Abstract
Background
The International Carotid Stenting Study (ICSS) was a multicentre randomised trial in which patients with symptomatic carotid artery stenosis were randomly allocated to treatment by carotid stenting or endarterectomy. Economic evidence comparing these treatments is limited and inconsistent.
Aims
We compared the cost-effectiveness of stenting versus endarterectomy using ICSS data.
Methods
We performed a cost-utility analysis estimating mean costs and quality-adjusted life years (QALYs) per patient for both treatments over a five-year time horizon based on resource use data and utility values collected in the trial. Costs of managing stroke events were estimated using individual patient data from a UK population-based study (Oxford Vascular Study).
Results
Mean costs per patient (95% CI) were US$10 477 ($9669 to $11 285) in the stenting group (N=853) and $9669 ($8835 to $10 504) in the endarterectomy group (N=857).There were no differences in mean QALYs per patient (3.247 (3.160 to 3.333) and 3.228 (3.150 to 3.306), respectively). There were no differences in adjusted costs between groups (mean incremental costs for stenting versus endarterectomy $736 (95% CI -$353 to $1826)) or adjusted outcomes (mean QALYs gained -0.010 (95% CI -0.117 to 0.097)). The incremental net monetary benefit for stenting versus endarterectomy was not significantly different from zero at the maximum willingness to pay for a QALY commonly used in the UK. Sensitivity analyses showed little uncertainty in these findings.
Conclusions
Economic considerations should not affect whether patients with symptomatic carotid stenosis undergo stenting or endarterectomy.
Keywords: cost factors, economics, carotid stenosis, carotid stenting, carotid endarterectomy, clinical trial
Introduction
Stroke is a leading cause of mortality and disability worldwide.1 In the UK there are 152 000 strokes and 49 000 stroke deaths each year. UK total annual health care costs of stroke are £1.8 billion (1% health care expenditure), and total annual societal costs are £3.7 billion.2 Carotid stenosis causes about 10% of all ischaemic strokes. Elective treatment of carotid stenosis by surgical endarterectomy or carotid artery stenting can prevent future stroke. The International Carotid Stenting Study (ICSS) was the largest randomised trial comparing stenting with endarterectomy for treatment of symptomatic carotid stenosis.3–5 ICSS was an international multicentre randomised clinical trial in which 1710 patients with symptomatic carotid stenosis were recruited and followed at 50 centres in Europe, Australia, New Zealand and Canada between May 2001 and October 2008. Participants were randomly assigned to stenting (n=853) or endarterectomy (n=857), and followed for up to ten years. The study found that long-term functional outcome and the risk of fatal or disabling stroke in patients with symptomatic carotid stenosis treated by stenting and endarterectomy were similar, concluding that stenting is an appropriate treatment choice for patients with symptomatic carotid stenosis if the risk of peri-procedural stroke is low.4–5The trial also showed an excess of procedural stroke (mainly non-disabling strokes) within 30 days of stenting compared to endarterectomy, while endarterectomy was associated with an excess of cranial nerve palsy and wound hematoma at time of surgery. The impact of these events on health care costs and quality of life was uncertain. Given the large number of patients eligible for these procedures, their cost and cost-effectiveness has implications for treatment selection. There have been several economic analyses of stenting versus endarterectomy for carotid stenosis, but many are observational or modelling studies with short time horizons; conclusions are mixed (Supporting Information).
Aims
We investigated the cost and cost-effectiveness of stenting versus endarterectomy using ICSS data.
Methods
Overview of Economic Evaluation
See Supporting Information for background details about ICSS. We undertook a cost-utility analysis to compare the costs and outcomes of stenting and endarterectomy for the 1710 patients in the intention-to-treat sample of ICSS. The outcome measure was quality-adjusted life years (QALYs), which combine length of life and quality of life, based on National Institute for Health and Care Excellence (NICE) recommendations.6 Cost-effectiveness was expressed as incremental net monetary benefits.6 The analysis took a UK National Health Service (NHS) and personal social services (PSS) perspective.6 Resource use data were included from all participating centers and UK unit costs were applied. Costs were calculated in 2013/14 UK£ and are presented in 2013/14 US$ using a purchasing power parity of £1=$1.43.7 The time horizon was five years, reflecting average follow-up in the trial. Extrapolation beyond the end of the trial was not undertaken because the within-trial analysis found no evidence of significant differences in costs or benefits between groups; five years was long enough to reflect all important differences in costs or outcomes between treatments. An annual discount rate of 3.5% was applied to costs and outcomes.6
Resource Use and Costs
For every patient we calculated the cost of the index procedure and of follow-up based on resource use data from the trial. Costs included: surgeon and radiologist time; operating theatre time including nursing staff, drugs, consumables and overheads; anaesthesia; materials and devices including stents, shunts, patches, cerebral protection devices, catheters, wires and sheaths; length of hospital stay in the intensive care unit (ICU) and inpatient ward; additional procedures; complications within 30 days of index procedure (fatal and non-fatal myocardial infarction, severe haematoma, disabling cranial nerve palsy); imaging tests (nine types); drug treatment (six types); and, subsequent non-disabling, disabling or fatal strokes.
Unit costs were obtained from published sources8–13 and local costs, inflated where appropriate.8 Unit costs of surgeon, radiologist and operating theatre time were hourly costs applied to procedure durations collected during the trial. Choice of stents was at the discretion of the interventionist. In the base case analysis each stent was assigned an acquisition cost of $1199 based on the local cost of the most commonly used stent (Carotid Wallstent (Boston Scientific)); this was varied in sensitivity analysis. Unit costs of hospital stays were daily costs applied to length of stay data collected in the trial. Length of stay in days on the ICU was not collected for individual patients, but mean values were collected by centre. We assumed patients admitted to the ICU post-operatively stayed for one day. Costs of additional carotid artery procedures were assumed to be equal to the mean cost of the index procedures. Costs of drug treatment were monthly costs applied to treatment durations collected in the trial. Stroke events were recorded in the trial, but the costs of managing them were not. These were obtained from supplementary analyses of a contemporaneous UK population-based study of all strokes, the Oxford Vascular (OXVASC) Study,14–15 including over 1,000 consenting transient ischaemic attack or stroke patients recruited from 1 April 2002 to 31 March 2007 in nine general practices across Oxfordshire, UK and followed for up to 60 months. These data were used to predict care home and hospital care costs for each stroke patient as a function of their sex, age, disability before stroke, previous history of cardiovascular disease, stroke severity and recurrent strokes. See Supporting Information for further details about analysis of OXVASC data.
Utilities and QALYs
Generic health status was described at baseline (randomisation), one and six months, and one, two, three, four and five years post-randomisation using the EQ-5D-3L descriptive system.16–17 EQ-5D-3L health states were converted into utility values using a formula that attaches weights to each level in each dimension based on valuations by general population samples. We used a value set for the UK population to calculate utility values at each time point for every participant.18 Utility values of one represent full health, values of zero are equivalent to death, negative values represent states worse than death. Patients who died were assigned a utility value of zero at their date of death. A utility profile was constructed for every patient assuming a straight line relation between their utility values at each measurement point. QALYs for every patient from baseline to five years were calculated as the area under the utility profile.
Dealing with Missing Data
The extent of missing data across all of the individual variables in the analysis ranged from 0% to 64% (Supplementary Information). Multiple imputation was used to impute missing data for cost of: surgeon time; radiologist time; operating theatre time; anaesthesia; stents; shunts; patches; cerebral protection devices; other materials used in stenting; length of hospital stay; non-fatal myocardial infarction; imaging test; drug treatment; and, strokes; plus, total cost; utility values at every time point; and total QALYs. Age, sex, study centre and treatment allocation were included in the imputation as additional explanatory variables. We used an iterative Markov chain Monte Carlo procedure based on multivariate normal regression, generating 20 imputed datasets.
Statistical Methods
Mean costs, outcomes and net monetary benefits (NMBs) were compared between all patients randomly assigned to stenting and endarterectomy, irrespective of which treatment was administered and whether or not they received additional carotid artery procedures. We calculated differences in mean costs, QALYs and incremental NMBs between groups. NMBs for stenting (S) and endarterectomy (E) were calculated as the mean QALYs per patient (Q) multiplied by the maximum willingness to pay for a QALY (R) minus the mean cost per patient (C), i.e., NMBi = Qi*R – Ci for i = S, E. The incremental NMB (iNMB) was calculated as the difference in mean QALYs per patient with stenting versus endarterectomy multiplied by the maximum willingness to pay for a QALY minus the difference in mean cost per patient, i.e., iNMB = (QS – QE)*R – (CS – CE). We used the cost-effectiveness threshold range recommended by NICE (£20 000 (approximately $29 000) to £30 000 ($43 000)6) as the lower and upper limits of the maximum willingness to pay for a QALY (R). If the incremental NMB is positive (negative) then stenting (endarterectomy) was preferred on cost-effectiveness grounds. QALYs gained were adjusted for age, sex, study centre and baseline utility values using regression analysis; incremental costs were adjusted for age, sex and study centre. For each of the 20 imputed datasets we ran 1000 bootstrap replications using non-parametric bootstrapping, resampling observations with replacement. Results were combined using equations described by Briggs et al19 to calculate standard errors around mean values accounting for uncertainty in imputed values, skewness of cost and utility data, and sampling variation. Standard errors were used to calculate 95% CIs around point estimates.
Sensitivity and Sub-Group Analyses
A cost-effectiveness acceptability curve20 showing the probability that stenting was cost-effective compared with endarterectomy at a range of values for the maximum willingness to pay for a QALY was generated based on the proportion of the bootstrap replications across all 20 imputed datasets with positive incremental NMBs.21 The probability that stenting was cost-effective at a maximum willingness to pay for a QALY of $29 000 and $43 000 was based on the proportion of bootstrap replications with positive incremental NMBs at these values. We undertook further sensitivity analyses: no adjustment for potential confounders; complete case analysis without imputing missing values; univariate analyses of high and low values for each cost component (50% higher and lower than the base case); and, discount rate (1.5%, 5%). No significant interactions were found in any sub-group analyses of the primary outcomes in ICSS. In post hoc sub-group analyses we investigated cost-effectiveness by sex and baseline age (≥70, <70 years).
Results
See Supporting Information for the resource use and unit cost data used in the analysis. Accounting for missing data, mean total costs per patient (95% CI) were $10 477 ($9669 to $11 285) in the stenting group (N=853) and $9669 ($8835 to $10 504) in the endarterectomy group (N=857; Table 1). In both groups approximately two-thirds of the total costs were for the index procedure and one-third for follow-up. Values were similar in the complete case analysis (Supporting Information Table S5).
Table 1. Mean Utility Values, QALYs and Costs per Patient.
| Endarterectomy (N=857) | Stenting (N=853) | |||
|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | |
| Cost of index procedure | 6499 | (6171, 6828) | 6820 | (6463, 7177) |
| Cost of follow-up | 3170 | (2466, 3870) | 3657 | (3032, 4283) |
| Total cost | 9669 | (8835, 10 504) | 10 477 | (9669, 11 285) |
| Utility values | ||||
| Baseline | 0.758 | (0.743, 0.774) | 0.776 | (0.761, 0.790) |
| 1 month | 0.779 | (0.763, 0.795) | 0.777 | (0.759, 0.795) |
| 6 months | 0.763 | (0.746, 0.780) | 0.754 | (0.735, 0.773) |
| 1 year | 0.739 | (0.721, 0.758) | 0.737 | (0.718, 0.757) |
| 2 years | 0.709 | (0.688, 0.729) | 0.710 | (0.689, 0.732) |
| 3 years | 0.677 | (0.655, 0.699) | 0.674 | (0.650, 0.698) |
| 4 years | 0.628 | (0.602, 0.653) | 0.648 | (0.622, 0.675) |
| 5 years | 0.594 | (0.563, 0.625) | 0.609 | (0.578, 0.641) |
| QALYs | 3.228 | (3.150, 3.306) | 3.247 | (3.160, 3.333) |
| Net monetary benefit | ||||
| $29 000 | 82 478 | (79 832, 85 124) | 82 262 | (79 447, 85 077) |
| $43 000 | 128 551 | (124 774, 132 328) | 128 632 | (124 580, 132 684) |
QALY = quality-adjusted life year. CI = confidence interval. Costs are in 2013/14 US$. Data include values imputed using multiple imputation (see text).
Mean utility values at each follow-up point were similar for the two groups and there was a decline over time (Table 1). Mean total QALYs per patient were 3.228 (3.150 to 3.306) in the endarterectomy group and 3.247 (3.160 to 3.333) in the stenting group. Values were similar for complete cases (Supporting Information).
Mean NMBs for endarterectomy and stenting were $82 478 ($79 832 to $85 124) and $82 262 ($79 447 to $85 077) at a maximum willingness to pay for a QALY of $29 000, and $128 551 ($124 774 to $132 328) and $128 632 ($124 580 to $132 684) at a maximum willingness to pay for a QALY of $43 000 (Table 1).
There were no significant differences in costs between the two groups (mean incremental costs for stenting versus endarterectomy $736 (95% CI -$353 to $1826)) or in outcomes (mean QALYs gained -0.010 (95% CI -0.117 to 0.097); Table 2). The incremental NMB for stenting versus endarterectomy was not significantly different from zero at a maximum willingness to pay for a QALY of $29 000 (mean -$991, 95% CI -$4475 to $2494) or $43 000 (mean -$1118, 95% CI -$6110 to $3875).
Table 2. Incremental cost-effectiveness of stenting vs. endarterectomy.
| Incremental cost | QALYs gained | Incremental net monetary benefit | Probability stenting cost-effective | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| $29 000 | $43 000 | |||||||||
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | $29 000 | $43 000 | |
| Base case* | 736 | (-353, 1826) | -0.010 | (-0.117, 0.097) | -991 | (-4475, 2494) | -1118 | (-6110, 3875) | 0.27 | 0.31 |
| No adjustment† | 808 | (-299, 1915) | 0.019 | (-0.098, 0.135) | -215 | (-4027, 3419) | 81 | (-5369, 5299) | 0.43 | 0.49 |
| Complete case analysis‡ | 761 | (-1193, 2715) | 0.006 | (-0.194, 0.206) | -592 | (-6665, 5482) | -507 | (-9333, 8318) | 0.42 | 0.45 |
| Sub-group analyses§ | ||||||||||
| Men | 481 | (-672, 1634) | -0.055 | (-0.185, 0.076) | -2043 | (-6107, 2023) | -2822 | (-8702, 3058) | 0.17 | 0.18 |
| Women | 1123 | (-1153, 3400) | 0.103 | (-0.098, 0.304) | 1793 | (-4759, 8371) | 3270 | (-6044, 12 583) | 0.71 | 0.75 |
| Age ≥70 years | 1112 | (-461, 2685) | -0.061 | (-0.219, 0.097) | -2845 | (-7814, 2126) | -3710 | (-10 859, 3437) | 0.13 | 0.16 |
| Age <70 years | 204 | (-1018, 1426) | 0.057 | (-0.094, 0.208) | 1416 | (-3189, 6023) | 2227 | (-4481, 8935) | 0.73 | 0.75 |
QALY = quality-adjusted life year. CI = confidence interval. Costs are in 2013/14 US$.
Data include values imputed using multiple imputation (see text). The QALYs gained, incremental cost and incremental net monetary benefit figures are for stenting minus endarterectomy and are adjusted for potential confounders (see text).
As for the base case analysis except the QALYs gained and the incremental costs are unadjusted.
As for the base case analysis except there is no multiple imputation of missing values and the 95% confidence intervals were derived from 1000 bootstrap replications of a single dataset containing the N=202 endarterectomy patients and N=254 stenting patients with no missing values.
As for the base case analysis but run on patients within each sub-group. Among the N=857 endarterectomy patients 606 are men, 251 are women, 453 are age ≥70 and 404 are <70. Among the N=853 stenting patients the figures are 601, 252, 458 and 395.
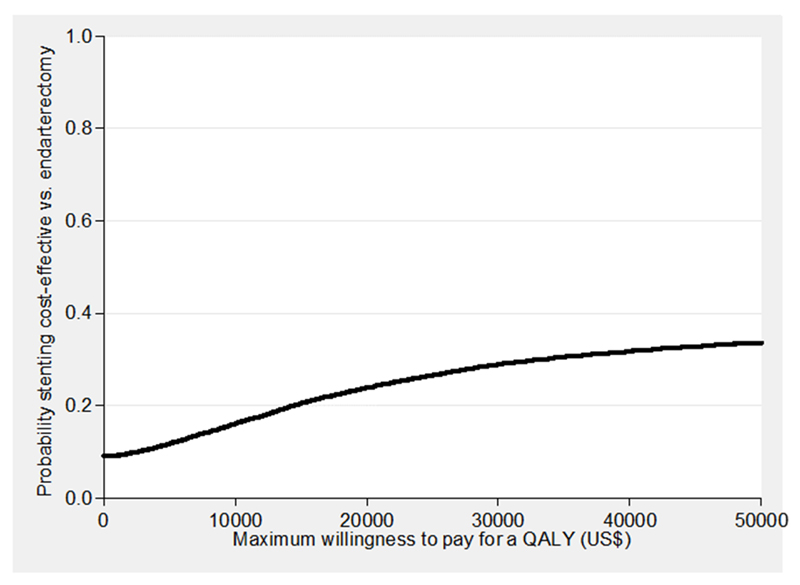
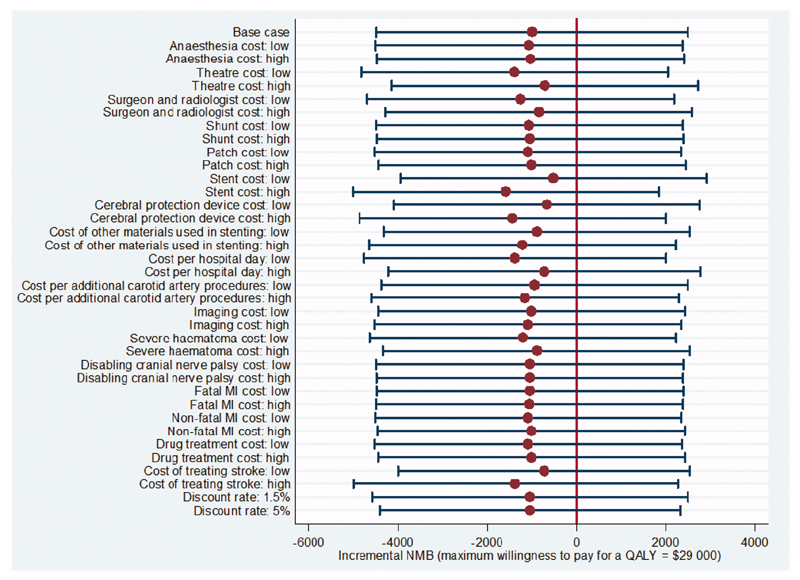
At a maximum willingness to pay for a QALY of $29 000 ($43 000) the probability that stenting is cost-effective was 0.27 (0.31; Table 2, Figure 1). Incremental costs, QALYs gained and incremental NMBs for stenting versus endarterectomy remained not significantly different from zero when rerunning the analysis without adjustment, and using complete cases (Table 2). The incremental NMB was most sensitive to the cost of stents (Figure 2), but in every case it was not significantly different from zero. In all sub-groups the incremental costs, QALYs gained and incremental NMBs were not significantly different from zero, though in men and those aged ≥70 years the probability that stenting is cost-effective compared with endarterectomy was lower than for women and those aged <70 years.
Figure 1. Cost-effectiveness acceptability curve showing the probability that stenting is cost-effective vs. endarterectomy at different values of the maximum willingness to pay for a QALY.
QALY = quality adjusted life year.
Figure 2. Univariate sensitivity analysis.
MI = myocardial infarction. NMB = net monetary benefit. All analyses are as for the base case analysis with univariate adjustment of the parameters listed (see text). Results are point estimates of the incremental net monetary benefit of stenting vs. endarterectomy (circles) and 95% confidence intervals (capped spikes). The incremental net monetary benefit is calculated at a maximum willingness to pay for a QALY of $29 000 (see Supporting Information for results calculated at a maximum willingness to pay for a QALY of $43 000).
Discussion
Our economic analysis of the ICSS showed that stenting and endarterectomy had similar costs and outcomes. This was despite the finding in ICSS of higher rates of non-disabling strokes in the stenting group. Sensitivity analyses showed little uncertainty in this finding. The findings mean there is no reason to prefer stenting or endarterectomy on the basis of differences in quality of life or on economic grounds; other factors should be taken into account when deciding which option to use to treat patients with symptomatic carotid stenosis, e.g., imaging features.
Previous economic analyses of stenting versus endarterectomy are mainly small single-centre observational cost studies with limited consideration of costs; they have drawn varying conclusions (see Supporting Information for a detailed review). Only one other randomised trial, the North-American-based CREST, has reported an economic analysis; this also found evidence of no differences in costs and QALYs between stenting and endarterectomy.22 CREST included patients with asymptomatic stenosis, which has a much lower rate of procedural stroke that might have influenced the analysis.
The main strength of our analysis is that it is based on a large international multicentre randomised trial with detailed information on resource use, utility values and mortality for a median follow-up period of 4.2 years. There are several limitations. First, data on costs of managing strokes were not collected in the trial. Rather than applying the same unit cost to every stroke, we used individual patient data from of the OXVASC Study to predict stroke costs at the patient-level. These were detailed contemporaneous UK-specific costs matched to patients in the trial, but are not the actual costs incurred. When we adjusted these costs in sensitivity analyses the findings did not change. Second, the analysis took a UK NHS/PSS perspective. Results may differ between countries depending on the relative value of unit costs (e.g., cost of stents). Third, a wider perspective (e.g., societal) could have been taken, including costs to patients, families and businesses. Given the trial found no differences in mortality or disability it is unlikely this would affect the incremental costs. Fourth, the time horizon was five years. We could have taken a longer time horizon, but there were no differences in costs or benefits between groups at this point so this would not have affected the incremental analyses. Fifth, we did not have complete data for every participant in the trial and used multiple imputation. Conclusions were the same for analyses using multiple imputation and complete cases. Sixth, ICSS started in the early days of carotid stenting, and stents and protection devices have also evolved since the trial started. It is possible that the costs and outcomes are not representative of routine clinical practice today.
ICSS showed that long-term functional outcome and the risk of fatal or disabling stroke of patients with symptomatic carotid stenosis allocated treatment by stenting is similar to endarterectomy. Our accompanying economic analysis has shown that despite stenting in the trial being associated with an excess of stroke, this did not translate into differences in quality of life or costs.
Supplementary Material
Funding
This study was funded by grants from the Medical Research Council, the Stroke Association, Sanofi-Synthélabo, and the European Union. MMB’s Chair in Stroke Medicine is supported by the Reta Lila Weston Trust for Medical Research. RLF was supported by a grant from Medical Research Council. The funding from the MRC was managed by NIHR on behalf of the MRC-NIHR partnership. Part of this work was undertaken at University College London, which received a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. The Oxford Vascular Study is funded by the Wellcome Trust, UK Medical Research Council, Dunhill Medical Trust, Stroke Association, National Institute for Health Research (NIHR), and the NIHR Biomedical Research Centre, Oxford. PMR is in receipt of an NIHR Senior Investigator Award and a Wellcome Trust Senior Investigator Award.
Footnotes
Author contributions
MMB was Principal Investigator. SM, MMB, JD, RLF and TR designed the analysis. MMB, JD and RLF analysed the ICSS data. PMR and RL-F analysed the OXVASC data. NVP identified unit costs and calculated the utility values. SM ran the analyses and drafted the paper. All authors contributed to data interpretation and the final version of the paper.
Conflicts of interest
None
References
- 1.Mackay J, Mensah GA. The atlas of heart disease and stroke. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Townsend N, Wickramasinghe K, Bhatnagar P, et al. Coronary heart disease statistics 2012 edition. London: British Heart Foundation; 2012. [Google Scholar]
- 3.Featherstone RL, Brown MM, Coward LJ. International carotid stenting study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69–74. doi: 10.1159/000078753. [DOI] [PubMed] [Google Scholar]
- 4.International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529–538. doi: 10.1016/S0140-6736(14)61184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence (NICE) Guide to the methods of technology appraisal 2013. London: NICE; 2013. [PubMed] [Google Scholar]
- 7.Organisation for Economic Co-operation and Development (OECD) Prices and Purchasing Power Parities. [Accessed 28 April 2015]; OECD website. http://www.oecd.org/std/prices-ppp/
- 8.Curtis L. Unit costs of health and social care 2013. Kent: Personal Social Services Research Unit; 2013. [Google Scholar]
- 9.Information Services Division Scotland. Theatre Services. Edinburgh: Information Services Division Scotland; 2012. [Google Scholar]
- 10.Gomes M, Soares MO, Dumville JC, et al. the GALA Collaborative Group Cost-effectiveness analysis of general anaesthesia versus local anaesthesia for carotid surgery (GALA Trial) Br J Surg. 2010;97:1218–1225. doi: 10.1002/bjs.7110. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health. National Schedule of Reference Costs - Year 2011- London: Department of Health; 2012. [Google Scholar]
- 12.Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14) doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 13.Joint Formulary Committee. British National Formulary, 66th edition. London: BMJ Group and Pharmaceutical Press; 2013. [Google Scholar]
- 14.Luengo-Fernandez R, Gray AM, Rothwell PM, on behalf of the Oxford Vascular Study A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke. 2012;43:3343–3351. doi: 10.1161/STROKEAHA.112.667204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luengo-Fernandez R, Paul NLM, Gray AM, et al. on behalf of the Oxford Vascular Study A population-based study of disability and institutionalisation after TIA and stroke: 10-year results of the Oxford Vascular Study. Stroke. 2013;44:2854–2861. doi: 10.1161/STROKEAHA.113.001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 18.Dolan P. Modelling valuations for health states. Medical care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Briggs A, Clark T, Wolstenholme J, Clarke P. Missing … presumed at random: cost-analysis of incomplete data. Health Econ. 2003;12:377–392. doi: 10.1002/hec.766. [DOI] [PubMed] [Google Scholar]
- 20.Briggs AH, Gray AM. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol Assess. 1999;3:1–134. [PubMed] [Google Scholar]
- 21.Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 22.Vilain KR, Magnuson EA, Li H, et al. Costs and cost-effectiveness of carotid stenting versus endarterectomy for patients at standard surgical risk: results from the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) Stroke. 2012;43:2408–2416. doi: 10.1161/STROKEAHA.112.661355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.