Abstract
Pethidine is a synthetic opioid with local anesthetic properties. Our goal was to evaluate the analgesic efficacy of pethidine for achieving the ultrasound-guided oblique subcostal transversus abdominis plane (OSTAP) block in laparoscopic cholecystectomy. This prospective, double-blind study included 79 patients of physical status I and II according to American Society of Anesthesiologists, scheduled for elective laparoscopic cholecystectomy. The patients were randomly allocated into three groups, depending on the drug used to achieve preoperative bilateral OSTAP block: 1) OSTAP-Placebo (treated with normal saline); 2) OSTAP-Bupivacaine (treated with 0.25% bupivacaine); and 3) OSTAP-Pethidine (treated with 1% pethidine). The efficacy of pethidine in achieving the OSTAP block was analyzed using visual analog scale (VAS), intraoperative opioid dose, opioid consumption in post anesthesia care unit, and opioid consumption in the first 24 postoperative hours. The pain scores assessed by VAS at 0, 2, 4, 6, 12, and 24 hours were significantly lower in OSTAP-Pethidine than in OSTAP-Placebo group (p < 0.001). The mean intraoperative opioid consumption was significantly lower in OSTAP-Pethidine compared to OSTAP-Placebo group (150 versus 400 mg, p < 0.001), as well as the mean opioid consumption in the first 24 hours (20.4 versus 78 mg, p < 0.001). Comparing VAS assessment between OSTAP-Bupivacaine and OSTAP-Pethidine groups, statistically significant differences were observed only for the immediate postoperative pain assessment (0 hours), where lower values were observed in OSTAP-Pethidine group (p = 0.004). There were no statistically significant differences in the incidence of postoperative nausea and vomiting (p = 0.131) between the groups. The use of 1% pethidine can be an alternative to 0.25% bupivacaine in achieving OSTAP block for laparoscopic cholecystectomy.
KEY WORDS: Pethidine, oblique subcostal transversus abdominis plane block, laparoscopic cholecystectomy, analgesia
INTRODUCTION
The laparoscopic cholecystectomy is a minimally invasive, widespread surgical procedure, associated with postoperative pain of moderate intensity in the early postoperative period. The pain reaches its peak within the first few hours after surgery and diminishes during the next two to three days [1-4]. There are several approaches to postoperative pain management after laparoscopic cholecystectomy, such as intravenous patient-controlled analgesia with opioids (IV-PCA), neuraxial blocks, intraperitoneal local anesthesia, and wound infiltration. All of these procedures are more or less effective and have specific side effects [5-7].
The transversus abdominis plane (TAP) block is a regional analgesia technique and an alternative to “classical” postoperative analgesia methods. First described by Rafi [8] and McDonnell et al. [9], this technique has undergone some changes over time, which increased its efficacy. Hebbard et al. [10] described the ultrasound-guided oblique subcostal transversus abdominis plane (OSTAP) block, which is used to provide analgesia to the upper and lower abdomen. This technique has a lower rate of complications due to the direct ultrasound visualization. Different studies confirmed the analgesic efficacy of the OSTAP block and the postoperative opioid sparing effect after laparoscopic cholecystectomy [11,12].
Traditionally, the TAP block is achieved with classical amino-amide local anesthetics, where bupivacaine, levobupivacaine, and ropivacaine are the most commonly used [13].
Pethidine is a synthetic opioid that binds to peripheral opioid receptors and it affects voltage-gated sodium channels [14,15]. The peripheral opioid receptors are associated with pain in inflamed tissues [16]. Studies showed that opioid antinociception was initiated by the activation of opioid receptors located outside the central nervous system, and that the analgesic effect, produced by systemically administered opioids, was largely mediated by the peripheral opioid receptors [17-19].
The electrophysiological studies showed that the administration of 1% pethidine is followed by sensory and motor block produced by the action on the peripheral nerves [14]. Pethidine is effective in many regional anesthesia techniques, such as subarachnoid, epidural, intra-articular, and regional intravenous anesthesia [20-22].
Based on the previous findings, in this study, we evaluated the efficacy of 1% pethidine in achieving the OSTAP block after laparoscopic cholecystectomy, and compared it with the results obtained for 0.25% bupivacaine and placebo.
The primary outcomes of the study were pethidine consumption during the first 24 hours postoperatively and VAS pain scores. The secondary outcomes were the intraoperative fentanyl consumption and the pethidine consumption in post anesthesia care unit (PACU), in all study groups. The duration of intervention, frequency of postoperative nausea and vomiting, and local and systemic complications due to OSTAP were also registered.
MATERIALS AND METHODS
This prospective, double-blind, randomized study was approved by the Institutional Ethics Committee of the Regional Institute of Gastroenterology and Hepatology Cluj-Napoca (2063/25.II.2015). This study was registered at www.clinicaltrials.gov (NCT02707250).
Patients
From 81 eligible patients, 79 patients of physical status I and II according to American Society of Anesthesiologists (ASA) scheduled for elective laparoscopic cholecystectomy, were randomized by a computer-generated random allocation sequence into three interventional groups (Figure 1). Written informed consent was obtained from all patients. In OSTAP- Placebo group, patients received general anesthesia and preoperative bilateral OSTAP block with 20 ml of sterile saline solution injected on each side. In OSTAP-Bupivacaine group, patients received general anesthesia and preoperative bilateral OSTAP block with 20 ml of 0.25% bupivacaine injected on each side. In OSTAP-Pethidine group, patients received general anesthesia and preoperative bilateral OSTAP block with 10 ml of 1% pethidine injected on each side. In the final analysis, data from 75 patients were included (Figure 1). Two patients refused to be included in the study, four patients were excluded because of the protocol violation, and in one patient the procedure was converted to open cholecystectomy. The visual analog scale (VAS) was explained to all patients before the surgery (i.e., how to quantify the pain intensity between 0 [no pain] and 10 [the strongest pain imaginable]).
FIGURE 1.
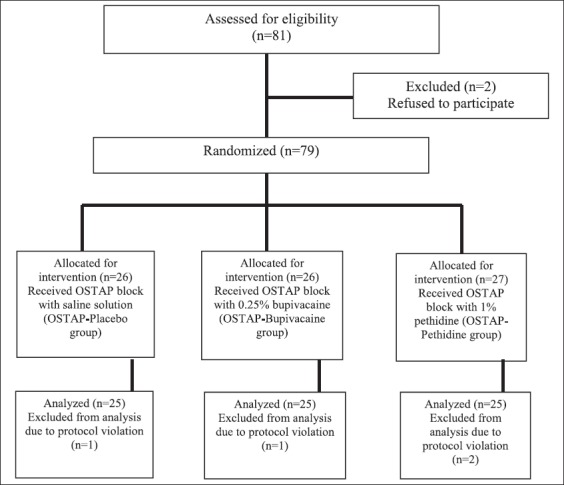
Organization of study groups is presented in the flowchart. OSTAP: Oblique subcostal transversus abdominis plane.
The exclusion criteria included patient’s refusal to participate in the study, allergy to local amino-amide anesthetics and pethidine, infection at the puncture site, acute cholecystitis, documented severe cardiovascular, renal, or liver diseases, neurological or psychiatric diseases, chronic pain syndrome, and surgery complications.
Anesthesia protocol
All patients received standard general anesthesia that included oral premedication with 7.5 mg of midazolam, 60 minutes before the surgery, followed by the induction with 2 mcg/kg of fentanyl, 2 mg/kg of propofol, 0.6 mg/kg of rocuronium or 0.5 mg/kg of atracurium to facilitate tracheal intubation. The maintenance of anesthesia was achieved with volatile sevoflurane 1-2 minimum alveolar concentration (MAC) in oxygen and air fraction of inspired oxygen (FiO2) 0.5. Patients were mechanically ventilated, maintaining EtCO2 between 35-45 mmHg. In all patients, SpO2 was between 96-100%. Intraoperative monitoring included ASA standard monitoring and train-of-four (TOF).
OSTAP block
After the induction and before surgery, the ultrasound-guided OSTAP block was performed. A high-frequency linear transducer (6-10 MHz) was used (Mindray DC-3 Biomedical Electronics, Shenzhen, China). The transducer was placed in a transverse orientation in the epigastric area, then moved along the subcostal edge to identify the rectus abdominis and transverse abdominis (TA) muscles. Once these structures were identified, a 21G x 150 mm needle (SonoTap cannula, PAJUNK Medizintechnologie, Germany) was introduced in-plane 2-3 cm lateral to the transducer under direct ultrasound visualization and 1-2 ml of solution was injected between the rectus abdominis and TA muscles. After confirming the correct placement of the needle and obtaining negative aspiration test results, the rest of the anesthetic drug was injected along the subcostal line into the transversus abdominis plane (20 ml of saline solution in OSTAP-Placebo group, 20 ml of 0.25% bupivacaine in OSTAP-Bupivacaine group, and 10 ml of 1% pethidine in OSTAP-Pethidine group) and the dissection of the plane was observed. The block was performed bilaterally with total of 40 ml solution for OSTAP-Placebo and OSTAP-Bupivacaine groups (20 ml of solution used for each side) and with total of 20 ml of 1% pethidine in OSTAP-Pethidine group. All blocks were performed by the same anesthesiologist who was aware of the type of solution injected, but was not involved in the postoperative data collection.
Laparoscopic cholecystectomy
The laparoscopic cholecystectomy started 15 minutes after the block was completed. Rescue fentanyl doses of 100 mcg were administered if mean arterial pressure and heart rate increased 15% above the baseline values, or if sweating or tears were observed. As intraoperative non-opioid analgesic, 1000 mg of acetaminophen was injected intravenously 30 minutes before the end of the surgery.
At the end of the surgery, the neuromuscular block was reversed with 0.04 mg/kg of neostigmine and 0.01 mg/kg of atropine. All patients were extubated when awake and when the TOF was ≥ 90%.
Postoperatively, patients were transferred to the PACU where bolus doses of 25-50 mg of pethidine were administered IV if the pain was described as moderate or severe (the pain was considered mild for VAS = 1-3, moderate for VAS = 4-6, or severe for VAS = 7-9). The discharge criteria from the PACU were the absence of pain or mild pain, the lack of nausea and vomiting, hemodynamic stability, and an Aldrete score of at least 9.
On the surgical ward, patients received 1 g of IV acetaminophen at 8 hours and, in the case of moderate or severe pain, 25-50 mg of IV pethidine was administrated until VAS < 3 was reported. If necessary, patients received 4 mg of IV ondansetron as antiemetic medication.
Nonsteroidal anti-inflammatory drugs were not included in the multimodal pain regimen, as their anti-inflammatory effects could have significantly influenced postoperative pain and the opioid requirements.
Pain evaluation
The severity of pain was evaluated postoperatively using VAS at 0 hours (in the PACU), 2, 4, 6, 12, and 24 hours. The pain evaluation was performed at rest and with movement (patients were asked to cough and to flex the knees). An anesthesiologist blinded to the study groups completed the pain evaluation and data recordings.
Sample size calculation
For the sample size calculation, a minimum difference of 2 between two VAS measurements was considered, assuming a standard deviation of 2, per group. The previous studies found a 1.3 standard deviation for TAP [23] and an interquartile range of 2 to 3 [4]. To randomize patients into the groups, a 1:1 allocation ratio was used for a power of 80%, and hypotheses tested at alpha level (p-value) of 0.017, using the Bonferroni correction to adjust the 0.05 level of confidence three times, since three comparisons for the three groups were done. The Student’s t-test was used for independent samples, with a two-sided p-value. We determined a total sample size of 69 subjects (i.e., 23 subjects per each group) and set the final sample size to 81, to allow possible loss to follow-up.
Statistical analysis
R- Environment for Statistical Computing version 3.2.1 was used for statistical analysis. Quantitative data with normal distribution were described as mean and standard deviation. The Shapiro-Wilk normality test was used to evaluate the normality of data distribution. The association between qualitative variables was tested using the χ2 test. To determine if there are differences between three or more independent groups of quantitative data, the Kruskal-Wallis test was used for data without a normal distribution and the one-way analysis of variance (ANOVA) was used for data with normal distribution. The Tukey-Kramer HSD (honestly significant difference) test was used post hoc to determine if there are differences between the study groups. The threshold for statistical significance was set at 0.017, considering the Bonferroni correction for the three comparisons.
RESULTS
From the 81 patients eligible to be enrolled in the study, 79 patients were randomized and data from 75 patients were included in the final analysis (Figure 1).
There were no statistically significant differences between the three groups related to age, weight, duration of surgery, and ASA (Table 1).
TABLE 1.
Demographic data of study groups
Statistically significant differences were observed between the three groups with regard to the quality of postoperative analgesia. OSTAP-Pethidine group had a statistically significant lower pain score than OSTAP-Placebo group at 0, 2, 4, 6, 12 and 24 hours postoperatively, both at rest and in movement (Tables 2 and 3). In OSTAP-Pethidine group, the VAS score in the PACU (0 hour) was significantly lower than the VAS score in OSTAP-Placebo and OSTAP-Bupivacaine groups (Table 2 and 3).
TABLE 2.
VAS score at rest
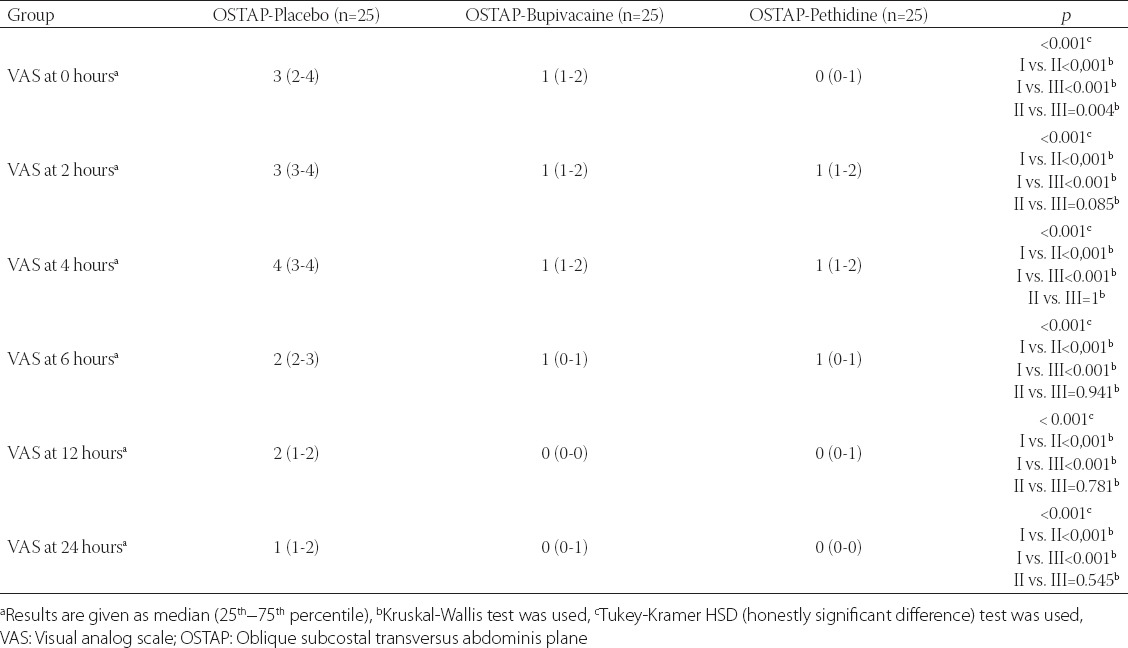
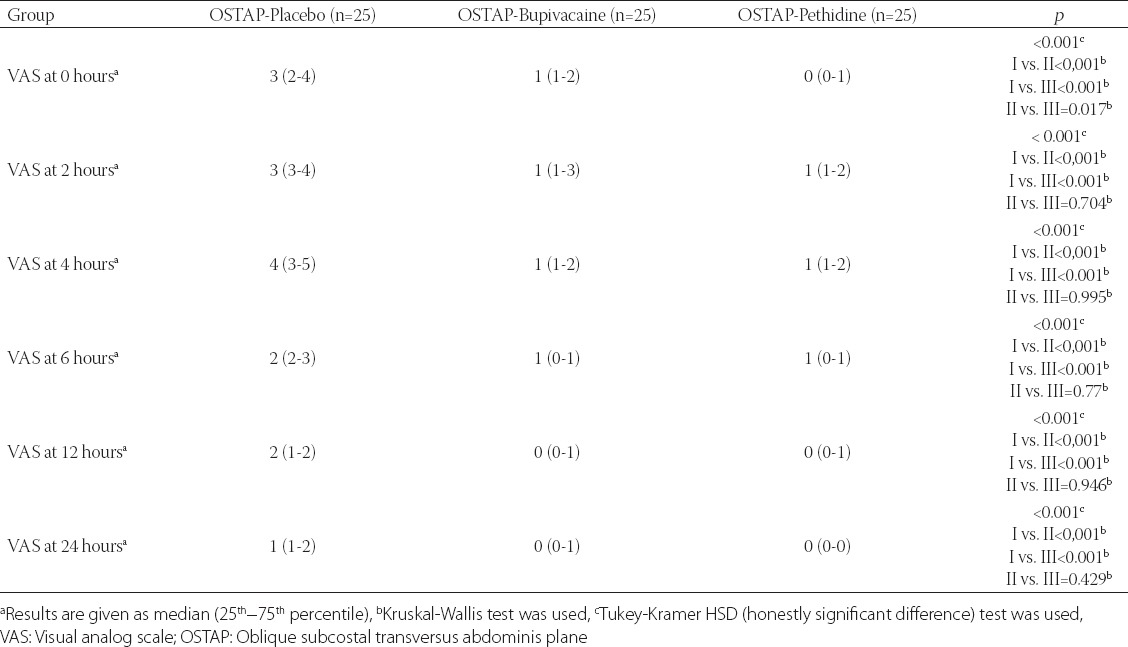
TABLE 3.
VAS pain scores in movement
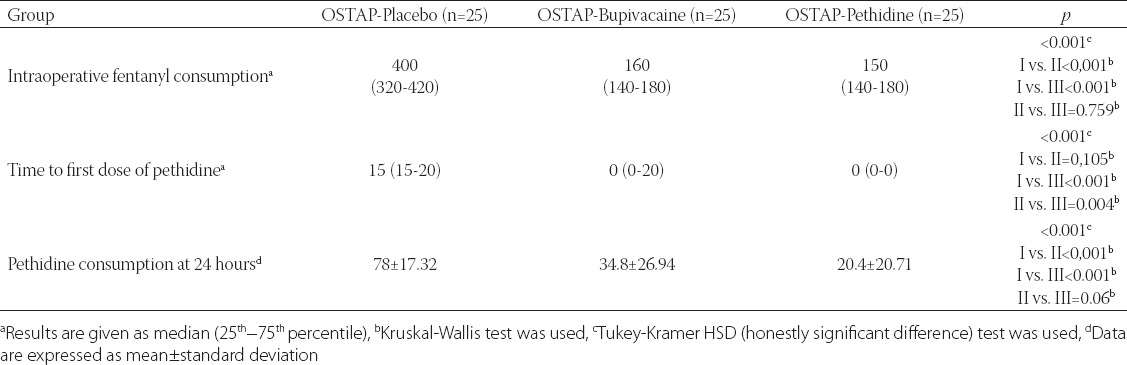
Intraoperative opioid consumption and opioid consumption at 24 hours were significantly lower in OSTAP-Pethidine and OSTAP-Bupivacaine groups compared to OSTAP-Placebo group (Table 4). The time to first opioid dose was significantly longer in OSTAP-Pethidine group compared with OSTAP-Placebo group (Table 4).
TABLE 4.
Intraoperative fentanyl consumption, time to first dose, and pethidine consumption at 24 hours
We found no difference in the incidence of postoperative nausea and vomiting in OSTAP-Bupivacaine and OSTAP-Pethidine groups compared with OSTAP-Placebo group. No complications or incidents were registered in the present study caused by the block. No side effects were reported due to peripheral administration of pethidine.
DISCUSSION
Our study evaluated prospectively the analgesic efficacy of pethidine in achieving the OSTAP block, in patients scheduled for elective laparoscopic cholecystectomy.
To our best knowledge, this is the first study in the literature that evaluated pethidine efficacy in achieving the OSTAP block and made a comparison with other local anesthetics, after laparoscopic cholecystectomy. The primary outcomes of the study were the pethidine consumption during the first 24 hours postoperatively and VAS pain scores. The secondary outcomes were the intraoperative fentanyl consumption and pethidine consumption in PACU, in all study groups.
The results of our study are consistent with previous research on the efficacy of ultrasound-guided OSTAP block in laparoscopic cholecystectomy interventions [13,24], in which bupivacaine was used for pain control. Our results showed that the efficacy of 1% pethidine was significantly higher than the efficacy of 0.25% bupivacaine for pain control in PACU, and that the efficacy of two anaesthetics was similar at 2, 4, 6, 12 and 24 hours, both at rest and in movement.
The analgesic effects of pethidine could be produced by at least three mechanisms: Systemic absorption, interaction with peripheral opioid receptors, and local anesthetic effects. Even though this was not a pharmacodynamic study, we can hypothesize [21,25] that the first two mechanisms (the systemic absorption and the interaction with peripheral opioid receptors) are responsible the most for the pain score results in the PACU. The third mechanism, local anesthetic effect of pethidine, may be responsible for the analgesic effect up to 24 hours, similar to the effect of 0.25% bupivacaine.
Our study also demonstrated significantly lower intraoperative opioid consumption, reduced pain scores in the first 24 hours after the surgery, and significantly lower opioid consumption in the first 24 hours, in OSTAP-Pethidine and OSTAP-Bupivacaine groups compared to OSTAP-Placebo group.
We did not register any hemodynamic instability during anesthesia, or postoperative complications such as respiratory depression, nausea, or vomiting. It can be assumed that the absorption of pethidine from TAP into the blood circulation is lower, to the extent that the side effects are prevented.
There are several studies evaluating the efficacy of opioids in achieving peripheral blocks. Jabalameli et al. [22] compared the efficacy of pethidine, tramadol, and bupivacaine administered intraincisionally for postoperative analgesia after caesarean sections. They found that subcutaneous administration of pethidine or tramadol, compared to bupivacaine, improved analgesia and significantly decreased the need for morphine [22]. Onutu et al. [26] showed that wound infiltration with 1% pethidine during hip arthroplasty decreased morphine consumption in the first 24 postoperative hours and ensured analgesia in the first 6 hours, when added to multimodal analgesia. Söderlund et al. [25] showed that intra-articular administration of pethidine before operation resulted in analgesia due to both, an increase in plasma level of pethidine followed by its central analgesic effect, and peripheral effects of pethidine due to synovial vascularization. Matkap et al. evaluated the port site infiltration of tramadol during laparoscopic cholecystectomy interventions and concluded that it reduced pain scores and the incidence of postoperative nausea and vomiting [27]. Our data are in accordance with the above-mentioned studies on the use of opioids with local anesthetic properties, such as tramadol and pethidine, in achieving peripheral blocks.
A debated issue in medical literature is the frequency of postoperative nausea and vomiting after the TAP block. Some authors demonstrated a decreased frequency of these side effects after the TAP block, but other studies found no significant benefit of TAP block in reducing the side-effects [28,29]. Our study did not observed any statistically significant difference between the three groups in the incidence of postoperative nausea and vomiting.
This study has several limitations. As the block was performed after the induction of general anesthesia, it was not possible to estimate the block installation time or its extension. We did not determine the plasma concentration of pethidine, to differentiate between the systemic and local effects of pethidine. We did not use patient-controlled analgesia (PCA) pethidine due to the shortage of pumps and also because of the reduced postoperative opioid requirements. Further studies are necessary to investigate the systemic absorption of pethidine injected into the transversus abdominis plane.
CONCLUSION
Our study showed that the use of pethidine in achieving the ultrasound-guided OSTAP block is an efficient alternative to conventional local anesthetics (e.g., bupivacaine) in laparoscopic cholecystectomy interventions. This alternative may be useful in patients with allergic reactions to local anesthetics and with increased analgesic requirements.
DECLARATION OF INTERESTS
Preliminary data of this study were published as an abstract at Euroanaesthesia 2016 (The European Anaesthesiology Congress).
REFERENCES
- [1].Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: A critical assessment of the evidence. Anesthesiology. 2006;104(4):835–46. doi: 10.1097/00000542-200604000-00030. http://dx.doi.org/10.1097/00000542-200604000-00030. [DOI] [PubMed] [Google Scholar]
- [2].Ekstein P, Szold A, Sagie B, Werbin N, Klausner JM, Weinbroum AA. Laparoscopic surgery may be associated with severe pain and high analgesia requirements in the immediate postoperative period. Ann Surg. 2006;243(1):41–6. doi: 10.1097/01.sla.0000193806.81428.6f. http://dx.doi.org/10.1097/01.sla.0000193806.81428.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kum CK, Wong CW, Goh PM, Ti TK. Comparative study of pain level and analgesic requirement after laparoscopic and open cholecystectomy. Surg Laparosc Endosc. 1994;4(2):139–41. [PubMed] [Google Scholar]
- [4].Tihan D, Totoz T, Tokocin M, Ercan G, Koc TC, Vartanoglu T, et al. Efficacy of laparoscopic transversus abdominis plane block for elective laparoscopic cholecystectomy in elderly patients. Bosn J Basic Med Sci. 2016;16(2):139–44. doi: 10.17305/bjbms.2015.841. http://dx.doi.org/10.17305/bjbms.2016.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hong D, Flood P, Diaz G. The side effects of morphine and hydromorphone patient - controlled analgesia. Anesth Analg. 2008;107(4):1384–9. doi: 10.1213/ane.0b013e3181823efb. http://dx.doi.org/10.1213/ane.0b013e3181823efb. [DOI] [PubMed] [Google Scholar]
- [6].Erol DD, Yilmaz S, Polat C, Arikan Y. Efficacy of thoracic epidural analgesia for laparoscopic cholecystectomy. Adv Ther. 2008;25(1):45–52. doi: 10.1007/s12325-008-0005-2. http://dx.doi.org/10.1007/s12325-008-0005-2. [DOI] [PubMed] [Google Scholar]
- [7].Boddy AP, Mehta S, Rhodes M. The effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy-a systemic review and meta-analysis. Anesth Analg. 2006;103(3):682–8. doi: 10.1213/01.ane.0000226268.06279.5a. http://dx.doi.org/10.1213/01.ane.0000226268.06279.5a. [DOI] [PubMed] [Google Scholar]
- [8].Rafi AN. Abdominal field block: A new approach via the lumbar triangle. Anaesthesia. 2001;56(10):1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. http://dx.doi.org/10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- [9].McDonnell JG, O’Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104(1):193–7. doi: 10.1213/01.ane.0000250223.49963.0f. http://dx.doi.org/10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- [10].Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35(4):616–7. [PubMed] [Google Scholar]
- [11].El-Dawlatly AA, Turkistani A, Kettner SC, Machata AM, Delvi MB, Thallaj A, et al. Ultrasound-guided transversus abdominis plane block: Description of a new technique and comparison with conventional systemic analgesia during laparoscopic cholecystectomy. Br J Anaesth. 2009;102(6):763–7. doi: 10.1093/bja/aep067. http://dx.doi.org/10.1093/bja/aep067. [DOI] [PubMed] [Google Scholar]
- [12].Ra YS, Kim CH, Lee GY, Han JI. The analgesic effect of the ultrasound-guided transverse abdominis plane block after laparoscopic cholecystectomy. Korean J Anesthesiol. 2010;58(4):362–8. doi: 10.4097/kjae.2010.58.4.362. http://dx.doi.org/10.4097/kjae.2010.58.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ripollés J, Mezquita SM, Abad A, Calvo J. Analgesic efficacy of the ultrasound-guided blockade of the transversus abdominis plane - a systematic review. [Article in Portuguese] Rev Bras Anestesiol. 2015;65(4):255–80. doi: 10.1016/j.bjan.2013.10.014. http://dx.doi.org/10.1016/j.bjan.2013.10.014. [DOI] [PubMed] [Google Scholar]
- [14].Beyazova M, Babacan A, Bilir E, Akcçabay M, Kaya K, Baysal AI. Perineural pethidine: Effects of different doses on nerve conduction. Eur J Anaesthesiol. 1993;10(5):353–6. [PubMed] [Google Scholar]
- [15].Armstrong PJ, Morton C, Nimmo F. Pethidine has a local anaesthetic action on peripheral nerves in vivo. Addition to prilocaine 0.25% for intravenous regional anaesthesia in volunteers. Anaesthesia. 1993;48(5):382–6. doi: 10.1111/j.1365-2044.1993.tb07008.x. http://dx.doi.org/10.1111/j.1365-2044.1993.tb07008.x. [DOI] [PubMed] [Google Scholar]
- [16].Cunha TM, Souza GR, Domingues AC, Carreira EU, Lotufo CM, Funez MI, et al. Stimulation of peripheral Kappa opioid receptors inhibits inflammatory hyperalgesia via activation of the PI3Kg/AKT/nNOS/NO signaling pathway. Mol Pain. 2012;8:10. doi: 10.1186/1744-8069-8-10. http://dx.doi.org/10.1186/1744-8069-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wagner LE, 2nd, Eaton M, Sabnis SS, Gingrich KJ. Meperidine and lidocaine block of recombinant voltage-dependent Na+channels: Evidence that meperidine is a local anesthetic. Anesthesiology. 1999;91(5):1481–90. doi: 10.1097/00000542-199911000-00042. http://dx.doi.org/10.1097/00000542-199911000-00042. [DOI] [PubMed] [Google Scholar]
- [18].Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332(25):1685–90. doi: 10.1056/NEJM199506223322506. http://dx.doi.org/10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- [19].Wood A. New method of treating neuralgia by the direct application of opiates to the painful points. Edinburgh Med Surg J. 1955;82:265. [PMC free article] [PubMed] [Google Scholar]
- [20].Hong JY, Lee IH. Comparison of the effects of intrathecal morphine and pethidine on shivering after Caesarean delivery under combined-spinal epidural anaesthesia. Anaesthesia. 2005;60(12):1168–72. doi: 10.1111/j.1365-2044.2005.04158.x. http://dx.doi.org/10.1111/j.1365-2044.2005.04158.x. [DOI] [PubMed] [Google Scholar]
- [21].Hashemi SJ, Soltani H, Heidari SM, Rezakohanfekr M. Preemptive analgesia with intra-articular pethidine reduces pain after arthroscopic knee surgery. Adv Biomed Res. 2013;2:9. doi: 10.4103/2277-9175.107971. http://dx.doi.org/10.4103/2277-9175.107971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jabalameli M, Safavi M, Honarmand A, Saryazdi H, Moradi D, Kashefi P. The comparison of intraincisional injection tramadol, pethidine and bupivacaine on postcesarean section pain relief under spinal anesthesia. Adv Biomed Res. 2012;1:53. doi: 10.4103/2277-9175.100165. http://dx.doi.org/10.4103/2277-9175.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petersen PL, Stjernholm P, Kristiansen VB, Torup H, Hansen EG, Mitchell AU, et al. The beneficial effect of transversus abdominis plane block after laparoscopic cholecystectomy in day-case surgery: A randomized clinical trial. Anesth Analg. 2012;115(3):527–33. doi: 10.1213/ANE.0b013e318261f16e. http://dx.doi.org/10.1213/ane.0b013e318261f16e. [DOI] [PubMed] [Google Scholar]
- [24].Tolchard S, Davies R, Martindale S. Efficacy of the subcostal transversus abdominis plane block in laparoscopic cholecystectomy: Comparison with conventional port-site infiltration. J Anaesthesiol Clin Pharmacol. 2012;28(3):339–43. doi: 10.4103/0970-9185.98331. http://dx.doi.org/10.4103/0970-9185.98331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Söderlund A, Boreus LO, Westman L, Engström B, Valentin A, Ekblom A. A comparison of 50, 100 and 200 mg of intra-articular pethidine during knee joint surgery, a controlled study with evidence for local demethylation to norpethidine. Pain. 1999;80(1-2):229–38. doi: 10.1016/s0304-3959(98)00207-3. http://dx.doi.org/10.1016/S0304-3959(98)00207-3. [DOI] [PubMed] [Google Scholar]
- [26].Onutu AH, Iacob IM, Todor A, Lucaciu DO, Acalovschi I. Wound infiltration with 1% pethidine provides an opioid-sparing effect after uncemented total hip arthroplasty: A prospective randomized study. Rom J Anesth Int Care. 2012;19(1):5–12. [Google Scholar]
- [27].Matkap E, Bedirli N, Akkaya T, Gümüş H. Preincisional local infiltration of tramadol at the trocar site versus intravenous tramadol for pain control after laparoscopic cholecystectomy. J Clin Anesth. 2011;23(3):197–201. doi: 10.1016/j.jclinane.2010.08.010. http://dx.doi.org/10.1016/j.jclinane.2010.08.010. [DOI] [PubMed] [Google Scholar]
- [28].Siddiqui MR, Sajid MS, Uncles DR, Cheek L, Baig MK. A meta-analysis on the clinical effectiveness of transversus abdominis plane block. J Clin Anesth. 2011;23(1):7–14. doi: 10.1016/j.jclinane.2010.05.008. http://dx.doi.org/10.1016/j.jclinane.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [29].Yu N, Long X, Lujan-Hernandez JR, Succar J, Xin X, Wang X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14:121. doi: 10.1186/1471-2253-14-121. http://dx.doi.org/10.1186/1471-2253-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]


