Abstract
Objective
Biomarkers are defined as anatomical, biochemical or physiological traits that are specific to certain disorders or syndromes. The objective of this paper is to summarise the current knowledge of biomarkers for anxiety disorders, obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD).
Methods
Findings in biomarker research were reviewed by a task force of international experts in the field, consisting of members of the World Federation of Societies for Biological Psychiatry Task Force on Biological Markers and of the European College of Neuropsychopharmacology Anxiety Disorders Research Network.
Results
The present article (Part II) summarises findings on potential biomarkers in neurochemistry (neurotransmitters such as serotonin, norepinephrine, dopamine or GABA, neuropeptides such as cholecystokinin, neurokinins, atrial natriuretic peptide, or oxytocin, the HPA axis, neurotrophic factors such as NGF and BDNF, immunology and CO2 hypersensitivity), neurophysiology (EEG, heart rate variability) and neurocognition. The accompanying paper (Part I) focuses on neuroimaging and genetics.
Conclusions
Although at present, none of the putative biomarkers is sufficient and specific as a diagnostic tool, an abundance of high quality research has accumulated that should improve our understanding of the neurobiological causes of anxiety disorders, OCD and PTSD.
Keywords: Anxiety disorders, neuroimaging, genetic, neurochemistry, neurobiology, review
Introduction
This consensus statement on biological markers of anxiety disorders was organised by members of the World Federation of Societies for Biological Psychiatry Task Force on Biological Markers and of the Anxiety Disorders Research Network (ADRN) within the European College of Neuropsychopharmacology Network Initiative (ECNP-NI; Baldwin et al. 2010), an initiative intended to meet the goal of extending current understanding of the causes of central nervous system (CNS) disorders, thereby contributing to improvements in clinical outcomes and reducing the associated societal burden.
The present article (Part II) summarises the findings on potential biomarkers in neurochemistry, neurophysiology, and neurocognition. Part I (Bandelow et al. 2016) focuses on neuroimaging and genetics.
Neurochemistry
Plasma appears to be a rational source for proteomic and metabolomic measurements in psychiatric conditions because it is easily accessible, and several molecules from the brain are transported across the blood–brain barrier and reach the peripheral circulation. However, it is difficult to draw inferences from the neurochemical composition of plasma on the situation in brain cells. Lumbar puncture is an invasive method, and the composition of cerebrospinal fluid (CSF) does not reflect exactly the neurochemistry in brain cells. Nevertheless, as a biomarker measure, such recourses are highly valuable, and several examples of evidence in the literature points to possible links between CNS and periphery. In the following sections, some of these findings are listed and described.
Neurotransmitters
Monoaminergic systems have long been suggested to play a major role in depression and anxiety disorders. While the “reward system” is modulated by endogenous dopamine and opioids (Barbano & Cador 2007; Berridge & Aldridge 2008; Le Merrer et al. 2009; Bandelow & Wedekind 2015), the “punishment system” is mainly driven by serotonin (5-HT; Stein 1971; Daw et al. 2002). Goal-directed behaviours are stimulated by dopamine (DA), and dopamine neurons have been suggested to be a substrate for intracranial self-stimulation (Wise & Bozarth 1982; Mason & Angel 1984; Aboitiz 2009). Norepinephrine (noradrenaline; NE) has been connected to “emotional memory” and the consolidation and retrieval of the emotional arousal induced by particular behaviours (van Praag et al. 1990; Goddard et al. 2010). NE neurons regulate vulnerability to social defeat through inhibitory control of ventral tegmental area DA neurones (Isingrini et al. 2016).
Serotonergic system
Findings on brain imaging and genetics of the serotonin system are summarised in Part I (Bandelow et al. 2016).
5-HT is a monoamine, found in the CNS, in blood platelets, and the gastrointestinal tract. The principal source of serotonin release in the brain are the raphe nuclei in the brainstem. They are hypothesised to have a dual role in aversive contingencies (Deakin & Graeff 1991; Deakin 2013). 5-HT can inhibit periaqueductal grey matter-medicated fight/flight responses from threats, while it can also facilitate amygdala-mediated anxiety responses. The latter mechanism has been demonstrated both in animals (Deakin & Graeff 1991; Deakin 2013) and humans (Blanchard et al. 2001; Mobbs et al. 2007; Feinstein et al. 2013). Such differences may explain partly the different types of emotions (Mobbs et al. 2007) and anxiety disorders seen in humans (Deakin & Graeff 1991). Therefore, reaction to threat, mediating periaquaeductal-grey-mediated threats, related to the emotion named “fear”, may be more closely related with phobic, escape-dominant behavioural syndromes, such as specific phobias, social anxiety disorder (SAD) and panic disorder with or without agoraphobia (PDA; Gray & McNaughton 2000; McNaughton & Corr 2004), while amygdala-mediated threats seem to be linked to the emotion named “anxiety” such as general anxiety disorder (GAD) and obsessive-compulsive disorder (OCD; Gray & McNaughton 2000; McNaughton & Corr 2004). Recently, a functional difference in 5-HT between fear and anxiety disorders was demonstrated using an acute tryptophan depletion technique that transiently lowers brain 5-HT (Corchs et al. 2015). In this study, decreasing the function of the 5-HT system, using tryptophan depletion in patients in clinical remission lead to psychological and physiological exacerbation in response to stressors in PDA, SAD and posttraumatic stress disorder (PTSD), but not in GAD or OCD. This difference might be due to long-lasting neuronal changes, needed in anxiety disorders after serotonin-mediated therapeutics, in which acute 5-HT depletion does not cause such effects (Graeff & Zangrossi 2010). Animal data and genetic and neuroimaging studies in humans point to a role of the 5HT1A receptor in the neural processing of anxiety (Akimova et al. 2009). Recently, a review of the 5HT2C receptor suggested that this receptor may play a crucial role in anxiety (Chagraoui et al. 2016).
In the following paragraphs, the 5-HT involvement in various disorders is discussed in more details.
PDA
5-HT plasma levels measured by high-performance liquid chromatography were found to be significantly lower in PDA patients compared with control volunteers (Schneider et al. 1987b). Furthermore, in a study of males with PDA, serum 5-HT concentrations were measured via enzyme-linked immunosorbent assay. The authors reported lower serum 5-HT in patients compared with control group at baseline, which was further decreased after treatment with the selective serotonin reuptake inhibitor (SSRI) paroxetine, although symptom improvements were observed (Shutov & Bystrova 2008).
Platelet 5-HT reuptake site binding was found to be decreased in PDA patients in two studies (Iny et al. 1994; Lewis et al. 1985), while most studies reported no difference comparing to controls (Innis et al. 1987; Nutt & Fraser 1987; Pecknold et al. 1987; Schneider et al. 1987a; Uhde et al. 1987; Norman et al. 1989a, 1989b; Butler et al. 1992;). Moreover, platelet 5-HT concentration was reported also not to change in PDA patients (Balon et al. 1987; McIntyre et al. 1989), except in one report, where decreased 5-HT concentrations were observed (Evans et al. 1985). Two studies have reported increased platelet 5-HT uptake in PDA patients (Norman et al. 1986; Norman et al. 1989b), while two studies reported decreased platelet 5-HT uptake in a PDA group, compared with controls (Pecknold et al. 1988; Butler et al. 1992). Moreover, platelet aggregation in response to 5-HT was significantly lower in panic patients compared with controls (Butler et al. 1992).
CSF levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) were not different between PDA patients and healthy controls; nevertheless, in a small study with PDA patients responding to clomipramine or imipramine for at least 2 months, CSF 5-HIAA levels decreased significantly compared with baseline levels (Eriksson et al. 1991). Nevertheless, in female patients with major depressive disorder (MDD) comorbid with PDA, CSF 5-HIAA levels were significantly higher than in MDD patients without PDA and in healthy volunteers (Sullivan et al. 2006). Higher CSF 5-HIAA in women with comorbid MDD and lifetime panic disorder was indicative of greater 5-HT release, increased 5-HT metabolism, and/or decreased 5-HIAA clearance in this group. Esler et al. (2004) measured brain 5-HT turnover via measurement of 5-HIAA levels in plasma from internal jugular veins that has a direct overflow from brain neurons and not from the cerebrovascular sympathetic nerves (Lambert et al. 1995). A significant increase in brain 5-HT turnover, estimated from the jugular venous overflow of 5-HIAA, was observed in non-medicated PDA patients compared with healthy subjects (Esler et al. 2004).
Another approach measuring 5-HT disruption is via measurement of antibodies directed at the 5-HT system, such as anti-serotonin and 5-HT anti-idiotypic antibodies (directed at the serotonin receptors). Using this approach, Coplan et al. (1999) showed significantly elevated levels of plasma anti-serotonin and serotonin anti-idiotypic antibodies in panic disorder patients compared with controls. These findings suggest an autoimmune mechanism interrupting the 5-HT system in PDA.
GAD
Platelet 5-HT reuptake site binding was found to be decreased in GAD patients (Iny et al. 1994). 5-HT binding in lymphocytes did not differ in GAD patients compared with controls (Hernandez et al. 2002). Moreover, both 5-HT and 5-HIAA in platelet-rich and - poor plasma as well as in lymphocytes did not differ between GAD patients and controls (Hernandez et al. 2002).
SAD
The therapeutic efficacy of SSRIs and serotonin norepinephrine reuptake inhibitors (SNRIs) strongly suggests that 5-HT plays a crucial role in SAD. Patients with SAD show an exaggerated cortisol response to the serotonin-releasing compound fenfluramine, indicating supersensitivity of the post-synaptic serotonin receptors (Tancer 1993). In a similar study, SAD patients underwent challenges for serotonergic (fenfluramine), dopaminergic (levodopa), and noradrenergic (clonidine) systems in a double-blind study. They had an increased cortisol response to fenfluramine administration, compared with healthy volunteers. Neither the prolactin response to fenfluramine, the growth hormone or norepinephrine response to clonidine, nor prolactin or eye-blink responses to levodopa, differed between patients with SAD and healthy volunteers (Tancer et al. 1994b).
Platelet 5-HT2 receptor density did not differentiate between the SAD patients and controls, but was associated with severity (Chatterjee et al. 1997).
Patients with SAD, healthy control subjects, and OCD control subjects were challenged with single doses of the partial serotonin agonist oral meta-chlorophenylpiperazine (mCPP) and placebo. SAD patients did not significantly differ from normal or OCD control subjects in prolactin response to mCPP. Female patients with SAD had more robust cortisol responses to mCPP challenge (Hollander et al. 1998).
SAD patients, who had been successfully treated with an SSRI, underwent a tryptophan depletion challenge combined with a public speaking task. Salivary α-amylase, a marker of autonomic nervous system response, and hypothalamic-pituitary-adrenal (HPA) axis response, as measured with salivary cortisol, were assessed. The tryptophan depletion group showed a significant larger salivary α-amylase response to the public speaking task as compared with the placebo group, whereas no differences were seen in cortisol responses (van Veen et al. 2009).
OCD
Measurement of peripheral serotonergic parameters, like whole-blood 5-HT concentration, CSF concentration, platelet 5-HT transporter (5-HTT), 5-HT2A receptor binding characteristics and platelet inositol 1,4,5-triphosphate content, is the oldest classical approach, which has identified some predictors of clinical outcome of the treatment in OCD patients medicated with SSRIs.
In an early study, Thoren et al. (1980) showed initially elevated 5-HIAA levels in the CSF and a decrease during treatment were associated with better clinical outcome in patients treated with clomipramine (Flament et al. 1985).
There was no difference in blood 5-HT content between children and adolescents with severe OCD and the normal controls. However, OCD patients with a family history of OCD had significantly higher blood 5-HT levels than did either the OCD patients without family history or the healthy controls (Hanna et al. 1991). Blood 5-HT levels were decreased after treatment with SSRIs (Kremer et al. 1990; Humble & Wistedt 1992; Humble et al. 2001), and higher 5-HT concentrations were associated with better outcome after treatment of OCD (Aymard et al. 1994; Delorme et al. 2004).
Serotonin reuptake binding capacity on platelets was found to be reduced in children and adolescents with OCD, but not in Tourette syndrome (Sallee et al. 1996). The binding capacity of the 5-HTT for SSRIs and the tricyclic antidepressant (TCA) imipramine decreased in untreated OCD patients (Marazziti et al. 1996; Sallee et al. 1996). After treatment with the TCA clomipramine, binding was decreased (Black et al. 1990), whereas another study has found increased binding after treatment with the SSRI with fluvoxamine and or clomipramine (Marazziti et al. 1992).
PTSD
In an early review of trauma-related studies involving epinephrine, norepinephrine, and serotonin, evidence of serotonergic dysregulation in PTSD was reported, including frequent symptoms of aggression, impulsivity, depression and suicidality, decreased platelet paroxetine binding, blunted prolactin response to fenfluramine, exaggerated reactivity to m-chlorophenylpiperazine (mCPP), and clinical efficacy of SSRIs (Southwick et al. 1999).
No change in 5-HT1A receptor binding was found in a study by Bonne et al. (2005). A lower number of platelet [3H]paroxetine binding sites and a lower dissociation constant for [3H]paroxetine binding in combat veterans with PTSD compared with normal control subjects was reported (Fichtner et al. 1995). Platelet 5-HT concentration was significantly lower in suicidal PTSD and non-PTSD patients compared with non-suicidal patients or healthy controls (Kovacic et al. 2008). Compared with the control subjects, the PTSD patients showed significantly lower platelet-poor plasma 5-HT levels, elevated platelet-poor plasma norepinephrine levels, and significantly higher mean 24-hour urinary excretion of all three catecholamines (norepinephrine, dopamine and homovanillic acid; HVA) (Spivak et al. 1999).
During presentation of a trauma-related video, CSF concentrations of 5-HIAA diminished, but there was only a trend for statistical significance for this finding (Geracioti et al. 2013).
Dopaminergic system
Dopamine is involved in reward-motivated behaviour and motor control. Findings on brain imaging and genetics of the dopamine system are summarised in Part I (Bandelow et al. 2016). Similarly as for the serotonergic system, current findings related to the dopaminergic system are described in the following paragraph.
PDA
Eriksson et al. (1991) reported no significant change in CSF levels of HVA, the major metabolite of dopamine in patients with PDA compared with healthy controls. Nevertheless, in another study in both PDA and SAD, low CSF HVA levels were observed (Johnson et al. 1994).
SAD
In a study evaluating eye-blink response to administered levodopa, no dysfunction of the dopaminergic system was reported (Tancer et al. 1994a). Another approach is to challenge with dopamine agents such as the antagonist sulpiride and the agonist pramipexole. Hood et al. (2010) found that patients with SAD responded with increased anxiety to both drugs but that the effect of treatment with SSRIs was to attenuate the impact of pramipexole, suggesting a degree of dopamine D3 receptor desensitisation after SSRI treatment.
OCD
Acute deep brain stimulation targeted at the nucleus accumbens of 15 OCD patients induced a decrease in binding potential to the dopamine D2/D3 receptor (measured via SPECT [123I]IBZM binding), and chronic stimulation induced an increase in HVA plasma levels, implying that deep brain stimulation induces striatal dopamine release in OCD patients (Figee et al. 2014).
PTSD
In the aforementioned study by Geracioti et al. (2013), CSF HVA concentrations diminished significantly after a traumatic video. Compared with control subjects, PTSD subjects showed significantly higher mean 24-h urinary excretion of dopamine (Spivak et al. 1999).
Noradrenergic system
NE is a catecholamine produced mainly in the locus coeruleus in the pons. It is an important neurotransmitter in the autonomic nervous system. The metabolism and functions of norepinephrine have been studied extensively in depression and anxiety disorders. Hypofunction is postulated for the former, and hyperfunction for the latter. Findings on brain imaging and genetics of the noradrenergic system are summarised in Part I (Bandelow et al. 2016).
PDA
Stimulation of noradrenergic systems produces abnormal changes in measures of anxiety, somatic symptoms, blood pressure and plasma NE metabolite and cortisol levels in patients with PDA but not in patients with GAD, OCD, depression or schizophrenia, indicating specificity of abnormality in the regulation of the NE system in patients with PDA (Boulenger & Uhde 1982; Heninger & Charney 1988).
There is a body of evidence for NE involvement in anxiety in humans; e.g., anxiety can be induced using NE neuronal activators such as piperoxane and yohimbine (Redmond & Huang 1979). In patients with PDA, peripheral markers, including platelet aggregation to NE and to 5-HT, platelet a2-receptor density, lymphocyte β-receptor density, [3H]ketanserin binding to platelet 5-HT2 receptors and [3H]5-HTT uptake into platelets, largely remained abnormal during 6 months treatment with either clomipramine or lofepramine, despite clinical improvement (Butler et al. 1992). Therefore, these peripheral markers have been suggested to be potential trait markers in patients with PDA. Adrenergic receptor function has been measured in several clinical studies. Platelet α2-adrenoceptors have been studied in PDA patients using clonidine and yohimbine binding assays and correlated to symptom ratings and measurement of lying and standing plasma adrenaline and NE levels (Cameron et al. 1996; Nutt & Fraser 1987). Tritiated clonidine binding was decreased and resting heart rate was increased in PDA patients before treatment (fluoxetine, tricyclics or alprazolam). The magnitude of decrease in receptor binding was correlated with symptom severity and standing plasma NE (Cameron et al. 1996). In a similar approach, Gurguis et al. (1999) showed that patients with PDA had high α2-adrenoceptor density in both conformational states.
Stimulation of the locus coeruleus, an area containing most of the noradrenergic cell bodies of the brain, has been shown to induce anxiety and to raise the concentration of the main central NE metabolite, 3-methoxy-4-hydroxyphenyl glycol (MHPG) in patients with panic attacks. The decrease in plasma MHPG concentrations was found to parallel the response of patients with PDA to treatment (Charney et al. 1983). However, this could not be confirmed in a study of the effects of imipramine in PDA by Nutt & Glue (1991). Similarly, CSF levels of MHPG were not changed significantly in patients with PDA (Eriksson et al. 1991). On the other hand, Lista (1989) reported short time urine sampling to measure NE excretion as a marker for monitoring sympathetic activity. NE excretion was highest in major depression, followed by “minor” depression, anxiety disorders and healthy controls. Although plasma catecholamines (NE and epinephrine), blood pressure and heart rate were only partially correlated with salivary α-amylases, Kang (2010) proposed α-amylase as a measure of stress sensitivity causing an increase in anxiety scores. Recently, it was shown that epinephrine (24-h urine collection) was positively correlated with anxiety but not with depression, whereas 24-h urinary NE excretion was neither correlated with anxiety nor depression (Paine et al. 2015).
A low pre-treatment β-adrenoceptor affinity was found to predict the treatment response to paroxetine in patients with PDA and was suggested as a biomarker of pharmacological outcome in PDA (Lee et al. 2008).
PTSD
Compared with control subjects, PTSD patients showed significantly elevated platelet-poor plasma NE levels, and significantly higher mean 24-h urinary excretion of all three catecholamines (NE, dopamine and HVA) (Spivak et al. 1999).
γ-Aminobutyric acid
There is ample evidence that the pathogenesis of anxiety disorders is in part linked to a dysfunction of central inhibitory mechanisms. With regard to neurotransmission, the γ-aminobutyric acid (GABA) system serves as the most important inhibitory neurotransmitter system (Domschke & Zwanzger 2008). According to both preclinical and clinical studies, this system has been suggested to be strongly involved in the pathophysiology of anxiety and anxiety disorders. For example, benzodiazepines, which act at the GABA system, are used to treat anxiety. GABA is synthesised by a specific enzyme – glutamate acid decarboxylase – from glutamate. Released in the synaptic cleft, it either binds on GABA receptors or is removed by the main degradative enzyme GABA-transaminase (GABA-T) (for a review, see Olson 2002).
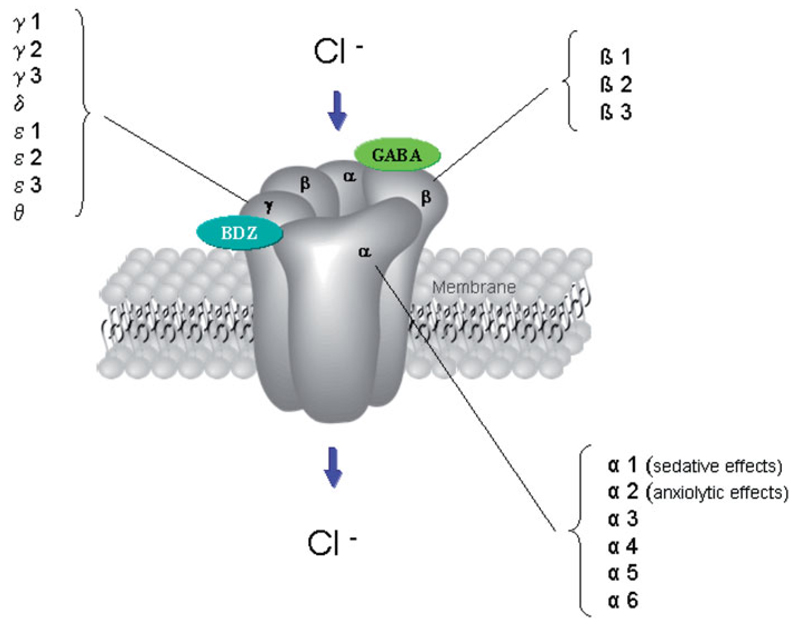
So far, three major subtypes of GABA receptors have been identified: GABAA, GABAB and GABAC receptors. GABAA and GABAC receptors belong to the class of ligand-gated ion channels, GABAB receptors serve as transmembrane receptors, coupled with G-proteins and activate second messenger systems (Chebib & Johnston 1999). However, the fast inhibitory action of the neurotransmitter GABA is mediated through GABAA receptors. A large variety of GABAA receptor subtypes has been characterised so far: α 1-6, β 1-3, γ 1-3, δ, ε 1-3, θ, π (Jacob et al. 2008); see Figure 1.
Figure 1.
GABA-A receptor and subunit structure; GABA and benzodiazepine (BZD) binding site (Domschke & Zwanzger 2008).
GABAA receptors consist of two α subunits, two β subunits and one γ or δ subunit (Jacob et al. 2008). Moreover, there are two distinct binding sites on the GABAA receptor: whereas GABA itself binds on the GABA binding site, which is located at the interface between the α and γ subunit, anxiolytic agents such as benzodiazepines bind at the benzodiazepine binding site at the interface between the α and the γ subunit. According to several preclinical studies, anxiolytic effects of benzodiazepines have been shown to be mostly mediated by the α2-subunit of the GABAA receptor (Low et al. 2000).
Therefore, a specific role of distinct GABAA receptor subunits can be hypothesised with regard to the pathogenesis of anxiety. Research on specific subunit selective psychopharmacological compounds targeting the α2-subunit of the GABAA receptor and lacking sedative or other associated side effects of benzodiazepines is ongoing.
PDA
Neurochemistry. An interesting approach investigating the role of GABAA receptors on the pathogenesis of panic attacks stems from Nutt et al. (1990) who suggested alterations in benzodiazepine receptor sensitivity in patients with PDA. After intravenous challenge, subjects with panic disorder exhibited panic attacks after flumazenil injection, a phenomenon which has been interpreted as a possible shift of the “receptor setpoint” (Nutt et al. 1990). However, these results have not been replicated (Strohle et al. 1999).
There is also evidence for a dysfunction of GABAA receptor modulatory neuroactive steroid regulation in panic disorder patients (Rupprecht 2003). It has been demonstrated that panic disorder patients show increased concentrations of GABA agonistic 3α-reduced neuroactive steroids (Strohle et al. 2002), which has been interpreted as a counter-regulatory mechanism against the occurrence of spontaneous panic attacks. In contrast, during experimentally induced panic induction with lactate or cholecystokinin-tetrapeptide (CCK-4) panic disorder patients show a significant decrease of GABA agonistic 3α-reduced neurosteroids along with an increase of the antagonistic 3α-reduced isomer, when compared with healthy controls (Strohle et al. 2003).
Translocator protein (TSPO) is an 18-kDa protein in the mitochondrial membrane which was first thought to be a peripheral binding site for benzodiazepines (Papadopoulos et al. 2006). However, recent research has found that it is not only expressed in the body but also in the brain. Ligands of this protein may promote the synthesis of endogenous neurosteroids. Some metabolites of progesterone are potent, positive allosteric modulators of GABAA receptors. Their concentrations are reduced during panic attacks in patients with PDA (Strohle et al. 2003). Unexpectedly, patients with PDA had significantly greater concentrations of the agonistic 3α-reduced neuroactive steroids (Strohle et al. 2002). The TSPO ligand XBD173 enhanced GABA-mediated neurotransmission and exerted antipanic activity in humans. In contrast to benzodiazepines, the drug did not cause withdrawal symptoms or sedation. Thus, TSPO ligands are promising candidates for novel anxiolytic drugs (Rupprecht et al. 2009), though a polymorphism of the binding site exists in humans that means around 10% have a low affinity variant (Owen et al. 2011).
Neuroimaging studies have found a reduction of GABA concentrations and benzodiazepine binding in patients with PDA (see chapter Neuroimaging, Part I; Bandelow et al. 2016). A few genetic studies have attempted to elucidate the role of GABA in anxiety disorders (see chapter Genetics, Part I (Bandelow et al. 2016). Pharmacological modulation of the GABA system. From a clinical point of view, the significance of the GABA system in the pathophysiology of panic and anxiety has also been derived from observing beneficial effects on symptoms following selective GABAergic treatment. In addition to the rapid and strong anxiolytic properties of benzodiazepines, targeting the benzodiazepine binding site of the GABAA receptor, modulation of GABA metabolism has also been shown to reduce anxiety and the occurrence of panic attacks. Among anticonvulsants, tiagabine and vigabatrin both increase GABA availability via a reduction of GABA degradation by inhibition of the GABA transaminase (vigabatrin) or inhibition of GABA reuptake via blockade of the GABA transporter GAT-I (tiagabine). For both compounds, anxiolytic action has been suggested through clinical studies and studies using pharmacological panic induction with CCK-4 (for a review, see Zwanzger & Rupprecht, 2005).
Other drugs that enhance GABAergic tone (e.g., barbiturates, ethanol, valproate) have anxiolytic effects, whereas negative modulators produce anxiogenic-like effects (Zwanzger et al. 2001; Kalueff & Nutt 2007; Zwanzger et al. 2009).
SepAD and benzodiazepines
Several studies favour the role of TSPO as a useful biological marker of adult separation anxiety disorder (A-SepAD). The TSPO is involved in the secretion of neurosteroids, whose levels are reported to be changed in several diseases and to be implicated in the pathogenic mechanisms of anxiety and mood disorders in humans. A reduction of platelet expression of TSPO density was found to relate specifically to the presence of A-SepAD in samples of patients with PDA (Pini et al. 2005) or major depression (Chelli et al. 2008) or bipolar depression (Abelli et al. 2010). Furthermore, Costa et al. (2012) found Ala147Thr substitution in TSPO to be associated with A-SepAD in patients with depression.
Neuropeptides
CCK
CCK is one of the most abundant neurotransmitter peptides in the brain and has been shown to induce excitation of central neurons as well as inhibitory post-synaptic effects (Bourin & Dailly 2004). CCK-1 and -2 receptors (G protein-coupled receptors) (recently reclassified as A and B) are widely distributed throughout the CNS. A large body of evidence suggests that the neuropeptide CCK might be an important modulator of the neuronal networks that are involved in anxiety, in particular in PDA.
PDA
In humans, CCK-induced anxiety may be mediated via CCK-B receptors (vs. CCK-B and -A in mice) (Li et al. 2013). Intravenous administration of exogenous CCK-4, -8 or the CCK agonist pentagastrin produced panic-like attacks in healthy volunteers within one minute, and these effects were attenuated by pre-treatment with benzodiazepines (de Montigny 1989; Bradwejn et al. 1991b). The most common clinical effects observed after administration of intravenous CCK-4 were dyspnoea, palpitations/tachycardia, chest pain/discomfort, faintness, dizziness, paresthaesia, hot flushes/cold chills, nausea/abdominal distress, anxiety/fear/apprehension and fear of losing control – a cluster of symptoms similar to those observed in spontaneous panic attacks in PDA.
In addition, the dose-response to intravenous CCK-4 reliably differentiates PDA patients from healthy controls with no personal or family history of panic attacks (Bradwejn et al. 1992). Furthermore, a relationship between dose and effect was found in healthy volunteers (Bradwejn et al. 1991a). While the panic rate after injection of 25 μg of CCK-4 was 91% for patients as compared with only 17% for controls, and 50 μg induced a full-blown panic attack in 100% of patients vs. 47% of controls.
In contrast to the findings in patients with PDA, in CCK-4-sensitive healthy volunteers, treatment with an antipanic SSRI did not cause a reduction of CCK-4-induced panic attacks beyond the effect of placebo (Toru et al. 2013). However, a significant reduction in CCK-induced anxiety was observed after administration of the benzodiazepine alprazolam and the GABAergic anticonvulsant vigabatrin (Zwanzger et al. 2001; Zwanzger et al. 2003). Baseline anxiety is a not a major determinant of the subjective panic response to CCK-4, emphasising the importance of neurobiological factors (Eser et al. 2008). It was proposed that benzodiazepine-mediated antagonism of CCK-induced excitation might be an important mechanism by which benzodiazepines exert their clinically relevant actions.
Moreover, in PDA patients, decreased concentrations of CCK-8 in the CSF have been reported compared with control subjects (Lydiard et al. 1992). Concentrations of CCK-8 in lymphocytes were also significantly reduced in patients with PDA compared with healthy controls (Brambilla et al. 1993). Finally, CCK-B receptor expression and binding are increased in animal models of anxiety. These findings are in favour of abnormalities in the CCK system in PDA patients.
The key regions of the fear network, such as basolateral amygdala (Del Boca et al. 2012), hypothalamus, periaqueductal grey, or cortical regions such as the anterior cingulate cortex (ACC), seem to be connected by CCK-ergic pathways (Dieler et al. 2008). Moreover, these effects seem to be modulated by molecular mechanisms, since neurochemical alterations were dependent on neuropeptide S genotype (Ruland et al. 2015). In humans, amygdala activation may be involved in the subjective perception of CCK-4-induced fear (Eser et al. 2009). In the amygdala, CCK may act in concordance with the endogenous cannabinoid system in the modulation of fear inhibition and extinction. In addition, CCK-4-induced panic is accompanied by a significant glutamate increase in the bilateral ACC (for a review, see Bowers et al., 2012). In contrast to placebo, alprazolam abolished the activation of the rostral ACC after challenge with CCK-4 and increased functional connectivity between the rostral ACC and other anxiety-related brain regions such as the amygdala and the prefrontal cortex (PFC). Moreover, the reduction in the CCK-4 induced activation of the rostral ACC correlated with the anxiolytic effect of alprazolam (Leicht et al. 2013). Finally, social stress-induced behavioural deficits are mediated partly by CCK-B receptors as a molecular target of ΔFosB in the medial prefrontal cortex (mPFC) and by molecular adaptations in the mPFC involving ΔFosB and CCK through cortical projections to distinct subcortical targets. In fact, CCK in mPFC-basolateral amygdala projections mediates anxiety symptoms (Vialou et al. 2014).
CCK also interacts with several anxiety-relevant neurotransmitters such as the serotonergic, GABAergic and noradrenergic systems, as well as with endocannabinoids, neuropeptides Y and S (for a review, see Zwanzger et al., 2012). For a review of CCK genes in anxiety disorders, see Part I (Bandelow et al. 2016).
In conclusion, experimental panic induction with CCK-4 has been established as a model to study the pathophysiology of PDA and might serve as a tool to assess the anti-panic potential of novel anxiolytic compounds if the challenge procedure is carried out according to strictly comparable conditions (Eser et al. 2007).
Neurokinins (tachykinins)
Central neurokinins (tachykinins) have been shown to play a role in the modulation of stress-related behaviours and anxiety. Different forms exist, termed neurokinins 1, 2 and 3. Substance P, a ligand of the neurokinin 1 (NK1) receptor, is released in response to stress, anxiety, and pain (Saria 1999; Carrasco & Van de Kar 2003; Ebner & Singewald 2006).
PDA
In a positron emission tomography (PET) study, decreased NK1 receptor binding was found in patients with PDA (Fujimura et al. 2009); see Part I (Bandelow et al. 2016). Attempts have been made to develop neurokinin antagonists for the treatment of anxiety disorders. The NK1 receptor antagonist vestipitant showed anxiolytic effects in a preliminary study (Poma et al. 2014). However, vofopitant, a NK1 antagonist, and onasetant, a NK3-receptor antagonist, were not effective (Kronenberg et al. 2005; Poma et al. 2014).
Specific phobia
In a PET study in women with specific phobias, uptake of the labelled NK1 receptor antagonist [11C]GR205171 was significantly reduced in the right amygdala during phobic stimulation (Michelgard et al. 2007).
Atrial natriuretic peptide
PDA
Atrial natriuretic peptide (ANP) is not only synthesised by atrial myocytes and released in the circulation (de Bold 1985), but is also found in various brain areas where specific receptors have been identified. ANP has been shown to inhibit the corticotropin-releasing hormone (CRH)-stimulated release of adrenocorticotropic hormone (ACTH; Kellner et al. 1992) and cortisol (Strohle et al. 1998a). Also, peripheral and central administration of ANP has an anxiolytic activity in different animal models of anxiety (Strohle et al. 1997). In patients with PDA, ANP reduced CCK-4-induced panic attacks (Strohle et al. 2001) and an activation of the HPA system (Wiedemann et al. 2001). Furthermore, a significantly accelerated ANP release has been described in patients with lactate-induced panic attacks (Kellner et al. 1995), and it has been suggested that this increase also contributes to the paradoxical blunting of ACTH and cortisol secretion during lactate-induced and possibly spontaneous panic attacks. As physical activity increases ANP concentrations, the anxiolytic activity of exercise might be associated with increased ANP concentrations. And indeed, the anxiolytic activity of a single exercise bout was correlated with the increased ANP concentrations (Strohle et al. 2006).
Although there have been major efforts to develop small-molecule, non-peptide receptor ligands acting as CRH1 antagonists, NK-antagonists or ANP agonists, we still lack convincing clinical proof-of-concept studies with peptidergic treatment approaches in patients with anxiety disorders.
Oxytocin
SAD
In humans, modulation of anxiety by oxytocin has been demonstrated by showing reduced amygdala responses to aversive stimuli. Moreover, intranasal oxytocin promotes trust, and reduces the level of anxiety, possibly at the level of the amygdala (Kirsch et al. 2005; Kosfeld et al. 2005; Zak et al. 2005; Heinrichs et al. 2009). The dysregulation of oxytocin as a putative mechanism underlying social attachment has been examined widely in animal studies (e.g., Williams et al. 1994), and recently has become of interest in human studies.
In a study examining oxytocin as add-on to exposure therapy in patients with SAD, participants administered with oxytocin showed improved positive evaluations of appearance and speech performance, but these effects did not generalise to improve overall treatment outcome from exposure therapy (Guastella et al. 2009).
The role of oxytocin in SAD has also been shown in neuroimaging studies (chapter Neuroimaging, Part I; Bandelow et al. 2016).
SepAD
Genetic studies have shown a possible role of oxytocin in SePAD (chapter Genetics, Part I; Bandelow et al. 2016).
PTSD
In Vietnam veterans with PTSD, no beneficial effects of intranasal oxytocin on physiological responses to combat imagery were observed (Pitman et al. 1993).
HPA axis
PDA
A growing number of studies has aimed to delineate the possible role of HPA axis function in the pathophysiology of the anxiety disorders, mainly through the use of plasma, urine, or saliva cortisol levels in basal conditions or after pharmacological or psychological challenge test as a potential biological marker (Elnazer & Baldwin 2014).
Basal levels
Baseline plasma levels of cortisol in PDA patients were reported to be elevated during the day (Nesse et al. 1984; Roy-Byrne et al. 1986; Goetz et al. 1989) or during the night (Abelson et al. 1996) by some authors, but to be normal by others (Brambilla et al. 1995; Cameron et al. 1987; Stein & Uhde 1988). Urinary free cortisol in PDA patients was found to be normal (Uhde et al. 1988), elevated (Bandelow et al. 1997) or elevated only in patients with complicated PDA (Lopez et al. 1990) when compared with healthy controls.
Baseline ACTH concentration in plasma was increased in patients compared with controls (Brambilla et al. 1992). HPA axis stimulation tests showed significantly lower ACTH responses to CRH in patients compared with normal control subjects in three studies (Roy-Byrne et al. 1986; Holsboer et al. 1987; Brambilla et al. 1992) and normal responses in one (Rapaport et al. 1989). Cortisol release after CRH was found to be lower in two (Roy-Byrne et al. 1986; Brambilla et al. 1992) and normal in two other studies (Holsboer et al. 1987; Rapaport et al. 1989).
HPA axis response during panic attacks
Cameron et al. (1987) measured cortisol during spontaneously occurring panic attacks while patients stayed at bed-rest with an indwelling venous catheter for sampling of blood. They found non-significantly elevated plasma cortisol levels during attacks.
During naturally occurring panic attacks, a significantly increased salivary cortisol secretion could be shown in PDA patients compared with values of the same individuals obtained at comparable daytime on panic-free days (Bandelow et al. 2000). The salivary method used in this study proved to be a useful non-invasive method to measure HPA function in anxiety disorders, and has often been used in subsequent research.
During exposure to feared situations, PDA patients did not show increased levels of concentrations of cortisol and ACTH (Siegmund et al. 2011). In order to investigate cortisol levels during panic attacks, panic provocation tests have been performed. In most studies, patients who panicked during lactate infusion did not show elevations in ACTH or cortisol (Carr et al. 1986; Levin et al. 1987; Den Boer et al. 1989; Gorman et al. 1989; Targum 1992; Strohle et al. 1998b). In a study by Liebowitz et al. (1985), only patients who rapidly developed panic attacks after lactate infusion had marginally higher cortisol levels than controls. By contrast, Hollander et al. (1998) found that cortisol levels fell significantly during lactate-induced panic in patients and controls. Interestingly, patients who panicked after lactate had higher plasma cortisol levels before the infusion than controls (Coplan et al. 1998).
Inhalation of carbon dioxide (CO2) did not induce a significant increase in plasma or salivary cortisol in panickers (Gorman et al. 1989; van Duinen et al. 2004). However, subsequent studies suggested that 35% CO2 significantly increases plasma levels of ACTH and cortisol in PDA patients (van Duinen et al. 2007) and of cortisol in healthy subjects (Argyropoulos et al. 2002). Nevertheless, in PDA patients, no specific association emerged between the 35% CO2-induced panic attacks and HPA axis activation observed after this challenge (van Duinen et al. 2007).
Patients reporting yohimbine-induced panic attacks had significantly larger increases in plasma cortisol than healthy subjects (Charney et al. 1987). mCPP or oral caffeine increased plasma cortisol in both patients and controls (Charney et al. 1985; Klein et al. 1991). However, a placebo-controlled study suggested that the significant increases in plasma cortisol, ACTH and dehydroepiandrosterone sulphate (DHEAS) observed after oral caffeine (400 mg) administration in PDA patients are not associated with the occurrence or non-occurrence of a panic attack at post-challenge (Masdrakis et al. 2015). Pentagastrin (CCK-4) induced panic attacks were associated with a pronounced rise of plasma cortisol levels (Abelson et al. 2007).
HPA axis response to treatment
Some studies investigated the effect of treatment on the HPA axis in patients with PDA. Nocturnal urinary cortisol excretion did not change during treatment with paroxetine vs. placebo combined with relaxation training or aerobic exercise (Wedekind et al. 2008). On the contrary, exercise training was associated with lowered salivary cortisol levels in PDA patients (Plag et al. 2014).
HPA axis suppression tests
Findings with the dexamethasone suppression test (DST) were summarised by Ising et al. (2012). Most studies found a normal reaction in the DST in PDA patients, e.g., Cameron & Nesse (1988), while cortisol non-suppression after dexamethasone was found in at least some patients in some other investigations (Avery et al. 1985; Erhardt et al. 2006; Petrowski et al. 2013). Results of studies employing the CRH stimulation test in PDA have been mixed. While two studies suggest an abnormal CRH response pattern in terms of a blunted ACTH response and a reduced ACTH/cortisol ratio, three studies were negative or showed inconsistent findings (Ising et al. 2012). Also, combined dexamethasone suppression/CRH tests supported the assumption of an impaired HPA axis regulation in PDA (Ising et al. 2012). Demiralay et al. (2012) found a blunted response of ACTH release following CCK-4 injection only after hydrocortisone pre-treatment.
HPA axis and neurotrophic factors
Early stressful life events may provoke alterations of the stress response and the HPA axis, which can endure until adulthood (Faravelli et al. 2012). Glucocorticoids suppress brain-derived neurotrophic factors (BDNF) at messenger ribonucleic acid and protein level. Activated glucocorticoid and mineralocorticoid receptors repress the transcription activity of the BDNF promoter site. Neurogenesis in the human brain is most prominent in the dentate gyrus of the hippocampus. Hypercortisolism caused by prolonged stress can suppress this neuroplasticity process. Acute stress, however, activates BDNF, stimulates neuroplasticity and hence improves learning and memory. Therefore, under chronic stress conditions such as in PDA, an increasing loss of neural plasticity may emerge and consequently the ability to appropriate coping (Bandelow & Wedekind 2006). The role of neurotrophic factors is reviewed in the next chapter (Neurotrophic factors, page 33).
GAD
Basal levels and HPA axis response to stressors
It remains uncertain whether untreated GAD is associated with abnormally increased cortisol levels. Thus, some studies suggest that GAD patients and controls demonstrate similar baseline cortisol levels and cortisol responses to challenge tests. More precisely, baseline urinary free cortisol levels between patients with “chronic moderate-to-severe anxiety” and normal controls did not differ significantly (Rosenbaum et al. 1983). Twenty GAD male adolescents and normal controls displayed similar cortisol plasma levels after a stressful test, but anxious subjects had demonstrated greater pre-stress ACTH concentrations (Gerra et al. 2000). In an extensive study with 1427 anxious patients and normal controls, GAD patients demonstrated significantly greater cortisol awakening response than controls, only when also suffering MDD (Vreeburg et al. 2010). Among 4256 Vietnam-era veterans, those suffering from GAD and normal controls showed similar cortisol and DHEAS plasma levels and cortisol/DHEAS ratio (Phillips et al. 2011). Corresponding to younger subjects, baseline cortisol levels of 201 elderly subjects with at least one anxiety disorder (including GAD and phobias) were comparable with those of normal controls. However, under stress, males showed a slower decline rate of post-stress cortisol increases compared with controls, while clinical severity was associated with larger post-stress cortisol increases and lower recovery capacity in females (Chaudieu et al. 2008). Administration of 7.5% CO2 did not significantly change salivary cortisol levels in medication-free GAD patients (Seddon et al. 2011). Finally, 7–11-year-old children with GAD did not differ from controls concerning pre-sleep salivary cortisol, despite the presence of sleep disturbances (Alfano et al. 2013).
On the contrary, other studies report abnormal – either increased or decreased – HPA axis activity in GAD. Thus, in elderly GAD patients, compared with non-anxious controls, cortisol levels were overall significantly more elevated, were higher during morning hours and were positively associated with GAD symptoms (Mantella et al. 2008). Moreover, not only untreated but also SNRI-treated GAD patients demonstrated significantly higher cortisol levels compared with normal controls (Hood et al. 2011).
A recent development is the analysis of hair cortisol concentrations, which reflect the long-term cortisol levels independently of the acute HPA axis responses in the laboratory context. GAD patients demonstrate up to 50–60% lower hair cortisol concentrations compared with healthy controls (Staufenbiel et al. 2013; Steudte et al. 2011). These results accord with the notion that chronic anxiety – an essential clinical feature of GAD – may result in down-regulation of HPA axis activity. Thus, older adults (≥65 years old) suffering from long-lasting anxiety disorders demonstrated a lower cortisol awakening response than normal controls. This association was most prominent in GAD patients, however, irrespectively of the duration of illness (Hek et al. 2013). Likewise, chronic anxiety may finally exhaust the capacity for increase in 5-HTT activity due to the chronically elevated plasma cortisol levels, e.g., GAD patients could not increase serotonin uptake in their lymphocytes after cortisol administration (Tafet et al. 2001).
HPA axis suppression tests
Non-suppression in the DST in GAD patients (up to 27%) is comparable to that of MDD outpatients, but seems to have little value in distinguishing between GAD and other disorders, including PDA, MDD and agoraphobia (Avery et al. 1985; Schweizer et al. 1986; Tiller et al. 1988; Okasha et al. 1994; Schittecatte et al. 1995).
HPA axis response to treatment
Some studies report that successful psychological or pharmacological treatment of GAD is associated with post-treatment cortisol level reductions. Thus, after successful cognitive-behavioural therapy (CBT) treatment for GAD, significant decreases in both anxiety symptoms and (the latter already elevated at baseline) plasma cortisol levels were observed (Tafet et al. 2005). GAD patients over 60 years of age displayed greater reductions in both peak and total salivary cortisol after escitalopram treatment, compared with placebo-treated patients (Lenze et al. 2011). Furthermore, cortisol reductions were positively associated with improvements in anxiety, although this was limited to subjects with elevated (above the median) baseline cortisol levels. Of note, genetic variability at the 5-HTT promoter predicted these cortisol changes. Furthermore, in the escitalopram (but not in the placebo) treatment group, salivary cortisol changes were significantly associated with changes in immediate and delayed memory tasks, suggesting that targeting HPA axis dysfunction may improve memory in older GAD patients (Lenze 2008). Tiller et al. (1988) reported that all GAD patients who were DST non-suppressors at pre-treatment were suppressors after successful behavioural treatment. Finally, refocusing GAD patients’ attention (and thus distracting them from their anxious thoughts) seems to reduce cortisol levels (Rosnick et al. 2013).
However, other studies report no association between a positive treatment outcome and post-treatment changes in cortisol levels, or no change of cortisol levels at all. Thus, effective treatment of GAD either with buspirone (Cohn et al. 1986) or with alprazolam (Klein et al. 1995) did not significantly alter cortisol levels. Intravenous administration of diazepam in eight GAD patients was associated with post-challenge reductions in cortisol (dose dependently) and ACTH (dose independently) (Roy-Byrne et al. 1991). There was no interaction with diagnosis for any of these endocrine measures, indicating no differential effects of diazepam on ACTH or cortisol in the GAD and control groups. Subsequently, in a larger study in GAD patients and healthy controls, diazepam reduced plasma cortisol levels both when acutely administered at baseline and during chronic treatment and this effect was most apparent in the elderly (60–79 years) compared with the young adults (19–35 years) (Pomara et al. 2005). However, this effect was not associated with the presence of GAD.
SAD
The HPA axis is an important stress system concerning social interaction. Primates with higher baseline HPA axis activity and greater reactivity to stressful stimuli demonstrate increased social avoidances (Sapolsky & Plotsky 1990; Kalin et al. 1998). Consequently, research concerning the pathophysiology of SAD has focussed on the potential role of cortisol in regulating cognitive processes and behavioural responses (e.g., avoidances) to social stressors (Sapolsky 1990; de Kloet et al. 1999; Roelofs et al. 2009; van Peer et al. 2010; Elnazer & Baldwin 2014).
Basal levels and HPA axis response to stressors
Some studies suggest that baseline cortisol levels or cortisol responses after pharmacological or psychological challenges are similar between SAD patients and controls. Thus, no evidence of HPA axis hyperactivity in SAD patients compared with healthy controls was observed, as this is reflected in urinary free cortisol levels or in the free cortisol/creatinine ratio (Potts et al. 1991), as well as in the 24-h excretion of urinary free cortisol and in post-dexamethasone cortisol levels (Uhde et al. 1994). In addition, diurnal saliva cortisol levels and cortisol increases observed both before attending school and before the Trier Social Stress Test were similar between 27 adolescent girls with SAD and healthy controls (Martel et al. 1999). Moreover, SAD patients, compared with controls, demonstrated significantly greater ACTH and cortisol responses to stress (Young et al. 2004) and a significantly greater cortisol awakening response (Vreeburg et al. 2010), only when suffering major depression as well. Intravenous administration of CCK-4 in SAD or OCD patients, or normal controls did not reveal any significant between-groups differences concerning post-challenge ACTH, cortisol, growth hormone and prolactin responses (Katzman et al. 2004). Intravenous administration of citalopram in SAD patients and healthy controls resulted in significantly greater increases in cortisol and prolactin plasma levels compared with placebo administration, but the changes were similar in patients and controls (Shlik et al. 2002). Although a rapid intravenous mCPP challenge resulted in significantly greater rate of panic attacks in PDA patients (85%) compared with generalised SAD patients (14%) and healthy controls (0%), post-challenge changes in cortisol levels were still comparable between these groups (van Veen et al. 2007).
In SAD patients evaluated at baseline and after dexamethasone, no differences were found concerning cortisol awakening response, post-dexamethasone and other cortisol measurements, in contrast to the observed elevations in diurnal and post-dexamethasone levels of salivary α-amylase, a marker of autonomic nervous system function (van Veen et al. 2008). Subsequently, SAD patients successfully treated with a SSRI underwent either a tryptophan depletion challenge or a placebo-test, combined with a public speaking-challenge. The tryptophan depletion group showed a significant larger salivary α-amylase response compared with the placebo group, but the two groups demonstrated similar salivary cortisol responses (van Veen et al. 2009). Accordingly, SAD patients who underwent an electrical stimulation test demonstrated significantly greater baseline and post-challenge salivary α-amylase levels compared with controls. Concerning salivary cortisol levels, neither within-subject nor group differences were observed (Tamura et al. 2013). These findings led some researchers to suggest that pathological vulnerability of the autonomic nervous system – and not of the HPA axis – may underlie SAD psychopathology (van Veen et al. 2008, 2009; Tamura et al. 2013). However, both salivary cortisol and α-amylase levels were similar between SAD children (aged 8–12 years) and healthy controls after undergoing the Trier Social Stress Test for Children, although the former demonstrated significantly higher reactivity compared with the latter (Kramer et al. 2012).
On the contrary, other studies suggest that SAD patients differ significantly from controls concerning baseline cortisol levels and/or cortisol responses to pharmacological or psychological challenges. Thus, in SAD patients, administration of fenfluramine (Tancer et al. 1994b) or mCPP (Hollander et al. 1998) resulted in significantly greater cortisol responses compared with controls. Furlan et al (2001) reported different dichotomies in magnitude and in distribution of cortisol responses to a speech-stressor between SAD patients and normal controls. Thus, seven patients and 14 controls demonstrated post-challenge cortisol increases (90 and 50%, respectively), while in the remaining 11 patients and three controls, cortisol decreased. Of note, both patient groups were significantly more anxious at post-challenge compared with controls. On the contrary, SAD patients and controls showed similar cortisol responses to a physical exercise challenge, suggesting that distinct biological processes underlie responses to different stressors in SAD (Furlan et al. 2001). Patients with SAD, compared with healthy controls, had a significantly larger cortisol response when performing an arithmetic/working memory task in front of an audience (Condren et al. 2002). Baseline ACTH and cortisol, as well as post-challenge ACTH responses were all similar between the two groups. Exaggerated cortisol response to a speech-stressor was suggested to be a potential neurobiological marker for pre-pubertal SAD children (van West et al. 2008). Moreover, an elevated afternoon salivary cortisol level at the age of 4.5 years was one of four risk factors (the others being female gender, early exposure to maternal stress and early manifestation of behavioural inhibition) mediating the association between chronic high inhibition in school age and SAD occurrence during adolescence (Essex et al. 2010). In addition, in adolescents, a higher baseline cortisol awakening response significantly predicted increased first onsets mainly of SAD (among other anxiety disorders) over a 6-year follow-up (Adam et al. 2014). Finally, recent data suggest that 8–12-year-old children with an anxiety disorder (including SAD, GAD, specific phobia and SePAD) demonstrate psychophysiological characteristics resembling those of chronic stress, i.e., a baseline pattern comprising reduced HPA axis functioning and elevated sympathetic and lowered parasympathetic activity compared with controls (Dieleman et al. 2015).
Increased cortisol stress-responsiveness may be linked to increased social avoidance behaviours in SAD patients. Indeed, SAD patients showed larger cortisol responses to a social stressor, compared with healthy controls. Most crucially, cortisol responses were correlated positively to avoidance behaviours displayed during the social stressor and, furthermore, predicted them irrespective of blood pressure and anxiety (Roelofs et al. 2009). The authors speculate that some studies failed to find an increased HPA axis response to social stressors in SAD patients due to protocol violations – e.g., manipulations that reduce a patient’s experimentally induced stress in order to avoid dropout of the patient – which might critically reduce their cortisol responses.
The potential role of cortisol in threat processing in SAD remains unclear. Event-related potential (ERP) analysis indicated that in SAD patients, cortisol administration prior to a social stress-related reaction time task increases the early processing of social stimuli (particularly angry faces) during avoidance (van Peer et al. 2009). A subsequent ERP study suggested a highly specific effect of cortisol on early motivated attention to social threat in SAD (van Peer et al. 2010).
HPA axis response to treatment
Clinical improvement after fluvoxamine treatment in SAD patients was not associated with baseline and post-treatment plasma cortisol responses to a speech-test (DeVane et al. 1999).
Glucocorticoids in the treatment of SAD
Elevated glucocorticoid levels might inhibit the retrieval of fear-related memories and, thereby, reduce phobic fear. Thus, in SAD patients, cortisone administered orally 1 h before a social stressor significantly reduced social fear (but not general anxiety) during the anticipation, exposure and recovery phase of the stressor. Moreover, the stress-induced release of cortisol in placebo-treated subjects correlated negatively with fear ratings, suggesting that endogenously released cortisol in a phobic context buffers fear symptoms (Soravia et al. 2006).
Specific Phobia
Basal levels and HPA axis response to stressors
Most studies suggest that specific phobia is characterised by exaggerated cortisol increases during exposure to phobic stimuli. Thus, in patients with specific phobia, exposure to phobic slides elicited larger cortisol excretion (as well as greater distress and skin-conductance responses), compared to neutral exposures (Fredrikson et al. 1985). Likewise, in women with animal phobias, cortisol levels (as well as levels of epinephrine, norepinephrine, growth hormone and insulin) significantly rose during in vivo exposure sessions, together with increases in anxiety, blood pressure and heart rate (Nesse et al. 1985). Moreover, in two patients who underwent exposure therapy for height phobia, increased cortisol responses remained over the course of treatment despite behavioural and subjective improvements (“desynchrony”) (Abelson & Curtis 1989). Subjects with driving phobia, compared to healthy controls, had significantly greater cortisol increases during driving and its anticipation one hour before driving. Cortisol levels were similar between the two groups on a non-driving day and on morning awakening (Alpers et al. 2003). Pregnant women with blood-injection phobia, when compared with healthy pregnant women, had a higher output of cortisol, although both groups demonstrated similar diurnal cortisol rhythms (Lilliecreutz et al. 2011).
Of note, van Duinen et al. (2010) reported that – although during exposure to phobic stimuli spider phobic patients demonstrated significantly stronger fear reaction compared with controls –cortisol levels were however similar between both groups, thereby suggesting a “desynchrony” in patients’ response systems.
HPA axis response to treatment
In army recruits with protective mask phobia, exaggerated salivary cortisol secretion was observed at both baseline and post-treatment, as well as in the morning. After successful 2-day intensive CBT, significant reductions in cortisol levels were observed (Brand et al. 2011). It has been suggested that phobic patients may not respond uniformly regarding HPA axis function when exposed to phobic stimuli and that this should be taken into consideration when tailoring individualised psychotherapeutic interventions. Hence, only two-thirds of women with spider phobia showed increased cortisol responses when exposed to spider photographs, while the rest, defined as “low-responsive”, showed lower cortisol responses compared with “medium-to-high responsive” non-phobic individuals (Knopf & Possel 2009).
Glucocorticoids in the treatment of specific phobia
Glucocorticoid treatment seems to reduce symptoms of specific phobia acutely and might have a prolonged effect concerning fear extinction, especially in combination with exposure therapy (de Quervain & Margraf 2008; Soravia et al. 2006). Thus, in subjects with spider phobia, repeated oral administration of cortisone (25 mg) 1 h before exposure to spider photographs reduced phobic (but not general) anxiety significantly more than placebo, and this effect was maintained for 2 days (Soravia et al. 2006). In addition, patients fearing heights who underwent a three-session virtual-reality exposure therapy after receiving cortisol (20 mg) 1 h before each session, demonstrated significant fear reduction, as well as reductions in acute anxiety and in skin conductance during exposures to phobic stimuli (de Quervain et al. 2011).
OCD
Basal levels and HPA axis response to stressors
Some studies found no difference in plasma and salivary levels of cortisol or circadian plasma cortisol variations (Brambilla et al. 1997a; Brambilla et al. 2000; Kawano et al. 2013; Millet et al. 1998), while one study found increased diurnal secretion of ACTH and cortisol in patients (Kluge et al. 2007).
After apomorphine infusion but also after saline infusion, OCD patients showed a higher rise in cortisol levels than healthy controls (Brambilla et al. 2000). Cortisol responses to administration of saline and of clonidine were the same in patients and controls (Brambilla et al. 1997a).
In a study with youth with OCD, higher early-morning cortisol values were found when compared with healthy controls. Cortisol levels in the OCD group diminished in response to a psychological stressor (exposure to a feared stimulus or a fire alarm), while an increase was found in healthy controls (Gustafsson et al. 2008). In a similar study, exposure with response prevention, was used as a stressor. Despite considerable psychological stress, no difference in increase of salivary cortisol was observed when compared with controls (Kellner et al. 2012).
In a study using deep brain stimulation for OCD, an increase in obsessive–compulsive and depressive symptoms correlated strongly with an increase in urinary free cortisol levels after the DBS device was switched off (de Koning et al. 2013).
PTSD
Some studies have found lower cortisol excretion in PTSD patients. According to a review by Yehuda (2005), most studies demonstrate alterations consistent with an enhanced negative feedback inhibition of cortisol on the pituitary, an overall hyper-reactivity of other target tissues (adrenal gland, hypothalamus), or both in PTSD. However, findings of low cortisol and increased reactivity of the pituitary in PTSD are also consistent with reduced adrenal output. The possible clinical applications of HPA biomarkers have been reviewed by Lehrner & Yehuda (2014).
Basal levels
Low urinary cortisol excretion was found in combat veterans with PTSD as compared with controls (Yehuda et al. 1990). Holocaust survivors with PTSD showed significantly lower mean urinary cortisol excretion than subjects without PTSD (Yehuda et al. 1995). In a small study, patients with PTSD were compared with patients with PDA and healthy controls. PTSD patients had lower cortisol and marginally reduced cortisol volatility compared with patients with panic disorder (Marshall et al. 2002). Low cortisol levels in the immediate aftermath of trauma were found to predict the development of PTSD (Delahanty et al. 2005; Delahanty et al. 2000; Yehuda et al. 1998). A meta-analysis of 47 studies revealed that daily cortisol output was lower for PTSD patients relative to healthy controls without trauma; subjects who were exposed to trauma but did not develop PTSD did not differ from healthy controls without trauma (Morris et al. 2012).
However, in a recent study assessing hair cortisol (which reflects long-term cortisol changes), PTSD patients and traumatised control subjects without PTSD exhibited lower hair cortisol concentrations than non-traumatised control subjects suggesting that trauma exposure per se, either in the absence or presence of PTSD is a correlate of long-term lower basal cortisol levels (Steudte et al. 2013).
Glucocorticoids in the treatment of PTSD
Based on the above-mentioned findings of decreased cortisol concentrations in PTSD, it has been hypothesised that glucocorticoid administration might benefit patients. Indeed, individuals who received a high dose of hydrocortisone within 6 h of a traumatic event had a reduced risk for the development of PTSD, compared with individuals who received placebo (Zohar et al. 2011).
In summary, although the clinical picture of anxiety disorders suggests the potential for a prominent role for disturbed stress response regulation, there are more inconsistencies than consistencies in the relevant research findings.
In PDA, findings are inconsistent regarding baseline cortisol and ACTH levels, response to spontananeously occurring panic attacks, response to exposure to feared situations, chemically provoked panic attacks or response to the dexamethasone suppression or CRH challenge.
In GAD, findings are inconsistent regarding whether baseline cortisol levels are normal or pathologically elevated, while findings from hair cortisol analysis – a recently developed technique, which reflects the long-term cortisol levels – suggest significantly lower hair cortisol concentrations. Although dexamethasone non-suppression in GAD patients is comparable to that of MDD outpatients, it seems to be of little value in the differential diagnosis of GAD from other mental disorders. Most, but not all, related studies suggest that successful psychotherapy or pharmacotherapy of GAD is associated with post-treatment reductions in cortisol concentrations.
With regard to patients with SAD, some, but not all, studies suggest that they differ significantly from healthy controls concerning baseline cortisol levels, and/or demonstrate exaggerated cortisol stress-responsiveness possibly linked to increased social avoidances.
Regarding specific phobia, most studies suggest inflated cortisol responses during exposure to phobic stimuli, which are however amenable to behaviour therapy.
Overall, it seems that various pathological findings are found in HPA axis function across the anxiety disorders. Nevertheless, it is not clear, as yet, whether this reflects reality, or is due to methodological weaknesses of current research. In order to more vigorously evaluate the potential role that HPA axis function plays in the pathophysiology of anxiety disorders, a number of strategies have previously been proposed, such as achieving greater consensus on study objectives and on clinical features of patient groups and designing meticulous methodological protocols (Baldwin et al. 2010; Elnazer & Baldwin 2014).
Neurotrophic factors
Neurotrophins are proteins involved in neurogenesis. Although most of the neurons in the brain are formed prenatally, some parts of the adult brain have the ability to form new neurons from neural stem cells, a process named neurogenesis. Neurotrophins include nerve growth factor (NGF), BDNF, neurotrophin-3, neurotrophin-4, and artemin.
Nerve Growth Factor (NGF)
NGF is a neuropeptide involved in the regulation of neuron growth. It may be involved in the alert mechanism associated with homeostatic adaptations (Cirulli & Alleva 2009), and might modulate sympathetic neurons, and therefore occupies a key position in controlling the responsiveness of immune-competent cells (Levi-Montalcini et al. 1995). Furthermore, NGF, via the hypothalamus (Scaccianoce et al. 1993), can activate the HPA axis (Otten et al. 1979) and plays a role in adaptive responses. More importantly, there is evidence that NGF might be an autocrine/paracrine factor for the development and regulation of immune cells (Levi-Montalcini et al. 1995). NGF is produced by T and B lymphocytes (Lambiase et al. 1997), which display functional NGF receptors (Franklin et al. 1995). Furthermore, NGF promotes the proliferation and differentiation of T and B lymphocytes (Brodie & Gelfand 1992), and acts as a survival factor for memory B lymphocytes (Torcia et al. 1996).
An association between trait anxiety and a genetic variation of NGF was found in healthy volunteers (Lang et al. 2008). In soldiers making their first parachute jump, NGF was increased during and after the jump (Aloe et al. 1994).
While a reduction of NGF in depression has been consistently reported (Wiener et al. 2015), NGF has not been studied widely in patients with anxiety disorders. In one GAD study, NGF was increased after successful CBT (Jockers-Scherubl et al. 2007).
BDNF
BDNF is a protein that acts on neurons in the brain and the peripheral nervous system, involved in neurogenesis and in the forming of new synapses. It has been assumed that BDNF is implicated in the aetiologies of depression and anxiety, but data on brain BDNF levels in anxiety disorders are inconsistent.
PDA
Serum BDNF levels of PDA patients with poor response to CBT were significantly lower than those of patients with good response (Kobayashi et al. 2005). Moreover, BDNF serum levels increased after 30 min of aerobic exercise in subjects with panic but not in healthy controls (Strohle et al. 2010).
GAD
In a treatment study with GAD patients, no significant association was found between baseline plasma BDNF levels and GAD severity. Patients who received the SNRI duloxetine had a significantly greater mean increase in plasma BDNF level, when compared with patients who had received placebo (Ball et al. 2013). In a sample of 393 patients with panic disorder, agoraphobia, GAD or SAD, no differences in BDNF levels were found when compared with 382 healthy controls (Molendijk et al. 2012).
A small study comparing patients with GAD or MDD to healthy subjects showed doubled levels of BDNF and artemin, a glial cell-line derived neurotrophic factor family member, in GAD patients compared with normal controls, while depressed patients showed a reduction (Pallanti et al. 2014).
In summary, neurotrophic factors seem to play a different role in mood disorders compared with anxiety disorders. While brain atrophy and growth factor reduction have been observed in mood disorders the opposite has been demonstrated in anxiety disorders. One hypothesis could be that the increase of neurotrophic factors and inflammatory factors observed in anxiety disorders are related to brain volume increase observed in brain areas such as the dorsal midbrain by some studies on anxiety disorders (Fujiwara et al. 2011; Uchida et al. 2008) (see also Chapter neuroimaging, Part I (Bandelow et al. 2016)).
Immunological markers
Neurobiological research on anxiety disorders has shown the possible relevance of neuroplasticity and inflammation processes in the pathophysiology of these disorders. The high rate of comorbidity between anxiety disorders and several inflammatory medical conditions has been interpreted as the result of specific inflammatory pathways. Anxiety has been linked to cardiovascular risk factors and diseases such as atherosclerosis (Seldenrijk et al. 2010), metabolic syndrome (Carroll et al. 2009), and coronary heart disease (Roest et al. 2010), which are also associated with low-grade systemic inflammation (Libby 2002). While depressive disorders, which are highly comorbid with anxiety disorders, have repeatedly been associated with the immune system (Kim et al. 2007; Myint & Kim 2014), only few studies have investigated the relationship between anxiety disorders and inflammation (Vogelzangs et al. 2013). These have suggested that certain inflammatory markers are elevated in anxiety disorders (Weik et al. 2008).
The immune system
The immune system is divided into the innate and the acquired immune system. The latter again is divided into the cellular and the humoral immune system. The humoral system is based on antibodies, while the cellular immune system involves the phagocytes, cytotoxic T-lymphocytes, and cytokines. Lymphocytes are white blood cells in the lymph that include thymus cells (T cells), which can produce enzymes that destroy pathogenic cells, bone marrow cells (B cells), which produce antibodies for the humoral immune system to fight bacteria and viruses, and natural killer cells, which defend the host from tumour cells and virus infections. Inflammatory responses are characterised by a complex interaction between pro- and anti-inflammatory cytokines (Pavlov & Tracey 2005). Cytokines are small proteins, including the interleukins (ILs) such IL1, -2, -6, -10, -18 and others, tumour necrosis factors (TNFs) and interferons (IFNs) such as IFNα, β and γ. Interferons are released by cells that have been infected by a virus, and are used as drugs (e.g., α-interferon for the treatment of hepatitis C or cancer, β-interferon for multiple sclerosis or interleukin 2 for cancer). Interferons also activate natural killer cells.
Epinephrine and norepinephrine modulate the release of cytokines and inflammation through α- and β-adrenoceptors on immune cells (Hasko & Szabo 1998). Results of in vitro and in vivo studies have suggested that norepinephrine enhances TNF production (Bertini et al. 1993; Spengler et al. 1994). TNF is an early cytokine mediator of local inflammatory response that causes inflammation and secondary tissue damage when released in excess (Tracey 2002). Both catecholamines have been reported to stimulate IL-6 release by immune cells and other peripheral cells (Chrousos 2000). NE augments macrophage phagocytosis and tumouricidal activity (Koff & Dunegan 1985). In contrast, acetylcholine dose-dependently inhibit the release of TNF and other pro-inflammatory cytokines such as IL1, IL6, and IL18, from endotoxin-activated primary human macrophages (Borovikova et al. 2000). However, the production of IL10, which is an anti-inflammatory cytokine, was unaffected by acetylcholine. Inhibition of acetyl-cholinesterase activity, which increases acetylcholine levels in the CNS, resulted in the suppression of the immune response, indicating that acetylcholine has an immunoinhibitory role in the brain (Pavlov et al. 2009). When stressful situations are prolonged, adrenergic agents can increase and acetylcholine can decrease, due to continuous sympathetic activation and the lack of parasympathetic counteractivation. Therefore, pro-inflammatory cytokines such as TNF, IL1, and IL6 can increase in prolonged stressful situations, such as anxiety disorders.
The autonomic nervous system and the immune system
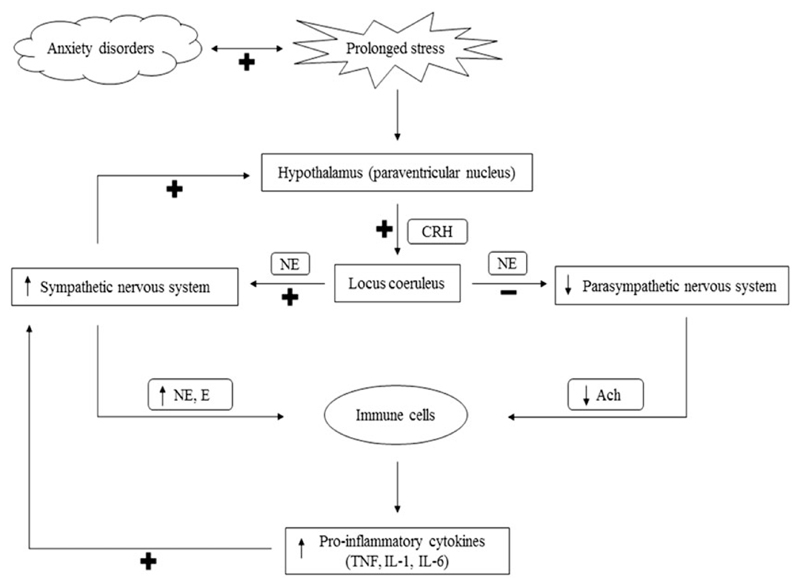
Although stress initially activates both the sympathetic nervous system and the HPA axis, the role of the autonomic nervous system and its interactions with stress and the immune system has received much less attention than the HPA axis (Elenkov et al. 2000). Stress-induced interactions between nervous, endocrine and immune systems are depicted in Figure 2.
Figure 2.
Stress-induced interactions between nervous, endocrine and immune systems. The hypothalamus secretes CRH in response to stress, and from the paraventricular nucleus of the hypothalamus. CRH-containing neurons have projections to the locus coeruleus. The locus coeruleus sends direct projections to the sympathetic and parasympathetic preganglionic neurons, increasing sympathetic activity and decreasing parasympathetic activity through the activation of adrenoceptors. In turn, the activation of the sympathetic nervous system stimulates the release of CRH. The products of sympathetic and parasympathetic nervous system activity are NE and E, and ACh, respectively. When stress is prolonged, as in anxiety disorders, the sympathetic nervous system continues to be activated with a lack of parasympathetic counteractivity. As a result, NE and E levels are increased and ACh levels are decreased, which leads to an increased release of pro-inflammatory cytokines from immune cells. Pro-inflammatory cytokines such as TNF, IL1 and IL6 then trigger the activation of the sympathetic nervous system. CRH, corticotropin-releasing hormone; NE, norepinephrine; E, epinephrine; ACh, acetylcholine, TNF, tumour necrosis factor; IL1, interleukin-1; IL6, interleukin-6; +, stimulation; −, inhibition.
Mental arithmetic and public speaking tasks applied as brief laboratory stressors induce increases in natural killer cell activity (Breznitz et al. 1998). These increases were potentiated in individuals who had greater cardiovascular reactivity to stress (Cacioppo et al. 1995). In other words, individuals who showed the greatest sympathetic nervous system and endocrine response to brief psychological stressors, also showed increased immune system alterations. Thus, the effect of stress on the neuroendocrine system and the mechanism by which that effect influences the immune system has become a subject of interest in recent years (Larson et al. 2001).
Cellular Immunity
PDA
In PDA patients, peripheral lymphocyte subsets did not differ initially from control subjects. However, after three months of treatment with the SSRI paroxetine, the percentages of some lymphocyte subsets were significantly increased, while others were decreased (Kim et al. 2004). This finding suggests that pharmacological treatment may affect immune function in panic disorder patients. In a study by Schleifer et al. (2002), drug-free patients with PDA showed decreased percentages and total circulating CD19+ B lymphocytes, but no differences in other lymphocyte measures. Natural killer cell activity did not differ between PDA patients and healthy control subjects in this study.
GAD
In a study by Wingo & Gibson (2015), anxiety as a symptom of GAD was associated with blood gene expression profiles in 336 community participants (157 anxious subjects and 179 controls). Findings did not show a significant differential expression in females, but 631 genes were differentially expressed between anxious male and healthy controls. Gene set-enrichment analysis revealed that genes with altered expression levels in anxious men were involved in response of various immune cells (B-cells, myeloid dendritic cells and monocytes) to vaccination and to acute viral and bacterial infection (peripheral blood mononuclear cells). In addition, this analysis also identified a network affecting traits of metabolic syndrome. These results suggest potential molecular pathways that can explain the negative effects of GAD on physical health that are observed in epidemiological studies. Remarkably, even mild anxiety, which most of the study participants had, was associated with observable changes in immune-related gene expression levels.
OCD
Studies in OCD have shown that circulating natural killer cells were either increased, decreased or not changed compared with controls. In one study, circulating natural killer cells were elevated predominantly in males which persisted after 12 weeks of SSRI treatment, possibly reflecting either characteristic of the illness, or a lack of true remission (Ravindran et al. 1999). Another study found that patients with childhood onset of OCD had significantly more natural killer cells than patients with late onset OCD (Denys et al. 2004). A subsequent study reported that the percentage and absolute numbers of natural killer cells measured as CD56 lymphocyte subpopulations, were unchanged (Marazziti et al. 1999). Patients with first-degree relatives with OCD also had significant lower natural killer cell activity compared with patients who had no relative with OCD (Denys et al. 2004). In a study by Marazziti et al. (1999), OCD patients had increased CD8+ T cells, both in terms of percent values and absolute number, and decreased CD4+ T cells. The CD3+, CD19+ and CD56+ lymphocyte subpopulations were unchanged.
Cytokines
PDA. Patients with PDA had reduced cell-mediated functions compared with healthy controls before pharmacological treatment, but after treatment, no significant differences were seen (Koh & Lee 2004). One study showed increased levels of 18 cytokines in subjects with PDA and PTSD, leading the authors to suggest that a generalised inflammatory state may be present in these diseases (Hoge et al. 2009). However, small studies on cytokines in PDA showed non-significant elevations of TNF-α, IL1-α, IL2 and IL3 but a significant increase of IL1 β (Brambilla et al. 1994; Rapaport & Stein 1994; Weizman et al. 1999). In a study conducted on PDA patients and healthy controls, plasma concentrations of TNF-α, IFN-γ, IL1β, IL2, IL6 and IL12 were measured. Decreased levels of IFN-γ and IL12 were observed, which suggested a correlation between levels of IFN-γ and anxiety-like behaviour, as seen in animal models (Tukel et al. 2012).
GAD
C-reactive protein (CRP) was found to be increased in some studies (Bankier et al. 2008; Copeland et al. 2012). A pilot study measured peripheral levels of relevant cytokines (α-MSH, IL2 and IL10) in small cohorts of GAD and MDD patients and compared them to healthy controls. They found increases in plasma concentrations of IL10 and α-MSH, but no significant variations in IL2 (Tofani et al. 2015). One study in patients with GAD and PDA measured cell-mediated immune functions through the lymphocyte proliferative response to phytohemagglutinin, IL2 production and natural killer cell activity. This study suggested a reduction in this function when compared with healthy controls (Koh & Lee 1998).
SAD
Among individuals with an anxiety disorder, those with SAD, females in particular, had lower levels of CRP and IL6. The highest CRP levels were found in those with an older age at anxiety disorder onset (Vogelzangs et al. 2013). CRP is an acute-phase protein produced in the liver that increases stimulated by IL6, which is in turn secreted by macrophages and T cells. OCD Different methodologies, including ex vivo production and peripheral blood or CSF measurements via a variety of techniques, make comparisons difficult. Several studies (Mittleman et al. 1997; Fluitman et al. 2010) have shown that cytokine levels may depend on factors such as age, and the content of obsessions. For example, a study by Fluitman et al. (2010) showed that norepinephrine levels increased while lipopolysaccharide-stimulated TNF-α and IL6 production by peripheral leucocytes decreased during exposure to disgust-related objects in OCD patients, but not in healthy controls. These data suggest that symptom provocation in OCD patients with contamination fear is accompanied by alterations in the immune and neuroendocrine systems, but does not affect cortisol levels.
In OCD, several studies have demonstrated diminished production of TNF-α (Brambilla et al. 1997b; Denys et al. 2004; Fluitman et al. 2010). One of the first studies in the field (Brambilla et al. 1997b) showed lower plasma concentrations of IL1β and TNF-α in OCD patients compared with controls, which has been related to hyperactivity of the noradrenergic system and of the HPA axis. In a study by Denys et al. (2004), the ex vivo production of TNF-α in whole blood cultures was significantly decreased in medication-free patients with OCD compared with controls. The same study showed reduced natural killer cells activity. The reduction in both TNF-α and natural killer cells activity suggests a potential role of altered immune function in the pathophysiology of OCD. Other studies have revealed normal cytokine production in OCD patients (Weizman et al. 1996). On the other hand, the possible involvement of the immune system in certain subtypes of OCD is supported by the relationship between the severity of the disorder and the IL6/IL6 receptor levels (Maes et al. 1994). However, childhood OCD appears to differ from that occurring at other ages, as increased CSF levels of cell-mediated cytokines have been reported in children with OCD, when compared with children with schizophrenia or attention deficit hyperactivity disorder (Mittleman et al. 1997). Hounie et al. (2008) reported a genetic association between the - 308 G/A and -238 G/A TNF-α polymorphisms and OCD in a Brazilian sample.
PTSD
Cytokine levels appear to be constantly elevated in PTSD. Some studies have reported higher plasma IL6 and TNF (von Kanel et al. 2007; Gill et al. 2008), and CSF IL6 levels (Baker et al. 2001) among PTSD. Higher levels of IL6 are linked to PTSD vulnerability following trauma (Sutherland et al. 2003; Pervanidou et al. 2007; Gill et al. 2009). Higher levels of stimulated TNF and IL6 were reported in PTSD patients. In a study by Rohleder et al. (2004), LPS-stimulated production of IL6, but not TNF-α, was markedly increased in patients. Spivak et al. (1997) showed that serum ILlβ levels (but not slL-2R) were significantly higher in PTSD patients than in controls. As these levels correlated significantly with the duration of PTSD symptoms, it was proposed that desensitisation of the HPA axis in chronic PTSD patients counteracted the stimulatory effect of ILlβ on cortisoI secretion. Another study showed that levels of TNF-α and of IL1β were higher in patients than in controls, while CRP, IL4 and IL10 were not significantly different (von Kanel et al. 2007). One study found higher IL1 β and lower IL2R levels in PTSD patients than in controls (Tucker et al. 2004). In all participants, TNF-α was correlated with PTSD severity. IL4 correlated with total hyperarousal symptoms, and PTSD total symptom score, after controlling for systolic blood pressure and smoking status. PTSD patients showed a low-grade systemic proinflammatory state that was related to disease severity, suggesting one mechanism by which PTSD could contribute to atherosclerotic disease. A study by Miller et al. (2001) reported a positive relationship between posttraumatic psychological disturbances and serum levels of receptors to interleukin 6 (sIL6r) and CRP, which provides the basis for further research on the effects of psychological disturbance on physical recovery after injury.
Humoral Immunity
PDA
Mannan-binding lectin (MBL) and MBL-associated serineprotease-2 (MASP-2) represent important arms of the innate immune system, and different deficiencies may result in infections or autoimmune diseases. Although PDA was associated with increased inflammatory response, infections and high comorbidity, the basis for these findings is not clear. A study by Foldager et al. (2014) investigated associations with MBL, MASP-2 or the gene MBL2 (which codes for MBL) with PDA. A large proportion (30%) of MBL deficient individuals was observed along with significantly lower levels of MBL and MASP-2 plus association with the MBL2 YA two-marker haplotype. Since MBL deficiency is highly heterogeneous and associated with both infectious and autoimmune states, more research is needed to identify which complement pathway components could be associated with PDA.
Antibodies
PANDAS (PANS/CANS). OCD is a clinically heterogeneous disorder with several possible subtypes. It has been hypothesised that one of these subtypes is associated with autoimmune disorders triggered by streptococcal infections (e.g., rheumatic fever and Sydenham’s chorea) (Miguel et al. 2005). Children who develop acute OCD after a group A β-haemolytic streptococci (GABHS) infection were first described by Swedo (2002), who coined the acronym PANDAS (Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococci). However, as the aetiology of the syndrome remains controversial, new descriptions have been proposed, including paediatric acute-onset neuropsychiatric syndrome (PANS) and idiopathic childhood acute neuropsychiatric syndrome (CANS; APA 2013).
Children with PANDAS showed OCD symptoms and tics, but did not have rheumatic fever or Sydenham’s chorea. It has also been reported that 4% of parents and grandparents of Sydenham’s chorea patients and 6.7% of the parents and grandparents of PANDAS patients had a history of rheumatic fever compared with 1.4% of parents and grandparents of controls. This suggests a common liability between rheumatic fever and OCD triggered by streptococcus infections (Swedo 2002). The presence of autoantibodies due to molecular mimicry mechanisms is one potential explanation for the association between OCD and rheumatic fever, following the autoimmune model for Sydenham’s chorea.
Infections with GABHS might result in PANDAS, and viral infections might trigger the autoimmune process that leads to OCD (Allen et al. 1995; Khanna et al. 1997). Furthermore, patients with rheumatic fever show a high level of antineural antibodies against the caudate (Husby et al. 1976). They also have high levels of a monoclonal antibody called D8/D17, which reacts with a particular antigen in B lymphocytes (Zabriskie 1986). The search for the trait marker for susceptibility (Singer & Loiselle 2003) showed that this antigen is also present in patients with childhood OCD, Tourette syndrome, and chronic tic disorder (Murphy et al. 1997). This D8/D17 antibody has expanded expression in individuals with Sydenham’s chorea (89%) compared with healthy children (17%). Preliminary studies of the D8/17 antibody in individuals with PANDAS also found that 85% of children with PANDAS compared with 17% of healthy children have this antibody (Swedo et al. 1997). The exact significance of these finding and how this marker is related to the disease process is remain unclear, especially since it has been reported in patients with other neuropsychiatric disorders of childhood onset, including autism (Hollander et al. 1999; Murphy et al. 1997).
An autoimmune hypothesis has been suggested for early onset OCD and Tourette syndrome. Antineural antibodies have been studied and found in the sera of some patients with these disorders, and they are thought to cross-react with streptococcal and basal ganglia antigens (Morer et al. 2008). Positive anti-basal ganglia antibodies were found in 64% of PANDAS patients but in only 9% of controls with a documented streptococcal infection but no neuropsychiatric symptoms (Pavone et al. 2004). Immunoblotting has identified multiple bands against the caudate supernatant fraction in PANDAS with primary tics that are different from the control group (Church et al. 2004). The presence of antibrain antibodies was reported in 42% of a group of children with OCD compared with rates between 2% and 10% in three different paediatric control (autoimmune, neurological and streptococcal) groups (Church et al. 2004). In addition, antibodies from a Sydenham’s chorea patient reacted against lysoganglioside and N-acetyl-beta-d-glucosamine, a neuronal antigen also found on the GABHS surface (Kirvan et al. 2003). In a second study of the same group (Kirvan et al. 2006), antibodies in PANDAS reacted with the neuronal cell surface and the caudate–putamen and induced calcium–calmodulin-dependent protein (CaM) kinase II activity in neuronal cells. Depletion of serum IgG abrogated CaM kinase II cell signalling and reactivity of CSF was blocked by streptococcal antigen N-acetyl-beta-d-glucosamine (GlcNAc). Antibodies against GlcNAc in PANDAS sera were inhibited by lysoganglioside GM1. Results suggest that antibodies from an infection may signal neuronal cells in some behavioural and movement disorders.
Dale et al. (2006) have identified antibodies against neuronal glycolytic enzymes (NGE) autoantigens (pyruvate kinase M1, aldolase C, neuronal-specific and non-neuronal enolase) in 20 unselected post-streptococcal patients with central nervous diseases compared with 20 controls. These enzymes are multifunctional proteins that are expressed both intracellularly and on the neuronal cell surface. On the neuronal plasma membrane, NGEs are involved in energy metabolism, cell signalling and synaptic neurotransmission. GABHS also expresses glycolytic enzymes on cell surfaces that have 0–49% identity with human NGE. This suggests molecular mimicry and autoimmune cross-reactivity may be the pathogenic mechanism in post-streptococcal CNS disease. Kansy et al. (2006) identified the M1 isoform of the glycolytic enzyme pyruvate kinase (PK) as an autoimmune target in Tourette syndrome and associated disorders. Antibodies to PK reacted strongly with surface antigens of infectious strains of streptococcus, and antibodies to streptococcal M proteins reacted with PK. Moreover, immunoreactivity to PK in patients with exacerbated symptoms who had recently acquired a streptococcal infection was 7-fold higher compared with patients with exacerbated symptoms and no evidence of a streptococcal infection. These data suggest that PK can also function as an autoimmune target and that this immunoreactivity may be associated with Tourette syndrome, OCD, and associated disorders.
Further support for an autoimmune hypothesis comes from evidence of induced stereotypic movements in rats after infusion of IgG of sera from patients with PANDAS (Taylor et al. 2002). The pathogenic role of these antibodies remains unclear. Specific binding with molecules from the GABHS surface, such as lysoganglioside or glucosamine, and more NGE as piruvate kinase, aldolase or enolase support the notion of an autoimmune brain disease (Kirvan et al. 2003; Dale et al. 2006). However, these antibodies might not be pathogenic, but may instead result from local damage.
However, some studies do not support an autoimmune hypothesis. If proved true, this hypothesis gives rise to new therapeutic approaches. In fact, some studies suggest that immuno-modulating strategies are effective in children with PANDAS (Garvey et al. 1999; Perlmutter et al. 1999; Murphy & Pichichero 2002; Snider et al. 2005). A study by Perlmutter et al. (1999) has demonstrated an improvement of obsessive–compulsive symptoms after plasmapheresis or intravenous immunoglobulin treatment. Twenty-nine children with PANDAS recruited from a nationwide search were randomised in a partially double-blind fashion (no sham apheresis) to an immunoglobulin, “immunoglobulin placebo” (saline), and plasmapheresis group. One month after treatment, the severity of obsessive-compulsive symptoms improved by 58 and 45% in the plasmapheresis and immunoglobulin groups, respectively, compared with only 3% in the saline control group. In contrast, tic scores significantly improved only after plasmapheresis treatment, but not in the immunoglobulin and the control group. Improvements in both tics and obsessive-compulsive behaviours were sustained for 1 year.
Even though PANDAS is by definition a paediatric disorder, patients with adult onset (after the age of 27) OCD or tic disorders related to streptococcal infections have also been described. These cases support the hypothesis that streptococcal disease may result in adult-onset OCD in some patients. It is possible that GABHS infection just serves as a trigger in childhood, and that autoimmune antibodies directed against neuronal structures later maintain obsessive–compulsive symptoms without new infections. In such cases, adult OCD with childhood onset may show anti-brain antibodies without elevated anti-streptolysin O (ASLO) titres or other signs of recent streptococcal infections. For a small proportion of OCD patients, autoimmune reactions towards neuronal structures are present, but further investigations are needed to demonstrate their aetiopathogenetic relevance (Maina et al. 2009). The vast majority of OCD patients are diagnosed and treated for the first time while they are already adults; the mean time from initial symptom manifestation to seeking professional care is approximately 10 years (Maina et al. 2009).
Immunological alterations appear to be different in paediatric and adult patients and probably reflect different pathophysiological mechanisms, such as primary processes in the first case, and perhaps, secondary alterations in adulthood (Marazziti et al. 1999).
A study by Maina et al. (2009) showed that the proportion of subjects with tic comorbidity or positive ASLO titre (>200 IU/ml) was significantly greater in OCD than in MDD patients. No other differences in antibody parameters were found. Four of 74 OCD patients (5.4%) and none of the controls were positive for anti-brain antibodies. The majority of adult OCD patients do not seem to have autoimmunity disturbances. However, a greater percentage of subjects with OCD have positive ASLO titres. For a small proportion of OCD patients, autoimmune reactions towards neuronal structures are present although further investigations are needed to demonstrate their etiopathogenetic relevance.
Two studies evaluated antineuronal antibodies or other markers of autoimmunity in samples of adult OCD patients; Black et al. (1998) found no humoral evidence of autoimmunity, but the study has certain limitations. The sample was small and heterogeneous, the severity of symptoms was not assessed at the time that blood was drawn, and an age- and gender-matched control group was not utilised. In a second study, child onset OCD was associated with higher mean ASLO titres and higher frequencies of tic disorders and tonsillitis in childhood, while no differences were found in D8/17 antibody titres or in other autoimmune parameters (Morer et al. 2006). This study suggested that OCD in adults is a heterogeneous disorder and that only childhood-onset OCD is related to an autoimmune aetiology. This topic needs further investigation, as the possible autoimmune aetiopathogenesis in some OCD patients could lead to new therapeutic scenarios for adults similar to those already suggested for the children. In fact, as a significant proportion of adult OCD patients do not respond to conventional treatment strategies, the search for alternative and hypothesis-driven treatments is critical.
Early detection of these conditions through serum search of antibodies against human brain enolase, neural tissue and Streptococcus can provide valuable information regarding etiopathogenesis and suitable therapies (Nicolini et al. 2015). While prophylactic antibiotic therapy is marginally helpful in preventing symptom exacerbation, intravenous immunoglobulin therapy, plasmapheresis and immunosuppressive doses of prednisone may be effective treatments in select individuals (Allen et al. 1995; Swedo et al. 2001; Nicolini et al. 2015).
In conclusion, elevated levels of pro-inflammatory cytokines such as TNF, IL1 and IL6 could serve as biological markers of anxiety disorders. TNF, IL1 and IL6 trigger the activation of both the HPA axis and the sympathetic nervous system (Chrousos 1995), which could prolong the inflammatory state. The effects of these cytokines are synergistic when produced in combination (Chrousos 2000). In accordance with our current understanding of how anxiety disorders represent a state of inflammation, previous studies have attempted to investigate whether anti-inflammatory drugs have treatment effects on anxiety disorders or other psychiatric disorders deeply related to stress and anxiety. Several human and animal studies have suggested that certain anti-inflammatory drugs might play an important adjunctive role in the treatment of major depression, bipolar disorder and OCD (Najjar et al. 2013). Although only few studies have reported positive results for the efficacy of anti-inflammatory drug treatment on anxiety disorders (Rodriguez et al. 2010; Sayyah et al. 2011), such results do illustrate the proinflammatory nature of anxiety disorders. As such, inflammatory conditions are considered to be triggered by an over-driven sympathetic nervous system together with an under-driven parasympathetic nervous system, treatments that increase parasympathetic tone and hence strengthen the cholinergic anti-inflammatory pathway (Pavlov 2008) could be useful in treating anxiety related disorders. This may explain why methods that increase parasympathetic tone, such as vagus nerve stimulation, may be effective in treating anxiety disorders (George et al. 2008).
CO2 hypersensitivity
Inhalation of air “enriched” with an increased proportion of CO2 can be used to induce anxiety in non-clinical (healthy volunteers) and clinical (patients) groups, and represents a human translational model aiding development of potential new treatments for anxiety disorders. CO2 inhalation has become one of the most frequently used experimental approaches to investigating panic, although studies employ variable challenge procedures, altering the CO2 concentration, the duration of inhalation, the population sample, and the range of outcome measures.
Anxiety induction via CO2 challenge was first performed in a small sample of patients with PDA undergoing 5% CO2 inhalation, and was found to induce panic attacks (Gorman et al. 1984). This finding was confirmed in a larger sample of PDA patients, who experienced a greater incidence of panic attacks during challenge than did healthy controls or patients with other anxiety disorders (Gorman et al. 1988). Brief inhalation of air with high concentrations of CO2 (such as single vital capacity inhalations of 35% CO2) is associated with the experience of acute severe anxiety, which often includes panic attacks. A single vital capacity breath of air enriched with 35% CO2 was found to induce panic and so was suggested as an approach for conducting exposure therapy in patients with PDA (Van den Hout & Griez 1984): the same group reported that patients with panic disorder were more sensitive to CO2 challenge than were healthy controls (Griez et al. 1987). Findings from subsequent studies in a range of diagnostic groups indicated that panic disorder patients were more sensitive to the panicogenic effects of CO2 challenge than were patients with other diagnoses (Leibold et al. 2015; Vollmer et al. 2015).
The mechanisms underlying the provocation of anxiety by CO2 challenge are not fully established, although findings from animal models and human pharmacological intervention studies provide many insights (Leibold et al. 2015; Vollmer et al. 2015). Twin studies suggest an association between genetic factors and CO2 hypersensitivity (Battaglia et al. 2007, 2008). Inhalation of air enriched with a high proportion (35%) of CO2 may be associated with increased cortisol secretion (Argyropoulos et al. 2002; Kaye et al. 2004), although it is unclear how specific the cortisol response is to CO2 challenge, rather than to other aspects of the experimental procedure (Leibold et al. 2015): most studies employing lower CO2 concentrations find no increase in cortisol levels, when compared with baseline (Woods et al. 1988; Coplan et al. 2002; Kaye et al. 2004). The potential role of disturbances in respiratory physiology in panic attack induction through CO2 inhalation is not fully clarified, but experimentally induced panic attacks are associated with low end-tidal CO2 and high ventilation variance at baseline (Papp et al. 1997). In a functional magnetic resonance imaging (fMRI) study, a greater activation in the brainstem during CO2 inhalation was found in patients with PDA compared with normal controls. Interestingly, the authors also showed that experienced divers showed the opposite, i.e., they were less sensitive than normals to increased CO2 (Goossens et al. 2014).
Serotonergic mechanisms may influence the panic response to CO2 challenge. Although tryptophan depletion does not have panicogenic effects (Goddard et al. 1994), depletion can enhance the panic response to CO2 inhalation (Schruers et al. 2000), and administration of the 5-HT precursor L-5-hydroxytryptophan can reduce the panic response (Schruers et al. 2002). Correlations between increases in subjective anxiety, heart rate and blood pressure in healthy volunteers following 35% CO2 challenge suggest a common and presumably noradrenergic-mediated mechanism underlying CO2 sensitivity (Bailey et al. 2003). Most norepinephrine (NE) in the brain is synthesised by neurones originating in the locus coeruleus, and afferent locus coeruleus neurones project to components of the limbic system that are known to be overactive in anxiety disorders (Martin et al. 2010). Changes in CO2 saturation may act upon pH or CO2-dependent chemoreceptors within the locus coeruleus and thereby increase the release of NE, as 5% CO2 increases locus coeruleus neuronal firing rate in rat brain slices (Martin et al. 2010). This CO2-induced release of NE may mediate autonomic and subjective features of anxiety through afferent projections to brain centres involved in cardiovascular control and the limbic system; and endocrine responses may be mediated by altered noradrenergic input into the paraventricular nucleus, thereby causing release of corticotrophin releasing factor (CRF) and anti-diuretic hormone, and triggering subsequent cortisol secretion.
There are limitations in an explanation of the anxiogenic effects of CO2 challenge which is based solely on altered NE function. For example, autonomic arousal is not consistently observed, and the effect of 7.0–7.5% CO2 on plasma cortisol is inconsistent. The attenuating effect of benzodiazepines and certain SSRIs on self-report anxiety but not on physiological markers suggest alterations in autonomic function may lie upstream of psychological anxious responding (Bailey et al. 2011a). Drugs which affect noradrenergic function have shown little effect on subjective responses to CO2 (Pinkney et al. 2014). Overall, it appears that while norepinephrine may be important in mediating anxiety provoked by 35% CO2 challenge, there is persisting uncertainty about the exact mechanism underlying 7.5% CO2-induced anxiety in humans.
Chemosensors within the amygdala are known to be directly linked to CO2 reactivity in mice (Ziemann et al. 2009). The most well-characterised chemosensor is the acid-sensing ion channel 1 (ASIC1a), which is a voltage-insensitive H+-gated cation channel, highly expressed in the amygdala, dentate gyrus, cortex, striatum and nucleus accumbens (Wemmie 2011). Inhalation of 2–20% CO2 elicits normal mouse fear behaviour in the presence of fully functioning acid-sensing ion channels (ASIC1a), which are expressed in the amygdala, but pharmacological blockade or elimination of ASIC1a in knockout mice impairs fear responses to CO2, whereas subsequent amygdala-localised re-expression restores fear behaviour.
Other potentially relevant chemosensitive structures include orexin neurones in the hypothalamus, serotonergic neurones in the medullary raphe (Wang et al. 1998), T cell death-associated gene-8 receptors in the subfornical organ, and hypoxia-sensitive chemosensory neurones in the periaqueductal grey (Vollmer et al. 2015). Perturbations in the activities of chemosensors may not fully explain the physiological effects of changes accompanying CO2 challenge and may not translate to humans, but suggest potential additional mechanisms, which operate alongside CO2-provoked alterations in noradrenergic activity.
Low dose (less than 15%) CO2 inhalation in healthy volunteers and patients
More prolonged (typically 15–20 min) inhalation of CO2 at lower concentration (between 5.0 and 7.5%) does not frequently result in panic, but reliably induces an experience which resembles the symptoms of GAD, with increased subjective and physiological features of anxiety, but no accompanying changes in cortisol secretion. Studies in healthy volunteers support the use of 20-min, 7.0–7.5% CO2 challenge to induce subjective and autonomic responses and neurocognitive changes which resemble the features of generalised anxiety. Increases in heart rate and systolic blood pressure are consistently seen, but an increase in diastolic blood pressure is less frequently observed.
Low dose (7.5%) but prolonged (20 min) CO2 inhalation was first found to induce anxiety in a double-blind, placebo-controlled trial involving healthy volunteers: when compared with normal (placebo) air inhalation, CO2 inhalation was associated with increased heart rate and blood pressure and heightened subjective anxiety (Bailey et al. 2005). A single-blind, placebo-controlled healthy volunteer study found that when compared with air, 7% CO2 inhalation increased respiratory rate, minute volume and endtidal CO2, skin conductance and subjective feelings of anxiety: a subgroup of participants who experienced marked anxiety underwent a subsequent identical inhalation with good test-retest repeatability. However, the study findings highlight potential limitations of the model, as 30% of participants were “non-responders”, and 10% of participants experienced significant anxiety during (placebo) air inhalation (Poma et al. 2005).
The effect of CO2 inhalation on attentional biases, which characterise anxiety states, has also been investigated. For example, 20-min 7.5% CO2 challenge is associated with performance deficits in an emotional anti-saccade task, similar to those seen in individuals with high levels of generalised trait anxiety (Garner et al. 2011). As 20 min of 7.5% CO2 inhalation has been found to significantly modulate attention, with increased alerting and orienting network function in the Attention Network Task, this suggests that CO2 challenge facilitates hypervigilance to threat and alters attention network function in a manner consistent with that seen in GAD (Garner et al. 2012).
Inhalation challenges with less than 15% CO2 provoke significantly more panic attacks in patients with PDA than in healthy controls (Bailey et al. 2011a), but it is uncertain whether altered sensitivity to “low dose” CO2 inhalation is also seen in patients with GAD. A single-blind, randomised, cross-over design study in medication-free GAD patients which employed a repeated 7.5%, 20-min inhalation paradigm found CO2 inhalation increased subjective anxiety and systolic blood pressure, when compared with air: a qualitative assessment indicated participants’ experiences resembled their usual symptoms, more closely for physiological rather than cognitive symptoms (Seddon et al. 2011). The findings should be viewed cautiously given the small sample (n = 12) and discontinuation of three participants due to panic responses.
Attenuation of CO2-induced anxiety by pharmacological interventions
The effectiveness of psychotropic medication (benzodiazepines, antidepressants, novel compounds) in attenuating CO2-evoked anxiety, has been assessed in a number of studies, with variable findings. In general terms, acute benzodiazepine administration reduces subjective CO2-provoked anxiety but has little impact on the physiological response. Administration of selective SSRIs, the SNRI venlafaxine, tricyclic antidepressants and the monoamine oxidase inhibitor toloxatone can all attenuate the panic response to CO2 challenge (Leibold et al. 2015). Administration of 2 mg of lorazepam was found to attenuate subjective anxiety (with no accompanying change in autonomic measures) when compared with placebo in healthy participants undergoing 20-min 7.5% CO2 inhalation (Bailey et al. 2007). These findings were replicated when lorazepam was employed as a control in studies using the same inhalation procedure to assess novel anxiolytic compounds (Bailey et al. 2011b; de Oliveira et al. 2012). Both alprazolam (1 mg) and the partial benzodiazepine receptor antagonist zolpidem (5 mg) attenuated subjective anxiety in healthy volunteers after 20 min of 7.5% CO2 inhalation (Bailey et al. 2009). However, a subsequent double-blind, placebo-controlled cross-over study which investigated dose-response relationships with lorazepam and which used the same experimental paradigm and measures found no attenuation of subjective or autonomic responses (Diaper et al. 2012).
Certain SSRIs and SNRIs are licenced for the treatment of GAD and their effect in attenuating the anxiogenic effects of CO2 inhalation is a marker of the predictive validity of the model. Investigations in small groups of patients with panic disorder found that treatment with different SSRIs and SNRIs reduced subjective anxiety following 5 and 7% CO2 challenge, when compared with baseline, pre-treatment inhalation (Gorman et al. 2004). However, a larger study involving 3 min of 5% CO2 in individuals “at high risk of panic disorder” found that 2-week administration of the SSRI escitalopram had no effect on self-report or autonomic indicators of anxiety (Coryell & Rickels 2009). Given that SSRIs typically take 2–4 weeks to exert notable therapeutic effects in GAD, longer drug administration may be needed to generate valid results.
Studies involving SSRI or SNRI administration in healthy volunteers using a 20-min 7.5% CO2 challenge have generated variable findings. Placebo-controlled administration of the SSRI paroxetine for 21 days reduced subjective anxiety (Bailey et al. 2007). A placebo-controlled investigation of 3-week administration of the SNRI venlafaxine or the anxiolytic pregabalin found no significant effect on change from baseline to post-treatment ratings of subjective anxiety or autonomic response in the venlafaxine group (Diaper et al. 2013). A 2-week randomised double-blind, placebo-controlled study of the SNRI duloxetine in healthy subjects found it had little attenuating effect on subjective anxiety or autonomic arousal following a 20-min, 7.5% CO2 challenge, though duloxetine administration was associated with improved accuracy in the anti-saccade task and reduction in negative thought intrusions (Pinkney et al. 2014).
As with benzodiazepines, SSRI or SNRI administration has a limited effect on physiological responses to CO2 challenge, and drugs within the same class may act variably on subjective anxiety, which raises questions about the validity of the model. However, a study involving the beta-blocker propranolol (40 mg) found it had no attenuating effect on self-report anxiety in healthy volunteers undergoing 20 min of 7.5% CO2 (Papadopoulos et al. 2010), which accords with its lack of efficacy in anxiety disorders (Gorman et al. 1988; Steenen et al. 2016). The same study also found the anti-histamine hydroxyzine (25 mg) had only limited effects.
From current knowledge to potential clinical applications
The response to CO2 inhalation could also be useful in predicting the likelihood of response to treatment, but this potential application has not been examined extensively. Investigation of the effects of double 35% CO2 vital capacity inhalations in a small sample of patients with PDA after 1 h, 2 weeks and 6 weeks of clonazepam treatment found that when compared with placebo both acute and chronic clonazepam administration reduced objectively rated panic attacks after CO2 inhalation (Valenca et al. 2002).
Inhalation of air “enriched” with 7.5% CO2 is an experimental tool for inducing anxiety without features of panic in healthy volunteers, the anxious response being composed of replicable changes in autonomic arousal (increased heart rate and systolic blood pressure), neurocognitive function (impaired performance in emotional antisaccade and attention control tasks) and subjective experience. The CO2 inhalation experimental model of anxiety disorders may therefore be useful for signalling the potential efficacy of novel therapeutic agents: and has been utilised in investigations of the CRF1 receptor antagonist R317573 (Bailey et al. 2011a) which did attenuate subjective effects, and the NK1 receptor antagonists vestipitant and vofopitant (Poma et al. 2014).
The model may be suitable for testing putative anxiolytics (Bailey et al. 2007), and compounds which are found to attenuate CO2-induced anxiety have potential clinical relevance. Studies with compounds which target chemosensory mechanisms may be informative in the development of anxiolytics with a novel mechanism of action: for example with the ASIC ion channel antagonist amiloride, which has been found to have neuroprotective effects (Arun et al. 2013); with orexin receptor antagonists, which can attenuate anxiety-like responses to CO2 challenge in rats (Johnson et al. 2012); and with the carbonic anhydrase inhibitor acetazolamide, which blocks the conversion of CO2 to carbonic acid and thence to hydrogen and bicarbonate ions (Vollmer et al. 2015).
SepAD
CO2 hypersensitivity was investigated in adult SepAD because children of adults with PDA experience elevated rates of SePAD and because childhood separation anxiety disorder (C-SepAD) was found to be associated with adult PDA (Bandelow et al. 2001). Support for this hypothesis comes from a study in which 104 children (aged 9–17 years), of whom 57 had an anxiety disorder, underwent 5% CO2 inhalation (Pine et al. 1998; Pine et al. 2000). In this study, CO2 hypersensitivity was clearly present for SepAD, as indicated by: (1) enhanced respiratory rate response during CO2 breathing; (2) elevated minute ventilation; and (3) lower end-tidal CO2 during room-air breathing. These correlates were also observed – albeit to a much lesser degree – in GAD, and were absent in SAD. Similarly, in a study of 212 offspring from 135 families, abnormal respiratory physiology in response to CO2 exposure was found in offspring with both SepAD and parental PDA relative to offspring with either of these features alone (Roberson-Nay et al. 2010). Given the common physiological perturbations of PDA and SepAD (i.e., physiological abnormalities, respiratory dysregulation, and reaction to inhaled CO2), the specificity of this biological correlate need further confirmatory research data.
Neurophysiology
Electroencephalography (EEG) and ERP
Basal instability of the cortical arousal system was reported in quantitative EEG (qEEG) studies as a common feature of most patients with anxiety disorders (Clark et al. 2009). This manifests as changed spectral power of specific EEG frequency bands in the theta (4–8 Hz) and alpha (8–13 Hz) ranges throughout most of the brain areas and beta range (above 13 Hz) especially in frontal and central brain regions. While none of the qEEG alterations are specific for anxiety disorders, they are regarded as related to anxiety symptoms and are targeted, e.g., by neurofeedback training (Simkin et al. 2014). Generally, sleep EEG (polysomnography; PSG) findings in anxiety disorders are in line with findings from wake EEG showing altered EEG-vigilance regulation in these patients. Patients with anxiety disorders typically have prolonged sleep latency, reduced sleep efficiency and shortened total sleep time. However, in contrast to patients with major depression, rapid eye movement (REM) sleep latency is usually not shortened in patients with anxiety disorders. Furthermore, a reduction of slow wave sleep is not as common as in some mental disorders, e.g., schizophrenia (Cox & Olatunji 2016).
PDA
Studies in patients with PDA showed increased cortical arousal in waking EEG, during sensory gating, and heightened cerebral processing of panic-relevant stimuli. This is reflected as increased beta power in qEEG and elevated contingent negative variation (CNV) and P3 components of ERP (Clark et al. 2009).
GAD
Electrophysiological studies in GAD studies did not report any ERP abnormalities (Clark et al. 2009).
SAD
In SAD, studies generally indicate tonic hyperarousal, as reflected in reduced low frequency (LF) and increased high frequency EEG power and an elevated PI component (Clark et al. 2009).
Specific phobias
In a few studies, cortical hypervigilance was reported in specific phobias, with indications of enhanced P3 and CNV components of ERP to phobic stimuli. One study has shown that the P3 amplitude can be normalised following successful behavioural therapy (Clark et al. 2009).
PTSD
Frontal asymmetry is a frequently studied biomarker in PTSD, and is calculated as the difference in mean alpha band power between the left and right frontal cortex over a time span of several minutes. Relatively greater left frontal activity is regarded as being related to appetitive motivation, and lower levels of depression and anxiety in PTSD patients (Meyer et al. 2015). However, this biomarker is not specific for PTSD, as it has also been reported in depression, premenstrual dysphoric disorder, and schizophrenia. Moreover, in some studies, no deviance in alpha asymmetry from healthy control groups was found in PTSD and anxiety disorders (Gordon et al. 2010).
Patients with PTSD, when compared with controls, were found to have decreased resting-state EEG frontal connectivity, which was significantly correlated with PTSD symptom severity, and with depressive and increased arousal symptoms (Lee et al. 2014). In a review, significant associations have been described with PTSD symptoms not only for alpha EEG rhythm but also for P200 and P300 ERP components (Lobo et al. 2015). Moreover, alterations of ERP components (N200 and P300 amplitudes) while performing an inhibitory control task (Stop Task) were reported to classify veterans with mild traumatic brain injury associated or not associated with the development of PTSD with high accuracy (Shu et al. 2014).
In PTSD, sleep disturbances shortly after trauma exposure predict the development of PTSD at follow-up assessment, however, the evidence is less clear regarding objective polysomnographic indices (Babson & Feldner 2010).
OCD
Over the past two decades, performance monitoring has been extensively studied in patients with OCD, using advanced methodologies, such as EEG source localisation, simultaneous EEG and MRI recording, intracerebral EEG recording, magnetoencephalography, EEG-informed fMRI and valuable results were obtained.
Research on “performance monitoring” and “error processing” has been undertaken extensively in OCD patients, who appear to monitor their thoughts and actions more carefully to avoid losing control or committing errors. Theoretically, error processing involves both recognising that an error has occurred and adjusting future responses. Deficits in either of these abilities could contribute to rigid, repetitive behaviour. Enlarged error signals have been consistently found in patients with OCD (Endrass & Ullsperger 2014). The introduction of specific task paradigms and emotional challenge conditions in such research has been shown to enhance individual differences, which can be more reliable than resting state measurements (Zambrano-Vazquez & Allen 2014).
Error processing is thought to be associated with activity in anterior/posterior medial frontal cortex, anterior insula/operculum, ventrolateral prefrontal cortex, dorsolateral prefrontal cortex and lateral parietal cortex (Grutzmann et al. 2014). The mid-cingulate cortex is specifically recognised to signal the need for adjustment of cognitive control to prevent subsequent errors (Ullsperger et al. 2014). In particular, the error-related negativity (ERN), a response-locked ERP, is defined as a negative voltage deflection that occurs 50–100 ms after an error or conflict response and is thought to specifically reflect activity of the response-monitoring system (Gehring et al. 1990).
Numerous EEG studies have found larger ERN amplitudes in patients with OCD, in adult (Gehring et al. 2000; Endrass et al. 2008; Endrass et al. 2010; Stern et al. 2010; Riesel et al. 2011; Xiao et al. 2011; Klawohn et al. 2014; Riesel et al. 2014) and paediatric (Hajcak et al. 2008; Hanna et al. 2012; Carrasco et al. 2013) samples. Enhancement of the ERN in OCD seems to be independent of pharmacological or psychological interventions (Endrass et al. 2010; Stern et al. 2010) and occurs among all major symptom dimensions (Riesel et al. 2014). Moreover, the same results have been obtained in individuals with subclinical OCD symptoms (Santesso et al. 2006; O’Toole et al. 2012) and non-affected first-degree relatives of patients with OCD (Riesel et al. 2011; Carrasco et al. 2013).
Globally, these findings have identified increased ERN amplitudes as a promising candidate vulnerability marker for OCD. However, to date, its sensitivity and specificity is not clearly defined (Manoach & Agam 2013). For example, some studies have also found an enhanced negativity on correct trials (sometimes referred to as the correct-related negativity), suggesting the presence of an overall hyperactivity during response monitoring in people with OCD (Ursu et al. 2003; Maltby et al. 2005). Broadly, amplified error signals in OCD might reflect hyperactive cortico-striatal circuitry during action monitoring (Agam et al. 2014; Grutzmann et al. 2014). Convergent results suggest the existence of a self-monitoring imbalance involving inhibitory deficits and executive dysfunctions in OCD (Melloni et al. 2012). In this model, the imbalance might be triggered by an excitatory role of the basal ganglia (associated with cognitive or motor actions without volitional control) and inhibitory activity of the orbitofrontal cortex (OFC) as well as excessive monitoring of the ACC to block excitatory impulses. This imbalance would simultaneously interact with the reduced activation of the parietal-dorsolateral prefrontal cortex network, leading to executive dysfunction (Melloni et al. 2012).
Further electrophysiological data suggest that the candidate network might be extended and include specific additional regions in the medial frontal cortex involved in performance monitoring, such as anterior insula or the pre-supplementary motor area (Bonini et al. 2014; Grutzmann et al. 2014; Ullsperger et al. 2014); posterior mid-cingulate regions (Agam et al. 2011); and sub-genual ACC regions, for which increased activity has been found in OCD (Agam et al. 2014). Thus, patients with OCD might tend to evaluate errors as being disproportionately salient. This would support the theory that inappropriate and exaggerated error signalling leads to a pervasive sense of incompleteness and self-doubt and triggers compulsions to repeat behaviours (Maltby et al. 2005). Other theories hypothesise that the ERN is not only associated with error detection, but may be modulated by the affective significance of an error (Hajcak et al. 2005). Hence, other factors that can potentially characterise the overactive response monitoring observed in individuals with OCD, such as error significance, have been also investigated. However, the results have been equivocal with some studies showing no difference in ERN amplitude between conditions with punishment and no punishment after error in participants with OCD but a significant difference in controls (Endrass et al. 2010); others have found that punishing errors leads to an enhanced ERN and, moreover, that it has long-lasting effect on the ERN (Riesel et al. 2012).
In the analysis of the activity of intracortical EEG sources in patients with OCD using low-resolution electromagnetic tomography and independent component analysis, both methods provided evidence for medial frontal hyperactivation in OCD (Koprivova et al. 2011).
Patients with OCD were also found to have frontal alpha rhythm asymmetry, compared with healthy controls, with frontal slow alpha power (8–10 Hz) being more dominant in the left hemisphere at rest and during presentation of neutral, aversive, and OCD-related pictures. These changes in hemispheric alpha band topography were proposed as biomarker for increased avoidance motivation in OCD patients (Ischebeck et al. 2014).
In sleep studies, OCD patients were reported to have significant disturbances of sleep continuity measures but in most cases, no abnormalities of slow wave sleep or REM sleep were found. Many of the sleep disturbances were characteristic for depression or related to depressive symptoms. Severe OCD symptoms were consistently associated with greater sleep disturbance (Paterson et al. 2013).
Other Obsessive-Compulsive-Related Disorders (OCRDs)
Electrophysiological studies in other OCRDs are still scarce. One study has attempted to explore the ERN as a measure of response monitoring capabilities in trichotillomania (Roberts et al. 2014). Results reported that individuals with hair pulling symptomatology might have significantly smaller ERNs than the control group, supporting the idea that trichotillomania is distinct from OCD. Smaller ERNs are believed to reflect deficits in error checking that contribute to difficulty monitoring one’s own actions, and such results might indicate that individuals with symptoms of trichotillomania have shortfalls in self-monitoring, perhaps related to more impulsive tendencies (Roberts et al. 2014). One other study has used meta-analysis to further characterise the ERN in OCD, and pooled data across studies to examine the ERN in OCD with or without hoarding (Mathews et al. 2012). When stratified, OCD showed a significantly enhanced ERN only in response conflict tasks. However, OCD with hoarding showed a marginally larger ERN than OCD without hoarding, but only for probabilistic learning tasks. These results suggest that the abnormal ERN in OCD might also be task-dependent, and that OCD with hoarding might show different ERN activity from OCD without hoarding, perhaps suggesting different pathophysiological mechanisms of error monitoring across these clinical dimensions.
In summary, as neurophysiological examinations are among the most sensitive tests in psychiatry, many alterations in EEG, ERP or PSG were found in patients with anxiety disorders. While some of these alterations can be used as biomarkers for specific research questions, especially in treatment studies looking at hyperarousal performance monitoring and information processing, they are not specific and cannot be used as diagnostic tests for anxiety disorders. Moreover, many of these reported neurophysiological findings are influenced by comorbid depressive symptoms and co-existing pharmacological treatment.
Heart rate variability
Cardiologists have long held the view that a heart rate which fluctuates over time, in contrast to a heart beating to a strict metronomic rhythm, is a marker of good cardiovascular health. Heart rate variability (HRV), the extent to which the interval between beats varies with time, is reduced in several cardiovascular disorders such as after myocardial infarction (Bigger et al. 1992; Carney et al. 2001), in coronary artery disease (Wennerblom et al. 2000) and in hypertension (Singh et al. 1998) and is a predictor of mortality (Dekker et al. 2000; La Rovere et al. 2003). As will be described in this section, heart rate variability is thought to be closely linked to the function of the autonomic nervous system and its sympathetic and inhibitory parasympathetic influences.
Anxiety, cardiovascular disorders and autonomic dysfunction
Anxiety disorders are associated with cardiovascular disease (Roest et al. 2010; Davies & Allgulander 2013) and may be a risk factor in sudden cardiac death (Kawachi et al. 1994). The leap from employing HRV as a marker in cardiovascular disorders to anxiety disorders relies on the hypothesis that there may be shared dysfunctions in the autonomic nervous system, which underlie, or at least are measurable in, many disorders in both fields.
PDA
An association of panic attacks or PDA with hypertension has been reported both in clinical samples (Davies et al. 1999) and in population-based data (Davies et al. 2012), and the possibility that this association is due to shared autonomic dysfunction has been explored (Davies et al. 2007). Symptoms of autonomic activation, such as racing heart, sweating and flushing are included in diagnostic criteria for PDA. Several authors have suggested that autonomic nervous system dysfunction may be an important aetiological factor in PDA, for instance, Klein (1993) categorised panic attacks into two distinct types; attacks caused by false suffocation alarms and those attributable to autonomic surges or HPA axis activation.
Esler’s group studied norepinephrine and adrenaline release (spill-over) from major organs in patients with PDA using invasive methods requiring cannulation of large vessels. Spill-over of adrenaline from the heart was significantly greater in patients with PDA than in controls at rest. During panic attacks, whole body adrenaline spill-over was markedly increased with proportionally smaller increases in norepinephrine spill-over (Wilkinson et al. 1998). This finding supports several studies which report evidence of sympathetic over-reactivity in PDA such as enhanced noradrenergic volatility during clonidine challenge (Coplan et al. 1997) and excess blood pressure overshoot on standing (Coupland et al. 1995). The latter effect was not observed in patients with autonomic failure (Mathias 2002) suggesting that the autonomic nervous system is essential in mediating this response.
Others have examined central autonomic system function and reported catecholamine or adrenoceptor function as being altered centrally in PDA (Nutt 1989; Tancer et al. 1993). Esler has demonstrated excess catecholamine spill-over in hypertension (Esler et al. 2001) and autonomic dysfunction is now understood to be a core aetiology of what was previously termed “essential” hypertension. PDA and hypertension may share a failure of control of sympathetic activation, perhaps through compromise of those centres which control the C1-adrenergic cell group in the rostral-ventrolateral medulla, which include the raphe pallidum and ventrolateral periaqueductal grey, the latter under the influence of the pre-frontal cortex (Johnson et al. 2004; Davies et al. 2007).
HRV measures
Heart rate variability allows an estimation of autonomic nervous system input to the heart to be ascertained speedily and non-invasively. There are both parasympathetic (cholinergic) and sympathetic (noradrenergic) influences on the heart. The sympathetic nervous system is linked to mobilisation behaviours, often in response to stressors, which may induce the classic “flight or fight response” requiring cardiac activation, whereas the parasympathetic system, mediated through the vagus nerve, is linked to immobilisation and disengagement (Porges 2001). Frequency of heart rate fluctuations are decreased when sympathetic tone is increased (Pagani et al. 1984) and with parasympathetic blockade (Akselrod et al. 1985).
The most commonly utilised measures HRV measures are “frequency-domain” and “time-domain” variables. Frequency-domain measures are based on power spectral analysis, which allows detection of LF and high frequency (HF) oscillation. HF oscillation relates to the activity of the parasympathetic system, mainly mediated through the vagus nerve, while LF oscillation is thought to be linked to variation in sympathetic tone. The LF/HF ratio was previously employed as a proxy measure of sympatheto-vagal balance (Pagani et al. 1984), having the advantage of being influenced by change in both sympathetic and parasympathetic nervous system cardiac input but the problem that simultaneous change in both parameters might be undetected.
Time-domain measures of HRV fall into two categories. The first are derived from the differences between adjacent beat intervals, the most frequently used being root mean square of successive differences (RMSSD) and pNN50 (mean occasions per hour where change in consecutive normal sinus (NN) intervals exceeds 50 ms (Ewing et al. 1984)). RMSSD and pNN50 are highly correlated with frequency domain derived HF oscillation (Stein et al. 1994). A second category, derived from observing beat to beat intervals over time, includes standard deviation of normal sinus intervals (SDNN) which represents the standard deviation of “NN” intervals (Sztajzel 2004). Since SDNN varies with the total recording time, comparisons between values obtained over widely differing time periods are problematic.
HRV: association of frequency domain and time domain measures with anxiety disorders
While the possibility of HRV being a biomarker in anxiety disorders has been considered for more than a decade (Gorman & Sloan 2000), a systematically organised meta-analysis of the relation of HRV to the presence of anxiety disorders has only recently been published. Chalmers et al. (2014) identified 36 studies meeting criteria requiring a comparison in HRV outcomes between patients with anxiety disorders and controls. The studies had 2086 participants with anxiety disorders and 2204 controls and employed a variety of methodologies. Recording periods ranged from 2 min to 24 h and studies used frequency domain measures such as LF and HF, time domain measures or other approaches including detection of respiratory sinus arrhythmia. The authors chose not to extract data on LF/HF ratio given its questionable utility and gave RMSSD preference over other time domain measures.
Across all anxiety disorders, the frequency domain HF oscillation variable (reported in 34 studies), was strongly and significantly associated with having an anxiety disorder. The association of time domain measures, reported in 20 studies, was of borderline significance but became highly significant after exclusion of one outlying study. The LF oscillation variable, reported in 22 studies, was a poor predictor of anxiety disorders. When specific anxiety disorders were considered, PDA featured in the most studies with 24 of the 34 papers having some participants with this disorder, in comparison to 13 for PTSD, five for GAD, four for SAD, two for OCD and one for specific phobia. The meta-analysis revealed that time domain measures were strong predictors of PDA, PTSD and GAD and weaker but still significant predictors of SAD and specific phobia. HF was strongly associated with GAD and SAD and had weaker but significant relations with PDA and PTSD. Neither measure was associated with OCD. LF was not associated with any of the anxiety disorders. The strength of association of both HF and time domain measures of HRV in generalised anxiety disorder, is of interest for the conceptualisation of this disorder. Although both analyses rely on only three studies, the results suggest that despite Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV and DSM-5 excluding clinical features suggestive of autonomic dysfunction from the list of symptoms contributing to the diagnosis, GAD may indeed be associated with autonomic dysfunction (Thayer et al. 1996).
Response of HRV to treatment and experimental neurotransmitter manipulation
Treatment of anxiety disorders may be associated with a restoration in HRV, especially when the treatment involves modulation of serotonin. Reduced HRV demonstrated in PDA was reversed by a serotonin promoting antidepressant (Yeragani et al. 1999) but not by nortriptyline, which primarily promotes central norepinephrine transmission (Tucker et al. 1997). However, serotonin-modulating drugs are not essential for improvement in HRV on treating anxiety CBT and SSRIs were equally capable of increasing HRV.
In healthy individuals, HRV is reduced during panic provoking challenges but SSRI treatment appears to blunt this response (Agorastos et al. 2015). The involvement of the serotonin system in the neurobiology of anxiety disorders has also been examined using the technique of tryptophan depletion (Hood et al. 2005). When this method is applied in subjects who have recovered from anxiety disorders, depletion is associated with a transient return of anxiety symptoms and exaggerated response to stress challenges (Davies et al. 2006). In one study in remitted patients with depression, HRV was measured before and during tryptophan depletion (Booij et al. 2006). Tryptophan depletion was associated with a significant reduction in HRV (ascertained using both time domain measures and the frequency domain HF measure) although this effect was limited to subjects who had experienced suicidal ideation. Notably, these patients experienced increased anxiety during the tryptophan depletion period.
The therapeutic effect of modulating serotonin in anxiety disorders appears, in the majority of studies, to ameliorate autonomic function as reflected in improving heart rate variability. One exception is a study reporting that CBT alone increased HRV in PDA, but that a CBT/SSRI combination did not (Garakani et al. 2009). Nevertheless, the potential for serotonin to influence autonomic function (and thereby HRV) has a neurobiological basis (Davies et al. 2007), since animal studies suggest that pH-dependent serotonergic neurons projecting to the RVLM may tonically inhibit sympathetic outflow (Richerson et al. 2001). Clinically, the enhanced noradrenergic volatility in PDA described during clonidine challenge was attenuated after successful treatment with SSRI antidepressants (Coplan et al. 1997).
Utility of HRV as a biomarker
Heart rate variability, whether ascertained using the frequency-domain measure of HF oscillation or by time domain measures, has advantages over other potential biomarkers of being non-invasive and easy to administer with valid data being obtainable in a matter of minutes. As such, it has potential use in case detection and in large population-based cohorts. As it is ameliorated by treatments that are effective in anxiety disorders and reduced by neurotransmitter manipulations known to provoke anxiety, it offers the possibility of identification of treatment response.
The proliferation of differing outcome measures is receding in importance as a disadvantage since the frequency domain HF measure, and time domain measures (RMSSD, pNN50 and SDNN) appear to be preferable to LF or the LF/HF ratio. However, several common disorders beyond the realm of anxiety are also associated with reduced HRV, including the cardiovascular disorders discussed earlier, depression, Alzheimer’s disease, fibromyalgia and diabetes, and indeed any disorder where autonomic nervous system dysfunction is typically present. This reduces specificity for detection of anxiety disorders. Furthermore, HRV is known to decrease with age (Liao et al. 1995), which may complicate its interpretation. Finally, standard HRV measurements cannot be used in subjects who are not in sinus rhythm (Sztajzel 2004).
In summary, HRV appears to offer a degree of sensitivity but limited specificity in anxiety disorders. Ease of ascertainment and the ability to detect treatment related changes are clear strengths. We await population-based longitudinal studies in larger sample sizes where more invasive approaches may be impractical.
Neurocognition
PDA
In a review of the literature investigating the neuropsychological disturbances PDA, limited support for impairment in short-term memory among individuals with PD was found in some but not all studies. Moreover, the studies did find some evidence for impairment in other areas of cognitive functioning, including executive function, long-term memory, visuospatial or perceptual abilities and working memory (O’Sullivan & Newman 2014). The review included 14 studies (total 439 patients, 510 healthy controls), the majority of which had average to high methodological quality. Studies with a sample size of less than 15 participants per group were excluded.
GAD
In a study including 112 patients with different anxiety disorders, no differences in neuropsychological functions were found in seven patients with GAD compared with healthy controls; of course, such a study would only have been powered to detect group differences with massive effect size (Airaksinen et al. 2005). Another study found that performance on executive and non-verbal memory tasks of GAD patients (n = 40) was largely worse than in healthy controls (n = 31). These cognitive deficits seemed to be more marked in patients taking antidepressants than in drug-naïve patients (Tempesta et al. 2013). However, the study was not randomised with regard to medication intake; therefore, it is problematic to assume a causal relationship between antidepressants and cognitive functioning.
SAD
Cognitive models of SAD assume that patients with SAD have cognitive biases regarding their interpretation of ambiguous social situations. A systematic review of 30 studies of the neuropsychological performance in SAD (698 patients) revealed that individuals with SAD consistently showed decreased performance on tests of verbal memory functions. In particular, the studies showed decreased performance regarding visual scanning and visuoconstructional ability as well as some indication for verbal memory difficulties (O’Toole & Pedersen 2011). Since this review was published, a study compared 25 subjects with SAD and 25 healthy controls and reported no significant between-group differences, based on a composite analysis of variance test (Sutterby & Bedwell 2012). In post hoc tests, patients had worse visual working memory performance than controls, but this finding did not withstand Bonferroni correction. In a subsequent study, SAD (n = 42 patients) performed worse than healthy controls (n = 42) on processing speed, visuospatial construction, visuospatial memory, verbal learning and word fluence (O’Toole et al. 2015).
OCD
Considerable evidence demonstrates that behavioural performance during cognitive tests, and related functional activations, are abnormal when OCD patients are probed on domains dependent upon the integrity of fronto-striatal circuitry.
Response inhibition
The ability of response inhibition can be measured by means of go/no-go tasks and stop signal reaction time (SSRT) tasks. Both types of paradigm require the participants to make a motor response on some trials and to withhold the response on some other trials, with the SSRT being more sophisticated in using stepwise tracking to measure inhibitory control. Deficits in response inhibition have been suggested as a candidate cognitive endophenotype for OCD (Chamberlain et al. 2007b). Moreover, impaired response inhibition was shown to be associated with reduced grey matter volume in the OFC and right inferior frontal regions, as well as increased grey matter volume in the cingulate, parietal and striatal regions in OCD patients and matched-relative groups, as compared with controls (Menzies et al. 2008); and these combined behavioural-structural MRI measures were significantly heritable. Inhibition difficulties were also pinpointed at the functional level, whereby successful inhibition on an SSRT task was associated with greater activation in the supplementary motor area in OCD patients (n = 41) and their siblings (n = 17), versus controls (n = 37) (de Wit et al. 2012). Impaired performance on response inhibition tasks was found to have a moderate effect size (0.49) in a meta-analysis on adult OCD patients as compared with control participants (Abramovitch et al. 2013). This meta-analysis comprised 115 studies (total 3452 patients) overall, although only a subset of these related to response inhibition.
Cognitive flexibility
The clinical manifestation of OCD is commonly represented by repetitive compulsive acts that might be linked to impaired cognitive flexibility (Chamberlain et al. 2005). The Intradimensional/Extradimensional set shifting paradigm allows a fine-grained examination of different cognitive processes germane to flexible responding including reversal learning, set formation and the ability to inhibit and shift attention between stimuli. By employing this multiple stage paradigm, it was shown that OCD patients were generally able to form an attentional set but impaired in their ability to switch their focus to a new, previously irrelevant dimension (extradimensional stage; ED shift) (Veale et al. 1996; Watkins et al. 2005; Chamberlain et al. 2006). Considering that impaired performance was unrelated to symptom severity and present irrespective of treatment, ED deficits might represent a trait marker of the disorder (Chamberlain et al. 2006). More conclusively, non-affected first-degree relatives (n = 20) exhibited impairments as well, versus controls (n = 20) (Chamberlain et al. 2007b).
Across species, the ability to flexibly adjust behavioural responses in face of negative feedback is subserved by the OFC and can be assessed by reversal learning tasks. As such reversal of responses is normally relatively easy for humans to manage, reversal learning abnormalities are mainly identified using imaging rather than behavioural tests, due to ceiling effects for the latter. Dampened OFC activation during reversal learning was reported in one fMRI study of OCD patients (n = 20), as compared with controls (n = 27) (Remijnse et al. 2006). Controlling for the potential confounding effect of comorbid depression, Chamberlain et al. (2008) showed that patients with OCD (n = 14) and unaffected relatives (n = 12) had extensive clusters of hypo-activation in the lateral OFC, lateral PFC and parietal cortices, versus controls (n = 15). Task switching abilities, strongly relying on the cross-talk between basal ganglia and PFC (Cools et al. 2004), have separately been assessed in OCD patients. Significantly higher error rates in task-switching trials and reduced activation of dorsolateral prefrontal cortex lateral OFC, ACC and caudate body were observed in 21 OCD patients versus 21 controls (Gu et al. 2008).
Planning
Executive planning entails the ability of attaining a goal through intermediate steps, which do not necessarily lead directly to that goal. It is tested by means of the Tower of London task and its variants, for which MRI versions are also often available. Studies in OCD patients revealed lengthened responses times (Veale et al. 1996; Nielen & Den Boer 2003) and, on more difficult task versions, impaired performance (Chamberlain et al. 2007a). Planning deficits have been linked with dorsolateral prefrontal cortex and basal ganglia (caudate, putamen) hypo-activation in OCD patients, in a study conducted in medication-free patients and healthy controls (van den Heuvel et al. 2005). Behavioural impairment – fewer correct responses and increased response times – was also found in unaffected relatives of OCD patients compared with normal participants (Delorme et al. 2007), suggesting that planning deficits constitute a vulnerability measure for OCD.
Goal-directed system and habit learning
Convergent evidence from the animal and human literature suggests that fronto-striatal loop circuits mediate the balance between purposeful, goal-directed actions and habitual, automatic behaviours. Considering the literature linking fronto-striatal loops to OCD symptomatology, it was proposed that OCD could be characterised as a disorder of maladaptive habit learning (Rauch et al. 2002). The hypothesis has been formally tested in a series of experiments that led to the conclusion that a defective “goal-directed system” may bias OCD patients to heavily rely on habits (Gillan & Robbins 2014). More specifically, it was first shown using an appetitive instrumental learning task that OCD patients (n = 21) were not able to refrain from responding to outcomes no longer associated with reward, as compared with controls (n = 30) (Gillan et al. 2011). Similarly, in an aversive context, OCD patients were trained to avoid mildly aversive electrical shocks by performing the correct response to a predictive stimulus. Following a training period, participants were instructed that the cable delivering the shock had been disconnected from one of their wrists. Patients (n = 25) on average made significantly more responses to the stimuli no longer associated with any shock than did controls (n = 25) (Gillan et al. 2014). An fMRI-compatible version of the task showed that excessive caudate activity was associated with increased performance of the avoidance habits in 37 OCD patients, compared with 33 healthy controls (Gillan et al. 2015). The finding that aberrant activation in the caudate nucleus occurred more in patients showing a bias towards the premature development of habits suggested that, in OCD, reliance on repetitive, habit-like behaviours might stem from dysfunction within goal-directed behaviour loci within the dorsal striatum (Yin & Knowlton 2006).
Despite the existence of some discordant findings, deficits related to behavioural inhibition, cognitive flexibility and executive functioning seem to represent core traits of OCD, and hold face validity considering the clinical manifestation of the disorder. Neuropsychological and imaging studies demonstrate that non-affected first-degree relatives show, to some extent, similar abnormalities to patients. On the one hand, these shared findings represent valuable tools for investigating the effect of specific genetic variants on both cognitive and neural substrates and importantly for investigating the disorder across species, possibly leading to better treatment. On the other hand, the similarity between affected and non-affected relatives demonstrates that our understanding of the steps leading from an “at risk” or vulnerable state to the development of “state” OCD is limited; as is our understanding of protective or resilience-related biological factors. Multi-modal investigation, providing convergent evidence and guided by specific theoretical hypotheses, might help to address these issues.
Other OCRDs
Trichotillomania has been associated with impaired stop-signal inhibitory control in multiple studies compared with controls, while set-shifting has generally been reported to be intact (Chamberlain et al. 2006; Odlaug et al. 2014). The sample sizes were 17 patients and 20 controls in the former study; and 12 patients and 14 controls in the latter study. However, there appear to be some differences in subtypes: in people with childhood onset trichotillomania (<11 years of age, n = 42), the neuropsychological profile appears to be more like OCD; i.e., impaired set-shifting and lesser stop-signal impairments; compared with later onset trichotillomania (n = 56) (Odlaug et al. 2012).
Patients with excoriation (skin-picking) disorder (n = 20) showed impaired stop-signal inhibition but intact set-shifting versus controls (n = 20) (Odlaug et al. 2010). Impaired response inhibition on a stop-signal task was found in patients with trichotillomania (n = 12) and their clinically asymptomatic first-degree relatives (n = 10) versus controls (n = 14) in a more recent study, suggesting that it may represent a vulnerability or predisposing factor (Odlaug et al. 2014). In a head-to-head comparison of skin-picking disorder (n = 31 patients) against trichotillomania (n = 39 patients), stop-signal impairments were more marked in the former group (Grant et al. 2011).
As is the case for imaging, cognitive studies in relation to compulsive hoarding have mostly been undertaken in the context of other disorders, rather than in “hoarding disorder” as a discrete entity. One exception to this is a recent study that compared cognition in people with hoarding disorder without OCD (n = 22), people with OCD plus hoarding (n = 24), and healthy controls (n = 28) (Morein-Zamir et al. 2014). Deficits in cognitive flexibility were common to both clinical groups, arguing against hoarding disorder having a distinct neuropsychological profile from that of OCD-hoarding, and highlighting the importance of cognitive rigidity in relation to these two disorders.
There are very few cognitive studies of body dysmorphic disorder (BDD). One study found that subjects with BDD exhibited deficits in cognitive flexibility in comparison to controls (Jefferies et al., submitted for publication). Consistent with this proposition, patients with comorbid skin-picking disorder and BDD (n = 16) had disproportionately impaired set-shifting compared with subjects with non-comorbid skin-picking disorder (n = 39) (Grant et al. 2015). Other research suggests that individuals with BDD may have abnormalities in visual processing (Feusner et al. 2010). The sample size was 17 patients and 16 controls. In sum, caution is warranted due to the small numbers of studies, but there is some evidence that the grooming disorders (trichotillomania, excoriation disorders) are commonly associated with impaired response inhibition; while hoarding disorder and BDD appear more OCD-like in their neuropsychological profiles.
PTSD
Research on the neuropsychology of PTSD has identified several neurocognitive deficits associated with the disorder (Everly & Horton 1989; Vasterling et al. 1998; Sachinvala et al. 2000; Levy-Gigi et al. 2012). In one study, subjects with PTSD (n = 38), trauma-exposed subjects without PTSD (n = 108) and healthy control subjects (n = 89) did not differ significantly on a number of neuropsychological tests; however, the study was done in a non-clinical sample of undergraduate students (Twamley et al. 2004). In a double-blind study with 18 PTSD patients, treatment with the SSRI paroxetine resulted in a significant increase in verbal declarative memory function (Fani et al. 2009). It remains unclear whether the memory deficits in PTSD can only be attributed to stress-related alterations. As there is a genetic vulnerability for developing PTSD, cognitive dysfunctions may have existed before the trauma and may have been, at least in part, the reason why vulnerable individuals develop PTSD after a trauma. Cognitive impairments in PTSD have also been attributed to comorbidity with substance abuse or other psychiatric disorders. However, in a study reporting memory function in rape victims with PTSD (n = 15), compared with rape victims without PTSD (n = 16), deficits were mild and not attributable to comorbid depression, anxiety or substance abuse (Jenkins et al. 1998).
One DSM-5 criterion for PTSD is the “inability to remember an important aspect of the traumatic event (typically due to dissociative amnesia)”. One may speculate that dissociative amnesia is associated with the memory impairments generally found in PTSD. However, it is contentious whether the phenomenon of dissociative amnesia exists at all (for a discussion, see McNally, 2007).
Gender issues
In international epidemiological surveys, the female to male ratio of the prevalence rates of anxiety disorders varied between 1.5:1 and 2.1:1% (Bandelow & Michaelis 2015). Psychosocial contributors (e.g., childhood sexual abuse and chronic stressors), but also genetic and neurobiological factors have been discussed as possible causes for the higher prevalence in women. Identification of the causes of gender-specific susceptibility for anxiety disorders may be useful for better understanding the aetiology of anxiety disorders in general. It is most likely that higher anxiety susceptibility in women is due to a delicate interplay between psychosocial and neurobiological factors. Hypotheses about the role of gender-specific stressors, and gender differences in the expression of fears warrant further investigation. Sex-specific variance has been identified in numerous neurotransmitter systems. The serotonin system may be of particular importance, as most drugs used in the treatment of anxiety disorders enhance serotonin neurotransmission and alterations in the serotonergic system have been found in anxiety patients relative to healthy controls. It seems likely that female sex hormones are involved, as periods of fluctuating levels of oestrogen and progesterone have been linked to increase or decrease of symptomatology in patients with PDA. Moreover, a plausible explanation for the gender-specific risk is a genetic one. For example, in PDA, the catechol-O-methyltransferase and monoamine oxidase (MAOA) genes have been associated with the higher risk of women to develop PDA (Bandelow & Domschke 2015).
Discussion
To our knowledge, there has been no comparable consensus initiative that put together all major research lines in the field of biomarkers for anxiety, OCD and PTSD. It is a challenge to summarise the incredible amount of findings collected in this paper and the accompanying article (see Part I; Bandelow et al. 2016) in a simple way.
First, a change in paradigms has been observed. In the 1980s and 1990s, “wet research” predominated, meaning that blood or CSF samples were taken from patients and healthy controls, either in resting state or after challenge tests with anxiety-provoking agents, e.g., lactate or carbon dioxide. Blood-based biomarkers of treatment response in psychiatric disorders remain in early stages of development and none has demonstrated reliability for predicting pharmacological outcome. Although research efforts in the past decades have definitely increased our knowledge of the neurobiological underpinnings of pathological anxiety, we still do not have the proof that a specific dysfunction of a neurotransmitter system, e.g., the serotonergic system, is the main cause for anxiety disorders. Still, the most robust evidence for an involvement of serotonin derives from the fact that a large number of drugs that are effective in anxiety disorders, OCD and PTSD have a common denominator, i.e., that they have an impact on serotonergic neurotransmission.
Serotonin reuptake inhibition is the main mechanism of action of these antidepressants but there also some drugs that have agonist or antagonist properties at serotonin receptors. Other medications that can treat anxiety act at the GABA binding site. However, as these binding sites are widespread in the brain and have non-specific inhibitory effects, the efficacy of benzodiazepines in anxiety disorders cannot be taken as evidence that a dysfunction of the GABA binding site is the cause of pathological anxiety.
Since the end of the 1990s, there has been a strong shift to neuroimaging and genetic studies – which are summarised in Part I of this consensus paper (Bandelow et al. 2016), while the publication output in neurochemistry studies seems to have declined.
Interpreting the abundant number of results of neuroimaging studies in anxiety disorders is a difficult task. The existing studies have found abnormalities in many different regions of the brain, and it is a challenge to synopsise the often contradictory findings in a uniform theory. A problem is the high number of statistical comparisons that are possible, and if the results are not corrected for multiple testing, there is a high chance for false-positive findings. The main methodological problem in most of the studies is the small sample size, making it difficult to reliably separate artefacts from substantiate findings.
Likewise, there is a plethora of genetic studies. In association studies, a large number of candidate genes have been investigated. The only clear result that we can derive from these studies is that anxiety disorders are not based on a single gene but are multigenic, while the contribution of single genes is only small. Genome-wide association studies may be a future possibility to separate relevant findings from findings by chance. Again, correction for multiplicity is crucial, and this again requires larger sample sizes that are often used in genetic research. International cooperation is needed to generate adequate sample sizes for this kind of research. Despite the manifold methodological shortcomings, the neuroimaging and genetics fields are two of the most promising areas for neurobiological research. In the future, neurochemistry, neurophysiology, neuropsychology, neuroimaging, genetics and other fields will have to be integrated in order to elucidate the neurobiological causes of anxiety. Increasing efforts are being made to find reliable biomarkers for diagnostic procedures or prediction of treatment outcome in anxiety disorders, OCD and PTSD. However, as with research in other mental disorders such as depression, there is still no biological or genetic predictor of sufficient clinical utility to inform the selection of a specific pharmacological compound for an individual patient, because of low sensitivity and specificity of the suggested biomarkers. Ideally, in the future, we will possibly be able to diagnose a mental disorder simply by taking a blood test and to choose a personalised medication or psychological treatment for a specific patient (“precision medicine”).
Acknowledgements
The present work was supported by the ADRN within the ECNP-NI.
Katherina Domschke’s work was supported by the German Research Foundation (DFG), Collaborative Research Centre “Fear, Anxiety, Anxiety Disorders” SFB-TRR-58, projects C02 and Z02.
Statement of interest
Prof. Bandelow has received research funding from European Commission (FP7) and was on the speakers’ and/or advisory board for Actelion, Glaxo, Janssen, Lundbeck, Meiji-Seika, Otsuka, Pfizer, and Servier.
Prof. Baldwin has attended advisory boards for Grunenthal, Eli Lilly, Lundbeck, Pfizer, and Servier. His university has received grants from Lundbeck and Pfizer to support research into anxiety disorders.
Dr. Chamberlain consults for Cambridge Cognition.
Dr. Fineberg has received financial support in various forms from the following: Otsuka, Lundbeck, Glaxo-SmithKline, Servier, Cephalon, Astra Zeneca, Jazz Pharmaceuticals, Bris-tol Myers Squibb, Novartis, Medical Research Council (UK), National Institute for Health Research (UK), Wellcome Foundation, European College of Neuropsychopharmacology, UK College of Mental Health Pharmacists, British Association for Psychopharmacology, International College of Obsessive-Compulsive Spectrum Disorders, International Society for Behavioural Addiction, World Health Organisation, Royal College of Psychiatrists.
Dr. Jarema has been on the speakers’ and/or advisory board for Angelini, Janssen, Lilly, Lundbeck, and Servier.
Prof. Ströhle: Research funding: German Federal Ministry of Education and Research (BMBF), German Research Foundation (DFG), European Commission (FP6), Lundbeck; speaker honoraria: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Lundbeck, Pfizer, Wyeth, UCB. Consultant for Actelion. Educational grants: Stifterverband für die Deutsche Wissenschaft, Berlin Brandenburgische Akademie der Wissenschaften, Boehringer Ingelheim Fonds, Eli Lilly International Foundation, Janssen-Cilag, Pfizer, and Lilly.
Prof. Thibaut is Editor-in-Chief of Dialogues in Clinical Neurosciences (grant by Servier).
Dr. Wichniak has been on the speakers’ and/or advisory board for Angelini, Janssen, Lundbeck, and Servier.
Prof. Zwanzger was on the speakers’ and/or advisory board for Lundbeck, Pfizer, Servier, Aristo, Merz and Hexal
Funding information
None.
Abbreviations
- 5-HIAA
5-Hydroxyindoleacetic acid
- 5-HT
Serotonin
- 5-HTP
Hydroxytryptophan
- 5-HTT
Serotonin transporter
- 5-HTTLPR
Serotonin-transporter-linked polymorphic region
- A-SepAD
Adult Separation Anxiety Disorder
- ACC
Anterior cingulate cortex
- ACTH
Adrenocorticotropic hormone or corticotropin
- ADRN
Anxiety Disorders Research Network
- ANP
Atrial natriuretic peptide
- ASLO
Anti-streptolysin O
- BDD
Body Dysmorphic Disorder
- BDNF
Brain-derived neurotrophic factor
- C-SepAD
Childhood Separation Anxiety Disorder
- CBT
Cognitive-behavioural therapy
- CCK
Cholecystokinin
- CNS
Central nervous system
- COMT
Catechol-O-methyltransferase
- CRH
Corticotropin-releasing hormone
- CRP
C-reactive protein
- CSF
Cerebro-spinal fluid
- DHEAS
Dehydroepiandrosterone sulphate
- DAT
Dopamine transporter
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- DST
Dexamethasone suppression test
- ECNP
European College of Neuropsychopharmacology
- EEG
Electroencephalography
- ELISA
Enzyme-linked immunosorbent assay
- ERN
Error-related negativity
- ERP
Event-related potential
- fMRI
Functional magnetic resonance imaging
- GABA
γ-Aminobutyric acid
- GABHS
Group A beta haemolytic streptococci
- GAD
Generalized Anxiety Disorder
- GWAS
Genome-wide association study
- HF
High frequency (high frequency oscillation is a frequency-domain heart rate variability measure)
- HPA axis
Hypothalamic-pituitary-adrenal axis
- HPLC
High-performance liquid chromatography
- HRV
Heart rate variability
- IL
Interleukin
- LF
Low frequency (low frequency oscillation is a frequency-domain heart rate variability measure)
- MAO
Monoamine oxidase
- MDD
Major Depressive Disorder
- mPFC
Medial prefrontal cortex
- mRNA
Messenger ribonucleic acid
- NE
Norepinephrine (noradrenalin)
- NET
Norepinephrine transporter
- NGF
Nerve growth factor
- NK
Neurokinin
- OCD
Obsessive-Compulsive Disorder
- OC-RD
Obsessive-Compulsive-Related Disorders
- OFC
Orbitofrontal cortex
- PANDAS
Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections
- PANS
Pediatric acute-onset neuropsychiatric syndrome
- PDA
Panic disorder with or without Agoraphobia
- PFC
Prefrontal cortex
- POMC
Proopiomelanocortin
- PSG
Polysomnography
- PTSD
Posttraumatic Stress Disorder
- RMSSD
Root mean square of successive differences
- SAD
Social Anxiety Disorder
- SDNN
Standard deviation of normal sinus intervals
- SNRI
Serotonin norepinephrine reuptake inhibitor
- SSRI
Selective serotonin reuptake inhibitor
- SSRT
Stop signal reaction task
- TNF
Tumor necrosis factor
- TSPO
Translocator protein
- WFSBP
World Federation of Societies for Biological Psychiatry
Footnotes
All other authors reported no conflicts of interest to declare.
ORCID
Borwin Bandelow http://orcid.org/0000-0003-2511-3768
Marianna Abelli http://orcid.org/0000-0002-3877-7048
Michel Bourin http://orcid.org/0000-0002-7268-4590
Samuel R. Chamberlain http://orcid.org/0000-0001-7014-8121
Eduardo Cinosi http://orcid.org/0000-0002-8903-181X
Naomi Fineberg http://orcid.org/0000-0003-1158-6900
Edna Grünblatt http://orcid.org/0000-0001-8505-7265
Marek Jarema http://orcid.org/0000-0001-5267-5315
Yong-Ku Kim http://orcid.org/0000-0001-5694-7840
Vasileios Masdrakis http://orcid.org/0000-0003-0197-9583
David Nutt http://orcid.org/0000-0002-1286-1401
Stefano Pallanti http://orcid.org/0000-0001-5828-4868
Stefano Pini http://orcid.org/0000-0001-9092-9144
Florence Thibaut http://orcid.org/0000-0002-0204-5435
Matilde M. Vaghi http://orcid.org/0000-0002-0999-9055
Eunsoo Won http://orcid.org/0000-0001-6825-032X
Peter Zwanzger http://orcid.org/0000-0002-5385-8188
References
- Abelli M, Chelli B, Costa B, Lari L, Cardini A, Gesi C, Muti M, Lucacchini A, Martini C, Cassano GB, et al. Reductions in platelet 18-kDa translocator protein density are associated with adult separation anxiety in patients with bipolar disorder. Neuropsychobiology. 2010;62:98–103. doi: 10.1159/000315440. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Curtis GC. Cardiac and neuroendocrine responses to exposure therapy in height phobics: desynchrony within the ‘physiological response system’. Behav Res Ther. 1989;27:561–567. doi: 10.1016/0005-7967(89)90091-0. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Curtis GC, Cameron OG. Hypothalamic-pituitary-adrenal axis activity in panic disorder: effects of alprazolam on 24 h secretion of adrenocorticotropin and cortisol. J Psychiatr Res. 1996;30:79–93. doi: 10.1016/0022-3956(95)00035-6. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Aboitiz F. Dynamics of neuromodulators–I. The role of dopaminergic signaling in goal-directed behavior. In: Aboitiz F, Cosmelli D, editors. From attention to goal-directed behavior. Heidelberg, Berlin: Springer-Verlag; 2009. pp. 187–204. [Google Scholar]
- Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. 2013;33:1163–1171. doi: 10.1016/j.cpr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up–2013 Curt Richter Award Winner. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y, Greenberg JL, Isom M, Falkenstein MJ, Jenike E, Wilhelm S, Manoach DS. Aberrant error processing in relation to symptom severity in obsessive-compulsive disorder: a multimodal neuroimaging study. Neuroimage Clin. 2014;5:141–151. doi: 10.1016/j.nicl.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y, Hamalainen MS, Lee AK, Dyckman KA, Friedman JS, Isom M, Makris N, Manoach DS. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc Nat Acad Sci USA. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Kellner M, Stiedl O, Muhtz C, Wiedemann K, Demiralay C. Blunted autonomic reactivity to pharmacological panic challenge under long-term escitalopram treatment in healthy men. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu053. pii: pyu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249:H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- Alfano CA, Reynolds K, Scott N, Dahl RE, Mellman TA. Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. J Affect Disord. 2013;147:379–384. doi: 10.1016/j.jad.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AJ, Leonard HL, Swedo SE. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1995;34:307–311. doi: 10.1097/00004583-199503000-00015. [DOI] [PubMed] [Google Scholar]
- Aloe L, Bracci-Laudiero L, Alleva E, Lambiase A, Micera A, Tirassa P. Emotional stress induced by parachute jumping enhances blood nerve growth factor levels and the distribution of nerve growth factor receptors in lymphocytes. Proc Nat Acad Sci USA. 1994;91:10440–10444. doi: 10.1073/pnas.91.22.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers GW, Abelson JL, Wilhelm FH, Roth WT. Salivary cortisol response during exposure treatment in driving phobics. Psychosom Med. 2003;65:679–687. doi: 10.1097/01.psy.0000073872.85623.0c. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Argyropoulos SV, Bailey JE, Hood SD, Kendrick AH, Rich AS, Laszlo G, Nash JR, Lightman SL, Nutt DJ. Inhalation of 35% CO(2) results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinology. 2002;27:715–729. doi: 10.1016/s0306-4530(01)00075-0. [DOI] [PubMed] [Google Scholar]
- Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, Gelineau-Morel R, Cavey A, Vergo S, Craner M, et al. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain. 2013;136:106–115. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- Avery DH, Osgood TB, Ishiki DM, Wilson LG, Kenny M, Dunner DL. The DST in psychiatric outpatients with generalized anxiety disorder, panic disorder, or primary affective disorder. Am J Psychiatry. 1985;142:844–848. doi: 10.1176/ajp.142.7.844. [DOI] [PubMed] [Google Scholar]
- Aymard N, Honore P, Carbuccia I. Determination of 5-hydroxytryptamine and tryptophan by liquid chromatography in whole blood. Its interest for the exploration of mental disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:77–86. doi: 10.1016/0278-5846(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Babson KA, Feldner MT. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24:1–15. doi: 10.1016/j.janxdis.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ. Behavioral and cardiovascular effects of 7.5% CO2 in human volunteers. Depress Anxiety. 2005;21:18–25. doi: 10.1002/da.20048. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Argyropoulos SV, Lightman SL, Nutt DJ. Does the brain noradrenaline network mediate the effects of the CO2 challenge? J Psychopharmacol (Oxford) 2003;17:252–259. doi: 10.1177/02698811030173002. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Dawson GR, Dourish CT, Nutt DJ. Validating the inhalation of 7.5% CO(2) in healthy volunteers as a human experimental medicine: a model of generalized anxiety disorder (GAD) J Psychopharmacol. 2011a;25:1192–1198. doi: 10.1177/0269881111408455. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Kendrick A, Diaper A, Potokar JP, Nutt DJ. A validation of the 7.5% CO2 model of GAD using paroxetine and lorazepam in healthy volunteers. J Psychopharmacol (Oxford) 2007;21:42–49. doi: 10.1177/0269881106063889. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt M, van der Ark P, Dourish CT, Dawson GR, Nutt DJ. Preliminary evidence of anxiolytic effects of the CRF(1) receptor antagonist R317573 in the 7.5% CO(2) proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011b;25:1199–1206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Seddon K, Nutt DJ. A comparison of the effects of a subtype selective and non-selective benzodiazepine receptor agonist in two CO(2) models of experimental human anxiety. J Psychopharmacol (Oxford) 2009;23:117–122. doi: 10.1177/0269881108089603. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD., Jr Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 2001;9:209–217. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Allgulander C, Altamura AC, Angst J, Bandelow B, den Boer J, Boyer P, Davies S, Dell’osso B, Eriksson E, et al. Manifesto for a European anxiety disorders research network. Eur Neuropsychopharmacol. 2010;20:426–432. doi: 10.1016/j.euroneuro.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ball S, Marangell LB, Lipsius S, Russell JM. Brain-derived neurotrophic factor in generalized anxiety disorder: results from a duloxetine clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2013;43:217–221. doi: 10.1016/j.pnpbp.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Balon R, Pohl R, Yeragani V, Rainey J, Oxenkrug GF. Platelet serotonin levels in panic disorder. Acta Psychiatr Scand. 1987;75:315–317. doi: 10.1111/j.1600-0447.1987.tb02794.x. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Alvarez Tichauer G, Spath C, Broocks A, Hajak G, Bleich S, Ruther E. Separation anxiety and actual separation experiences during childhood in patients with panic disorder. Can J Psychiatry. 2001;46:948–952. doi: 10.1177/070674370104601007. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Baldwin D, Abelli M, Altamura C, Dell’Osso B, Domschke K, Fineberg N, Grünblatt E, Jarema M, Maron E, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part I: neuroimaging and genetics. World J Biol Psychiatry. 2016 doi: 10.1080/15622975.2016.1181783. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Domschke K. Panic disorder. In: Stein D, Vythilingum B, editors. Anxiety disorders and gender. New York: Springer; 2015. [Google Scholar]
- Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17:327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Sengos G, Wedekind D, Huether G, Pilz J, Broocks A, Hajak G, Ruther E. Urinary excretion of cortisol, norepinephrine, testosterone, and melatonin in panic disorder. Pharmacopsychiatry. 1997;30:113–117. doi: 10.1055/s-2007-979494. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Wedekind D. Angst-neurobiologie. In: Förstl H, Hautzinger M, Roth G, editors. Neurobiologie psychischer Störungen. Heidelberg, Berlin: Springer; 2006. pp. 483–522. [Google Scholar]
- Bandelow B, Wedekind D. Possible role of a dysregulation of the endogenous opioid system in antisocial personality disorder. Hum Psychopharmacol. 2015;30:393–415. doi: 10.1002/hup.2497. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Wedekind D, Pauls J, Broocks A, Hajak G, Ruther E. Salivary cortisol in panic attacks. Am J Psychiatry. 2000;157:454–456. doi: 10.1176/appi.ajp.157.3.454. [DOI] [PubMed] [Google Scholar]
- Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL. Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur Heart J. 2008;29:2212–2217. doi: 10.1093/eurheartj/ehn326. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Harris J, Spatola CA, Pesenti-Gritti P, Reichborn-Kjennerud T, Torgersen S, Kringlen E, Tambs K. A genetic study of the acute anxious response to carbon dioxide stimulation in man. J Psychiatr Res. 2007;41:906–917. doi: 10.1016/j.jpsychires.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Pesenti-Gritti P, Spatola CA, Ogliari A, Tambs K. A twin study of the common vulnerability between heightened sensitivity to hypercapnia and panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:586–593. doi: 10.1002/ajmg.b.30647. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Aldridge JW. Decision utility, the brain, and pursuit of hedonic goals. Soc Cogn. 2008;26:621–646. doi: 10.1521/soco.2008.26.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R, Garattini S, Delgado R, Ghezzi P. Pharmacological activities of chlorpromazine involved in the inhibition of tumour necrosis factor production in vivo in mice. Immunology. 1993;79:217–219. [PMC free article] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Black DW, Kelly M, Myers C, Noyes R., Jr Tritiated imipramine binding in obsessive-compulsive volunteers and psychiatrically normal controls. Biol Psychiatry. 1990;27:319–327. doi: 10.1016/0006-3223(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Black JL, Lamke GT, Walikonis JE. Serologic survey of adult patients with obsessive-compulsive disorder for neuron-specific and other autoantibodies. Psychiatry Res. 1998;81:371–380. doi: 10.1016/s0165-1781(98)00120-6. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neurosci Biobehav Rev. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- Bonini F, Burle B, Liegeois-Chauvel C, Regis J, Chauvel P, Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343:888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Bonne O, Bain E, Neumeister A, Nugent AC, Vythilingam M, Carson RE, Luckenbaugh DA, Eckelman W, Herscovitch P, Drevets WC, et al. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162:383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- Booij L, Swenne CA, Brosschot JF, Haffmans PM, Thayer JF, Van der Does AJ. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biol Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Boulenger JP, Uhde TW. Biological peripheral correlates of anxiety. Encephale. 1982;8:119–130. [PubMed] [Google Scholar]
- Bourin M, Dailly E. Cholecystokinin and panic disorder. Acta Neuropsychiatr. 2004;16:85–93. doi: 10.1111/j.1601-5215.2004.0076.x. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol Behav. 2012;107:699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Bourin M. Dose ranging study of the effects of cholecystokinin in healthy volunteers. J Psychiatry Neurosci. 1991a;16:91–95. [PMC free article] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, du Tertre AC, Bourin M, Palmour R, Ervin F. The cholecystokinin hypothesis of panic and anxiety disorders: a review. J Psychopharmacol (Oxford) 1992;6:345–351. doi: 10.1177/026988119200600301. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Koszycki D, Shriqui C. Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Clinical and behavioral findings. Arch Gen Psychiatry. 1991b;48:603–610. doi: 10.1001/archpsyc.1991.01810310021005. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Arancio C, Bertani A, Perini G, Carraro C, Gava F. Noradrenergic receptor sensitivity in obsessive compulsive disorder: II. cortisol response to acute clonidine administration. Psychiatry Res. 1997a;69:163–168. doi: 10.1016/s0165-1781(96)03009-0. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Battaglia M, Sciuto G, Diaferia G, Petraglia F, Panerai A, Sacerdote P. Psychoimmunoendocrine aspects of panic disorder. Neuropsychobiology. 1992;26:12–22. doi: 10.1159/000118890. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res. 1994;54:135–142. doi: 10.1016/0165-1781(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G, Garberi A, Panerai A, Sacerdote P. Lymphocyte cholecystokinin concentrations in panic disorder. Am J Psychiatry. 1993;150:1111–1113. doi: 10.1176/ajp.150.7.1111. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Perna G, Bellodi L, Arancio C, Bertani A, Perini G, Carraro C, Gava F. Plasma interleukin-1 beta and tumor necrosis factor concentrations in obsessive-compulsive disorders. Biol Psychiatry. 1997b;42:976–981. doi: 10.1016/s0006-3223(96)00495-7. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Perna G, Bussi R, Bellodi L. Dopamine function in obsessive compulsive disorder: cortisol response to acute apomorphine stimulation. Psychoneuroendocrinology. 2000;25:301–310. doi: 10.1016/s0306-4530(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Perna G, Garberi A, Nobile P, Bellodi L. Alpha 2-adrenergic receptor sensitivity in panic disorder: I. GH response to GHRH and clonidine stimulation in panic disorder. Psychoneuroendocrinology. 1995;20:1–9. doi: 10.1016/0306-4530(94)e0021-z. [DOI] [PubMed] [Google Scholar]
- Brand S, Annen H, Holsboer-Trachsler E, Blaser A. Intensive two-day cognitive-behavioral intervention decreases cortisol secretion in soldiers suffering from specific phobia to wear protective mask. J Psychiatr Res. 2011;45:1337–1345. doi: 10.1016/j.jpsychires.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Breznitz S, Ben-Zur H, Berzon Y, Weiss DW, Levitan G, Tarcic N, Lischinsky S, Greenberg A, Levi N, Zinder O. Experimental induction and termination of acute psychological stress in human volunteers: effects on immunological, neuroendocrine, cardiovascular, and psychological parameters. Brain Behav Immun. 1998;12:34–52. doi: 10.1006/brbi.1997.0511. [DOI] [PubMed] [Google Scholar]
- Brodie C, Gelfand EW. Functional nerve growth factor receptors on human B lymphocytes. Interaction with IL-2. J Immunol. 1992;148:3492–3497. [PubMed] [Google Scholar]
- Butler J, O’Halloran A, Leonard BE. The Galway study of panic disorder. II: changes in some peripheral markers of noradrenergic and serotonergic function in DSM III-R panic disorder. J Affect Disord. 1992;26:89–99. doi: 10.1016/0165-0327(92)90039-9. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Malarkey WB, Kiecolt-Glaser JK, Uchino BN, Sgoutas-Emch SA, Sheridan JF, Berntson GG, Glaser R. Heterogeneity in neuroendocrine and immune responses to brief psychological stressors as a function of autonomic cardiac activation. Psychosom Med. 1995;57:154–164. doi: 10.1097/00006842-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Lee MA, Curtis GC, McCann DS. Endocrine and physiological changes during “spontaneous” panic attacks. Psychoneuroendocrinology. 1987;12:321–331. doi: 10.1016/0306-4530(87)90061-8. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Nesse RM. Systemic hormonal and physiological abnormalities in anxiety disorders. Psychoneuroendocrinology. 1988;13:287–307. doi: 10.1016/0306-4530(88)90054-6. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Smith CB, Nesse RM, Hill EM, Hollingsworth PJ, Abelson JA, Hariharan M, Curtis GC. Platelet alpha 2-adrenoreceptors, catecholamines, hemodynamic variables, and anxiety in panic patients and their asymptomatic relatives. Psychosom Med. 1996;58:289–301. doi: 10.1097/00006842-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sheehan DV, Surman OS, Coleman JH, Greenblatt DJ, Heninger GR, Jones KJ, Levine PH, Watkins WD. Neuroendocrine correlates of lactate-induced anxiety and their response to chronic alprazolam therapy. Am J Psychiatry. 1986;143:483–494. doi: 10.1176/ajp.143.4.483. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depress Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Thomas GN, Gale CR, Deary I, Batty GD. Generalized anxiety disorder is associated with metabolic syndrome in the Vietnam experience study. Biol Psychiatry. 2009;66:91–93. doi: 10.1016/j.biopsych.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M. 5-HT2C receptors in psychiatric disorders: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:120–135. doi: 10.1016/j.pnpbp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. doi: 10.3389/fpsyt.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007a;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Blackwell AD, Robbins TW, Sahakian BJ. Motor inhibition and cognitive flexibility in obsessive-compulsive disorder and trichotillomania. Am J Psychiatry. 2006;163:1282–1284. doi: 10.1176/ajp.2006.163.7.1282. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007b;164:335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Jatlow PI. Increased anxiogenic effects of caffeine in panic disorders. Arch Gen Psychiatry. 1985;42:233–243. doi: 10.1001/archpsyc.1985.01790260027003. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Charney DS, Woods SW, Goodman WK, Heninger GR. Neurobiological mechanisms of panic anxiety: biochemical and behavioral correlates of yohimbine-induced panic attacks. Am J Psychiatry. 1987;144:1030–1036. doi: 10.1176/ajp.144.8.1030. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Sunitha TA, Velayudhan A, Khanna S. An investigation into the psychobiology of social phobia: personality domains and serotonergic function. Acta Psychiatrica Scandinavica. 1997;95:544–550. doi: 10.1111/j.1600-0447.1997.tb10144.x. [DOI] [PubMed] [Google Scholar]
- Chaudieu I, Beluche I, Norton J, Boulenger JP, Ritchie K, Ancelin ML. Abnormal reactions to environmental stress in elderly persons with anxiety disorders: evidence from a population study of diurnal cortisol changes. J Affect Disord. 2008;106:307–313. doi: 10.1016/j.jad.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Chebib M, Johnston GA. The ’ABC’ of GABA receptors: a brief review. Clin Exp Pharmacol Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- Chelli B, Pini S, Abelli M, Cardini A, Lari L, Muti M, Gesi C, Cassano GB, Lucacchini A, Martini C. Platelet 18 kDa translocator protein density is reduced in depressed patients with adult separation anxiety. Eur Neuropsychopharmacol. 2008;18:249–254. doi: 10.1016/j.euroneuro.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. spector lecture. Ann NY Acad Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Church AJ, Dale RC, Giovannoni G. Anti-basal ganglia antibodies: a possible diagnostic utility in idiopathic movement disorders? Arch Dis Child. 2004;89:611–614. doi: 10.1136/adc.2003.031880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Alleva E. The NGF saga: from animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol. 2009;30:379–395. doi: 10.1016/j.yfrne.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Clark CR, Galletly CA, Ash DJ, Moores KA, Penrose RA, McFarlane AC. Evidence-based medicine evaluation of electrophysiological studies of the anxiety disorders. Clin EEG Neurosci. 2009;40:84–112. doi: 10.1177/155005940904000208. [DOI] [PubMed] [Google Scholar]
- Cohn JB, Wilcox CS, Meltzer HY. Neuroendocrine effects of buspirone in patients with generalized anxiety disorder. Am J Med. 1986;80:36–40. doi: 10.1016/0002-9343(86)90330-x. [DOI] [PubMed] [Google Scholar]
- Condren RM, O’Neill A, Ryan MC, Barrett P, Thakore JH. HPA axis response to a psychological stressor in generalised social phobia. Psychoneuroendocrinology. 2002;27:693–703. doi: 10.1016/s0306-4530(01)00070-1. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med. 2012;42:2641–2650. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Goetz R, Klein DF, Papp LA, Fyer AJ, Liebowitz MR, Davies SO, Gorman JM. Plasma cortisol concentrations preceding lactate-induced panic. Psychological, biochemical, and physiological correlates. Arch Gen Psychiatry. 1998;55:130–136. doi: 10.1001/archpsyc.55.2.130. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Moreau D, Chaput F, Martinez JM, Hoven CW, Mandell DJ, Gorman JM, Pine DS. Salivary cortisol concentrations before and after carbon-dioxide inhalations in children. Biological Psychiatry. 2002;51:326–333. doi: 10.1016/s0006-3223(01)01250-1. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Pine DS, Papp LA, Gorman JM. A view on noradrenergic, hypothalamic-pituitary-adrenal axis and extrahypothalamic corticotrophin-releasing factor function in anxiety and affective disorders: the reduced growth hormone response to clonidine. Psychopharmacol Bull. 1997;33:193–204. [PubMed] [Google Scholar]
- Coplan JD, Tamir H, Calaprice D, DeJesus M, de la Nuez M, Pine D, Papp LA, Klein DF, Gorman JM. Plasma anti-serotonin and serotonin anti-idiotypic antibodies are elevated in panic disorder. Neuropsychopharmacology. 1999;20:386–391. doi: 10.1016/S0893-133X(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Corchs F, Nutt DJ, Hince DA, Davies SJ, Bernik M, Hood SD. Evidence for serotonin function as a neurochemical difference between fear and anxiety disorders in humans? J Psychopharmacol (Oxford) 2015;29:1061–1069. doi: 10.1177/0269881115590603. [DOI] [PubMed] [Google Scholar]
- Coryell W, Rickels H. Effects of escitalopram on anxiety and respiratory responses to carbon dioxide inhalation in subjects at high risk for panic disorder: a placebo-controlled, crossover study. J Clin Psychopharmacol. 2009;29:174–178. doi: 10.1097/JCP.0b013e31819a8d96. [DOI] [PubMed] [Google Scholar]
- Costa B, Pini S, Abelli M, Gabelloni P, Da Pozzo E, Chelli B, Calugi S, Lari L, Cardini A, Lucacchini A, et al. Role of translocator protein (18 kDa) in adult separation anxiety and attachment style in patients with depression. Curr Mol Med. 2012;12:483–487. [PubMed] [Google Scholar]
- Coupland NY, Bailey JE, Potokar JP. Abnormal cardioascular responses to standing in panic disorder and social phobia. J Psychopharmacol. 1995;9:A73. [Google Scholar]
- Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. 2016;37:104–129. doi: 10.1016/j.janxdis.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Dale RC, Candler PM, Church AJ, Wait R, Pocock JM, Giovannoni G. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2006;172:187–197. doi: 10.1016/j.jneuroim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Bjerkeset O, Nutt DJ, Lewis G. A U-shaped relationship between systolic blood pressure and panic symptoms: the HUNT study. Psychol Med. 2012;42:1969–1976. doi: 10.1017/S0033291711003047. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Ghahramani P, Jackson PR, Noble TW, Hardy PG, Hippisley-Cox J, Yeo WW, Ramsay LE. Association of panic disorder and panic attacks with hypertension. Am J Med. 1999;107:310–316. doi: 10.1016/s0002-9343(99)00237-5. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Hood SD, Argyropoulos SV, Morris K, Bell C, Witchel HJ, Jackson PR, Nutt DJ, Potokar JP. Depleting serotonin enhances both cardiovascular and psychological stress reactivity in recovered patients with anxiety disorders. J Clin Psychopharmacol. 2006;26:414–418. doi: 10.1097/01.jcp.0000227704.79740.c0. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Lowry CA, Nutt DJ. Panic and hypertension: brothers in arms through 5-HT? J Psychopharmacol (Oxford) 2007;21:563–566. doi: 10.1177/0269881107082359. [DOI] [PubMed] [Google Scholar]
- Davies SJC, Allgulander C. Anxiety and cardiovascular disease. In: Baldwin DS, Lennard BE, editors. Modern trends in pharmacopsychiatry: anxiety disorders. Vol. 29. Basel, Switzerland: Karger Publishers; 2013. pp. 85–97. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Koning PP, Figee M, Endert E, Storosum JG, Fliers E, Denys D. Deep brain stimulation for obsessive-compulsive disorder is associated with cortisol changes. Psychoneuroendocrinology. 2013;38:1455–1459. doi: 10.1016/j.psyneuen.2012.12.006. [DOI] [PubMed] [Google Scholar]
- de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 1989;46:511–517. doi: 10.1001/archpsyc.1989.01810060031006. [DOI] [PubMed] [Google Scholar]
- de Oliveira DC, Chagas MH, Garcia LV, Crippa JA, Zuardi AW. Oxytocin interference in the effects induced by inhalation of 7.5% CO(2) in healthy volunteers. Hum Psychopharmacol. 2012;27:378–385. doi: 10.1002/hup.2237. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proc Nat Acad Sci USA. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- de Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, van Balkom AJ, Veltman DJ, van den Heuvel OA. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169:1100–1108. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- Deakin J. The origins of ’5-HT and mechanisms of defence’ by Deakin and Graeff: a personal perspective. J Psychopharmacol (Oxford) 2013;27:1084–1089. doi: 10.1177/0269881113503508. [DOI] [PubMed] [Google Scholar]
- Deakin JWF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacology. 1991;5:305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis risk in communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- Del Boca C, Lutz PE, Le Merrer J, Koebel P, Kieffer BL. Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience. 2012;218:185–195. doi: 10.1016/j.neuroscience.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Delorme R, Chabane N, Callebert J, Falissard B, Mouren-Simeoni MC, Rouillon F, Launay JM, Leboyer M. Platelet serotonergic predictors of clinical improvement in obsessive compulsive disorder. J Clin Psychopharmacol. 2004;24:18–23. doi: 10.1097/01.jcp.0000104905.75206.50. [DOI] [PubMed] [Google Scholar]
- Delorme R, Gousse V, Roy I, Trandafir A, Mathieu F, Mouren-Simeoni MC, Betancur C, Leboyer M. Shared executive dysfunctions in unaffected relatives of patients with autism and obsessive-compulsive disorder. Eur Psychiatry. 2007;22:32–38. doi: 10.1016/j.eurpsy.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralay C, Jahn H, Kellner M, Yassouridis A, Wiedemann K. Differential effects to CCK-4-induced panic by dexamethasone and hydrocortisone. World J Biol Psychiatry. 2012;13:526–534. doi: 10.3109/15622975.2011.604351. [DOI] [PubMed] [Google Scholar]
- Den Boer JA, Westenberg HG, Klompmakers AA, van Lint LE. Behavioral biochemical and neuroendocrine concomitants of lactate-induced panic anxiety. Biol Psychiatry. 1989;26:612–622. doi: 10.1016/0006-3223(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Denys D, Fluitman S, Kavelaars A, Heijnen C, Westenberg H. Decreased TNF-alpha and NK activity in obsessive-compulsive disorder. Psychoneuroendocrinology. 2004;29:945–952. doi: 10.1016/j.psyneuen.2003.08.008. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Ware MR, Emmanuel NP, Brawman-Mintzer O, Morton WA, Villarreal G, Lydiard RB. Evaluation of the efficacy, safety and physiological effects of fluvoxamine in social phobia. Int Clin Psychopharmacol. 1999;14:345–351. doi: 10.1097/00004850-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Diaper A, Osman-Hicks V, Rich AS, Craig K, Dourish CT, Dawson GR, Nutt DJ, Bailey JE. Evaluation of the effects of venlafaxine and pregabalin on the carbon dioxide inhalation models of generalised anxiety disorder and panic. J Psychopharmacol (Oxford) 2013;27:135–145. doi: 10.1177/0269881112443742. [DOI] [PubMed] [Google Scholar]
- Diaper A, Papadopoulos A, Rich AS, Dawson GR, Dourish CT, Nutt DJ, Bailey JE. The effect of a clinically effective and non-effective dose of lorazepam on 7.5% CO2-induced anxiety. Hum Psychopharmacol. 2012;27:540–548. doi: 10.1002/hup.2261. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, Verhulst FC. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology. 2015;51:135–150. doi: 10.1016/j.psyneuen.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Dieler AC, Samann PG, Leicht G, Eser D, Kirsch V, Baghai TC, Karch S, Schule C, Pogarell O, Czisch M, et al. Independent component analysis applied to pharmacological magnetic resonance imaging (phMRI): new insights into the functional networks underlying panic attacks as induced by CCK-4. Curr Pharm Des. 2008;14:3492–3507. doi: 10.2174/138161208786848801. [DOI] [PubMed] [Google Scholar]
- Domschke K, Zwanzger P. GABAergic and endocannabinoid dysfunction in anxiety - future therapeutic targets? Curr Pharm Des. 2008;14:3508–3517. doi: 10.2174/138161208786848784. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Curr Top Behav Neurosci. 2014;18:191–216. doi: 10.1007/7854_2014_299. [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia. 2008;46:1877–1887. doi: 10.1016/j.neuropsychologia.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, Kathmann N. Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biol Psychol. 2010;84:257–263. doi: 10.1016/j.biopsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Endrass T, Ullsperger M. Specificity of performance monitoring changes in obsessive-compulsive disorder. Neurosci Biobehav Rev. 2014;46(Pt 1):124–138. doi: 10.1016/j.neubiorev.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Ising M, Unschuld PG, Kern N, Lucae S, Putz B, Uhr M, Binder EB, Holsboer F, Keck ME. Regulation of the hypothalamic-pituitary-adrenocortical system in patients with panic disorder. Neuropsychopharmacology. 2006;31:2515–2522. doi: 10.1038/sj.npp.1301168. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Westberg P, Alling C, Thuresson K, Modigh K. Cerebrospinal fluid levels of monoamine metabolites in panic disorder. Psychiatry Res. 1991;36:243–251. doi: 10.1016/0165-1781(91)90023-i. [DOI] [PubMed] [Google Scholar]
- Eser D, Leicht G, Lutz J, Wenninger S, Kirsch V, Schule C, Karch S, Baghai T, Pogarell O, Born C, et al. Functional neuroanatomy of CCK-4-induced panic attacks in healthy volunteers. Hum Brain Mapp. 2009;30:511–522. doi: 10.1002/hbm.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Schule C, Baghai T, Floesser A, Krebs-Brown A, Enunwa M, de la Motte S, Engel R, Kucher K, Rupprecht R. Evaluation of the CCK-4 model as a challenge paradigm in a population of healthy volunteers within a proof-of-concept study. Psychopharmacology (Berl) 2007;192:479–487. doi: 10.1007/s00213-007-0738-7. [DOI] [PubMed] [Google Scholar]
- Eser D, Wenninger S, Baghai T, Schule C, Rupprecht R. Impact of state and trait anxiety on the panic response to CCK-4. J Neural Transm (Vienna) 2008;115:917–920. doi: 10.1007/s00702-008-0047-2. [DOI] [PubMed] [Google Scholar]
- Esler M, Alvarenga M, Lambert G, Kaye D, Hastings J, Jennings G, Morris M, Schwarz R, Richards J. Cardiac sympathetic nerve biology and brain monoamine turnover in panic disorder. Ann NY Acad Sci. 2004;1018:505–514. doi: 10.1196/annals.1296.062. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction? Am J Hypertens. 2001;14:139S–146S. doi: 10.1016/s0895-7061(01)02081-7. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am J Psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L, Schneider P, Ross-Lee L, Wiltshire B, Eadie M, Kenardy J, Hoey H. Plasma serotonin levels in agoraphobia. Am J Psychiatry. 1985;142:267. doi: 10.1176/ajp.142.2.267a. [DOI] [PubMed] [Google Scholar]
- Everly GS, Horton AM., Jr Neuropsychology of posttraumatic stress disorder: a pilot study. Percept Mot Skills. 1989;68:807–810. doi: 10.2466/pms.1989.68.3.807. [DOI] [PubMed] [Google Scholar]
- Ewing DJ, Neilson JM, Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Br Heart J. 1984;52:396–402. doi: 10.1136/hrt.52.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Kitayama N, Ashraf A, Reed L, Afzal N, Jawed F, Bremner JD. Neuropsychological functioning in patients with posttraumatic stress disorder following short-term paroxetine treatment. Psychopharmacol Bull. 2009;42:53–68. [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, Godini L, Benni L, Fioravanti G, Talamba GA, Castellini G, et al. The role of life events and HPA axis in anxiety disorders: a review. Curr Pharm Des. 2012;18:5663–5674. doi: 10.2174/138161212803530907. [DOI] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA. Fear and panic in humans with bilateral amygdala damage. Nat Neurosci. 2013;16:270–272. doi: 10.1038/nn.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner CG, O’Connor FL, Yeoh HC, Arora RC, Crayton JW. Hypodensity of platelet serotonin uptake sites in posttraumatic stress disorder: associated clinical features. Life Sci. 1995;57:PL37–PL44. doi: 10.1016/0024-3205(95)00262-5. [DOI] [PubMed] [Google Scholar]
- Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P, Schuurman R, van Wingen G, van Amelsvoort T, Booij J, et al. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Flament MF, Rapoport JL, Berg CJ, Sceery W, Kilts C, Mellstrom B, Linnoila M. Clomipramine treatment of childhood obsessive-compulsive disorder. A double-blind controlled study. Arch Gen Psychiatry. 1985;42:977–983. doi: 10.1001/archpsyc.1985.01790330057007. [DOI] [PubMed] [Google Scholar]
- Fluitman SB, Denys DA, Heijnen CJ, Westenberg HG. Disgust affects TNF-alpha, IL-6 and noradrenalin levels in patients with obsessive-compulsive disorder. Psychoneuroendocrinology. 2010;35:906–911. doi: 10.1016/j.psyneuen.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Foldager L, Kohler O, Steffensen R, Thiel S, Kristensen AS, Jensenius JC, Mors O. Bipolar and panic disorders may be associated with hereditary defects in the innate immune system. J Affect Disord. 2014;164:148–154. doi: 10.1016/j.jad.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Franklin RA, Brodie C, Melamed I, Terada N, Lucas JJ, Gelfand EW. Nerve growth factor induces activation of MAP-kinase and p90rsk in human B lymphocytes. J Immunol. 1995;154:4965–4972. [PubMed] [Google Scholar]
- Fredrikson M, Sundin O, Frankenhaeuser M. Cortisol excretion during the defense reaction in humans. Psychosom Med. 1985;47:313–319. doi: 10.1097/00006842-198507000-00001. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Yasuno F, Farris A, Liow JS, Geraci M, Drevets W, Pine DS, Ghose S, Lerner A, Hargreaves R, et al. Decreased neurokinin-1 (substance P) receptor binding in patients with panic disorder: positron emission tomographic study with [18F]SPA-RQ. Biol Psychiatry. 2009;66:94–97. doi: 10.1016/j.biopsych.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara A, Yoshida T, Otsuka T, Hayano F, Asami T, Narita H, Nakamura M, Inoue T, Hirayasu Y. Midbrain volume increase in patients with panic disorder. Psychiatry Clin Neurosci. 2011;65:365–373. doi: 10.1111/j.1440-1819.2011.02219.x. [DOI] [PubMed] [Google Scholar]
- Furlan PM, DeMartinis N, Schweizer E, Rickels K, Lucki I. Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biol Psychiatry. 2001;50:254–259. doi: 10.1016/s0006-3223(00)01126-4. [DOI] [PubMed] [Google Scholar]
- Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, Gorman JM. Effect of medication and psychotherapy on heart rate variability in panic disorder. Depress Anxiety. 2009;26:251–258. doi: 10.1002/da.20533. [DOI] [PubMed] [Google Scholar]
- Garner M, Attwood A, Baldwin DS, James A, Munafo MR. Inhalation of 7.5% carbon dioxide increases threat processing in humans. Neuropsychopharmacology. 2011;36:1557–1562. doi: 10.1038/npp.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M, Attwood A, Baldwin DS, Munafo MR. Inhalation of 7.5% carbon dioxide increases alerting and orienting attention network function. Psychopharmacology (Berl) 2012;223:67–73. doi: 10.1007/s00213-012-2690-4. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, Witowski ME, Dubbert B, Swedo SE. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry. 1999;45:1564–1571. doi: 10.1016/s0006-3223(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Gehring W, Coles MGH, Meyer DE, Donchin E. The error-related negativity: an event-related potential accompanying errors. Psychophysiology. 1990;27:S34. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- George MS, Ward HE, Jr, Ninan PT, Pollack M, Nahas Z, Anderson B, Kose S, Howland RH, Goodman WK, Ballenger JC. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul. 2008;1:112–121. doi: 10.1016/j.brs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Jefferson-Wilson L, Strawn JR, Baker DG, Dashevsky BA, Horn PS, Ekhator NN. Effect of traumatic imagery on cerebrospinal fluid dopamine and serotonin metabolites in posttraumatic stress disorder. J Psychiatr Res. 2013;47:995–998. doi: 10.1016/j.jpsychires.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M, Reali N, Bernasconi S, Brambilla F. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology. 2000;42:82–92. doi: 10.1159/000026677. [DOI] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- Gillan CM, Apergis-Schoute AM, Morein-Zamir S, Urcelay GP, Sule A, Fineberg NA, Sahakian BJ, Robbins TW. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry. 2015;172:284–293. doi: 10.1176/appi.ajp.2014.14040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Morein-Zamir S, Urcelay GP, Sule A, Voon V, Apergis-Schoute AM, Fineberg NA, Sahakian BJ, Robbins TW. Enhanced avoidance habits in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:631–638. doi: 10.1016/j.biopsych.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, de Wit S. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Robbins TW. Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc London B: Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0475. 20130475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Sholomskas DE, Walton KE, Augeri FM, Charney DS, Heninger GR, Goodman WK, Price LH. Effects of tryptophan depletion in panic disorder. Biol Psychiatry. 1994;36:775–777. doi: 10.1016/0006-3223(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Goetz RR, Gorman JM, Dillon DJ, Papp LA, Hollander E, Fyer AJ, Liebowitz MR, Klein DF. Do panic disorder patients indiscriminately endorse somatic complaints? Psychiatry Res. 1989;29:207–213. doi: 10.1016/0165-1781(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Goossens L, Leibold N, Peeters R, Esquivel G, Knuts I, Backes W, Marcelis M, Hofman P, Griez E, Schruers K. Brainstem response to hypercapnia: a symptom provocation study into the pathophysiology of panic disorder. J Psychopharmacol (Oxford) 2014;28:449–456. doi: 10.1177/0269881114527363. [DOI] [PubMed] [Google Scholar]
- Gordon E, Palmer DM, Cooper N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin EEG Neurosci. 2010;41:178–183. doi: 10.1177/155005941004100404. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM, Klein DF. Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry. 1984;141:857–861. doi: 10.1176/ajp.141.7.857. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Battista D, Goetz RR, Dillon DJ, Liebowitz MR, Fyer AJ, Kahn JP, Sandberg D, Klein DF. A comparison of sodium bicarbonate and sodium lactate infusion in the induction of panic attacks. Arch Gen Psychiatry. 1989;46:145–150. doi: 10.1001/archpsyc.1989.01810020047008. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ, Kinney J, Klein DF. Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry. 1988;45:31–39. doi: 10.1001/archpsyc.1988.01800250035006. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biol Psychiatry. 2004;56:862–867. doi: 10.1016/j.biopsych.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. Am Heart J. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Zangrossi H., Jr The dual role of serotonin in defense and the mode of action of antidepressants on generalized anxiety and panic disorders. Cent Nerv Syst Agents Med Chem. 2010;10:207–217. doi: 10.2174/1871524911006030207. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR. A cognitive comparison of pathological skin picking and trichotillomania. J Psychiatr Res. 2011;45:1634–1638. doi: 10.1016/j.jpsychires.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Grant JE, Redden SA, Leppink EW, Odlaug BL. Skin picking disorder with co-occurring body dysmorphic disorder. Body Image. 2015;15:44–48. doi: 10.1016/j.bodyim.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Gray J, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of septo-hippocampal system. 2nd ed. Oxford (UK): Oxford University Press; 2000. p. 440. [Google Scholar]
- Griez EJ, Lousberg H, van den Hout MA, van der Molen GM. CO2 vulnerability in panic disorder. Psychiatry Res. 1987;20:87–95. doi: 10.1016/0165-1781(87)90001-1. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Endrass T, Kaufmann C, Allen E, Eichele T, Kathmann N. Presupplementary motor area contributes to altered error monitoring in obsessive-compulsive disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.12.010. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Blakeley JE, Antai-Otong D, Vo SP, Orsulak PJ, Petty F, Rush AJ. Adrenergic receptor function in panic disorder II. Neutrophil beta 2 receptors: Gs protein coupling, effects of imipramine treatment and relationship to treatment outcome. J Psychiatr Res. 1999;33:309–322. doi: 10.1016/s0022-3956(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Ivarsson T, Nelson N. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. 2008;57:14–21. doi: 10.1159/000123117. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, Simons RF. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. Am J Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Carrasco M, Harbin SM, Nienhuis JK, LaRosa CE, Chen P, Fitzgerald KD, Gehring WJ. Error-related negativity and tic history in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:902–910. doi: 10.1016/j.jaac.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna GL, Yuwiler A, Cantwell DP. Whole blood serotonin in juvenile obsessive-compulsive disorder. Biol Psychiatry. 1991;29:738–744. doi: 10.1016/0006-3223(91)90193-p. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJ, Mulder CL, Tiemeier H. Anxiety disorders and salivary cortisol levels in older adults: a population-based study. Psychoneuroendocrinology. 2013;38:300–305. doi: 10.1016/j.psyneuen.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Charney DS. Monoamine receptor systems and anxiety disorders. Psychiatr Clin North Am. 1988;11:309–326. [PubMed] [Google Scholar]
- Hernandez E, Lastra S, Urbina M, Carreira I, Lima L. Serotonin, 5-hydroxyindoleacetic acid and serotonin transporter in blood peripheral lymphocytes of patients with generalized anxiety disorder. Int Immunopharmacol. 2002;2:893–900. doi: 10.1016/s1567-5769(02)00025-5. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Hollander E, DelGiudice-Asch G, Simon L, Schmeidler J, Cartwright C, DeCaria CM, Kwon J, Cunningham-Rundles C, Chapman F, Zabriskie JB. B lymphocyte antigen D8/17 and repetitive behaviors in autism. Am J Psychiatry. 1999;156:317–320. doi: 10.1176/ajp.156.2.317. [DOI] [PubMed] [Google Scholar]
- Hollander E, Kwon J, Weiller F, Cohen L, Stein DJ, DeCaria C, Liebowitz M, Simeon D. Serotonergic function in social phobia: comparison to normal control and obsessive-compulsive disorder subjects. Psychiatry Res. 1998;79:213–217. doi: 10.1016/s0165-1781(98)00041-9. [DOI] [PubMed] [Google Scholar]
- Holsboer F, von Bardeleben U, Buller R, Heuser I, Steiger A. Stimulation response to corticotropin-releasing hormone (CRH) in patients with depression, alcoholism and panic disorder. Horm Metab Res Suppl. 1987;16:80–88. [PubMed] [Google Scholar]
- Hood SD, Bell CJ, Nutt DJ. Acute tryptophan depletion. Part I: rationale and methodology. Aust NZ J Psychiatry. 2005;39:558–564. doi: 10.1080/j.1440-1614.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- Hood SD, Melichar JK, Taylor LG, Kalk N, Edwards TR, Hince DA, Lenox-Smith A, Lingford-Hughes AR, Nutt DJ. Noradrenergic function in generalized anxiety disorder: impact of treatment with venlafaxine on the physiological and psychological responses to clonidine challenge. J Psychopharmacol (Oxford) 2011;25:78–86. doi: 10.1177/0269881109359099. [DOI] [PubMed] [Google Scholar]
- Hood SD, Potokar JP, Davies SJ, Hince DA, Morris K, Seddon KM, Nutt DJ, Argyropoulos SV. Dopaminergic challenges in social anxiety disorder: evidence for dopamine D3 desensitisation following successful treatment with serotonergic antidepressants. J Psychopharmacol (Oxford) 2010;24:709–716. doi: 10.1177/0269881108098144. [DOI] [PubMed] [Google Scholar]
- Hounie AG, Cappi C, Cordeiro Q, Sampaio AS, Moraes I, Rosario MC, Palacios SA, Goldberg AC, Vallada HP, Machado-Lima A, et al. TNF-alpha polymorphisms are associated with obsessive-compulsive disorder. Neurosci Lett. 2008;442:86–90. doi: 10.1016/j.neulet.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Humble M, Bejerot S, Bergqvist PB, Bengtsson F. Reactivity of serotonin in whole blood: relationship with drug response in obsessive-compulsive disorder. Biol Psychiatry. 2001;49:360–368. doi: 10.1016/s0006-3223(00)00956-2. [DOI] [PubMed] [Google Scholar]
- Humble M, Wistedt B. Serotonin, panic disorder and agoraphobia: short-term and long-term efficacy of citalopram in panic disorders. Int Clin Psychopharmacol. 1992;6:21–39. [PubMed] [Google Scholar]
- Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC., Jr Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Charney DS, Heninger GR. Differential 3H-imipramine platelet binding in patients with panic disorder and depression. Psychiatry Res. 1987;21:33–41. doi: 10.1016/0165-1781(87)90059-x. [DOI] [PubMed] [Google Scholar]
- Iny LJ, Pecknold J, Suranyi Cadotte BE, Bernier B, Luthe L, Nair NP, Meaney MJ. Studies of a neurochemical link between depression, anxiety, and stress from [3H]imipramine and [3H]paroxetine binding on human platelets. Biol Psychiatry. 1994;36:281–291. doi: 10.1016/0006-3223(94)90625-4. [DOI] [PubMed] [Google Scholar]
- Ischebeck M, Endrass T, Simon D, Kathmann N. Altered frontal EEG asymmetry in obsessive-compulsive disorder. Psychophysiology. 2014;51:596–601. doi: 10.1111/psyp.12214. [DOI] [PubMed] [Google Scholar]
- Ising M, Hohne N, Siebertz A, Parchmann AM, Erhardt A, Keck M. Stress response regulation in panic disorder. Curr Pharm Des. 2012;18:5675–5684. doi: 10.2174/138161212803530880. [DOI] [PubMed] [Google Scholar]
- Isingrini E, Perret L, Rainer Q, Amilhon B, Guma E, Tanti A, Martin G, Robinson J, Moquin L, Marti F, et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci. 2016;19:560–563. doi: 10.1038/nn.4245. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis D, Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. Am J Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Zubraegel D, Baer T, Linden M, Danker-Hopfe H, Schulte-Herbruggen O, Neu P, Hellweg R. Nerve growth factor serum concentrations rise after successful cognitive-behavioural therapy of generalized anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:200–204. doi: 10.1016/j.pnpbp.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Lydiard RB, Zealberg JJ, Fossey MD, Ballenger JC. Plasma and CSF HVA levels in panic patients with comorbid social phobia. Biol Psychiatry. 1994;36:425–427. doi: 10.1016/0006-3223(94)91217-3. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 2012;37:1911–1922. doi: 10.1038/npp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Rickman M, Davidson RJ. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav Neurosci. 1998;112:251–254. doi: 10.1037//0735-7044.112.1.251. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kang Y. Psychological stress-induced changes in salivary alpha-amylase and adrenergic activity. Nurs Health Sci. 2010;12:477–484. doi: 10.1111/j.1442-2018.2010.00562.x. [DOI] [PubMed] [Google Scholar]
- Kansy JW, Katsovich L, McIver KS, Pick J, Zabriskie JB, Lombroso PJ, Leckman JF, Bibb JA. Identification of pyruvate kinase as an antigen associated with Tourette syndrome. J Neuroimmunol. 2006;181:165–176. doi: 10.1016/j.jneuroim.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman MA, Koszycki D, Bradwejn J. Effects of CCK-tet-rapeptide in patients with social phobia and obsessive-compulsive disorder. Depress Anxiety. 2004;20:51–58. doi: 10.1002/da.20012. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89:1992–1997. doi: 10.1161/01.cir.89.5.1992. [DOI] [PubMed] [Google Scholar]
- Kawano A, Tanaka Y, Ishitobi Y, Maruyama Y, Ando T, Inoue A, Okamoto S, Imanaga J, Kanehisa M, Higuma H, et al. Salivary alpha-amylase and cortisol responsiveness following electrical stimulation stress in obsessive-compulsive disorder patients. Psychiatry Res. 2013;209:85–90. doi: 10.1016/j.psychres.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J, Nutt D, Lightman S. Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol. 2004;16:256–264. doi: 10.1111/j.0953-8194.2004.01158.x. [DOI] [PubMed] [Google Scholar]
- Kellner M, Herzog L, Yassouridis A, Holsboer F, Wiedemann K. Possible role of atrial natriuretic hormone in pituitary-adrenocortical unresponsiveness in lactate-induced panic. Am J Psychiatry. 1995;152:1365–1367. doi: 10.1176/ajp.152.9.1365. [DOI] [PubMed] [Google Scholar]
- Kellner M, Wiedemann K, Holsboer F. Atrial natriuretic factor inhibits the CRH-stimulated secretion of ACTH and cortisol in man. Life Sci. 1992;50:1835–1842. doi: 10.1016/0024-3205(92)90543-x. [DOI] [PubMed] [Google Scholar]
- Kellner M, Wiedemann K, Yassouridis A, Muhtz C. Non-response of cortisol during stressful exposure therapy in patients with obsessive-compulsive disorder-preliminary results. Psychiatry Res. 2012;199:111–114. doi: 10.1016/j.psychres.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Khanna S, Ravi V, Shenoy PK, Chandramuki A, Channabasavanna SM. Cerebrospinal fluid viral antibodies in obsessive-compulsive disorder in an Indian population. Biol Psychiatry. 1997;41:883–890. doi: 10.1016/S0006-3223(96)00174-6. [DOI] [PubMed] [Google Scholar]
- Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kim YR, Park Q, Yu BH. Changes in lymphocyte subsets after short-term pharmacotherapy in patients with panic disorder. Psychiatry Res. 2004;128:183–190. doi: 10.1016/j.psychres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Klawohn J, Riesel A, Grutzmann R, Kathmann N, Endrass T. Performance monitoring in obsessive-compulsive disorder: a temporo-spatial principal component analysis. Cogn Affect Behav Neurosci. 2014;14:983–995. doi: 10.3758/s13415-014-0248-0. [DOI] [PubMed] [Google Scholar]
- Klein DF. Panic disorder with agoraphobia [letter] Br J Psychiatry. 1993;163:835–837. doi: 10.1192/bjp.163.6.835a. [DOI] [PubMed] [Google Scholar]
- Klein E, Zinder O, Colin V, Zilberman I, Levy N, Greenberg A, Lenox RH. Clinical similarity and biological diversity in the response to alprazolam in patients with panic disorder and generalized anxiety disorder. Acta Psychiatr Scand. 1995;92:399–408. doi: 10.1111/j.1600-0447.1995.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Klein E, Zohar J, Geraci MF, Murphy DL, Uhde TW. Anxiogenic effects of m-CPP in patients with panic disorder: comparison to caffeine’s anxiogenic effects. Biol Psychiatry. 1991;30:973–984. doi: 10.1016/0006-3223(91)90119-7. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schussler P, Kunzel HE, Dresler M, Yassouridis A, Steiger A. Increased nocturnal secretion of ACTH and cortisol in obsessive compulsive disorder. J Psychiatr Res. 2007;41:928–933. doi: 10.1016/j.jpsychires.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Knopf K, Possel P. Individual response differences in spider phobia: comparing phobic and non-phobic women of different reactivity levels. Anxiety Stress Coping. 2009;22:39–55. doi: 10.1080/10615800802169358. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Shimizu E, Hashimoto K, Mitsumori M, Koike K, Okamura N, Koizumi H, Ohgake S, Matsuzawa D, Zhang L, et al. Serum brain-derived neurotrophic factor (BDNF) levels in patients with panic disorder: as a biological predictor of response to group cognitive behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:658–663. doi: 10.1016/j.pnpbp.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Koff WC, Dunegan MA. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J Immunol. 1985;135:350–354. [PubMed] [Google Scholar]
- Koh KB, Lee BK. Reduced lymphocyte proliferation and interleukin-2 production in anxiety disorders. Psychosom Med. 1998;60:479–483. doi: 10.1097/00006842-199807000-00015. [DOI] [PubMed] [Google Scholar]
- Koh KB, Lee Y. Reduced anxiety level by therapeutic interventions and cell-mediated immunity in panic disorder patients. Psychother Psychosom. 2004;73:286–292. doi: 10.1159/000078845. [DOI] [PubMed] [Google Scholar]
- Koprivova J, Congedo M, Horacek J, Prasko J, Raszka M, Brunovsky M, Kohutova B, Hoschl C. EEG source analysis in obsessive-compulsive disorder. Clin Neurophysiol. 2011;122:1735–1743. doi: 10.1016/j.clinph.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacic Z, Henigsberg N, Pivac N, Nedic G, Borovecki A. Platelet serotonin concentration and suicidal behavior in combat related posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:544–551. doi: 10.1016/j.pnpbp.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Kramer M, Seefeldt WL, Heinrichs N, Tuschen-Caffier B, Schmitz J, Wolf OT, Blechert J. Subjective, autonomic, and endocrine reactivity during social stress in children with social phobia. J Abnorm Child Psychol. 2012;40:95–104. doi: 10.1007/s10802-011-9548-9. [DOI] [PubMed] [Google Scholar]
- Kremer HP, Goekoop JG, Van Kempen GM. Clinical use of the determination of serotonin in whole blood. J Clin Psychopharmacol. 1990;10:83–87. doi: 10.1097/00004714-199004000-00002. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Berger P, Tauber RF, Bandelow B, Henkel V, Heuser I. Randomized, double-blind study of SR142801 (Osanetant). A novel neurokinin-3 (NK3) receptor antagonist in panic disorder with pre- and posttreatment cholecystokinin tetrapeptide (CCK-4) challenges. Pharmacopsychiatry. 2005;38:24–29. doi: 10.1055/s-2005-837768. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Cox HS, Vaz M, Turner AG, Jennings GL, Esler MD. Regional 5-hydroxyindoleacetic acid production in humans. Life Sci. 1995;57:255–267. doi: 10.1016/0024-3205(95)00269-c. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Bracci-Laudiero L, Bonini S, Bonini S, Starace G, D’Elios MM, De Carli M, Aloe L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–414. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Bajbouj M, Gaus V, Sander T, Gallinat J. Gender-dependent association of a functional NGF polymorphism with anxiety-related personality traits. Pharmacopsychiatry. 2008;41:196–199. doi: 10.1055/s-0028-1082070. [DOI] [PubMed] [Google Scholar]
- Larson MR, Ader R, Moynihan JA. Heart rate, neuroendocrine, and immunological reactivity in response to an acute laboratory stressor. Psychosom Med. 2001;63:493–501. doi: 10.1097/00006842-200105000-00020. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IS, Kim KJ, Kang EH, Yu BH. beta-adrenoceptor affinity as a biological predictor of treatment response to paroxetine in patients with acute panic disorder. J Affect Disord. 2008;110:156–160. doi: 10.1016/j.jad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Lee SH, Yoon S, Kim JI, Jin SH, Chung CK. Functional connectivity of resting state EEG and symptom severity in patients with post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:51–57. doi: 10.1016/j.pnpbp.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Lehrner A, Yehuda R. Biomarkers of PTSD: military applications and considerations. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23797. 23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold NK, van den Hove DL, Esquivel G, De Cort K, Goossens L, Strackx E, Buchanan GF, Steinbusch HW, Lesch KP, Schruers KR. The brain acid-base homeostasis and serotonin: a perspective on the use of carbon dioxide as human and rodent experimental model of panic. Prog Neurobiol. 2015;129:58–78. doi: 10.1016/j.pneurobio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Leicht G, Mulert C, Eser D, Samann PG, Ertl M, Laenger A, Karch S, Pogarell O, Meindl T, Czisch M, et al. Benzodiazepines counteract rostral anterior cingulate cortex activation induced by cholecystokinin-tetrapeptide in humans. Biol Psychiatry. 2013;73:337–344. doi: 10.1016/j.biopsych.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19:482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130:119–127. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- Levin AP, Doran AR, Liebowitz MR, Fyer AJ, Gorman JM, Klein DF, Paul SM. Pituitary adrenocortical unresponsiveness in lactate-induced panic. Psychiatry Res. 1987;21:23–32. doi: 10.1016/0165-1781(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Levy-Gigi E, Keri S, Myers CE, Lencovsky Z, Sharvit-Benbaji H, Orr SP, Gilbertson MW, Servatius RJ, Tsao JW, Gluck MA. Individuals with posttraumatic stress disorder show a selective deficit in generalization of associative learning. Neuropsychology. 2012;26:758–767. doi: 10.1037/a0029361. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Noyes R, Jr, Coryell W, Clancy J. Tritiated imipramine binding to platelets is decreased in patients with agoraphobia. Psychiatry Res. 1985;16:1–9. doi: 10.1016/0165-1781(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Li H, Ohta H, Izumi H, Matsuda Y, Seki M, Toda T, Akiyama M, Matsushima Y, Goto Y, Kaga M, et al. Behavioral and cortical EEG evaluations confirm the roles of both CCKA and CCKB receptors in mouse CCK-induced anxiety. Behav Brain Res. 2013;237:325–332. doi: 10.1016/j.bbr.2012.09.051. [DOI] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability-the ARIC study. Atherosclerosis risk in communities. Am J Cardiol. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer AJ, Levitt M, Dillon D, Levy G, Appleby IL, Anderson S, Palij M, Davies SO, et al. Lactate provocation of panic attacks. II. Biochemical and physiological findings. Arch Gen Psychiatry. 1985;42:709–719. doi: 10.1001/archpsyc.1985.01790300077010. [DOI] [PubMed] [Google Scholar]
- Lilliecreutz C, Theodorsson E, Sydsjo G, Josefsson A. Salivary cortisol in pregnant women suffering from blood and injection phobia. Arch Womens Ment Health. 2011;14:405–411. doi: 10.1007/s00737-011-0234-2. [DOI] [PubMed] [Google Scholar]
- Lista AL. Differential rates of urinary noradrenalin excretion in affective disorders: utility of a short time sampling procedure. Psychiatry Res. 1989;30:253–258. doi: 10.1016/0165-1781(89)90016-4. [DOI] [PubMed] [Google Scholar]
- Lobo I, Portugal LC, Figueira I, Volchan E, David I, Garcia Pereira M, de Oliveira L. EEG correlates of the severity of posttraumatic stress symptoms: A systematic review of the dimensional PTSD literature. J Affect Disord. 2015;183:210–220. doi: 10.1016/j.jad.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Lopez AL, Kathol RG, Noyes R., Jr Reduction in urinary free cortisol during benzodiazepine treatment of panic disorder. Psychoneuroendocrinology. 1990;15:23–28. doi: 10.1016/0306-4530(90)90043-9. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lydiard RB, Ballenger JC, Laraia MT, Fossey MD, Beinfeld MC. CSF cholecystokinin concentrations in patients with panic disorder and in normal comparison subjects. Am J Psychiatry. 1992;149:691–693. doi: 10.1176/ajp.149.5.691. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E. Psychoimmune investigation in obsessive-compulsive disorder: assays of plasma transferrin, IL-2 and IL-6 receptor, and IL-1 beta and IL-6 concentrations. Neuropsychobiology. 1994;30:57–60. doi: 10.1159/000119136. [DOI] [PubMed] [Google Scholar]
- Maina G, Albert U, Bogetto F, Borghese C, Berro AC, Mutani R, Rossi F, Vigliani MC. Anti-brain antibodies in adult patients with obsessive-compulsive disorder. J Affect Disord. 2009;116:192–200. doi: 10.1016/j.jad.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Front Hum Neurosci. 2013;7:350. doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Hollander E, Lensi P, Ravagli S, Cassano GB. Peripheral markers of serotonin and dopamine function in obsessive-compulsive disorder. Psychiatry Res. 1992;42:41–51. doi: 10.1016/0165-1781(92)90037-4. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Presta S, Pfanner C, Gemignani A, Rossi A, Sbrana S, Rocchi V, Ambrogi F, Cassano GB. Immunological alterations in adult obsessive-compulsive disorder. Biol Psychiatry. 1999;46:810–814. doi: 10.1016/s0006-3223(98)00371-0. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Rossi A, Gemignani A, Giannaccini G, Pfanner C, Milanfranchi A, Presta S, Lucacchini A, Cassano GB. Decreased platelet 3H-paroxetine binding in obsessive-compulsive patients. Neuropsychobiology. 1996;34:184–187. doi: 10.1159/000119308. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Blanco C, Printz D, Liebowitz MR, Klein DF, Coplan J. A pilot study of noradrenergic and HPA axis functioning in PTSD vs. panic disorder. Psychiatry Res. 2002;110:219–230. doi: 10.1016/s0165-1781(02)00126-9. [DOI] [PubMed] [Google Scholar]
- Martel FL, Hayward C, Lyons DM, Sanborn K, Varady S, Schatzberg AF. Salivary cortisol levels in socially phobic adolescent girls. Depress Anxiety. 1999;10:25–27. doi: 10.1002/(sici)1520-6394(1999)10:1<25::aid-da4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin Lab Med. 2010;30:865–891. doi: 10.1016/j.cll.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Masdrakis VG, Markianos M, Oulis P. Lack of specific association between panicogenic properties of caffeine and HPA-axis activation. A placebo-controlled study of caffeine challenge in patients with panic disorder. Psychiatry Res. 2015;229:75–81. doi: 10.1016/j.psychres.2015.07.069. [DOI] [PubMed] [Google Scholar]
- Mason ST, Angel A. Chronic and acute administration of typical and atypical antidepressants on activity of brain noradrenaline systems in the rat thiopentone anaesthesia model. Psychopharmacology (Berl) 1984;84:304–309. doi: 10.1007/BF00555203. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Badner JA, Andresen JM, Sheppard B, Himle JA, Grant JE, Williams KA, Chavira DA, Azzam A, Schwartz M, et al. Genome-wide linkage analysis of obsessive-compulsive disorder implicates chromosome 1p36. Biol Psychiatry. 2012;72:629–636. doi: 10.1016/j.biopsych.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CJ. To stand on one’s own legs. Clin Med (Lond) 2002;2:237–245. doi: 10.7861/clinmedicine.2-3-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre IM, Judd FK, Burrows GD, Norman TR. Serotonin in panic disorder: platelet uptake and concentration. Int Clin Psychopharmacol. 1989;4:1–6. doi: 10.1097/00004850-198901000-00001. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Dispelling confusion about traumatic dissociative amnesia. Mayo Clin Proc. 2007;82:1083–1090. doi: 10.4065/82.9.1083. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Melloni M, Urbistondo C, Sedeno L, Gelormini C, Kichic R, Ibanez A. The extended fronto-striatal model of obsessive compulsive disorder: convergence from event-related potentials, neuropsychology and neuroimaging. Front Hum Neurosci. 2012;6:259. doi: 10.3389/fnhum.2012.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Smeets T, Giesbrecht T, Quaedflieg CW, Smulders FT, Meijer EH, Merckelbach HL. The role of frontal EEG asymmetry in post-traumatic stress disorder. Biol Psychol. 2015;108:62–77. doi: 10.1016/j.biopsycho.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Michelgard A, Appel L, Pissiota A, Frans O, Langstrom B, Bergstrom M, Fredrikson M. Symptom provocation in specific phobia affects the substance P neurokinin-1 receptor system. Biol Psychiatry. 2007;61:1002–1006. doi: 10.1016/j.biopsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, Chacon P, Pauls DL. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. 2005;10:258–275. doi: 10.1038/sj.mp.4001617. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Sutherland AG, Hutchison JD, Alexander DA. C-reactive protein and interleukin 6 receptor in post-traumatic stress disorder: a pilot study. Cytokine. 2001;13:253–255. doi: 10.1006/cyto.2000.0825. [DOI] [PubMed] [Google Scholar]
- Millet B, Touitou Y, Poirier MF, Bourdel MC, Hantouche E, Bogdan A, Olie JP. Plasma melatonin and cortisol in patients with obsessive-compulsive disorder: relationship with axillary temperature, physical activity, and clinical symptoms. Biol Psychiatry. 1998;44:874–881. doi: 10.1016/s0006-3223(97)00512-x. [DOI] [PubMed] [Google Scholar]
- Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. 1997;159:2994–2999. [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Prickaerts J, Oude Voshaar RC, Elzinga BM. Gender specific associations of serum levels of brain-derived neurotrophic factor in anxiety. World J Biol Psychiatry. 2012;13:535–543. doi: 10.3109/15622975.2011.587892. [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Papmeyer M, Pertusa A, Chamberlain SR, Fineberg NA, Sahakian BJ, Mataix-Cols D, Robbins TW. The profile of executive function in OCD hoarders and hoarding disorder. Psychiatry Res. 2014;215:659–667. doi: 10.1016/j.psychres.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morer A, Lazaro L, Sabater L, Massana J, Castro J, Graus F. Antineuronal antibodies in a group of children with obsessive-compulsive disorder and Tourette syndrome. J Psychiatr Res. 2008;42:64–68. doi: 10.1016/j.jpsychires.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Morer A, Vinas O, Lazaro L, Calvo R, Andres S, Bosch J, Gasto C, Massana J, Castro J. Subtyping obsessive-compulsive disorder: clinical and immunological findings in child and adult onset. J Psychiatr Res. 2006;40:207–213. doi: 10.1016/j.jpsychires.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS) Arch Pediatr Adolesc Med. 2002;156:356–361. doi: 10.1001/archpedi.156.4.356. [DOI] [PubMed] [Google Scholar]
- Murphy TK, Goodman WK, Fudge MW, Williams RC, Jr, Ayoub EM, Dalal M, Lewis MH, Zabriskie JB. B lymphocyte antigen D8/17: a peripheral marker for childhood-onset obsessive-compulsive disorder and Tourette’s syndrome? Am J Psychiatry. 1997;154:402–407. doi: 10.1176/ajp.154.3.402. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Cameron OG, Curtis GC, McCann DS, Huber-Smith MJ. Adrenergic function in patients with panic anxiety. Arch Gen Psychiatry. 1984;41:771–776. doi: 10.1001/archpsyc.1984.01790190045005. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Curtis GC, Thyer BA, McCann DS, Huber-Smith MJ, Knopf RF. Endocrine and cardiovascular responses during phobic anxiety. Psychosom Med. 1985;47:320–332. doi: 10.1097/00006842-198507000-00002. [DOI] [PubMed] [Google Scholar]
- Nicolini H, Lopez Y, Genis-Mendoza AD, Manrique V, Lopez-Canovas L, Niubo E, Hernandez L, Bobes MA, Riveron AM, Lopez-Casamichana M, et al. Detection of antistreptococcal, antienolase, and anti-neural antibodies in subjects with early-onset psychiatric disorders. Actas Esp Psiquiatr. 2015;43:35–41. [PubMed] [Google Scholar]
- Nielen MM, Den Boer JA. Neuropsychological performance of OCD patients before and after treatment with fluoxetine: evidence for persistent cognitive deficits. Psychol Med. 2003;33:917–925. doi: 10.1017/s0033291703007682. [DOI] [PubMed] [Google Scholar]
- Norman TR, Judd FK, Burrows GD, McIntyre IM. Platelet serotonin uptake in panic disorder patients: a replication study. Psychiatry Res. 1989a;30:63–68. doi: 10.1016/0165-1781(89)90172-8. [DOI] [PubMed] [Google Scholar]
- Norman TR, Judd FK, Gregory M, James RH, Kimber NM, McIntyre IM, Burrows GD. Platelet serotonin uptake in panic disorder. J Affect Disord. 1986;11:69–72. doi: 10.1016/0165-0327(86)90062-5. [DOI] [PubMed] [Google Scholar]
- Norman TR, Sartor DM, Judd FK, Burrows GD, Gregory MS, McIntyre IM. Platelet serotonin uptake and 3H-imipramine binding in panic disorder. J Affect Disord. 1989b;17:77–81. doi: 10.1016/0165-0327(89)90026-8. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Altered central alpha 2-adrenoceptor sensitivity in panic disorder. Arch Gen Psychiatry. 1989;46:165–169. doi: 10.1001/archpsyc.1989.01810020067011. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Fraser S. Platelet binding studies in panic disorder. J Affect Disord. 1987;12:7–11. doi: 10.1016/0165-0327(87)90055-3. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Glue P. Imipramine in panic disorder 1. Clinical response and pharmacological changes. J Psychopharmacol (Oxford) 1991;5:56–64. doi: 10.1177/026988119100500108. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Glue P, Lawson C, Wilson S. Flumazenil provocation of panic attacks. Evidence for altered benzodiazepine receptor sensitivity in panic disorder. Arch Gen Psychiatry. 1990;47:917–925. doi: 10.1001/archpsyc.1990.01810220033004. [DOI] [PubMed] [Google Scholar]
- O’Sullivan K, Newman EF. Neuropsychological impairments in panic disorder: a systematic review. J Affect Disord. 2014;167:268–284. doi: 10.1016/j.jad.2014.06.024. [DOI] [PubMed] [Google Scholar]
- O’Toole MS, Pedersen AD. A systematic review of neuropsychological performance in social anxiety disorder. Nord J Psychiatry. 2011;65:147–161. doi: 10.3109/08039488.2011.565801. [DOI] [PubMed] [Google Scholar]
- O’Toole MS, Pedersen AD, Hougaard E, Rosenberg NK. Neuropsychological test performance in social anxiety disorder. Nord J Psychiatry. 2015;69:444–452. doi: 10.3109/08039488.2014.997288. [DOI] [PubMed] [Google Scholar]
- O’Toole SA, Weinborn M, Fox AM. Performance monitoring among non-patients with obsessive-compulsive symptoms: ERP evidence of aberrant feedback monitoring. Biol Psychol. 2012;91:221. doi: 10.1016/j.biopsycho.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Derbyshire KL, Leppink EW, Grant JE. Impaired response inhibition and excess cortical thickness as candidate endophenotypes for trichotillomania. J Psychiatr Res. 2014;59:167–173. doi: 10.1016/j.jpsychires.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Grant JE. Motor inhibition and cognitive flexibility in pathologic skin picking. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:208–211. doi: 10.1016/j.pnpbp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Odlaug BL, Chamberlain SR, Harvanko AM, Grant JE. Age at onset in trichotillomania:clinical variables and neurocognitive performance. Prim Care Companion CNS Disord. 2012;14(4) doi: 10.4088/PCC.12m01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okasha A, Bishry Z, Khalil AH, Darwish TA, el Dawla AS, Shohdy A. Panic disorder. An overlapping or independent entity? Br J Psychiatry. 1994;164:818–825. doi: 10.1192/bjp.164.6.818. [DOI] [PubMed] [Google Scholar]
- Olson R. GABA. In: Davis KL, Charney D, Coyle JT, editors. Neuropsychopharmacology The Fifth Generation of Progress; An Official Publication of the American College of Neuropsychopharmacology. Philadelphia, PA: LWW (PE); 2002. pp. 150–168. [Google Scholar]
- Otten U, Baumann JB, Girard J. Stimulation of the pituitary-adrenocortical axis by nerve growth factor. Nature. 1979;282:413–414. doi: 10.1038/282413a0. [DOI] [PubMed] [Google Scholar]
- Owen DR, Lewis AJ, Reynolds R, Rupprecht R, Eser D, Wilkins MR, Bennacef I, Nutt DJ, Parker CA. Variation in binding affinity of the novel anxiolytic XBD173 for the 18 kDa translocator protein in human brain. Synapse. 2011;65:257–259. doi: 10.1002/syn.20884. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Sandrone G, Rimoldi O, Malfatto G, Cerutti S, Malliani A. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl. 1984;2:S383–S385. [PubMed] [Google Scholar]
- Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM, Sherwood A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom Med. 2015;77:136–144. doi: 10.1097/PSY.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S, Tofani T, Zanardelli M, Di Cesare Mannelli L, Ghelardini C. BDNF and Artemin are increased in drug-naïve non-depressed GAD patients: preliminary data. Int J Psychiatry Clin Pract. 2014;18:255–260. doi: 10.3109/13651501.2014.940051. [DOI] [PubMed] [Google Scholar]
- Papadopoulos A, Rich A, Nutt DJ, Bailey JE. The effects of single dose anxiolytic medication on the CO2 models of anxiety: differentiation of subjective and objective measures. J Psychopharmacol (Oxford) 2010;24:649–656. doi: 10.1177/0269881108097716. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papp LA, Martinez JM, Klein DF, Coplan JD, Norman RG, Cole R, de Jesus MJ, Ross D, Goetz R, Gorman JM. Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatry. 1997;154:1557–1565. doi: 10.1176/ajp.154.11.1557. [DOI] [PubMed] [Google Scholar]
- Paterson JL, Reynolds AC, Ferguson SA, Dawson D. Sleep and obsessive-compulsive disorder (OCD) Sleep Med Rev. 2013;17:465–474. doi: 10.1016/j.smrv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Pavlov VA. Cholinergic modulation of inflammation. Int J Clin Exp Med. 2008;1:203–212. [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, Chavan S, Al-Abed Y, Tracey KJ. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, Trifiletti RR. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30:107–110. doi: 10.1016/S0887-8994(03)00413-2. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, Chang H, Fleury D, Koszychi D, Quirion R, Nair NP, Suranyi-Cadotte BE. Platelet imipramine binding in patients with panic disorder and major familial depression. J Psychiatr Res. 1987;21:319–326. doi: 10.1016/0022-3956(87)90034-3. [DOI] [PubMed] [Google Scholar]
- Pecknold JC, Suranyi-Cadotte B, Chang H, Nair NP. Serotonin uptake in panic disorder and agoraphobia. Neuropsychopharmacology. 1988;1:173–176. doi: 10.1016/0893-133x(88)90010-3. [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou C, Papassotiriou I, Hindmarsh P, Bakoula C, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder (PTSD) after motor vehicle accidents: progressive divergence of noradrenaline and cortisol concentrations over time. Biol Psychiatry. 2007;62:1095–1102. doi: 10.1016/j.biopsych.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Petrowski K, Wintermann GB, Schaarschmidt M, Bornstein SR, Kirschbaum C. Blunted salivary and plasma cortisol response in patients with panic disorder under psychosocial stress. Int J Psychophysiol. 2013;88:35–39. doi: 10.1016/j.ijpsycho.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Gale CR, Lord JM, Arlt W, Carroll D. Major depressive disorder, generalised anxiety disorder, and their comorbidity: associations with cortisol in the Vietnam Experience Study. Psychoneuroendocrinology. 2011;36:682–690. doi: 10.1016/j.psyneuen.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Pine DS, Coplan JD, Papp LA, Klein RG, Martinez JM, Kovalenko P, Tancer N, Moreau D, Dummit ES, 3rd, Shaffer D, et al. Ventilatory physiology of children and adolescents with anxiety disorders. Arch Gen Psychiatry. 1998;55:123–129. doi: 10.1001/archpsyc.55.2.123. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, Coplan JD, Papp LA, Hoven CW, Martinez J, Kovalenko P, Mandell DJ, Moreau D, Klein DF, et al. Differential carbon dioxide sensitivity in childhood anxiety disorders and nonill comparison group. Arch Gen Psychiatry. 2000;57:960–967. doi: 10.1001/archpsyc.57.10.960. [DOI] [PubMed] [Google Scholar]
- Pini S, Martini C, Abelli M, Muti M, Gesi C, Montali M, Chelli B, Lucacchini A, Cassano GB. Peripheral-type benzodiazepine receptor binding sites in platelets of patients with panic disorder associated to separation anxiety symptoms. Psychopharmacology (Berl) 2005;181:407–411. doi: 10.1007/s00213-005-2247-x. [DOI] [PubMed] [Google Scholar]
- Pinkney V, Bamford S, Baldwin DS, Munafo MR, Garner M. The effects of duloxetine on subjective, autonomic and neurocognitive response to 7.5% carbon dioxide challenge. European Neuropsychopharmacology. 2014;24:S579–S579. [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Plag J, Gaudlitz K, Schumacher S, Dimeo F, Bobbert T, Kirschbaum C, Strohle A. Effect of combined cognitive-behavioural therapy and endurance training on cortisol and salivary alpha-amylase in panic disorder. J Psychiatr Res. 2014;58:12–19. doi: 10.1016/j.jpsychires.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Poma SZ, Merlo-Pich E, Bettica P, Bani M, Fina P, Ziviani L, Milleri S. Anxiolytic effects of vestipitant in a sub-group of healthy volunteers known to be sensitive to CO2 challenge. J Psychopharmacol. 2014;28:491–497. doi: 10.1177/0269881113507641. [DOI] [PubMed] [Google Scholar]
- Poma SZ, Milleri S, Squassante L, Nucci G, Bani M, Perini GI, Merlo-Pich E. Characterization of a 7% carbon dioxide (CO2) inhalation paradigm to evoke anxiety symptoms in healthy subjects. J Psychopharmacol (Oxford) 2005;19:494–503. doi: 10.1177/0269881105056533. [DOI] [PubMed] [Google Scholar]
- Pomara N, Willoughby LM, Sidtis JJ, Cooper TB, Greenblatt DJ. Cortisol response to diazepam: its relationship to age, dose, duration of treatment, and presence of generalized anxiety disorder. Psychopharmacology (Berl) 2005;178:1–8. doi: 10.1007/s00213-004-1974-8. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Potts NL, Davidson JR, Krishnan KR, Doraiswamy PM, Ritchie JC. Levels of urinary free cortisol in social phobia. J Clin Psychiatry. 1991;52(Suppl):41–42. [PubMed] [Google Scholar]
- Rapaport MH, Risch SC, Golshan S, Gillin JC. Neuroendocrine effects of ovine corticotropin-releasing hormone in panic disorder patients. Biol Psychiatry. 1989;26:344–348. doi: 10.1016/0006-3223(89)90049-8. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Stein MB. Serum interleukin-2 and soluble interleukin-2 receptor levels in generalized social phobia. Anxiety. 1994;1:50–53. doi: 10.1002/anxi.3070010203. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Fischman AJ, Jenike MA. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: a PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–791. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Griffiths J, Merali Z, Anisman H. Circulating lymphocyte subsets in obsessive compulsive disorder, major depression and normal controls. J Affect Disord. 1999;52:1–10. doi: 10.1016/s0165-0327(98)00072-x. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Huang YH. Current concepts. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol. 2001;129:175–189. doi: 10.1016/s0034-5687(01)00289-4. [DOI] [PubMed] [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, Kathmann N. Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. Am J Psychiatry. 2011;168:317–324. doi: 10.1176/appi.ajp.2010.10030416. [DOI] [PubMed] [Google Scholar]
- Riesel A, Kathmann N, Endrass T. Overactive performance monitoring in obsessive-compulsive disorder is independent of symptom expression. Eur Arch Psychiatry Clin Neurosci. 2014;264:707–717. doi: 10.1007/s00406-014-0499-3. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Kathmann N, Hajcak G. Punishment has a lasting impact on error-related brain activity. Psychophysiology. 2012;49:239–247. doi: 10.1111/j.1469-8986.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, Klein DF, Klein RG, Mannuzza S, Moulton JL, 3rd, Guardino M, Pine DS. Carbon dioxide hypersensitivity in separation-anxious offspring of parents with panic disorder. Biol Psychiatry. 2010;67:1171–1177. doi: 10.1016/j.biopsych.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K, Stanley EM, Franklin ME, Simons RF. Decreased response monitoring in individuals with symptoms of trichotillomania. Psychophysiology. 2014;51:706–713. doi: 10.1111/psyp.12205. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Bender J, Jr, Marcus SM, Snape M, Rynn M, Simpson HB. Minocycline augmentation of pharmacotherapy in obsessive-compulsive disorder: an open-label trial. J Clin Psychiatry. 2010;71:1247–1249. doi: 10.4088/JCP.09l05805blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K, van Peer J, Berretty E, Jong P, Spinhoven P, Elzinga BM. Hypothalamus-pituitary-adrenal axis hyperresponsiveness is associated with increased social avoidance behavior in social phobia. Biol Psychiatry. 2009;65:336–343. doi: 10.1016/j.biopsych.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Rosenbaum AH, Schatzberg AF, Jost FA, 3rd, Cross PD, Wells LA, Jiang NS, Maruta T. Urinary free cortisol levels in anxiety. Psychosomatics. 1983;24:835–837. doi: 10.1016/S0033-3182(83)73157-9. [DOI] [PubMed] [Google Scholar]
- Rosnick CB, Rawson KS, Butters MA, Lenze EJ. Association of cortisol with neuropsychological assessment in older adults with generalized anxiety disorder. Aging Ment Health. 2013;17:432–440. doi: 10.1080/13607863.2012.761673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne PP, Cowley DS, Hommer D, Ritchie J, Greenblatt D, Nemeroff C. Neuroendocrine effects of diazepam in panic and generalized anxiety disorders. Biol Psychiatry. 1991;30:73–80. doi: 10.1016/0006-3223(91)90072-t. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW, Post RM, Gallucci W, Chrousos GP, Gold PW. The corticotropin-releasing hormone stimulation test in patients with panic disorder. Am J Psychiatry. 1986;143:896–899. doi: 10.1176/ajp.143.7.896. [DOI] [PubMed] [Google Scholar]
- Ruland T, Domschke K, Schutte V, Zavorotnyy M, Kugel H, Notzon S, Vennewald N, Ohrmann P, Arolt V, Pfleiderer B, et al. Neuropeptide S receptor gene variation modulates anterior cingulate cortex Glx levels during CCK-4 induced panic. Eur Neuropsychopharmacol. 2015;25:1677–1682. doi: 10.1016/j.euroneuro.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Sachinvala N, von Scotti H, McGuire M, Fairbanks L, Bakst K, McGuire M, Fairbanks L, Bakst K, McGuire M, Brown N. Memory, attention, function, and mood among patients with chronic posttraumatic stress disorder. J Nerv Ment Dis. 2000;188:818–823. doi: 10.1097/00005053-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Sallee FR, Richman H, Beach K, Sethuraman G, Nesbitt L. Platelet serotonin transporter in children and adolescents with obsessive-compulsive disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1996;35:1647–1656. doi: 10.1097/00004583-199612000-00017. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol. 2006;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. A. E. Bennett Award paper. Adrenocortical function, social rank, and personality among wild baboons. Biol Psychiatry. 1990;28:862–878. doi: 10.1016/0006-3223(90)90568-m. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Plotsky PM. Hypercortisolism and its possible neural bases. Biol Psychiatry. 1990;27:937–952. doi: 10.1016/0006-3223(90)90032-w. [DOI] [PubMed] [Google Scholar]
- Saria A. The tachykinin NK1 receptor in the brain: pharmacology and putative functions. Eur J Pharmacol. 1999;375:51–60. doi: 10.1016/s0014-2999(99)00259-9. [DOI] [PubMed] [Google Scholar]
- Sayyah M, Boostani H, Pakseresht S, Malayeri A. A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res. 2011;189:403–406. doi: 10.1016/j.psychres.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Cigliana G, Nicolai R, Muscolo LA, Porcu A, Navarra D, Perez-Polo JR, Angelucci L. Hypothalamic involvement in the activation of the pituitary-adrenocortical axis by nerve growth factor. Neuroendocrinology. 1993;58:202–209. doi: 10.1159/000126534. [DOI] [PubMed] [Google Scholar]
- Schittecatte M, Garcia-Valentin J, Charles G, Machowski R, Pena-Othaitz MJ, Mendlewicz J, Wilmotte J. Efficacy of the ’clonidine REM suppression test (CREST)’ to separate patients with major depression from controls; a comparison with three currently proposed biological markers of depression. J Affect Disord. 1995;33:151–157. doi: 10.1016/0165-0327(94)00059-i. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Bartlett JA. Panic disorder and immunity: few effects on circulating lymphocytes, mitogen response, and NK cell activity. Brain Behav Immun. 2002;16:698–705. doi: 10.1016/s0889-1591(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Munjack D, Severson JA, Palmer R. Platelet [3H]imipramine binding in generalized anxiety disorder, panic disorder, and agoraphobia with panic attacks. Biol Psychiatry. 1987a;22:59–66. doi: 10.1016/0006-3223(87)90130-2. [DOI] [PubMed] [Google Scholar]
- Schneider P, Evans L, Ross-Lee L, Wiltshire B, Eadie M, Kenardy J, Hoey H. Plasma biogenic amine levels in agoraphobia with panic attacks. Pharmacopsychiatry. 1987b;20:102–104. doi: 10.1055/s-2007-1017084. [DOI] [PubMed] [Google Scholar]
- Schruers K, Klaassen T, Pols H, Overbeek T, Deutz NE, Griez E. Effects of tryptophan depletion on carbon dioxide provoked panic in panic disorder patients. Psychiatry Res. 2000;93:179–187. doi: 10.1016/s0165-1781(00)00117-7. [DOI] [PubMed] [Google Scholar]
- Schruers K, van Diest R, Overbeek T, Griez E. Acute L-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatry Res. 2002;113:237–243. doi: 10.1016/s0165-1781(02)00262-7. [DOI] [PubMed] [Google Scholar]
- Schweizer EE, Swenson CM, Winokur A, Rickels K, Maislin G. The dexamethasone suppression test in generalised anxiety disorder. Br J Psychiatry. 1986;149:320–322. doi: 10.1192/bjp.149.3.320. [DOI] [PubMed] [Google Scholar]
- Seddon K, Morris K, Bailey J, Potokar J, Rich A, Wilson S, Bettica P, Nutt DJ. Effects of 7.5% CO2 challenge in generalized anxiety disorder. J Psychopharmacol (Oxford) 2011;25:43–51. doi: 10.1177/0269881110364270. [DOI] [PubMed] [Google Scholar]
- Seldenrijk A, Vogelzangs N, van Hout HP, van Marwijk HW, Diamant M, Penninx BW. Depressive and anxiety disorders and risk of subclinical atherosclerosis findings from the Netherlands study of depression and anxiety (NESDA) J Psychosom Res. 2010;69:203–210. doi: 10.1016/j.jpsychores.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Shlik J, Maron E, Aluoja A, Vasar V, Toru I. Citalopram challenge in social anxiety disorder. Euro Neuropsychopharmacol. 2002;12:S339–S340. doi: 10.1017/S146114570300405X. [DOI] [PubMed] [Google Scholar]
- Shu IW, Onton JA, O’Connell RM, Simmons AN, Matthews SC. Combat veterans with comorbid PTSD and mild TBI exhibit a greater inhibitory processing ERP from the dorsal anterior cingulate cortex. Psychiatry Res. 2014;224:58–66. doi: 10.1016/j.pscychresns.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Shutov AA, Bystrova OV. Serum-blood serotonin level as a marker of panic attacks severity and effectiveness of their treatment. Zh Nevrol Psikhiatr Im S S Korsakova. 2008;108:49–54. [PubMed] [Google Scholar]
- Siegmund A, Koster L, Meves AM, Plag J, Stoy M, Strohle A. Stress hormones during flooding therapy and their relationship to therapy outcome in patients with panic disorder and agoraphobia. J Psychiatr Res. 2011;45:339–346. doi: 10.1016/j.jpsychires.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Simkin DR, Thatcher RW, Lubar J. Quantitative EEG and neurofeedback in children and adolescents: anxiety disorders, depressive disorders, comorbid addiction and attention-deficit/hyperactivity disorder, and brain injury. Child Adolesc Psychiatr Clin N Am. 2014;23:427–464. doi: 10.1016/j.chc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Singer HS, Loiselle C. PANDAS: a commentary. J Psychosom Res. 2003;55:31–39. doi: 10.1016/s0022-3999(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham heart study. Hypertension. 1998;32:293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- Snider LA, Lougee L, Slattery M, Grant P, Swedo SE. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry. 2005;57:788–792. doi: 10.1016/j.biopsych.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proc Nat Acad Sci USA. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, 3rd, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994;152:3024–3031. [PubMed] [Google Scholar]
- Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1 beta in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:345–348. doi: 10.1016/S0006-3223(96)00375-7. [DOI] [PubMed] [Google Scholar]
- Spivak B, Vered Y, Graff E, Blum I, Mester R, Weizman A. Low platelet-poor plasma concentrations of serotonin in patients with combat-related posttraumatic stress disorder. Biol Psychiatry. 1999;45:840–845. doi: 10.1016/s0006-3223(98)00231-5. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38:1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: Systematic review and meta-analysis. J Psychopharmacol (Oxford) 2016;30:128–139. doi: 10.1177/0269881115612236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. Neurochemistry of reward and punishment: some implications for the etiology of schizophrenia. J Psychiatr Res. 1971;8:345–361. doi: 10.1016/0022-3956(71)90030-6. [DOI] [PubMed] [Google Scholar]
- Stein MB, Uhde TW. Cortisol response to clonidine in panic disorder: comparison with depressed patients and normal controls. Biol Psychiatry. 1988;24:322–330. doi: 10.1016/0006-3223(88)90201-6. [DOI] [PubMed] [Google Scholar]
- Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J. 1994;127:1376–1381. doi: 10.1016/0002-8703(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Stern ER, Liu Y, Gehring WJ, Lister JJ, Yin G, Zhang J, Fitzgerald KD, Himle JA, Abelson JL, Taylor SF. Chronic medication does not affect hyperactive error responses in obsessive-compulsive disorder. Psychophysiology. 2010;47:913–920. doi: 10.1111/j.1469-8986.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schonfeld S, Hoyer J, Stalder T. Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biol Psychiatry. 2013;74:639–646. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res. 2011;186:310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Strohle A, Feller C, Strasburger CJ, Heinz A, Dimeo F. Anxiety modulation by the heart? Aerobic exercise and atrial natriuretic peptide. Psychoneuroendocrinology. 2006;31:1127–1130. doi: 10.1016/j.psyneuen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Strohle A, Jahn H, Montkowski A, Liebsch G, Boll E, Landgraf R, Holsboer F, Wiedemann K. Central and peripheral administration of atriopeptin is anxiolytic in rats. Neuroendocrinology. 1997;65:210–215. doi: 10.1159/000127274. [DOI] [PubMed] [Google Scholar]
- Strohle A, Kellner M, Holsboer F, Wiedemann K. Atrial natriuretic hormone decreases endocrine response to a combined dexamethasone-corticotropin-releasing hormone test. Biol Psychiatry. 1998a;43:371–375. doi: 10.1016/s0006-3223(97)00200-x. [DOI] [PubMed] [Google Scholar]
- Strohle A, Kellner M, Holsboer F, Wiedemann K. Behavioral, neuroendocrine, and cardiovascular response to flumazenil: no evidence for an altered benzodiazepine receptor sensitivity in panic disorder. Biol Psychiatry. 1999;45:321–326. doi: 10.1016/s0006-3223(98)00295-9. [DOI] [PubMed] [Google Scholar]
- Strohle A, Kellner M, Holsboer F, Wiedemann K. Anxiolytic activity of atrial natriuretic peptide in patients with panic disorder. Am J Psychiatry. 2001;158:1514–1516. doi: 10.1176/appi.ajp.158.9.1514. [DOI] [PubMed] [Google Scholar]
- Strohle A, Kellner M, Yassouridis A, Holsboer F, Wiedemann K. Effect of flumazenil in lactate-sensitive patients with panic disorder. Am J Psychiatry. 1998b;155:610–612. doi: 10.1176/ajp.155.5.610. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, Rupprecht R. Induced panic attacks shift gamma-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–168. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. GABA(A) receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry. 2002;159:145–147. doi: 10.1176/appi.ajp.159.1.145. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Graetz B, Scheel M, Wittmann A, Gallinat J, Lang UE, Dimeo F, Hellweg R. Acute exercise ameliorates reduced brain-derived neurotrophic factor in patients with panic disorder. Psychoneuroendocrinology. 2010;35:364–368. doi: 10.1016/j.psyneuen.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Oquendo MA, Huang YY, Mann JJ. Elevated cerebrospinal fluid 5-hydroxyindoleacetic acid levels in women with comorbid depression and panic disorder. Int J Neuropsychopharmacol. 2006;9:547–556. doi: 10.1017/S1461145705006231. [DOI] [PubMed] [Google Scholar]
- Sutherland AG, Alexander DA, Hutchison JD. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine. 2003;24:219–225. doi: 10.1016/j.cyto.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Sutterby SR, Bedwell JS. Lack of neuropsychological deficits in generalized social phobia. PLoS One. 2012;7:e42675. doi: 10.1371/journal.pone.0042675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) Mol Psychiatry. 2002;7:S24–S25. doi: 10.1038/sj.mp.4001170. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Garvey M, Snider L, Hamilton C, Leonard HL. The PANDAS subgroup: recognition and treatment. CNS Spectr. 2001;6:419–422. doi: 10.1017/s1092852900021799. 425-6. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Mittleman BB, Allen AJ, Rapoport JL, Dow SP, Kanter ME, Chapman F, Zabriskie J. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry. 1997;154:110–112. doi: 10.1176/ajp.154.1.110. [DOI] [PubMed] [Google Scholar]
- Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Feder DJ, Abulafia DP, Roffman SS. Regulation of hypothalamic-pituitary-adrenal activity in response to cognitive therapy in patients with generalized anxiety disorder. Cogn Affect Behav Neurosci. 2005;5:37–40. doi: 10.3758/cabn.5.1.37. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Idoyaga-Vargas VP, Abulafia DP, Calandria JM, Roffman SS, Chiovetta A, Shinitzky M. Correlation between cortisol level and serotonin uptake in patients with chronic stress and depression. Cogn Affect Behav Neurosci. 2001;1:388–393. doi: 10.3758/cabn.1.4.388. [DOI] [PubMed] [Google Scholar]
- Tamura A, Maruyama Y, Ishitobi Y, Kawano A, Ando T, Ikeda R, Inoue A, Imanaga J, Okamoto S, Kanehisa M, et al. Salivary alpha-amylase and cortisol responsiveness following electrical stimulation stress in patients with the generalized type of social anxiety disorder. Pharmacopsychiatry. 2013;46:225–260. doi: 10.1055/s-0033-1353157. [DOI] [PubMed] [Google Scholar]
- Tancer ME. Neurobiology of social phobia. J Clin Psychiatry. 1993;54:26–30. [PubMed] [Google Scholar]
- Tancer ME, Mailman RB, Stein MB, Mason GA, Carson SW, Golden RN. Neuroendocrine responsivity to monoaminergic system probes in generalized social phobia. Anxiety. 1994a;1:216–223. [PubMed] [Google Scholar]
- Tancer ME, Stein MB, Black B, Uhde TW. Blunted growth hormone responses to growth hormone-releasing factor and to clonidine in panic disorder. Am J Psychiatry. 1993;150:336–337. doi: 10.1176/ajp.150.2.336. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Stein MB, Uhde TW. Lactic acid response to caffeine in panic disorder: comparison with social phobics and normal controls. Anxiety. 1994b;1:138–140. doi: 10.1002/anxi.3070010307. [DOI] [PubMed] [Google Scholar]
- Targum SD. Cortisol response during different anxiogenic challenges in panic disorder patients. Psychoneuroendocrinology. 1992;17:453–458. doi: 10.1016/0306-4530(92)90003-p. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, King RA, Leckman JF, Lombroso PJ. An animal model of Tourette’s syndrome. Am J Psychiatry. 2002;159:657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- Tempesta D, Mazza M, Serroni N, Moschetta FS, Di Giannantonio M, Ferrara M, De Berardis D. Neuropsychological functioning in young subjects with generalized anxiety disorder with and without pharmacotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:236–241. doi: 10.1016/j.pnpbp.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thoren P, Asberg M, Cronholm B, Jornestedt L, Traskman L. Clomipramine treatment of obsessive-compulsive disorder. I. A controlled clinical trial. Arch Gen Psychiatry. 1980;37:1281–1285. doi: 10.1001/archpsyc.1980.01780240079009. [DOI] [PubMed] [Google Scholar]
- Tiller JW, Biddle N, Maguire KP, Davies BM. The dexamethasone suppression test and plasma dexamethasone in generalized anxiety disorder. Biol Psychiatry. 1988;23:261–270. doi: 10.1016/0006-3223(88)90037-6. [DOI] [PubMed] [Google Scholar]
- Tofani T, Mannelli LD, Zanardelli M, Ghelardini C, Pallanti S. An immunologic profile study in drug-naive generalized anxiety non depressed patients: a pilot study. Eur Neuropsychopharmacol. 2015;25:S226. [Google Scholar]
- Torcia M, BracciLaudiero L, Lucibello M, Nencioni L, Labardi D, Rubartelli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Toru I, Maron E, Raag M, Vasar V, Nutt DJ, Shlik J. The effect of 6-week treatment with escitalopram on CCK-4 challenge: a placebo-controlled study in CCK-4-sensitive healthy volunteers. Eur Neuropsychopharmacol. 2013;23:645–652. doi: 10.1016/j.euroneuro.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tucker P, Adamson P, Miranda R, Jr, Scarborough A, Williams D, Groff J, McLean H. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370–376. doi: 10.1097/00004714-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, Wyatt DB. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Tukel R, Arslan BA, Ertekin BA, Ertekin E, Oflaz S, Ergen A, Kuruca SE, Isbir T. Decreased IFN-γ and IL-12 levels in panic disorder. J Psychosom Res. 2012;73:63–67. doi: 10.1016/j.jpsychores.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Hami S, Stein MB. Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Res. 2004;126:265–274. doi: 10.1016/j.psychres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Uchida RR, Del-Ben CM, Busatto GF, Duran FL, Guimaraes FS, Crippa JA, Araujo D, Santos AC, Graeff FG. Regional gray matter abnormalities in panic disorder: a voxel-based morphometry study. Psychiatry Res. 2008;163:21–29. doi: 10.1016/j.pscychresns.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Uhde TW, Berrettini WH, Roy-Byrne PP, Boulenger JP, Post RM. Platelet [3H]imipramine binding in patients with panic disorder. Biol Psychiatry. 1987;22:52–58. doi: 10.1016/0006-3223(87)90129-6. [DOI] [PubMed] [Google Scholar]
- Uhde TW, Joffe RT, Jimerson DC, Post RM. Normal urinary free cortisol and plasma MHPG in panic disorder: clinical and theoretical implications. Biol Psychiatry. 1988;23:575–585. doi: 10.1016/0006-3223(88)90004-2. [DOI] [PubMed] [Google Scholar]
- Uhde TW, Tancer ME, Gelernter CS, Vittone BJ. Normal urinary free cortisol and postdexamethasone cortisol in social phobia: comparison to normal volunteers. J Affect Disord. 1994;30:155–161. doi: 10.1016/0165-0327(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Valenca AM, Nardi AE, Nascimento I, Zin WA, Lopes FL, Mezzasalma MA, Versiani M. Early carbon dioxide challenge test may predict clinical response in panic disorder. Psychiatry Res. 2002;112:269–272. doi: 10.1016/s0165-1781(02)00222-6. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–309. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- Van den Hout MA, Griez E. Panic symptoms after inhalation of carbon dioxide. Br J Psychiatry. 1984;144:503–507. doi: 10.1192/bjp.144.5.503. [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Griez EJ. Desynchrony of fear in phobic exposure. J Psychopharmacol (Oxford) 2010;24:695–699. doi: 10.1177/0269881108098821. [DOI] [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Jaegers E, Maes M, Griez EJ. Salivary cortisol in panic: are males more vulnerable? Neuro Endocrinol Lett. 2004;25:386–390. [PubMed] [Google Scholar]
- van Duinen MA, Schruers KR, Maes M, Griez EJ. CO2 challenge induced HPA axis activation in panic. Int J Neuropsychopharmacol. 2007;10:797–804. doi: 10.1017/S1461145706007358. [DOI] [PubMed] [Google Scholar]
- van Peer JM, Spinhoven P, Roelofs K. Psychophysiological evidence for cortisol-induced reduction in early bias for implicit social threat in social phobia. Psychoneuroendocrinology. 2010;35:21–32. doi: 10.1016/j.psyneuen.2009.09.012. [DOI] [PubMed] [Google Scholar]
- van Peer JM, Spinhoven P, van Dijk JG, Roelofs K. Cortisol-induced enhancement of emotional face processing in social phobia depends on symptom severity and motivational context. Biol Psychol. 2009;81:123–130. doi: 10.1016/j.biopsycho.2009.03.006. [DOI] [PubMed] [Google Scholar]
- van Praag HM, Asnis GM, Kahn RS, Brown SL, Korn M, Friedman JM, Wetzler S. Monoamines and abnormal behaviour. A multi-aminergic perspective. Br J Psychiatry. 1990;157:723–734. doi: 10.1192/bjp.157.5.723. [DOI] [PubMed] [Google Scholar]
- van Veen JF, Van der Wee NJ, Fiselier J, Van Vliet IM, Westenberg HG. Behavioural effects of rapid intravenous administration of meta-chlorophenylpiperazine (m-CPP) in patients with generalized social anxiety disorder, panic disorder and healthy controls. Eur Neuropsychopharmacol. 2007;17:637–642. doi: 10.1016/j.euroneuro.2007.03.005. [DOI] [PubMed] [Google Scholar]
- van Veen JF, van Vliet IM, de Rijk RH, van Pelt J, Mertens B, Fekkes D, Zitman FG. Tryptophan depletion affects the autonomic stress response in generalized social anxiety disorder. Psychoneuroendocrinology. 2009;34:1590–1594. doi: 10.1016/j.psyneuen.2009.05.007. [DOI] [PubMed] [Google Scholar]
- van Veen JF, van Vliet IM, Derijk RH, van Pelt J, Mertens B, Zitman FG. Elevated alpha-amylase but not cortisol in generalized social anxiety disorder. Psychoneuroendocrinology. 2008;33:1313–1321. doi: 10.1016/j.psyneuen.2008.07.004. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Sulon J, Deboutte D. Hypothalamic-pituitary-adrenal reactivity in prepubertal children with social phobia. J Affect Disord. 2008;111:281–290. doi: 10.1016/j.jad.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Veale DM, Sahakian BJ, Owen AM, Marks IM. Specific cognitive deficits in tests sensitive to frontal lobe dysfunction in obsessive-compulsive disorder. Psychol Med. 1996;26:1261–1269. doi: 10.1017/s0033291700035984. [DOI] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, Fallon B, Mazei-Robison M, Ku SM, Harrigan E, et al. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Strawn JR, Sah R. Acid-base dysregulation and chemosensory mechanisms in panic disorder: a translational update. Transl Psychiatry. 2015;5:e572. doi: 10.1038/tp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Hoogendijk WJ, Smit JH, Penninx BW. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72:340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LH, Sahakian BJ, Robertson MM, Veale DM, Rogers RD, Pickard KM, Aitken MR, Robbins TW. Executive function in Tourette’s syndrome and obsessive-compulsive disorder. Psychol Med. 2005;35:571–582. doi: 10.1017/s0033291704003691. [DOI] [PubMed] [Google Scholar]
- Wedekind D, Sprute A, Broocks A, Huther G, Engel K, Falkai P, Bandelow B. Nocturnal urinary cortisol excretion over a randomized controlled trial with paroxetine vs. placebo combined with relaxation training or aerobic exercise in panic disorder. Curr Pharm Des. 2008;14:3518–3524. doi: 10.2174/138161208786848757. [DOI] [PubMed] [Google Scholar]
- Weik U, Herforth A, Kolb-Bachofen V, Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosom Med. 2008;70:906–912. doi: 10.1097/PSY.0b013e3181835bf3. [DOI] [PubMed] [Google Scholar]
- Weizman R, Laor N, Barber Y, Hermesh H, Notti I, Djaldetti M, Bessler H. Cytokine production in obsessive-compulsive disorder. Biol Psychiatry. 1996;40:908–912. doi: 10.1016/0006-3223(95)00520-X. [DOI] [PubMed] [Google Scholar]
- Weizman R, Laor N, Wiener Z, Wolmer L, Bessler H. Cytokine production in panic disorder patients. Clin Neuropharmacol. 1999;22:107–109. doi: 10.1097/00002826-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Wemmie JA. Neurobiology of panic and pH chemosensation in the brain. Dialogues Clin Neurosci. 2011;13:475–483. doi: 10.31887/DCNS.2011.13.4/jwemmie. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerblom B, Lurje L, Tygesen H, Vahisalo R, Hjalmarson A. Patients with uncomplicated coronary artery disease have reduced heart rate variability mainly affecting vagal tone. Heart. 2000;83:290–294. doi: 10.1136/heart.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann R, Ghofrani HA, Weissmann N, Schermuly R, Quanz K, Grimminger F, Seeger W, Olschewski H. Atrial natriuretic peptide in severe primary and nonprimary pulmonary hypertension: response to iloprost inhalation. J Am Coll Cardiol. 2001;38:1130–1136. doi: 10.1016/s0735-1097(01)01490-5. [DOI] [PubMed] [Google Scholar]
- Wiener CD, de Mello Ferreira S, Pedrotti Moreira F, Bittencourt G, de Oliveira JF, Lopez Molina M, Jansen K, de Mattos Souza LD, Rizzato Lara D, Portela LV, et al. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J Affect Disord. 2015;184:245–248. doi: 10.1016/j.jad.2015.05.067. [DOI] [PubMed] [Google Scholar]
- Wilkinson DJC, Thompson JM, Lambert GW, Jennings GL, Schwarz RG, Jefferys D, Turner AG, Esler MD. Sympathetic activity in patients with panic disorder at rest, under laboratory mental stress, and during panic attacks. Arch Gen Psychiatry. 1998;55:511–520. doi: 10.1001/archpsyc.55.6.511. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Gibson G. Blood gene expression profiles suggest altered immune function associated with symptoms of generalized anxiety disorder. Brain Behav Immun. 2015;43:184–191. doi: 10.1016/j.bbi.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Action of drugs of abuse on brain reward systems: an update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17:239–243. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Woods SW, Charney DS, Goodman WK, Heninger GR. Carbon dioxide-induced anxiety. Behavioral, physiologic, and biochemical effects of carbon dioxide in patients with panic disorders and healthy subjects. Arch Gen Psychiatry. 1988;45:43–52. doi: 10.1001/archpsyc.1988.01800250051007. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, Fan Q, Fromson JA. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005;169:371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, Shalev AY. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44:1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Jampala VC, Sobelewski E, Kay J, Igel G. Effects of paroxetine on heart period variability in patients with panic disorder: a study of holter ECG records. Neuropsychobiology. 1999;40:124–128. doi: 10.1159/000026608. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Zabriskie JB. Rheumatic fever: a model for the pathological consequences of microbial-host mimicry. Clin Exp Rheumatol. 1986;4:65–73. [PubMed] [Google Scholar]
- Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Zambrano-Vazquez L, Allen JJ. Differential contributions of worry, anxiety, and obsessive compulsive symptoms to ERN amplitudes in response monitoring and reinforcement learning tasks. Neuropsychologia. 2014;61:197–209. doi: 10.1016/j.neuropsychologia.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, Yehuda R, Cohen H. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, Schwarz M, Moller HJ, Rupprecht R. Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers. Neuropsychopharmacology. 2001;25:699–703. doi: 10.1016/S0893-133X(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Domschke K, Bradwejn J. Neuronal network of panic disorder: the role of the neuropeptide cholecystokinin. Depress Anxiety. 2012;29:762–774. doi: 10.1002/da.21919. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Eser D, Aicher S, Schule C, Baghai TC, Padberg F, Ella R, Moller HJ, Rupprecht R. Effects of alprazolam on cholecystokinin-tetrapeptide-induced panic and hypothalamic-pituitary-adrenal-axis activity: a placebo-controlled study. Neuropsychopharmacology. 2003;28:979–984. doi: 10.1038/sj.npp.1300131. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Eser D, Nothdurfter C, Baghai TC, Moller HJ, Padberg F, Rupprecht R. Effects of the GABA-reuptake inhibitor tiagabine on panic and anxiety in patients with panic disorder. Pharmacopsychiatry. 2009;42:266–269. doi: 10.1055/s-0029-1241798. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Rupprecht R. Selective GABAergic treatment for panic? Investigations in experimental panic induction and panic disorder. J Psychiatry Neurosci. 2005;30:167–175. [PMC free article] [PubMed] [Google Scholar]