Abstract
Background
Decellularized heart valves are emerging as a potential alternative to current bioprostheses for valve replacement. While techniques of decellularization have been thoroughly examined, terminal sterilization techniques have not received the same scrutiny.
Methods
This study evaluated low dose gamma irradiation as a sterilization method for decellularized heart valves. Incubation of valves and transmission electron microscopy evaluation after different doses of gamma irradiation were used to determine the optimal dose of gamma irradiation. Quantitative evaluation of mechanical properties was done by tensile mechanical testing of isolated cusps. Sterilize decellularized heart valves were tested in a sheep model (n=3, 1 1,500 Gy and 2 3,000 Gy) of pulmonary valve replacement.
Results
Valves sterilized with gamma radiation between 1,000 Gy and 3,000 Gy were found to be optimal with in-vitro testing. However, with in-vivo showed deteriorating valve function within 2 months. On explant the valve with 1,500 Gy gamma irradiation showed signs of endocarditis with neutrophils on hematoxylin and eosin staining, positive gram stain resembling streptococcus infection. The 3,000 Gy valves had no evidence of infection, but the hematoxylin and eosin staining showed evidence of wound remodeling with macrophages and fibroblasts. Tensile strength testing showed decreased strength (0 Gy-2.53±0.98 MPa, 1,500 Gy-2.03±1.23 MPa, 3,000 Gy-1.26±0.90 MPa) with increasing levels of irradiation.
Conclusions
Low dose gamma irradiation does not maintain the mechanical integrity of valves and the balance between sterilization and damage may not be able to be achieved with gamma irradiation. Other methods of terminal sterilization must be pursued and evaluated.
Keywords: gamma irradiation, porcine tissue, scaffold, xenotransplantation
Maintenance of mechanical integrity and achievement of decontamination are both important when choosing the terminal sterilization procedure for decellularized tissues such as heart valves. Low-dose (<10,000 gray [Gy]) gamma irradiation has the potential to achieve both aims. Tissue banks have ubiquitously used gamma irradiation for sterilization of bone [1], tendon, and even teeth [2]. Before bioprosthetic valve manufacturing techniques became proprietary, gamma irradiation was a promising way of sterilizing bioprosthetic valves for clinical use [3]. Gamma irradiation to sterilize decellularized porcine pulmonary valves has been reported recently, but the specifics of the sterilization process were not given [4].
The effect of gamma irradiation on the mechanical properties of scaffolds has been dose dependent in studied tissues, including bone [5]. Thus, we hypothesized that, at low doses, gamma irradiation would have the potential to sterilize while minimally altering the mechanical properties of heart valves.
Materials and Methods
Heart Valve Processing
Whole porcine hearts were obtained from a slaughterhouse abattoir. The aortic valve was meticulously dissected from the rest of the heart. The anterior leaflet of the mitral valve was left intact, and minimal myocardium was left on the valve construct. Decellularization was performed with 1% sodium dodecyl sulfate (SDS) and 2% DNAse for 4 days changing every day followed by 2 days of 2% DNAse, 1M MgCl2, and 1M Tris Buffer.
Gamma Irradiation Dose
Different doses of gamma irradiation were chosen to test for both sterility and extracellular matrix integrity. Each decellularized porcine heart valve was individually sealed and subjected to gamma irradiation (no irradiation [control], 1,000 Gy, 3,000 Gy, or 10,000 Gy). These doses were chosen because they represent the range of the effect of gamma irradiation from none to high-dose gamma irradiation. Specimens from the cusps, sinuses, and root of each sample were then cultured in DMEM (Dulbecco’s Modified Eagle’s Medium) for 30 days or until contamination was evident. Additional cusp specimens were examined with transmission electron microscopy to qualitatively determine extracellular matrix damage by evaluating matrix breakage and orientation.
In Vitro Sterility Testing
The decellularized porcine heart valves that were gamma irradiated at 1,500 Gy (n=4) and 3,000 Gy (n=4) were subjected to further in vitro sterility testing. Specific tests conducted were Gram stain, fungal stain, and incubation in 3 different types of culture broth meant to induce bacterial growth (tryptic soy broth, thioglycollate broth, and Sabouraud dextrose broth) at 37°C.
In Vitro Tensile Strength Testing
Cusps of porcine heart valves were gamma irradiated at 3,000 Gy (n=12) and at 1,500 Gy (n=12). They were then isolated and punched into dog bone shapes 8 mm wide at the ends and 2 mm wide at the center, with the long axis in the circumferential direction. Digital calipers were used to measure the sample thickness at the narrow region. Samples were clamped within custom grips on a Bose ElectroForce 3200 tensile tester (Bose Corp, Eden Prairie, Minnesota) with the aid of cyanoacrylate adhesive and sandpaper applied at each end. A uniaxial tensile displacement of 0.1667 mm/second was applied while force and displacement were recorded. Ultimate stress was calculated as the force per cross-sectional area at failure which gives an indication of the strength of the tissue (i.e. higher ultimate stress corresponds to higher strength). Stiffness was calculated as the slope of the linear region of the stress-strain relationship which gives an indication of the elasticity of the tissue (i.e. higher stiffness corresponds to lower elasticity).
Animals and Operative Model
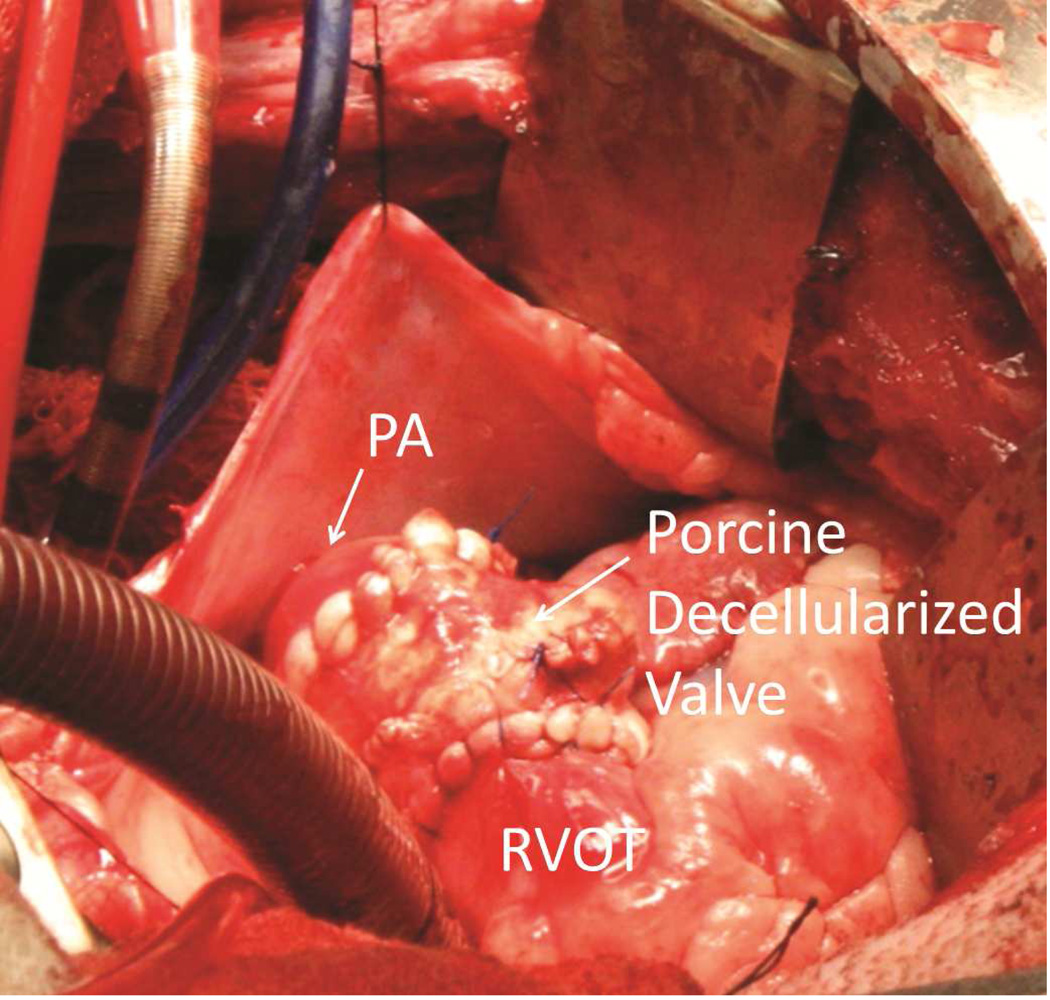
Animal experiments were conducted conforming to the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals, and were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Three juvenile sheep that were 3 to 4 months of age and weighed 30 kg to 40 kg underwent pulmonary valve replacement. One sheep received a valve that had been gamma irradiated with 1,500 Gy. The other 2 sheep received valves that had been gamma irradiated with 3,000 Gy. The operations were performed through a left thoracotomy. After the sheep were heparinized, normothermic cardiopulmonary bypass was conducted without cardiac arrest or aortic cross-clamping. The main pulmonary artery was excised 1 cm above the pulmonary valve and just below the bifurcation of the pulmonary artery. The native pulmonary valve cusps were excised. The decellularized porcine aortic valve was interposed in the pulmonary position with running sutures of 4-0 polypropylene proximally and distally (Figure 1).
Figure 1.
Completed Pulmonary Valve Replacement in a Juvenile Sheep Model. The pericardium is shown reflected with silk sutures. Also visible are the venous and aortic cannulas for cardiopulmonary bypass. The porcine decellularized valve is shown after completion of both anastomoses. PA indicates pulmonary artery; RVOT, right ventricular outflow tract.
After the sheep were weaned from cardiopulmonary bypass, heparin was reversed with protamine sulfate, hemostasis was achieved, and the wounds were closed. Intercostal nerve blocks were performed with a mixture of 0.5% bupivacaine hydrochloride and epinephrine prior to closure of the thoracotomy. Ceftiofur sodium 5 mg/kg was given intramuscularly the day before the surgery and was repeated on the third postoperative day. Cefazolin 50 mg/kg was given intravenously 15 minutes prior to incision.
Before incision, all 3 sheep underwent transthoracic echocardiography (GE Vivid 7; GE Healthcare, Wauwatosa, Wisconsin), and after incision all 3 underwent epicardial echocardiography prior to cardiopulmonary bypass. Valve size and right ventricular function were assessed. After each animal was weaned from cardiopulmonary bypass, epicardial echocardiography was again performed to assess valve hemodynamics and ventricular function. Valve competence was assessed by standard color Doppler imaging, and gradients by continuous wave Doppler.
Postoperative Follow-Up
Animals were kept indoors for 1 week postoperatively and given 25 U/kg heparin twice a day for 2 days postoperatively. Vital signs were monitored daily for 1 week postoperation. Blood was drawn at 1 week postoperation to determine complete blood cell count. At 1 week postoperation, a transthoracic echocardiogram was performed to assess valve hemodynamics, and animals were transferred to our farm facility for long-term follow-up.
Macroscopic Analysis and Valve Processing
Animals were anesthetized, valves were imaged with transthoracic echocardiography, and animals were euthanized with intravenous pentobarbital. The heart was harvested and dissected by a cardiac pathologist. All native valves were examined, as were the right and left ventricles. The decellularized heart valve was then examined and photographed. One cusp and both distal and proximal anastomoses were placed in optimal cutting temperature compound embedding, and the remaining tissue was placed in 10% formalin or Trump fixative. Explanted heart valve specimens were analyzed by Brown and Brenn stain (modified Gram stain), fungal stain (periodic acid– Schiff), and hematoxylin-eosin stain. Additional specimens from the explanted valve cusps were cultured in DMEM at 37°C.
Statistical Analysis
Statistical analysis was done using GraphPad Prism (GraphPad Software Inc; La Jolla, California). Analysis of variance was utilized for comparisons of 3 or more groups, with the Tukey modification for pairwise analysis. For tensile strength analysis, data are represented as means with standard error of the mean shown in the error bars. Values of P<.05 were considered statistically significant.
Results
Gamma Irradiation Dose Experiments
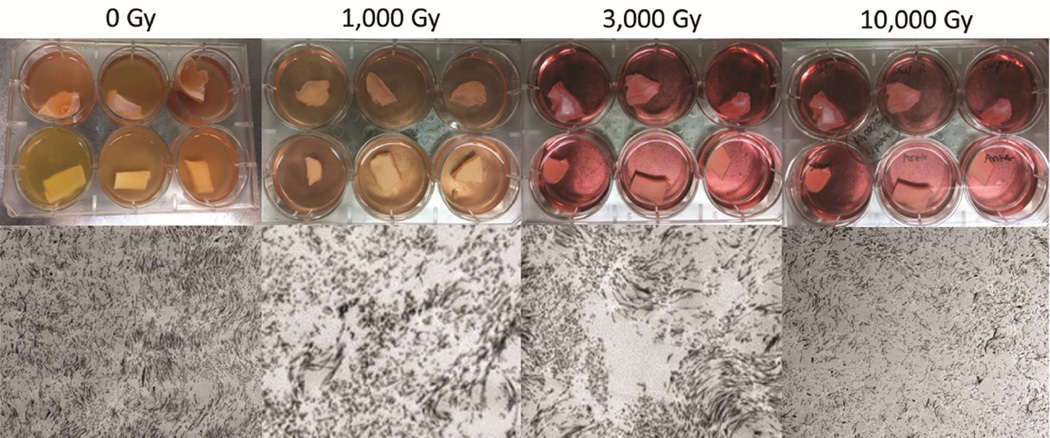
Valve cusps that were either not sterilized with gamma irradiation or that were exposed to only 1,000 Gy of gamma irradiation became turbid when cultured as described above; thus, gamma irradiation of 1,000 Gy or lower was considered to have not sterilized the decellularized heart valves. Cusps exposed to 3,000 Gy or higher of gamma irradiation were not cloudy and remained clear for 30 days of culturing; thus, they were deemed to have been effectively sterilized. Transmission electron microscopy imaging showed extracellular matrix damage at 1,000 Gy that was increased with higher doses. On the basis of the evaluation of qualitative destruction of extracellular matrix, using transmission electron microscopy and the culture results, we determined that 3,000 Gy of gamma irradiation was the optimal dosage. This dose adequately sterilized the decellularized heart valves with the minimum amount of damage to the extracellular matrix (Figure 2).
Figure 2.
Testing Different Doses of Gamma Irradiation. The top panel shows culture results of cusp, sinus, and root of the valve construct. Nonsterilized valves and those exposed to gamma irradiation at 1,000 Gy show contamination in tissue culture (note cloudy culture media [B]) whereas those irradiated at 3,000 and 10,000 Gy remained sterile (note clear culture media [D]).The bottom panel shows dose-corresponding transmission electron microscopy images of cusps at an acceleration voltage of 80 kilovolts and at a magnification of 4,000 × for all images.
In Vitro Sterility Testing
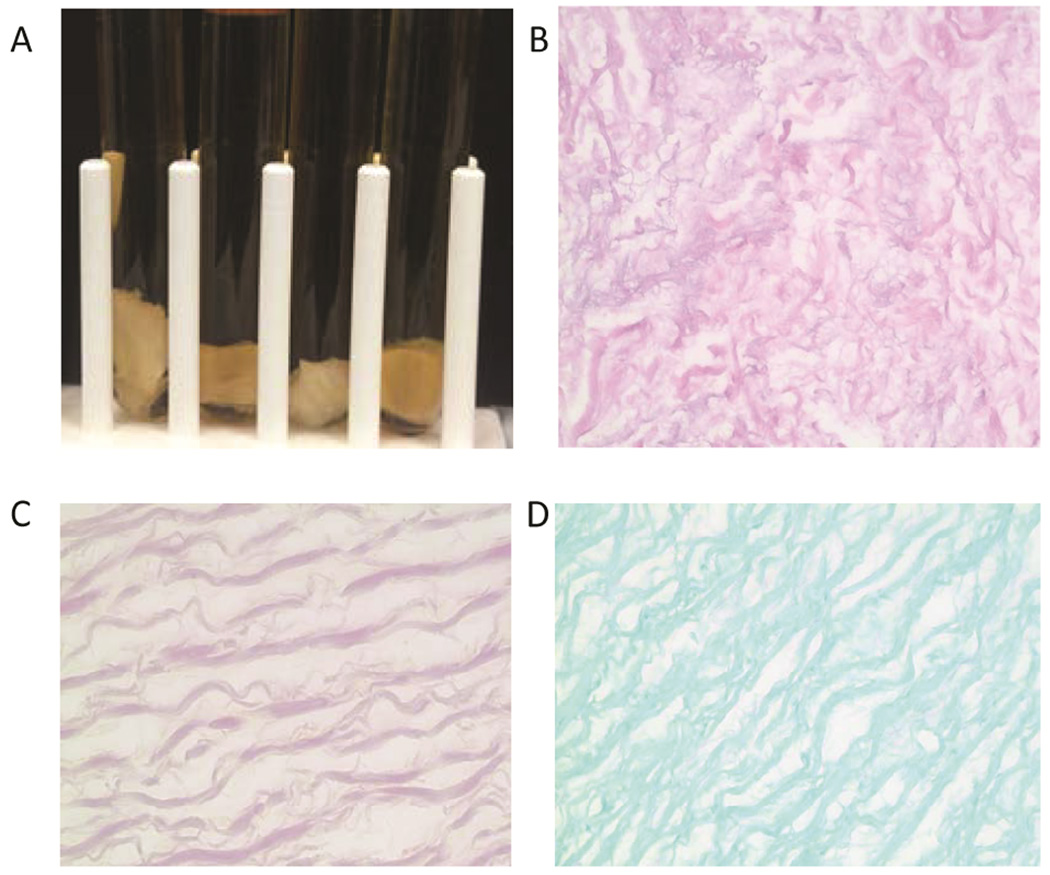
Valves exposed to 1,500 Gy and 3,000 Gy of gamma irradiation underwent further stringent sterility trials. Fungal and Gram stains were negative for valves treated with 3,000 Gy gamma irradiation(Figure 3). Cultures of cusps in all 3 broths showed no contamination or growth in 3,000 Gy valves. However, in valves that underwent 1,500 Gy gamma irradiation, there was turbidity at 3 days in both aerobic and anerobic broth but not fungal broths..).
Figure 3.
Sterility Testing of Valves Gamma Irradiated With 3,000 Gy of Gamma Irradiation. A, Culture of sinuses in tryptic soy broth, thioglycollate broth (duplicates), and Sabouraud dextrose broth showing no contamination. B, Hematoxylin-eosin (H&E) staining showing the lack of cells in the decellularized heart valves after gamma irradiation. C, Modified Gram staining (Brown and Brenn) of an irradiated sinus exposed to 3,000 Gy gamma irradiation showing no bacteria. D, Fungal stain of an irradiated sinus exposed to 3,000 Gy gamma irradiation showing no evidence of fungal contamination.
In Vitro Mechanical Testing
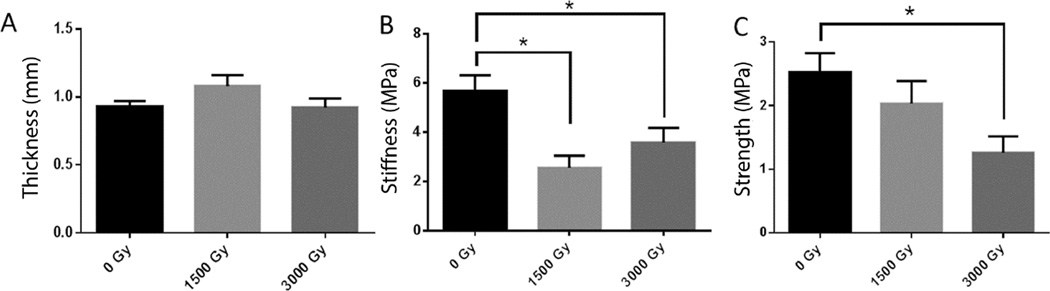
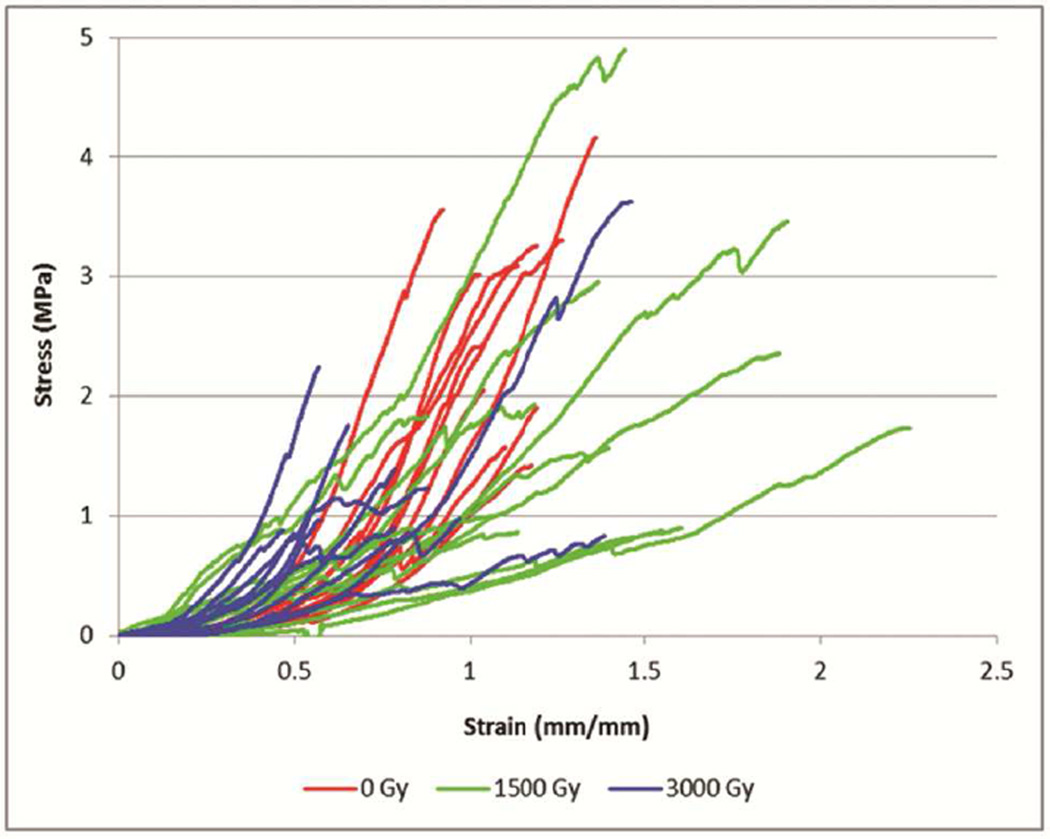
Tensile strength testing showed decreased stiffness and ultimate strength in cusps of gamma-irradiated valves (Figure 4). Stiffness was significantly decreased both in the valves gamma irradiated with 1,500 Gy and in the valves gamma irradiated with 3,000 Gy compared to control valves that received no gamma irradiation (P=.002 and P=.04, respectively), but there was a dose-dependent trend (0 Gy=5.67±2.14 [megapascals] MPa; 1,500 Gy=2.56±1.68 MPa; 3,000 Gy=3.58±2.09 MPa). Ultimate strength was significantly decreased in the cusps that were gamma irradiated with 3,000 Gy compared to the control cusps (P=.02). There was also a trend of decreasing ultimate strength with increasing doses of gamma irradiation (0 Gy=2.53±0.98 MPa; 1,500 Gy=2.03±1.23 MPa; 3,000 Gy=1.26±0.89 MPa). There was no statistically significant difference between the thickness of the cusps in the 3 groups, which indicated that the differences in stiffness and ultimate strength were not secondary to the thickness of the cusps (0 Gy=0.95±0.15 mm; 1,500 Gy=1.08±0.27 mm; 3,000 Gy=0.92±0.23 mm; P=.2).
Figure 4.
Tensile Strength Testing of Valve Cusps Not Exposed to Either Low-Dose or High-Dose Gamma Irradiation. Asterisk (*) indicates P values <.5 when comparing the 2 groups (1,500 Gy and 3,000 Gy gamma irradiation). A, The thickness of each group of cusps was comparable. B, The stiffness of the cusps decreased with each dose of gamma irradiation compared to the control. C, The ultimate strength of cusps with 3,000 Gy gamma irradiation was decreased significantly. D, Tensile stress versus strain curves for decellularized porcine cusps sterilized with 0 Gy (n=12), 1500 Gy (n=12), or 3000 Gy (n=12) of gamma irradiation. Samples were tested in the circumferential direction.
Animals and Macroscopic Analysis of Explanted Valves
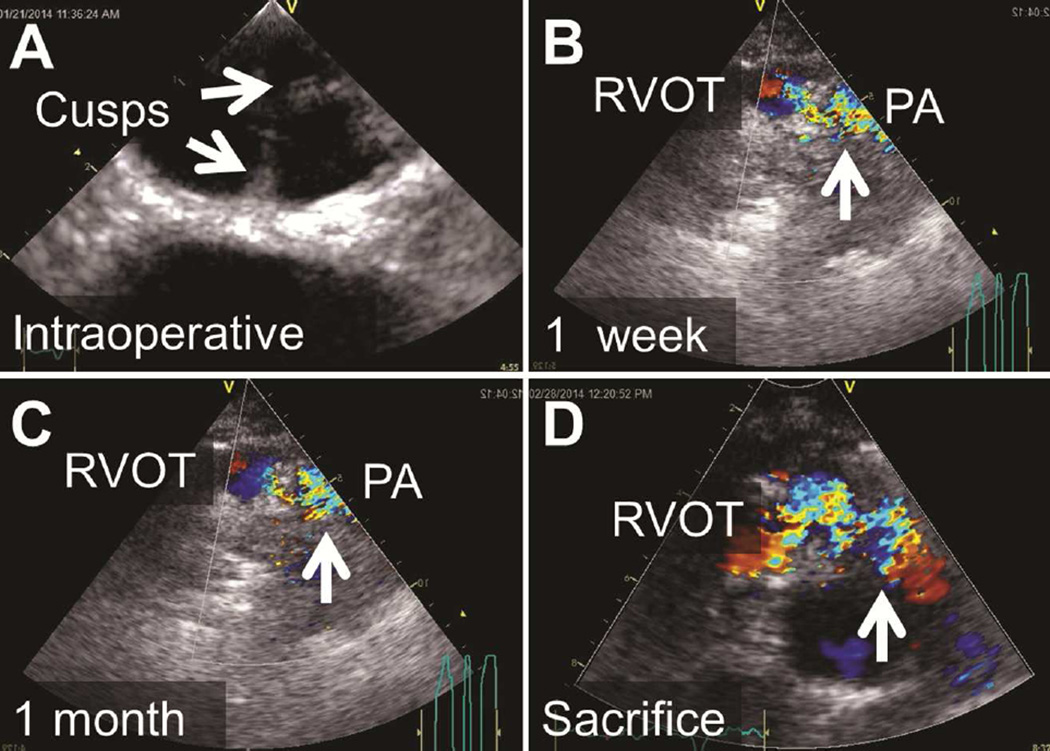
The cardiac valves implanted into the 3 sheep were studied. Signs of valve malfunction were visible at 1 week postimplantation. At implantation, there was no regurgitation in any animal and no stenosis; mean gradients were 7±3 mm Hg. At 1 week postimplantation, the valves had no signs of regurgitation, but did show some signs of stenosis, with a mean gradient of 22±5 mm Hg. Echocardiograms at 2-month follow-up showed regurgitation and stenosis of all valves, with a mean gradient of 30±7 mm Hg (Figure 5). All animal experimentation was approved by the Institutional Animal Care and Use Committee and care of animals was in compliance with the NIH guidelines.
Figure 5.
Images of Echocardiography of the Pulmonary Valve in Vivo. A, Immediately postoperatively; B, at 1 week postimplantation; C, at 1 month postimplantation; and, D, immediately prior to sacrifice. Note progressively worsening turbulent flow (arrows), starting in the right ventricular outflow tract (RVOT) just below the xenograft and extending into the main pulmonary artery (PA). Increasing gradients were noted by continuous wave Doppler consistent with progressive xenograft valve stenosis.
Qualitative Analysis of Explanted Valves
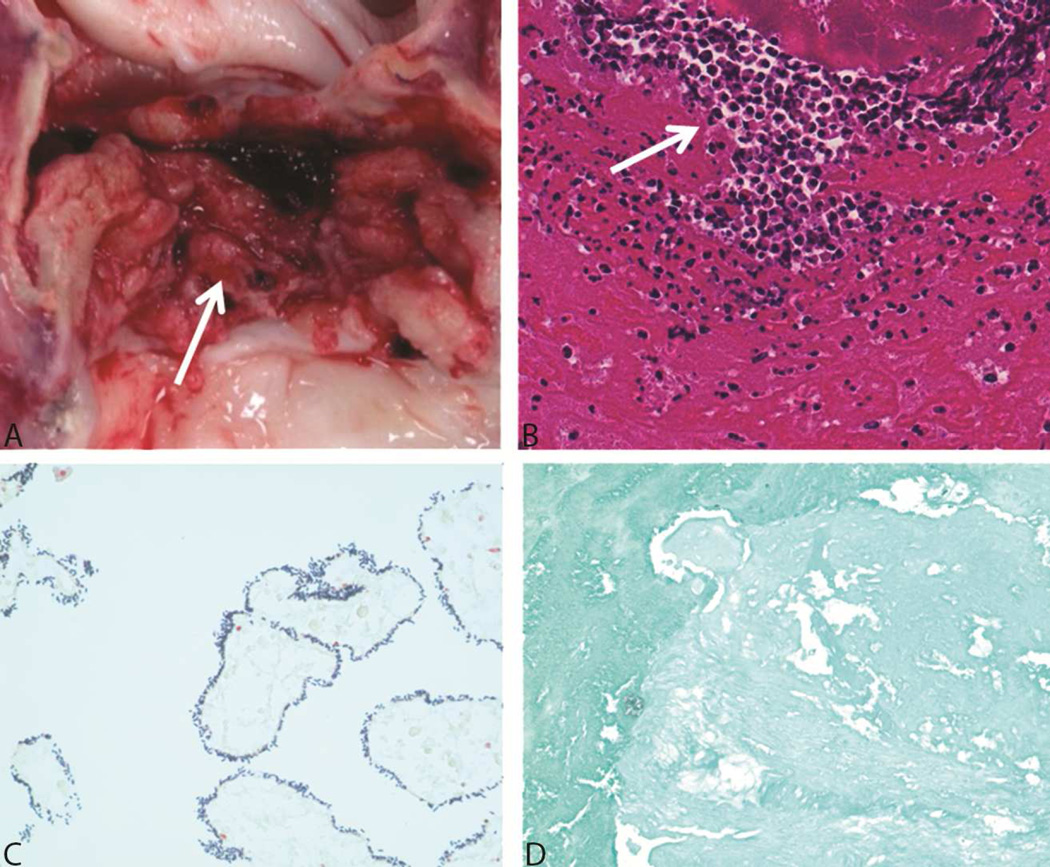
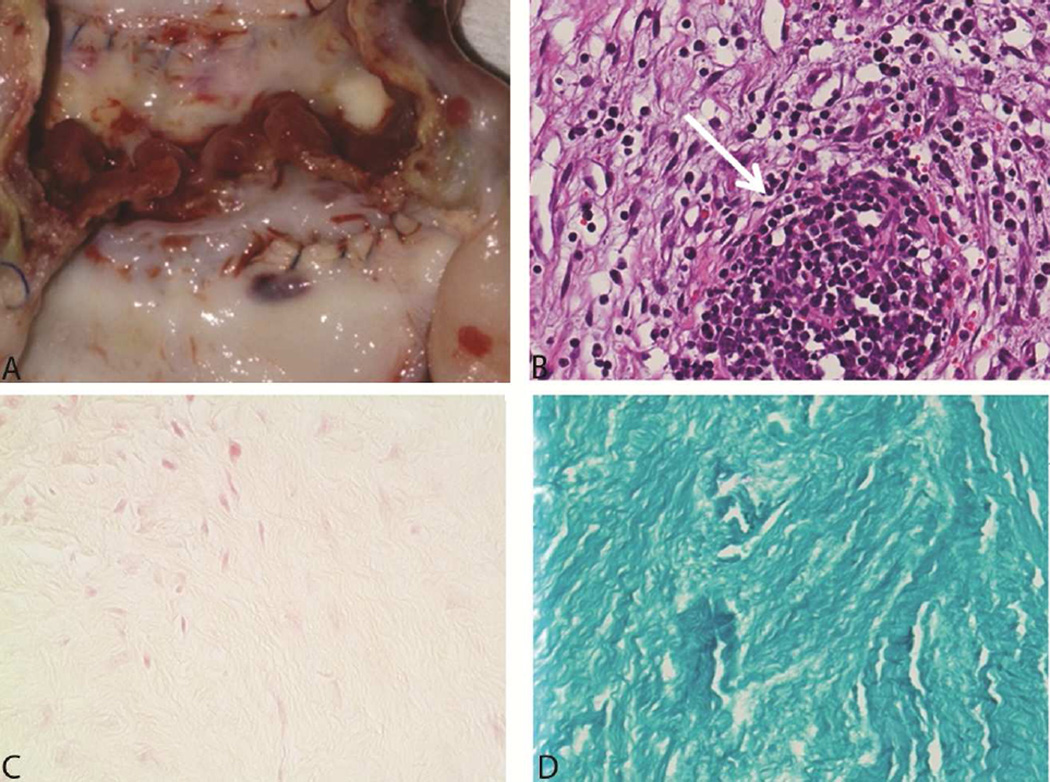
The explanted porcine cardiac valve exposed to 1,500 Gy gamma irradiation exhibited clear signs of infection. The valve cusps were thickened and brittle. However, the aortic xenograft root at the anastomosis was similar to that in preimplantation valves and showed no subjective signs of damage. Brown and Brenn–stained slides had abundant Gram-positive cocci. The PAS-stained slides were negative for fungal organisms. Hematoxylin-eosin stained slides had large areas of suppuration consistent with bacterial endocarditis (Figure 6). In contrast, the explanted valves that had been treated with 3,000 Gy of gamma irradiation showed no signs of infection (Figure 7). Abundant fibroplasia infiltrated by numerous macrophages and a few neutrophils were noted in the valve tissue sections. The special stains for bacteria and fungi were negative. Valve cusps that were cultured revealed no growth.
Figure 6.
An Explanted Valve Irradiated With 1,500 Gy Gamma Irradiation. A, Gross image of the valve at explantation shows friable, thick cusps with vegetations (arrow). B, Staining with hematoxylin-eosin shows abundant neutrophils (arrow). C, Brown and Brenn stain shows bacteria characteristic of streptococcus. D, Fungal stain shows no contamination with fungus.
Figure 7.
An Explanted Valve Irradiated With 3,000 Gy Gamma Irradiation. A, Gross image of the valve at explantation shows firm, rubbery cusps. B, Staining with hematoxylin-eosin (H&E) shows giant cell formation and abundant fibroblasts and macrophages (arrow) but few neutrophils. C, Brown and Brenn stain shows no bacteria. D, Fungal stain shows no contamination with fungus.
Comment
Our findings demonstrated for the first time the dose-dependent effect of gamma irradiation in vivo on physical parameters for sterilization of decellularized heart valves. Results showed that the doses that effectively sterilized the decellularized structure caused significant structural and functional changes in the valve leaflets. Moreover, the function of the decellularized valves sterilized with gamma irradiation was compromised. Thus, our data suggest that the use of gamma irradiation may not be the ideal method for sterilization of decellularized heart valves.
The failure of the valves was secondary either to ineffective sterilization from using 1,500 Gy of gamma irradiation or to damage from using 3,000 Gy of gamma irradiation. An acceptable balance between sterility and damage will likely not be achieved with gamma irradiation. The adverse effect of gamma irradiation on mechanical integrity has been shown in bone, cartilage, and ligament allografts [6,7]. Gamma irradiation of polymers, including chitosan and other carbohydrate-based films, has also been shown to accelerate their degradation rate [8]. Gamma irradiation is known to cause scission of polypeptide chains such as collagen [9]. This results in a significant weakening of the tissue and our data support a dose-dependent effect. This also results in a significant loss of stiffness although our data do not support a dose-dependent effect. A possible explanation is the loss in stiffness due to scission is partially offset by a gain in stiffness due to crosslinking as a result of the formation of highly reactive hydroxyl radicals by gamma irradiation-induced radiolysis of water molecules [10].
The decellularization process alone likely damages heart valves, but decellularization with SDS has been shown to retain the most strength in aortic valve cusps compared to other decellularization procedures [9]. The added damage with gamma irradiation is substantial and contributed to the maladaptive wound healing observed in the explanted valves in our study.
More stringent in vitro sterility testing of decellularized heart valves must be conducted prior to in vivo testing. The most common technique described in the literature is to grow portions of heart valve cusps in DMEM culture media in the incubator for up to 30 days, checking for time of contamination [11,12,13]. Erdbrugger et al [14] went a step further and checked their sterilization method against a select few bacteria and viruses. In this vein, we subjected our porcine cardiac valves to Gram stain, fungal stain, hematoxylin-eosin stain, and to incubation in bacterial growth-inducing culture medium. Since sterilization techniques can have a large impact on the mechanical strength of decellularized structures, part of the evaluation of any sterilization process must be mechanical testing of the product after sterilization.
In this in vivo study using decellularized porcine heart valves, our results showed that sterilization techniques are integral not only to experimental study design but also to the outcome and the integrity of the performance of the valve. The lessons we have learned serve both as a cautionary tale for future experiments and to highlight the need for detailed descriptions of sterilization techniques in published reports.
The choice of sterilization technique, along with dosage and protocol of each sterilization method, has the potential to alter a decellularized heart valve tremendously. This topic has currently been explored only in reviews [15]; future research must focus on head-to-head comparisons of sterilization methods that do not cross-link or fix tissue, in order to move the field toward clinical use. Terminal sterilization procedures are necessary for xenograft material–derived tissue-engineered heart valves. Insufficient sterilization may have led to the cases of endocarditis reported in the literature [16] and to the early failure of such valves.
Our study is limited by its small sample size in regards to the in vivo data. Further animal trials could not be justified, in our opinion, and were ended in light of such clinically significant results. Comprehensive in vitro data complements our limited in vivo data.
Conclusions
In this report, we have described our preliminary results in what we believe to be a novel study evaluating sterilization with low-dose gamma irradiation of decellularized porcine heart valves in an in vivo model. Gamma irradiation with 3,000 Gy produced a valve free from infection but damaged it and compromised the function of the valve. Low-dose gamma irradiation is not a sterilization procedure that maintains the mechanical integrity of heart valve cusps. Thus, an optimal balance between sterilization and damage may not be achievable with gamma irradiation. Other methods of terminal sterilization must be pursued and evaluated while the field moves forward.
Abbreviations
- DMEM
Dulbecco’s Modified Eagle’s Medium
- Gy
gray
- MPa
megapascals
- SDS
sodium dodecyl sulfate
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank. 2007;8(2):81–91. doi: 10.1007/s10561-006-9019-7. Epub 2006 Jul 5. [DOI] [PubMed] [Google Scholar]
- 2.White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res. 1994;73(9):1560–1567. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- 3.Jordan JE, Williams JK, Lee SJ, Raghavan D, Atala A, Yoo JJ. Bioengineered self-seeding heart valves. J Thorac Cardiovasc Surg. 2012;143(1):201–208. doi: 10.1016/j.jtcvs.2011.10.005. Epub 2011 Nov 1. [DOI] [PubMed] [Google Scholar]
- 4.Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13(6):898–906. doi: 10.1002/jor.1100130614. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly RJ, Aparicio SR, Dexter F, Deverall PB, Watson DA. Gamma-radiation of heart valves at 4 degrees C: a comparative study using techniques of histochemistry and electron and light microscopy. Thorax. 1973;28(1):95–101. doi: 10.1136/thx.28.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seto AU, Gatt CJ, Jr, Dunn MG. Sterilization of tendon allografts: a method to improve strength and stability after exposure to 50 kGy gamma radiation. Cell Tissue Bank. 2013;14(3):349–357. doi: 10.1007/s10561-012-9336-y. Epub 2012 Aug 24. [DOI] [PubMed] [Google Scholar]
- 7.Berry DJ, Currier BH, Mayor MB, Collier JP. Gamma-irradiation sterilization in an inert environment: a partial solution. Clin Orthop Relat Res. 2012;470(7):1805–1813. doi: 10.1007/s11999-011-2150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Li J, Xia J, Kennedy JF, Yie X, Liu TG. Effect of gamma irradiation on the condensed state structure and mechanical properties of konjac glucomannan/chitosan blend films. Carbohydr Polym. 2011;83(1):44–51. [Google Scholar]
- 9.Sun WQ, Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4(4):817–826. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Grant RA, Cox RW, Kent CM. The effects of gamma irradiation on the structure and reactivity of native and cross-linked collagen fibres. J Anat. 1973;115(Pt 1):29–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29(8):1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhyari P, Kamiya H, Gwanmesia P, Aubin H, Tschierschke R, Hoffmann S, et al. In vivo functional performance and structural maturation of decellularised allogenic aortic valves in the subcoronary position. Eur J Cardiothorac Surg. 2010;38(5):539–546. doi: 10.1016/j.ejcts.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Quinn RW, Hilbert SL, Bert AA, Drake BW, Bustamante JA, Fenton JE, et al. Performance and morphology of decellularized pulmonary valves implanted in juvenile sheep. Ann Thorac Surg. 2011;92(1):131–137. doi: 10.1016/j.athoracsur.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Erdbrugger W, Konertz W, Dohmen PM, Posner S, Ellerbrok H, Brodde OE, et al. Decellularized xenogenic heart valves reveal remodeling and growth potential in vivo. Tissue Eng. 2006;12(8):2059–2068. doi: 10.1089/ten.2006.12.2059. [DOI] [PubMed] [Google Scholar]
- 15.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. Epub 2011 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo M, Naso F, Poser H, Rossi A, Franci P, Bianco R, et al. Physiological performance of a detergent decellularized heart valve implanted for 15 months in Vietnamese pigs: surgical procedure, follow-up, and explant inspection. Artif Organs. 2012;36(6):E138–E150. doi: 10.1111/j.1525-1594.2012.01447.x. Epub 2012 Apr 18. [DOI] [PubMed] [Google Scholar]