Highlights
-
•
Few women received the OCV unknowingly while pregnant during a large vaccine trial.
-
•
There is limited data on the safety of OCVs in pregnancy.
-
•
We evaluated the effect of a killed OCV, Shanchol™, on pregnancy outcomes.
-
•
Study showed no evidence of exposure to Shanchol™ on adverse pregnancy outcomes.
Keywords: Shanchol™, Oral cholera vaccine, Pregnancy, Vaccination
Abstract
Background
Pregnant women are vulnerable to complications of cholera. Killed oral cholera vaccines (OCV) are not recommended for pregnant women though there is no evidence of harmful effects during pregnancy. We evaluated the effect of a killed OCV, Shanchol™, on pregnancy outcomes during an effectiveness trial of the vaccine in urban Bangladesh.
Methodology
Individuals ⩾1 year were invited to participate in the trial, conducted in 2011 in Dhaka, Bangladesh. Pregnancy by history was an exclusion criterion and all women of reproductive age (15–49 years) were verbally questioned about pregnancy at enrollment and prior to vaccination. Out of 48,414 women of reproductive age 286 women received the OCV unknowingly while pregnant. Out of these, we could recruit 69 women defined as exposed to OCV. Accordingly, we selected 69 pregnant women randomly from those who did not take the OCV (non-exposed to OCV). We evaluated adverse pregnancy outcome (spontaneous miscarriages, still births, or congenital malformations) between those who were exposed to OCV and those who were not exposed to OCV.
Results
About 16% of pregnant women exposed to OCV had pregnancy loss, as compared to 12% of unvaccinated pregnant women (P = 0.38). One congenital anomaly was observed and occurred in women non-exposed to OCV group. Models that adjusted for baseline characteristics that were unbalanced between the exposed and non-exposed groups, revealed a no elevation of risk of adverse pregnancy outcomes in vaccinees versus non-vaccinees (Adj. OR (95% CI): 0.45 (0.11–1.88).
Conclusions
No excess of adverse fetal outcomes associated with receipt of OCV was observed in this study.
Trial registration: Clinical Trials.gov number NCT01339845.
1. Background
Women of child-bearing age are at high risk of cholera in endemic settings [1]. Moreover, cholera during pregnancy has been associated with abortion, premature childbirth and maternal death [2], [3], [4]. Thus, prevention of cholera in pregnancy constitutes an important public health priority. One such preventive approach is administration of killed oral cholera vaccine (OCV), as two such vaccines represent the only cholera vaccines currently prequalified by WHO for purchase by UN agencies.
According to WHO, those who are at high risk for severe cholera and for which the vaccines are not contraindicated, such as pregnant women and HIV-infected individuals, may also be targeted for receipt of killed OCVs [5]. However, administration of OCVs to pregnant women presents a dilemma, since there is limited data on the safety of OCVs in pregnancy. The data that do exist show no evidence of a harmful effect. One recent study in Guinea showed that there was no significant increase in adverse fetal or newborn outcomes among pregnant women who received killed OCV [6]. Another study conducted in Zanzibar showed that there was no statistically significant evidence of a harmful effect of gestational exposure to a killed OCV [7].
Moreover, there are several reasons to believe that killed OCVs are unlikely to have a harmful effect on fetal development and survival. Firstly, the bacteria in the vaccine are killed and do not replicate. Secondly, the vaccine antigens act locally on the gastro-intestinal mucosa, and are not absorbed systemically. Thirdly, these types of vaccines do not generally trigger systemic reactions (e.g. fever) linked to abortion early in pregnancy. Finally, there are several examples of inactivated vaccines given parenterally, such as tetanus, acellular pertussis, and influenza vaccines, that are currently recommended for use in pregnancy, with substantial records of safety, albeit with recommendations that the vaccines be given in the third trimester of pregnancy [8].
A cluster-randomized, effectiveness trial of the killed whole cell OCV, Shanchol™ (Shantha Biotechnics, Hyderabad, India/Sanofi), was initiated in February 2011 in Dhaka, Bangladesh [9]. The package insert in the Shanchol vaccine states ‘‘No specific clinical studies have been performed to evaluate the safety and immunogenicity of Shanchol in pregnant women and for the fetus. The vaccine is therefore not recommended for pregnant women.” Accordingly, individuals who were one year and older and non-pregnant were invited to receive the vaccine in this feasibility study. Pregnancy status was screened verbally for all married women of child bearing age (15–49 years) during a baseline census as well as immediately before vaccination. Because this screening could have missed some women who were in fact pregnant at the time of vaccination, we identified women who were likely pregnant at the time of dosing and compared the occurrence of fetal loss (miscarriage and still birth) or malformation between these women and a control group of women who were deemed ineligible for the trial because of their pregnancies. Herein, we present the results of our evaluation.
2. Methods
2.1. The study area and population
As previously described [10], the study was conducted in six wards of Mirpur, an urban area of Dhaka City, Bangladesh with a population of about 269,000. In brief, a census was conducted between September and December 2010 to register the population judged to be at high risk for cholera in the study area, by virtue of lower socioeconomic status and poor sanitation and hygiene practices [9]. After acquisition of verbal informed consent from the head of the household, demographic, socioeconomic, and water use-hygiene data were collected. Data were directly entered into the handheld computers, also known as personal digital assistants (PDA) [11]. A unique identification number (ID) was assigned to each individual registered in the census, and ID cards containing identification about censused persons were distributed to the residents of the study area.
2.2. The mass vaccination campaign
During the study, residents of the study area who were one year of age or older were invited to participate in the mass vaccination campaign and all healthy, non-pregnant residents were issued study identification cards. The residents were requested to bring their ID cards when coming to the vaccination centre.
The study had three arms: one receiving only vaccine, one receiving vaccine plus water-hygiene behavior change communication (BCC) to improve water quality and hand washing practices, and a non-intervention arm. The mass vaccination campaign was targeted to the residents of vaccine and vaccine plus BCC arms. Each 1.5 ml dose of the liquid bivalent Shanchol™ vaccine contained inactivated whole-cell heat-killed and formalin-killed Vibrio cholerae O1 and O139 [12]. The vaccine was given in two doses; between 17 February 2011 and 16 April 2011 with a gap of at least 14 days between the doses. Details of the study procedures and the mass vaccination campaign along with the result have been published [10]. Verbal histories of pregnancy were obtained from married women aged 15–49 years at the time of each dose, as well as in the census, and women judged to be pregnant were excluded from dosing. We defined pregnancy as a statement by the woman that she is pregnant, or gave a history of having the onset of her last menstrual period (LMP) 28 days or more prior to the interview. We also excluded the women from dosing who were uncertain about the timing of their LMP.
2.3. Assembly of the final cohorts and ascertainment of pregnancy outcomes
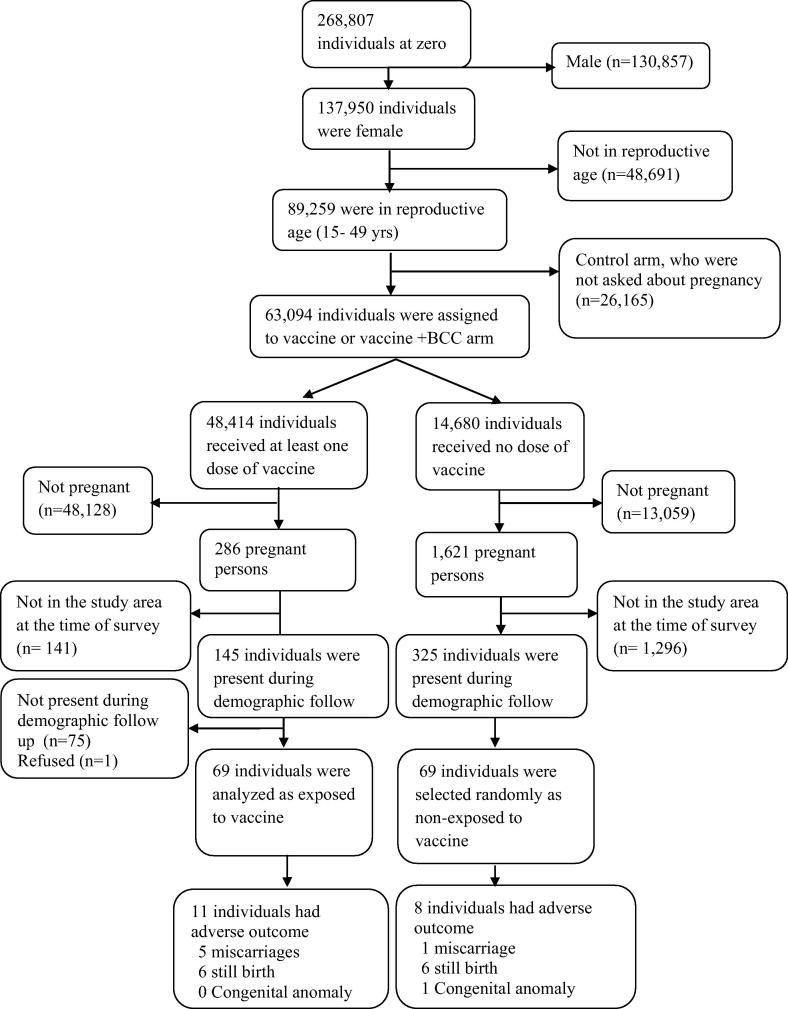
We enumerated 89,259 married women of reproductive age at the time of the vaccination campaign in our study area. Of them, 63,094 individuals were assigned to receive vaccine, and 48,414 women received at least one dose of OCV. Among them, 286 women took the 1st dose (based on vaccination record book) while pregnant, as ascertained at the time of the second dose. To assemble a control group of non-vaccinated women for follow-up interviews, a list of 1621 pregnant women was produced from the baseline census survey data. These women were ineligible for receiving the vaccine due to their pregnancy and did not take the vaccine. Surveillance for pregnancy outcomes was carried out between 14 Jan 2013 and 13 Jun 2013—about 21 months after the vaccination. The mean (SD) time of interview from “zero time” (defined as date of dosing while pregnant for exposed individuals and the median/mid-point of dosing interval for the entire population for non-exposed women) for the vaccinated group was 467 (88) days while for the non-vaccinated group was 554 (50) days. Interview of the non-vaccinated women was delayed because we reach them after completion of exposed women to match equal number of exposed and non-exposed group as we planned. Out of these 286 pregnant, vaccinated women, only 69 women were found present in the study area at the time of the interviews. Information on pregnancy and dosing were reconfirmed by taking the history from the participants. Among the non-vaccinated, pregnant women, only 325 women were present in the study area at the time of physician visits for this study. Of those, 69 women were randomly selected (to keep the exposure and non-exposure ratio 1:1, Fig. 1).
Fig. 1.
Assembling of the exposed and non-exposed to oral cholera vaccine (OCV) for the study.
Following training on study procedures, the physicians with the help from field staff visited the residences of the listed women, and, after acquisition of informed consent, interviewed the women and examined index newborns to collect information about the duration, outcome, and antenatal care received in their pregnancies, as well as additional information about exposures during pregnancy and past obstetric history.
2.4. Study endpoints
The study endpoint included the adverse fetal outcomes of miscarriage, stillbirth and congenital anomaly. A gross congenital malformation was defined as a physical defect present in a baby at birth and evident on the naked baby on physical examination (e.g. cleft lip or palate, Down syndrome, spina bifida, club foot). We classified miscarriage as spontaneous, induced, or accidental abortion. A spontaneous abortion was defined as termination of a pregnancy without a known external cause within 28 weeks of gestation (duration of gestation was estimated as the interval between the LMP and the date of pregnancy termination). Induced abortion was defined as termination of pregnancy by drug or any medical intervention within 28 weeks of gestation. Accidental abortion was defined as abortion caused unintentionally by a fall, blow or any other accidental injury (there was only one accidental abortion from the vaccinees). In the study endpoint definition we considered only spontaneous abortions. A stillbirth was defined as delivery of a dead fetus after 28 weeks of gestation.
2.5. Statistical analysis
In simple analyses we compared characteristics of the vaccinated and non-vaccinated groups with the Chi-square test (or Fisher’s exact test for sparse data) and Student's t-test (or the Mann-Whitney test for non-normal data) for binary and continuous variables, respectively, and expressed the association between receipt of OCV and the occurrence of an adverse fetal outcome as odds ratios. To adjust for confounders, we used multivariable logistic models with the occurrence of an adverse fetal outcome as the dependent variable. Since we recorded relatively few adverse fetal outcomes, we avoided over fitting the models by computing propensity scores [13] using the variables that were unbalanced in the vaccinated and non-vaccinated groups to adjust for confounding variables [13], [14], [15]. We calculated the propensity score using a logistic regression model regressing exposure to OCV on the covariates that were associated with exposure to OCV at p ⩽ 0.20 in bivariate analyses, which included gestational age at zero time, age at zero time, education, sanitary toilet use, sharing of kitchen with other household(s) and having one or more children prior to the index pregnancy. All p-values and 95% confidence intervals were interpreted in a two-tailed fashion. The analyses were performed using R3.2.3.
2.6. Ethics
This study was approved by the research review committee and ethical review committee of icddr,b. During census enumeration and census updates of the study population, verbal informed consent was obtained prior to interviewing the participants. During the mass vaccination, written informed consent was obtained from each participant or from his or her guardian, if they were less than 18 years of age. In addition, verbal assent from children 11 to 17 years of age was obtained. The participants received information regarding the vaccine, including advice for children less than 1 year of age and pregnant women not to receive the vaccine. The follow up of pregnant women and the ascertainment of pregnancy outcomes were added as addendum to the approved protocol and was approved by Institutional Review Board of icddr,b. Written informed consent was obtained before interviewing the women to ascertain their pregnancy outcomes.
3. Results
Table 1 compares the two groups for several features. Only gestational age at zero time and recall period of pregnancy outcome from zero time were significantly different between two groups (both at p < 0.01), while important, but non-significant differences were observed for literacy (higher in vaccinees); having a sanitary toilet (higher in vaccinees); living a household with a shared kitchen (higher in non-vaccinees); living in a house with more than four people per room (higher in non-vaccinees); and having had one or more children prior to the index pregnancy (higher in vaccinees). The average age of vaccinees and non-vaccinees at zero time was 25 and 24 years, respectively, and the median of gestational age of vaccinees and non-vaccinees at zero time was 4 and 14 weeks, respectively.
Table 1.
Characteristics of vaccinated and non-vaccinated participants at zero time.
| Characteristics | Exposed to OCV (n = 69) N (%) | Non-exposed to OCV (n = 69) N (%) | p-value |
|---|---|---|---|
| Median (IQR) of gestational age (in weeks) at zero time | 4 (4, 8) | 14 (12, 20) | <0.01 |
| Mean (SD) of age (years) at zero time | 25 (5) | 24 (5) | 0.20 |
| Literate (at least 6 years of schooling) | 36 (52.174) | 25 (36) | 0.09 |
| Living in a household had sanitary toilet | 54 (78.26) | 46 (67) | 0.18 |
| Living in a household had improved water source (own tap) for drinking | 7 (10.15) | 9 (13) | 0.79 |
| Living in a household had shared kitchen | 10 (14.49) | 17 (25) | 0.20 |
| Living in a household had more than 4 people sharing in a room | 24 (34.78) | 31 (45) | 0.30 |
| Household income < BDT. 10000.00a | 34 (49.28) | 36 (52 | 0.87 |
| Having one or more children | 48 (69.56) | 39 (57) | 0.16 |
| Mean (SD) of the recall period of pregnancy outcome (days) | 467 (88) | 554 (50) | <0.01 |
1USD = BDT80.00.
Out of 69 exposed women, 3 pregnancies were terminated by induced abortion and one by accidental abortion were excluded in primary analysis, resulting in 65 women in the exposed group and 69 women in the non-exposed group. The distribution of study endpoints is reported in Table 2. There were 11 adverse fetal outcomes born to vaccinated mothers (5 spontaneous abortions and 6 stillbirths) and 8 adverse fetal outcomes in non-vaccinated mothers (1spontaneous abortions 6 stillbirths and 1 congenital anomaly). The only identified child with a congenital anomaly had congenital heart disease, evident as a cardiac murmur on auscultation and confirmed by doctor’s note. The unadjusted analyses found a somewhat elevated risk of adverse outcomes in the vaccinated group, though the difference in risk between vaccinees and non-vaccinees was not statistically significant (odds ratio (OR): 2.88; 95% CI: 0.87–6.44 for primary analysis and 2.28; 95% CI: 0.87–6.44 for a secondary analysis in which induced abortions were included (see Table 3).
Table 2.
Pregnancy outcome by vaccination status.
| Outcome | Exposed to OCV n (%) | Non-exposed to OCV n (%) |
|---|---|---|
| N | 69 (100) | 69 (100) |
| Live birth | 54 (78) | 62 (90) |
| Induced abortion | 3 (4) | 0 (0) |
| Accidental abortion | 1 (1) | 0 (0) |
| Spontaneous abortion | 5 (7) | 1 (1) |
| Still birth | 6 (8) | 6 (9) |
| Congenital malformation | 0 (0) | 1 (1) |
| All fetal adverse outcomes, excluding therapeutic abortions | 11 (16) | 8 (12) |
| Overall fetal adverse outcomes including therapeutic abortions | 15 (22) | 8 (12) |
| Crude OR of having adverse outcomea | 1.55 (95% CI: 0.59–4.28; p = 0.38) | |
| Crude OR of having adverse outcomeb | 2.11 (95% CI: 0.85–5.62; p = 0.12) | |
Overall fetal adverse outcomes include spontaneous abortion, still birth, intrauterine death and congenital malformation.
Overall fetal adverse outcome 2 include spontaneous abortion, still birth, intrauterine death and congenital malformation along with induced abortion.
Table 3.
Associations between exposure to oral cholera vaccine (OCV) and occurrence of adverse pregnancy outcomes in Dhaka, Bangladesh.
| Exposed to OCV (cases/total samples) | Non-exposed to OCV (cases/total samples) | Odds ratio (95% CI) | p-Value | |
|---|---|---|---|---|
| Model 1 | 11/65 | 8/69 | 1.63 (0.58–4.77) | 0.36 |
| Model 2 | 15/69 | 8/69 | 2.28 (0.87–6.44) | 0.10 |
| Model 3 | 11/65 | 8/69 | 0.45 (0.11–1.88) | 0.27 |
| Model 4 | 15/69 | 8/69 | 0.59 (0.15–2.34) | 0.22 |
Model 1: Adverse pregnancy outcome (without induced abortion) was modeled on the exposure to OCV adjusted for propensity scorea.
Model 2: Adverse pregnancy outcome (including induced abortion) was modeled on the exposure to OCV adjusted for propensity scorea.
Model 3: Adverse pregnancy outcome (without induced abortion) was modeled on the exposure to OCV adjusted for propensity scoreb.
Model 4: Adverse pregnancy outcome (including induced abortion) was modeled on the exposure to OCV adjusted for propensity scoreb.
Propensity score includes age at zero time, education, sanitary toilet use, sharing of kitchen with other household(s) and having one or more children prior to the index pregnancy.
Propensity score additionally includes gestational age at zero time.
In adjusted primary analyses, a multivariable model (Model 1) that adjusted for the propensity score (gestational age excluded) showed statistically non-significant association between vaccine exposure and adverse pregnancy outcome (OR: 1.63; 95% CI: 0.58–4.77). A similar association was observed (Model 2) if induced abortion was also considered as adverse outcome (OR: 2.28; 95% CI: 0.87–6.44). On the other hand, with gestational age at zero time added to the propensity score, the adjusted analysis displayed no elevation of risk for either the analysis without (OR: 0.45; 95% CI: 0.11–1.88) or with (OR: 0.59; 95% CI: 0.15–2.34) inclusion of induced abortions as outcomes.
4. Discussion
The results of the present study showed no evidence that exposure to Shanchol™ vaccine had adverse outcomes in pregnancy. Our data are also concordant with an analysis of pregnancy outcomes among women who received Shanchol while pregnant during a vaccine campaign in Guinea [6]. Similar findings were observed for the Dukoral™ cholera vaccine in Zanzibar [7]. Pregnant women who did not receive OCV due to pregnancy were interviewed as control to compare with the women who receive OCV as case. Use of a concurrent cohort of non-vaccinated pregnant women, selected from the same geographic locale as vaccinees and with a socioeconomic status, was strength of this study. However, the study had a number of limitations. A major limitation of this study was the small number of women who received OCV while pregnant (n = 69), with a small number of detected adverse fetal outcomes, which reduced our ability to detect an elevated risk of adverse fetal outcomes if one existed in the exposure group. Moreover, the substantial loss to follow-up of a large proportion of the cohort of vaccinated women could have led to a bias. In urban Bangladesh, where the study took place, pregnant women are likely to stay for a long period of time in their rural home villages after the delivery, which could be the reason for the low rate of not finding delivered women during the time of surveillance. As well, exposure or non-exposure to the vaccine was not randomized, and factors related to the risk of adverse pregnancy outcome could have been imbalanced between the compared groups and biased our results. We controlled for measured factors that were imbalanced using propensity scores. As expected, women in the vaccinated group were followed from an earlier stage of pregnancy than non-vaccinated women, making them inherently more likely to have reported spontaneous or therapeutic abortions. Adjustment for gestational age and zero time revealed no elevation of risk of adverse pregnancy outcome. Nonetheless, residual confounding due to unmeasured variables could have affected our results. Thirdly, bias from differential recall of pregnancy outcomes in the two groups cannot be ruled out, since the interviews were carried out retrospectively, with longer post-outcome recall intervals for unvaccinated than vaccinated women, although the direction of such a bias is difficult to predict.
5. Interpretation
The results of the study showed no evidence of an elevated risk of adverse fetal outcomes in pregnant women who receive the killed OCV, Shanchol™ and is concordant with the findings on the risk of adverse pregnancy outcomes in women who received killed OCVs during pregnancy. While more studies are needed, this study supports a recommendation that killed OCVs may be given to pregnant women, for whom a judgment is made that the benefit outweighs the risk, taking account of the epidemiological context of cholera vaccination [16], [17].
Contributors
FQ, JC, AC, AIK, FC, IAK, AS, AK, MA contributed to the study design and implementation. FQ, AIK, FC, AS, IAK contributed to the implementation and supervision of the study. YAY, NCD, AK involved in data cleaning and analysis. MA supervises the data analysis and took responsibility for the accuracy of the data analysis. All others (AK, AA, MA) supported the study in the different components. All authors participated in the writing of the manuscript and had full access to the data in the study. All authors saw and approved the final version of the manuscript.
Role of the funding source
The funding agency of this study is the Bill & Melinda Gates Foundation. The Foundation had no role in data collection, data analysis, data interpretation, or writing this manuscript.
Conflicts of interest
There are no conflicts of interest by authors.
Financial support
This work was funded by the Bill and Melinda Gates Foundation (Grant No. OPP50419).
Funding
Bill & Melinda Gates Foundation.
Acknowledgements
This study was supported by a Grant OPP50419 from the Bill & Melinda Gates Foundation Additionally the study was also supported by core grants to the icddr,b. icddr,b also gratefully acknowledges the following donors who provide unrestricted support: Government of the People’s Republic of Bangladesh; Global Affairs Canada (GAC); Swedish International Development Cooperation Agency (Sida) and the Department for International Development, (UKAid). Shantha-Sanofi provided vaccine for the study at a discretionary price. We are grateful to the people of Mirpur where our study is being undertaken; to the field, laboratory and data management staff who provided tremendous effort to make the study successful; and to the people who provided valuable support in our study.
Footnotes
Informed consent was obtained from the study participants. The research was approved by the Research and Ethical Review Committees of the International Centre of Diarrheal Disease Research, Dhaka, Bangladesh and IRB of the International Vaccine Institute.
References
- 1.Glass R.I. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol. 1982;116(6):959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 2.Diop S. Cholera and pregnancy: epidemiological, clinical, and evolutionary aspects. Medecine et maladies infectieuses. 2007;37(12):816–820. doi: 10.1016/j.medmal.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Hirschhorn N., Chowdhury A.A., Lindenbaum J. Cholera in pregnant women. Lancet. 1969;293(7608):1230–1232. doi: 10.1016/s0140-6736(69)92115-1. [DOI] [PubMed] [Google Scholar]
- 4.Ayangade O. The significance of cholera outbreak in the prognosis of pregnancy. Int J Gynecol Obstetr. 1981;19(5):403–407. doi: 10.1016/0020-7292(81)90025-4. [DOI] [PubMed] [Google Scholar]
- 5.WHO, WHO position paper. Wkly Epidemiol Rec, 2010:85(13): 117–28. [PubMed]
- 6.Grout L. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine in Guinea: a retrospective cohort study. PLoS Negl Trop Dis. 2015;9(12):e0004274. doi: 10.1371/journal.pntd.0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashim R. Safety of the recombinant cholera toxin B subunit, killed whole-cell (rBS-WC) oral cholera vaccine in pregnancy. PLoS Negl Trop Dis. 2012;6(7):e1743. doi: 10.1371/journal.pntd.0001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sur D.K., Wallis D.H., O’Connell T.X. Vaccinations in pregnancy. Am Fam Physician. 2003;68(2):299–304. [PubMed] [Google Scholar]
- 9.Khan I.A. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka, Bangladesh. Vaccine. 2013;31(51):6058–6064. doi: 10.1016/j.vaccine.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Qadri F. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet. 2015;386(10001):1362–1371. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 11.Ali M. Paperless registration during survey enumerations and large oral cholera mass vaccination in Zanzibar, the United Republic of Tanzania. Bull World Health Organ. 2010;88(7):556–559. doi: 10.2471/BLT.09.070334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahalanabis D. A randomized, placebo-controlled trial of the bivalent killed, whole-cell, oral cholera vaccine in adults and children in a cholera endemic area in Kolkata, India. PLoS ONE. 2008;3(6):e2323. doi: 10.1371/journal.pone.0002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 14.Peduzzi P. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 15.Patorno E. Studies with many covariates and few outcomes: selecting covariates and implementing propensity-score–based confounding adjustments. Epidemiology. 2014;25(2):268–278. doi: 10.1097/EDE.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 16.WHO, Technical Note: evidence of the risks and benefits of vaccinating pregnant women with WHO pre-qualified cholera vaccines during mass campaigns; 2016.
- 17.CHOLERA, S., Cholera and the use of oral cholera vaccines in pregnant women; 2016.