Abstract
Background and Purpose
Vascular dementia is the second most common form of dementia but reliable evidence on age-specific associations between blood pressure and risk of vascular dementia is limited and some studies have reported negative associations at older ages.
Methods
In a cohort of 4.28 million individuals, free of known vascular disease and dementia and identified from linked electronic primary care health records in the UK (Clinical Practice Research Datalink), we related blood pressure to time to physician-diagnosed vascular dementia. We further determined associations between blood pressure and dementia in a prospective population-based cohort of incident TIA and stroke (Oxford Vascular Study).
Results
Over a median follow-up of 7.0 years, 11 114 initial presentations of vascular dementia were observed in the primary care cohort after exclusion of the first four years of follow-up. The association between usual systolic blood pressure (SBP) and risk of vascular dementia decreased with age (HR per 20 mm Hg higher SBP = 1.62, 95% CI 1.13-2.35 at 30-50 years; 1.26, 1.18-1.35 at 51-70 years; 0.97, 0.92-1.03 at 71-90 years; p trend = 0.006). Usual SBP remained predictive of vascular dementia after accounting for effect mediation by stroke and TIA. In the population-based cohort, prior SBP was predictive of 5-year risk of dementia with no evidence of negative association at older ages.
Conclusions
Blood pressure is positively associated with risk of vascular dementia, irrespective of preceding TIA or stroke. Previous reports of inverse associations in old age could not be confirmed.
Keywords: hypertension, epidemiology, dementia
Subject Codes: Etiology: epidemiology, High blood pressure
Introduction
Vascular dementia is the second most common cause of dementia1 and is increasing in prevalence in developing countries.2 Vascular dementia usually occurs in association with vascular risk factors or after transient ischaemic attack (TIA) or stroke3 and commonly manifests with a decline in cognitive function, apathy and depression, and can eventually result in death.1,4
Blood pressure, a known causal risk factor for stroke5, has also been identified as a potential risk factor for vascular dementia.6 However, studies have reported conflicting results on the relationship between blood pressure and vascular dementia (Supp. Table I).6 Previous analyses of healthy cohorts and patients with previous diagnosis of TIA or stroke have suggested a positive association between midlife blood pressure (age 40-50 years) and future development of dementia, including vascular dementia.7–9 However, several other studies have suggested that low blood pressure in old age is associated with an increased risk of dementia (Supp. Table I).10,11
The reported negative associations between blood pressure and risk of vascular dementia at older ages may be due to “reverse causality”, that is, vascular dementia causing low blood pressure, due to loss of sympathetic drive, rather than low blood pressure causing vascular dementia.12 Null or negative associations could also result from the prior prescription of BP lowering drugs. Previous studies have been relatively small, limiting their ability to study these interactions reliably (Supp. Table I).
To clarify these uncertainties, we sought to determine the associations between blood pressure and risk of vascular dementia both in individuals without prior TIA or stroke and in individuals after TIA and stroke. We therefore undertook an analysis of 4.28 million individuals free from vascular disease and dementia in a contemporary United Kingdom primary care population, supplemented with an analysis of a prospective population-based cohort of patients with TIA and stroke in the Oxfordshire region.
Methods
Data sources, population and exposure
We utilized linked electronic health records which have been validated for epidemiological research13 and previously used to examine the relationship between blood pressure and vascular events. We used data from the Clinical Practice Research Datalink (CPRD, primary care records), linked to Hospital Episode Statistics (HES, hospitalization records) and Office of National Statistics Mortality data (ONS, cause-specific mortality records). All baseline and exposure covariates were defined through the primary care database. Outcomes (i.e. diagnoses of vascular dementia) were defined as the first event from any of the linked data sources, as previously performed.14
Patients were eligible for inclusion if they were between the ages of 30 and 90, inclusive, and had a blood pressure measurement performed between 01/01/1990 and 01/01/2013 at their general practice. We excluded individuals above the age of 90 at baseline who were viewed as being more likely to have undiagnosed vascular dementia causing lower blood pressure (that is, more likely to suffer from reverse causality). Patients additionally had to have their age recorded and registered at a research-standard general practice for at least one year. Baseline covariates, including body mass index (BMI), smoking status, total cholesterol, HDL cholesterol, diabetes were defined using existing phenotyping algorithms.15 The baseline value for each covariate was defined as the closest measurement within two years of the baseline blood pressure measurement of the covariate. To minimize the risk of reverse causality and confounding (e.g. pre-existing cardiovascular disease causing a reduced blood pressure while predisposing individuals to an increased risk of vascular dementia), we excluded all individuals with pre-existing cardiovascular disease. This was defined as any diagnosis (either during hospitalization or in primary care) prior to the baseline blood pressure measurement of ischemic heart disease (including angina and myocardial infarction), cerebrovascular disease, peripheral arterial disease, heart failure, atrial fibrillation or chronic kidney disease.
Endpoints
We utilized an inclusive definition of vascular dementia (ICD 10 code: F01, Read codes provided in Supp. Table II), defining it as any form of dementia with a physician-diagnosed vascular component. Therefore, for our primary analysis, we considered vascular dementia to be all cases of vascular dementia regardless of the co-existence of Alzheimer’s disease (i.e. mixed dementia). As a secondary analysis, we excluded all individuals diagnosed with Alzheimer’ diseases as well as individuals prescribed donepezil, galantamine, memantine and rivastagime, which are largely used to treat Alzheimer’s disease.16 For all analyses, participants were censored at the earliest of an occurrence of the primary outcome, transfer out of practice, death or last collection date of practice. To minimize the risk of reverse causality17, that is, undiagnosed dementia causing low blood pressure, the first four years of follow-up were excluded in the primary analysis.
Statistical Analysis
Cox models, stratified by practice, were used to determine hazard ratios for the association between blood pressure and risk of vascular dementia, while accounting for clustering of patients by practice. Blood pressure was analysed both as a categorical variable, which does not assume any particular shape of the association between blood pressure and vascular risk, and as a continuous variable, which assumes a linear relationship. The primary analysis was adjusted for age, sex, body mass index and smoking status. Proportional hazards assumption was tested by plotting Schoenfeld residuals. Multiple imputation using chained equations, was used to impute missing covariates (BMI and smoking for the primary analysis, total cholesterol and HDL cholesterol for the secondary analysis); five imputations were generated. Regression dilution bias was adjusted for using serial measurements of blood pressure (Supp. Methods).
Sensitivity analyses
Five sensitivity analyses were conducted. First, models were further adjusted for total cholesterol, HDL cholesterol and the presence of diabetes. Second, models were further adjusted for socioeconomic status and for year of the initial blood pressure measurement, as a categorical variable (1990-1994, 1995-1999, 2000-2004, 2005-2009, 2010-2013). Third, individuals prescribed antihypertensive medication or lipid lowering drugs at baseline or during follow-up were excluded. Fourth, individuals with mixed dementia (i.e. diagnosed with both Alzheimer’s disease and vascular dementia) and individuals diagnosed with vascular dementia, but also prescribed donepezil, galantamine, memantine and rivastagime were excluded. Fifth, events within the first zero to two, two to four, four to six, six to eight, and over eight years of follow-up for individuals aged 71 to 90 were examined to determine if previously described inverse associations between blood pressure and risk of dementia in old age were due to reverse causality.6
To investigate to what extent the potential association between blood pressure and vascular dementia was mediated by stroke or TIA, we adjusted for occurrence of stroke or TIA during follow up (Supplementary Methods).
Oxford Vascular Study Cohort
To confirm findings independently, we studied the association between prior BP and subsequent risk of dementia in a population-based cohort of all individuals with a first TIA or stroke (Oxford Vascular Study; OXVASC) recruited from 1/4/2002 to 31/3/2012. The methods of ascertainment and follow-up in OXVASC have been reported previously18, and are summarized in the supplementary appendix. This cohort has two advantages in relation to the described primary care cohort. First, all patients underwent cognitive screening after their initial TIA or stroke to exclude pre-morbid or baseline dementia and the subsequent diagnosis of dementia was made by a dementia specialist physician, based mainly on prospective face-to-face follow-up in clinic or at home using a multi-domain screening tool (Supp. Methods). These assessments were made without knowledge of the data on prior blood pressure, which were collected by independent investigators from the primary care records, thereby reducing the potential risk of biased outcome reporting and classification because of differences in BP. Second, since the predominant type of dementia in this cohort is vascular, we were able to study the risk of all dementia on follow-up, thereby eliminating any potential diagnostic bias whereby cases of dementia were more likely to be diagnosed as “vascular dementia” because of a history of hypertension or in light of recent BP readings. The five-year risk of new dementia during follow-up in OXVASC was related to mean pre-morbid blood pressure based on all readings recorded in primary care records since 1990 using a Cox model with and without adjustment for age, sex, smoking and BMI.
Results
In the primary care cohort, 4.7 million individuals had at least one acceptable blood pressure measurement at baseline and were eligible for inclusion in the analysis (Supp. Figure I). 12 449 individuals were excluded for a prior diagnosis of dementia and 405 516 individuals were excluded for prior vascular disease, leaving a cohort of 4.28 million individuals, free of vascular disease and dementia. Baseline characteristics are provided (Supp. Table III).
In total, 14 934 patients were reported to have vascular dementia during follow-up. 3820 of these presentations were recorded in the first four years of follow-up and excluded from the primary analysis. Therefore, 11 114 cases of vascular dementia were included in the primary analysis.
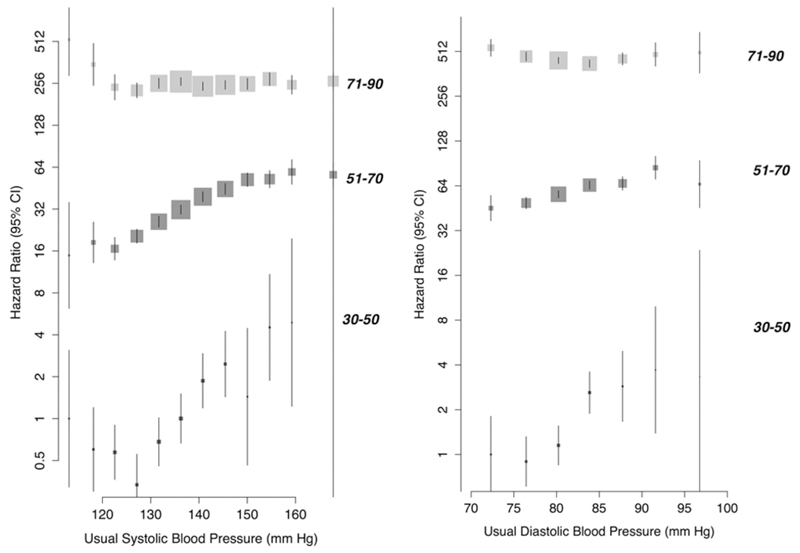
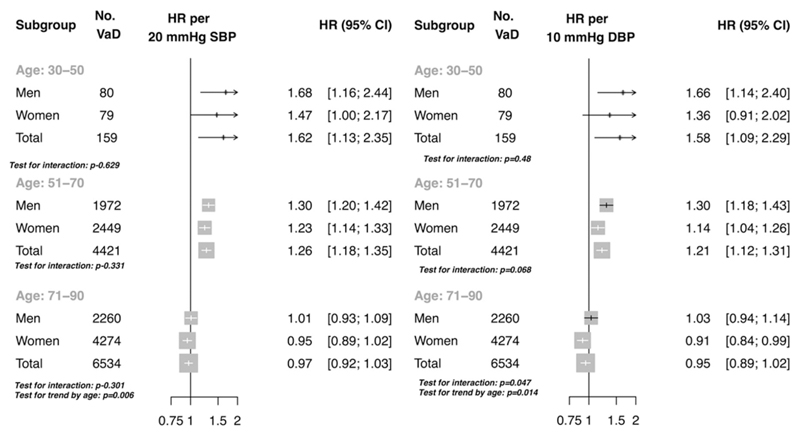
The association between usual SBP and risk of vascular dementia appeared broadly linear within the age groups of 30-50 and 51-70 (Figure 1). No clear evidence of a nadir was observed, although floating risks appeared to plateau at approximately 120 mm Hg SBP in both the 30-50 year and 51-70 year age groups. In contrast, in the age group of 71-90 years, floating risks of vascular dementia showed no significant association with baseline SBP. The strength of association per 20 mm Hg higher usual SBP diminished with increasing age category, from an HR of 1.62 (95% CI 1.13, 2.35) in the age group 30 to 50 years, to an HR of 1.26 (95% CI 1.18, 1.35) in the age group of 51 to 70 years, to an HR of 0.97 (95% CI 0.92, 1.03) in the age group 71 to 90 years (p trend =0.006, Figure 1 and Figure 2). There was no evidence of interaction by sex (Figure 1). Estimates for usual baseline diastolic blood pressure (DBP) were similar (Figure 1 and Figure 2) to usual SBP.
Figure 1.
Floating absolute risk of new onset dementia in the primary care cohort by systolic and diastolic blood pressure, stratified by age category (30-50,51-70,71-90). Model adjusted for BMI, smoking status, sex and an interaction term between age category and categorical blood pressure (plotted).
Figure 2.
Hazard ratio per 20 mm Hg higher usual systolic blood pressure and 10 mm Hg higher usual diastolic blood pressure in the primary care cohort. Model adjusted for BMI, smoking status, sex, age category, an interaction term between age category and continuous age and an interaction term between age category and blood pressure (plotted). No. VaD refers to the number of vascular dementia events.
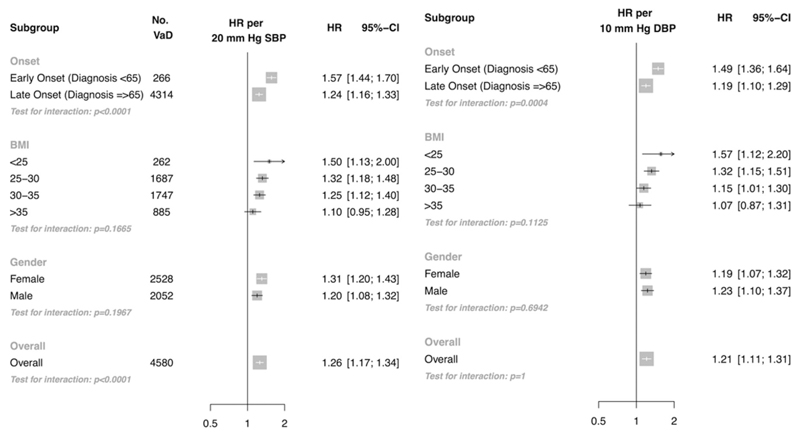
Estimates per 20 mm Hg higher SBP were similar when models were further adjusted for total cholesterol, HDL cholesterol and baseline diabetes (Supp. Figure II) and baseline BP measurement period (Supp. Figure III). When individuals prescribed antihypertensive and lipid lowering therapies at baseline or during follow-up were excluded, the HR per 20 mm Hg higher SBP was strengthened in the age group 51-70 years (HR 1.56 CI 1.31, 1.85), although there were too few events to reliably ascertain the association in the age group 31-50 years (Supp. Figure IV). Estimates were similar when individuals diagnosed with Alzheimer’s disease or prescribed anti-dementia drugs (in addition to a diagnosis of vascular dementia) were excluded (Supp. Figure V). Overall for individuals aged 70 years or less at baseline, 20 mm Hg higher usual SBP was associated with a 26% higher risk of vascular dementia (HR 1.26 CI 1.17, 1.34, Figure 3).
Figure 3.
Risk of vascular dementia by onset of dementia (early onset: age at diagnosis or end of follow-up <65 years, late onset: age at diagnosis or end of follow-up ≥ 65 years), BMI and sex for individuals 70 years or less at baseline in the primary care cohort. Models adjusted for age, BMI, sex and smoking status. Models in subgroups further adjusted for an interaction term between BP and age at event (plotted, Onset subgroup), BMI categories and an interaction term between BP and BMI categories (plotted, BMI subgroup), an interaction term between BP and sex (plotted, sex subgroup). No. VaD refers to the number of vascular dementia events.
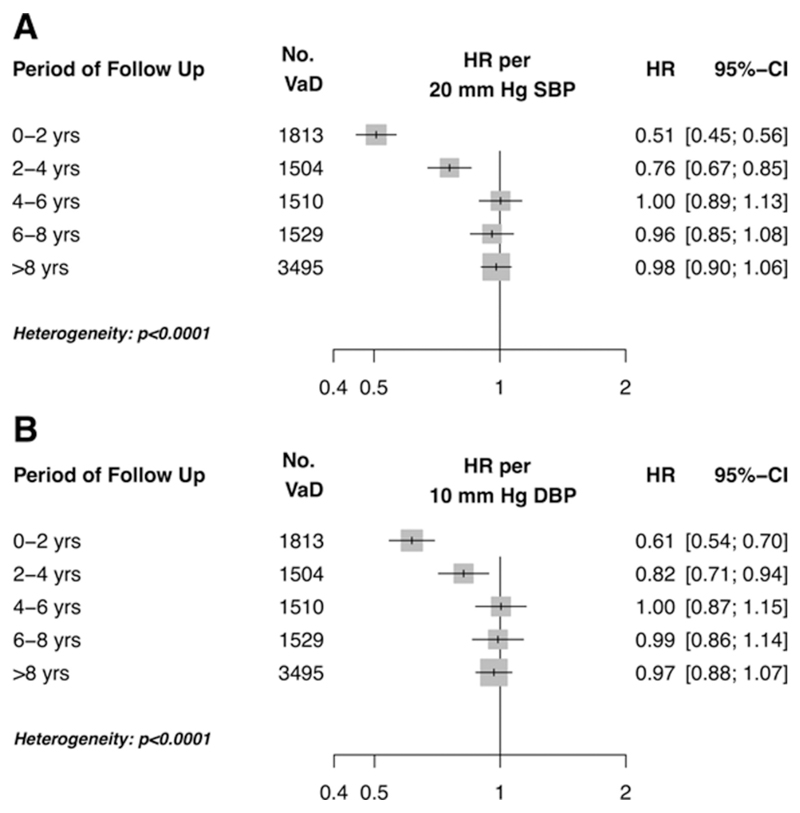
When only events in the first 2 years were considered, significant negative associations with both systolic (HR 0.51 CI 0.45, 0.56 per 20 mm Hg higher usual SBP) and diastolic blood pressures (HR 0.61 CI 0.54, 0.70 per 10 mm Hg higher usual DBP) were observed for the age group 71-90 (Figure 4). However, progressive examination of greater lengths of follow-up reduced the measured negative association for both systolic and diastolic blood pressure. Indeed, after exclusion of the first eight years of follow-up, no significant association with systolic blood pressure or diastolic blood pressure was observed. Tests for interaction by period of follow-up were highly significant (p heterogeneity < 0.0001).
Figure 4.
Risk of vascular dementia for individuals aged 71 to 90 years, by period of follow-up for (A) systolic blood pressure and (B) diastolic blood pressure in the primary care cohort. Model adjusted for blood pressure (plotted), age, sex, BMI and smoking status.
In the primary care cohort, a total of 27 316 TIA events and 38 199 stroke events were observed after exclusion of the first four years of follow-up. Adjustment for both TIA and stroke reduced the HR to 1.18 (CI 1.10, 1.26), indicating that 30% of excess risk of vascular dementia per 20 mm Hg higher SBP is mediated through future stroke and TIA (Supp. Figure VI).
In the OXVASC cohort, 1680 patients with a recent first TIA or stroke did not have dementia at baseline assessment. Mean (IQR) age was 74 (64-82) years, 823 (49.2%) were women, 232 (13.8%) were diabetic, 739 (44.0%) had never smoked, and 985 (58.6%) were on prior BP-lowering medication. During 5-year follow-up 314 patients developed new dementia. Risk of new dementia was unrelated to the most recent pre-morbid SBP or DBP in patients (Table 1). However, there were significant positive associations with both DBP and SBP in 5-9 years prior to the TIA/stroke and particularly 10-20 years prior (Table 1). These associations were stronger in patients aged<75 years and those not on prior BP lowering drugs (Supp. Table IV).
Table 1.
Risk of dementia during follow-up (excluding premorbid and baseline dementia) in relation to mean pre-morbid blood pressure (hazard ratio per 20/10mmHg increase in mean SBP/DBP) readings in cohort of patients with a first TIA or stroke (OXVASC cohort) stratified by measurement period (years prior to TIA/stroke), with two sensitivity analyses: 1. excluding patients on BP-lowering medication prior to the first TIA/stroke; 2. excluding dementia with onset after recurrent stroke.
| All patients (n=1680; 314 events) |
Patients not on prior BP-lowering drugs (n=695; 92 events) |
All patients (censored at recurrent stroke) (n=1680; 287 events) |
||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Mean SBP | ||||||
| 10-20 years ago | 1.61 (1.40-1.84) | 1.25 (1.07-1.46) | 2.30 (1.72-3.10) | 1.57 (1.12-2.19) | 1.58 (1.37-1.82) | 1.22 (1.04-1.43) |
| 5-9 years ago | 1.64 (1.42-1.88) | 1.28 (1.10-1.48) | 1.80 (1.38-2.35) | 1.22 (0.91-1.64) | 1.60 (1.39-1.85) | 1.25 (1.07-1.46) |
| <5 years ago | 1.16 (1.06-1.27) | 1.03 (0.95-1.12) | 1.22 (1.05-1.43) | 1.06 (0.93-1.21) | 1.17 (1.07-1.29) | 1.04 (0.95-1.13) |
| Most recent | 1.11 (0.99-1.24) | 0.98 (0.87-1.10) | 1.42 (1.15-1.76) | 1.11 (0.89-1.39) | 1.12 (0.99-1.26) | 0.99 (0.88-1.12) |
| Mean DBP | ||||||
| 10-20 years ago | 1.27 (1.10-1.46) | 1.19 (1.03-1.39) | 1.73 (1.27-2.34) | 1.58 (1.14-2.19) | 1.23 (1.07-1.43) | 1.16 (0.99-1.35) |
| 5-9 years ago | 1.13 (0.98-1.31) | 1.20 (1.03-1.40) | 1.28 (0.95-1.73) | 1.26 (0.92-1.72) | 1.12 (0.96-1.30) | 1.18 (1.00-1.39) |
| <5 years ago | 1.02 (0.96-1.09) | 1.03 (0.95-1.11) | 1.09 (0.98-1.21) | 1.07 (0.94-1.21) | 1.03 (0.96-1.10) | 1.03 (0.95-1.12) |
| Most recent | 0.89 (0.80-1.00) | 1.02 (0.91-1.13) | 1.06 (0.86-1.31) | 1.17 (0.94-1.45) | 0.88 (0.79-0.99) | 1.00 (0.90-1.13) |
Discussion
In our main analysis of 4.28 million individuals and 11 114 presentations of vascular dementia, blood pressure was found to be continuously associated with future risk of vascular dementia, with a possible plateau at 120 mm Hg. The association between blood pressure and risk of vascular dementia declined with increasing age, from a 62% higher risk per 20 mm Hg higher SBP in the age group 30 to 50 years, to a non-significant association in the age group of 71 to 90 years when early events after start of follow-up were excluded. A large proportion of the association between blood pressure and risk of vascular dementia in the age group 30 to 70 years was mediated by future stroke and transient ischemic attack.
Findings were broadly similar in our analysis of the risk of new dementia in the OXVASC patients with TIA or stroke. Indeed, a major strength of our study is the combination of the “big data” from the CPRD with the more deeply phenotyped prospective population-based data from OXVASC. Risk of dementia within 5-years of recruitment into OXVASC was unrelated to the most recent pre-morbid blood pressure, but no statistically significant negative associations were observed even in this group. Significant positive associations with both DBP and SBP were observed with measurements 5-9 years and 10-20 years prior to the TIA or stroke.
Previous studies (Supp. Table I) have been limited by relatively small sample sizes and have provided conflicting results on the relationship between blood pressure and vascular dementia. In an analysis of the relationship between midlife blood pressure and vascular dementia, with 38 presentations of vascular dementia, SBP was significantly and positively related to the risk of vascular dementia (OR 1.33 CI 1.14, 1.56 per 10 mm Hg higher SBP).9 In contrast, in an analysis of 6668 participants aged 55 years and older with 46 presentations of vascular dementia, no significant association between SBP and risk of vascular dementia was observed.10 An inverse association was observed in a cross-sectional analysis of 1642 individuals, with hypertensive individuals (SBP ≥ 140 mm Hg) having a 50% lower odds (OR 0.5 CI 0.27, 0.92) of developing vascular dementia than non-hypertensive individuals.11
Our results characterize the age-dependent relationship between blood pressure and risk of vascular dementia and support the previously reported positive associations between blood pressure in mid-life and vascular dementia. Our estimate of an HR 1.62 (CI 1.13, 2.35) in the age group 30 to 50 years in the primary care cohort is consistent with that of Yamada et al., who estimated an OR of 1.77 (CI 1.30, 2.43) per 20 mm Hg higher SBP in a population where the large majority of participants were at age 30 to 50 years at baseline.9 However, contrary to previous analyses suggesting that late-life blood pressure has either no association or an inverse association with risk of vascular dementia, our results demonstrate that elevated blood pressure in the age range of 51 to 70 years is a significant a risk factor for vascular dementia and refutes earlier reports of an inverse association between blood pressure and risk of vascular dementia in the age range of 71 to 90 years. Although we observed a negative association between systolic and diastolic blood pressure and risk of vascular dementia within the first two years of follow-up, consistent with previous analyses11, this association disappeared after excluding the first four years of follow-up. Similarly, in the OxVASC cohort of patients with symptomatic cerebrovascular disease, there was no suggestion of a negative association even at age>75 years. These results suggest that previously reported inverse associations between blood pressure and risk of vascular dementia are due to reverse causality, and are not due to reduced cerebral perfusion causing vascular dementia, as has been previously suggested.6,12,19
Recent studies have suggested that elevated blood pressure may be unassociated with incident Alzheimer’s disease20, and or may be inversely associated. 21 These findings, which differ from the current analysis, may be due to methodological differences or may reflect genuine differences in the pathophysiology of vascular dementia relative to Alzheimer’s disease. In favor of the former hypothesis is evidence of a shared pathology between Alzheimer’s disease and vascular dementia, which suggests that classification of either disease is not rigid.22 Furthermore, vascular dementia may represent a heterogeneous group of disorders with differing pathophysiologies and courses depending on the source of dementia (e.g. large infarction versus small vessel disease). 23 Advances in classification and clinical diagnosis of subtypes of dementia may allow for more consistent estimation of associations with risk factors in epidemiological studies.
Assuming causality, our results suggest that individual or population based methods to lower blood pressure, even late in life, may further reduce the incidence of vascular dementia. As our results also indicate that a large proportion of the association of blood pressure with risk of vascular dementia is mediated through stroke and TIA, efforts to prevent these forms of cerebrovascular disease, as well as efforts to rapidly treat stroke and TIA, may further reduce the future burden of vascular dementia in developed and developing countries. However, when considering that only 30% of the excess risk of vascular dementia associated with a 20 mm Hg higher SBP was mediated by future stroke or TIA in the CPRD analysis, efforts to reduce blood pressure are likely to have a larger impact in reducing the risk of vascular dementia than that expected simply from prevention and treatment of overt stroke or TIA.
Our analysis of the primary care cohort has a number of strengths. It is the largest analysis to date of the association between blood pressure and risk of vascular dementia (Supp. Table I). It is also contemporary, with many individuals prescribed antihypertensive medication and statins. However, our use of the primary care cohort does have several limitations. First, recording of dementia in primary care records has low sensitivity. 24,25 Second, it is possible that the coding of vascular dementia would be influenced by prior hypertension since hypertension is included in most diagnostic criteria for vascular dementia.26,27 Given the consequent potential circularity of associations between blood pressure and vascular dementia, we studied the risk of all new dementia in the OXVASC TIA and stroke cohort and found strong confirmatory associations between prior blood pressure and dementia.
In conclusion, blood pressure is continuously related to risk of vascular dementia at baseline age of 70 years and less and prior blood pressure is a strong risk factor for dementia during follow-up after TIA and stroke. Although the strength of these associations declines with increasing age, previous reports of an inverse association between blood pressure and risk of dementia in the ages 71 to 90 years was refuted. Assuming causality, individual and population based efforts to reduce blood pressure and reduce the complications of cerebrovascular disease may further reduce the incidence of vascular dementia.
Supplementary Material
Acknowledgements
KR is supported by a UK National Institute for Health Research (NIHR) Career Development Fellowship. CE is supported by the Rhodes Trust. MW is supported by a Principal Research Fellowship from the Australian Health and Medical Research Council and is a consultant for Amgen and Novartis. The work of the George Institute is supported by the Oxford Martin School and the NIHR Oxford Biomedical Research Centre. SA is an Academic Clinical Lecturer in Cardiology and is funded by the National Institute of Health Research. STP and ZM are funded by the NIHR Oxford Biomedical Research Centre (BRC). PMR is in receipt of Senior Investigator Awards from the Wellcome Trust and from the NIHR.
The CPRD study was funded by the UK NIHR and Oxford NIHR BRC. The OXVASC Study is funded by Wellcome Trust, UK Medical Research Council, the Dunhill Medical Trust, the Stroke Association, the NIHR, and the Oxford NIHR BRC. We acknowledge the help of Mrs Linda Bull in collecting blood pressure readings from primary care records of patients recruited in the OXVASC study.
Footnotes
Contributors
CE and KR conceived of the CPRD study, acquired, analysed and interpreted the data, and drafted and critically revised the report. CE and KR had full access to CPRD data in the study and take responsibility for the integrity of the CPRD data and the accuracy of the data analysis. PMR planed and directed the OXVASC Study, interpreted the OXVASC data, critically revised the report, and takes responsibility for the integrity of the OXVASC data. STP assessed dementia outcomes in the OXVASC study and ZM analysed the data. All authors interpreted the data and critically revised the report.
Declaration of Interests
We declare no competing interests.
References
- 1.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, Luchsinger JA, Ogunniyi A, Perry EK, Potocnik F, Prince M, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 4.Ballard C, Neill D, O'Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000;59:97–106. doi: 10.1016/s0165-0327(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 5.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 7.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 8.Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58:22–28. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B, van Harskamp F, Hofman A, Breteler MM. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12:33–39. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ. 1996;312:805–808. doi: 10.1136/bmj.312.7034.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4:395–402. doi: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, Timmis A, Hemingway H. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. 2013;346:f2350–f2350. doi: 10.1136/bmj.f2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2014;0 doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denaxas SC, George J, Herrett E, Shah AD, Kalra D, Hingorani AD, Kivimäki M, Timmis AD, Smeeth L, Hemingway H. Data resource profile: cardiovascular disease research using linked bespoke studies and electronic health records (CALIBER) Int J Epidemiol. 2012;41:1625–1638. doi: 10.1093/ije/dys188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien JT, Burns A, BAP Dementia Consensus Group Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:997–1019. doi: 10.1177/0269881110387547. [DOI] [PubMed] [Google Scholar]
- 17.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 19.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and dementia - a comprehensive review. Ther Adv Neurol Disord. 2009;2:241–260. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Østergaard SD, Mukherjee S, Sharp SJ, Proitsi P, Lotta LA, Day F, Perry JRB, Boehme KL, Walter S, Kauwe JS, Gibbons LE, et al. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLoS medicine. 2015;12:e1001841–discussion e1001841. doi: 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S115–23. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta-analysis of physician accuracy. Acta Psychiatr Scand. 2011;124:165–183. doi: 10.1111/j.1600-0447.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- 25.Russell P, Banerjee S, Watt J, Adleman R, Agoe B, Burnie N, Carefull A, Chandan K, Constable D, Daniels M, Davies D, et al. Improving the identification of people with dementia in primary care: evaluation of the impact of primary care dementia coding guidance on identified prevalence. BMJ Open. 2013;3:e004023–e004023. doi: 10.1136/bmjopen-2013-004023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachinski V, Oveisgharan S, Romney AK, Shankle WR. Optimizing the Hachinski Ischemic Scale. Arch Neurol. 2012;69:169–175. doi: 10.1001/archneurol.2011.1698. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev P, Kalaria R, O'Brien J, Skoog I, Alladi S, Black SE, Blacker D, Blazer DG, Chen C, Chui H, Ganguli M, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.