Abstract
Availability of adequate bone structure for dental implants is still a problem in dentistry. Alloplastic grafts, which promote bone regeneration, are used as bone substitutes in orthopedic and oral surgical procedures. The aim of this study was to evaluate the radiopacity of three different synthetic bone grafts in rabbit calvaria, over 3 months, using cone beam computed tomography (CBCT). Four critical-size defects were made on the calvaria of 11 rabbits. The lesions were classified into three groups according to the alloplastic grafts they received: Osteon® 70/30, Osteon collagen®, and Osteon II® groups. The fourth group received blood clot, and served as a control. The bone samples were collected and analyzed with CBCT after the 1st, 2nd, and 3rd month. One month after surgery, the lesions that received Osteon® 70/30 and Osteon collagen® grafts showed the highest radiopacity compared to the lesions with Osteon II® and blood clot. After the 2nd month, the radiopacity values between the three groups that received the grafts were more similar compared to the group with blood clot. After the 3rd month, the lesions with Osteon® 70/30 graft showed the highest radiopacity values, followed by Osteon collagen® and Osteon II® groups. The group that received blood clot showed the lowest radiopacity values. In conclusion, the grafts used in this study had higher radiopacity values compared to blood clot. Among the grafts used, the Osteon® 70/30 graft showed the highest radiopacity values in the 3-month period.
KEY WORDS: Alloplastic bone grafts, cone beam computed tomography, critical bone defects
INTRODUCTION
Alloplastic grafts are commonly used as bone substitutes in orthopedic and oral surgeries to treat osseous defects [1,2], due to their capability to promote bone regeneration within an acceptable time frame [3]. In particular, calcium phosphate based ceramics have similar characteristics as the crystal structure and chemistry of bone mineral phase [2,3]. Among synthetic calcium phosphate ceramics are hydroxyapatites (HAs), tricalcium phosphates (TCP), and calcium phosphate cements [4]. These bone grafts need to be osteoconductive to provide a scaffold structure, as well as, to have bioactive properties, which means being capable of adhering to the bone [5].
After graft placement, approximately 70% is resorbed and replaced by newly formed bone [6]. Different methods have been used to measure the resorption rates [7,8]. Although histological analysis is necessary to determine the actual nature of the tissue of interest, in most cases, such analysis is not performed due to economical and practical reasons. In this sense, the cone beam computed tomography (CBCT) is a less-invasive method, and highly used by surgeons and dentists. It provides the three-dimensional (3D) analysis of bone and other tissues [9,10], as well as the means to determine their radiopacity.
The radiopacity is a representation of tissue mineral density [11]. The radiopacity of an alloplastic bone graft vary over time due to the fraction of TCP and calcium phosphate that is resorbed and replaced by osteoid tissue [4]. However, because of the low resolution and limitations of conventional radiographs, the change in radiopacity during this time is not visible until 50% of the biomaterial is replaced by bone [4]. Current literature lacks studies that use CBCT to quantify the variations in radiopacity that occur in a particular time period after alloplastic bone graft implantation.
The present study aimed to evaluate the radiopacity of three different synthetic bone grafts in rabbit calvaria over 3-month period, using CBCT.
MATERIALS AND METHODS
Surgical procedure
The following study was approved by the Scientific Ethics Committee from the Universidad de La Frontera (Protocol 015/13) and performed according to the Declaration of Helsinki.
Eleven adult New Zealand rabbits (Oryctolagus cuniculus - weights of 3.9 ± 0.2 kg) were selected from the Experimental Surgery Center of the Universidad de La Frontera.
The animals were premedicated according to their weight with ketamine (35 mg/kg) and xylazine (2 mg/kg). In addition, the head was shaved, and a local anesthetic (0.4 ml lidocaine 2%) was applied to this region. A median lineal incision was made from the frontal to occipital region, elevating the periosteum, and exposing both parietal bones. Subsequently, four bicortical defects were created with an 8 mm external diameter trephine bur.
The samples were divided into four groups depending on the material used to fill in the defects:
Osteon® 70/30 group - 70% HA + 30% β-TCP (Genoss, Suwon, Korea);
Osteon II® group - Biphasic calcium phosphate coated with β-TCP (Genoss, Suwon, Korea);
Osteon collagen® group - HA + β-TCP + collagen (Genoss, Suwon, Korea);
Blood clot group - control.
The periosteum and skin were separately sutured, with a 5-0 absorbable Vicryl® suture (Ethicon, Novartis Animal Health US, Inc., Greensboro, NC, USA). Following the surgery, the animals were medicated for the first 3 days with amoxicillin (50 mg/kg) and metamizole (0.2 ml/5 kg, twice a day; Drag Pharma, Chile) and maintained with ad libitum feeding in closed cages with controlled temperature.
After healing period, the animals were euthanized with an overdose of pentobarbital (150 mg/kg) after the 1st (n = 3), 2nd (n = 4), and 3rd months (n = 4), and the defects were extracted immediately with safety margins of 2 mm and perfused with 10% paraformaldehyde.
CBCT evaluation
After the rabbits were euthanized, the cranium was analyzed using CBCT (Pax Zenith, Vatech, Korea) with an exposition time of 23 s, 100 kvp, and 5 mA. The window size used in all samples was 12 cm × 9 cm, and voxel size was 0.12 mm. Data were imported in DICOM format and analyzed with specific software (EZ3D2009, E-Woo Technology Co., Korea).
Image analysis
Each defect was analyzed separately by locating the course at the center of defect, and the views were readjusted to perform a multiplanar reconstruction. Subsequently, tracings were performed parallel to the bone with an interval of 0.5 and 1 mm thickness. From all the images obtained, the main central images were selected for the analysis, and the other images were discarded.
APFill Ink Coverage Calculator software (V.5.2, Insight, AZ, USA) was used to measure the radiopacity of each defect, using a scale from 0 (black) to 250 (white). The radiopacity of each defect corresponded to the mean of radiopacity values of the three cuts or central planes.
Statistical analysis
Statistical analysis was performed using SigmaPlot v.12.0 software (Systat Software, Inc., CA, USA) and a value of p < 0.05 was considered statistically significant. The Shapiro–Wilk test was used to test the normality of data distribution. After determining the normal distribution of the sample, one-way analysis of variance (ANOVA) was used with the Holm-Sidak post-test, which allows the simultaneous analysis of multiple groups. All data are presented as mean ± standard deviation.
RESULTS
Clinically, no allergic reactions or other local complications were observed in the three groups.
Figure 1A and B show the radiopacity values and tendency, respectively, of the blood clot and alloplastic bone grafts over the three time periods. The radiopacity of all defects filled in with the alloplastic bone grafts was the highest after the 3rd month. The Osteon® 70/30 grafts demonstrated the highest radiopacity, while the blood clot showed the lowest radiopacity during the three time periods (Figure 2). The statistical analysis for each time period is summarized in Figure 3A-C.
FIGURE 1.
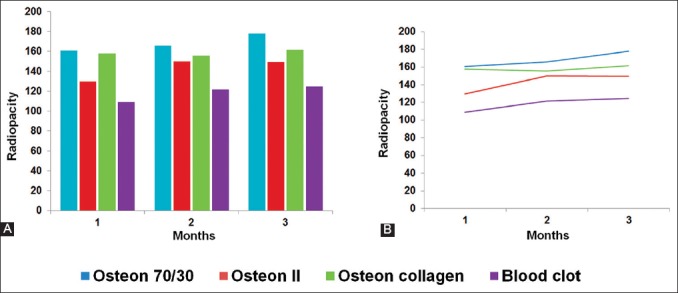
Radiopacity values of three bone grafts and blood clot. (A) Radiopacity data grouped by each month. (B) Radiopacity values over the 3-month period.
FIGURE 2.
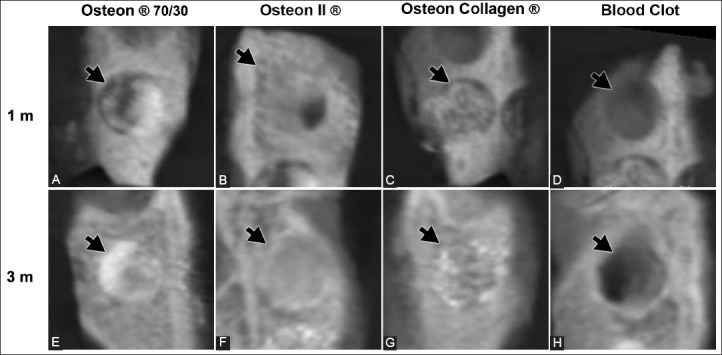
Cone beam computed tomography image showing the radiopacity (black arrows) of the three biomaterials and blood clot. (A-D) After the 1st month and (E-H) after 3 months of application.
FIGURE 3.
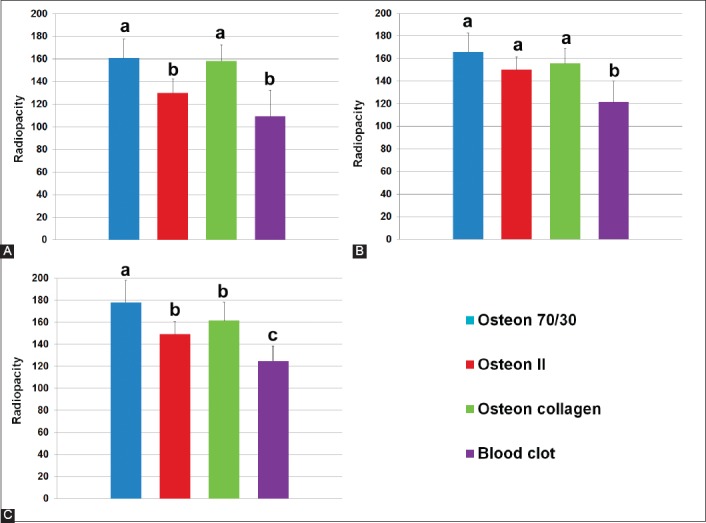
Statistical analysis of radiopacity values for each month. (A) 1st month; (B) 2nd month; and (C) 3rd month. a, b, and c – statistically significant difference (p < 0.05).
After the 1st month of healing, similar radiopacity values were detected in Osteon® 70/30 (162.17 ± 20.37) and Osteon collagen® groups (160 ± 14.03) (p = 0.745). In addition, similar radiopacity values were detected in Osteon II® (136.17 ± 8.93) and blood clot groups (124.17 ± 5.56). However, the first two groups showed significant differences in the radiopacity values compared to the second two groups (p = 0.003).
After the 2nd month of healing, similar radiopacity values were detected in Osteon® 70/30 (165.72 ± 16.81), Osteon collagen (155.72 ± 13.2), and Osteon II® groups (150.09 ± 11.6). In addition, the radiopacity values in these groups were significantly higher than the values in blood clot group (121.76 ± 18.4) (p = 0.001).
After the 3rd month of healing, Osteon® 70/30 group (177.92 ± 20.18) showed significantly higher radiopacity values than the other three groups (p = 0.001). Osteon collagen® (161.42 ± 16.47) and Osteon II® (149.3 ± 11.53) groups showed similar radiopacity values which were higher than the radiopacity values in blood clot group (124.71 ± 13.99) (p = 0.012).
DISCUSSION
The calcium phosphate grafts are biodegradable, bioactive, and osteoconductive, with a similar composition as the mineral phase of bone [2]. However, a highly soluble graft is not recommended because a high release of calcium may inhibit the osteoclast function [5].
Because of the increasing radiopacity values observed over the study period, the three bone grafts used in this study appear to be osteoconductive. In a reconstructive 3D study from 2015, Dadsetan et al. [12] observed that the three coatings (magnesium-substituted β-tricalcium phosphate, carbonated HA, and biphasic calcium phosphate) loaded in combination with recombinant human bone morphogenetic protein-2 were osteoconductive in critical-size defects, after 6 weeks of implantation.
The Osteon® and Osteon collagen® grafts showed similar radiopacity values at 1st month after the implantation. Furthermore, 2 months after the implantation, Osteon II® graft showed radiopacity values similar to the values observed for the first two groups, without statistically significant differences between the three grafts. This result might be explained by the fact that Osteon® and Osteon collagen® have the same composition, except for the addition of bovine collagen in Osteon collagen®. The lower radiopacity values of Osteon II® during the first two months could be due to a slow resorption rate when compared with Osteon® and Osteon collagen®. After the 3rd month, the Osteon II® and Osteon collagen® grafts showed similar radiopacity values, while Osteon® showed significantly higher radiopacity, indicating that collagen or the block presentation of this graft could affect the mineralization of bone graft when compared with the pure Osteon® graft. The graft composition and its presentation directly affect its solubility and the ability to differentiate the osteoclast precursor cells into mature osteoclasts [2,5]. These mature osteoclasts have the ability to release cytokines that influence osteoblasts and bone formation [2]. Furthermore, the bone graft presentation may be influenced by the particle geometry, porosity, and interconnectivity [13].
β-TCP is a resorbable ceramic that bonds to the bone directly. The β-TCP granules are rapidly dissolved and newly formed bone fill in the area of dissolved granules [14]. The graft releases calcium and phosphate ions that stimulate the bone healing [15]. However, if the graft resorption takes place before the new bone is formed, the bone healing rate may be affected [16]. When this ceramic is mixed with HA, which is a low resorption rate graft [2,17], it improves the formation and remodeling of new bone by providing adequate mechanical resistance [18].
The difference in the resorption rate between β-TCP and HA is due to the fact that HA adheres directly to the bone through a collagen-free layer interface with no preferred orientation [14], and thus is more stable than β-TCP. This collagen-free layer has an active role absorbing extracellular proteins such as growth factors [19]. Thus, bone grafts containing more HA show more radiopacity during the time of healing [18]. The effect of Osteon® (70% of HA) graft observed in our study seems to be related to this process.
The blood clot used as the control exhibited the lowest radiopacity over the study period. It might have acted as a critical size-defect, which is a defect that will not heal spontaneously during the lifetime of an animal [20]. The critical size defect used in this study concurs with defects having an external diameter of 8 mm, as suggested by other authors [7,8,16,21]. Despite some controversies regarding the critical-size defect, the results of this study confirmed that the prepared area was sufficient for analysis. The same results for blood clot were observed in a two-dimensional radiographic study by Oporto et al. [8] for a follow-up period of 3 months, where they observed <10% of ossification.
CBCT allows a more accurate analysis of bone compared to conventional radiographs [22,23], making it a useful method to quantify bone density [9,24]. Each slice of a CBCT image is composed of pixels that represent the tissue density, thus providing better accuracy [25].
A limitation of this study is lack of information on the percentage and distribution of HA and β-TCP in the Osteon II® graft. This is a limitation because the chemical properties of a graft depend on the HA/β-TCP ratio [5]. Moreover, a histological analysis is necessary to determine the possible causes of differences in radiopacity values between defects filled with different bone grafts [8]; however, this analysis was not the main objective of our study.
As assessed by CBCT, the grafts used in this study had higher radiopacity values compared to blood clot. Among the grafts applied to the bone lesions, the Osteon® 70/30 graft showed the highest radiopacity values over the 3-month period.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
ACKNOWLEDGMENTS
Funded by Universidad de La Frontera, Project DI15-0009.
REFERENCES
- [1].Ogose A, Hotta T, Kawashima H, Kondo N, Gu W, Kamura T, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res B Appl Biomater. 2005;72(1):94–101. doi: 10.1002/jbm.b.30136. http://dx.doi.org/10.1002/jbm.b.30136. [DOI] [PubMed] [Google Scholar]
- [2].Shiwaku Y, Neff L, Nagano K, Takeyama K, de Bruijn J, Dard M, et al. The crosstalk between osteoclasts and osteoblasts is dependent upon the composition and structure of biphasic calcium phosphates. PLoS One. 2015;10(7):e0132903. doi: 10.1371/journal.pone.0132903. DOI: 10.1371/journal.pone.0132903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Denry I, Kuhn LT. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater. 2016;32(1):43–53. doi: 10.1016/j.dental.2015.09.008. http://dx.doi.org/10.1016/j.dental.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Larsson S. Calcium phosphates: What is the evidence? J Orthop Trauma. 2010;24(Suppl 1):S41–5. doi: 10.1097/BOT.0b013e3181cec472. DOI: 10.1097/BOT.0b013e3181cec472. [DOI] [PubMed] [Google Scholar]
- [5].Yamada S, Heymann D, Bouler JM, Daculsi G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/beta-tricalcium phosphate ratios. Biomaterials. 1997;18(15):1037–41. doi: 10.1016/s0142-9612(97)00036-7. http://dx.doi.org/10.1016/S0142-9612(97)00036-7. [DOI] [PubMed] [Google Scholar]
- [6].Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29(9):1177–88. doi: 10.1016/j.biomaterials.2007.11.034. http://dx.doi.org/10.1016/j.biomaterials.2007.11.034. [DOI] [PubMed] [Google Scholar]
- [7].Borie E, Fuentes R, Del Sol M, Oporto G, Engelke W. The influence of FDBA and autogenous bone particles on regeneration of calvaria defects in the rabbit: A pilot study. Ann Anat. 2011;193(5):412–7. doi: 10.1016/j.aanat.2011.06.003. http://dx.doi.org/10.1016/j.aanat.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [8].Oporto VG, Fuentes R, Borie E, Del Sol M, Orsi IA, Engelke W. Radiographical and clinical evaluation of critical size defects in rabbit calvaria filled with allograft and autograft: A pilot study. Int J Clin Exp Med. 2014;7(7):1669–75. [PMC free article] [PubMed] [Google Scholar]
- [9].Ahmad R, Abu-Hassan MI, Li Q, Swain MV. Three dimensional quantification of mandibular bone remodeling using standard tessellation language registration based superimposition. Clin Oral Implants Res. 2013;24(11):1273–9. doi: 10.1111/j.1600-0501.2012.02566.x. DOI: 10.1111/j.1600-0501.2012.02566.x. [DOI] [PubMed] [Google Scholar]
- [10].Fernandes TM, Adamczyk J, Poleti ML, Henriques JF, Friedland B, Garib DG. Comparison between 3D volumetric rendering and multiplanar slices on the reliability of linear measurements on CBCT images: An in vitro study. J Appl Oral Sci. 2015;23(1):56–63. doi: 10.1590/1678-775720130445. http://dx.doi.org/10.1590/1678-775720130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marquezan M, Osório A, Sant’Anna E, Souza MM, Maia L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin Oral Implants Res. 2012;23(7):767–74. doi: 10.1111/j.1600-0501.2011.02228.x. http://dx.doi.org/10.1111/j.1600-0501.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- [12].Dadsetan M, Guda T, Runge MB, Mijares D, LeGeros RZ, LeGeros JP, et al. Effect of calcium phosphate coating and rhBMP-2 on bone regeneration in rabbit calvaria using poly(propylene fumarate) scaffolds. Acta Biomater. 2015;18:9–20. doi: 10.1016/j.actbio.2014.12.024. http://dx.doi.org/10.1016/j.actbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- [13].Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, et al. Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials. 2008;29(3):266–71. doi: 10.1016/j.biomaterials.2007.09.035. http://dx.doi.org/10.1016/j.biomaterials.2007.09.035. [DOI] [PubMed] [Google Scholar]
- [14].Fujita R, Yokoyama A, Nodasaka Y, Kohgo T, Kawasaki T. Ultrastructure of ceramic-bone interface using hydroxyapatite and beta-tricalcium phosphate ceramics and replacement mechanism of beta-tricalcium phosphate in bone. Tissue Cell. 2003;35(6):427–40. doi: 10.1016/s0040-8166(03)00067-3. http://dx.doi.org/10.1016/S0040-8166(03)00067-3. [DOI] [PubMed] [Google Scholar]
- [15].LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev. 2008;108(11):4742–53. doi: 10.1021/cr800427g. http://dx.doi.org/10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- [16].Lee JH, Ryu MY, Baek HR, Lee KM, Seo JH, Lee HK. Fabrication and evaluation of porous beta-tricalcium phosphate/hydroxyapatite (60/40) composite as a bone graft extender using rat calvarial bone defect model. Scientific World J. 2013;2013:481789. doi: 10.1155/2013/481789. http://dx.doi.org/10.1155/2013/481789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Farzadi A, Solati-Hashjin M, Bakhshi F, Aminian A. Synthesis and characterization of hydroxyapatite/β-tricalcium phosphate nanocomposites using microwave irradiation. Ceram Int. 2011;37(1):65–71. http://dx.doi.org/10.1016/j.ceramint.2010.08.021. [Google Scholar]
- [18].Bansal S, Chauhan V, Sharma S, Maheshwari R, Juyal A, Raghuvanshi S. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion. Indian J Orthop. 2009;43(3):234–9. doi: 10.4103/0019-5413.49387. http://dx.doi.org/10.4103/0019-5413.49387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hench LL. Bioactive ceramics: Theory and clinical applications. In: Anderson ÖH, Happonen RP, Yli-Urpo A, editors. Bioceramics. Oxford: Butterworth-Heinemann; 1994. pp. 3–14. http://dx.doi.org/10.1016/B978-0-08-042144-5.50005-4. [Google Scholar]
- [20].Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299–308. http://dx.doi.org/10.1097/00003086-198604000-00036. [PubMed] [Google Scholar]
- [21].Kitayama S, Wong LO, Ma L, Hao J, Kasugai S, Lang NP, et al. Regeneration of rabbit calvarial defects using biphasic calcium phosphate and a strontium hydroxyapatite-containing collagen membrane. Clin Oral Implants Res. 2015 doi: 10.1111/clr.12605. http://dx.doi.org/10.1111/clr.12605. [DOI] [PubMed] [Google Scholar]
- [22].Creanga AG, Geha H, Sankar V, Teixeira FB, McMahan CA, Noujeim M. Accuracy of digital periapical radiography and cone-beam computed tomography in detecting external root resorption. Imaging Sci Dent. 2015;45(3):153–8. doi: 10.5624/isd.2015.45.3.153. http://dx.doi.org/10.5624/isd.2015.45.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Saidi A, Naaman A, Zogheib C. Accuracy of cone-beam computed tomography and periapical radiography in endodontically treated teeth evaluation: A five-year retrospective study. J Int Oral Health. 2015;7(3):15–9. [PMC free article] [PubMed] [Google Scholar]
- [24].Ehrhart N, Kraft S, Conover D, Rosier RN, Schwarz EM. Quantification of massive allograft healing with dynamic contrast enhanced-MRI and cone beam-CT: A pilot study. Clin Orthop Relat Res. 2008;466(8):1897–904. doi: 10.1007/s11999-008-0293-5. http://dx.doi.org/10.1007/s11999-008-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Azeredo F, de Menezes LM, Enciso R, Weissheimer A, de Oliveira RB. Computed gray levels in multislice and cone-beam computed tomography. Am J Orthod Dentofacial Orthop. 2013;144(1):147–55. doi: 10.1016/j.ajodo.2013.03.013. http://dx.doi.org/10.1016/j.ajodo.2013.03.013. [DOI] [PubMed] [Google Scholar]


