Abstract
The microvasculature plays a primary role in the interchange of substances between tissues and the circulation. In visceral organs that undergo considerable distension upon filling, the microvasculature appears to display intrinsic contractile properties to maintain their flow. Submucosal venules in the bladder or gastrointestinal tract generate rhythmic spontaneous phasic constrictions and associated Ca2+ transients. These events are initiated within either venular pericytes or smooth muscle cells (SMCs) arising from spontaneous Ca2+ release from the sarcoplasmic reticulum (SR) and the opening of Ca2+‐activated chloride channels (CaCCs) that trigger Ca2+ influx through L‐type voltage‐dependent Ca2+ channels (VDCCs). L‐type VDCCs also play a critical role in maintaining synchrony within the contractile mural cells. In the stomach myenteric layer, spontaneous Ca2+ transients originating in capillary pericytes appear to spread to their neighbouring arteriolar SMCs. Capillary Ca2+ transients primarily rely on SR Ca2+ release, but also require Ca2+ influx through T‐type VDCCs for their synchrony. The opening of T‐type VDCCs also contribute to the propagation of Ca2+ transients into SMCs. In visceral microvasculature, pericytes act as either spontaneously active contractile machinery of the venules or as pacemaker cells generating synchronous Ca2+ transients that drive spontaneous contractions in upstream arterioles. Thus pericytes play different roles in different vascular beds in a manner that may well depend on the selective expression of T‐type and L‐type Ca2+ channels.
Abbreviations
- α‐SMA
alpha smooth muscle actin
- ACh
acetylcholine
- CaCC
Ca2+‐activated chloride channel
- CGRP
calcitonin gene‐related peptide
- EFS
electrical field stimulation
- CICR
Ca2+‐induced Ca2+ release
- GI
gastrointestinal
- InsP3
inositol trisphosphate
- ICC
interstitial cells of Cajal
- NCX
sodium–calcium exchangers
- NO
nitric oxide
- PKG
protein kinase G
- PLC
phospholipase C
- SERCA
sarco‐endoplasmic reticulum Ca2+‐ATPase
- sGC
soluble guanylate cyclase
- SMC
smooth muscle cell
- SOC
store‐operated Ca2+ entry channel
- SR
sarcoplasmic reticulum
- VDCC
voltage‐dependent Ca2+ channel
Introduction
The microvasculature consisting of precapillary arterioles, capillaries and postcapillary venules regulates the transport of nutrients to tissues and removal of their excreta. Arterioles control the blood flow into the tissues to meet the demands of individual organs, while transfer of substances predominately occurs across the wall of the capillaries. Since capillary filtration and reabsorption is a function of the hydrostatic pressure that is determined by arteriolar and venular pressures and the ratio of post‐to‐precapillary resistance, the different components of the microvasculature play a critical role in regulating the microcirculation. The relative importance of these different units may vary amongst organs depending on extravascular factors, e.g. extraluminal tissue pressure and stretch or compression associated with tissue volume changes.
In visceral organs that undergo considerable wall distention or rises in the luminal pressure upon filling, e.g. urinary bladder (Hashitani et al. 2011, 2012; Shimizu et al. 2014), stomach (Mitsui & Hashitani, 2015 a; Hashitani et al. 2015) and colon (Mitsui et al. 2013), the microvasculature appears to display intrinsic contractile properties to maintain their flow. Previous studies on the contractile properties of the microvasculature in visceral organs have mostly focused on the arterioles, particularly those in the gastrointestinal tract (Hirst, 1977; Kotecha & Neild, 1995). The properties of the mural cells, i.e. SMCs and/or pericytes, in capillaries and venules have been less explored. Since even small rises in the intraluminal pressure and/or associated organ wall distension may diminish blood flow in capillaries or venules more readily than in arterioles, the intrinsic contractile properties of these microvessels should be further investigated. In addition, since contractility of microvasculature is regulated by both neural activity and intrinsic ‘myogenic’ activity, it is reasonable to assume that spontaneous contractile activity of microvasculature in visceral organs plays a fundamental role in the maintenance of the microcirculation.
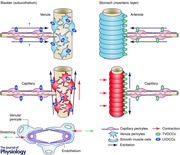
Arterioles or venules are surrounded by densely packed vascular SMCs, while capillaries and pre‐ and post‐capillary microvessles are covered by morphologically distinct pericytes. Pericytes in precapillary arterioles and postcapillary venules express α‐smooth muscle actin (α‐SMA) and are contractile, while capillary pericytes are often negative for α‐SMA and non‐contractile (Burdyga & Borysova, 2014). However, the role of pericytes in regulating capillary blood flow has been recently revealed (Peppiatt et al. 2006; Femández‐Klett et al. 2010; Hall et al. 2014). In several vascular beds, capillary pericytes have been shown to display Ca2+ transients, membrane depolarisations and contractile responses upon stimulation with neurotransmitters or humoral substances, e.g. noradrenaline (Hall et al. 2014), acetylcholine (Wu et al. 2003), angiotensin‐II (Zhang et al. 2008) or endothelin‐1 (Borysova et al. 2013). Pericyte properties have been investigated mostly in the cerebral and retinal microvasculature (Peppiatt et al. 2006; Femández‐Klett et al. 2010; Hall et al. 2014), only a few studies have been made in visceral organs (Borysova et al. 2013). In particular, it has not been established whether capillary pericytes develop any spontaneous activity that contributes to the regulation of capillary blood blow, or whether venular pericytes in visceral organs exhibit any spontaneous electrical, Ca2+ and contractile activity.
This article summarises recent examinations of how spontaneous activity in the microvasculature of visceral organs can be modulated by neurohumoral substances. Spontaneous phasic constrictions resulting from spontaneous Ca2+ transients and associated depolarisations appear to be primarily initiated by Ca2+ release from SR within mural cells. Different types of VDCCs contribute to not only the amplification of these Ca2+ transients but also to the synchrony amongst mural cells. Importantly, pericytes may act as originators for spontaneous activity in the microvasculature tree.
Suburothelial venule of the bladder
Spontaneous venular constrictions
The urinary bladder is capable of accommodating large volumes of urine with remarkably little increase in intravesical pressure. Since intravesical pressure during bladder filling does not exceed capillary pressure, normal bladders maintain their circulation, so that the blood supply only transiently drops during voiding (Greenland & Brading, 1996). Blood vessels in the bladder wall are characterized by their winding arrangement, which prevents them from being stretched in their longitudinal direction during the filling phase (Sarma, 1981). Thus, they are capable of maintaining their diameter so that the resistance against blood flow is not increased.
Besides this structural characteristic, suburothelial venules in the bladder of rat (Hashitani et al. 2011; Shimizu et al. 2014) and mouse (Hashitani et al. 2012) develop spontaneous phasic constrictions, suggesting that the venules actively contribute to the regulation of the microcirculation. In contrast, suburothelial arterioles are not spontaneously active in both species, and their constrictions are exclusively mediated by sympathetic transmission.
Contractile mural cells
In the rat bladder, venular constrictions predominately arise through contractions of circumferentially arranged ‘slender’ SMCs. However, stellate‐shaped pericytes also exhibited spontaneous Ca2+ transients and constrictions. Where these SMCs and pericytes are co‐localised, they display synchronous Ca2+ transients, suggesting that they are coupled with each other. The contractile mural cells generating spontaneous Ca2+ transients in the mouse bladder are stellate‐shaped pericytes, while spindle‐shaped SMCs are distributed only in the proximal, larger venules. Consistent with this vigorous contractility of the venular pericytes and SMCs, immunohistochemistry reveals that both mural cell types express α‐SMA. Interestingly, venular pericytes are not immunoreactive against the pericyte marker NG2 (Ozerdem et al. 2001), while pericytes in capillaries and arteriolar SMCs both express NG2 (Mitsui & Hashitani, 2013). The morphological characteristics of suburothelial venular pericytes are consistent with those of other vascular beds, having stellate‐shaped cell bodies with extensive processes (Higuchi et al. 2000; Borysova et al. 2013) and a NG2−, α‐SMA+ phenotype (see Burdyga & Borysova, 2014). Pericytes have been considered to be multipotent precursors for several different cell types, including SMCs (Hirschi & D'Amore, 1996) and thus there seems to be a transition from pericyte‐enveloped to SMC‐enveloped venules, presumably to meet functional demands.
Role of L‐type VDCCs
Spontaneous action potentials with a slow upstroke and decay precede phasic constrictions. Blockers for L‐type voltage‐dependent Ca2+ channels (LVDCCs) suppress action potentials and associated constrictions leaving small fluctuations of the membrane potential, indicating that electro‐mechanical coupling upon the opening of LVDCCs plays a fundamental role in venular constriction. The predominant role of LVDCCs in Ca2+ influx of SMCs is well established. Ca2+ currents arising from the opening of LVDCCs have also been demonstrated in freshly isolated retinal pericytes of the rat (Sakagami et al. 1999).
Blockers of LVDCCs reduce the amplitude of venule spontaneous Ca2+ transients and also disrupts their synchrony amongst the contractile mural cells, indicating that LVDCCs play a critical role in maintaining multicellular coupling (Fig. 1; Hashitani et al. 2011, 2012). Because of the regenerative nature of LVDCC activation, spontaneous action potentials generated in any mural cell within their network spreads to ‘electrically‐coupled’ neighbouring cells irrespective of their length constant. Pericytes in the suburotheial venules appear to make close appositions with themselves as well as the endothelium via gap junctions (Cuevas et al. 1984). Since endothelial cells are well coupled to each other and act as a low resistance path of electrical transmission (Yamamoto et al. 2001), pericyte depolarisation may even be effectively transmitted to electrically remote pericytes via the endothelium.
Figure 1. Role of VDCCs in ‘coupled’ oscillators in the microvasculature .
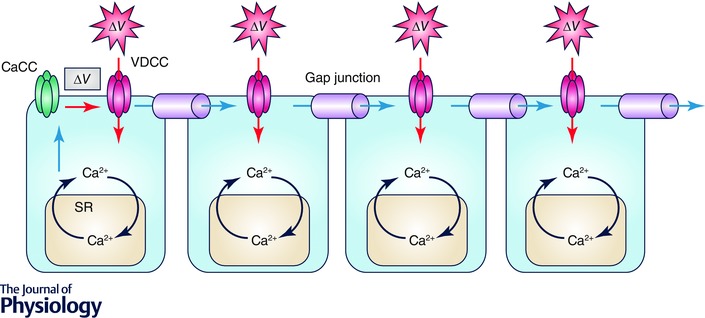
Mural cells in the microvasculature are electrically coupled via gap junctions. Adjacent monolayers of endothelial cells may act as a low resistant pathway for intercellular communications. Spontaneous SR Ca2+ release in a mural cell causes the opening of Ca2+ activated Cl− channels (CaCCs) and membrane depolarization (ΔV in grey box). This depolarisation triggers the opening of either L‐ or T‐type VDCCs to cause the firing of ‘regenerative’ action potentials (ΔV in red exploded polygons). These regenerative action potentials spread to coupled mural cells, and the Ca2+ influx via VDCCs triggers Ca2+‐induced Ca2+ release (CICR).
Role of SR Ca2+ release
After the blockade of LVDCCs, both pericytes and SMCs are capable of generating asynchronous spontaneous Ca2+ transients. These residual Ca2+ transients are abolished by CPA, an inhibitor for sarco‐endoplasmic reticulum Ca2+‐ATPase (SERCA), 2‐APB, a blocker for inositol trisphosphate (InsP3)‐induced Ca2+ release, or U73122, a phospholipase C (PLC) inhibitor, but not ryanodine, suggesting a primary role of InsP3‐induced Ca2+ release from SR in their generation. Spontaneous SR Ca2+ release opens Ca2+‐activated chloride channels (CaCCs), the resultant depolarization triggering the opening of LVDCCs and associated Ca2+ influx to cause venular constrictions (Fig. 2). Such a primary role of CaCCs in electro‐mechanical coupling in pericytes has been demonstrated in other vascular beds (Sakagami et al. 1999; Zhang et al. 2008).
Figure 2. Mechanisms of cytosolic Ca2+ oscillator in mural cells of the microvasculature .
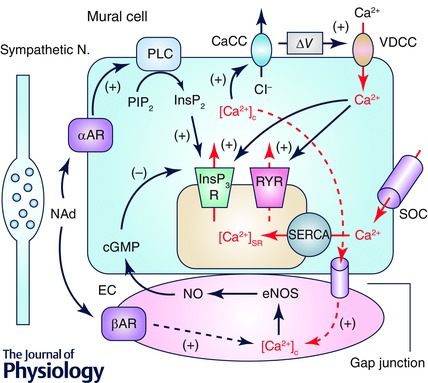
Mural cells, i.e. pericytes and venular SMCs, operate a ‘cytosolic Ca2+ oscillator’ primarily arising from Ca2+ release from SR via inositol trisphosphate receptors (InsP3Rs). Ryanodine receptor (RYRs) may also contribute to Ca2+‐induced Ca2+ release (CICR) from SR. Increased cytosolic Ca2+ concentration ([Ca2+]c) causes openings of Ca2+‐activated Cl− channels (CaCCs) to depolarise the membrane. The depolarisation triggers the opening of voltage‐dependent Ca2+ channels (VDCCs) to induce Ca2+ influx that may be amplified by CICR. Reduction in [Ca2+]SR triggers Ca2+ influx via store‐operated Ca2+ channels (SOCs) that will be taken up via sarco‐endoplasmic reticulum Ca2+‐ATPase (SERCA) to refill SR. Noradrenaline (NAd) released from sympathetic nerves acting on α‐adrenoceptors (αARs) stimulate the production of InsP3 to facilitate SR Ca2+ release via InsP3R. NAd binding to β‐adrenoceptors (βARs) on endothelial cells stimulates the production of NO. NO increases cGMP in mural cells to inhibit SR Ca2+ release via InsP3R. Arrows and dotted arrows in red indicate flows of Ca2+. Dashed arrows indicate proposed or unidentified signalling pathways. eNOS, endothelial NO synthase; EC, endothelium; ∆V, membrane depolarization; PLC, phospholipase C.
SR Ca2+ stores have been shown to function through InsP3‐induced Ca2+ release in interstitial cells of Cajal (ICC) of the GI tract (Suzuki et al. 2000; van Helden et al. 2000), and lymphatic SMCs (van Helden, 1993; Imtiaz et al. 2007). In comparison, some blood vessels and interstitial cells of the urethra and renal pelvis have Ca2+ stores that function through both ryanodine‐ and InsP3‐receptors (Sergeant et al. 2001; Haddock & Hill, 2005; Lang et al. 2007).
Besides the periodical cycle of SR Ca2+ handling, Ca2+ influx via store‐operated Ca2+ entry channels (SOCs) but not sodium calcium exchangers (NCXs) is required in maintaining spontaneous activity of the suburothelial venules of the bladder. This is in contrast to the pacemaking mechanism of interstitial cells of the urethra that relies on Ca2+ influx via NCXs but not SOCs (Bradley et al. 2005, 2006). In addition, Ca2+ handling by mitochondria may also be involved in the operation of cytosolic Ca2+ oscillators underlying spontaneous venular constrictions, as has been demonstrated in ICC in the GI tract (Ward et al. 2000), interstitial cells in the urethra (Sergeant et al. 2008) or atypical smooth muscle cells in the renal pelvis (Hashitani et al. 2009).
Modulation of spontaneous activity
Spontaneous constrictions of the suburothelial venules are accelerated upon the activation of α‐adrenoceptors by neutrally released noradrenaline (Shimizu et al. 2014). After the blockade of α‐adrenocetors, sympathetic nerve stimulation abolishes spontaneous constrictions or reduces their frequency upon the activation β‐adrenoceptors. This inhibition of spontaneous constrictions is largely diminished upon blockade of nitric oxide (NO) production, suggesting that β‐adrenoceptors stimulate NO production presumably within the endothelium. Since suburothelial venules are surrounded by a monolayer of interconnecting stellate‐shaped pericytes connecting via thin processes with each other (Hashitani et al. 2012), neurally released noradrenaline may readily have access to endothelial receptors through these inter‐process spaces. Alpha‐adrenoceptors are coupled to Gq/11 proteins which activates PLC to produce InsP3, while cGMP, a second messenger of NO, is known to inhibit InsP3R‐induced Ca2+ release (Kannan et al. 1997). Therefore, the adrenoceptors‐mediated changes in the frequency of spontaneous constrictions results from the modulation of InsP3R‐induced Ca2+ release (Fig. 2). In pericytes of several vascular beds, binding of neurohumoral substances to G‐protein coupled receptors, including α‐adrenoceptors, is reported to induce InsP3‐induced Ca2+ release from SR (Zhang et al. 2008; Borysova et al. 2013).
Blockade of NO production in the bladder increases the frequency of spontaneous venular constrictions. Pericytes in other vascular beds express soluble guanylate cyclase (sGC) and protein kinase G (PKG), and NO donor‐induced dilatation is prevented by sGC inhibition (Hamilton et al. 2010). Electron microscopy reveals that numerous fusions or close appositions of pericyte and endothelial membranes, especially in their processes; although gap junctions or other junctional structures are not evident (Hashitani et al. 2012). Therefore, spontaneous Ca2+ transients in pericytes may well spread to the endothelium to increase [Ca2+]i resulting in the production and release of NO (Fig. 2). In the descending vasa recta of the rat, angiotensin II induces similar depolarisations in pericytes and the endothelium, suggesting electrical coupling via myoendothelial gap junctions (Zhang et al. 2014). However, corresponding increases in [Ca2+]i in pericytes did not spread to the endothelium (Zhang et al. 2008). Thus, it may be possible that pericyte depolarisation transmits to the endothelium to activate T‐type VDCCs (TVDCCs) to cause Ca2+‐dependent NO production as suggested in other vascular preparations (Kuo et al. 2011). Nevertheless, the interaction between pericytes and the endothelium in the suburothelial venules may act as self‐limiting mechanism to prevent excessive venular constrictions.
Submucosal venule of the GI tract
Spontaneous activity
Submucosal venules of gastric antrum (Mitsui & Hashitani, 2015 a) and distal colon (Mitsui et al. 2013) also develop spontaneous phasic constrictions. Unlike the suburothelial venules of the bladder, the contractile mural cells in the submucosal venules of the stomach and colon are circumferentially arranged SMCs, not stellate‐shaped pericytes. Stellate‐shaped pericytes in post‐capillary venules of the gastric antrum have recently been found to exhibit properties similar to those of venular SMCs (Mitsui & Hashitani, 2015 b). Venules in the wall of in the stomach and colon are likely to be chronically stretched and/or compressed during accommodation of the luminal content. Thus, rhythmic constrictions of these venules may be beneficial in preventing blood stagnation in venules and/or capillaries to maintain submucosal microcirculation.
Intracellular mechanisms
Blockade of LVDCCs suppresses spontaneous Ca2+ transients and also disrupts synchrony amongst the venular SMCs of the stomach submucosa (Mitsui & Hashitani, 2015 a), indicating LVDCCs play a critical role in promoting intercellular coupling (Fig. 1). As a consequence, blockers for LVDCCs abolish spontaneous venular constrictions and causes dilatation. Thus, LVDCCs contribute to not only the generation of phasic constrictions but also basal tone.
Spontaneous Ca2+ transients and associated constrictions are also blocked upon inhibition of SERCA or InsP3‐induced Ca2+ release, but not Ca2+‐induced Ca2+ release (CICR) via ryanodine receptors, indicating that mechanisms underlying spontaneous activity of the submucosal venules are basically similar to those of suburothelial venules of the bladder (Fig. 2). In addition, blockers for CaCCs channels suppress phasic constrictions associated with a dilatation, suggesting that depolarisations resulting from the opening of CaCCs are required to activate LVDCCs.
Spontaneous venular constrictions are accelerated upon blockade of NO production, while acetylcholine (ACh) suppresses constrictions in an NO‐dependent manner, suggesting that endothelium‐derived NO increases cGMP in venular SMCs to inhibit InsP3‐induced Ca2+ release and cytosolic Ca2+ oscillations (Fig. 2).
Neuronal modulation
Noradrenaline released from sympathetic nerves induces long‐lasting vasoconstriction in GI venules that is blocked by phentolamine, indicating that neutrally mediated venular constriction is exclusively mediated upon the activation of α‐adrenoceptors. Thus, unlike arteriolar constrictions, purinergic transmission is not involved in nerve‐mediated venular constriction. Consistent with this, immunoreactivity of P2X purinoceptors is detected in the arterioles, but not in neighbouring venules. Spontaneous venular constrictions are suppressed upon activation of primary afferents with capsaicin which is attenuated by a calcitonin gene‐related peptide (CGRP) receptor antagonist. Immunohistochemical data reveal that both tyrosine hydroxylase‐positive sympathetic nerves and CGRP‐expressing nerves project to venules.
Myenteric microvasculatur of the stomach
In the gastrointestinal tract, the myenteric layer, comprising an extensive nerve plexus and networks of ICC and platelet‐derived growth factor receptor α (PDGFRα)‐positive (PDGFRα+) fibroblast‐like interstitial cells, plays a central role in regulating gastrointestinal motility (Sanders et al. 2014). Despite the importance of this layer, previous studies have primarily focused on the functional and morphological characteristics of the microcirculation in the submucosal layer, where another neural plexus is located that plays a critical role in regulating mucosal blood supply (Hirst, 1977; Kotecha & Neild, 1995).
Role of L‐type VDCCs
In marked contrast to spontaneous Ca2+ transients in mural cells of the venules in the bladder or the submucosa of stomach (Hashitani et al. 2011, 2012; Mitsui & Hashitani, 2015 a), blockade of LVDCCs does not disrupt the generation or synchrony of Ca2+ transients in a majority of myenteric capillary pericytes or arteriolar SMCs (Hashitani et al. 2015). LVDCCs also play a minimal role in establishing the basal Ca2+ concentration of capillary pericytes, (cf. mural cells in the proximal retinal microvasculature; Ishizaki et al. 2009). Thus, the relative contribution of LVDCCs to Ca2+ transients in the microvasculature appears to vary amongst vascular beds and also exhibit regional differences even within a microvascular network (see Burdyga & Borysova, 2014).
Role of T‐type VDCCs
Synchronous mural cell Ca2+ transients in the myenteric microvasculature are abolished by blockers of TVDCCs, indicating that these spontaneous Ca2+ transients predominately rely on TVDCCs (Fig. 1). TVDCCs are low threshold channels that open at membrane potentials as negative as −70 mV (Perez‐Reyes, 2003). The window current for TVDCCs usually occurs over membrane potentials which are more hyperpolarised than that of LVDCCs. (e.g. T‐type, −65 mV to −45 mV cf. L‐type, −30 mV to 0 mV; Hirano et al. 1989). Since the resting membrane potential of retinal or renal pericytes ranged between −60 mV and −50 mV (Sakagami et al. 1999; Zhang et al. 2001), window currents for TVDCCs in pericytes may well be contribute to these cells exhibiting pacemaker function.
Differences in the contribution of L‐ and TVDCCs to spontaneous activity are evident in various vascular beds or regions within a microvasculature tree. TVDCCs are functionally expressed in arterial and arteriolar SMCs and contribute to vascular tone (Hansen et al. 2001; Jensen et al. 2004; Kuo et al. 2010, Abd El‐Rahman et al. 2013). In cerebral arteries, nifedipine‐resistant, mibefradil‐sensitive vascular tone increases with decreasing vessel size, suggesting that TVDCCs play a larger role in smaller arteries (Kuo et al. 2010). Furthermore, the percentage of TVDCC currents in freshly isolated vascular SMCs increases dramatically along the lower branches of the mesenteric artery, rising to almost 100% in submucosal arterioles (Morita et al. 1999). Conducted vasoconstriction in rat mesenteric arterioles <40 μm in vivo also relies exclusively on TVDCCs (Gustafsson et al. 2001).
Role of SR Ca2+ release
While the generation of synchronous Ca2+ transients in the myenteric microvasculature relies on TVDCCs, Ca2+ transients are also readily abolished by CPA, caffeine or tetracaine, suggesting that both InsP3‐ and ryanodine‐receptors are involved (Parker & Ivorra, 1991). SR store Ca2+ release may be the primary event in generating spontaneous Ca2+ transients in capillary pericytes, since the inhibition of SERCA with CPA abolishes asynchronous Ca2+ transients of pericytes when L‐ and TVDCCs are previously blocked (Fig. 2). Extracellular Ca2+ influx via a pathway other than L‐ and TVDCCS is also required to maintain the cytosolic Ca2+ oscillator in these pericytes, as both synchronous and asynchronous Ca2+ transients are abolished by nominally Ca2+‐free solution.
A common mechanism underlying pericyte pacemaking and synchronisation is coupled oscillators arising from the reciprocal interaction between Ca2+ release from SR and membrane depolarisations (Fig. 1). This has been proposed in lymphatics (Imtiaz et al. 2007), arteries and arterioles (Peng et al. 2001; Haddock & Hill, 2005) and in the distal gastric antrum (van Helden & Imtiaz, 2003). SR Ca2+ release can cause depolarisation by opening CaCCs or cation channels. Pericytes in the descending vasa recta and retina have CaCCs associated with cyclic oscillations in [Ca2+]i (Sakagami et al. 1999; Zhang et al. 2008). ICC in the GI tract are known to express TMEM16A/Ano1 CaCCs (Gomez‐Pinilla et al. 2009; Hwang et al. 2009). In the myenteric microvasculature of the stomach, TMEM16A/Ano1 immunofluorescence is detected in myenteric ICC (ICC‐MY) but not in the microvasculature. In the mouse small intestine, pericytes associated with the microvasculature near the myenteric plexus express cation permeable, maxi‐anion channels and are not immunoreactive for Ano1 (Parsons et al. 2012). Thus the Ca2+‐activated inward currents that trigger the activation of TVDCCs in myenteric pericytes remain to be established.
Coupled oscillator mechanisms require membrane depolarisation paired with SR Ca2+ release (Fig. 1). In pericytes of suburothelial venules of the bladders or venular SMCs in the stomach submucosa, this appears to be mediated by depolarisation associated with the opening of LVDCCs that cause Ca2+ influx to activate SR Ca2+ release through CICR. In contrast, TVDCCs undertake this role in myenteric pericytes or arteriolar SMCs. Functional coupling between TVDCCs and SR Ca2+ release to drive pacemaker mechanisms has been reported in sinoatrial pacemaker cells (Háuser et al. 2000) or ICC in the GI tract (Kito & Suzuki, 2003; Zheng et al. 2014). Interestingly, in interstitial cells of the guinea‐pig prostate, TVDCCs but not LVDCCs stimulate CaCCs, suggesting that TVDCCs are functionally coupled with SR Ca2+ release (Lang et al. 2014).
Pericytes act as pacemaker cells
Synchronous spontaneous Ca2+ transients in the myenteric microvasculature are initiated in the capillaries where the pericytes are distributed. Imaging the cerebral microvasculature in vivo shows that capillary dilatation in response to the excitation of vasodilatory neurons precedes arteriolar dilatation, suggesting that hyperpolarising signals initiated in capillaries may spread to arterioles (Hall et al. 2014).
Capillary Ca2+ transients sometimes fail to spread into neighbouring arterioles, suggesting that there may be gating functions at the junctions between capillaries and arterioles. In addition, active capillaries that initiate spontaneous Ca2+ transients do not generally maintain their dominance, often switching randomly from one capillary to another with time. Consistently, studies on the retinal microvasculature indicate that the axial electrotonic voltage transmission is highly efficient, particularly in capillaries, but significant voltage dissipation occurs at branching points (Zhang et al. 2011). Although specialised units of SMCs located at arterial branching points may serve as originators of rhythmic vasomotion in the microvasculature of kidney and skin (Colantuoni et al. 1985; Goligorsky et al. 1995), this is not the case in the myenteric microvasculature tree. Thus, it is reasonable to suggest that capillary pericytes may act as pacemaker cells generating spontaneous Ca2+ transients and associated depolarising signals that entrain arterioles to develop highly synchronous Ca2+ transients in mural cells of the microvasculature.
Comparison of spontaneous Ca2+ transients in ICC‐MY with those in neighbouring microvessel indicate there are no temporal correlations (Hashitani et al. 2015). Thus, Ca2+ transients in capillary pericytes or arteriolar SMCs occur independently of ICC‐MY activity. Furthermore, pericytes or SMCs of the myenteric microvasculature and perivascular interstitial cells are not immunoreactive for antibodies against Kit receptor, the universal marker for ICC. Therefore, neither ICC nor Kit‐positive interstitial cells found in other visceral organs act as pacemakers driving the spontaneous Ca2+ transients in the viseceral microvasculature.
Spontaneous Ca2+ transients in the myenteric microvasculature are not affected by tetrodotoxin or electrical field stimulation (EFS). Consistently, PGP9.5‐positive perivascular nerve fibres are not found along the myenteric microvasculature, whereas perivascular nerve fibres containing many varicosities, the sites of neurotransmission, are adjacent to the intramuscular microvasculature. This is in contrast to the microvasculature in the submucosal layer of the GI tract where both arterioles and venules are functionally innervated by sympathetic nerves (Hirst, 1977; Mitsui et al. 2013; Mitsui & Hashitani, 2015 a). Recent 3‐D imaging of the myenteric layer of mouse small intestine with a technique termed ‘vessel painting’ has revealed that the periganglionic capillary network is in close contact with ganglionic cells, as well as glial cells (Fu et al. 2013). Nevertheless, spontaneous Ca2+ transients in the myenteric microvasculature occur independently from neural activity.
Outlook and concluding remarks
Vasomotion in arterioles has been considered to reduce vascular resistance and enhance blood flow to facilitate tissue oxygenation (Nilsson & Aalkjaer, 2003). Similarly, active venular constrictions produce an increase in the blood flow and exhibit temporal vessel diameter‐blood velocity and pressure relationships characteristic of a peristaltic pump (Dongaonkar et al. 2012). Although it was believed that valves are absent in fine veins or venules, microvalves in venules that restrict flow from postcapillary venules back into the capillary bed have been demonstrated (Caggiati et al. 2006). The presence of valves further enhances the efficiency of the venular peristaltic pump by preventing retrograde flow. Since capillary filtration and reabsorption is a function of hydrostatic pressure that is determined by the ratio of post‐to‐precapillary resistance, regulation of contractility of ‘upstream’ arterioles and ‘downstream’ venules by capillary pericytes seems to be ideal mechanism to adjust microcirculation to meet tissue demands.
In the bladder suburothelium, capillary pericytes express NG2 but not α‐SMA, questioning their ability to contract. More recently, NG2‐DeRed mice allowed us to visualise spontaneous Ca2+ transients in capillary pericytes, but the Ca2+ transients are apparently not associated with a reduction in capillary diameter, suggesting that these capillary pericytes are non‐contractile (H Hashitani, unpublished data). Similarly, capillary pericytes in myenteric microcirculation of the stomach appear to act as ‘non‐contractile’ pacemaker cells to drive ‘upstream’ arterioles. In contrast, pericytes in retinal and cerebral capillaries contract upon electrical stimulation or application of excitatory neurotransmitters (Peppiatt et al. 2006; Fernández‐Klett et al. 2010; Hall et al. 2014). Such differences in the role of pericytes may reflect characteristics of different microcirculatory beds. Since both the retinal and cerebral microvasculature are located within organs that are relatively inflexible in terms of their architecture and limited volumes, fine regulation and/or distribution of blood supply may be required. On the other hands, more dynamic rearrangements of microvasculature architecture as well as blood flow distribution may well be possible in distensible visceral organs. Therefore, pericytes may play different roles in different microcirculatory beds to meet the characteristics of individual organs.
Hyperpolarising signals initiated in capillary pericytes may spread to arterioles in retinal and cerebral microcirculation and result in the closure of LVDCCs and therefore vasodilatation (Puro, 2012; Hall et al. 2014). In contrast, pericyets in visceral organs appear to generate periodical transient depolarisations to drive spontaneous rhythmic vasoconstrictions in ‘upstream’ arterioles or ‘downstream’ venules. During progressive distension of the wall in visceral organs that are usually associated with only small rises in transmural pressure, suppressions of contractility of microvessels as seen in the retina and cerebral microcirculation would result in compression or collapse of microvasculature, particularly in venules where the intraluminal pressure is the lowest. Thus, periodical vasomotions rather than simple vasodilatation may be required to maintain microcirculation blood flow. Distension of visceral organs may facilitate pericyte‐driven vasomotion by opening stretch‐activated channels.
Dysfunction of ‘pacemaking’ mechanisms of capillary pericytes and/or transmission of ‘pacemaking’ depolarisations to precapillary arterioles would result in insufficient tissue oxygenation due to the increased resistance against blood flow into capillaries. Disturbances in the spread of ‘pacemaking’ depolarisations to post‐capillary venules would diminish the pumping functions of venules, resulting in stagnation of tissue excreta.
In the normal bladder intravesical pressure rises are minimal, so that it is likely that blood flow of arteries or arterioles is well maintained. However, even small rises in the intravesical pressure may readily attenuate the spontaneous venular constrictions, resulting in the disturbance of suburothelial microcirculation. In addition, the multipotency of pericytes may contribute to the remodelling of the bladder suburothelium that is commonly seen in an overactive bladder. Increased pericyte numbers have been reported in pulmonary microvessels in patients with pulmonary hypertension and a murine retinal angiogenesis model (Ricard et al. 2014). Thus, increased pericyte expression may alter contractility of microvasculature and simply reduce substance exchange across capillary walls by reducing the capillary surface area.
Ischaemic and reperfusion damage to the cells in the bladder wall has been proposed as a major cause of overactive bladder (Gosling et al. 2000) which accompanies ageing and atherosclerosis (Azadzoi et al. 1999; Yoshida et al. 2010). The beneficial effects of α‐adrenoceptor antagonists on bladder storage symptoms may be attributed to an improvement in bladder microcirculation. Similarly, β3‐adrenoceptor agonists and PDE5 inhibitors may also exert their action by improving the bladder microcirculation. Since these pharmacological agents improve bladder storage function without improving the narrowed luminal diameter of the feeding arteries (Andersson et al. 2014), their therapeutic effects may also be attributed to their actions on the contractile and/or morphological properties of microvasculature within the bladder wall.
Further understanding of microvasculature functions in visceral organs, particularly the role of pericytes and VDCCs in the generation of ‘spreading’ spontaneous activity, may highlight novel therapeutic strategies for functional disorders in visceral organs.
Additional information
Competing interests
The authors state no conflict of interest.
Acknowledgements
This study was supported by Grant‐in‐Aid for Scientific Research (B) (No. 22390304) and Grant‐in‐Aid for Challenging Exploratory Research (Nos 21659377 and 23659763) from Japan Society for the Promotion of Science (JSPS) to H.H.
Biographies
Hikaru Hashitani has been Professor of Cell Physiology at Nagoya City University since 2010. He received his medical degree from Kyushu University, completed his PhD at Nagoya City University, and then studied at Melbourne University and University of Oxford. His research interests have primarily focused on cellular mechanisms underlying spontaneous electrical and calcium signalling in smooth muscle of the genitourinary, gastrointestinal and microvascular systems.

Richard Lang received his PhD Monash University in 1979. He was an MRC Research Officer and Fellow 1978–1986 in the Department of Pharmacology, St George's Hospital Medical School, London. He returned to Monash University as a Vice‐Chancellor's Research Fellow (1986–1987), an NHRMC Senior Research Officer (1988–1990) and a Senior Research Fellow (1991–). Throughout, he has been interested in the role membrane channels and calcium play in pacemaker and tone generation in both gastrointestinal and urogenital organs.
References
- Abd El‐Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D & Welsh DG (2013). Identification of L‐ and T‐type Ca2+ channels in rat cerebral arteries: role in myogenic tone development. Am J Physiol Heart Circ Physiol 304, H58–H71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Nomiya M, Sawada N & Yamaguchi O (2014). Pharmacological treatment of chronic pelvic ischemia. Ther Adv Urol 6, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadzoi KM, Tarcan T, Siroky MB & Krane RJ (1999). Atherosclerosis‐induced chronic ischemia causes bladder fibrosis and non‐compliance in the rabbit. J Urol 161, 1626–1635. [PubMed] [Google Scholar]
- Burdyga T & Borysova L (2014). Calcium signalling in pericytes. J Vasc Res 51, 190–199. [DOI] [PubMed] [Google Scholar]
- Borysova L, Wray S, Eisner DA & Burdyga T (2013). How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 54, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, Johnston L, Large RJ, Matsuda T, Baba A, McHale NG, Thornbury KD & Sergeant GP (2006). Contribution of reverse Na+–Ca2+ exchange to spontaneous activity in interstitial cells of Cajal in the rabbit urethra. J Physiol 574, 651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, McHale NG, Thornbury KD & Sergeant GP. (2005). Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am J Physiol Cell Physiol 289, C625–C632. [DOI] [PubMed] [Google Scholar]
- Caggiati A, Phillips M, Lametschwandtner A, Allegra C (2006). Valves in small veins and venules. Eur J Vasc Endovasc Surg 32, 447–452. [DOI] [PubMed] [Google Scholar]
- Colantuoni A, Bertuglia S & Intaglietta M (1985). Variations of rhythmic diameter changes at the arterial microvascular bifurcations. Pflugers Arch 403, 289–295. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Gutierrez‐Diaz JA, Reimers D, Dujovny M, Diaz FG & Ausman JI (1984). Pericyte endothelial gap junctions in human cerebral capillaries. Anat Embryol (Berl) 170, 155–159. [DOI] [PubMed] [Google Scholar]
- Dongaonkar RM, Quick CM, Vo JC, Meisner JK, Laine GA, Davis MJ & Stewart RH (2012). Blood flow augmentation by intrinsic venular contraction in vivo . Am J Physiol Regul Integr Comp Physiol 302, R1436–R1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández‐Klett F, Offenhauser N, Dirnagl U, Priller J & Lindauer U (2010). Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA 107, 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YY, Peng SJ, Lin HY, Pasricha PJ & Tang SC (2013). 3‐D imaging and illustration of mouse intestinal neurovascular complex. Am J Physiol Gastrointest Liver Physiol 304, G1–G11. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS, Colflesh D, Gordienko D & Moore LC (1995). Branching points of renal resistance arteries are enriched in L‐type calcium channels and initiate vasoconstriction. Am J Physiol Renal Physiol 268, F251–F257. [DOI] [PubMed] [Google Scholar]
- Gomez‐Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T & Farrugia G. (2009). Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296, G1370–G1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C & Levim RM (2000). Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol 163, 1349–1356. [PubMed] [Google Scholar]
- Greenland JE & Brading AF (1996). Urinary bladder blood flow changes during the micturition cycle in a conscious pig. J Urol 156, 1858–1861. [PubMed] [Google Scholar]
- Gustafsson F, Andreasen D, Salomonsson M, Jensen BL & Holstein‐Rathlou N (2001). Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine‐insensitive Ca2+ channels. Am J Physiol Heart Circ Physiol 280, H582–H590. [DOI] [PubMed] [Google Scholar]
- Haddock RE & Hill CE (2005). Rhythmicity in arterial smooth muscle. J Physiol 566, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M & Attwell D (2014). Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D & Hall CN (2010). Pericyte‐mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PB, Jensen BL, Andreasen D & Skøtt O (2001). Differential expression of T‐ and L‐type voltage‐dependent calcium channels in renal resistance vessels. Circ Res 89, 630–638. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Lang RJ, Mitsui R, Mabuchi Y & Suzuki H (2009). Distinct effects of CGRP on typical and atypical smooth muscle cells involved in generating spontaneous contractions in the mouse renal pelvis. Br J Pharmacol 158, 2030–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Mitsui R, Masaki S & Van Helden DF (2015). Pacemaker role of pericytes in generating synchronized spontaneous Ca2+ transients in the myenteric microvasculature of the guinea‐pig gastric antrum. Cell Calcium 58, 442–456. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Mitsui R, Shimizu Y, Higashi R & Nakamura K (2012). Functional and morphological properties of pericytes in suburothelial venules of the mouse bladder. Br J Pharmacol 167, 1723–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Takano H, Fujita K, Mitsui R & Suzuki H (2011). Functional properties of suburothelial microvessels in the rat bladder. J Urol 185, 2382–2391. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Hashizume H, Aizawa Y & Ushiki T (2000). Scanning electron microscopic studies of the vascular smooth muscle cells and pericytes in the rat heart. Arch Histol Cytol 63, 115–126. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA & January CT (1989). Characteristics of L‐ and T‐type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol 256, H1478–H1492. [DOI] [PubMed] [Google Scholar]
- Hirschi KK & D'Amore PA (1996). Pericytes in the microvasculature. Cardiovasc Res. 32, 687–698. [PubMed] [Google Scholar]
- Hirst GDS (1977). Neuromuscular transmission in arterioles of guinea‐pig submucosa. J Physiol 273, 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser J, Blatter LA & Lipsius SL (2000). Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J Physiol 524, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM & Ward SM (2009). Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587, 4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MS1 Imtiaz, Zhao J, Hosaka K, von der Weid PY, Crowe M & van Helden DF (2007). Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J 92, 3843–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki E, Fukumoto M & Puro DG (2009). Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol 587, 2233–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LJ, Salomonsson M, Jensen BL & Holstein‐Rathlou NH (2004). Depolarization‐induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil‐sensitive calcium channels. Br J Pharmacol 142, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan MS, Prakash YS, Johnson DE & Sieck GC (1997). Nitric oxide inhibits calcium release from sarcoplasmic reticulum of porcine tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 272, L1–L7. [DOI] [PubMed] [Google Scholar]
- Kito Y & Suzuki H (2003). Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553, 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha N & Neild TO (1995). Actions of vasodilator nerves on arteriolar smooth muscle and neurotransmitter release from sympathetic nerves in the guinea‐pig small intestine. J Physiol 489, 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, Ellis A, Seymour VA, Sandow SL & Hill CE (2010). Dihydropyridine‐insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab 30, 1226–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, Wölfle SE & Hill CE (2011). T‐type calcium channels and vascular function: the new kid on the block? J Physiol 589, 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Hashitani H, Tonta MA, Suzuki H & Parkington HC (2007). Role of Ca2+ entry and Ca2+ stores in atypical smooth muscle cell autorhythmicity in the mouse renal pelvis. Br J Pharmacol 152, 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Tonta MA, Takano H & Hashitani H (2014). Voltage‐operated Ca2+ currents and Ca2+ ‐activated Cl− currents in single interstitial cells of the guinea‐pig prostate. BJU Int 114, 436–446. [DOI] [PubMed] [Google Scholar]
- Mitsui R & Hashitani H (2013). Immunohistochemical characteristics of suburothelial microvasculature in the mouse bladder. Histochem Cell Biol 140, 189–200. [DOI] [PubMed] [Google Scholar]
- Mitsui R & Hashitani H (2015. a). Functional properties of submucosal venules in the rat stomach. Pflugers Arch 467, 1327–1342. [DOI] [PubMed] [Google Scholar]
- Mitsui R & Hashitani H (2015. b). Mechanisms underlying spontaneous constrictions of postcapillary venules in the rat stomach. Pflugers Arch. DOI:10.1007/s00424-015-1752-y. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Miyamoto S, Takano H & Hashitani H (2013). Properties of submucosal venules in the rat distal colon. Br J Pharmacol 170, 968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Cousins H, Onoue H, Ito Y & Inoue R (1999). Predominant distribution of nifedipine‐insensitive, high voltage‐activated Ca2+ channels in the terminal mesenteric artery of guinea pig. Circ Res 85, 596–605. [DOI] [PubMed] [Google Scholar]
- Nilsson H & Aalkjaer C (2003). Vasomotion: mechanisms and physiological importance. Mol Interv. 3, 79–89. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin‐Huppe K, Monosov E & Stallcup WB (2001). NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 222, 218–227. [DOI] [PubMed] [Google Scholar]
- Parker I & Ivorra I (1991). Caffeine inhibits inositol trisphosphate‐mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol 433, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SP, Kunze WA & Huizinga JD (2012). Maxi‐channels recorded in situ from ICC and pericytes associated with the mouse myenteric plexus. Am J Physiol Cell Physiol 302, C1055–C1069. [DOI] [PubMed] [Google Scholar]
- Peng H, Matchkov V, Ivarsen A, Aalkjaer C & Nilsson H (2001). Hypothesis for the initiation of vasomotion. Circ Res 88, 810–815. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P & Attwell D (2006). Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Reyes E. (2003). Molecular physiology of low‐voltage‐activated T‐type calcium channels. Physiol Rev 83, 117–161. [DOI] [PubMed] [Google Scholar]
- Puro DG (2012). Retinovascular physiology and pathophysiology: new experimental approach/new insights. Prog Retin Eye Res 31, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, Dorfmüller P, Humbert M & Guignabert C (2014). Increased pericyte coverage mediated by endothelial‐derived fibroblast growth factor‐2 and interleukin‐6 is a source of smooth muscle‐like cells in pulmonary hypertension. Circulation 129, 1586–1597. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Wu DM & Puro DG (1999). Physiology of rat retinal pericytes: modulation of ion channel activity by serum‐derived molecules. J Physiol 521, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM & Koh SD (2014). Interstitial cells: regulators of smooth muscle function. Physiol Rev 94, 859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma KP (1981). Microangiography of the bladder in health. Br J Urol 53, 237–240. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Bradley E, Thornbury KD, McHale NG & Hollywood MA (2008). Role of mitochondria in modulation of spontaneous Ca2+ waves in freshly dispersed interstitial cells of Cajal from the rabbit urethra. J Physiol. 586, 4631–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG & Thornbury KD (2001). Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol 280, C1349–C1356. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Mochizuki S, Mitsui R & Hashitani H (2014). Neurohumoral regulation of spontaneous constrictions in suburothelial venules of the rat urinary bladder. Vascul Pharmacol 60, 84–94. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K & Mikoshiba K (2000). Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol 525, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden DF (1993). Pacemaker potentials in lymphatic smooth muscle of the guinea‐pig mesentery. J Physiol 471, 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden DF & Imtiaz MS (2003). Ca2+ phase waves: a basis for cellular pacemaking and long‐range synchronicity in the guinea‐pig gastric pylorus. J Physiol 548, 271–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P‐Y, Dosen PJ (2000). Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea‐pig gastric pylorus. J Physiol 524, 245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K & Sanders KM (2000). Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol.525, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M & Puro DG (2003). Cholinergic regulation of pericyte‐containing retinal microvessels. Am J Physiol Heart Circ Physiol 284, H2083–H2090. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Klemm MF, Edwards FR & Suzuki H (2001). Intercellular electrical communication among smooth muscle and endothelial cells in guinea‐pig mesenteric arterioles. J Physiol 535, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Nagata T, Satoji Y & Shiomi M (2010). The effects of chronic hyperlipidemia on bladder function in myocardial infarction‐prone Watanabe heritable hyperlipidemic (WHHLMI) rabbits. Neurourol Urodyn 29, 1350–1354. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Cao C, Zhang Z, Wier WG, Edwards A & Pallone TL (2008). Membrane current oscillations in descending vasa recta pericytes. Am J Physiol Renal Physiol 294, F656–F666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Huang JM, Turner MR, Rhinehart KL, Pallone TL (2001). Role of chloride in constriction of descending vasa recta by angiotensin II. Am J Physiol Regul Integr Comp Physiol 280, R1878–R1886. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu DM, Xu GZ & Puro DG (2011). The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol 589, 2383–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Payne K, Pallone TL (2014). Syncytial communication in descending vasa recta includes myoendothelial coupling. Am J Physiol Renal Physiol 307, F41–F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Park KS, Koh SD & Sanders KM (2014). Expression and function of a T‐type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Physiol Cell Physiol 306, C705–C713. [DOI] [PMC free article] [PubMed] [Google Scholar]


