Abstract
Cerebral infarction is a type of ischemic stroke and is one of the main causes of irreversible brain damage. Although multiple neuroprotective agents have been investigated recently, the potential of DL-2-amino-3-phosphonopropionic acid (DL-AP3) in treating oxygen-glucose deprivation (OGD)-induced neuronal injury, has not been clarified yet. This study was aimed to explore the role of DL-AP3 in primary neuronal cell cultures. Primary neurons were divided into four groups: (1) A control group that was not treated; (2) DL-AP3 group treated with 10 µM of DL-AP3; (3) OGD group, in which neurons were cultured under OGD conditions; (4) OGD + DL-AP3 group, in which OGD model was first established and then the cells were treated with 10 µM of DL-AP3. Neuronal viability and apoptosis were measured using Cell Counting Kit-8 and flow cytometry. Expressions of phospho-Akt1 (p-Akt1) and cytochrome C were detected using Western blot. The results showed that DL-AP3 did not affect neuronal viability and apoptosis in DL-AP3 group, nor it changed p-Akt1 and cytochrome C expression (p > 0.05). In OGD + DL-AP3 group, DL-AP3 significantly attenuated the inhibitory effects of OGD on neuronal viability (p < 0.001) and reduced OGD induced apoptosis (p < 0.01). In addition, the down-regulation of p-Akt1 and up-regulation of cytochrome C, induced by OGD, were recovered to some extent after DL-AP3 treatment (p < 0.05 or p < 0.001). Overall, DL-AP3 could protect primary neurons from OGD-induced injury by affecting the viability and apoptosis of neurons, and by regulating the expressions of p-Akt1 and cytochrome C.
KEY WORDS: DL-2-amino-3-phosphonopropionic acid, cerebral infarction, oxygen-glucose deprivation, neuron viability, apoptosis
INTRODUCTION
Cerebral infarction is a type of ischemic stroke and is one of the main causes of irreversible brain damage [1]. Risk factors of cerebral infarction include high blood pressure, diabetes mellitus, tobacco smoking, obesity, and dyslipidemia [2]. Although great efforts have been made to improve the therapeutic strategies for the treatment of cerebral infarction, including the application of recombinant tissue plasminogen activator, thrombolytic therapy, and surgery, an optimal method has not been developed yet [3,4]. Therefore, establishing the means to treat cerebral infarction and focal cerebral ischemia/reperfusion injury would be highly beneficial [5].
Excitatory amino acids (EAAs) have been identified to play a role in causing irreversible ischemic brain damage [6]. The EAA glutamate is the major excitatory neurotransmitter in central nervous system. An increasing number of studies has demonstrated a vital role of glutamate in a focal ischemic model of the human brain [7,8]. High concentrations of glutamate are associated with neurotoxicity and may be involved in several neurodegenerative disorders [9]. Glutamate receptors, especially the N-methyl-D-aspartate receptors, control neuronal survival by affecting the function of mitochondria and playing a role in oxidative stress [7,10,11]. An in vitro study indicated that glutamate receptors antagonists have neuroprotective abilities in primary cultures of rat cerebellar granule cells, briefly exposed to glutamate [12]. Ke et al. have found that MK-801 may alleviate ischemia/reperfusion injury of rat sciatic nerve by inhibiting the activation of tumor necrosis factor-α [13]. Adachi et al. found that phencyclidine-induced the decrease of synaptic connectivity by inhibiting the secretion of brain-derived neurotrophic factor in cultured cortical neurons [14].
DL-2-amino-3-phosphonopropionic acid (DL-AP3) is one of the glutamate receptor antagonists. However, little is known about the potential neuroprotective effects of DL-AP3. In this study, primary neuronal cell cultures were exposed to oxygen-glucose deprivation (OGD) and were treated with DL-AP3 to explore the effects of DL-AP3 on OGD-induced neuronal damage. In addition, the changes in protein expression of phospho-Akt1 (p-Akt1) and cytochrome C in neurons were monitored, to reveal the possible molecular mechanism of DL-AP3. This study may help understand the potential role of DL-AP3 in cerebral infarction.
MATERIALS AND METHODS
Primary neuronal cell culture
Pregnant, specific pathogen-free Sprague-Dawley rats on day 15 of gestation were provided by the Animal Center of Academy of Military Science of the Chinese People’s Liberation Army. The animals were anesthetized with ether. Bilateral hippocampi of fetal rats were cut, minced, dissolved, filtered, and suspended in DMEM/F-12 medium (Sigma, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; Gibco, USA). The suspended cells were then seeded on plates coated with polylysine (Sigma-Aldrich, St Louis, MO, USA) and cultured at 37°C in humidified air with 5% CO2 [15]. After a 4-hour incubation, the medium was replaced by serum-free Neurobasal A medium (Life Technologies, Gaithersburg, MD, USA) supplemented with B27 (Gibco, Carlsbad, CA, USA) [16]. This study was approved by the Local Ethics Committee. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Precautions were taken to minimize the suffering and number of animals used in each experiment.
OGD model and DL-AP3 treatment
Primary neurons were divided into four groups: (1) A control group that was not treated; (2) DL-AP3 group treated with 10 µM of DL-AP3 for 6 hours; (3) OGD group, in which neurons were cultured under OGD conditions for 12 hours; (4) OGD + DL-AP3 group, in which OGD model was first established and then the cells were treated with 10 µM of DL-AP3 for 6 hours [17]. OGD model was established as previously described [18,19]. Briefly, neurons were washed with glucose-free Earle’s Balanced Salt Solution (Bioleaf, Shanghai, China) and then were placed in a modular incubator chamber (Billups-Rothenberg, Del Mar, CA, USA, MC-101) filled with gas mixture (95% N2 and 5% CO2) at 37°C. To terminate OGD, neurons were incubated under normal conditions.
Cell viability analysis
Neuronal viability was determined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kyushu, Japan), according to the manufacturer’s instructions. Briefly, primary neurons in the four groups were collected and seeded in 96-well plates with 2 × 103 cells/well. After 24-72-hour incubation, 20 µL of CCK-8 was added to each well and incubated for another 3 hours. Finally, the absorbance was measured by a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 450 nm [20].
Apoptosis analysis
Cell apoptosis was performed by Annexin V-FITC Apoptosis Detection Kit (Sigma, St. Louis, MO), according to the manufacturer’s instructions. Briefly, neurons in the four groups were collected and re-suspended in 200 µL of binding buffer containing 10 µL of Annexin-V-FITC. After 30 minutes of incubation in the dark at room temperature, 300 µL of phosphate buffered saline and 5 µL of propidium iodide solution were added into each sample and then the apoptotic cells were analyzed using flow cytometry (Becton Dickinson, Mountain View, CA, USA) [21].
Western blot analysis
Cells in the four groups were collected and lysed in the lysis buffer (Beyotime, Beijing, China). The protein concentration of the supernatant was determined using the BCA Protein Assay Kit (Beyotime, Beijing, China), according to the manual. Equal amounts of proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes [22]. Then, the membranes were stained with primary antibodies: p-Akt1 (1:1000, ab66138), cytochrome C (1:5000; ab133504) or actin (1:1000; ab1801) (Abcam, Cambridge, MA, USA) overnight at 4°C. Subsequently, the blots were incubated with the horseradish peroxidase conjugated secondary antibodies for 1 hour at room temperature. The bands were visualized with the enhanced chemiluminescence detection kit (Amersham Biosciences, Uppsala, Sweden), and data were analyzed using Image Lab (Bio-Rad Laboratories, Hercules, CA, USA) software [23].
Statistical analysis
All data were expressed as mean ± standard derivation from five independent experiments. The data were analyzed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA) and the Student’s t-test. A statistically significant difference was considered at p < 0.05.
RESULTS
DL-AP3 alleviated OGD-induced injury
To explore the effects of DL-AP3 on neurons under hypoxic conditions, neurons were cultured under OGD conditions and treated with DL-AP3. DL-AP3 did not affect neuronal viability (p > 0.05) in DL-AP3 group. In OGD group, OGD significantly reduced neuronal viability after 24 and 72 hours (p < 0.001). However, DL-AP3 significantly attenuated the inhibitory effect of OGD on neuronal viability (p < 0.001), in OGD + DL-AP3 group. The results of cell viability assay in all groups are shown in Figure 1A.
FIGURE 1.
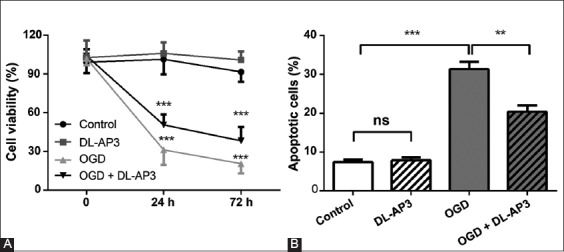
(A) The effects of DL-2-amino-3-phosphonopropionic acid (DL-AP3) on cell viability in four groups of neuronal cells, measured by CCK-8 kit. DL-AP3 significantly attenuated the inhibitory effect of oxygen-glucose deprivation (OGD) on neuronal viability in OGD + DL-AP3 group. (B) The effects of DL-AP3 on apoptosis in four groups of neuronal cells determined using flow cytometry. OGD significantly increased the apoptotic cell rate in OGD group, but this effect was significantly attenuated in OGD + DL-AP3 group. DL-AP3: DL-2-amino-3-phosphonopropionic acid; OGD: oxygen-glucose deprivation; CCK-8: Cell counting kit-8; Control: Control group; DL-AP3: DL-AP3 group was treated with 10 µM of DL-AP3 for 6 hours; OGD: OGD group in which neurons were cultured under OGD conditions for 12 hours; OGD + DL-AP3: OGD + DL-AP3 group, in which OGD model was first established and then the cells were treated with 10 µM of DL-AP3 for 6 hours; ns: no significance; **p < 0.01; ***p < 0.001.
In DL-AP3 group, DL-AP3 had no significant effect on apoptosis (p > 0.05). However, OGD significantly increased the apoptotic cell rate in OGD group (p < 0.001), but this effect was significantly attenuated in OGD + DL-AP3 group (p < 0.01). The results of cell apoptosis in the four groups are presented in Figure 1B.
DL-AP3 protected neurons against OGD-induced injury by regulating p-Akt1 and cytochrome C expression
To further investigate the molecular mechanism underlying the effect of DL-AP3 on OGD-induced injury, the protein expressions of p-Akt1 and cytochrome C in neurons were determined. In DL-AP3 group, DL-AP3 did not change the protein levels of p-Akt1 and cytochrome C (p > 0.05). However, OGD significantly down-regulated the level of p-Akt1 (p < 0.001) and up-regulated the level of cytochrome C (p < 0.001) in OGD group. Nevertheless, DL-AP3 significantly recovered the decreased levels of p-Akt1 and the increase of cytochrome C in OGD + DL-AP3 group (p < 0.001 and p < 0.05, respectively). The results of p-Akt1 and cytochrome C protein expression in the four groups are illustrated in Figure 2A and B.
FIGURE 2.
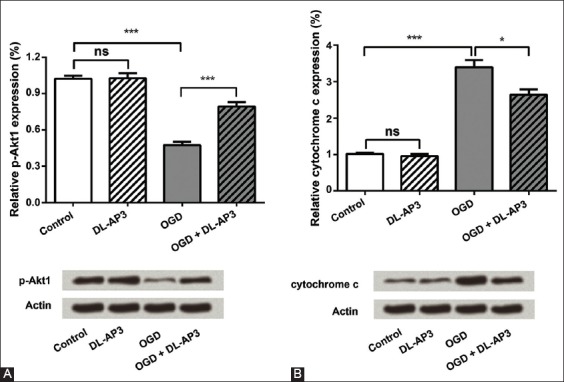
The analysis of (A) phospho-Akt1 (p-Akt1) and (B) cytochrome c protein expressions in four groups of neuronal cells, using Western blot analysis. Oxygen-glucose deprivation (OGD) significantly down-regulated the level of p-Akt-1 and up-regulated the level of cytochrome c in OGD group. Nevertheless, DL-2-amino-3-phosphonopropionic acid (DL-AP3) significantly recovered the decreased levels of p-Akt1 and the increase of cytochrome c in OGD + DL-AP3 group. DL-AP3: DL-2-amino-3-phosphonopropionic acid; OGD: Oxygen-glucose deprivation; Control: Control group; DL-AP3: DL-AP3 group was treated with 10µM of DL-AP3 for 6 hours; OGD: OGD group in which neurons were cultured under OGD conditions for 12 hours; OGD + DL-AP3: OGD + DL-AP3 group, in which OGD model was first established, and then, the cells were treated with 10 µM of DL-AP3 for 6 hours; ns: No significance; *p < 0.05; ***p < 0.001.
DISCUSSION
Cerebral infarction is a type of ischemic stroke and one of the leading causes of death [1,6,24]. Recently, multiple neuroprotective agents have been in the focus of research. However, the potential role of DL-AP3 in ischemia-injured neurons has not been elucidated so far. In the present study, we found that OGD significantly decreased neuronal viability and induced apoptosis in these cells. The protein expression level of p-Akt1 was down-regulated, and the level of cytochrome C was up-regulated in OGD group. However, DL-AP3 could significantly attenuate the inhibitory effects of OGD on neuronal viability and reduce the apoptosis induced by OGD. Moreover, the expression of p-Akt1 and cytochrome C proteins was regulated by DL-AP3.
Neuronal necrosis and apoptosis are the major indicators of cerebral injury [25,26]. Thus, neuronal proliferation and apoptosis could be used as the indices for measuring cerebral infarction. Glutamate can induce either necrosis or apoptosis in neuronal cells [27]. Recent studies have shown that multiple glutamate receptor antagonists have the ability to protect neurons from injury. Bai et al. found that ketamine enhanced human neural stem cell proliferation and induced neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway [28]. In addition, Zhang et al. have found that MK-801 is able to reduce neuronal death by preventing apoptosis [29]. In this study, DL-AP3 could significantly attenuate the decreased neuronal viability and increased apoptosis induced by OGD. Our data for the first time suggested that DL-AP3 acts as a neuroprotective agent via modulating neuronal proliferation and apoptosis.
Neuronal cell proliferation and apoptosis are associated with various factors that induce neurological damage [1]. The Akt signaling pathway is one of the main pathways involved in cell proliferation. Molecules inducing cellular proliferation often increase the phosphorylation of Akt protein [30]. Akt has been consistently shown to mediate the effect of several agents that specifically promote neuronal cell proliferation [31]. In addition, the suppression of Akt could attenuate neural stem cell self-renewal [32]. Cytochrome C is the only protein in the electron transport chain that is mobile and is known to have a regulatory role in apoptosis [33]. The release of cytochrome C induces DNA fragmentation and apoptotic neuronal cell death [25]. In this study, we found that DL-AP3 could recover the down-regulation effects of OGD on the protein expression of p-Akt1 and could suppress the release of cytochrome C induced by OGD. These findings imply that the possible mechanism of DL-AP3 in protecting neurons from injury might be via up-regulating the expression of p-Akt1 and suppressing the release of cytochrome C.
CONCLUSION
Our results demonstrated that DL-AP3 could attenuate OGD-induced injury by controlling neuronal viability and apoptosis. In addition, the protective effects of DL-AP3 on neurons might be associated with the expression of p-Akt1 and cytochrome C proteins. Our study may provide a theoretical basis for the possible application of DL-AP3 in treating cerebral infarction. However, whether DL-AP3 can be used in clinical treatment of cerebral infarction still requires long-term investigations in in vivo models.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Liu JJ, Pan SY. Protective effects of estrogen combined with sevoflurane in an experimental model of cerebral infarction and focal cerebral ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2016;20(9):1839–44. [PubMed] [Google Scholar]
- [2].Hankey GJ. Potential new risk factors for ischemic stroke: What is their potential? Stroke. 2006;37(8):2181–8. doi: 10.1161/01.STR.0000229883.72010.e4. https://doi.org/10.1161/01.STR.0000229883.72010.e4. [DOI] [PubMed] [Google Scholar]
- [3].Liu N, Zhang Y, Fan L, Yuan M, Du H, Cheng R, et al. Effects of transplantation with bone marrow-derived mesenchymal stem cells modified by Survivin on experimental stroke in rats. J Transl Med. 2011;9:105. doi: 10.1186/1479-5876-9-105. https://doi.org/10.1186/1479-5876-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang S, Zhou J, Kang W, Dong Z, Wang H. Tocilizumab inhibits neuronal cell apoptosis and activates STAT3 in cerebral infarction rat model. Bosn J Basic Med Sci. 2016;16(2):145–50. doi: 10.17305/bjbms.2016.853. https://doi.org/10.17305/bjbms.2016.853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [5].Onteddu SR, Goddeau RP, Jr, Minaeian A, Henninger N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J Neurol Sci. 2015;359(1-2):418–23. doi: 10.1016/j.jns.2015.10.005. https://doi.org/10.1016/j.jns.2015.10.005. [DOI] [PubMed] [Google Scholar]
- [6].Slomka M, Kuszczyk M, Lazarewicz JW, Makarewicz D. NMDA receptor antagonists MK-801 and memantine induce tolerance to oxygen and glucose deprivation in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2014;74(4):396–404. doi: 10.55782/ane-2014-2002. [DOI] [PubMed] [Google Scholar]
- [7].Kanthan R, Shuaib A, Griebel R, Miyashita H. Intracerebral human microdialysis In vivo study of an acute focal ischemic model of the human brain. Stroke. 1995;26(5):870–3. doi: 10.1161/01.str.26.5.870. https://doi.org/10.1161/01.STR.26.5.870. [DOI] [PubMed] [Google Scholar]
- [8].Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28(4):866–72. doi: 10.1161/01.str.28.4.866. https://doi.org/10.1161/01.STR.28.4.866. [DOI] [PubMed] [Google Scholar]
- [9].Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–95. doi: 10.1126/science.7901908. https://doi.org/10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- [10].Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–4. doi: 10.1126/science.283.5398.70. https://doi.org/10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- [11].Yun BR, Yang HJ, Weon JB, Lee J, Eom MR, Ma CJ. Neuroprotective properties of compounds extracted from Dianthus superbus L. Against glutamate-induced cell death in HT22 cells. Pharmacogn Mag. 2016;12(46):109–13. doi: 10.4103/0973-1296.177905. https://doi.org/10.4103/0973-1296.177905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuszczyk M, Slomka M, Antkiewicz-Michaluk L, Salinska E, Lazarewicz JW. 1-methyl-1,2,3,4-tetrahydroisoquinoline and established uncompetitive NMDA receptor antagonists induce tolerance to excitotoxicity. Pharmacol Rep. 2010;62(6):1041–50. doi: 10.1016/s1734-1140(10)70366-2. https://doi.org/10.1016/S1734-1140(10)70366-2. [DOI] [PubMed] [Google Scholar]
- [13].Ke T, Li R, Chen W. Inhibition of the NMDA receptor protects the rat sciatic nerve against ischemia/reperfusion injury. Exp Ther Med. 2016;11(5):1563–72. doi: 10.3892/etm.2016.3148. https://doi.org/10.3892/etm.2016.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adachi N, Numakawa T, Kumamaru E, Itami C, Chiba S, Iijima Y, et al. Phencyclidine-induced decrease of synaptic connectivity via inhibition of BDNF secretion in cultured cortical neurons. Cereb Cortex. 2013;23(4):847–58. doi: 10.1093/cercor/bhs074. https://doi.org/10.1093/cercor/bhs074. [DOI] [PubMed] [Google Scholar]
- [15].Xu SY, Wu YM, Ji Z, Gao XY, Pan SY. A modified technique for culturing primary fetal rat cortical neurons. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/803930. 803930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao XY, Huang JO, Hu YF, Gu Y, Zhu SZ, Huang KB, et al. Combination of mild hypothermia with neuroprotectants has greater neuroprotective effects during oxygen-glucose deprivation and reoxygenation-mediated neuronal injury. Sci Rep. 2014;4:7091. doi: 10.1038/srep07091. https://doi.org/10.1038/srep07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Z, Yang P, Qi Y. Role of microRNA-134 in the neuroprotective effects of propofol against oxygen-glucose deprivation and related mechanisms. Int J Clin Exp Med. 2015;8(11):20617–23. [PMC free article] [PubMed] [Google Scholar]
- [18].Vieira M, Fernandes J, Carreto L, Anuncibay-Soto B, Santos M, Han J, et al. Ischemic insults induce necroptotic cell death in hippocampal neurons through the up-regulation of endogenous RIP3. Neurobiol Dis. 2014;68:26–36. doi: 10.1016/j.nbd.2014.04.002. https://doi.org/10.1016/j.nbd.2014.04.002. [DOI] [PubMed] [Google Scholar]
- [19].Sheng R, Liu XQ, Zhang LS, Gao B, Han R, Wu YQ, et al. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8(3):310–25. doi: 10.4161/auto.18673. https://doi.org/10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]
- [20].Pan XW, Zhao XH. In Vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19) Int J Mol Sci. 2015;16(6):13908–20. doi: 10.3390/ijms160613908. https://doi.org/10.3390/ijms160613908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang N, Su Y, Xu L. Targeting PKCe by miR-143 regulates cell apoptosis in lung cancer. FEBS Lett. 2013;587(22):3661–7. doi: 10.1016/j.febslet.2013.09.018. https://doi.org/10.1016/j.febslet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- [22].Nam K, Oh S, Lee KM, Yoo SA, Shin I. CD44 regulates cell proliferation, migration, and invasion via modulation of c-Src transcription in human breast cancer cells. Cell Signal. 2015;27(9):1882–94. doi: 10.1016/j.cellsig.2015.05.002. https://doi.org/10.1016/j.cellsig.2015.05.002. [DOI] [PubMed] [Google Scholar]
- [23].Trapé AP, Liu S, Cortes AC, Ueno NT, Gonzalez-Angulo AM. Effects of CDK4/6 inhibition in hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer cells with acquired resistance to paclitaxel. J Cancer. 2016;7(8):947–56. doi: 10.7150/jca.14441. https://doi.org/10.7150/jca.14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–8. doi: 10.1016/j.neuropharm.2008.01.005. https://doi.org/10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aboutaleb N, Shamsaei N, Rajabi H, Khaksari M, Erfani S, Nikbakht F, et al. Protection of hippocampal cA1 neurons against ischemia/reperfusion injury by exercise preconditioning via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. Basic Clin Neurosci. 2016;7(1):21–9. [PMC free article] [PubMed] [Google Scholar]
- [26].Chalmers-Redman RM, Fraser AD, Ju WY, Wadia J, Tatton NA, Tatton WG. Mechanisms of nerve cell death: Apoptosis or necrosis after cerebral ischaemia. Int Rev Neurobiol. 1997;40:1–25. doi: 10.1016/s0074-7742(08)60713-8. https://doi.org/10.1016/S0074-7742(08)60713-8. [DOI] [PubMed] [Google Scholar]
- [27].Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–73. doi: 10.1016/0896-6273(95)90186-8. https://doi.org/10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- [28].Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, et al. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116(4):869–80. doi: 10.1213/ANE.0b013e3182860fc9. https://doi.org/10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Q, Shao Y, Zhao C, Cai J, Sun S. N-methyl-D-aspartate receptor antagonist MK-801 prevents apoptosis in rats that have undergone fetal spinal cord transplantation following spinal hemisection. Exp Ther Med. 2014;8(6):1731–6. doi: 10.3892/etm.2014.2029. https://doi.org/10.3892/etm.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Migita H, Kominami K, Higashida M, Maruyama R, Tuchida N, McDonald F, et al. Activation of adenosine A1 receptor-induced neural stem cell proliferation via MEK/ERK and Akt signaling pathways. J Neurosci Res. 2008;86(13):2820–8. doi: 10.1002/jnr.21742. https://doi.org/10.1002/jnr.21742. [DOI] [PubMed] [Google Scholar]
- [31].Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. https://doi.org/10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103(1):111–6. doi: 10.1073/pnas.0509939103. https://doi.org/10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jung SR, Kuok IT, Couron D, Rizzo N, Margineantu DH, Hockenbery DM, et al. Reduced cytochrome C is an essential regulator of sustained insulin secretion by pancreatic islets. J Biol Chem. 2011;286(20):17422–34. doi: 10.1074/jbc.M110.202820. https://doi.org/10.1074/jbc.M110.202820. [DOI] [PMC free article] [PubMed] [Google Scholar]


