Abstract
Cardiac hypertrophy is often initiated as an adaptive response to haemodynamic stress or myocardial injury, and allows the heart to meet an increased demand for oxygen. Although initially beneficial, hypertrophy can ultimately contribute to the progression of cardiac disease, leading to an increase in interstitial fibrosis and a decrease in ventricular function. Metabolic changes have emerged as key mechanisms involved in the development and progression of pathological remodelling. As the myocardium is a highly oxidative tissue, mitochondria play a central role in maintaining optimal performance of the heart. ‘Mitochondrial dynamics’, the processes of mitochondrial fusion, fission, biogenesis and mitophagy that determine mitochondrial morphology, quality and abundance have recently been implicated in cardiovascular disease. Studies link mitochondrial dynamics to the balance between energy demand and nutrient supply, suggesting that changes in mitochondrial morphology may act as a mechanism for bioenergetic adaptation during cardiac pathological remodelling. Another critical function of mitochondrial dynamics is the removal of damaged and dysfunctional mitochondria through mitophagy, which is dependent on the fission/fusion cycle. In this article, we discuss the latest findings regarding the impact of mitochondrial dynamics and mitophagy on the development and progression of cardiovascular pathologies, including diabetic cardiomyopathy, atherosclerosis, damage from ischaemia–reperfusion, cardiac hypertrophy and decompensated heart failure. We will address the ability of mitochondrial fusion and fission to impact all cell types within the myocardium, including cardiac myocytes, cardiac fibroblasts and vascular smooth muscle cells. Finally, we will discuss how these findings can be applied to improve the treatment and prevention of cardiovascular diseases.
Abbreviations
- ΔΨm
mitochondrial membrane potential
- DRP1
dynamin‐related protein 1
- FIS1
mitochondrial fission 1 protein
- I/R
ischaemia/reperfusion
- KO
knockout
- MFF
mitochondrial fission factor
- MFN
mitofusin
- mPTP
mitochondrial permeability transition pore
- mtDNA
mitochondrial DNA
- OPA1
optic atrophy protein 1
- PDGF
platelet‐derived growth factor
- PINK1
PTEN‐induced putative kinase 1
- ROS
reactive oxygen species
- T2DM
type 2 diabetes mellitus
- VSMCs
vascular smooth muscle cells
Mitochondrial dynamics as a therapeutic target in cardiovascular disease
Maintenance of mitochondrial function and integrity is crucial for normal cell physiology, particularly in cells with high energy demands. This is particularly certain in the heart, where mitochondria occupy approximately 30% of the total cell volume – and produce an astounding 6 kg of ATP per day through oxidative phosphorylation – in order to sustain cardiac mechanical function (Hall et al. 2014). In addition to ATP production, mitochondria regulate cell death and survival by integrating a range of cellular signals; they are also the primary source of reactive oxygen species (ROS), which can trigger oxidative stress, thereby affecting cell survival and death (Pellegrini & Scorrano, 2007).
Although mitochondria are often depicted as isolated organelles, they actually form highly dynamic networks whose structure and distribution have a potential effect on metabolic function. The nature of these intricate networks depends on the balance between the opposing processes of mitochondrial fission and fusion (Westermann, 2002; Parra et al. 2011). In mammalian cells, the primary regulators of mitochondrial fusion are dynamin‐related GTPases termed mitofusins (MFN1 and MFN2) and optic atrophy protein 1 (OPA1). Conversely, mitochondrial fission 1 protein (FIS1) and the dynamin‐related protein 1 (DRP1) are involved in mitochondrial fission (Ong & Hausenloy, 2010; Parra et al. 2011).
Given the unique and highly dynamic configuration of mitochondria in the adult heart, the effects of changes in mitochondrial morphology in this organ are complex. Indeed, emerging data suggest that the dynamics of mitochondria are relevant to various aspects of cardiovascular biology: these include cardiac development, responses to ischaemia/reperfusion (I/R) injury, heart failure, type 2 diabetes mellitus (T2DM) and apoptosis. In this article, we review how changes in mitochondrial morphology, and mitochondrial fission and fusion proteins, impact heart function.
Mitochondrial fusion and fission in cardiovascular cells
Fusion and fission machinery
The molecular machinery that controls the processes of mitochondrial fusion and fission is highly regulated. As noted above, mitochondrial fusion in mammals is controlled by MFN1 and MFN2, proteins situated in the outer mitochondrial membrane, and OPA1, which is located in the inner mitochondrial membrane (Cipolat et al. 2004) (Fig. 1). MFN1 and MFN2 are necessary for mitochondrial fusion, and cells lacking either of these GTPases display a significant decrease in the fusion process. Also, the proteins are partially redundant in their function; thus when MFN1 expression is decreased, mitochondrial fusion can be rescued by MFN2 overexpression and vice versa (Chen & Chan, 2005; Chan, 2006). Additionally, MFN2 has been implicated in several other physiological functions, such as modulation of energetic processes, endoplasmic reticulum–mitochondria coupling, and regulation of mitophagy, a process through which mitochondria are engulfed by autophagosomes and delivered to lysosomes for degradation (Pich et al. 2005; Zorzano et al. 2009; Chen & Dorn, 2013).
Figure 1. Complex interplay between mitochondrial fusion and fission supports cell homeostasis .
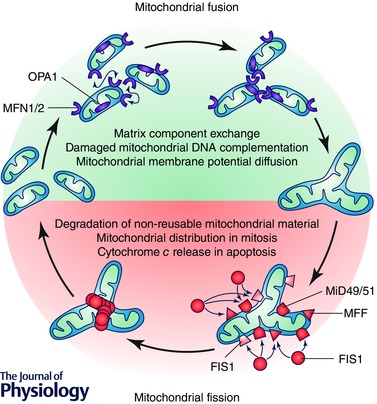
Mitochondrial dynamics sustains a balance between the processes of mitochondrial fusion and fission to maintain the proper function of this complex organelle. Mitochondrial fusion is regulated by mitofusin (MFN) 1 and 2 and optical atrophy protein 1 (OPA1). This allows for exchange of material (matrix components, damaged mitochondrial DNA), as well as promoting a balance in bioenergetic properties (e.g. mitochondrial membrane potential). On the other hand, DRP1 and its adapter proteins FIS1, MFF and MiD49/51 control mitochondrial fission. This process is necessary for various processes throughout the life of the cell, such as redistribution of mitochondria in mitosis, as well as release of cytochrome c during cell death by apoptosis. Moreover, mitochondrial fission is required for selective mitochondrial degradation (mitophagy).
The second component regulating mitochondrial fusion is OPA1, a transmembrane protein tightly associated with the mitochondrial inner membrane. The transcript coding for OPA1 undergoes alternative splicing, giving rise to eight variants that are expressed in a variety of patterns across different tissues (Delettre et al. 2001). Moreover, OPA1 also undergoes proteolytic processing, resulting in short (s) and long (l) isoforms, both of which are necessary for mitochondrial fusion (Song et al. 2007). Also, uncoupling of mitochondrial membrane potential (ΔΨm) leads to OPA1 processing (Griparic et al. 2007) through the action of the OMA1 metalloprotease (Head et al. 2009). Additional studies have implicated other proteases in the processing of OPA1, including presenilin‐associated rhomboid‐like (PARL) protein, paraplegin and the mAAA protease complex ATPase family gene‐3, yeast‐like‐1 (AFG3L1) (McBride & Soubannier, 2010).
Mitochondrial fission in mammalian cells is controlled by the GTPases DRP1, FIS1, mitochondrial fission factor (MFF), and mitochondrial dynamic proteins of 49 and 51 kDa (MiD49/51) (Hall & Hausenloy, 2012) (Fig. 1). DRP1, like dynamins, functions as a mechanoenzyme, serving to constrict the mitochondrion physically, an early step in fission. Noteworthy, DRP1 lacks a mitochondrial destination sequence; as a result, it requires that FIS1 be located in the mitochondrial outer membrane to form the fission complex (Bossy‐Wetzel et al. 2003). However, silencing FIS1 in mammalian cells has little effect on DRP1 translocation to mitochondria (Lee et al. 2004). In that context, MFF appears to be the protein acting as the DRP1 mitochondrial receptor (Gandre‐Babbe & van der Bliek, 2008). Reduction of MFF levels induces mitochondrial elongation and decreases DRP1 translocation to mitochondria (Gandre‐Babbe & van der Bliek, 2008). Likewise, MiD49 and MiD51 are involved in the mammalian fission machinery (Otera et al. 2010). Currently, it is widely accepted that multiple receptors are capable of recruiting DRP1 to mitochondria to induce fission.
DRP1 activity is also modified by post‐translational modifications. The kinases Cdk1/cyclin B (Taguchi et al. 2007) and CaMKIα (Han et al. 2008) increase DRP1 fission activity; conversely, protein kinase A phosphorylation decreases DRP1 function (Chang & Blackstone, 2007). Specifically, this phosphate residue can be removed by the Ca2+–calmodulin‐dependent phosphatase calcineurin, promoting mitochondrial fission (Cereghetti et al. 2008).
Mitochondrial fusion, fission and mitophagy work together to maintain mitochondrial and cellular homeostasis. Perturbations in mitochondrial dynamics that alter the normal balance between fission and fusion in either direction can lead to the accumulation of damaged and inefficient organelles. Mitochondrial fission is essential for maintenance and repair as it facilitates the removal of damaged components by partitioning them to a daughter that can then be targeted for removal and degradation by mitophagy. However, excessive mitochondrial fission and mitophagy can compromise the metabolic capacity of a cell (Twig & Shirihai, 2011). Thus, maintaining an appropriate balance in the fission/fusion cycle is essential.
As will be discussed in detail later, emerging key players in the regulation of mammalian mitophagy are the serine/threonine kinase PTEN‐induced putative kinase 1 (PINK1) and the E3 ubiquitin ligase Parkin, which selectively promote the degradation of impaired mitochondria (Narendra et al. 2008). In healthy mitochondria, PINK1 is preferentially imported to the mitochondrial internal membrane, where it is cleaved by the protease PARL. Although Parkin‐induced mitophagy has been shown to be dependent on the activity of PINK1, this process depends on DRP1‐mediated mitochondrial fission (Tanaka et al. 2010; Fischer et al. 2012). A full understanding of the regulatory mechanisms involved in these processes has yet to be developed, as well as their connection with the onset of cardiovascular diseases.
Cardiac myocytes
In cardiac myocytes, unremitting mitochondrial function is essential, owing to the high energy demands of this ever‐pumping muscle. Interestingly, the distribution and metabolic function of these organelles varies depending on the developmental stage of the myocardium (Lopaschuk & Jaswal, 2010). In neonatal cardiac myocytes, the heart preferentially obtains its energy from glycolysis and glucose oxidation (Lopaschuk et al. 1991). During this period, mitochondria manifest a characteristic reticular distribution in the cytosol that allows them to move freely. However, in adult cardiac myocytes, energy derives mainly from the oxidation of fatty acids (Stanley et al. 2005), and the motility of mitochondria is restricted.
Mitochondria in adult cardiac myocytes localize within three subcellular distributions: interfibrillar, subsarcolemmal and perinuclear (Makinde et al. 1998; Piquereau et al. 2012; Ong et al. 2013). Interfibrillar mitochondria are relatively uniform in size and shape. They align longitudinally between the myofibrils and are situated at places where Ca2+ release occurs. Their main function is to provide ATP for muscle contraction. Subsarcolemmal and perinuclear mitochondria are less well organized and have greater variation in their shape and size (Iglewski et al. 2010). These mitochondria are involved in the transport of metabolites and electrolytes through the sarcolemma, and perinuclear mitochondria probably have a role in nuclear transcription (Shimada et al. 1984; Ong & Hausenloy, 2010).
Although mitochondrial fusion and fission are most evident in neonatal cardiac myocytes due to the features described above (Chen et al. 2011), there is strong evidence that these processes are important in the adult cardiac tissue (Coleman et al. 1987; Delettre et al. 2001; Santel et al. 2003). For example, the proteins involved in fusion and fission are highly expressed in the adult heart (Delettre et al. 2001; Santel et al. 2003). Ultrastructural analysis of murine left ventricle subjected to physical training reveals the presence of giant mitochondria, thought to be the result of multiple fusion events (Coleman et al. 1987).
More evidence for the importance of mitochondrial dynamics in the heart comes from loss‐of‐function studies of proteins involved in the process. Papanicolaou et al. demonstrated that Mfn1 knockout (KO) mice harbour small and spherical mitochondria within cardiac myocytes, although cardiac function remained normal (Papanicolaou et al. 2011 b). In contrast to MFN1 deletion, Mfn2 KO mice harbour enlarged mitochondria, which protects cardiac myocytes from proapoptotic stimuli (Papanicolaou et al. 2011 a). Cardiac myocytes isolated from murine models of an inducible ablation of Mfn‐1/2 harbour fragmented mitochondria with abnormal cristae (Papanicolaou et al. 2011 a). These morphological changes were accompanied by alterations in mitochondrial respiration, mitophagy and mitochondrial biogenesis which ultimately culminated in cardiomyopathy (Chen et al. 2011; Papanicolaou et al. 2012).
Abnormalities in mitochondrial morphology were also observed in mice heterozygous for Opa1. In this model, large mitochondria with abnormal cristae were found. However, decreased expression of OPA1 did not alter mitochondrial respiration (Piquereau et al. 2012). Mitochondria within these cardiac myocytes also manifest increased ability to accumulate Ca2+ and are marked by a delayed opening of the mitochondrial permeability transition pore (mPTP), coupled with increased sensitivity to prolonged mechanical stress (Piquereau et al. 2012). Chen et al. also analysed cardiac myocytes from Opa1+/− mice and reported that mitochondria showed a pattern of abnormal cristae and disruption in mitochondrial organization (Chen et al. 2012). Alterations in functional parameters included increased production of ROS and a decrease in mitochondrial DNA (mtDNA) content, indicating that mitochondrial function was impaired.
Cardiac fibroblasts
Cardiac fibroblasts are the most abundant cell in the myocardium, accounting for 60–70% of the total population of cells. They are essential for the maintenance of the extracellular matrix (ECM) and hence, structural and mechanical properties of the heart (Porter & Turner, 2009). Cardiac fibroblast function is mediated by the differentiation of cardiac fibroblasts into myofibroblasts, cells with enhanced capacity for proliferation, migration and secretion (Porter & Turner, 2009). In addition, cardiac myofibroblasts are primarily reactive to mechanical stress, hormones, proinflammatory cytokines and vasoactive peptides (angiotensin II). In response, these cells synthesize and secrete ECM proteins, along with a variety of signalling molecules.
Despite all these relevant functions in the heart, only a single study has emerged exploring mitochondrial dynamics in cardiac fibroblasts, Samant et al. showed that SIRT3 binds directly to OPA1 and activates it by deacetylation (Samant et al. 2014). Adult Sirt3 KO cardiac fibroblasts manifest reduced mitochondrial fusion and loss of ΔΨm (Samant et al. 2014). Given the importance of mitochondrial dynamics in cardiac function and metabolism, additional research is warranted, focusing on establishing links between mitochondrial dynamics and the function of cardiac fibroblasts and myofibroblasts.
Vascular smooth muscle cells
The principal function of vascular smooth muscle cells (VSMCs) is the regulation of vascular tone and consequently blood pressure and blood flow. Unlike cardiac myocytes, these cells are highly plastic and undergo reversible changes in phenotype in response to environmental stimuli (Jay et al. 2008; Martin‐Garrido et al. 2013). Differentiated VSMCs have a contractile phenotype, which is characterized by little proliferation, minimal secretion of extracellular matrix, and expression of specific contractile proteins, such as smooth muscle myosin heavy chain, smooth muscle α‐actin and calponin (Owens, 1995). However, when a vessel is damaged, these cells transform into a synthetic phenotype in which the cells proliferate and migrate towards the injury site (Johnson et al. 2001). These events are accompanied by increased secretion of ECM components and expression of other proteins, such as vimentin and tropomyosin‐4 (Owens, 1995; Abouhamed et al. 2003).
VSMC proliferation can be induced by several factors, including platelet‐derived growth factor (PDGF), insulin‐like growth factor 1 (IGF‐1), angiotensin II and endothelin‐1 (Janakidevi et al. 1992; Pukac et al. 1998; Watanabe et al. 2014). Proliferation is inhibited by other molecules, such as transforming growth factor‐β (TGF‐β) and heparin (Björkerud, 1991; Lindner et al. 1992).
Recently, a relationship has been proposed between the VSMC proliferative phenotype and mitochondrial dynamics (Chalmers et al. 2012; Marsboom et al. 2012). For instance, pulmonary artery smooth muscle cells from hypertensive mice manifest hyperproliferation along with a fragmented mitochondrial phenotype (Marsboom et al. 2012). Additional studies show that PDGF promotes mitochondrial fragmentation and changes the metabolic profile of VSMCs, increasing fatty acid oxidation and decreasing glucose oxidation (Salabei & Hill, 2013). PDGF induces mitochondrial fragmentation reducing MFN2 levels (Salabei & Hill, 2013), without changing the levels of other proteins that regulate mitochondrial dynamics, such as DRP1, FIS1 and OPA1. Using a pharmacological inhibitor of DRP1 (mdivi‐1) to inhibit mitochondrial fission, PDGF stimulation of VSMC proliferation was prevented as well as changes in glucose and fatty acid oxidation, suggesting that mitochondrial fission is required for these processes (Chalmers et al. 2012; Salabei & Hill, 2013). Thus, mitochondrial fission is an important event in the development of the synthetic phenotype of VSMCs (Salabei & Hill, 2013).
Mitochondrial fission is also necessary for oxygen‐dependent constriction of the ductus arteriosus. Prolonged exposure of ductus arteriosus smooth muscle cells (DASMCs) to oxygen, significantly increased the levels of DRP1 and triggered mitochondrial fragmentation, thereby increasing oxygen consumption and oxidative metabolism (Hong et al. 2013). When DRP1 was inhibited, proliferation of DASMCs and closure of the ductus arteriosus were each prevented (Hong et al. 2013).
In summary, although research in this area is limited, there is some background information regarding the relationship between VSMC phenotypes and mitochondrial dynamics. This relationship is probably significant, given the established role of the VSMC synthetic phenotype in the development of diseases such as pulmonary hypertension and atherosclerosis (Lacolley et al. 2012; Marsboom et al. 2012).
Mitochondrial dynamics and cardiovascular disease
Cardiac hypertrophy and failure
Cardiac hypertrophy is a response triggered by a wide variety of stimuli, including haemodynamic overload, neurohormonal activation and ischaemia. Thought to be initially adaptive, this response involves structural, morphological and functional changes in cardiac myocytes, including increases in cell size, thereby provoking an overall growth in heart mass (Frey et al. 2004; Barry et al. 2008; Hill & Olson, 2008).
Hypertrophic growth begins as a compensatory process that normalizes wall stress and oxygen demand. However, prolonged exposure to disease‐related stimuli leads to pathological cardiac myocyte growth, cell death and heart failure (Burchfield et al. 2013). Throughout, mitochondrial metabolism remains essential for adequate myocardial pump function, because cardiac myocytes require vast amounts of energy in this state in order to maintain contractile performance, Ca2+ homeostasis and ion transport (Huss & Kelly, 2005).
In adult cardiac myocytes, mitochondria are tightly packed between myofibrils (Vendelin et al. 2005) and it has been reported that this subpopulation of mitochondria do not undergo mitochondrial dynamics events (Beraud et al. 2009). However, there is evidence from models of both cardiac hypertrophy (Fang et al. 2007) and heart failure (Chen et al. 2009) for robust mitochondrial dynamics‐related changes. Chen et al. described small and fragmented mitochondria in both human and rat models of heart failure, which were associated with decreased OPA1 levels (Chen et al. 2009). Other studies in neonatal rat cardiac myocytes exposed to phenylephrine to induce hypertrophy (Fang et al. 2007), as well as in vivo models of cardiac hypertrophy, also described decreases in Mfn2 mRNA levels (Fang et al. 2007).
Calcineurin is an essential regulator of cardiac hypertrophy and heart failure (Heineke & Molkentin, 2006) and participates in the regulation of mitochondrial fission by DRP1 dephosphorylation (Cereghetti et al. 2008). Wang et al. showed that both the A and B isoforms of the calcineurin catalytic subunit are direct targets of microRNA (miR)‐499, increasing DRP1 phosphorylation at residue Ser656, thereby reducing mitochondrial fission (Wang et al. 2011). Interestingly, miR‐499 transgenic mice manifested a decline in hypertrophic parameters assessed by heart/body weight ratio, cardiac myocyte cross‐sectional area, collagen content, heart chamber dimensions and cardiac function after ischaemia–reperfusion (I/R). Conversely, knockdown of endogenous miR‐499 aggravated maladaptive cardiac remodelling (Wang et al. 2011).
We recently reported that noradrenaline (norepinephrine) triggers mitochondrial fission in a calcineurin‐ and DRP1‐dependent manner (Pennanen et al. 2014). Adenovirus‐mediated expression of dominant‐negative DRP1 prevented mitochondrial fission and hypertrophic cardiac myocyte growth in response to noradrenaline. Furthermore, an adenovirus‐expressed antisense sequence to MFN2 was sufficient to increase mitochondrial fission and elicit a hypertrophic response in cultured cardiac myocytes (Pennanen et al. 2014) (Fig. 2). Consistent with these results, Papanicolaou et al. reported that Mfn2‐deficient mice display modest cardiac hypertrophy accompanied by a slight functional deterioration (Papanicolaou et al. 2011 a). In a more recent work, wild‐type mice were subjected to ascending aortic banding to generate heart failure and then treated with the DRP1 inhibitor mdivi‐1. Left ventricular dysfunction was ameliorated by treatment with mdivi‐1, and these effects were associated with decreased expression of the autophagy markers LC3 and p62 (Givvimani et al. 2012).
Figure 2. Mitochondrial dynamics and cardiovascular diseases .
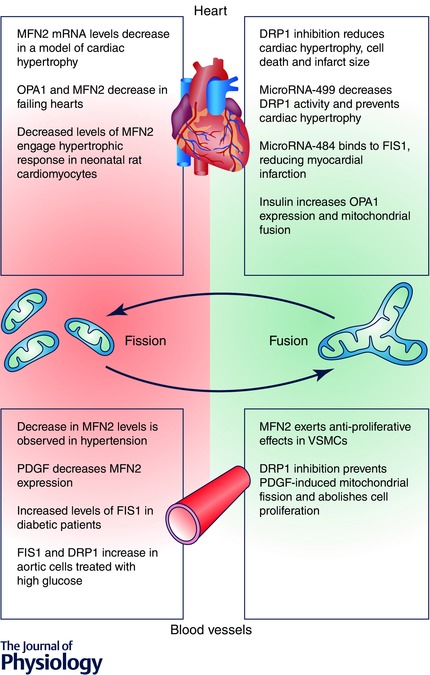
Summary of important molecular events in cardiovascular diseases associated with mitochondrial fission (red zone) and therapeutic interventions or molecular processes related to mitochondrial fusion and linked with an improved cardiovascular function (green zone).
The findings described in this review suggest a predominant role for mitochondrial fission in pathological cardiac remodelling. However, in a DRP1‐mutant mouse model that alters protein interactions and prevents mitochondrial fission events, elongated mitochondrial networks associated with reduced levels of mitochondrial enzyme complexes, cardiac ATP depletion and heart failure were observed (Ashrafian et al. 2010). One possible explanation is that disruption of mitochondrial fission prevents appropriate mitochondrial quality control, leading to accumulation of damaged and dysfunctional mitochondria. This idea, on which we elaborate later, is consistent with observations in a Parkin knockout Drosophila model, in which disruption of mitophagy triggered accumulation of enlarged mitochondria in heart tubes and dilated cardiomyopathy (Bhandari et al. 2014).
Diabetic cardiomyopathy
Cardiovascular complications are the primary cause of morbidity and mortality in patients with type 2 diabetes mellitus (T2DM) and mitochondrial dysfunction has been implicated in the pathogenesis and almost all the complications observed in T2DM (Bugger & Abel, 2014). An important contributory factor linked to the increased risk of cardiovascular disease is hyperglycaemia‐induced mitochondrial oxidative stress which can cause cellular injury and dysfunction (Green et al. 2004). Consistently, in vitro data from H9c2 cells suggest that hyperglycaemia induces mitochondrial fragmentation (Yu et al. 2006). In this report, H9c2 cells (a myoblast cell line, derived from embryonic rat heart) exposed to sustained hyperglycaemia manifested mitochondrial fragmentation and mitochondrial ROS accumulation resulting in cell death by apoptosis (Yu et al. 2006). Importantly, these events were prevented by over‐expression of DRP1K38A, a fission‐inactive mutant of DRP1, suggesting that this process was DRP1 dependent (Yu et al. 2006).
Moreover, in a recent article, Watanabe et al. presented evidence from H9c2 cells for a functional interaction between DRP1 and ROS promoting mitochondrial dysfunction and inhibition of insulin signalling. This effect was partially overcome when cells were treated with the superoxide dismutase (SOD) mimetic TMPyP (Watanabe et al. 2014). In a different model, Makino et al. reported that coronary endothelial cells from murine diabetic hearts displayed mitochondrial fragmentation associated with reduced OPA1 levels and increased levels of DRP1. In this work, although mitochondrial fragmentation was abolished by 4 weeks of pre‐treatment with antioxidants, protein levels of OPA1 and DRP1 were not restored (Makino et al. 2010). In aggregate, these data suggest a role for oxidative stress as a mediator of mitochondrial fragmentation, a notion that has been confirmed by the fact that oxidative stress induces additional mitochondrial fragmentation in coronary endothelial cells (Makino et al. 2010).
Following this same line of investigation, exposure of neonatal cardiac myocytes to high levels of glucose (35 mm) increased mitochondrial fragmentation and reduced mitochondrial membrane potential (ΔΨm) and electron transport chain activity. High glucose treatment decreased mitochondrial OPA1 and MFN1 protein levels whereas FIS1 levels were significantly increased. Overexpression of OPA1 was able to reverse some of the negative effects of high glucose on cardiac myocytes (Makino et al. 2011). Atrial tissue sections from T2DM patients show an increase in mitochondrial fragmentation and a reduction in MFN1 protein levels (Montaigne et al. 2014).
Recently, our group demonstrated a link between mitochondrial dynamics and insulin's impact on cardiac myocyte metabolism (Parra et al. 2014). Insulin treatment of either cardiac myocytes or skeletal muscle cells increased OPA1 levels and triggered mitochondrial fusion, enhancing ATP production and the rate of oxygen consumption (Parra et al. 2014) (Fig. 2). These findings are consistent with those reported by Quirós et al. (2012) who showed that alterations in the proteolytic processing of OPA1 in Oma1 knockout mice leads to insulin resistance, impaired glucose homeostasis, and altered thermogenesis. Oma1–/– mice develop metabolic defects similar to those seen with high‐fat feeding, highlighting the importance of OPA1 and the relative stoichiometry of its l and s isoforms for maintaining mitochondrial function (Griparic et al. 2007; McBride & Soubannier, 2010). In our experimental model, the effect of insulin on mitochondrial metabolism was impaired in cells deficient for OPA1 and MFN2 (Parra et al. 2014). These findings are also consistent with the clinical observation of impaired mitochondrial function and diminished levels of OPA1 and MFN2 in individuals with diabetes (Kelley et al. 2002; Lowell & Shulman, 2005; Zorzano et al. 2009).
Mitochondrial dynamics directly influence pancreatic function as well. In ob/ob mice, OPA1 levels decrease in pancreatic islet cells before the onset of diabetes (Keller et al. 2008). Silencing Opa1 in pancreatic β cells using a Cre‐loxP system yields similar results (Zhang et al. 2011). β cells lacking OPA1 maintained normal mtDNA copy number, but electron transport chain complex IV levels and activity were significantly reduced, leading to impaired glucose stimulation of ATP production and insulin secretion.
Whether mitochondrial fission contributes to the cardiac dysfunction associated with diabetic cardiomyopathy is unknown. Similarly, it is not known whether mitochondrial fusion acts as a direct mediator of mitochondrial metabolism and cardiac function. However, recent evidence suggests that mitochondrial fragmentation is a ‘starting point’ of numerous events participating in cardiac metabolic disease (Montaigne et al. 2014). Contractile dysfunction is observed in T2DM patients but not in ‘metabolically healthy’ obese patients at early stages of the insulin‐resistant state (Montaigne et al. 2014). Based on this, we speculate that declines in ventricular performance occurring in the transition from obesity to diabetes mellitus are caused, at least in part, by deterioration of cardiac myocyte mitochondrial function. Further studies are required to test whether modulation of mitochondrial dynamics could emerge as a means to enhance mitochondrial function and ultimately cardiac performance.
Myocardial infarction and ischaemia/reperfusion
Ischaemic heart disease is the leading cause of death and disability worldwide (Writing Group Members et al. 2006). Its clinical manifestations derive from the detrimental effects of acute I/R on the myocardium. During myocardial infarction, cardiac myocyte metabolism is severely perturbed owing to deprivation of oxygen and nutrients coupled with intracellular acidification (Thygesen et al. 2008). In this context, susceptibility to, and recovery from, I/R injury are critically dependent on mitochondrial function. Mitochondrial dysfunction in response to acute I/R and the subsequent opening of the mPTP during reperfusion are critical determinants of cell death (Davidson et al. 2014). Therefore, preservation of mitochondrial health and the prevention of mPTP opening during acute I/R are two important therapeutic targets for cardioprotection.
Whether changes in mitochondrial dynamics occur in the heart in response to I/R was first addressed by Brady et al. who described extensive mitochondrial fission in HL‐1 cells (a murine atrial myocyte‐derived cell line) in response to sustained ischaemia, changes which persisted during reperfusion and were inhibited by the p38MAPK inhibitor SB203580 (Brady et al. 2006). Furthermore, Ong et al. reported that inhibition of mitochondrial fission by mdivi‐1 protects cardiac myocytes from I/R injury in both in vitro and in vivo models (Ong et al. 2010).
Inhibition of DRP1 with mdivi‐1 increased the proportion of adult cardiac myocytes with elongated mitochondria and protected them against simulated I/R by inhibiting mPTP opening and resulted in decreased infarct size (Ong et al. 2010). In this same context, we recently reported that DRP1 inhibition reduces I/R injury through declines in cardiomyocyte oxygen dependence while increasing proton leak‐associated oxygen consumption (Zepeda et al. 2014). Moreover, Sharp et al. reported that DRP1 inhibition has therapeutic benefits even when administered after ischaemia (Sharp et al. 2014). Inhibition of DRP1 dephosphorylation at Ser637 in a Langendorff model conferred cardioprotection by preserving mitochondrial morphology and cytosolic calcium levels, and diminishing cell death (Sharp et al. 2014). Using a different strategy, Din et al. suppressed DRP1 translocation to mitochondria (and thereby mitochondrial fission) in simulated I/R through the over‐expression of Pim1, implicating this kinase as another potential mechanism of cardioprotection (Din et al. 2013). Finally, in a more recent study, a particular peptide inhibitor of DRP1, P110, was employed, revealing that inhibition of mitochondrial fission at reperfusion reduces myocardial infarct size and prevents adverse left ventricular remodelling post‐myocardial infarction in the adult rat heart (Disatnik et al. 2013).
Mechanisms described to this point suggest that cytosolic Ca2+ overload and ROS may be contributory factors to mitochondrial fragmentation observed during I/R. In this way, mitochondrial fragmentation interferes with cellular respiration and can result in ROS overproduction. Consistently, Plotnikov et al. (2008) showed that pre‐treatment with an antioxidant could prevent ischaemia‐induced mitochondrial fission (Plotnikov et al. 2008). Interestingly, in this study, insulin was also reported to prevent mitochondrial fragmentation, although the mechanism was not investigated. These findings agree with our recent report describing cardiac myocyte mitochondrial fusion triggered by insulin (Parra et al. 2014).
Other works have uncovered beneficial effects of inhibiting mitochondrial fission using cardiac microRNAs (Wang et al. 2011, 2012). In the first study, Wang et al. reported that modulation of miR‐499 levels affects apoptosis and the extent of myocardial infarction and cardiac dysfunction induced by I/R by targeting calcineurin‐mediated DRP1 activation (Wang et al. 2011). However, two other groups have expressed miR‐499 in mouse hearts and found that, rather than being protective, the intervention predisposed to heart failure (Shieh et al. 2011; Dorn et al. 2012). Also, calcineurin A has not yet been fully confirmed as a valid in vitro target of miR‐499 in the myocardium (Dorn et al. 2012). Thus, the conclusion about miR‐499 and its potentially cardioprotective properties remains unclear.
More recently, a second paper also associated a miR with mitochondrial dynamics and heart protection during I/R. In this report, Wang et al. showed that miR‐484, a microRNA which binds to the amino acid coding region of FIS1 transcripts can repress FIS1 expression and thereby inhibit mitochondrial fission (Wang et al. 2012). These investigators also reported that FoxO3a transactivates miR‐484 expression. FoxO3a transgenic or knockout mice exhibit, respectively, a high or low level of miR‐484 and a reduced or enhanced mitochondrial fission, apoptosis and myocardial infarction (Wang et al. 2012).
Reduced levels of OPA1 have been reported in samples from human hearts with ischaemic cardiomyopathy (Chen et al. 2009). However, the role of the mitochondrial fusion proteins (MFN1, MFN2 and OPA1) as targets of cardioprotection remains in flux, as unexpected and contradictory findings have emerged. In HL‐1 cells, Ong et al. reported that over‐expression of MFN1 or MFN2 prevented the opening of the mPTP and reduced cell death after I/R (Ong et al. 2010). However, Papanicolaou et al. in a parallel study showed that small interfering RNA (siRNA) knockdown of MFN2 delayed mPTP opening, thus rendering cardiac myocytes more susceptible to ROS (Papanicolaou et al. 2011 a). Similarly, partial genetic ablation of Opa1 has been shown to delay mPTP opening, although the effect on acute I/R has not been studied (Piquereau et al. 2012). In summary, the interplay between mitochondrial fusion proteins and I/R is complex and requires additional in‐depth analysis.
Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that predisposes to I/R injury to the heart, brain and other tissues and is a significant risk factor for premature death (Libby, 2002). In this complex disorder, arterial endothelium expresses elevated levels of adhesion molecules, which promote monocyte infiltration, differentiation and transformation into highly active lipid‐loaded foam cells, accompanied by migration of VSMCs (that also have the potential to become foam cells) toward the intima (Libby, 2002). Mitochondrial generation of ROS is the most thoroughly documented relationship between mitochondria and vascular disease (Cho et al. 2013; Wang & Tabas, 2014). Conversely, ROS molecules also target this organelle, where mitochondrial DNA is probably the most sensitive cellular target (Anan et al. 1995; Wallace, 1999). Emerging evidence indicates that mitochondrial DNA damage can directly promote atherosclerosis. Ballinger et al., working with human aortic specimens and a murine model of early atherogenesis (apolipoprotein E null mice), found that mitochondrial DNA damage correlated with the extent of atherosclerosis in both models (Ballinger et al. 2002). In the case of apolipoprotein E null mice, it was associated with deficiency of manganese SOD, a mitochondrial antioxidant enzyme, with early increases in mitochondrial DNA damage, and a phenotype of accelerated atherogenesis (Ballinger et al. 2002).
Increased mitochondrial ROS production also causes endothelial dysfunction, along with proliferation and apoptosis of VSMCs and macrophages, with ensuing atherosclerosis lesion progression and possible plaque rupture (Madamanchi & Runge, 2007). Directly related to this, Shenouda et al. reported mitochondrial fragmentation and increased levels of FIS1 protein in venous endothelial cells from patients with T2DM, as well as increased abundance of FIS1 and DRP1 proteins in cultured human aortic endothelial cells incubated with high glucose medium (Shenouda et al. 2011). Altered mitochondrial dynamics were associated with increased mitochondrial ROS production, and silencing FIS1 or DRP1 expression with siRNA prevented high glucose‐induced alterations in mitochondrial networks and ROS production (Shenouda et al. 2011).
In atherosclerosis, VSMCs develop a highly proliferative and synthetic phenotype in a process triggered by PDGF (Yu et al. 2001; Perez et al. 2010). Exposure of VSMCs to PDGF resulted in mitochondrial fragmentation and decreases in MFN2 protein levels (Salabei & Hill, 2013). Treatment of VSMCs with mdivi‐1 inhibited PDGF‐induced mitochondrial fragmentation and abolished increases in cell proliferation (Salabei & Hill, 2013). Collectively, these data demonstrate the importance of mitochondrial fission machinery in the pathogenesis of vascular disease.
Mitophagy in cardiovascular cells and its role in cardiovascular diseases
In response to various environmental stresses, cellular ATP synthesis can be disrupted. Mitochondria then become major producers of ROS, which causes cellular damage and can initiate the apoptotic pathway (Kubli & Gustafsson, 2012). However, cells have developed a defence mechanism against ROS production by aberrant mitochondria (Levine & Kroemer, 2012). Fundamental among these is the selective sequestration and degradation of dysfunctional mitochondria before they cause damage or trigger cell death, through a process called mitochondrial autophagy or mitophagy (Kubli & Gustafsson, 2012).
Macroautophagy, hereafter referred to as autophagy, is a highly conserved mechanism for the degradation of cytoplasmic components, including damaged organelles, toxic protein aggregates and intracellular pathogens (Mizushima et al. 2008; Gatica et al. 2015). Basal autophagy plays a key role in eukaryotic cells, degrading long half‐life macromolecules and large supramolecular structures, including organelles such as mitochondria, peroxisomes and endoplasmic reticulum (Levine & Kroemer, 2012). Autophagy initiates with formation of the autophagosome, a double‐membrane intracellular structure of reticular origin that engulfs cytoplasmic contents and ultimately fuses with lysosomes for cargo degradation. Materials degraded within these newly formed autolysosomes are recruited to anabolic reactions to maintain energy levels and provide macromolecules for the synthesis of higher‐order structures (nucleic acids, proteins or organelles), thereby sustaining cell metabolism, homeostasis, and survival (Mizushima, 2007; Gatica et al. 2015). Initial phagophore formation requires the assembly of a complex consisting of BECLIN1, vacuolar protein sorting (VPS) 34 and VPS15 (Kang et al. 2011). Next, the expansion of the membrane is mediated by two ubiquitin‐like conjugation systems, microtubule‐associated protein 1 light chain 3 (LC3) and autophagy protein (ATG) 12‐ATG5, that promote assembly of the ATG16L complex and the conjugation of LC3 with phosphatidylethanolamine (Mizushima et al. 1999; Kabeya et al. 2004). The phagophore expands until its borders fuse around its target(s), forming a structure with double membrane, called the autophagosome. Finally, the autophagosome fuses with a lysosome and the contents are degraded by lysosomal enzymes (Mizushima et al. 2008).
The same stimuli that trigger mitochondrial‐dependent cell death can trigger mitophagy (Kubli & Gustafsson, 2012). The balance between these two highly regulated processes will then determine whether a cell lives or dies. Understanding the role of mitochondria and mitophagy in various cardiovascular pathologies is currently an area of great interest. Ensuring the proper removal of dysfunctional mitochondria is critical as mitochondrial damage has been linked to diverse cardiovascular pathologies (Lavandero et al. 2015).
PINK1/Parkin mitophagy pathway
Mitophagy in mammalian cells is mainly regulated by the cytosolic E3 ubiquitin ligase Parkin (Kitada et al. 1998) and the outer mitochondrial membrane kinase PINK1 (Valente et al. 2004), two genes altered in Parkinson's disease. Upon mitochondrial impairment or loss of mitochondrial potential (for example using a mitochondrial uncoupler, such as CCCP), Parkin translocates from the cytosol (Fig. 3) to damaged or uncoupled mitochondria, promoting ubiquitination of several mitochondrial outer membrane proteins, such as MFN1, MFN2, VDAC, MIRO1, etc. (Chan et al. 2011). Parkin recruitment to damaged mitochondria is selective and requires the participation of PINK1 (Kawajiri et al. 2010; Narendra et al. 2010; Vives‐Bauza et al. 2010). In healthy mitochondria, PINK1 is rapidly degraded by proteolysis and maintained at low levels. In the setting of mitochondrial damage, however, PINK1 cleavage is inhibited, allowing PINK1 accumulation selectively within damaged organelles (Matsuda et al. 2010). Thus, Parkin recruitment is specific to mitochondria that have high levels of accumulated PINK1.
Figure 3. Mitophagy .
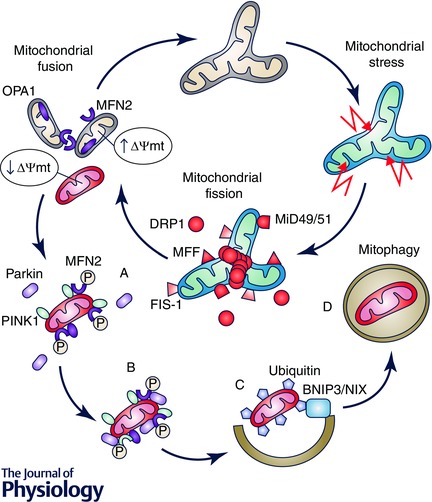
The process of selective targeting of mitochondria by autophagy (mitophagy) is coordinated with mitochondrial fusion and fission to ensure proper mitochondrial quality control. Mitochondrial fission isolates damaged mitochondria (usually with low membrane potential) to initiate the process of mitophagy. Under conditions of low mitochondrial membrane potential, PINK1 kinase accumulates on a mitochondrion and phosphorylates MFN2 (A). Parkin E3 ligase binds phosphorylated MFN2 and localizes to the mitochondrion (B). To catalyse the ubiquitination of several proteins on the mitochondrial surface, various adapter proteins, such as BNIP3 and NIX, are associated with the autophagy machinery (C), ultimately facilitating elimination of mitochondrial content (D).
PINK1 phosphorylates MFN2 and promotes Parkin translocation to mitochondria (Chen & Dorn, 2013) (Fig. 3). In mouse cardiac myocytes, MFN2 removal prevented depolarization‐induced recruitment of Parkin to damaged mitochondria, triggering progressive cardiomyopathy (Chen & Dorn, 2013). This phenomenon highlights the relevance of the PINK1/Parkin pathway in the quality control of cardiac mitochondria. Indeed, PINK1 is essential for healthy heart function. Pink1−/− mice develop early left ventricular dysfunction and pathological cardiac hypertrophy (Billia et al. 2011). This phenotype is accompanied by increased oxidative stress and mitochondrial impairment in cardiac myocytes. Supporting these data, PINK1 protein levels are also notably reduced in advanced human heart failure (Billia et al. 2011).
On the contrary, Parkin‐deficient mice manifest normal myocardial function (Kubli et al. 2013). Parkin−/ − hearts harbour altered mitochondrial networks with small mitochondria, although mitochondrial function is unaffected. Despite this, Parkin−/− mice are more susceptible to myocardial infarction damage induced by coronary artery ligation (Kubli et al. 2013). Collectively, these data suggest a non‐redundant function for PINK1 in mammalian cardiac tissue, in contrast to Parkin. This idea is supported by an interesting study from the Dorn group (Bhandari et al. 2014). In Parkin knockout mouse heart, there is a compensatory up‐regulation of several Parkin‐related E3 ubiquitin ligases of the RING families (Bhandari et al. 2014). Studying heart tubes of Parkin‐KO Drosophila mutants (because, in contrast with mice, Drosophila lacks Parkin‐redundant genes), Dorn et al. observed enlarged mitochondria accompanied by dilated cardiomyopathy. Parkin‐deficient mitochondria are depolarized and generate enhanced ROS. This abnormal phenotype was rescued by suppressing mitochondrial fusion (by silencing MARF, the MFN2 Drosophila orthologue), normalizing mitochondrial morphology and function and preventing the cardiomyopathic phenotype (Bhandari et al. 2014). Additionally, a new study from the same group showed that Parkin mRNA and protein are present at very low levels in normal mouse hearts, but are upregulated after cardiac myocyte‐specific deletion of the Drp1 gene in adult mice ). Thus, Drp1 deficiency appears to trigger Parkin‐dependent over‐activation of mitophagy leading to a severe myopathic phenotype. The authors propose that DRP1 helps in maintaining mitochondrial quality control by promoting mitochondrial fission to segregate dysfunctional mitochondria that can then be targeted by mitophagy (Song et al. 2015). These data highlight the central role of mitochondrial dynamics in cardiac mitochondria quality control, ensuring proper elimination of damaged and dysfunctional organelles (Cao et al. 2015). In support of this notion, Sadoshima's group recently highlighted the participation of DRP1 in mitophagy. DRP1 down‐regulation induces accumulation of damaged mitochondria due to suppressed mitochondrial autophagy (Ikeda et al. 2015). Also, Drp1‐CKO (cardiac myocyte‐specific KO) mice develop mitochondrial and cardiac dysfunction and are prone to I/R injury (Ikeda et al. 2015; Song et al. 2015).
NIX and BNIP3 during cardiac mitochondrial clearance
In mammalian cells, NIX/BNIP3 and mitochondrial pro‐apoptotic BH3‐only domain protein (BNIP3) are involved in the selective mitochondrial clearance. The canonical function of BNIP3 and NIX in cardiac myocytes is to sense myocardial damage and initiate the apoptotic programme through activation of the pro‐apoptotic proteins Bax and Bak (Youle & Strasser, 2008). Although BNIP3 and NIX perform similar functions regulating cardiac myocyte death, both are controlled by stimuli of a different nature. BNIP3 is up‐regulated in myocardium during hypoxia (Regula, 2002), while NIX appears to be up‐regulated in pathological cardiac hypertrophy (Gálvez et al. 2006).
Additionally, Dorn et al. (2010) showed that both BNIP3 and NIX are mitophagy regulators in adult heart. Mitochondrial distribution in Bnip3/Nix DKO hearts was markedly perturbed, and mitochondrial morphology manifested an atypical phenotype characterized by loss and dilatation of mitochondrial cristae. These results showed that BNIP3 and NIX play constitutive roles in the elimination of damaged cardiac mitochondria. However, under sustained stress conditions, such as hypoxia or pathological hypertrophy, BNIP3 and NIX trigger cardiac myocyte death.
Concluding remarks
Mitochondrial dynamics play a fundamental role in homeostasis of the cardiovascular system. This process is associated with important cellular functions such as metabolism and quality control. Specifically, the balance between mitochondrial fusion and fission is linked with mitochondria energy production. Given the never‐ceasing mechanical demands on the cardiovascular system, provision of energy must be continuous, especially in the heart. Metabolic remodelling occurs in a diversity of cardiovascular diseases. Changes in mitochondrial dynamics can be directly linked to a decline in energy efficiency, as increased mitochondrial fission is associated with a decrease in ATP production. For example, mitochondrial dynamics are frequently altered in examples of either metabolic overload, such as in diabetes, or nutrient deprivation, such as in periods of prolonged ischaemia (Fig. 4). These changes in mitochondrial structure and function lead to an imbalance in energy production. It will be important for future research to fully define the mechanisms that trigger and carry out adaptive and pathological changes in mitochondrial dynamics (protein levels and function).
Figure 4. Mitochondrial dynamics and cardiovascular remodelling .
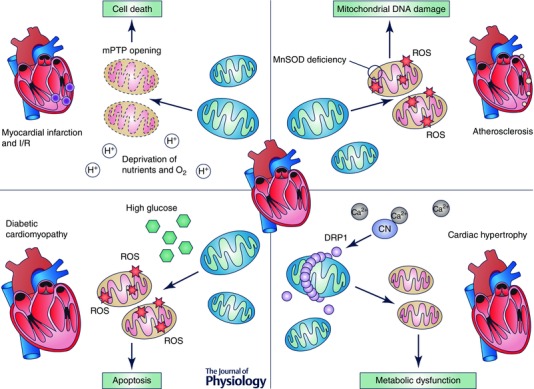
Alterations in mitochondrial dynamics have a significant role in cardiovascular remodelling. Cardiac hypertrophy, diabetic cardiomyopathy, myocardial infarction and atherosclerosis share common pathological mechanisms and features related to fragmentation of the mitochondrial network. Mitochondrial morphological alterations finally lead to metabolic dysfunction, damage of mitochondrial components (mtDNA) and/or cell death.
Of equal importance is the roles of mitochondrial dynamics and mitophagy in quality control and repair. It is of particular interest to investigate the participation of mitophagy in cardiovascular disease, as current evidence indicates that it can be both an essential protective mechanism when repairing damage and a pathological one when it progresses unrestrained. Thus, if mitophagy is to emerge as a useful therapeutic target, it will be critical to determine where and when manipulating mitophagy activity can prevent, counteract or reverse a specific cardiovascular disease.
In summary, we highlight here evidence for novel interventions related to mitochondrial dynamics with relevance to certain cardiovascular diseases. We speculate that further research into mitochondrial dynamics will uncover novel approaches to therapeutic manipulation of cardiac myocyte energetics, mitochondrial function and quality control.
Additional information
Competing interests
None declared.
Funding
This work was supported by grants from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT), Chile (FONDECYT 1120212 to S.L. and 3130749 to C.P.; FONDAP 15130011 to S.L., Becas Chile Postdoctoral fellowship to V.P.), NIH (HL‐120732 and HL‐100401 to J.A.H., HL097768 to B.A.R.), AHA (14SFRN20740000 to J.A.H.; Postdoctoral fellowship to V.P.), the Cancer Prevention and Research Institute of Texas (CPRIT) (RP110486P3 to J.A.H.) and the Leducq Foundation (11CVD04 to J.A.H.). C.V.‐T. and I.G.‐C. are both recipients of a PhD fellowship from CONICYT, Chile.
Biographies
Joseph A. Hill is Professor in the Departments of Internal Medicine (Division of Cardiology) and Molecular Biology at UT Southwestern Medical Centre, Dallas. He is Chief of UT Southwestern Medical Centre's Division of Cardiology and Director of the Harry S. Moss Heart Centre. Dr Hill's research is focused on molecular mechanisms involved in cardiac hypertrophy, heart failure and diabetic cardiomyopathy.

Beverly A. Rothermel is Associate Professor in the Departments of Internal Medicine (Division of Cardiology) and Molecular Biology at UT Southwestern Medical Centre, Dallas. Her research centres on calcium‐regulated signal transduction pathways with a focus on the protein phosphatase calcineurin.
Sergio Lavandero is Professor at the University of Chile and Adjunct Professor in Internal Medicine (Division of Cardiology) at UT Southwestern Medical Centre, Dallas. Currently, he is Director and PI of the Advanced Centre for Chronic Diseases. He has a long‐standing interest in the molecular mechanisms involved in the genesis and progression of cardiovascular and metabolic diseases.
References
- Abouhamed M, Reichenberg S, Robenek H & Plenz G (2003). Tropomyosin 4 expression is enhanced in dedifferentiating smooth muscle cells in vitro and during atherogenesis. Eur J Cell Biol 82, 473–482. [DOI] [PubMed] [Google Scholar]
- Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, Osame M & Tanaka H (1995). Cardiac involvement in mitochondrial diseases: A study on 17 patients with documented mitochondrial DNA defects. Circulation 91, 955–961. [DOI] [PubMed] [Google Scholar]
- Ashrafian H , Docherty L, Leo V, Towlson C, Neilan M, Steeples V, Lygate CA, Hough T, Townsend S, Williams D, Wells S, Norris D, Glyn‐Jones S, Land J, Barbaric I, Lalanne Z, Denny P, Szumska D, Bhattacharya S, Griffin JL, Hargreaves I, Fernandez‐Fuentes N, Cheeseman M, Watkins H & Dear TN (2010). A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet 6, e1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger SW, Patterson C, Knight‐Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K & Runge MS (2002). Mitochondrial integrity and function in atherogenesis. Circulation 106, 544–549. [DOI] [PubMed] [Google Scholar]
- Barry SP, Davidson SM & Townsend PA (2008). Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol 40, 2023–2039. [DOI] [PubMed] [Google Scholar]
- Beraud N, Pelloux S, Usson Y, Kuznetsov A, Ronot X, Tourneur Y & Saks V (2009). Mitochondrial dynamics in heart cells: Very low amplitude high frequency fluctuations in adult cardiomyocytes and flow motion in non beating HL‐1 cells. J Bioenerg Biomembr 41, 195–214. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Song M, Chen Y, Burelle Y & Dorn GW (2014). Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res 114, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billia F, Hauck L & Konecny F (2011). PTEN‐inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci USA 108, 9572–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkerud S (1991). Effects of transforming growth factor‐β1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb Vasc Biol 11, 892–902. [PubMed] [Google Scholar]
- Bossy‐Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R & Lipton SA (2003). Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol 15, 706–716. [DOI] [PubMed] [Google Scholar]
- Brady NR, Hamacher‐Brady A & Gottlieb RA (2006). Proapoptotic BCL‐2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL‐1 cells and GFP biosensors. Biochim Biophys Acta 1757, 667–678. [DOI] [PubMed] [Google Scholar]
- Bugger H & Abel ED (2014). Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57, 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield JS, Xie M & Hill JA (2013). Pathological ventricular remodeling: mechanisms: Part 1 of 2. Circulation 128, 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DJ, Lavandero S & Hill JA (2015). Parkin gone wild: Unbridled ubiquitination. Circ Res 117, 311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, de Brito OM, Chang CR, Blackstone C, Bernardi P & Scorrano L (2008). Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105, 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers S, Saunter C, Wilson C, Coats P, Girkin JM & McCarron JG (2012). Mitochondrial motility and vascular smooth muscle proliferation. Arterioscler Thromb Vasc Biol 32, 3000–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC (2006). Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22, 79–99. [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S & Chan DC (2011). Broad activation of the ubiquitin‐proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20, 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C‐R & Blackstone C (2007). Cyclic AMP‐dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282, 21583–21587. [DOI] [PubMed] [Google Scholar]
- Chen H & Chan DC (2005). Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet 14, R283–R289. [DOI] [PubMed] [Google Scholar]
- Chen L, Gong Q, Stice JP & Knowlton AA (2009). Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu T, Tran A, Lu X, Tomilov AA, Davies V, Cortopassi G, Chiamvimonvat N, Bers DM, Votruba M & Knowlton AA (2012). OPA1 mutation and late‐onset cardiomyopathy: Mitochondrial dysfunction and mtDNA instability. J Am Heart Assoc 1, e003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y & Dorn GW (2013). PINK1‐phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liu Y & Dorn GW (2011). Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109, 1327–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y‐E, Basu A, Dai A, Heldak M & Makino A (2013). Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol 305, C1033–C1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B & Scorrano L (2004). OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101, 15927–15932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R, Silbermann M, Gershon D & Reznick A (1987). Giant mitochondria in the myocardium of aging and endurance‐trained mice. Gerontology 33, 34–39. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Lopaschuk GD, Spedding M & Beart PM (2014). Mitochondrial pharmacology: energy, injury and beyond. Br J Pharmacol 171, 1795–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Griffoin J‐M, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P & Hamel C (2001). Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet 109, 584–591. [DOI] [PubMed] [Google Scholar]
- Din S, Mason M, Völkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, Konstandin MH & Sussman MA (2013). Pim‐1 preserves mitochondrial morphology by inhibiting dynamin‐related protein 1 translocation. Proc Natl Acad Sci USA 110, 5969–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disatnik M, Ferreira JCB, Campos JC, Gomes KS, Dourado PMM, Qi X & Mochly‐Rosen D (2013). Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long‐term cardiac dysfunction. J Am Heart Assoc 2, e000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW 2nd (2010). Mitochondrial pruning by Nix and BNip3: an essential function for cardiac‐expressed death factors. J Cardiovasc Transl Res 3, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn GW 2nd, Matkovich SJ, Eschenbacher WH & Zhang Y (2012). A human 3′ miR‐499 mutation alters cardiac mRNA targeting and function. Circ Res 110, 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Moore X‐L, Gao X‐M, Dart AM, Lim YL & Du X‐J (2007). Down‐regulation of mitofusin‐2 expression in cardiac hypertrophy in vitro and in vivo. Life Sci 80, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Fischer F, Hamann A & Osiewacz HD (2012). Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 37, 284–292. [DOI] [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN & Hill JA (2004). Hypertrophy of the heart: A new therapeutic target? Circulation 109, 1580–1589. [DOI] [PubMed] [Google Scholar]
- Gálvez AS, Brunskill EW, Marreez Y, Benner BJ, Regula KM, Kirschenbaum LA & Dorn GW 2nd (2006). Distinct pathways regulate proapoptotic Nix and BNip3 in cardiac stress. J Biol Chem 281, 1442–1448. [DOI] [PubMed] [Google Scholar]
- Gandre‐Babbe S & van der Bliek AM (2008). The novel tail‐anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19, 2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatica D, Chiong M, Lavandero S & Klionsky DJ (2015). Molecular mechanisms of autophagy in the cardiovascular system. Circ Res 116, 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N & Tyagi SC (2012). Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One 7, e32388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K, Brand MD & Murphy MP (2004). Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53, S110–S118. [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T & van der Bliek AM (2007). Regulation of the mitochondrial dynamin‐like protein Opa1 by proteolytic cleavage. J Cell Biol 178, 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AR, Burke N, Dongworth RK & Hausenloy DJ (2014). Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol 171, 1890–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AR & Hausenloy DJ (2012). The shape of things to come: mitochondrial fusion and fission in the adult heart. Cardiovasc Res 94, 391–392. [DOI] [PubMed] [Google Scholar]
- Han X‐J, Lu Y‐F, Li S‐A, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H & Matsushita M (2008). CaM kinase Iα‐induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 182, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre‐Babbe S & van der Bliek AM (2009). Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187, 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J & Molkentin JD (2006). Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7, 589–600. [DOI] [PubMed] [Google Scholar]
- Hill JA & Olson EN (2008). Cardiac plasticity. N Engl J Med 358, 1370–1380. [DOI] [PubMed] [Google Scholar]
- Hong Z, Kutty S, Toth PT, Marsboom G, Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, Trivedi K, Weir EK & Archer SL (2013). Role of dynamin‐related protein 1 (Drp1)‐mediated mitochondrial fission in oxygen sensing and constriction of the ductus arteriosus. Circ Res 112, 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss JM & Kelly DP (2005). Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski M, Hill J, Lavandero S & Rothermel B (2010). Mitochondrial fission and autophagy in the normal and diseased heart. Curr Hypertens Rep 12, 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M & Sadoshima J (2015). Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116, 264–278. [DOI] [PubMed] [Google Scholar]
- Janakidevi K, Fisher MA, Del Vecchio PJ, Tiruppathi C, Figge J & Malik AB (1992). Endothelin‐1 stimulates DNA synthesis and proliferation of pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 263, C1295–C1301. [DOI] [PubMed] [Google Scholar]
- Jay DB, Papaharalambus CA, Seidel‐Rogol B, Dikalova AE, Lassègue B & Griendling KK (2008). Nox5 mediates PDGF‐induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, van Eys GJ, Angelini GD & George SJ (2001). Injury induces dedifferentiation of smooth muscle cells and increased matrix‐degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol 21, 1146–1151. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani‐Okamoto S, Ohsumi Y & Yoshimori T (2004). LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form‐II formation. J Cell Sci 117, 2805–2812. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT & Tang D (2011). The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri S, Saiki S, Sato S, Sato F, Hatano T, Eguchi H & Hattori N (2010). PINK1 is recruited to mitochondria with parkin and associates with LC3 in mitophagy. FEBS Lett 584, 1073–1079. [DOI] [PubMed] [Google Scholar]
- Keller MP, Choi Y, Wang P, Belt Davis D, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C & Attie AD (2008). A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res 18, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV & Ritov VB (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S & Hattori N (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 169, 166–169. [DOI] [PubMed] [Google Scholar]
- Kubli DA & Gustafsson ÅB (2012). Mitochondria and mitophagy: The yin and yang of cell death control . Circ Res 111, 1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN & Gustafsson AB (2013). Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem 288, 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley P, Regnault V, Nicoletti A, Li Z & Michel J‐B (2012). The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res 95, 194–204. [DOI] [PubMed] [Google Scholar]
- Lavandero S, Chiong M, Rothermel BA & Hill JA (2015). Autophagy in cardiovascular biology. J Clin Invest 125, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Jeong S‐Y, Karbowski M, Smith CL & Youle RJ (2004). Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15, 5001–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B & Kroemer G (2012). Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ 16, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P (2002). Inflammation in atherosclerosis. Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- Lindner V, Olson NE, Clowes AW & Reidy MA (1992). Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest 90, 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD & Jaswal JS (2010). Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56, 130–140. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Spafford MA & Marsh DR (1991). Glycolysis is predominant source of myocardial ATP production immediately after birth. Am J Physiol Hear Circ Physiol 261, H1698–H1705. [DOI] [PubMed] [Google Scholar]
- Lowell BB & Shulman GI (2005). Mitochondrial dysfunction and type 2 diabetes. Science 307, 384–387. [DOI] [PubMed] [Google Scholar]
- McBride H & Soubannier V (2010). Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol 20, R274–R276. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR & Runge MS (2007). Mitochondrial dysfunction in atherosclerosis. Circ Res 100, 460–473. [DOI] [PubMed] [Google Scholar]
- Makinde A, Kantor P & Lopaschuk G (1998). Maturation of fatty acid and carbohydrate metabolism in the newborn heart. Mol Cell Biochem 188, 49–56. [PubMed] [Google Scholar]
- Makino A, Scott BT & Dillmann WH (2010). Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53, 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT & Dillmann WH (2011). Regulation of mitochondrial morphology and function by O‐GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol 300, R1296–R1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang Y‐H, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J & Archer SL (2012). Dynamin‐related protein 1‐mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110, 1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Garrido A, Williams HC, Lee M, Seidel‐Rogol B, Ci X, Dong J‐T, Lassègue B, Martín AS & Griendling KK (2013). Transforming growth factor β inhibits platelet derived growth factor‐induced vascular smooth muscle cell proliferation via Akt‐independent, Smad‐mediated Cyclin D1 downregulation. PLoS One 8, e79657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou Y‐S, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N & Tanaka K (2010). PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N (2007). Autophagy: process and function. Genes Dev 21, 2861–2873. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM & Klionsky DJ (2008). Autophagy fights disease through cellular self‐digestion. Nature 451, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Noda T & Ohsumi Y (1999). Apg16p is required for the function of the Apg12p–Apg5p conjugate in the yeast autophagy pathway. EMBO J 18, 3888–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy‐Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P & Staels B (2014). Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130, 554–564. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen D‐F & Youle RJ (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen D‐F, Gautier CA, Shen J, Cookson MR & Youle RJ (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8, e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S‐B, Hall AR & Hausenloy DJ (2013). Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal 19, 400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S‐B & Hausenloy DJ (2010). Mitochondrial morphology and cardiovascular disease. Cardiovasc Res 88, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S‐B, Subrayan S, Lim SY, Yellon DM, Davidson SM & Hausenloy DJ (2010). Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121, 2012–2022. [DOI] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ & Mihara K (2010). Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191, 1141–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK (1995). Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75, 487–517. [DOI] [PubMed] [Google Scholar]
- Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC & Walsh K (2011. a). Mitofusin‐2 maintains mitochondrial structure and contributes to stress‐induced permeability transition in cardiac myocytes. Mol Cell Biol 31, 1309–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC & Walsh K (2012). Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res 111, 1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou KN, Ngoh GA, Dabkowski ER, Connell KA, Ribeiro RF, Stanley WC & Walsh K (2011. b). Cardiomyocyte deletion of mitofusin‐1 leads to mitochondrial fragmentation and improves tolerance to ROS‐induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol 302, H167–H179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra V, Verdejo H, del Campo A, Pennanen C, Kuzmicic J, Iglewski M, Hill J, Rothermel B & Lavandero S (2011). The complex interplay between mitochondrial dynamics and cardiac metabolism. J Bioenerg Biomembr 43, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra V, Verdejo HE, Iglewski M, del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez‐Crisosto C, Jaña F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A & Lavandero S (2014). Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt‐mTOR‐NFκB‐Opa‐1 signaling pathway. Diabetes 63, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L & Scorrano L (2007). A cut short to death: Parl and Opa1 in the regulation of mitochondrial morphology and apoptosis. Cell Death Differ 14, 1275–1284. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Parra V, López‐Crisosto C, Morales PE, del Campo A, Gutierrez T, Rivera‐Mejías P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA & Lavandero S (2014). Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+‐calcineurin signaling pathway. J Cell Sci 127, 2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J, Hill BG, Benavides GA, Dranka BP & Darley‐Usmar VM (2010). Role of cellular bioenergetics in smooth muscle cell proliferation induced by platelet‐derived growth factor. Biochem J 428, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacín M & Zorzano A (2005). The Charcot–Marie–Tooth type 2A gene product, Mfn2, up‐regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, Huynh LH, Nicolas V, Alavi MV, Brenner C, Ventura‐Clapier R, Veksler V & Joubert F (2012). Down‐regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94, 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Vasileva AK, Arkhangelskaya AA, Pevzner IB, Skulachev VP & Zorov DB (2008). Interrelations of mitochondrial fragmentation and cell death under ischemia/reoxygenation and UV‐irradiation: Protective effects of SkQ1, lithium ions and insulin. FEBS Lett 582, 3117–3124. [DOI] [PubMed] [Google Scholar]
- Porter KE & Turner NA (2009). Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther 123, 255–278. [DOI] [PubMed] [Google Scholar]
- Pukac L, Huangpu J & Karnovsky MJ (1998). Platelet‐derived growth factor‐BB, insulin‐like growth factor‐I, and phorbol ester activate different signaling pathways for stimulation of vascular smooth muscle cell migration. Exp Cell Res 242, 548–560. [DOI] [PubMed] [Google Scholar]
- Quirós PM, Ramsay AJ, Sala D, Fernández‐Vizarra E, Rodríguez F, Peinado JR, Fernández‐García MS, Vega JA, Enríquez JA, Zorzano A & López‐Otín C (2012). Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31, 2117–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regula KM (2002). Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia‐mediated cell death of ventricular myocytes. Circ Res 91, 226–231. [DOI] [PubMed] [Google Scholar]
- Salabei JK & Hill BG (2013). Mitochondrial fission induced by platelet‐derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol 1, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC & Gupta MP (2014). SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34, 807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle RJ & Fuller MT (2003). Mitofusin‐1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116, 2763–2774. [DOI] [PubMed] [Google Scholar]
- Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ & Archer SL (2014). Dynamin‐related protein 1 (Drp1)‐mediated diastolic dysfunction in myocardial ischemia‐reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J 28, 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess M‐A, Levit A, Kim B, Hartman M‐L, Joseph L, Shirihai OS & Vita JA (2011). Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124, 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh JTC, Huang Y, Gilmore J & Srivastava D (2011). Elevated miR‐499 levels blunt the cardiac stress response. PLoS One 6, e19481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Horita K, Murakami M & Ogura R (1984). Morphological studies of different mitochondrial populations in monkey myocardial cells. Cell Tissue Res 238, 577–582. [DOI] [PubMed] [Google Scholar]
- Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ & Dorn GW 2nd (2015). Interdependence of Parkin‐mediated mitophagy and mitochondrial fission in adult mouse hearts. Circ Res 117, 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C & Chan DC (2007). OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol 178, 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA & Lopaschuk GD (2005). Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85, 1093–1129. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T & Mihara K (2007). Mitotic phosphorylation of dynamin‐related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282, 11521–11529. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen D‐F, Karbowski M & Youle RJ (2010). Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191, 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS & White HD (2008). Diagnostic application of the universal definition of myocardial infarction in the intensive care unit. Curr Opin Crit Care 14, 543–548. [DOI] [PubMed] [Google Scholar]
- Twig G & Shirihai OS (2011). The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM , Abou‐Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González‐Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G & Wood NW (2004). Hereditary early‐onset Parkinson's disease caused by mutations in PINK1 . Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- Vendelin M, Béraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L & Saks VA (2005). Mitochondrial regular arrangement in muscle cells: a ‘crystal‐like’ pattern. Am J Physiol Cell Physiol 288, C757–C767. [DOI] [PubMed] [Google Scholar]
- Vives‐Bauza C, Zhou C, Huang Y, Cui M, de Vries RLA, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K & Przedborski S (2010). PINK1‐dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC (1999). Mitochondrial diseases in man and mouse. Science 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Wang J‐X, Jiao J‐Q, Li Q, Long B, Wang K, Liu J‐P, Li Y‐R & Li P‐F (2011). miR‐499 regulates mitochondrial dynamics by targeting calcineurin and dynamin‐related protein‐1. Nat Med 17, 71–78. [DOI] [PubMed] [Google Scholar]
- Wang K, Long B, Jiao J‐Q, Wang J‐X, Liu J‐P, Li Q & Li P‐F (2012). miR‐484 regulates mitochondrial network through targeting Fis1. Nat Commun 17, 781. [DOI] [PubMed] [Google Scholar]
- Wang Y & Tabas I (2014). Emerging roles of mitochondria ROS in atherosclerotic lesions: causation or association? J Atheroscler Thromb 21, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Saotome M, Nobuhara M, Sakamoto A, Urushida T, Katoh H, Satoh H, Funaki M & Hayashi H (2014). Roles of mitochondrial fragmentation and reactive oxygen species in mitochondrial dysfunction and myocardial insulin resistance. Exp Cell Res 323, 314–325. [DOI] [PubMed] [Google Scholar]
- Westermann B (2002). Merging mitochondria matters. EMBO Rep 3, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group Members: Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, Lloyd‐Jones D, Goff DC Jr & Hong Y; Members of the Statistics Committee and Stroke Statistics Subcommittee: Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel‐Smoller S, Wilson M & Wolf P (2006). Heart disease and stroke statistics–2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113, e85–e151. [DOI] [PubMed] [Google Scholar]
- Youle RJ & Strasser A (2008). The BCL‐2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9, 47–59. [DOI] [PubMed] [Google Scholar]
- Yu J‐C, Lokker NA, Hollenbach S, Apatira M, Li J, Betz A, Sedlock D, Oda S, Nomoto Y, Matsuno K, Ide S, Tsukuda E & Giese NA (2001). Efficacy of the novel selective platelet‐derived growth factor receptor antagonist CT52923 on cellular proliferation, migration, and suppression of neointima following vascular injury. J Pharmacol Exp Ther 298, 1172–1178. [PubMed] [Google Scholar]
- Yu T, Robotham JL & Yoon Y (2006). Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103, 2653–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, Pedrozo Z, Chiong M, Sánchez G & Lavandero S (2014). Drp1 loss‐of‐function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia‐reperfusion injury. J Cardiovasc Pharmacol 63, 477–487. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wakabayashi N, Wakabayashi J, Tamura Y, Song W‐J, Sereda S, Clerc P, Polster BM, Aja SM, Pletnikov MV, Kensler TW, Shirihai OS, Iijima M, Hussain MA & Sesaki H (2011). The dynamin‐related GTPase Opa1 is required for glucose‐stimulated ATP production in pancreatic beta cells. Mol Biol Cell 22, 2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M & Palacín M (2009). Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol 41, 1846–1854. [DOI] [PubMed] [Google Scholar]


