Abstract
The DAPI structure has been modified by replacing the phenyl group with substituted phenyl or heteroaryl rings. Twelve amidines were synthesized and their DNA binding, fluorescence properties, in vitro and in vivo activities were evaluated. These compounds are shown to bind in the DNA minor groove with high affinity, and exhibit superior in vitro antitrypanosomal activity to that of DAPI. Six new diamidines (5b, 5c, 5d, 5e, 5f and 5j) exhibit superior in vivo activity to that of DAPI and four of these compounds provide 100% animal cure at a low dose of 4 × 5 mg/kg i.p. in T. b. rhodesiense infected mice. Generally, the fluorescence properties of the new analogues are inferior to that of DAPI with the exception of compound 5i which shows a moderate increase in efficacy while compound 5k is comparable to DAPI.
Keywords: Diamidines, Stille coupling, Pinner reaction, DAPI, DNA minor groove binders, Antitrypanosomal activity, Fluorescence properties
Graphical abstract
Highlights
-
•
Twelve amidines were synthesized as analogues to the known antitrypanosomal agent DAPI.
-
•
The DNA binding, fluorescence properties, in vitro and in vivo activities of the new amidines were evaluated.
-
•
These compounds are shown to bind in the DNA minor groove with higher affinity than DAPI.
-
•
Four new diamidines provided 100% animal cure which is superior to that of DAPI.
1. Introduction
Human African trypanosomiasis also known as sleeping sickness is a neglected tropical disease in Sub-Saharan Africa caused by two subspecies of Trypanosoma brucei (T. b. gambiense and T. b. rhodesiense). It threatens over 65 million people among the poorest in the world in 36 countries [1a]. The trypanosomes are transmitted mainly by blood-feeding tsetse flies. Once infected, the disease generally progresses through two stages which are defined by the location of the parasites in the patient. In the first hemolymphatic stage the parasites reside in the blood and lymph system whereas in the second meningoencephalitic stage the parasites invade additionally the central nervous system [1b]. It is generally accepted that without an effective treatment the disease is lethal. Drugs currently in use for sleeping sickness are pentamidine, suramin, melarsoprol, and a combination of nifurtimox and eflornithine (NECT). The treatment selection is based on the subspecies of T. brucei and on the disease stage. All drugs have disadvantages such as difficult administration, serious side effects or resistance issues. Better drugs are needed to control or eliminate sleeping sickness. There are two new molecules, fexinidazole and the oxaborole SCYX-7158 at the stage of clinical development [1c]. However, due to the high attrition rate in drug development it is essential to continue with drug discovery to find new drugs to cure sleeping sickness.
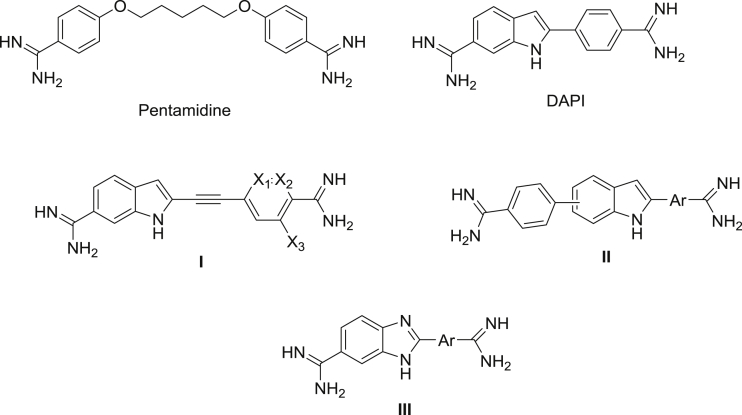
Dicationic amidines that bind in the DNA minor groove are promising agents against African trypanosomiasis. So far, pentamidine (Fig. 1) is the only one of this class which has seen significant human clinical use [2]. However, it is the most frequently used drug for the treatment of first stage sleeping sickness since almost 8 decades and it is still effective by a parenteral route of application.
Fig. 1.
Antitrypanosomal diamidines.
DAPI (4′, 6-diamidino-2-phenylindole) (Fig. 1), was developed as a compound related to diminazene and stilbamidine, to be used as an antitrypanosomal agent [3]. DAPI has been subsequently found to exhibit a variety of other biological effects, including antifungal, antibacterial, and antiviral activities [3], [4]. Also, DAPI is a fluorescent dye which exhibits several binding modes to DNA [5] and it has been widely utilized as a DNA specific probe for flow cytometry, chromosome staining, DNA visualization and quantitation [6], and has become now an important tool in molecular biology. We previously have reported some phenyl acetylene derivatives of DAPI (compound I, Fig. 1), that showed strong DNA binding and exhibited potent in vitro activity superior to that of DAPI [7]. In a related investigation, we extended the overall length of DAPI by adding one phenyl group at the 5 or 6 position of indole and replacing the phenyl group of DAPI with different aryl and heteroaryls (compound II, Fig. 1), these diamidines showed strong DNA binding comparable to that of DAPI and showed stronger in vitro antitrypanosomal activity with a comparable or stronger in vivo activity than that of DAPI [8]. Moreover, we recently studied the replacement of the indole ring of DAPI with a benzimidazole ring (compound III, Fig. 1), these benzimidazole derivatives showed good in vitro activity but exhibited very limited in vivo activity [9], which demonstrated the importance of the indole ring of DAPI for the antitrypanosomal activity. Here we report the replacement of the phenyl ring of DAPI with different substituted phenyl and heteroaryl rings in anticipation of finding enhanced in vivo activity and physicochemical properties.
2. Results and discussion
2.1. Chemistry
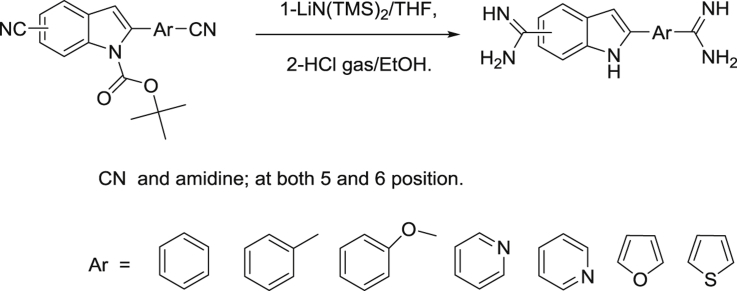
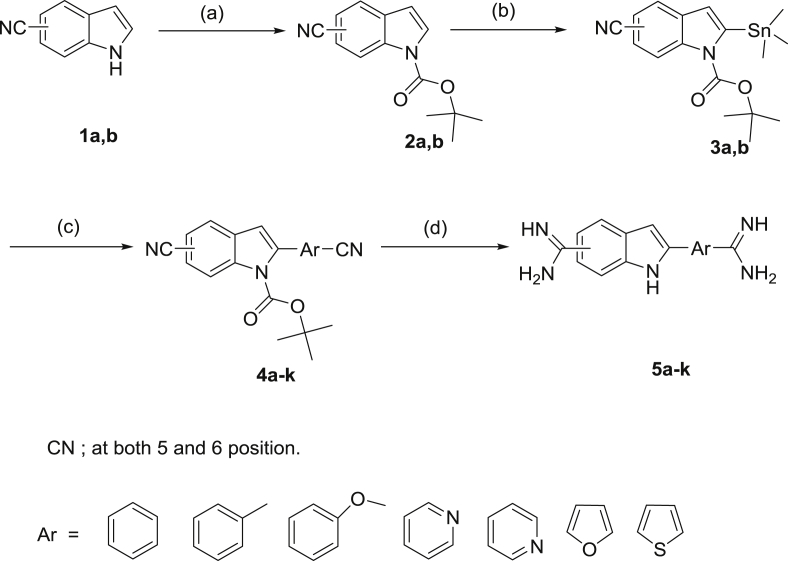
Scheme 1 describes the preparation of the diamidines 5a-k in good yield. 5 (6)-Cyanoindoles 1a,b were protected by employing di-tert-butyldicarbonate in dichloromethane using 4-(dimethylamino)pyridine as a catalyst. Lithiation of the Boc-protected indoles 2a,b was readily achieved with lithium diisopropylamide (LDA) in anhydrous tetrahydrofuran at −20 °C. Subsequent reaction of the lithioindole intermediate with trimethyltin chloride gave the indole stannanes 3a,b in good yield [10]. The stannane intermediates were allowed to react with different bromoaryl and heteroaryl benzonitriles under Stille coupling conditions in the presence of 5 mol % Pd(PPh3)4 using dioxane as a solvent to afford the bisnitriles 4a-k in good yield. The bisnitriles 4a-k were allowed to react with lithium bis(trimethylsilyl)amide [11] in THF, followed by deprotection of the silylated amidines with ethanolic HCl to furnish the dihydrochloride salts of the diamidines 5a-k.
Scheme 1.
Reagents and conditions. (a) Boc2O, DMAP, CH2Cl2; (b) ClSn(CH3)3, LDA/THF; (c) Br-Ar-CN, Pd(PPh3)4, Dioxane (d) 1-LiN(TMS)2/THF,2-HCl gas/EtOH.
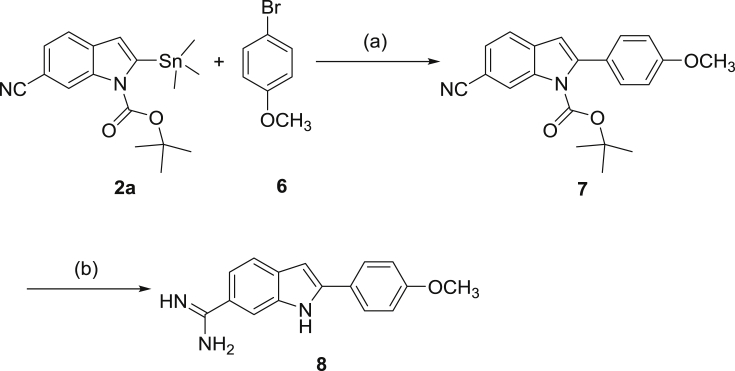
Scheme 2 illustrates the synthesis of the monoamidine 8. The commercially available 1-bromo-4-methoxybenzene was coupled with the indole stannane 2a utilizing Stille coupling conditions to afford the mononitrile 7 in high yield. This mononitrile was converted to the monoamidine hydrochloride 8 by utilizing the Pinner methodology [12] by stirring in dry ethanolic HCl to yield the imidate ester hydrochloride which was separated, dried and suspended in ethanolic ammonia to furnish the monoamidine 8.
Scheme 2.
Reagents and conditions: (a) Pd(PPh3)4, Dioxane (b) i-HCl gas/EtOH.ii-NH3/EtOH.
2.2. Biology
Table 1 summarizes the DNA binding affinities for the twelve new amidino analogues of DAPI as well as both the in vitro and in vivo activities of these compounds against Trypanosoma brucei rhodensiense (T. b. r.). For comparative purposes, data for DAPI are included. The thermal melting increase ΔTm (Tm of DNA-ligand complex – Tm of free DNA) is a rapid and reliable method for ranking binding affinities for large numbers of aryldiamidines [13]. The ΔTm values for the complexes between poly (dA-dT) and the new analogues vary with the change in the substitution of the 2- aryl group and ranges from 10 to >26 °C. The methyl 5b, methoxy 5d and the o-pyridyl 5e analogues exhibit high ΔTm values (>26 °C) comparable to that of DAPI. These three compounds have the amidine group at the 6-position of indole, while their isomers with the amidine group at 5-position (5a, 5c, 5f) of indole exhibit lower ΔTm values. We hypothize that this decrease in DNA affinity is likely due to the fact that in the complex 6- amidino indole can form a strong hydrogen bond involving the indole NH to a thymine carbonyl as has been previously reported [7]. While in the analogous complex for the 5-amidino indole such is not possible as the 3-position C—H is pointed into the groove. Removal of the amidine groups from DAPI as in the monoamidine 8 results in a big reduction of binding affinity consistent with the loss of one key cationic binding center.
Table 1.
DNA binding, in vitro and in vivo antitrypanosomal activity of the DAPI analogues.
| Compound | Code | ΔTma (°C) | Cytotoxb (μM) | T. b. rc (nM) | SId | In vivof(cures) |
|---|---|---|---|---|---|---|
 |
DAPI | >27 | 0.86 | 18 | 48 | 2/4 |
 |
5a | 19.2 | 1.7 | 3 | 566 | 0/4 |
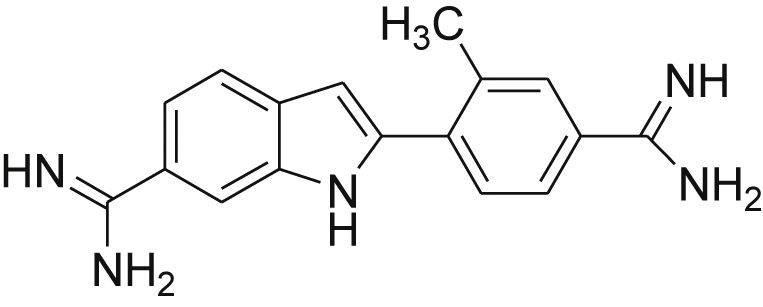 |
5b | >26 | 15.7 | 5 | 3140 | 3/4 |
 |
5c | 15.3 | 23.5 | 3 | 7833 | 3/4 |
 |
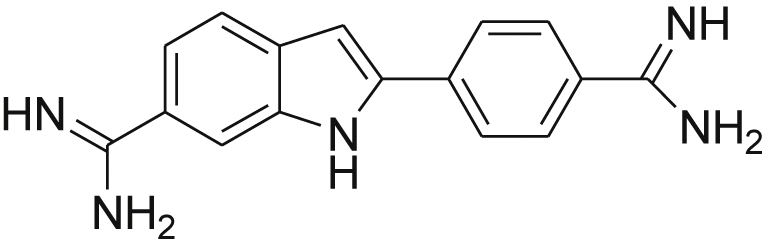
5d | >26 | 29.6 | 6 | 4876 | 4/4 |
 |
5e | 22.1 | 3.2 | 24 | 133 | 4/4 |
 |
5f | >26 | 21.3 | 4 | 5325 | 4/4 |
 |
5g | 15.3 | 2.0 | 3 | 666.7 | 1/4 |
 |
5h | 12.1 | 1.8 | 5 | 394 | 1/4 |
 |
5i | 11 | 7.2 | 9 | 800 | 0/2 |
 |
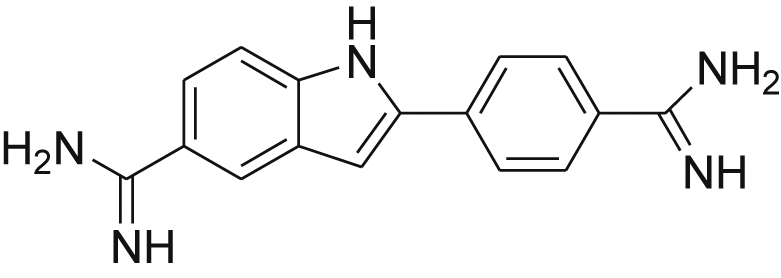
5j | 21.4 | 2.7 | 3 | 900 | 4/4 |
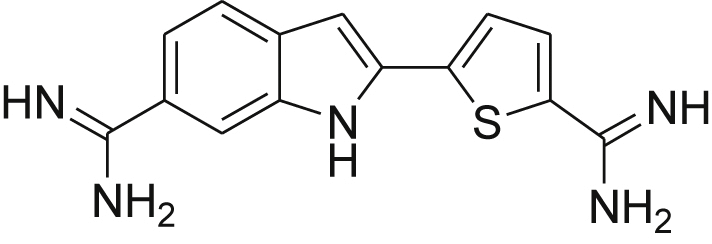 |
5k | 10 | 4.5 | 11 | 409 | 0/4 |
 |
8 | 12.6 | 29 | 4869 | 6 | NDe |
Increase in thermal melting of poly (dA-dT)n.
Cytotoxicity was evaluated using cultured L6 rat myoblast cells.
The T. b. r. (Trypanosoma brucei rhodesiense) strain was STIB900. The values are the average of duplicate determinations.
Selectivity Index for T. b. r. expressed as the ratio: IC50 (L6)/IC50 (T.b.r.).
Not determined as in vitro inactive.
In vivo efficacy was determined in T. b. rhodesiense (STIB900) infected mice at 4 × 5 mg/kg i.p. dose.
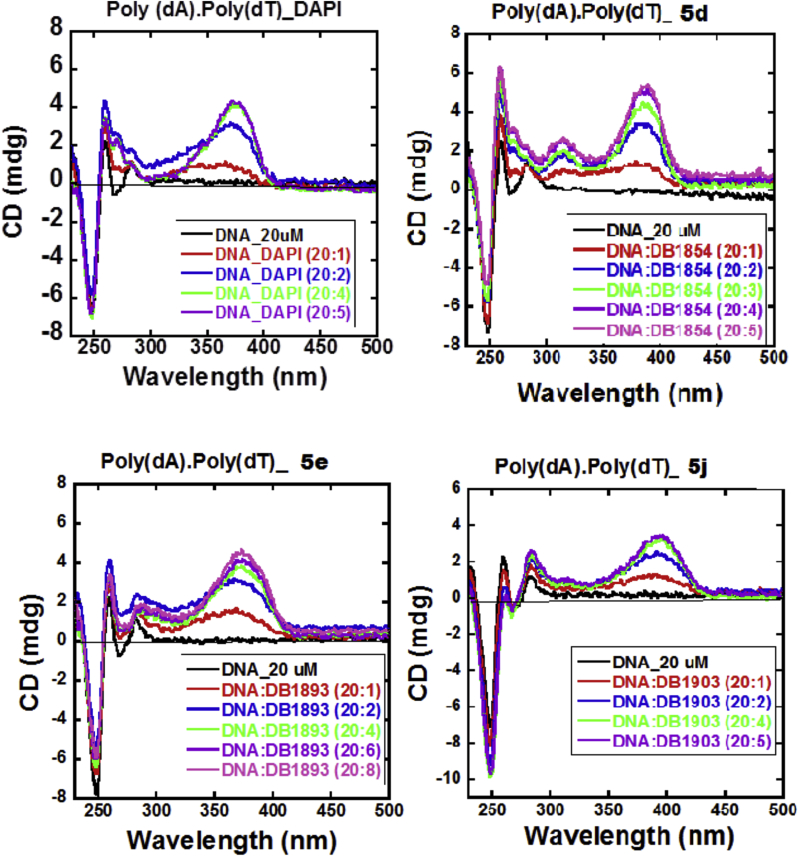
Generally, heterocyclic diamidines have been found to bind in the DNA minor groove in AT base pair sequences of DNA [14].Circular dichroism (CD) spectroscopy is a valuable technique for characterizing minor groove interactions. In order to determine if these new analogues bind in the DNA minor groove, we observed the CD spectroscopy of complex formation. Fig. 2 shows the CD data for the interaction between DNA and 5d, 5e and 5j along with that of DAPI as a reference compound. Minor groove binders yield a large positive induced CD signal on binding to AT sequences and only exhibit small changes in the CD pattern of the DNA spectrum. The induced CD spectrum of DAPI is shown as a reference in Fig. 2(a). The expected positive signal in the compound absorption region with relatively small changes in the DNA region are observed. The results shown in Fig. 2 shows that the CD data are consistent with the Δ Tm results for 5d, 5e and 5j. Reflecting strong binding to DNA, compound 5d exhibits large induced CD spectra in its absorption regions that are higher than that for DAPI while 5e and 5j exhibit comparable CD spectra to that of DAPI. This data demonstrates the DNA minor groove binding of these compounds.
Fig. 2.
Circular dichroism spectral profiles on the addition of representative compounds, with Poly (dA).Poly (dT) duplex DNA sequences in buffer (10 mM MES, 100 mM NaCl, 1 mM EDTA, at pH, 7.4).
DAPI exhibits excellent in vitro activity against T. b. r (18 nM). All of the newly synthesized compounds, except 5e and 8, showed an in vitro activity stronger than that of DAPI ranging from a 2–6 fold increase in activity. All the newly synthetized compounds showed good selectivity indices ranging from 133 to 7833 indicating that they are clearly less cytotoxic than DAPI (SI = 48) with exception of compound 8 (SI = 6), which was in vitro inactive versus T. b. r.
In view of the high in vitro activity and selectivity of these new amidines, they were evaluated for efficacy in the acute stage of infection in the rigorous T. b. r. STIB900 mouse model (Table 1). The compounds were tested by daily intraperitoneal dosage of 5 mg/kg for four consecutive days. Four of the twelve new compounds (5d, 5e, 5f and 5j) cured all 4 infected mice and gave 100% cure rate. Two compounds (5b, 5c) cured 3 out of 4 mice and gave 75% cure rate, while 5g and 5h gave 25% cure rate. Compound 5i was toxic killing 2 out of 4 mice and did not cure any of the two remaining mice. The in vivo results for six compounds (5b, 5c, 5d, 5e, 5f and 5j) are superior to that of DAPI and pentamidine that is in clinical use for 1st stage HAT since 80 years (both cure 2 out of 4 mice at 4 × 5 mg/kg i.p.) in the STIB900 acute mouse model and therefore the six new compounds merit further evaluation as potential drug candidates for HAT.
The widespread use of DAPI in molecular biology is in large part due to its fluorescence properties. During the study of these new indole amidines we observed that several of the compounds were fluorescent in the visible region. Therefore we decided to record the fluorescence data for the new compounds and compare the results with that of DAPI. These data are presented in Table 2.
Table 2.
Fluorescence data for the new amidines.
| Compound | λexa,b (nm) | λema,b (nm) | Em. intensityc |
|---|---|---|---|
| DAPI | 343 | 458 | 821 |
| 5a | 322 | 481 | 176 |
| 5b | 351 | 463 | 611 |
| 5c | 341 | 461 | 33 |
| 5d | 351 | 493 | 452 |
| 5e | 348 | 470 | 461 |
| 5f | 339 | 462 | 260 |
| 5g | 342 | 480 | 53 |
| 5h | 358 | 475 | 200 |
| 5i | 322 | 418 | 875 |
| 5j | 348 | 460 | 680 |
| 5k | 370 | 481 | 810 |
| 8 | 312 | 433 | 690 |
Wavelengths (λ)are indicated for excitation (ex) and emission (em).
Measurements were made with 0.001 M solutions in distilled water.
Emission intensities were measured at excitation slit width of 5 and emission slit width of 20.
The absorption maximum for the new amidines ranges from 312 to 370 nm; that for DAPI is 343 nm. The emission maximum ranges from 418 to 493 nm in comparison with a value of 458 nm for DAPI. A qualitative comparison of quantum yield of emission for the indoles shows that comp 5i shows a moderate increase in efficacy compared to that of DAPI while compound 5k is comparable to DAPI. Both new diamidines have the advantage of being less cytotoxic than DAPI (Table 1). Compounds 5b, 5j and 8 shows 11–18% decrease from that of DAPI, the rest of the compounds shows significantly lower quantum yield by a factor of 2–25.
3. Conclusion
The two diamidines, DAPI and pentamidine are highly active against African trypanosomes. Substitutions on the phenyl ring of DAPI or its replacement with heterocyclic rings results in compounds that bind in the DNA minor groove with high affinity and exhibit superior in vitro antitrypanosomal activity to that of DAPI. In vivo efficacy of six new diamidines (5b, 5c, 5d, 5e, 5f and 5j) was further improved compared to that of DAPI, four of them gave 100% animal cures at the low dose of 4 × 5 mg/kg i.p. Generally, there is a decline in the fluorescence properties of the new analogues compared to that of DAPI with the exception of compound 5i that shows a moderate increase in efficacy whereas compound 5k is comparable to DAPI.
4. Experimental
4.1. Biology
4.1.1. Efficacy studies
The in vitro viability assays [15] with the T. b. r. strain STIB 900 as well as the efficacy studies in the STIB900 acute mouse model for T. b. r. at a 4 × 5 mg/kg i.p. dosage were carried out as previously reported [16].
4.1.2. Tm measurements
Thermal melting experiments were conducted with a Cary 300 spectrophotometer. Cuvettes for the experiment were mounted in a thermal block and the solution temperatures monitored by a thermistor in the reference cuvette. Temperatures were maintained under computer control and increased at 0.5 °C/min. The experiments were conducted in 1 cm path length quartz cuvettes in CAC 10 buffer (cacodylic acid 10 mM, EDTA 1 mM, NaCl 100 mM with NaOH added to give pH = 7.0). The concentrations of DNA were determined by measuring its absorbance at 260 nm. A ratio of 0.3 mol compound per mole of DNA was used for the complex and DNA alone was used as a control [13]. ΔTm values were determined by the peak in first derivative curves (dA/dT).
4.1.3. Circular dichroism (CD)
CD spectra were collected employing a Jasco J-810 spectrometer at different ratios of compound to DNA at 25 °C in MES 10 buffer (10 mM MES, 100 mM NaCl, I mM EDTA). A DNA solution in a 1-cm quartz cuvette was first scanned over the desired wavelength range. DAPI, 5d, 5e and 5j at increasing ratios, were titrated into the same cuvette and the complexes rescanned under the same conditions [17].
4.2. Chemistry
All commercial reagents were used without purification. Melting points were determined on a Mel-Temp 3.0 melting point apparatus, and are uncorrected. TLC analysis was carried out on silica gel 60 F254 precoated aluminum sheets using UV light for detection. 1H and 13C NMR spectra were recorded on a Bruker 400 MHz spectrometer (except as noted) using the indicated solvents. Mass spectra were obtained from the Georgia State University Mass Spectrometry Laboratory, Atlanta, GA. If the compounds reported as salts contain a fraction of water and/or solvents, these fractions are seen in HNMR spectra. Elemental analysis were performed by Atlantic Microlab Inc., Norcross, GA. Compounds 5a [18] and 5d [19] were previously reported.
5. General procedure for the synthesis of the bisnitriles 4a-k and 7
Tetrakistriphenylphosphine palladium (0.288 gm, 0.25 mmol) was added to a stirred mixture of the indole stannane (3a or 3b) (5 mmol) and the aryl halide (5 mmol) in de-aireated dioxane (15 mL) under a nitrogen atmosphere. The vigorously stirred mixture was heated at 90–100 °C for 24 h. The solvent was evaporated under reduced pressure, the resulting solid was partitioned between ethyl acetate (200 mL) and 5 mL of concentrated ammonia to remove the palladium complex, washed with water, passed through celite to remove the catalyst, dried over sodium sulfate and evaporated. Purification by column chromatography on silica gel, using hexanes/ethyl acetate (75/25, v/v).
5.1. 1-(tert-Butoxycarbonyl)-2-(2-methyl-4-cyanophenyl)-1H-indole-6-carbonitrile (4b)
White solid, yield (1.98 gm, 75%). mp 164–164.5 °C; 1HNMR (CDCl3, 400 MHz) δ 8.66 (s, 1H), 7.67 (d, 1H, J = 8 Hz)760–7.58 (m, 2H), 7.55 (br d, 1H, J = 8 Hz), 7.41 (d, 1H, J = 8 Hz), 6.57 (s, 1H), 2.23 (s, 3H), 1.31 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 156.4, 151.1, 149.2, 140.4, 137, 136.1, 132.5, 131.2, 130.0, 129.8, 127.6, 126.2, 125.1, 121.2, 121.1, 119.2, 112.8109.1, 104.2, 27.5, 21.5; ESI-MS: m/z calculated for C22H19N3O2: 357.41, found: 358.2 (M++1); Anal. Calcd. For C22H19N3O2: C, 73.93; H, 5.36; N, 11.76. Found: C, 73.69; H, 5.32; N, 11.99.
5.2. 1-(tert-Butoxycarbonyl)-2-(2-methyl-4-cyanophenyl)-1H-indole-5-carbonitrile (4c)
White solid, yield (0.85 gm, 66%), mp 191–193 °C; 1HNMR (DMSO-d6, 400 MHz) δ 8.11 (d, 1H, J = 1.5 Hz), 7.83 (s, 1H), 7.79 (dd, 1H, J = 1.5 Hz, J = 8.1 Hz), 7.74 (d, 1H, J = 8.1 Hz), 7.58 (d, 1H, J = 8.4 Hz), 7.47 (dd, 1H, J = 1.5 Hz, J = 8.4 Hz), 6.88 (d, 1H, J = 1.5 Hz), 2.52 (s, 3H), 1.29 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 138.2, 137.7, 137.1, 135.9, 134.2, 129.6, 129.4, 127.7, 125.8, 124.5, 120.2, 118.4, 112.4, 110.4, 103.9, 101.5, 27.1, 21.3; ESI-MS: m/z calculated for C17H11N3: 257 (M+).
5.3. 1-(tert-Butoxycarbonyl)-2-(5-cyanopyridin-2-yl)-1H-indole-6-carbonitrile (4e)
White solid, yield (2.21 gm, 87%). mp 171–171.5 °C; 1HNMR (CDCl3, 400 MHz) δ 8.96 (d, 1H, J = 1.2 Hz), 8.51 (s, 1H), 8.08 (dd, 1H, J = 2.0 Hz, J = 8.0 Hz), 7.71 (d, 1H, J = 8 Hz), 7.69 (d, 1H, J = 8 Hz), 7.53 (dd, 1H, J = 1.2 Hz, J = 8 Hz), 6.95 (s, 1H), 1.44 (s, 9H); 13CNMR (CDCl3, 400 MHz) δ 155.2, 151.7, 148.8, 140.6, 139.4, 136.9, 131.6, 126.2, 123.1, 122.2, 119.8, 116.4, 112.3, 108.6, 109, 85.6, 27.6; ESI-MS: m/z calculated for C20H16N4O2: 344.37, found: 345.2 (M++1); Anal. Calcd. For C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.49; H, 4.58; N, 16.08.
5.4. 1-(tert-Butoxycarbonyl)-2-(5-cyanopyridin-2-yl)-1H-indole-5-carbonitrile (4f)
White solid, yield (2.06 gm, 81%). mp 180–181 °C; 1HNMR (CDCl3, 400 MHz) δ 8.65 (s, 1H), 7.67 (s, 1H, J = 8.0 Hz), 7.61–7.58 (m, 2H), 7.55 (dd, 1H, J = 1.6 Hz, J = 8.0 Hz), 7.41 (d, 1H, J = 8.0 Hz), 6.57 (s, 1H), 1.31 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 150.7, 148.9, 139.4, 137.0, 136.5, 133.3, 128.7, 128.5, 127.6, 125.9, 119.3, 117.0, 116.7112.0, 107.2, 86.2, 27.7; ESI-MS: m/z calculated for C20H16N4O2: 344.37, found: 345.2 (M++1); Anal. Calcd. For C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.69; H, 4.81; N, 15.99.
5.5. 1-(tert-Butoxycarbonyl)-2-(6-cyanopyridin-3-yl)-1H-indole-6-carbonitrile (4g)
White solid, yield (2.14 gm, 84%). mp 168–168.5 °C; 1HNMR (CDCl3, 400 MHz) δ 8.81 (s, 1H), 8.35 (d, 1H, J = 8.8 Hz), 7.97 (s, 1H), 7.92 (br d, 1H, J = 8.0 Hz), 7.81 (d, 1H, J = 8.0 Hz), 7.66 (d,1H, J = 8.8 Hz), 6.79 (s, 1H), 1.45 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 155.0, 151.8, 148.9, 140.7, 139.5, 137.0, 131.2, 126.7, 122.9, 122.4, 119.3, 116.5, 112.4, 108.7, 108.7, 108.4, 85.6, 27.7; ESI-MS: m/z calculated for C20H16N4O2: 344.37, found: 345.2 (M++1); Anal. Calcd. For C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.66; H, 4.62; N, 16.21.
5.6. 1-(tert-Butoxycarbonyl)-2-(6-cyanopyridin-3-yl)-1H-indole-5-carbonitrile (4h)
White solid, yield (2.11 gm, 83%). mp 197–197.5 °C; 1HNMR (CDCl3, 400 MHz) δ 8.81 (s, 1H), 8.34 (d, 1H, J = 8.8 Hz), 7.97 (s, 1H), 7.93 (br d, 1H, J = 8.0 Hz), 7.81 (d, 1H, J = 8.8 Hz), 7.66 (d, 1H, J = 8.0 Hz), 6.79 (s, 1H), 1.44 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 150.8, 149.1, 139.7, 137.4, 136.3, 132.9, 128.9, 128.3, 127.5, 126.3, 119.2, 116.9, 116.8, 112.0, 107.2, 86.3, 27.7; ESI-MS: m/z calculated for C20H16N4O2: 344.37, found: 345.2 (M++1); Anal. Calcd. For C20H16N4O2: C, 69.76; H, 4.68; N, 16.27. Found: C, 69.72; H, 4.66; N, 16.17.
5.7. 1-(tert-Butoxycarbonyl)-2-(5-cyanofuran-2yl)-1H-indole-6-carbonitrile (4i)
Yellow solid, yield (1.58 gm, 64%). mp 154–155 °C; 1HNMR (CDCl3, 400 MHz) δ 8.3–8.15 (m, 2H), 7.81–7.74 (m, 2H), 7.20 (s, 1H), 7.14 (br s, 1H), 1.45 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 150.7, 148.8, 148.6, 139.0, 129.3, 129.2, 128.3, 127.4, 125.4, 119.6, 116.3, 113.3, 112.7, 112.0, 106.4, 85.9, 27.5; ESI-MS: m/z calculated for C19H15N3O3: 333.34, found: 334.2 (M++1); Anal. Calcd. For C19H15N3O3: C, 68.46; H, 4.54; N, 12.61. Found: C, 68.32; H, 4.61; N, 12.53.
5.8. 1-(tert-Butoxycarbonyl)-2-(5-cyanofuran-2yl)-1H-indole-5-carbonitrile (4j)
Yellow solid, yield (1.7 gm, 69%). mp 162–162.5 °C; 1HNMR (CDCl3, 400 MHz) δ 8.32 (d, 1H, J = 8.4 Hz), 7.94 (s, 1H), 7.66–7.62 (m, 2H), 7.18 (d, 1H, J = 3.6 Hz), 6.84 (s, 1H), 1.5 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 148.8, 141.5, 139.2, 136.9, 132.0, 128.5, 128.4, 128.3, 125.8, 119.3, 116.6, 113.7, 110.5, 112.9, 107.0, 86.0, 27.7; ESI-MS: m/z calculated for C19H15N3O3: 333.34, found: 334.2 (M++1); Anal. Calcd. For C19H15N3O3: C, 68.46; H, 4.54; N, 12.61. Found: C, 68.45; H, 4.56; N, 12.6.
5.9. 1-(tert-Butoxycarbonyl)-2-(5-cyanothiophen-2yl)-1H-indole-6-carbonitrile (4k)
Dark yellow solid, yield (1.83 gm, 71%). mp 181–181.3 °C; 1HNMR (CDCl3, 400 MHz) δ 8.31 (d, 1H, J = 8.8 Hz), 7.95 (s, 1H), 7.64 (d, 1H, J = 8.8 Hz), 7.23 (d, 1H, J = 3.6HZ), 6.95 (s, 1H), 6.76 (d, 1H, J = 3.6 Hz), 1.55 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 158.8, 150.1, 148.6, 139.2, 129.3, 128.7, 128.1, 126.1, 123.1, 123.1, 119.2, 116.5, 112.6, 112.4, 111.2, 107.0, 85.8, 27.8; ESI-MS: m/z calculated for C19H15N3O2S: 349.41, found: 349.3 (M++1); Anal. Calcd. For C19H15N3O2S: C, 65.31; H, 4.33; N, 12.03. Found: C, 65.28; H, 4.41; N, 11.99.
5.10. 1-(tert-Butoxycarbonyl)-2-(4-methoxyphenyl)-1H-indole-6-carbonitrile (7)
White solid, yield (1.42 gm, 82%). mp 206–207 °C as reported [20]; 1HNMR (CDCl3, 400 MHz) δ 8.56 (s, 1H), 7.61 (dd,1H, J = 2.0 Hz, J = 8.0 Hz), 7.51 (d, 1H, J = 8 Hz), 7.36 (d, 2H, J = 8.8HZ), 6.99 (d, 2H, J = 8.8HZ), 6.58 (d, 1H, J = 2 Hz), 3.89 (s, 3H), 1.38 (s, 9H); 13CNMR (CDCl3, 100 MHz) δ 159.9, 149.6, 144, 136.3, 132.6, 130, 126.2, 126.1, 121, 119.9, 113.5, 109.2, 106, 84.7, 55.4, 27.6; ESI-MS: m/z calculated for C21H20N2O3: 348.4, found: 349.3 (M++1); Anal. Calcd. For C21H20N2O3: C, 72.40; H, 5.79; N, 8.04. Found: C, 72.35; H, 5.68; N, 8.12.
6. General procedure for the synthesis of the diamidines 5a-k
The dinitriles (4a-k) (0.66 mmol) were suspended in freshly distilled THF (5 ml), and treated with lithium trimethylsilylamide 1 M solution in tetrahydrofuran (4 ml, 3.98 mmol), the mixture was stirred for three days at room temperature. The reaction mixture was then cooled to zero oC and HCl saturated ethanol (2 ml) was added. The mixture was stirred for two days, diluted with ether and the resultant solid was collected by filtration. The diamidine was purified by neutralization with 1 N sodium hydroxide solution followed by filtration of the resultant solid and washing with water and dried. Finally, the free base was stirred with ethanolic HCl for one week to make sure that the (Boc)2O group was completely removed, diluted with ether, and the solid formed was filtered and dried to give the diamidines salt.
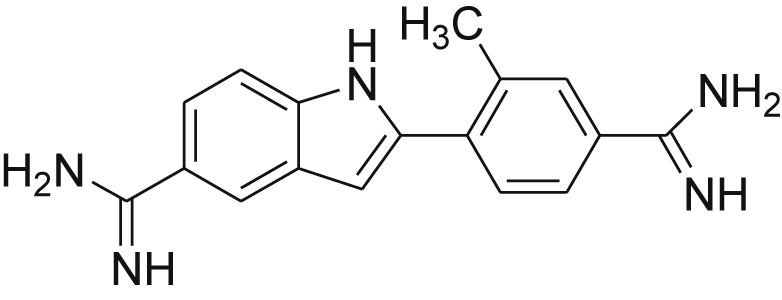
6.1. 2-(4-Amidino-3-methylphenyl)-1H-indole-6-amidine (5b)
Yellow solid, yield (0.149 gm, 61%), mp 277–279 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.32 (s, 1H), 9.47 (s,2H), 9.32 (s,2H), 9.20 (s,2H), 8.96 (s,2H), 8.01 (s, 1H), 7.92 (s, 1H), 7.83–7.78 (m, 3H), 7.49 (d, 1H, J = 8.4 Hz), 6.93 (s, 1H), 2.59 (s, 3H);; 13CNMR (DMSO-d6, 100 MHz) δ 167.0, 165.6, 140.4, 137.1, 136.2, 132.6, 131.2, 130.0, 127.6, 126.2, 121.2, 121.1, 119.2, 112.8, 104.2, 21.6; ESI-MS: m/z calculated for C17H17N5: 291.35, found: 292 (amidine base M++1); Anal. Calcd. For C17H17N5+2HCl + H2O: C, 53.41; H, 5.53; N, 18.31. Found: C, 53.15; H, 5.67; N, 18.12.
6.2. 2-(4-Amidino-3-methylphenyl)-1H-indole-5-amidine (5c)
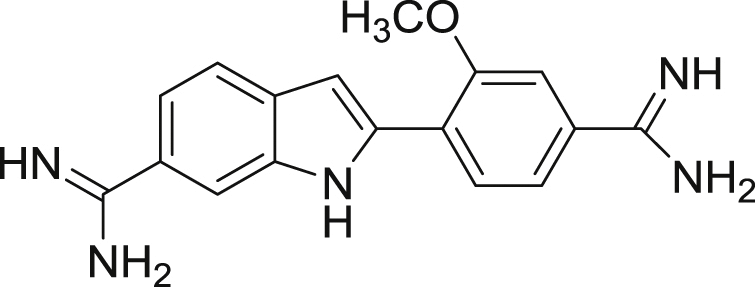
pale solid, yield (0.130 gm, 53%), mp 285–287 °C dec.; 1HNMR (DMSO-d6, 400 MHz) δ 12.39 (s, 1H), 9.55 (s, 2H), 9.35 (s, 2H), 9.32 (s, 2H), 9.12 (s, 2H), 8.23 (s, 1H), 7.93 (s, 1H), 7.87–7.82 (br s, 2H), 7.64 (br s, 2H), 6.95 (s, 1H), 2.59 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.5, 165.1, 139.5138.1, 136.5, 136.4, 130.8, 129.3, 127.7, 126.9, 125.8, 121.7, 121.4, 118.7, 112.0, 104.5, 21.3; ESI-MS: m/z calculated for C17H17N5: 291.35, found: 292 (amidine free base M++1); Anal. Calcd. For C17H17N5+2HCl+0.25H2O: C, 55.37; H, 5.39; N, 18.99. Found: C, 55.46; H, 5.43; N, 18.72.
6.3. 2-(5-Amidinopyridine-2-yl)-1H-indole-6-amidine (5e)
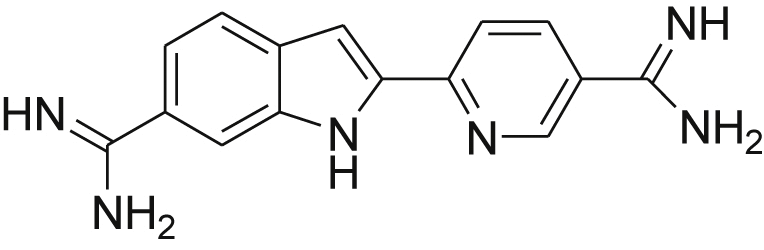
Yellow solid, yield (0.171 gm, 71%), mp > 300 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.65 (s, 1H), 9.70 (s,2H), 9.39 (s,2H), 9.13 (s,1H), 9.08 (s, 2H), 8.38 (br s,2H), 8.01 (s, 1H), 7.85 (d, 1H, J = 8.4 Hz), 7.51 (s, 1H), 7.46 (d, 1H, J = 8.4 Hz); 13CNMR (DMSO-d6, 100 MHz) δ 166.9, 162.0, 146.1, 143.1, 141.3, 136.1, 134.0, 132.3, 127.3, 124.5, 122.1, 118.9, 113.2, 104.3; ESI-MS: m/z calculated for C15H14N6: 278.31, found: 279.2 (amidine base M++1); Anal. Calcd. For C15H14N6+2HCl+1.35H2O+ 0.2 Et2O: C, 48.61; H, 5.34; N, 21.52. Found: C, 48.29; H, 4.95; N, 21.24.
6.4. 2-(5-Amidinopyridine-2-yl)-1H-indole-5-amidine (5f)
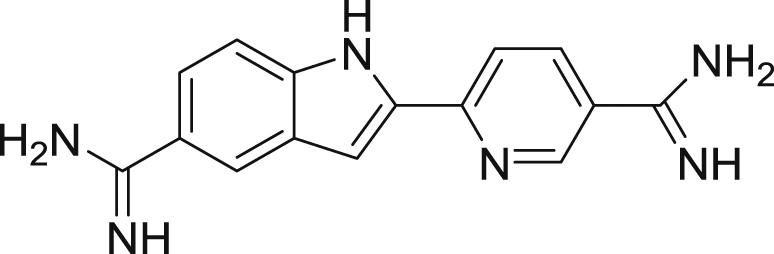
Yellow solid, yield (0.157 gm, 65%), mp > 300 °C; 1HNMR (DMSO-d6, 400 MHz) δ 13.0 (s, 1H), 9.70 (s, 2H), 9.50 (s, 2H), 9.48(s, H), 9.33 (s, 2H), 9.09 (s, 2H), 8.76 (dd,1H, J = 2.0 Hz, J = 8.0 Hz), 7.51 (d, 1H, J = 8.0 Hz), 8.26 (s, 1H), 7.69 (d, 1H, J = 8.8 Hz), 7.67 (d, 1H, J = 8.8 Hz), 7.55 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.7, 161.9, 146.9, 142.6, 140.9, 136.0, 134.7, 131.9, 128.1, 124.0, 122.6, 119.7, 112.7, 104.0; ESI-MS: m/z calculated for C15H14N6: 278.31, found: 279.4 (amidine base M++1); Anal. Calcd. For C15H14N6+2HCl+1.65H2O+0.45Et2O: C, 48.7; H, 5.79; N, 20.28. Found: C, 48.81; H, 5.93; N, 19.99.
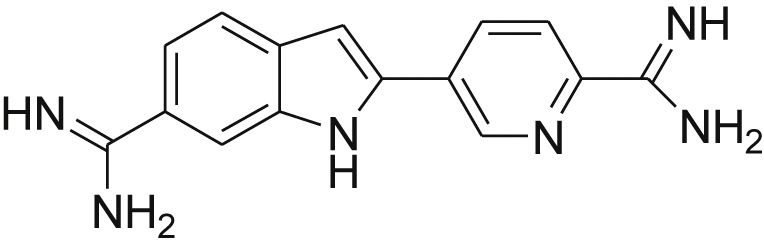
6.5. 2-(6-Amidinopyridine-3-yl)-1H-indole-6-amidine (5g)
Yellow solid, yield (0.171 gm, 71%), mp > 300 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.85 (s, 1H), 9.68 (s,2H), 9.48 (br s, 3H), 9.32 (s, 2H), 9.07 (s,2H), 8.74 (d,1H, J = 8.4 Hz), 7.49 (d, 1H, J = 8.4 Hz), 8.25 (s, 1H), 7.70 (d, 1H, J = 8.4 Hz), 7.66 (d, 1H, J = 8.4 Hz), 7.56 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.4, 162.9, 146.9, 142.7, 140.9, 136.0, 134.4, 131.9, 128.2, 124.0, 122.7, 119.7, 112.7, 104.3; ESI-MS: m/z calculated for C15H14N6: 278.31, found: 279.2 (amidine base M++1); Anal. Calcd. For C15H14N6+2HCl+0.35H2O: C, 50.4; H, 4.7; N, 23.5. Found: C, 50.72; H, 4.65; N, 23.16.
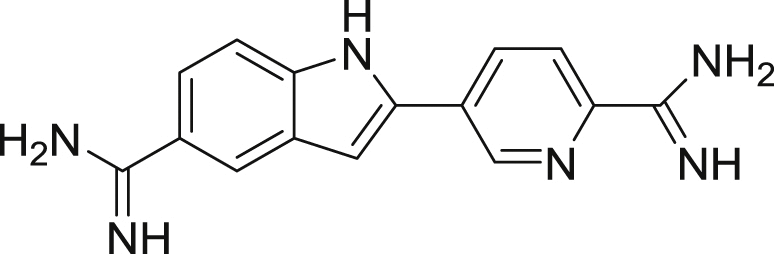
6.6. 2-(6-Amidinopyridine-3-yl)-1H-indole-5-amidine (5h)
Yellow solid, yield (0.166 gm, 69%), mp > 300 °C; 1HNMR (DMSO-d6, 400 MHz) δ 13.12 (s, 1H), 9.76 (s,2H), 9.51 (s,2H), 9.48 (s,1H), 9.33 (s, 2H), 9.10 (s,2H), 8.75 (d,1H, J = 8.4 Hz), 7.51 (d, 1H, J = 8.4 Hz), 8.26 (s, 1H), 7.71 (d, 1H, J = 8.8 Hz), 7.6 (d, 1H, J = 8.8 Hz), 7.56 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.9, 162.1, 147.0, 142.7, 141.0, 136.0, 134.2, 132.0, 127.7, 124.1, 122.7, 119.9, 112.8, 104.3; ESI-MS: m/z calculated for C15H14N6: 278.31, found: 279.2 (amidine base M++1); Anal. Calcd. For C15H14N6+2HCl+0.3H2O+ 0.2 Et2O: C, 51.08; H, 5.04; N, 22.62. Found: C, 50.94; H, 4.73; N, 22.94.
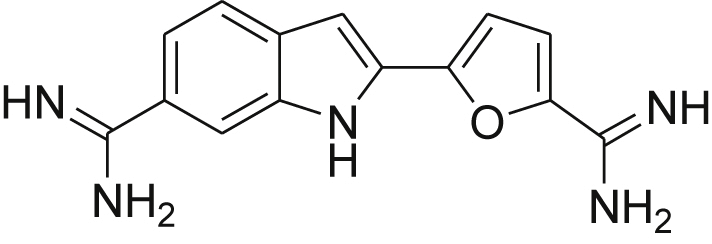
6.7. 2-(5-Amidinofuran-2-yl)-1H-indole-6-amidine (5i)
Yellow solid, yield (0.173 gm, 49%), mp 277–279 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.98 (s, 1H), 9.70 (s,2H), 9.39 (s,2H), 9.30 (s,2H), 9.07 (s,2H), 8.25 (s, 1H), 7.99 (d, 1H, J = 3.6 Hz), 7.73 (d, 1H, J = 8.8 Hz), 7.64(b, 1H, J = 8.8 Hz), 7.36 (d, 1H, J = 3.6 Hz), 7.32 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.7, 153.7, 151.6, 140.3, 140.1, 139.1, 130.0, 127.9, 122.7, 121.4, 119.7, 112.4, 110.0, 102.6,; ESI-MS: m/z calculated for C14H13N5O: 267.29, found: 268.4 (amidine base M++1); Anal. Calcd. For C14H13N5O +2HCl + H2O+0.23Et2O: C, 47.75; H, 5.18; N, 18.66. Found: C, 47.62; H, 5.48; N, 18.28.
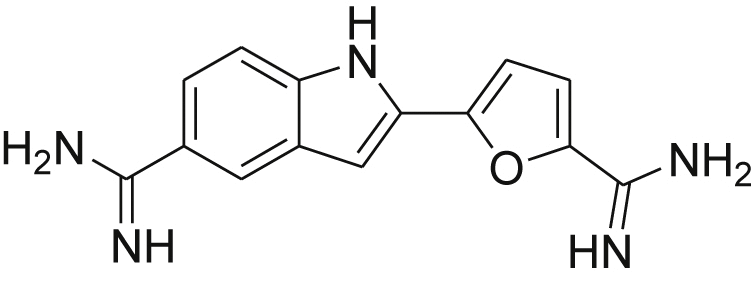
6.8. 2-(5-Amidinofuran-2-yl)-1H-indole-5-amidine (5j)
Yellow solid, yield (0.159 gm, 45%), mp 263–265 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.85 (s, 1H), 9.54 (s,2H), 9.42 (s,2H), 9.31 (s,2H), 9.17 (s,2H), 8.24 (s, 1H), 8.17 (d, 1H, J = 4 Hz), 7.91 (d, 1H, J = 4 Hz), 7.64 (br s, 2H), 7.11 (br s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.8, 158.9, 142.2, 140.5, 135.7, 133.3, 128.1, 127.7, 126.1, 122.6, 122.3, 119.8, 112.5, 102.6; ESI-MS: m/z calculated for C14H13N5O: 267.29, found: 267.2 (amidine base M+); Anal. Calcd. For C14H13N5O +2HCl+2H2O+0.24Et2O: C, 45.6; H, 5.47; N, 17.77. Found: C, 45.49; H, 5.62; N, 17.37.
6.9. 2-(5-Amidinothiophen-2-yl)-1H-indole-6-amidine (5k)
Yellow solid, yield (0.14 gm, 58%), mp 281–283 °C; 1HNMR (DMSO-d6, 400 MHz) δ 13.0 (s, 1H), 9.61 (s,2H), 9.30 (s,2H), 9.20 (s,2H), 9.03 (s,2H), 8.25 (s,1H), 8.02 (d, 1H, J = 4 Hz), 7.76 (dd, 1H, J = 1.2 Hz, J = 8.4 Hz), 7.61 (d, 1H, J = 8 Hz), 7.36 (d, 1H, J = 4 Hz), 7.33 (br s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 166.8, 153.2, 151.9, 140.4, 140.1, 140.0, 130.1, 127.1, 122.9, 121.4, 120.1, 112.5, 110.3, 102.7; ESI-MS: m/z calculated for C14H13N5S: 283.35, found: 284.1 (amidine base M++1); Anal. Calcd. For C14H13N5S +2HCl+0.02H2O: C, 47.14; H, 4.25; N, 19.63. Found: C, 46.96; H, 4.43; N, 19.39.
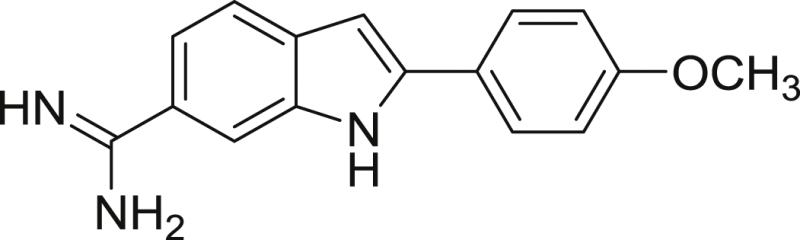
6.10. 2-(4-methoxyphenyl)-1H-indole-6-amidine (8)
The mononitrile 7 (0.23 g, 0.66 mmol) was dissolved in saturated ethanolic HCl (5 mL) and stirred at room temperature for 1 week, isolated from air and moisture. Dry ether was added, the crystals which formed were filtered, dried under vacuum for 1 h, dissolved in absolute ethanol, ammonia gas was passed into the solution for 30 min while cooling and the resulting solution was stirred for 4 days at room temperature. Dry ether was added, the precipitated crystals (HCl salt) were filtered. The diamidine was purified by neutralization with 1 N sodium hydroxide solution followed by filtration of the resultant solid and washing with water and dried. Finally, the free base was stirred with ethanolic HCl (5 mL) for 2 days at room temperature, diluted with ether, and the formed solid was filtered and dried.
White solid, yield (0.11 gm, 54%), mp 281–283 °C; 1HNMR (DMSO-d6, 400 MHz) δ 12.03 (s, 1H), 9.26 (s, 2H), 8.97 (s, 2H), 7.92 (m, 3H), 7.69 (d, 1H, J = 8.4 Hz), 7.44 (d, 1H, J = 8.4 Hz), 7.08 (d, 2H, J = 8.4 Hz), 6.95 (s, 1H); 13CNMR (DMSO-d6, 100 MHz) δ 167, 142.8, 137.2, 136.4, 133.5, 129.7, 127.7, 124.3, 120.3, 120, 119.2, 115, 112.2, 55.8; ESI-MS: m/z calculated for C16H15N3O: 265.31, found: 266.1 (amidine base M++1); Anal. Calcd. For C16H15N3O +1HCl+1.15H2O: C, 59.59; H, 5.71; N, 13.03. Found: C, 59.35; H, 5.78; N, 13.01.
Acknowledgements
This work was supported by an award from the Bill and Melinda Gates Foundation through the Consortium for Parasitic Drug Development (RB, WDW, DWB).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech.2017.01.037.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.a) http://www.who.int/mediacentre/factsheets/fs259/en/.; b) Brun R., Blum J., Chappuis F., Burri C. Lancet. 2010;375:148. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]; c) Maeser P., Wittlin S., Rottmann M., Wenzler T., Kaiser M., Brun R. Curr. Opin. Pharmacol. 2012;5:562. doi: 10.1016/j.coph.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Bray P.G., Barrett M.P., Ward S.A., de Koning H.P. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 2003;19:232. doi: 10.1016/s1471-4922(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 3.Dann O., Bergen G., Demant E., Vol G. Trypanosomicidal diamidines of 2-phenylbenzofuran, 2-phenylindene, and 2-phenylindole. Liebigs Ann. Chem. 1971;749:68. [Google Scholar]
- 4.Anne J., De Clercq E., Eyssen H., Dann O. Antifungal and antibacterial activities of diarylamidine derivatives. Antimicrob. Agents Chemother. 1980;18:231. doi: 10.1128/aac.18.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson W.D., Tanious F.A., Barton H.G., Jones R.L., Fox K., Wydra R.L., Strekowski L. DNA sequence dependant binding modes of 4’, 6-diamidino-2-phenylindole (DAPI) Biochemistry. 1990;29:8452. doi: 10.1021/bi00488a036. [DOI] [PubMed] [Google Scholar]
- 6.Kapuscinski J. DAPI : a DNA-specific fluorescent probe. Biotech. Histochem. 1995;70:220. doi: 10.3109/10520299509108199. [DOI] [PubMed] [Google Scholar]
- 7.Farahat A.A., Kumar A., Say M., Barghash A.M., Goda F.E., Eisa H.M., Wenzler T., Brun R., Liu Y., Mickelson L., Wilson W.D., Boykin D.W. Synthesis, DNA binding, fluorecence measurments and antiparasitic activity of DAPI related diamidines. Bioorg. Med. Chem. 2010;18:557. doi: 10.1016/j.bmc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Farahat A.A., Kumar A., Paliakova E., Barghash A.M., Goda F.E., Eisa H.M., Wenzler T., Brun R., Liu Y., Wilson W.D., Boykin D.W. Exploration of larger central ring linkers in furamidine analogues: synthesis and evaluation of their DNA binding, antiparasitic and fluorescence properties. Bioorg. Med. Chem. 2011;19:2156. doi: 10.1016/j.bmc.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Farahat A.A., Kumar A., Paliakova E., Barghash A.M., Goda F.E., Eisa H.M., Wenzler T., Brun R., Liu Y., Wilson W.D., Boykin D.W. Synthesis, DNA binding and antitrypanosomal activity of benzimidazole analogues of DAPI. Bioorg. Med. Chem. Lett. 2016;26:5607. doi: 10.1016/j.bmcl.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A., Say M., Boykin D.W. Synthesis of new substituted 2-(trimethylstannyl)indoles. Synthesis. 2008;5:707. [Google Scholar]
- 11.(a) Guo P., Paul A., Kumar A., Farahat A.A., Kumar D., Wang S., Boykin D.W., Wilson W.D. The thiophene sigma hole as a concept for Preorganized, specific recognition of G.C base pairs in the DNA minor groove. Chem. Eur. J. 2016;22:15404. doi: 10.1002/chem.201603422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Abou-ELkhair R.A.I., Hassan A.E.A., Boykin D.W., Wilson W.D. Lithium hexamethyldisilazane transformation of transiently protected 4-aza/benzimidazole nitriles to amidines and their dimethyl sulfoxide mediated imidazole ring formation. Org. Lett. 2016;18:4714. doi: 10.1021/acs.orglett.6b02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughlin S., Wang S., Kumar A., Farahat A.A., Boykin D.W., Wilson W.D. Resolution of mixed site DNA complexes with dimer-forming minor-groove binders by using electrospray ionization Mass Spectrometry: compound structure and DNA sequence effects. Chem. Eur. J. 2015;21:5528. doi: 10.1002/chem.201406322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson W.D., Tanious F.A., Fernandez-Saiz M., Rigl C.T. Evaluation of drug–nucleic acid interactions by thermal melting curves. In: Fox K., editor. Methods in Mol. Biol., Drug–DNA Interaction Protocols. vol. 90. 1997. p. 219. [DOI] [PubMed] [Google Scholar]
- 14.Wilson W.D., Tanious F.A., Mathis A., Tevis D., Hall J.E., Boykin D.W. Antiparasitic compounds that target DNA. Biochimie. 2008;90:999. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakunov S.A., Bakunova S.M., Wenzler T., Ghebru M., Werbovetz K.A., Brun R., Tidwell R.R. Synthesis and antiprotozoal activity of cationic 1,4-Diphenyl-1-H-1,2,3-triazoles. J. Med. Chem. 2010;53:254. doi: 10.1021/jm901178d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzler T., Boykin D.W., Ismail M.A., Hall J.E., Tidwell R.R., Brun R. New treatment option for second-stage African sleeping sickness: in vitro and in vivo efficacy of aza analogues of DB289. Antimicrob. Agents Chemother. 2009;53:4185. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mc Coubrey A., Latham H.C., Cook P.R., Rodger A., Lowe G. 4-Picoline-2,2': 6',2”-terpyridine-platinum(II)- a potent intercalator of DNA. FEBS Lett. 1996;380:73. doi: 10.1016/0014-5793(95)01537-x. [DOI] [PubMed] [Google Scholar]
- 18.Tidwell R.R., Geratz J.D., Dann O., Volz G., Zeh D., Loewe H. J. Med. Chem. 1978;21:613. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein Steven W., Mylari Banauara L., Perez Jose R., Glazer Edward A. 2004. Preparation of Bisamidinophenylindoles as Antiproliferative DNA Methyltransferase Inhibiting agents.U.S. US 6699862 B1 20040302. [Google Scholar]
- 20.Li B., Pai R., Cardinale S.C., Butler M., Peet N.P., Moir D.T., Sina Bavari B., Bowlin T.L. Synthesis and biological evaluation of botulinum neurotoxin a protease inhibitors. J. Med. Chem. 2010;53:2264. doi: 10.1021/jm901852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.