate, 3 severe and 4 very severe). From this, a 7 day sum of hot flushes provided a weekly hot flush score. A daily severity score was calculated by the sum of hot flushes recorded in each severity rating: e.g. (3 × mild(1))+(4 × moderate(2))+(1 × severe(3))+(1 × very severe(4)) = daily severity score of 14. A hot flush severity index was then calculated by the total sum of daily severity scores over the 7 day period. The use of self‐report diaries is a valid approach to obtaining data on patient symptoms and perceptions of hot flushes (Sloan et al. 2001). The self‐reported hot flush and cardiorespiratory fitness data are included in the current manuscript for comparison purposes, and have also been reported by Bailey et al. (2015) who focused on the impact of improving thermoregulatory efficiency during heat stress on self‐reported hot flush frequency.
Physiological hot flush assessment
Participants were placed in a tube‐lined jacket and trousers (Med‐Eng, Ottawa, Canada), which covered the entire body except for the head, feet and the right forearm. Participants rested quietly in a semi‐recumbent position while water (34°C) was perfused through the suit for a baseline period of 30 min. Participants were then exposed to a mild heat stress by perfusing ∼46°C water through the suit to induce a hot flush (Kronenberg, 1990; Freedman, 2001; Lucas et al. 2013). HR was obtained from a three‐lead electrocardiogram (PowerLab, AD Instruments, Oxford, UK), alongside continuous beat‐by‐beat finger arterial BP (Finapress, Amsterdam, Netherlands). Stroke volume (SV) and cardiac output (CO) were calculated using the BP waveform using the Modelflow method, incorporating age, height, sex and weight (Beatscope 1.0 software, TNO, Biomedical Instruments, Amsterdam, the Netherlands). To verify continuous BP measured at the finger an automated BP (Dinamap, Germany) reading was collected at regular intervals. Core body temperature was measured from an ingestible pill telemetry system taken ∼5 h before data collection began (CoreTemp, HQInc., Palmetto, FL, US), with the ingestion time recorded and repeated for each participant's post‐intervention assessment.
Local sweat rate was recorded continuously from the dorsal forearm and the mid‐sternum (not covered by the water‐perfused suit) using capacitance hygrometry. Dry 100% nitrogen gas was supplied through acrylic capsules (surface area = 2.32 cm2) attached to the skin's surface at a flow rate of 150 ml min−1, with the humidity of the gas flowing out of the capsules measured by a capacitance hygrometer (Viasala HMP155, Helsinki, Finland). Local skin blood flow was also measured at the chest and the forearm, using laser Doppler flowmetry (Periflux System 5001, Perimed AB, Järfälla, Sweden). Laser Doppler flow probes were affixed with an adhesive heating ring in close proximity to the ventilated sweat rate capsule. Cutaneous vascular conductance (CVC) was calculated as the ratio of laser Doppler flux units to mean arterial pressure (MAP) and expressed as both CVC and a percentage of maximum CVC (%CVCmax).
Middle cerebral artery blood velocity (MCAv, 1 cm distal to the MCA–anterior cerebral artery bifurcation) was measured continuously through the temporal window using transcranial Doppler ultrasonography. A 2 MHz Doppler probe (Spencer Technologies, Seattle, WA, USA) was adjusted until an optimal signal was identified, as described in detail previously (Willie et al. 2011), and held in place using a headband strap to prevent subtle movement of the Doppler probe and maintain insonation angle accuracy. Once the optimal MCA signal was attained in the temporal window, the probe location and machine settings (depth, gain and power) were recorded to identify the same imaging site during post‐intervention assessments. An index of cerebrovascular conductance (CBVC) was calculated from the ratio of MCAv to MAP. All data were sampled at 50 Hz with a data acquisition system (PowerLab).
Following the passive heat stress, local skin heating was performed simultaneously at the chest and forearm laser Doppler flowmetry sites to assess maximal cutaneous blood flow. Temperature of the local Doppler flowmetry units on the chest and forearm was increased at a rate of 0.5°C every 5 s to a temperature of 42°C (Tew et al. 2011). This resulted in an increase in skin temperature to ∼42°C at the heating probe–skin surface interface. The protocol was complete once flux at both sites had reached a stable plateau (∼30 min).
Physiological hot flush analysis
A hot flush was objectively recorded in 18 participants (11 exercise and 7 control participants) in both normothermia (spontaneous) and during heating. Hot flushes recorded in participants both at baseline and following the exercise training (n = 9) and control (n = 7) interventions were used for analysis. The onset of a hot flush was objectively identified as a transient and pronounced increase in sternal sweat rate (>0.002 mg cm−2 min−1) as has been used previously (Freedman, 2001; Low et al. 2008, 2010). Participants also informed the research team of a self‐reported feeling of a hot flush and once the feeling had dissipated. The end of each hot flush was objectively recorded as the return of sweat rate to pre‐hot flush baseline values. Because of the variance in the length of hot flushes, each hot flush episode was divided into eight equal segments, with each segment representing 12.5% of hot flush duration (Low et al. 2010; Lucas et al. 2013). Five second periods of data at the end of each segment, and over a period of 2 min before, i.e. baseline, and 2 min following the hot flush, i.e. post‐hot flush recovery, were used for data analysis.
Supervised exercise training intervention
Before commencing the exercise intervention, all the participants in this study arm attended a thorough familiarisation session. Participants were required to attend the University gym on a weekly basis during which time they wore an HR monitor (Polar Fitness, Polar Electro Oy, Kempele, Finland) and were provided with full exercise supervision and guidance from a trained exercise physiologist. During these sessions, participants were issued with a weekly progressive exercise programme that was specific to their own rate of progression (Pugh et al. 2013; Sprung et al. 2013). On the basis of individual fitness levels, participants underwent 30 min of moderate intensity aerobic exercise three times per week (30% heart rate reserve (HRR)), which progressed weekly based on HR responses and included treadmill walking/running, cycling, cross‐training and rowing. At week 12, participants were exercising 4–5 times per week for 45 min at 60% HRR. To facilitate compliance throughout the 16 week intervention, participants were monitored via the Wellness Key system, a software program that enables remote and accurate tracking of exercise activity. This type of moderate intensity programme was informed by NHS guidelines and our previous studies in which improvements in cardiorespiratory fitness were documented (NHS, 2011; Pugh et al. 2013; Sprung et al. 2013).
Control participants
After consent and physiological hot flush assessment, women who preferred to participate in the control group had no contact with the research team throughout the 16 weeks. This type of control reflects the current absence of non‐pharmacological treatment for hot flushes in the UK. As such, the research team did not influence any lifestyle factors during the 16 week period.
Statistical analysis
For self‐reported hot flush frequency, severity index, duration and cardiorespiratory fitness (the variables measured once at baseline and once at follow‐up), the raw changes from baseline were calculated and intervention versus control differences were quantified with general linear models, with baseline data entered as a covariate. This approach adjusts properly for any study arm imbalance at the pre‐intervention point (Vickers & Altman, 2001) and is superior to quantifying intervention effects using percentage changes (Vickers, 2001).
For physiological measures during the hot flushes, data were acquired continuously at 50 Hz throughout baseline and passive heating protocol (PowerLab) and hot flushes were analysed in a blinded fashion by the same observer. A three‐factor (intervention, time and segment) linear mixed model was employed for the analysis of hot flush outcomes (BP, HR, sweating skin and cerebral blood flow) during each 12.5% segment, following exercise training or control. Statistically significant interaction terms were followed up with a simple main effects analysis and the least significant difference (LSD) approach to multiple comparisons (Perneger, 1998). Point estimates of each outcome at each time point during the protocol are reported using the mean and standard deviation. Intervention effects are quantified by reporting the difference between exercise and control with associated 95% confidence limits in parentheses, i.e. (lower limit – upper limit) (Gardner & Altman, 1986).
Results
Compliance to the exercise sessions was 93% over the 16 week period. Following adjustment for baseline values, the body mass‐normalised change in was 4.5 (1.9–8.2) ml kg−1 min−1 greater in the exercise group versus control group (P = 0.04). The absolute change was 21.0 (0.4–41.5) ml min−1 greater in the exercise group versus control group (P = 0.05). Exercise training reduced self‐reported hot flush frequency by 48 (39–56) flushes per week (P < 0.001), and hot flush severity index by 109 (80–121) AU (P < 0.001) versus the changes in the control group.
Physiological hot flush assessment
Hot flush duration
Exercise training reduced hot flush duration compared to control [61 s (−9, 112)], but this did not reach statistical significance (P = 0.34).
Haemodynamics
HR significantly increased by 8 beats min−1 (6, 11; P < 0.001) during hot flushes. There was no main effect of intervention, time or intervention × time interaction in HR during hot flushes (P > 0.05). MAP decreased significantly by 5 mmHg (1, 10; P < 0.001) during the hot flushes with no main effect of intervention, time or intervention × time interaction (P > 0.05). The changes in CO and SV were negligible and not statistically significant during the hot flushes or between study groups (P > 0.05, Table 1).
Table 1.
Haemodynamic responses during hot flushes before (pre) and after (post) exercise and control intervention
| Pre hot flush (%) | Post hot flush (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time × | |||||||||||
| Baseline | Post | Baseline | Post | segment | |||||||
| Variable | (2 min) | 0 | 50 | 100 | (+2 min) | (2 min) | 0 | 50 | 100 | (+2 min) | interaction |
| Exercise | |||||||||||
| HR (beatsmin−1)* | 68 | 76 | 74 | 72 | 69 | 66 | 74 | 73 | 71 | 67 | P > 0.05 |
| (9) | (10) | (9) | (8) | (10) | (7) | (8) | (9) | (12) | (7) | ||
| MAP (mmHg)* | 75 | 70 | 71 | 73 | 73 | 74 | 71 | 70 | 72 | 73 | P > 0.05 |
| (7) | (9) | (10) | (8) | (7) | (7) | (8) | (9) | (8) | (7) | ||
| CO (l min–1) | 7.4 | 7.9 | 7.6 | 7.2 | 7.5 | 7.8 | 8 | 7.8 | 7.6 | 7.3 | P > 0.05 |
| (2.8) | (2.6) | (2.6) | (2.4) | (2.2) | (2.9) | (2.3) | (2.2) | (2.5) | (2.2) | ||
| SV (ml) | 109 | 108 | 106 | 110 | 107 | 112 | 110 | 107 | 109 | 108 | P > 0.05 |
| (12) | (14) | (15) | (14) | (14) | (12) | (12) | (12) | (17) | (18) | ||
| CBVC (cm s−1mmHg–1)*, † | 0.66 | 0.62 | 0.59 | 0.6 | 0.63 | 0.7 | 0.69 | 0.67 | 0.71 | 0.72 | P < 0.05 |
| (0.08) | (0.1) | (0.09) | (0.13) | (0.09) | (0.06) | (0.06) | (0.12) | (0.08) | (0.1) | ||
| Control | |||||||||||
| HR (beats min−1)* | 69 | 77 | 76 | 72 | 70 | 68 | 77 | 74 | 72 | 70 | P > 0.05 |
| (10) | (8) | (12) | (11) | (13) | (7) | (11) | (13) | (12) | (14) | ||
| MAP (mmHg)* | 75 | 70 | 71 | 72 | 73 | 75 | 69 | 71 | 72 | 74 | P > 0.05 |
| (5) | (4) | (7) | (7) | (5) | (6) | (5) | (5) | (7) | (4) | ||
| CO (l min–1) | 7.2 | 7.3 | 7.4 | 7.2 | 7.3 | 7.4 | 7.6 | 7.4 | 7.2 | 7.2 | P > 0.05 |
| (2.4) | (2.4) | (2.5) | (2.2) | (2.3) | (2.4) | (2.8) | (2.6) | (2.3) | (2.6) | ||
| SV (ml) | 103 | 107 | 104 | 106 | 105 | 106 | 110 | 108 | 109 | 108 | P > 0.05 |
| (11) | (20) | (13) | (11) | (17) | (18) | (12) | (12) | (14) | (16) | ||
| CBVC (cm s−1 mmHg–1)*, † | 0.67 | 0.65 | 0.62 | 0.68 | 0.66 | 0.68 | 0.65 | 0.63 | 0.67 | 0.65 | P < 0.05 |
| (0.06) | (0.05) | (0.07) | (0.09) | (0.05) | (0.06) | (0.07) | (0.11) | (0.1) | (0.05) | ||
Data are presented as mean (SD).
*Significant main effect of time.
†Significant intervention × time interaction (P < 0.05).
NB. Hot flushes were statistically analysed over eight segments, but are represented over three time segments (0, 50, 100%) above.
Core temperature
Core body temperature tended to decrease following hot flushes [by 0.06°C (0.02, 0.11; P = 0.15)]. There was no intervention × time interaction for core body temperature (Table 2).
Table 2.
Thermoregulatory responses during hot flushes before (pre) and after (post) the exercise and control intervention
| Pre hot flush (%) | Post hot Flush (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time × | |||||||||||
| segment | |||||||||||
| Variable | Baseline | 0 | 50 | 100 | Post | Baseline | 0 | 50 | 100 | Post | interaction |
| Exercise | |||||||||||
| Core temperature (°C) | 37.1 | 37.1 | 37.1 | 37 | 37 | 37 | 37 | 37 | 36.9 | 36.9 | P > 0.05 |
| (0.4) | (0.3) | (0.4) | (0.4) | (0.3) | (0.3) | (0.3) | (0.3) | (0.4) | (0.3) | ||
| CVCchest (AU mmHg–1)*, † | 1.3 | 2.5 | 1.8 | 1.7 | 1.4 | 1.1 | 1.9 | 1.4 | 1.3 | 1.2 | P < 0.05 |
| (0.7) | (0.9) | (1.1) | (0.9) | (0.8) | (0.8) | (0.9) | (0.9) | (0.9) | (0.8) | ||
| CVCarm (AU mmHg–1)* | 0.5 | 1.4 | 0.9 | 0.8 | 0.8 | 0.4 | 1.3 | 0.7 | 0.6 | 0.6 | P > 0.05 |
| (0.4) | (0.5) | (0.7) | (0.6) | (0.6) | (0.3) | (0.5) | (0.6) | (0.5) | (0.4) | ||
| Control | |||||||||||
| Core temperature (°C) | 37 | 37 | 37 | 36.9 | 36.9 | 37 | 37 | 37 | 36.9 | 36.9 | P > 0.05 |
| (0.3) | (0.3) | (0.2) | (0.2) | (0.3) | (0.2) | (0.2) | (0.3) | (0.3) | (0.3) | ||
| CVCchest (AU mmHg–1)* | 1.6 | 2.7 | 1.9 | 1.8 | 1.3 | 1.3 | 2.6 | 1.7 | 1.6 | 1.3 | P > 0.05 |
| (1.0) | (1.2) | (1.2) | (1.1) | (0.4) | (0.8) | (0.7) | (0.9) | (1.0) | (0.9) | ||
| CVCarm (AU mmHg–1)* | 0.7 | 1.4 | 0.9 | 0.8 | 0.9 | 0.8 | 1.5 | 0.8 | 0.7 | 0.7 | P > 0.05 |
| (0.4) | (0.3) | (0.5) | (0.4) | (0.5) | (0.2) | (0.4) | (0.3) | (0.9) | (0.3) | ||
Data are presented as mean (SD).
*Significant main effect of time (P < 0.05).
†Significant interaction between time and hot flush segment (P < 0.05).
NB. Hot flushes were statistically analysed over eight segments, but are represented over three time segments (0, 50, 100%) above.
Skin blood flow
Skin blood flow increased during hot flushes (P < 0.001; Fig. 1). At the chest, there was an intervention × time interaction with a reduction of 9% CVCmax (6, 11; P = 0.01) during hot flushes following exercise training compared to 4% CVCmax (−6, 8; P = 0.34) in control. Similarly, at the forearm there was an intervention × time interaction with a reduction of 7% CVCmax (4, 9; P = 0.05) during hot flushes following exercise training compared to a 3% CVCmax (−3, 6; P = 0.44) change following control.
Figure 1. Changes in chest and forearm skin blood flow during hot flushes before and after exercise training and control .
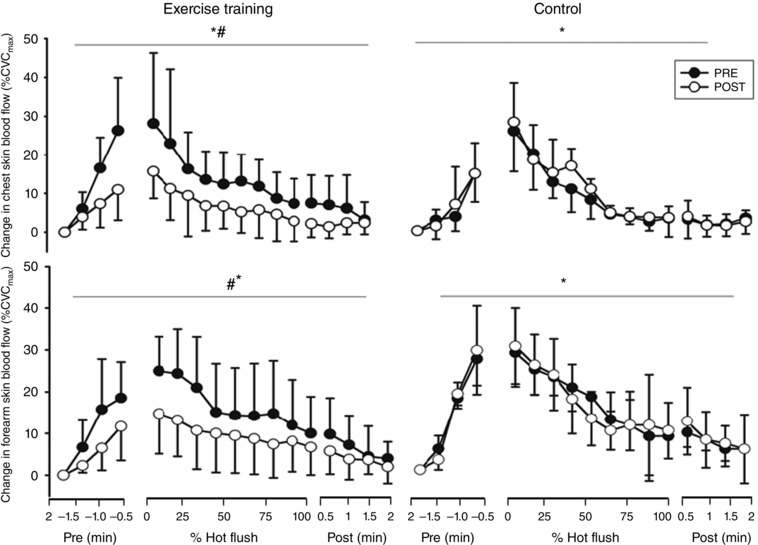
Error bars are SD. *Significant change in skin blood flow during a hot flush; #significant interaction between intervention and time (P < 0.05).
Sweating
Sweat rate increased at the chest and forearm during hot flushes (P < 0.001; Fig. 2). There was an intervention × time interaction with a reduction of 0.04 mg cm2 min−1 (0.02, 0.06; P = 0.01) in chest sweat rate following exercise training compared to 0.01 mg cm2 min−1 (−0.02, 0.03; P = 0.19) change in control. There was an intervention × time interaction with a reduction of 0.03 mg cm2 min−1 (0.02, 0.05; P = 0.01) in forearm sweat rate following exercise training compared a 0.01 mg cm2 min−1 (−0.01, 0.02; P = 0.78) change in control.
Figure 2. Changes in sweat rate during hot flushes before and after exercise training and control .
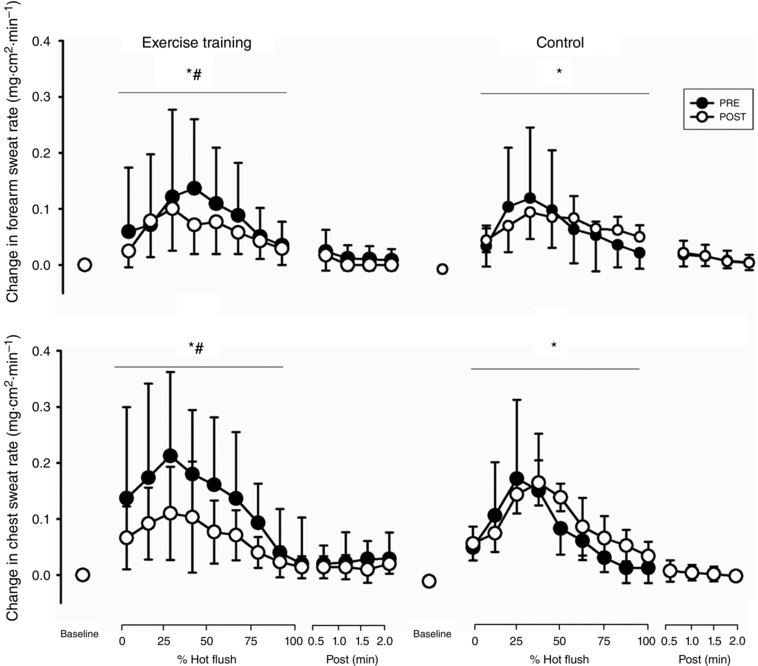
Error bars are SD. *Significant change in sweat rate during a hot flush; #significant interaction between intervention and time (P < 0.05).
Cerebral blood flow
MCAv decreased significantly during hot flushes (P < 0.001; Fig. 3). There was an intervention × time interaction in MCAv, with the size of the decrease reduced by 3.4 cm s−1 (0.7, 5.1; P < 0.001) following exercise training compared to 0.6 cm s−1 (−0.7, 1.8; P = 0.41) in control. When expressed as CBVC, similar findings were evident (P = 0.04; Table 2).
Figure 3. Changes in cerebral blood flow during hot flushes before and after exercise training and control .
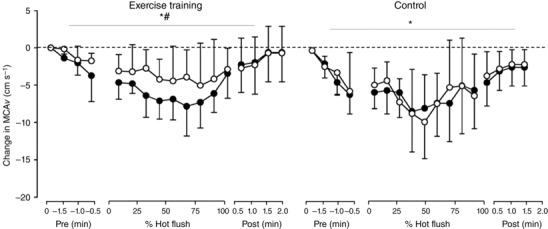
Error bars are SD. MCAv, middle cerebral artery velocity. *Significant change in MCAv during hot flush; #significant interaction between intervention and time (P < 0.05).
Discussion
This is the first study to quantify the effects of an exercise training intervention on the physiological responses observed during hot flushes in symptomatic post‐menopausal women. The novel findings of this preference‐controlled trial were that a 16‐week exercise training intervention reduced the sweating and cutaneous vasodilatation response typically observed during a hot flush episode. In addition, the acute reduction in cerebral blood flow observed during a hot flush was attenuated with exercise training. Taken together, these objective physiological responses which define self‐reported hot flush severity in post‐menopausal women provide direct evidence that exercise training reduces the physiological hot flush severity by improving thermoregulatory and cerebrovascular responses during a hot flush.
Previous exercise training studies investigating changes in hot flush severity involve self‐report questionnaires that rely on self‐reported selection of descriptions based on physical symptoms (e.g. Sloan et al. 2001). The impact of exercise training on hot flush severity has been inconsistent, with some studies reporting reductions in self‐reported severity (Lindh‐Astrand et al. 2004; Karacan, 2010; Luoto et al. 2012; Moilanen et al. 2012; Reed et al. 2014) and others either reporting no change or have relied solely on hot flush frequency scores (Sternfeld et al. 2014; Daley et al. 2015). Nevertheless, the physical symptoms on which the self‐reported hot flush severity scale is rated are defined by the magnitude of sweating, cutaneous vasodilatation, dizziness and HR changes during a hot flush (Sloan et al. 2001). For the first time, our data provide direct evidence that exercise training reduces the objective physiological severity of menopausal hot flushes. The results of the current study suggest exercise training mediates acute amelioration of physiological perturbations (sweating, cutaneous vasodilatation and changes in cerebral blood flow) observed during hot flushes, which potentially explains the improvements in self‐reported hot flush severity that corresponds with reduced skin reddening, sweating and dizziness as described on the self‐reported severity scale following exercise training in this study, and others.
A previous study by our research group recently examined the potential physiological mechanisms for exercise training‐mediated improvements in the self‐reported frequency and severity of menopausal hot flushes by assessing the thermoregulatory and systemic vascular responses to passive heating (i.e. a protocol to examine thermoregulatory control rather than examine responses during hot flush) before and after an exercise training intervention (Bailey et al. 2015). We found that exercise training improves thermoregulatory control and enhances the function of the cerebral and cutaneous circulations to passive heating alongside improvements in the frequency of menopausal hot flushes (Bailey et al. 2015). More specifically, exercise training mediated a reduction in resting core temperature that enabled an earlier onset of sweating and cutaneous vasodilatation, improved sensitivity of sweating and attenuated reductions in cerebral blood flow during passive heating. Given that the mechanisms causing hot flushes have often been attributed to a dysfunctional thermoregulatory control system (Freedman, 2014) and/or vascular dysfunction (Bechlioulis et al. 2010, 2012; Sassarini et al. 2012, 2014), it is possible that improvements in thermoregulatory control and cerebrovascular function also explain the attenuations in sweating, cutaneous vasodilatation and alterations in cerebral blood flow that occur during a hot flush per se in the current study. Whilst an increase in core body temperature has previously been proposed as the trigger for hot flushes, the reported elevations are minor (∼0.04°C) (Freedman & Wooodward, 1996; Freedman, 2014) and in the current study we did not observe a core body temperature increase during (or immediately prior to) a hot flush. Therefore, the trigger for a hot flush and importantly the exercise training‐mediated reductions in thermoregulatory intensity during a hot flush are unlikely to be mediated by alterations in core temperature, unlike the typical cutaneous and sudomotor responses that occur during passive heating.
An alternative mechanistic explanation that must be considered is an exercise‐mediated reduction in sympathetic nerve activity. Although we did not measure sympathetic nerve activity in the current study, there is some evidence to suggest that (muscle) sympathetic nerve activity is lower following exercise training in post‐menopausal women (Oneda et al. 2014). Cutaneous vasodilatation during a hot flush is neurally mediated by a large transient increase in skin sympathetic nerve activity (Low et al. 2010), and it is therefore probable that the reduced cutaneous vasodilatation (and potentially sweating) may be a consequence of a smaller engagement of the sympathetic cholinergic system, potentially reducing the direct or more likely the acetylcholine‐induced release of nitric oxide (NO) from the endothelium during hot flushes (Hubing et al. 2010; Sassarini et al. 2012). Exercise training has a well‐established impact on NO‐dependent vasodilatation in both large and small vessels, with repeated episodic increases in shear stress during acute exercise causing enhanced NO‐mediated endothelial function (Green et al. 2004). It is important to highlight that enhanced NO‐mediated endothelial function in response to shear stress would cause an increase in cutaneous vasodilatation to the same stimulus, whereas cutaneous vasodilatation was attenuated during a hot flush in the present study, suggesting that the cutaneous NO response is mediated directly by the sympathetic cholinergic system, and not shear stress.
The attenuation of a reduction in cerebral blood flow during a hot flush was not explained by alterations in BP (e.g. a smaller reduction in BP during a hot flush was not evident following training), which is in agreement with the observations of Lucas et al. (2013). Other modulators of cerebral control are CO, arterial CO2 content and sympathetic nerve activity (Ainslie & Duffin, 2009). CO (along with SV) remained unchanged during hot flushes following exercise training in the current study. Whilst a hyperventilatory‐induced reduction in arterial CO2 during hot flushes is possible, this was not measured during hot flushes in the current or previous studies (Lucas et al. 2013) and warrants further investigation. Nevertheless, a reduction in basal or hot flush‐induced sympathetic nerve activity (as discussed above) may also reduce cerebral vasoconstriction and potentially self‐reported feelings of faintness.
The current study employs objective physiological measurements of hot flush severity that cannot be influenced by the participant and were analysed in a blinded fashion. Nonetheless, one limitation was that participants only rated weekly self‐reported severity and did not also rate severity during the acute hot flushes assessed in the laboratory. Exercise training did reduce weekly severity, which supports the physiological acute hot flush data obtained in the laboratory and supports the notion that exercise training reduces the severity of post‐menopausal hot flushes.
In summary, we have shown that exercise training improves objective physiological markers (sweating and cutaneous vasodilatation) of hot flush severity in symptomatic post‐menopausal women. These findings confirm that exercise training has a direct influence on thermoregulatory and cerebrovascular responses during hot flushes per se, which may explain reductions in self‐reported hot flush severity symptoms following exercise training.
Additional information
Competing interests
The authors have no conflicts of interest.
Author contributions
T.B.: conception and design; provision of study materials or patients; collection and assembly of data; data analysis and interpretation; manuscript writing. N.T.C.: conception and design; data analysis and interpretation; manuscript writing. N.A.: conception and design; provision of study materials or patients. G.A.: conception and design; data analysis and interpretation; manuscript writing. D.C., H.J.: manuscript writing. D.L.: conception and design; collection and assembly of data; data analysis and interpretation; manuscript writing. H.J.: conception and design; provision of study materials or patients; collection and assembly of data; data analysis and interpretation. All authors approved the final version of the manuscript.
Funding
This work was funded by Liverpool Primary Care Trust and NHS Liverpool Clinical Commissioning Group.
References
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ & Atkinson G (2008). Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586, 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN & Duffin J (2009). Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296, R1473–1495. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S & Ainslie PN (2013). Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44, 3235–3238. [DOI] [PubMed] [Google Scholar]
- Bailey TG, Cable NT, Aziz N, Atkinson G, Low D & Jones H (2015). “Exercise training reduces the frequency of menopausal hot flushes by improving themroregulatory control”: A‐38 Free Communication/Poster ‐ Clinical Populations I. Med Sci Sports Exerce 47, 54–57. [Google Scholar]
- Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, Chrousos GP & Michalis LK (2010). Endothelial function, but not carotid intima‐media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab 95, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Bechlioulis A, Naka KK, Kalantaridou SN, Kaponis A, Papanikolaou O, Vezyraki P, Kolettis TM, Vlahos AP, Gartzonika K, Mavridis A & Michalis LK (2012). Increased vascular inflammation in early menopausal women is associated with hot flush severity. J Clin Endocrinol Metab 97, E760–764. [DOI] [PubMed] [Google Scholar]
- Borg G (1970). [Physical training. 3. Perceived exertion in physical work]. Lakartidningen 67, 4548–4557. [PubMed] [Google Scholar]
- Charkoudian N & Stachenfeld NS (2014). Reproductive hormone influences on thermoregulation in women. Comp Physiol 4, 793–804. [DOI] [PubMed] [Google Scholar]
- Daley AJ, Thomas A, Roalfe AK, Stokes‐Lampard H, Coleman S, Rees M, Hunter MS & MacArthur C (2015). The effectiveness of exercise as treatment for vasomotor menopausal symptoms: randomised controlled trial. BJOG 122, 565–575. [DOI] [PubMed] [Google Scholar]
- Freedman RR (2001). Physiology of hot flashes. Am J Hum Biol 13, 453–464. [DOI] [PubMed] [Google Scholar]
- Freedman RR (2002). Core body temperature variation in symptomatic and asymptomatic postmenopausal women: brief report. Menopause 9, 399–401. [DOI] [PubMed] [Google Scholar]
- Freedman RR (2014). Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol 142c, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR & Wooodward S (1996). Core body temperature during menopausal hot flushes. Fertil Steril 65, 1141–1144. [PubMed] [Google Scholar]
- Gardner MJ & Altman DG (1986). Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J (Clin Res Ed) 292, 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O'Driscoll G & Taylor R (2004). Effect of exercise training on endothelium‐derived nitric oxide function in humans. J Physiol 561, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubing KA, Wingo JE, Brothers RM, Del Coso J, Low DA & Crandall CG (2010). Nitric oxide synthase inhibition attenuates cutaneous vasodilation during postmenopausal hot flash episodes. Menopause 17, 978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi‐Engbeaya C, Abbara A, Ratnasabapathy R, Mogford J, Ng N, Sarang Z, Ghatei MA, Bloom SR, Hunter MS & Dhillo WS (2015). Neurokinin B administration induces hot flushes in women. Sci Rep 5, 8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karacan S (2010). Effects of long‐term aerobic exercise on physical fitness and postmenopausal symptoms with menopausal rating scale. Sci Sports 25, 39–46. [Google Scholar]
- Kowalski CJ & Mrdjenovich AJ (2013). Patient preference clinical trials: why and when they will sometimes be preferred. Perspect Biol Med 56, 18–35. [DOI] [PubMed] [Google Scholar]
- Kronenberg F (1990). Hot flashes: epidemiology and physiology. Ann N Y Acad Sci 592, 52–86. [DOI] [PubMed] [Google Scholar]
- Lindh‐Astrand L, Nedstrand E, Wyon Y & Hammar M (2004). Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas 48, 97–105. [DOI] [PubMed] [Google Scholar]
- Low D, Hubing K, Del Coso J & Crandall C (2010). Mechanisms of cutaneous vasodilation during the menopausal hot flash. Menopause 18, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low DA, Davis SL, Keller DM, Shibasaki M & Crandall CG (2008). Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause 15, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RA, Ganio MS, Pearson J & Crandall CG (2013). Brain blood flow and cardiovascular responses to hot flashes in postmenopausal women. Menopause 20, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto R, Moilanen J, Heinonen R, Mikkola T, Raitanen J, Tomas E, Ojala K, Mansikkamaki K & Nygard CH (2012). Effect of aerobic training on hot flushes and quality of life–a randomized controlled trial. Ann Med 44, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen JM, Mikkola TS, Raitanen JA, Heinonen RH, Tomas EI, Nygard CH & Luoto RM (2012). Effect of aerobic training on menopausal symptoms–a randomized controlled trial. Menopause 19, 691–696. [DOI] [PubMed] [Google Scholar]
- Murrell C, Cotter J, Thomas K, Lucas SE, Williams MA & Ainslie P (2013). Cerebral blood flow and cerebrovascular reactivity at rest and during sub‐maximal exercise: effect of age and 12‐week exercise training. AGE 35, 905–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS (2011). Physical Activity Guidelines for Adults (19–64 years) Department of Health, London. [Google Scholar]
- Oneda B, Cardoso CG, Jr , Forjaz CL, Araujo TG, Bernardo FR, de Gusmao JL, Pinto LG, Labes E, Abrahao SB, Mion D, Jr , Fonseca AM & Tinucci T (2014). Effects of estrogen therapy and aerobic training on sympathetic activity and hemodynamics in healthy postmenopausal women: a double‐blind randomized trial. Menopause 21, 369–375. [DOI] [PubMed] [Google Scholar]
- Perneger TV (1998). What's wrong with Bonferroni adjustments. BMJ 316, 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CJ, Cuthbertson DJ, Sprung VS, Kemp GJ, Richardson P, Umpleby AM, Green DJ, Cable NT & Jones H (2013). Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 305, E50–58. [DOI] [PubMed] [Google Scholar]
- Reed SD, Guthrie KA, Newton KM, Anderson GL, Booth‐LaForce C, Caan B, Carpenter JS, Cohen LS, Dunn AL, Ensrud KE, Freeman EW, Hunt JR, Joffe H, Larson JC, Learman LA, Rothenberg R, Seguin RA, Sherman KJ, Sternfeld BS & LaCroix AZ (2014). Menopausal quality of life: RCT of yoga, exercise, and omega‐3 supplements. Am J Obstet Gynecol 210, 244.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassarini J, Fox H, Ferrell W, Sattar N & Lumsden MA (2012). Hot flushes, vascular reactivity and the role of the α‐adrenergic system. Climacteric 15, 332–338. [DOI] [PubMed] [Google Scholar]
- Sassarini J, Krishnadas R, Cavanagh J, Nicol A, Pimlott SL, Ferrell W & Lumsden MA (2014). Venlafaxine alters microvascular perfusion, [123I]‐β ‐CIT binding and BDI scores in flushing postmenopausal women. Maturitas 77, 267–273. [DOI] [PubMed] [Google Scholar]
- Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI & Windschitl H (2001). Methodologic lessons learned from hot flash studies. J Clin Oncol 19, 4280–4290. [DOI] [PubMed] [Google Scholar]
- Sprung VS, Cuthbertson DJ, Pugh CJ, Daousi C, Atkinson G, Aziz NF, Kemp GJ, Green DJ, Cable NT & Jones H (2013). Nitric oxide‐mediated cutaneous microvascular function is impaired in polycystic ovary sydrome but can be improved by exercise training. J Physiol 591, 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns V, Beebe KL, Iyengar M & Dube E (2003). Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 289, 2827–2834. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Guthrie KA, Ensrud KE, LaCroix AZ, Larson JC, Dunn AL, Anderson GL, Seguin RA, Carpenter JS, Newton KM, Reed SD, Freeman EW, Cohen LS, Joffe H, Roberts M & Caan BJ (2014). Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 21, 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew GA, Saxton JM, Klonizakis M, Moss J, Ruddock AD & Hodges GJ (2011). Aging and aerobic fitness affect the contribution of noradrenergic sympathetic nerves to the rapid cutaneous vasodilator response to local heating. J Appl Physiol 110, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Thurston RC, El Khoudary SR, Sutton‐Tyrrell K, Crandall CJ, Gold EB, Sternfeld B, Joffe H, Selzer F & Matthews KA (2012. a). Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol 119, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston RC, El Khoudary SR, Sutton‐Tyrrell K, Crandall CJ, Sternfeld B, Joffe H, Gold EB, Selzer F & Matthews KA (2012. b). Vasomotor symptoms and insulin resistance in the study of women's health across the nation. J Clin Endocrinol Metab 97, 3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AJ (2001). The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AJ & Altman DG (2001). Statistics notes: analysing controlled trials with baseline and follow up measurements. BMJ 323, 1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK & Ainslie PN (2011). Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196, 221–237. [DOI] [PubMed] [Google Scholar]
- Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M & Crandall CG (2010). Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol 109, 1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]


